Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Macias V, Kajdacsy-Balla A. Intraductal carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/prostateidc.html. Accessed April 1st, 2025.
Definition / general
- An intra-acinar or intraductal neoplastic epithelial proliferation that has some features of high grade prostatic intraepithelial neoplasia (HGPIN) but exhibits much greater architectural or cytological atypia, typically associated with high grade, high stage prostate carcinoma (Eur Urol 2016;70:106)
Essential features
- High degree of cytological atypia, commonly with necrosis that fills prostatic ducts and acini
- Usually associated with high grade and high stage invasive carcinoma
- Appropriate to use immunohistochemistry to demonstrate presence of basal cells
- Pure intraductal carcinoma of the prostate (IDC-P) without the presence of invasive carcinoma should not be graded (Adv Anat Pathol 2021;28:1)
- IDC-P should be noted when observed with invasive prostate cancer
- Incorporation of IDC-P into grading in cases with concomitant invasive carcinoma remains controversial (J Clin Transl Pathol 2023;3:26)
Terminology
- Intraductal carcinoma of the prostate (IDC-P): currently recognized term
- Ductal carcinoma in situ: not recommended
- Acinar carcinoma in situ: not recommended
- Low grade intraductal carcinoma: not recommended
ICD coding
- ICD-O: 8500/2 - intraductal carcinoma
- ICD-11: 2E67.5 & XH1H31 - carcinoma in situ of prostate & intraductal carcinoma, noninfiltrating, NOS
Epidemiology
- Present in ~14% of prostate biopsies and 15 - 30% of radical prostatectomy specimens with concurrent invasive carcinoma (Int J Clin Exp Pathol 2014;7:2518, Eur Urol 2017;72:492, Eur Urol 2022;82:469)
- Rare finding in biopsy or prostatectomy specimens without associated invasive disease (0.06 - 0.26%) (Mod Pathol 2006;19:1528, Arch Pathol Lab Med 2007;131:1122, J Urol 2010;184:1328, Histopathology 2013;63:574, Int J Clin Exp Pathol 2014;7:2518, J Pathol 2019;249:79)
Sites
- Prostate ducts and acini
Pathophysiology
