Table of Contents
Mucinous metaplasia | Paneth cell-like change | Squamous metaplasia | Urothelial metaplasia | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Iczkowski KA. Nonneoplastic metaplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/prostatenonneoplasticmet.html. Accessed December 22nd, 2024.
Mucinous metaplasia
Definition / general
Essential features
Epidemiology
Sites
Pathophysiology
Etiology
Clinical features
Diagnosis
Treatment
Microscopic (histologic) description
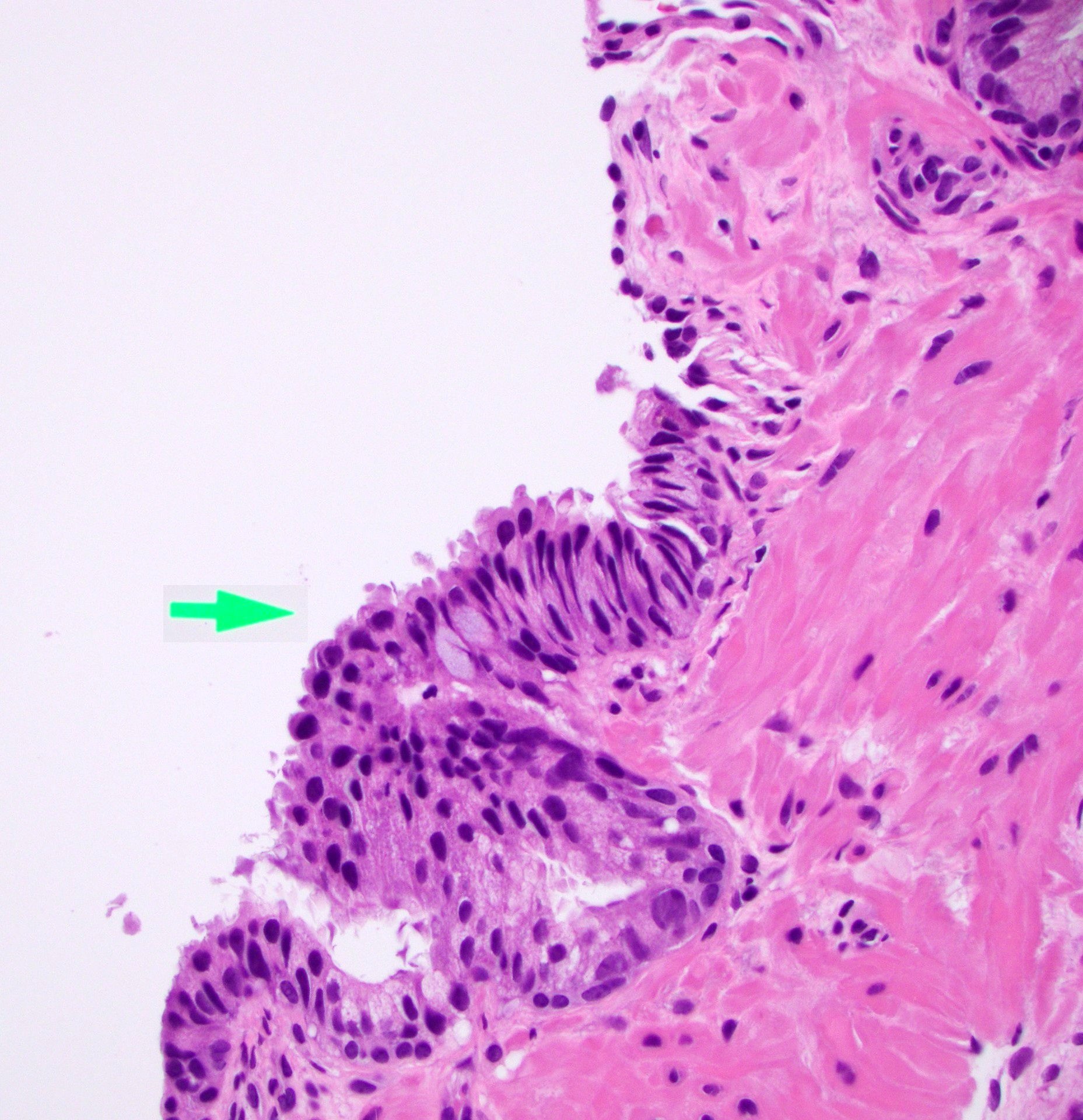
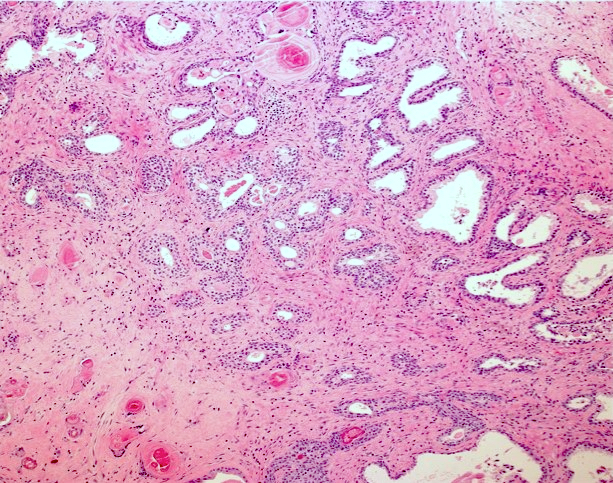
Microscopic (histologic) images
Contributed by Kenneth A. Iczkowski, M.D.