- May represent 2 distinct entities (regular versus precursor-like) and 2 putative mechanisms of pathogenesis (Int J Clin Exp Pathol 2014;7:2518, J Pathol 2019;249:79, Int J Surg Pathol 2023;31:184, Eur Urol Focus 2021;7:955, Mod Pathol 2023;36:100130)
- Retrograde spread of invasive carcinoma into benign ducts and acini
- In situ transformation and de novo intraductal proliferation of malignant cells in benign ducts and acini
Etiology
- No known causes to date
Diagnosis
- Prostate core needle biopsy, prostatectomy or transurethral resection specimens
Laboratory
- Elevated prostate specific antigen (PSA)
Radiology description
- In situ disease not identified by imaging; microscopic diagnosis only
Prognostic factors
- Associated with higher Gleason grade (patterns 4 or 5) invasive prostatic acinar adenocarcinoma (Urol Oncol 2017;35:673.e9, Pathologica 2020;112:17)
- Associated with large tumor volume (J Urol 2010;184:1328, Eur Urol Oncol 2018;1:21)
- Associated with higher stage, including extraprostatic extension and seminal vesicle invasion (Urol Oncol 2017;35:673.e9, Pathologica 2020;112:17)
- Higher rate of recurrence compared to same grade grouping without IDC-P (Virchows Arch 2019;474:525, Prostate 2019;79:1065)
- Associated with lower biochemical recurrence free survival and cancer specific survival in localized prostate cancer (J Urol 2020;204:909)
- Associated with lower overall survival in advanced prostate cancer (J Urol 2020;204:909, Asian J Androl 2021;23:103)
- Higher risk of prostate cancer mortality in localized and metastatic disease when IDC-P is present on diagnostic biopsy (Prostate 2017;77:859)
Case reports
- 55 year old man with elevated PSA and 80 year old man with hematuria and bladder tumor detected under cystoscopy (Int J Surg Pathol 2020;28:918)
- 56 year old man with firm right lobe of prostate and PSA of 2.4 ng/mL (Arch Pathol Lab Med 2007;131:1122)
- 2 patients with primary prostatic adenocarcinoma, metastasis and intraductal carcinoma (Eur Urol 2015;67:819)
Treatment
- Immediate rebiopsy (within 3 months) or definitive treatment by radiation or radical prostatectomy if only intraductal tumor is present (Yonsei Med J 2016;57:1054, Virchows Arch 2019;474:525, Radiat Oncol 2019;14:60, Eur Urol Focus 2021;7:955)
- Patients with diagnostic IDC-P biopsy should be excluded from active surveillance (J Urol 2020;204:909, Eur Urol 2021;79:243, J Urol 2020;203:311)
Gross description
- Not seen on gross examination
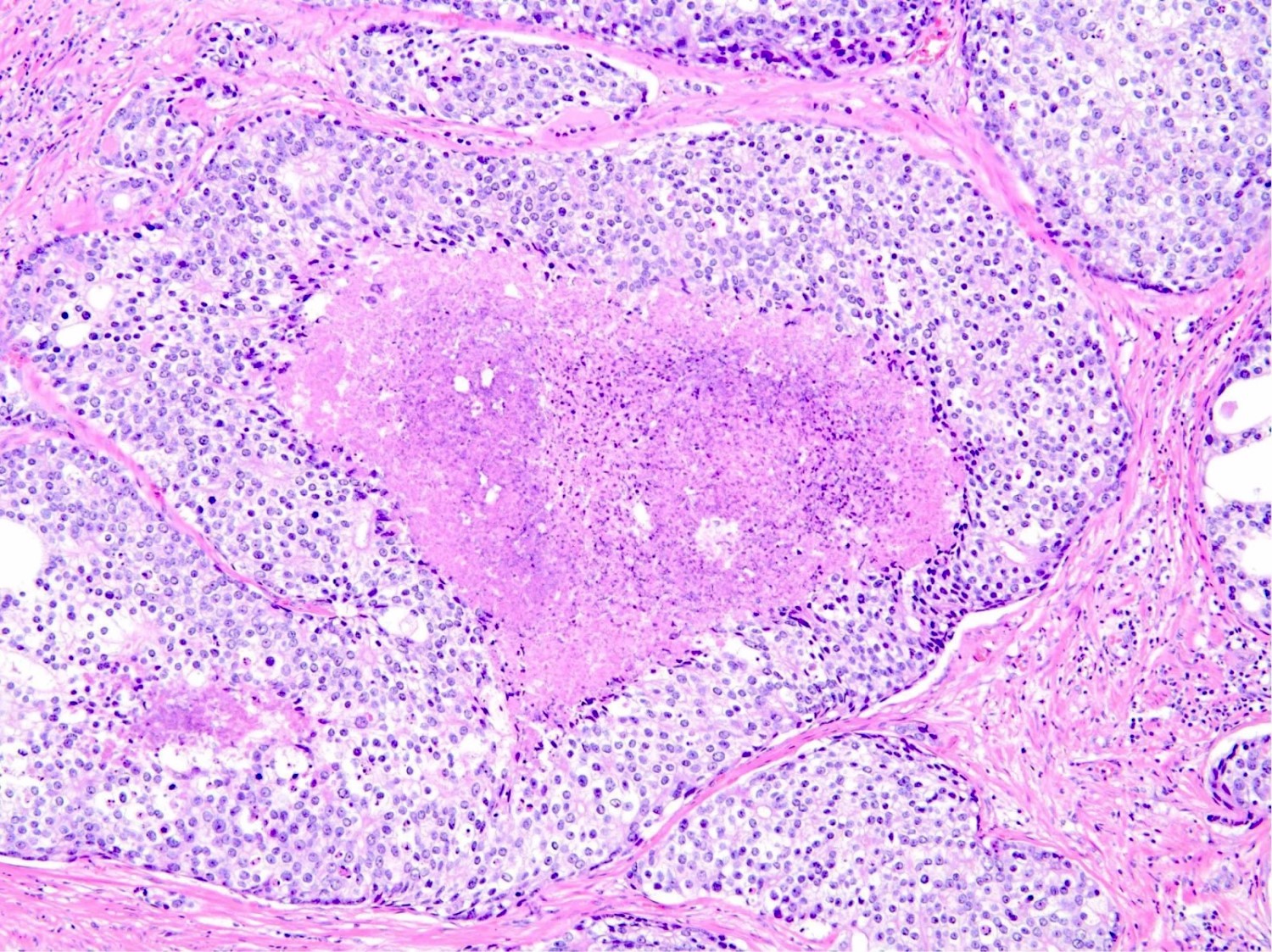
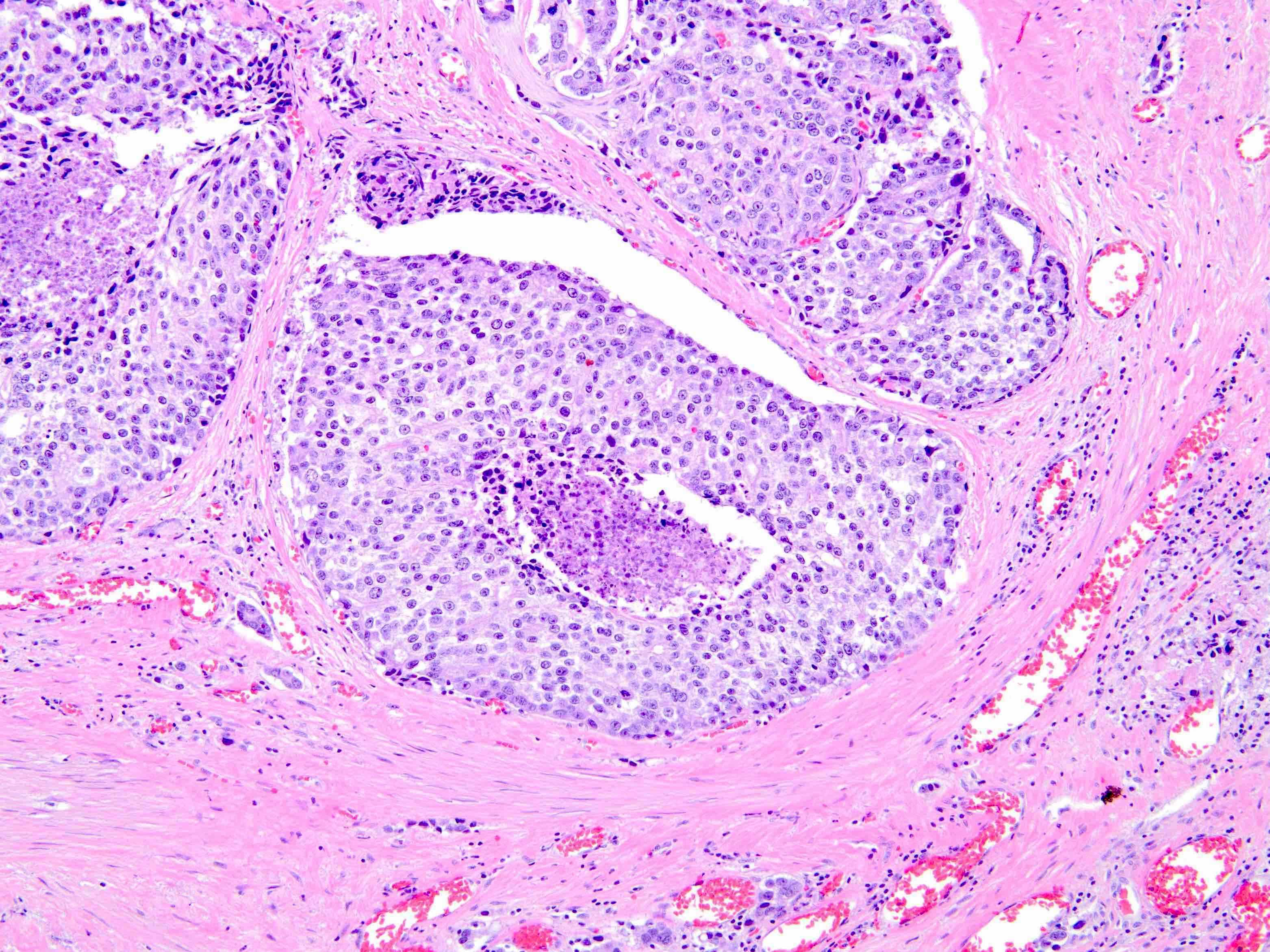
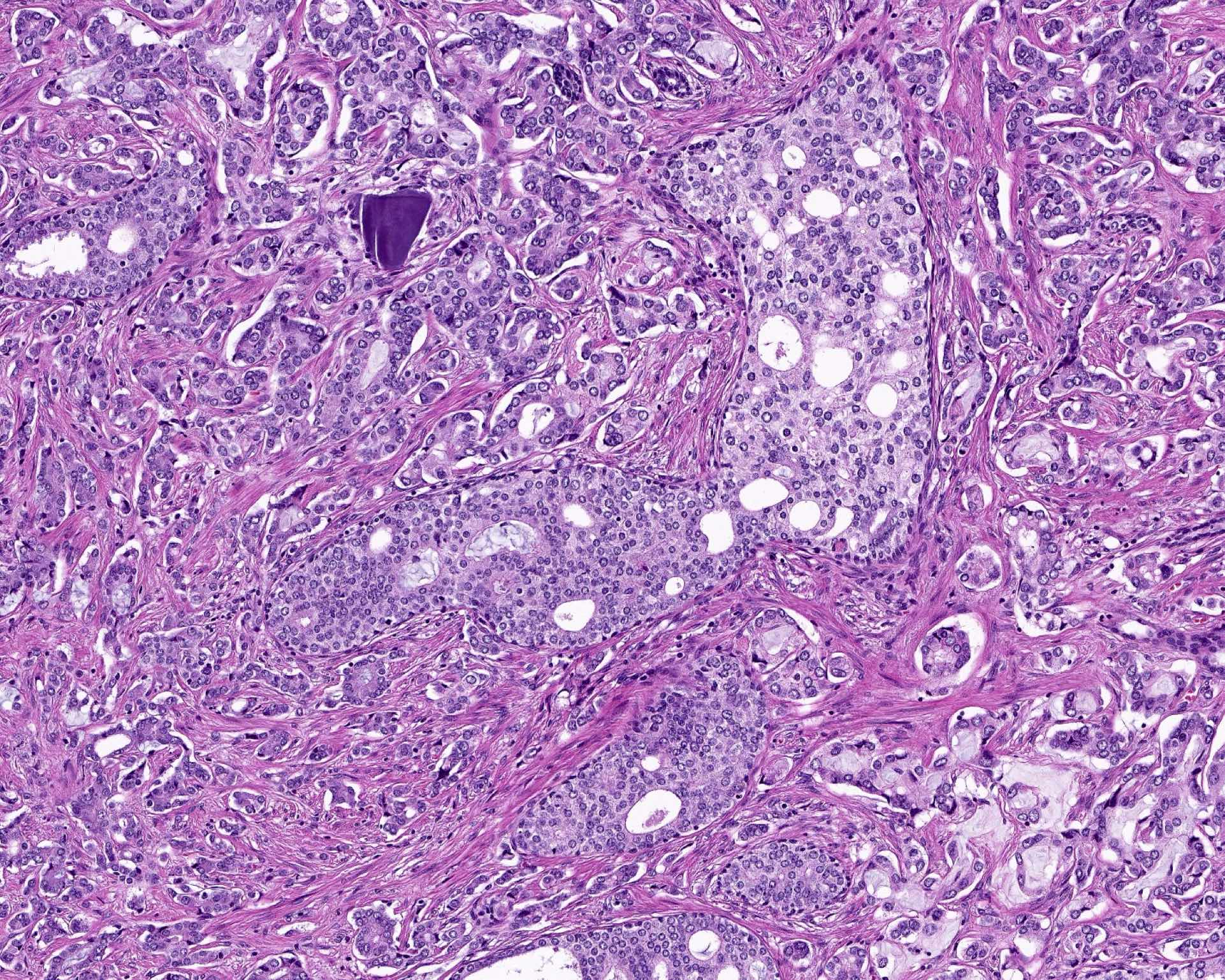
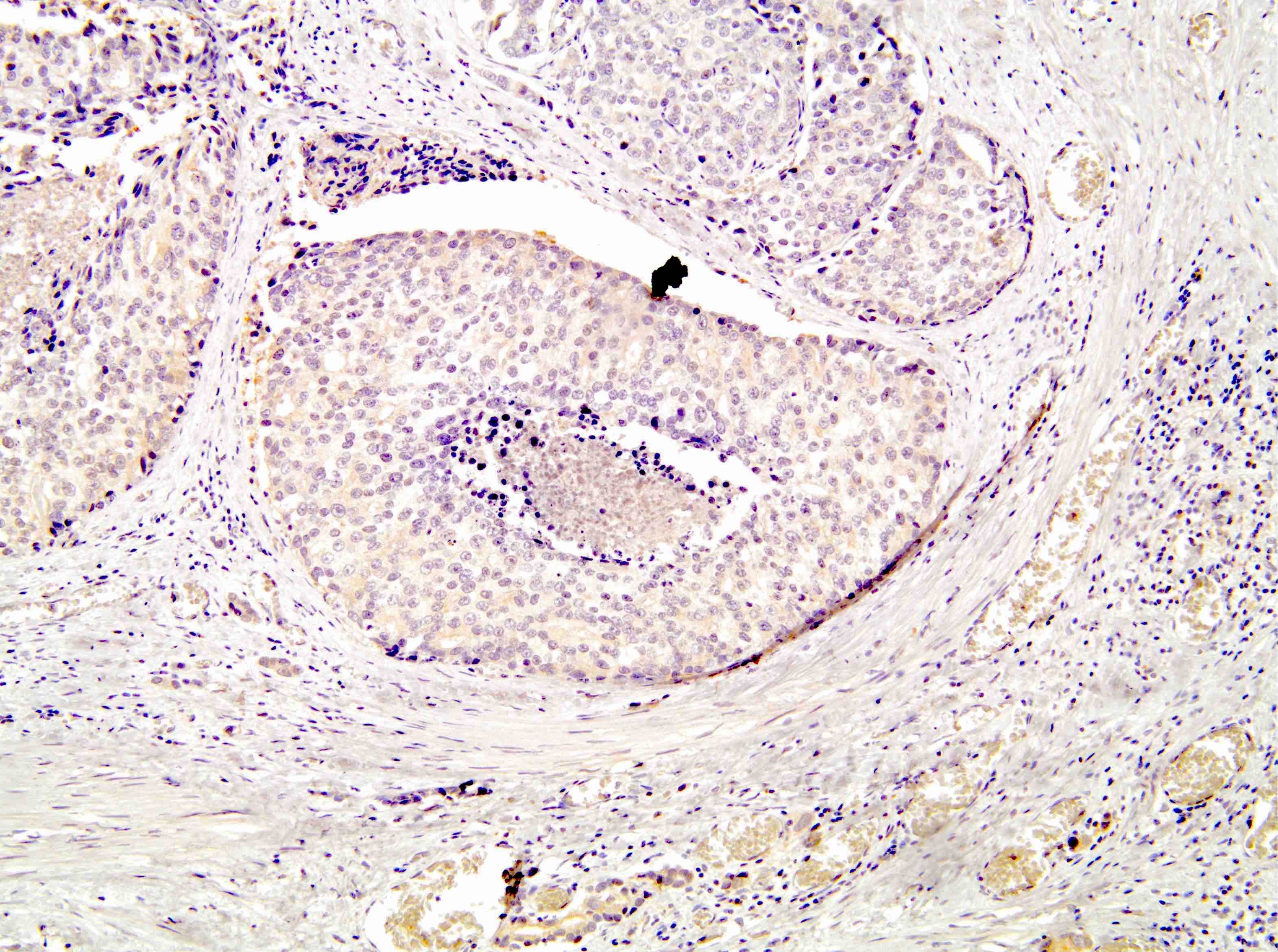
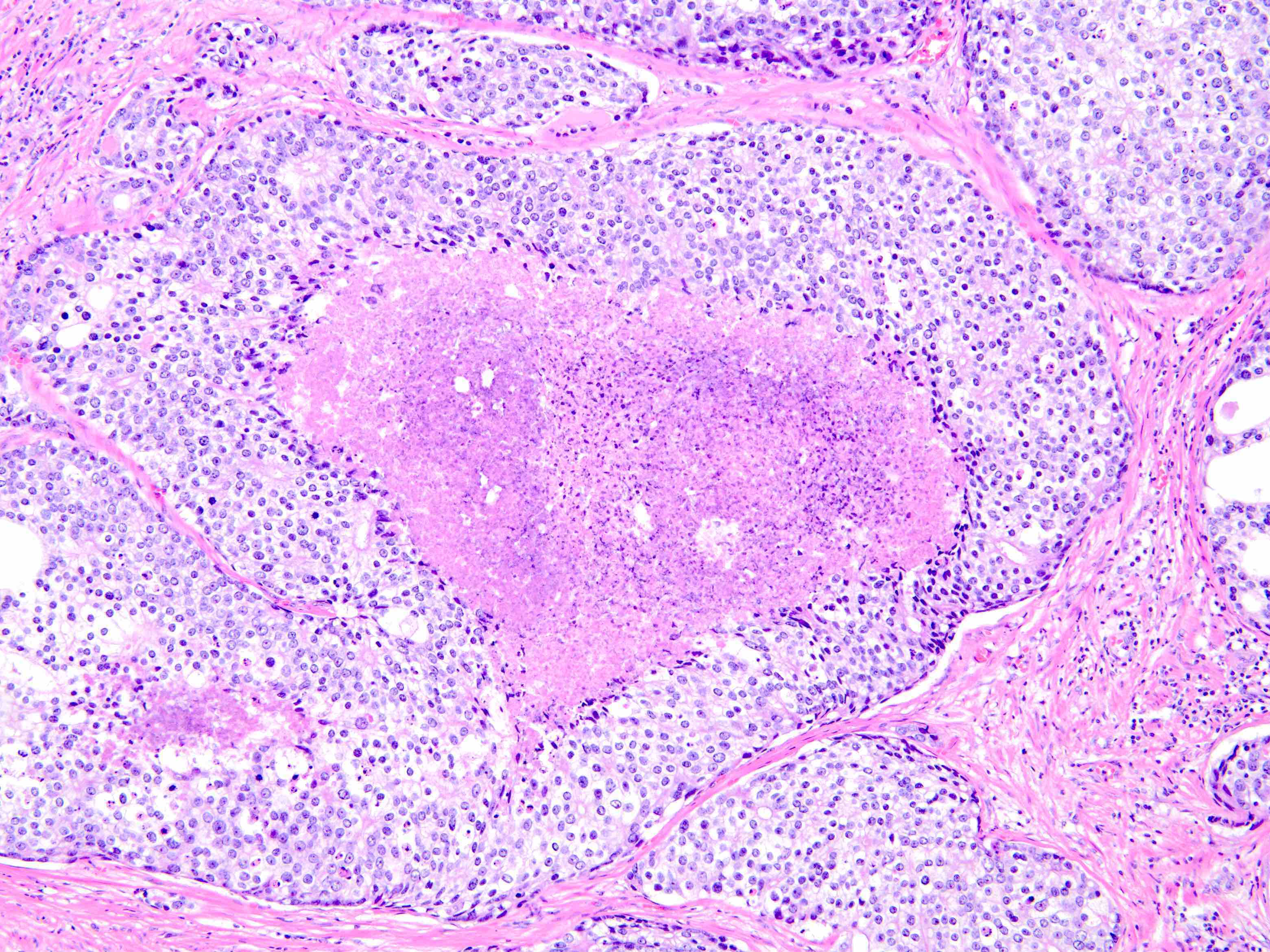
Microscopic (histologic) description
- Diagnostic criteria (Mod Pathol 2006;19:1528, Arch Pathol Lab Med 2007;131:1103, Am J Surg Pathol 2010;34:470, Arch Pathol Lab Med 2015;139:1234, Arch Pathol Lab Med 2021;145:461, Adv Anat Pathol 2021;28:276, Histopathology 2022;81:447)
- Architecture
- Solid growth
- Dense cribriform pattern, characterized by more solid than luminal areas (> 50% epithelium relative to luminal spaces)
- Inclusion of patterns with less complexity (i.e., loose cribriform and micropapillary, remain controversial)
- Histologic features
- Expansile epithelial proliferation in the preexisting duct acinar system
- Retention of basal cell layer: continuous or interrupted
- Enlarged nuclei