Positive stains
Negative stains
Differential diagnosis
Additional references
- Mucinous metaplasia is a form of glandular differentiation
Essential features
- Acini with tall, mucin filled cells with flat, basal pyknotic nuclei
- May occur as a group of glands or single cells within glands
- More common in the periurethral area
- May mimic cancer but not associated with malignancy
- Resembles Cowper glands
Epidemiology
- One autopsy study described a frequency of mucinous metaplasia of ~1% (Am J Surg Pathol 1993;17:618)
- However, in another study with entirely sampled prostates, up to ~50% of cases showed a focus of it (Br J Urol 1996;77:113)
- In 2 cases, mucinous metaplasia was associated with postatrophic hyperplasia (Am J Surg Pathol 1995;19:1068)
Sites
- Prostate
Pathophysiology
- Pathophysiology and etiology are unknown
Etiology
- Mucinous metaplasia in 21% of prostatic biopsies after radiation therapy (Am J Surg Pathol 1999;23:1021)
Clinical features
- The only clinical significance is that it may mimic cancer in a needle biopsy
Diagnosis
- Mucin filled vacuoles distend the cytoplasm of benign cells
Treatment
- Not required
- Mucinous metaplasia is a morphologic variant of prostatic acini
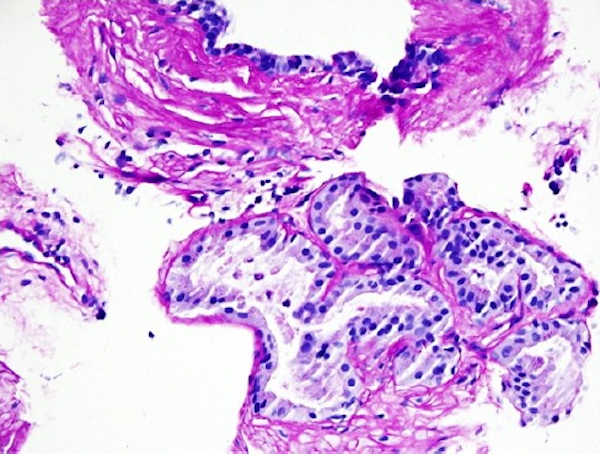
Microscopic (histologic) description
- Acini with tall mucin filled cells with flat, basal pyknotic nuclei
Microscopic (histologic) images
Contributed by Kenneth A. Iczkowski, M.D.
Positive stains
Negative stains
- Cytokeratins and prostatic markers PSA, PSAP in the mucinous cells (Br J Urol 1996;77:113)
Differential diagnosis
- Tall columnar cells bear a resemblance to prostatic ductal adenocarcinoma:
- In contrast to adenocarcinoma, mucinous metaplasia does not show an infiltrative growth pattern, usually does not display prominent nucleoli and is positive for basal cell markers p63 and high molecular weight cytokeratin by immunohistochemistry
Additional references
Paneth cell-like change
Definition / general
Essential features
Terminology
Epidemiology
Pathophysiology
Etiology
Clinical features
Diagnosis
Laboratory
Case reports
Treatment
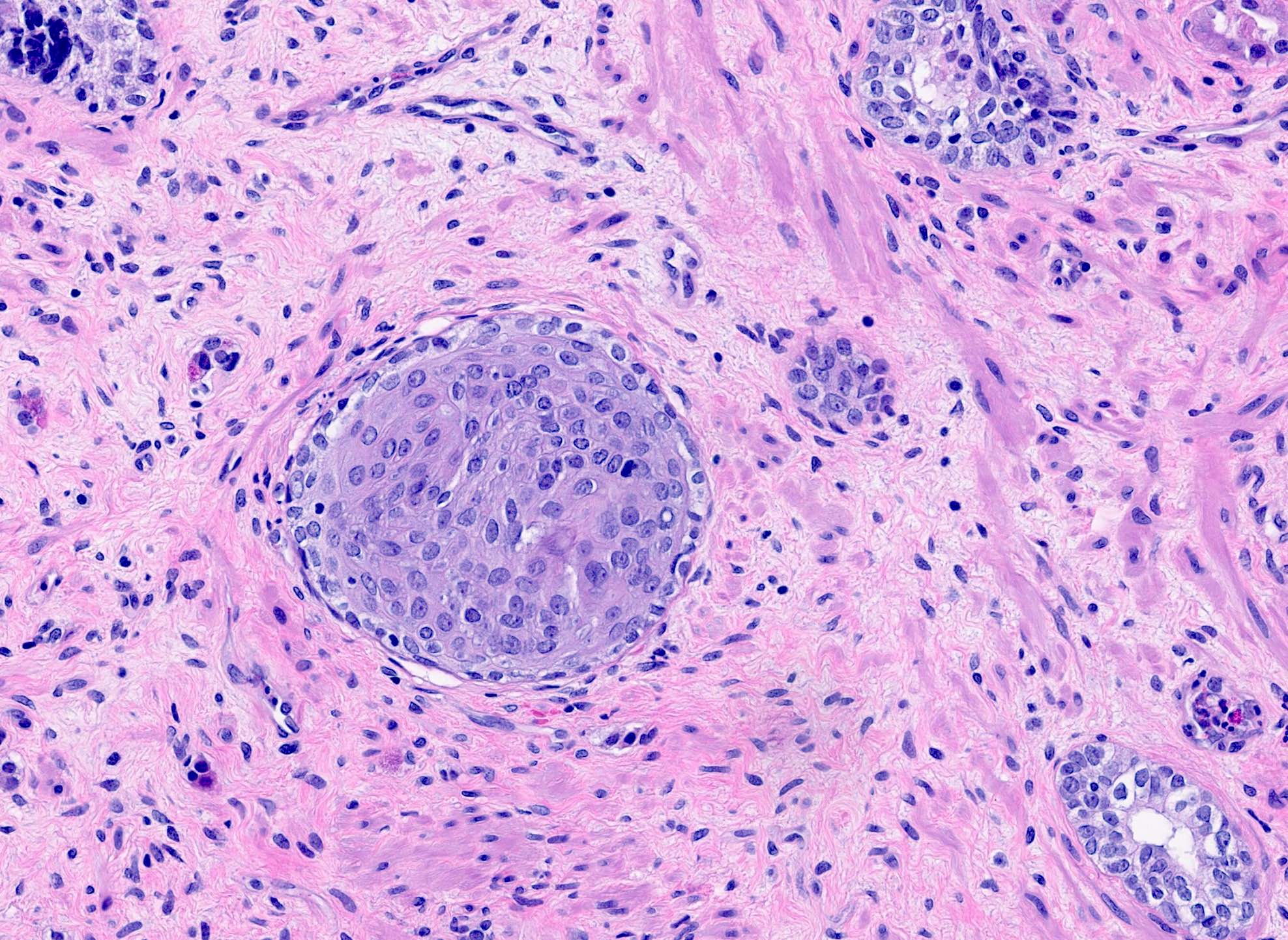
Microscopic (histologic) description
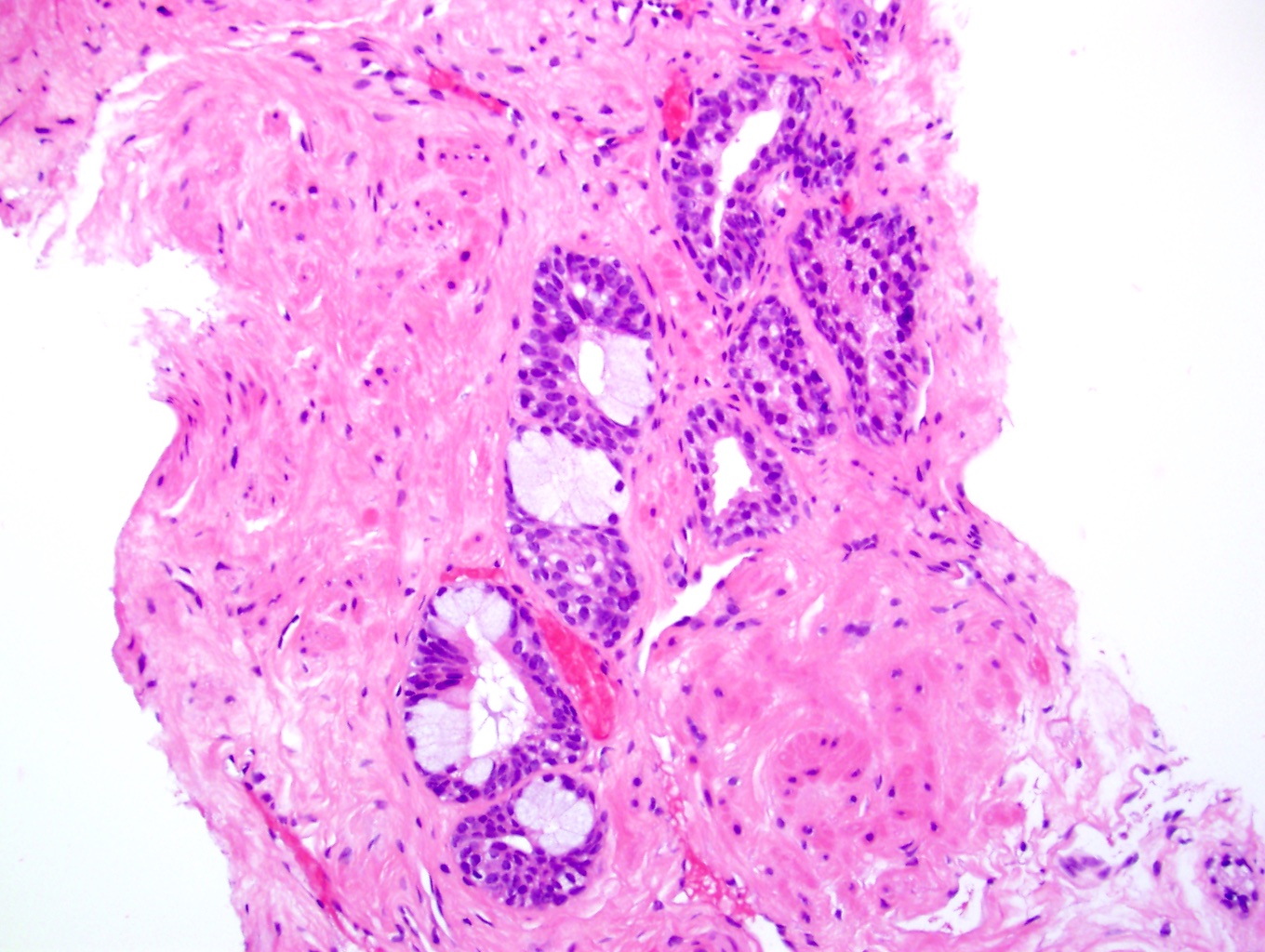
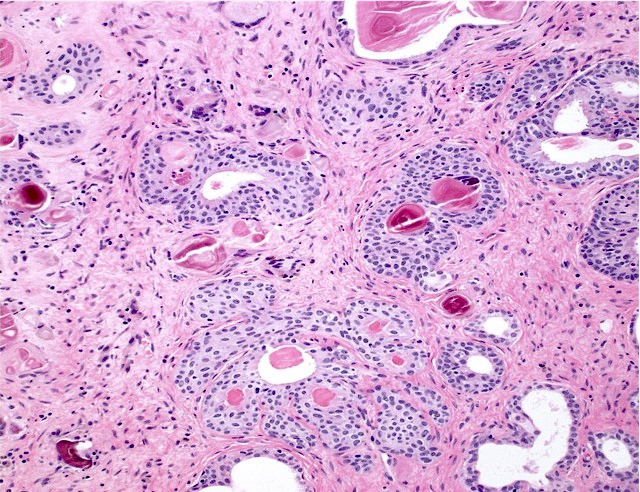
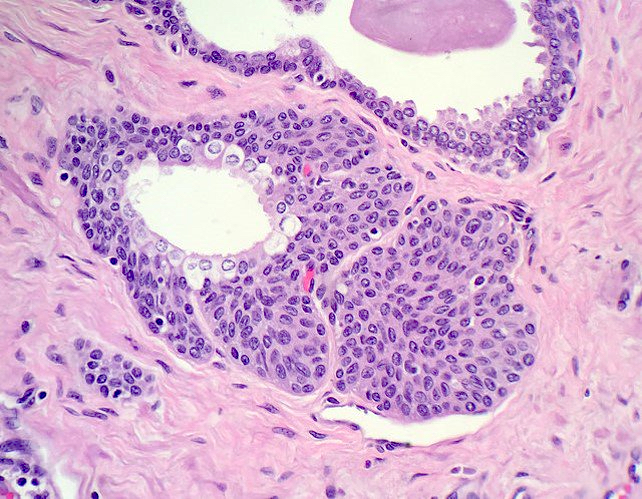
Microscopic (histologic) images
Contributed by Kenneth A. Iczkowski, M.D.