- Nuclear atypia
- Frequent, easily identifiable mitosis
- Comedonecrosis
- Architecture
Microscopic (histologic) images
Cytology description
- Cytology is not performed for the diagnosis of this disease entity
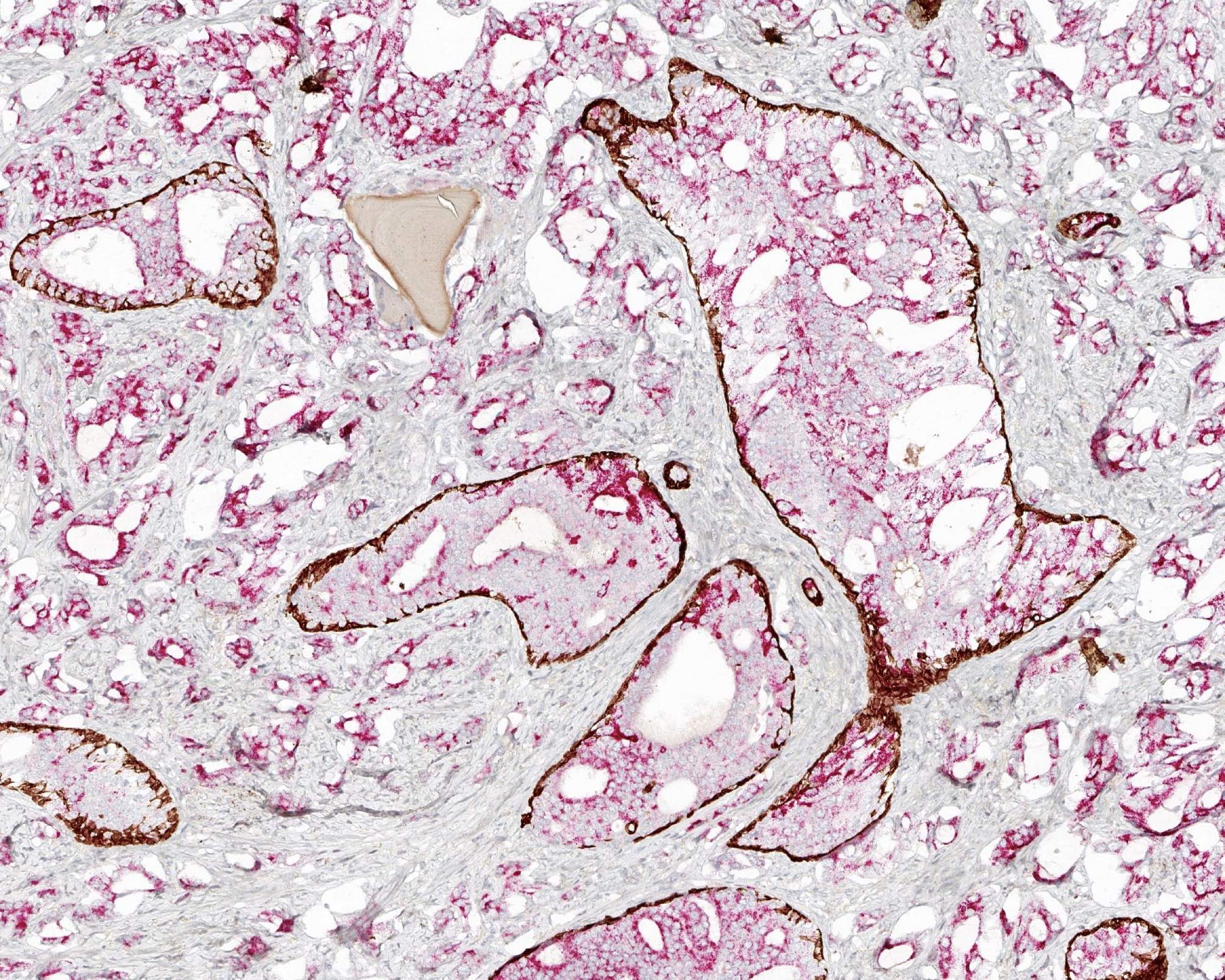
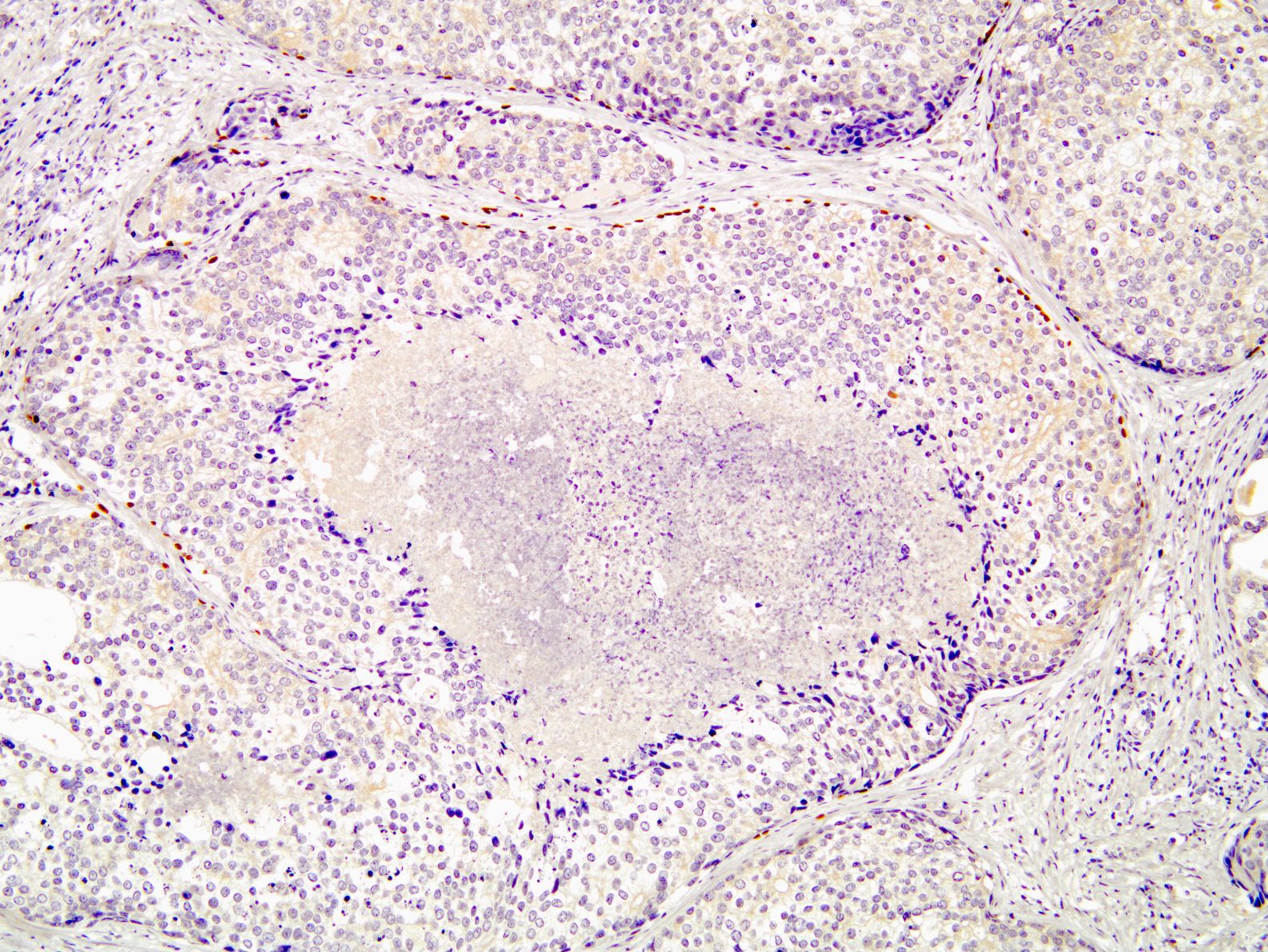
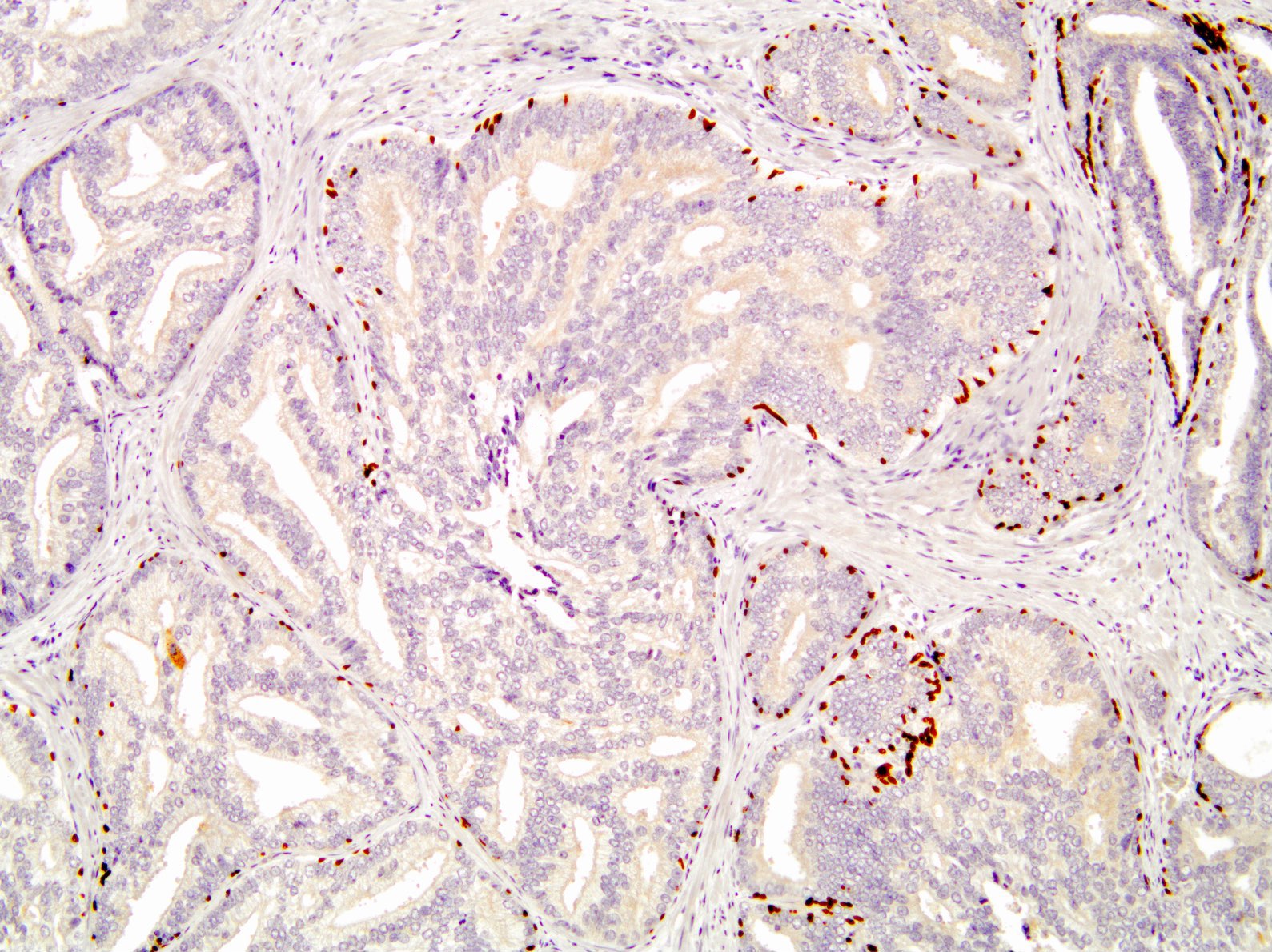
Positive stains
- p63, CK5/6 and CK903 highlight basal cells
- AMACR, PSA and PSAP stain IDC-P cells
- Ki67 proliferation index is high in lesional cells (Prostate 2000;43:11, BJU Int 2018;121:971)
Negative stains
- PTEN: cytoplasmic loss in 69 - 89% of cases (Mod Pathol 2013;26:587, Prostate 2016;76:394, Prostate 2019;79:1267, Histopathology 2021;79:1061)
Electron microscopy description
- Electron microscopy is not used to diagnose this disease entity
Molecular / cytogenetics description
- Loss of heterozygosity (LOH) of TP53, RB1 and PTEN is more common in IDC-P compared to invasive prostate carcinoma (Cancer Med 2024;13:e6939)
- LOH of TP53 found in 60% versus 40% (IDC-P versus invasive carcinoma) (Genes Chromosomes Cancer 2008;47:565)