Positive stains
Negative stains
Electron microscopy description
Differential diagnosis
- Associated with both benign and malignant lesions (Arch Pathol Lab Med 1992;116:274)
- Collections of prostatic cells with eosinophilic granules resembling intestinal Paneth cells (Am J Surg Pathol 1992;16:1013, Int J Clin Exp Pathol 2014;7:3454)
- Represents either (a) PAS positive and diastase resistant eosinophilic cytoplasmic granular change in benign prostatic epithelium or (b) endocrine differentiation with neuroendocrine granules in dysplastic and malignant prostatic epithelium (Am J Surg Pathol 1992;16:62, Hum Pathol 1994;25:135, Am J Surg Pathol 2006;30:980)
Essential features
- Small focus of cells with bright pink granules in cytoplasm
- No cytologic atypia
Terminology
- Eosinophilic metaplasia (Prostate 2019;79:622, Indian J Pathol Microbiol 2017;60:409)
Epidemiology
- Noted in 32% of prostatic biopsies after irradiation (Am J Surg Pathol 1999;23:1021)
- Another study found it in ~20% of both biopsy and prostatectomy cases (Anal Quant Cytol Histol 2008;30:226)
- In transurethral resections, it may be as high as 56% (Indian J Pathol Microbiol 2020;63:423)
Pathophysiology
- It is a reactive change of the prostatic epithelium, often seen with inflammation
Etiology
- Radiation therapy
- Granulomatous prostatitis
- Possibly a phenotypic adaptive mechanism directed against intraluminal antigens or lipids (APMIS 2005;113:564, Indian J Pathol Microbiol 2017;60:409)
Clinical features
- Hormone therapy, radiotherapy, inflammation
Diagnosis
- Morphology
Laboratory
- Serum prostate specific antigen (PSA) is elevated if associated with inflammation or benign prostatic hyperplasia (BPH)
Case reports
- 49 and 52 year old men with serum PSAs of 4 - 5 ng/mL and incidental findings in benign biopsies (Int J Clin Exp Pathol 2014;7:3454)
- 68 year old man with nonspecific granulomatous prostatitis in association with eosinophilic epithelial metaplasia and prostatic adenocarcinoma (Indian J Pathol Microbiol 2017;60:409)
- 71 year old man with serum PSA of 10 ng/mL in association with nonspecific granulomatous prostatitis and BPH (APMIS 2005;113:564)
Treatment
- Not required
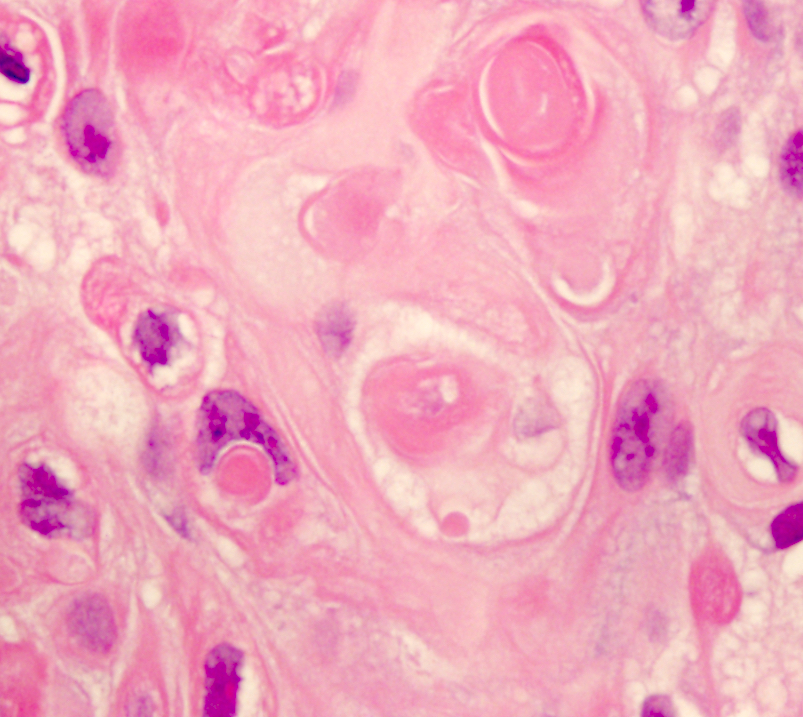
Microscopic (histologic) description
- Nests of squamous epithelial cells with eosinophilic cytoplasm and focal keratin
- Mitoses may be seen but should not be frequent
Microscopic (histologic) images
Contributed by Kenneth A. Iczkowski, M.D.
Positive stains
- P504S (also called AMACR) marker has been reported as strongly positive, a finding that overlaps with cancer, high grade prostatic intraepithelial neoplasia (HGPIN) and nephrogenic adenoma (Int J Clin Exp Pathol 2014;7:3454)
- PAS+, diastase resistant (benign epithelium)
- Neuroendocrine markers (in malignant epithelium), PSA (Am J Surg Pathol 1992;16:62)
- MUC1 (Prostate 2019;79:622)
Negative stains
- Lysozyme
- Neuroendocrine markers (in benign epithelium) (Am J Surg Pathol 1992;16:62)
Electron microscopy description
- Large exocrine type or lysosomal-like electron dense granules in the apical cytoplasm of benign epithelium that measure 240 - 480 nm (Anal Quant Cytol Histol 2008;30:226, APMIS 2005;113:564)
Differential diagnosis
- Possible differential diagnosis is prostatic adenocarcinoma with Paneth cell differentiation:
- Those lesions have infiltrating, angulated glands, often with collapsed gland lumina, although they tend to be of low grade and low stage (Hum Pathol 2020;102:7)
- In contrast, benign Paneth cell metaplasia has well rounded, uniform and circumscribed groups of glands without atypia
- Cancer of no specific type:
- Also has strong P504S reactivity but would have atypia and lack a granular pink cytoplasm
Squamous metaplasia
Definition / general
Essential features
Epidemiology
Pathophysiology
Etiology
Diagnosis
Treatment
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Kenneth A. Iczkowski, M.D.
Positive stains
Negative stains
Differential diagnosis
Additional references
- Squamous metaplasia is usually an incidental finding in the setting of infarction in the nodules of benign prostatic hyperplasia (BPH) or secondary to treatment (hormonal therapy and radiation)
Essential features