- LOH of RB1 in 81% versus 60% (IDC-P versus organ confined invasive tumor) (Genes Chromosomes Cancer 2008;47:565)
- ERG fusions detected in 7% versus 55 - 75% (isolated versus concurrent IDC-P) (Adv Anat Pathol 2013;20:117, Histopathology 2017;71:693, J Pathol 2019;249:79, Cancers (Basel) 2022;14:820, Cancers (Basel) 2023;15:5512)
- PTEN deletions detected by molecular analysis in ~45% of cases (Genes Chromosomes Cancer 2008;47:565, Mod Pathol 2013;26:587, Virchows Arch 2019;474:525, J Pathol 2019;249:79)
- Familial prostatic adenocarcinoma with BRCA2 mutations have higher proportion of IDC-P (Eur Urol 2015;67:496, Nat Commun 2017;8:13671)
Sample pathology report
- Prostate, core needle biopsy:
- 1 out of 2 cores with intraductal carcinoma, measuring 10 mm out of 15 mm continuously and involving 66% of the core (see comment)
- Negative for evidence of invasive carcinoma
- Comment: Intraductal carcinoma without evidence of invasive carcinoma is highly associated with high grade invasive prostatic carcinoma at prostatectomy or rebiopsy. Rebiopsy may be clinically indicated.