- Usually an incidental finding in the setting of inflammation, acute infarction of benign prostatic hyperplasia or secondary to hormone / radiation therapy or following prostatic artery embolization therapy for benign prostatic hyperplasia
- Can present with cytologic atypia that is reactive to the underlying process
- Key to avoiding misinterpretation as cancer is to identify the underlying cause (i.e., adjacent hemorrhage or obvious necrosis indicating infarction, clinical history of hormone therapy or radiation)
Epidemiology
- ~0.3% incidence in unselected series (Urol J 2009;6:109)
- Seen in transurethral resection of the prostate (TURP) specimens, some with benign prostatic hyperplasia, particularly with infarct of nodules of BPH; also associated with radiation
- Feminizing hormone therapy for gender transition (Arch Pathol Lab Med 2022;146:252)
Pathophysiology
- It is a reactive change of the prostatic epithelium
Etiology
- Squamous metaplasia was found in 6% of prostatic biopsies after radiation therapy (Am J Surg Pathol 1999;23:1021)
- Also with ischemia / infarction, hormone therapy, inflammation, schistosomiasis (Neoplasma 1985;32:613)
Diagnosis
- Cells are nonneoplastic without atypia
- They should have a pavemented configuration with crisp cell borders, intracellular keratin, extracellular keratin pearls or intercellular bridges (tonofilaments) visible on high power
Treatment
- Not required
Microscopic (histologic) description
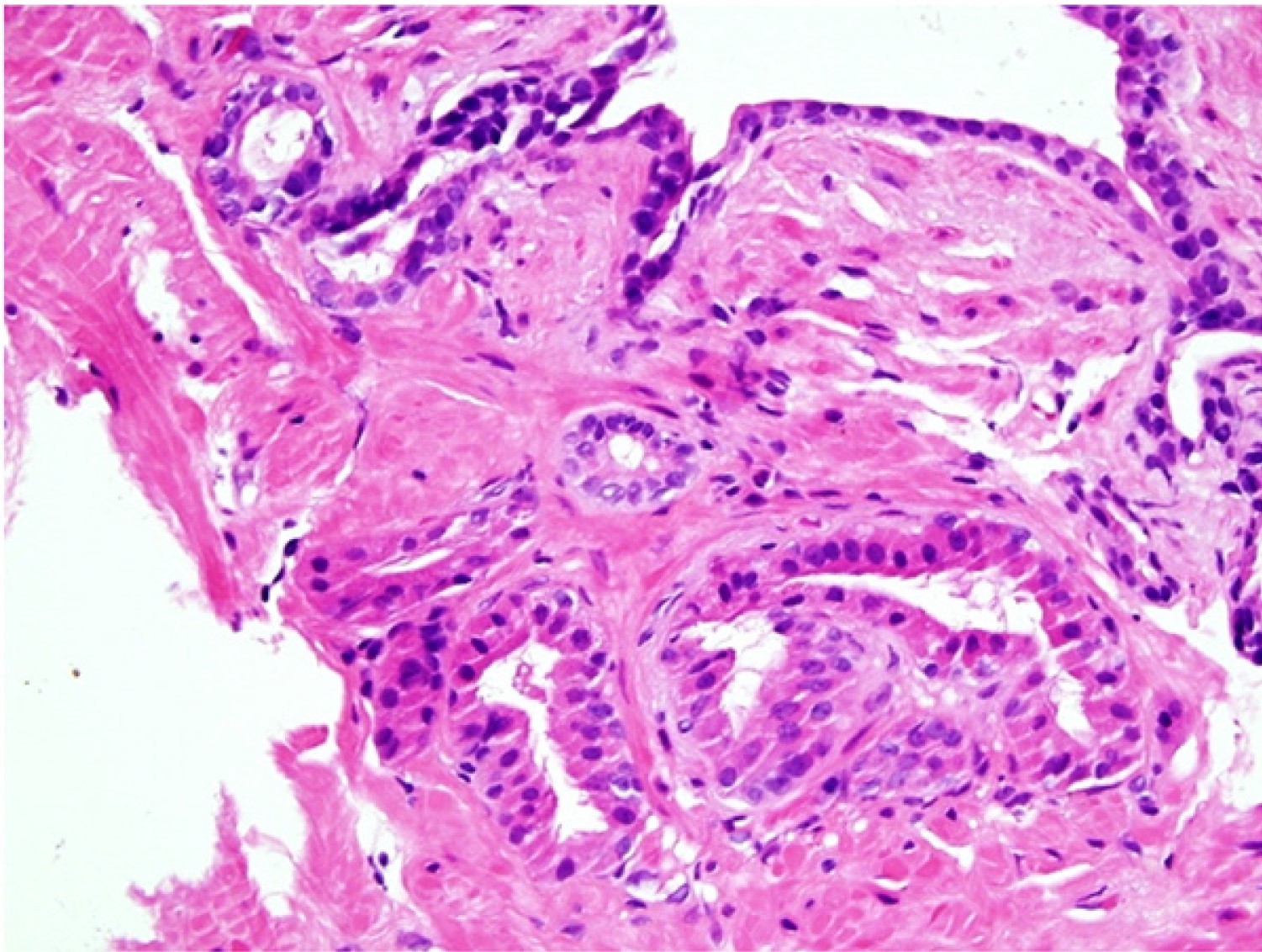
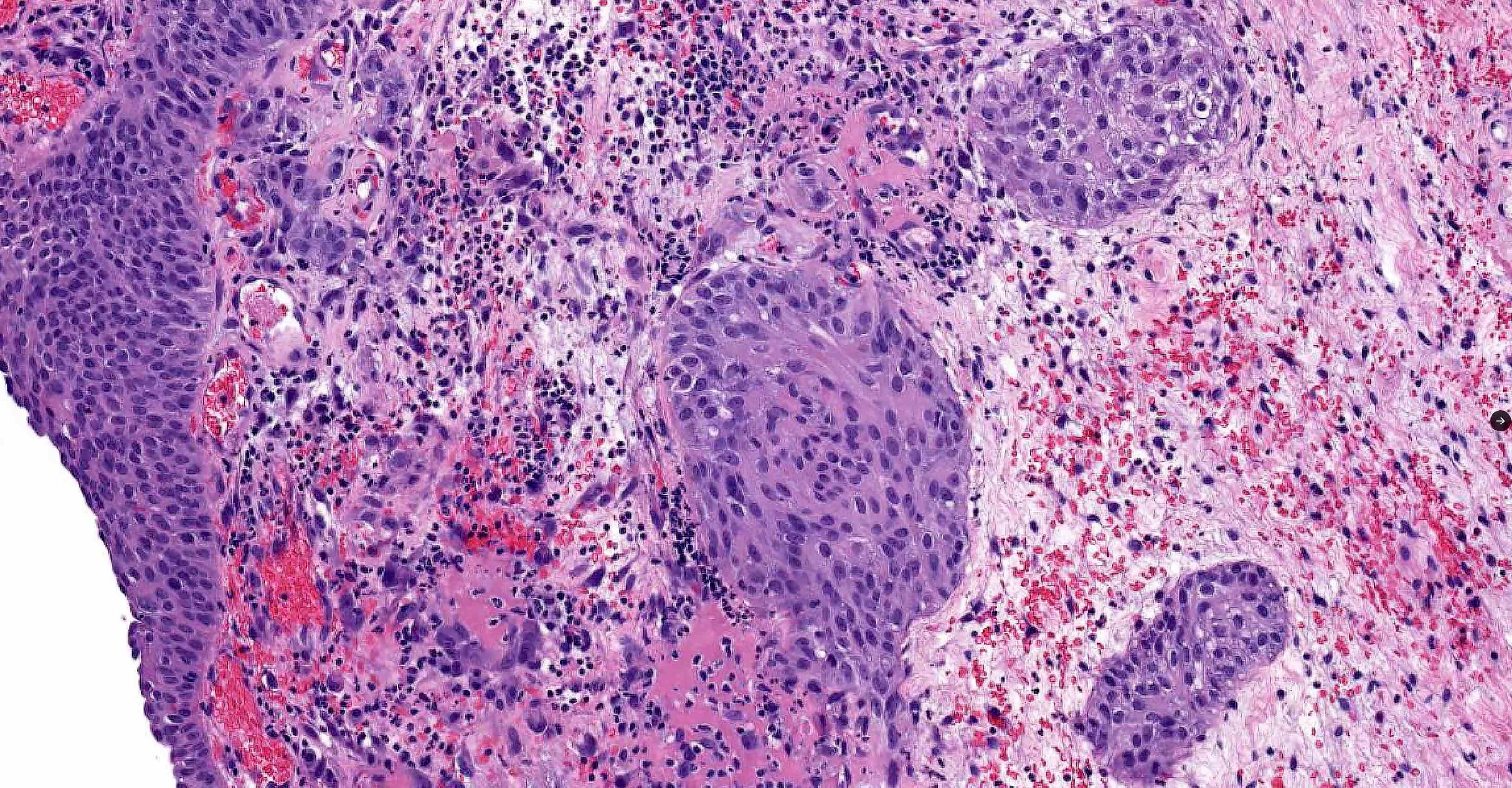
- Nests of squamous epithelial cells with eosinophilic cytoplasm and focal keratinization
- Mitoses can be seen but should not be frequent
Microscopic (histologic) images
Contributed by Kenneth A. Iczkowski, M.D.
Positive stains
Negative stains
Differential diagnosis
- Main differential diagnoses are squamous cell carcinoma and urothelial carcinoma
- Differential diagnosis is based on the degree of cytologic atypia and the presence of stromal invasion
- Squamous cell carcinoma has intercellular bridges and intracellular or extracellular keratin while urothelial carcinoma does not
Additional references
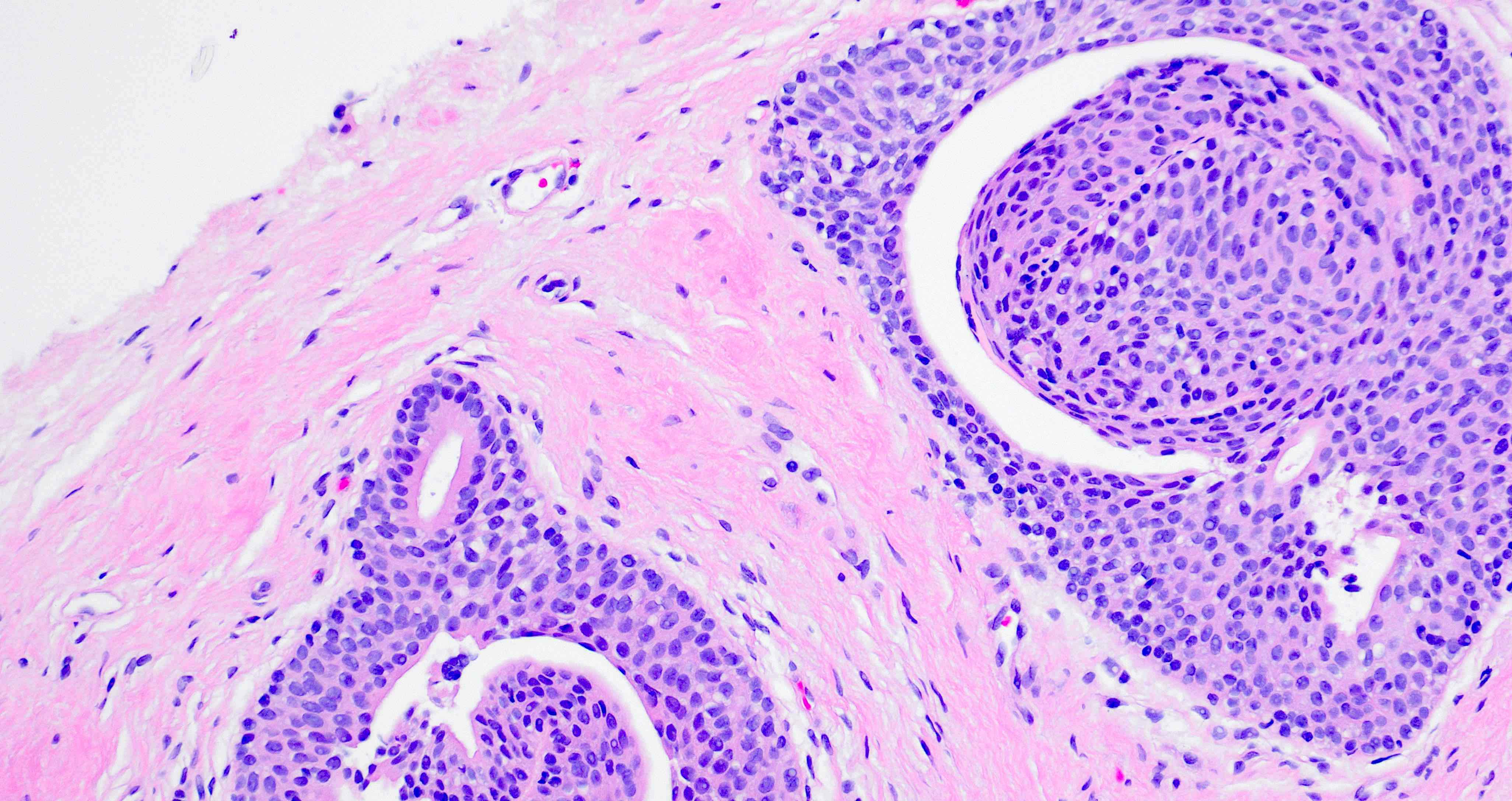
Urothelial metaplasia
Definition / general
Essential features
Epidemiology
Sites
Clinical features
Diagnosis
Treatment
Gross description
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Andres Matoso, M.D., Ziad El-Zaatari, M.D. and Kenneth A. Iczkowski, M.D.
Positive stains
Negative stains
Differential diagnosis
- Urothelium normally lines the glands and ducts of the prostate
Essential features
- Epithelium contains oval or spindle cells with occasional nuclear grooves
- Urothelial cells may be located underneath the prostatic luminal epithelium
- Prostatic ducts may also show the spread of urothelial carcinoma but in contrast to urothelial metaplasia, carcinoma has high grade nuclear atypia, similar to carcinoma in situ in the bladder
Epidemiology
- Urothelial metaplasia is an exceedingly common finding, at any age
Sites
- Peripheral prostate acini and ducts
Clinical features
- Urothelial metaplasia is a histologic finding and has no clinical manifestations
Diagnosis
- Diagnosis of urothelial metaplasia is morphologic
Treatment
- Not required
Gross description
- Urothelial metaplasia is not seen grossly
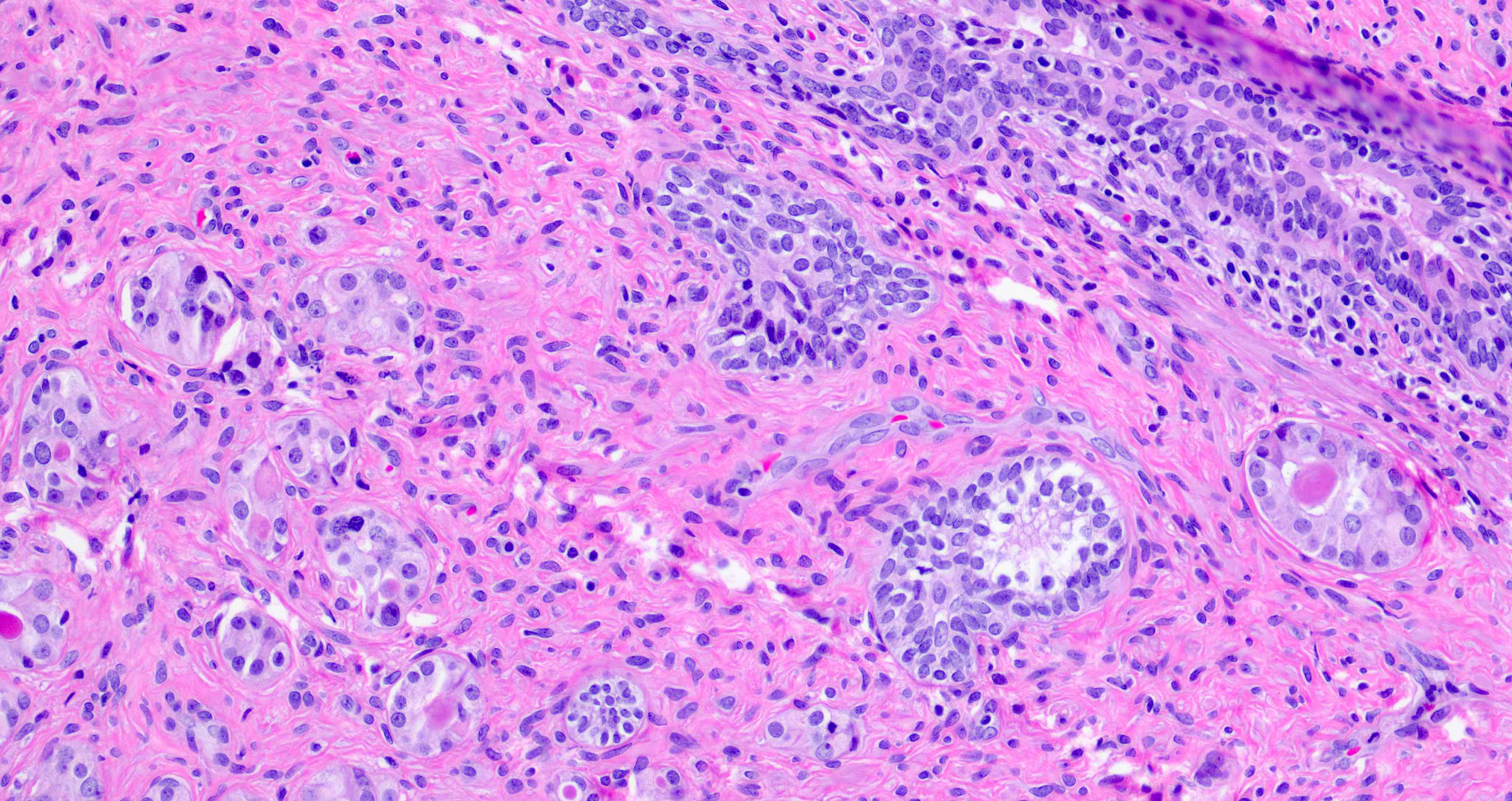
Microscopic (histologic) description
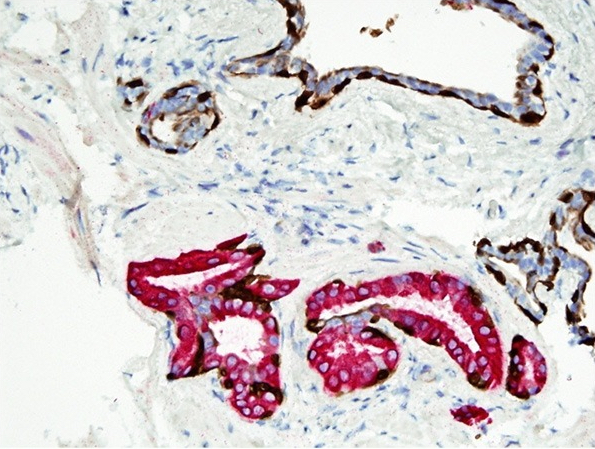
- Epithelium is multilayered, with oval or spindle cells with occasional nuclear grooves (Ann Diagn Pathol 2019;38:11)
- Focally, the cells may be oriented parallel to the basement membrane
- Urothelial cells may be located underneath a prostatic luminal epithelium
Microscopic (histologic) images
Contributed by Andres Matoso, M.D., Ziad El-Zaatari, M.D. and Kenneth A. Iczkowski, M.D.
Positive stains
Negative stains