- Prostate, core needle biopsy:
- Prostatic adenocarcinoma, grade group 2, Gleason score 3+4 involving 2 of 2 cores, measuring 8 mm of 12 mm in core #1 (66% core length involvement) and 2 mm of 14 mm in core #2 (14% core length involvement)
- Intraductal carcinoma present
Differential diagnosis
- Clear cell cribriform hyperplasia (Adv Anat Pathol 2021;28:276):
- Prominent complete basal cell layer easily identified under brightfield microscopy
- Usually seen in the transition zone
- No nuclear atypia
- No comedonecrosis
- High grade prostatic intraepithelial neoplasm (HGPIN) (Mod Pathol 2006;19:1528, Eur Urol Oncol 2018;1:21, Arch Pathol Lab Med 2021;145:461, Histopathology 2022;81:447, Int J Urol 2024;31:7):
- Neoplastic in situ disease of acini with cytologic atypia and retained basal cell layer
- No cribriform or solid pattern in general (J Clin Transl Pathol 2023;3:26)
- Cytologic atypia is less pronounced than in IDC-P
- Clear / vesicular nuclei
- Smaller nuclear size
- ERG expression and PTEN loss rarely seen (Am J Surg Pathol 2015;39:169, Adv Anat Pathol 2021;28:276)
- Not usually associated with high grade invasive carcinoma
- Atypical intraductal proliferation (AIP) (Arch Pathol Lab Med 2021;145:461, Histopathology 2022;81:447, J Clin Transl Pathol 2023;3:26):
- Mainly loose cribriform architecture
- Other patterns: solid or dense cribriform
- Also has retained basal cell layer
- More nuclear atypia than high grade intraepithelial neoplasm
- Less nuclear atypia than intraductal carcinoma of prostate
- Lack of intraluminal necrosis
- Ductal carcinoma of prostate (Mod Pathol 2006;19:1528, Arch Pathol Lab Med 2012;136:418, Adv Anat Pathol 2021;28:276, Int J Urol 2024;31:7):
- Lacks basal cell layer
- Peripheral basal cells seen when intraductal extension is present
- Cribriform architecture with slit-like spaces
- Complex papillary architecture with fibrovascular cores
- Pseudostratified columnar epithelium with oval nuclei, more cytoplasm and tufting
- Infiltrative growth and pagetoid spread common
- High grade invasive acinar prostatic adenocarcinoma (Mod Pathol 2006;19:1528, Arch Pathol Lab Med 2012;136:418, Int J Urol 2024;31:7):
- Lacks basal cell layer
- Can have necrosis and cribriform architecture like IDC-P
- Can be intimately associated with IDC-P
- Cribriform invasive prostate cancer may have ERG expression and PTEN loss (Adv Anat Pathol 2021;28:276)
- Urothelial carcinoma (Mod Pathol 2006;19:1528, Arch Pathol Lab Med 2012;136:418):
- Solid growth pattern with central necrosis
- May show comedonecrosis and pseudocribriform architecture (Adv Anat Pathol 2021;28:276)
- Greater cytologic atypia and pleomorphism
- More nuclear pleomorphism; nucleoli are less uniform; more frequent mitoses
- Diffusely positive for CK7, p63, GATA3, uroplakin III, uroplakin II; negative for PSA (Hum Pathol 2002;33:1136, Eur Urol Oncol 2018;1:21)
Additional references
Board review style question #1
Which of the following is true regarding intraductal carcinoma of the prostate (IDC-P)?
- Basal cell layer is not present in intraductal carcinoma
- Intraductal carcinoma is a common finding in prostate biopsies
- Intraductal carcinoma should be graded based on the Gleason pattern scoring system
- PTEN mutations are common in intraductal carcinoma
Board review style answer #1
D. PTEN mutations are common in intraductal carcinoma. Intraductal carcinoma commonly has PTEN deletions seen by IHC. PTEN loss is seen in 69 - 89% of IDC-P cases and loss of heterozygosity (LOH) of PTEN is seen in ~45% of IDC-P cases (Prostate 2016;76:394, Genes Chromosomes Cancer 2008;47:565).
Answer C is incorrect because IDC-P without the presence of invasive carcinoma should not be assigned a Gleason grade. 2 differing recommendations exist regarding whether to grade or not when associated with invasive carcinoma: the 2019 Genitourinary Pathology Society (GUPS) does not favor including IDC-P into the Gleason score, while the 2019 ISUP favors it. The 2022 5th edition of the WHO classification of tumors of the urinary and male genital systems has not endorsed either of these positions (Eur Urol 2021;79:3, Eur Urol 2022;82:469).
Answer B is incorrect because IDC-P is found in ~14% of prostate biopsies that harbor concomitant invasive carcinoma. The incidence of IDC-P in prostate biopsies without associated invasive carcinoma has been reported to be 0.06 - 0.26% (Mod Pathol 2006;19:1528, Histopathology 2013;63:574, Eur Urol 2017;72:492, Eur Urol 2022;82:469).
Answer A is incorrect because IDC-P is characterized by the presence of peripheral basal cells that can be highlighted by IHC when appropriate (Mod Pathol 2006;19:1528, J Clin Transl Pathol 2023;3:26).
Comment Here
Reference: Intraductal carcinoma
Comment Here
Reference: Intraductal carcinoma
Board review style question #2
A 65 year old man had a radical prostatectomy for invasive carcinoma previously found on prostate biopsy. What do the above histology findings predict about this patient's clinical course?
- Patient has a higher probability of biochemical recurrence
- Patient is cured with prostatectomy alone
- Patient was overtreated as he did not need a prostatectomy
- Patient will do the same as other patients within his same grade group without this finding
Board review style answer #2
A. Patient has a higher probability of biochemical recurrence. Patients with intraductal carcinoma have higher stage, higher grade tumors with higher likelihood of recurrence and do worse when matched for grade group. IDC-P is strongly associated with biochemical recurrence after radical prostatectomy (Eur Urol 2022;82:469).
Answer B is incorrect because rebiopsy within 3 months or definitive therapy is recommended in isolated IDC-P without invasive carcinoma and in IDC-P with invasive low grade carcinoma. In IDC-P cases associated with a high grade invasive tumor, current guidelines recommend surgery and adjuvant therapy (Yonsei Med J 2016;57:1054, Virchows Arch 2019;474:525).
Answer C is incorrect because, given the clinical significance of the presence of IDC-P and its association with aggressive disease, radical therapy is the current recommendation (Curr Oncol Rep 2021;23:110).
Answer D is incorrect because IDC-P is considered an independent adverse prognostic factor and is frequently associated with high grade and high stage invasive prostatic adenocarcinoma. Comedonecrosis has been associated with worse histopathological features on prostatectomies and is an independent predictor of poorer clinical outcome (Yonsei Med J 2016;57:1054, Virchows Arch 2019;474:525, Histopathology 2022;81:447, Arch Pathol Lab Med 2023;147:94).
Comment Here
Reference: Intraductal carcinoma
Comment Here
Reference: Intraductal carcinoma