Differential diagnosis
- Urothelial carcinoma spreading in prostatic ducts:
- In contrast to urothelial metaplasia, urothelial carcinoma is characterized by high grade nuclear atypia
- Intraductal prostatic adenocarcinoma:
- This is another multilayered epithelium but it would be solid to cribriform growth, with marked nuclear enlargement and prominent nucleoli, surrounded by basal cells
- High grade prostatic intraepithelial neoplasia:
- Multilayering of epithelium but usually with a tufted or micropapillary pattern
- Atypia is present but to a lesser degree than in intraductal prostatic carcinoma
- Urothelial metaplasia has been reported in the seminal vesicle (Ann Diagn Pathol 2012;16:145)
- In that location, golden-brown pigmented granules and a widely branched pattern of epithelium would be distinctive
Board review style question #1
Board review style answer #1
B. Paneth cell-like metaplasia. Paneth cell-like metaplasia is positive for P504S, similar to cancer. Answer A is incorrect because although mucinous metaplasia is PAS positive and diastase resistant, it is not positive for P504S (AMACR). Answer C is incorrect because squamous metaplasia is negative for both of these markers. Answer D is incorrect because urothelial metaplasia is also negative for these markers.
Comment Here
Reference: Nonneoplastic metaplasia
Comment Here
Reference: Nonneoplastic metaplasia
Board review style question #2
Board review style answer #2
C. Ischemia or infarction. The figure shows squamous metaplasia, which is the only metaplasia associated with ischemia or infarct. Answer A is incorrect because inflammation can be associated with Paneth cell-like metaplasia as well as with squamous metaplasia. Answer B is incorrect because granulomatous inflammation has been associated with Paneth cell-like metaplasia. Answer D is incorrect because radiotherapy can be associated with mucinous, Paneth cell-like or squamous metaplasias. Meanwhile, urothelial metaplasia has no specific associations and is very common.
Comment Here
Reference: Nonneoplastic metaplasia
Comment Here
Reference: Nonneoplastic metaplasia