Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Immunohistochemistry & special stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Talibi S, Smith SC. Sarcomatoid adenocarcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/prostatecarcinosarcoma.html. Accessed December 27th, 2024.
Definition / general
- WHO recognized variant subtype of prostatic adenocarcinoma
- Malignant monophasic or biphasic neoplasm including a spindle cell or pleomorphic sarcomatoid component
- Represents dedifferentiation from (usually high grade acinar) prostatic adenocarcinoma
- Clinical, histologic, immunophenotypic or molecular evidence of origin from prostatic adenocarcinoma
Essential features
- Malignant sarcomatoid neoplasm, usually admixed with recognizable prostatic adenocarcinoma but rarely monophasic
- Monophasic cases have history, immunophenotypic or molecular evidence relating the sarcomatoid malignancy to prostatic adenocarcinoma
- Most cases are associated with prior radiotherapy or hormone therapy
- Usually clinically aggressive, high stage, poor prognosis
Terminology
- Formerly carcinosarcoma
ICD coding
- ICD-O: 8572/3 - adenocarcinoma with spindle cell metaplasia
- ICD-10: C61 - malignant neoplasm of prostate
- ICD-11: 2C82 & XH2QL8 - malignant neoplasms of prostate and adenocarcinoma with spindle cell metaplasia
Epidemiology
- Rare, < 200 cases reported
- Age range: 49 - 88 years (Urology 2015;86:539)
- > 60% have history of prior acinar prostatic adenocarcinoma (Am J Surg Pathol 2006;30:1316)
- 9 - 22 years after initial diagnosis (Urology 2015;86:539)
- 90% have history of prior treatment by androgen deprivation or radiotherapy
Sites
- Prostate gland
- Frequent invasion of the bladder
- Systemic metastases to lungs and bone
Pathophysiology
- Arises via dedifferentiation from precursor conventional prostatic adenocarcinoma
- Preexisting carcinoma diagnosed may be low grade (grade group 1, ~50%) or higher (grade group 2, ~50%)
- Molecular features shared between sarcomatoid and carcinomatous component (Histopathology 2015;66:898)
- ERG rearrangements shared between components
Etiology
- Risk factors related to precursor conventional prostatic adenocarcinoma
- Strong association with prior hormone or radiation therapy
Clinical features
- Organ confined disease has best prognosis
- Definitive treatment recommended
- Increased aggression in locally advanced and metastatic disease (Urology 2015;86:539)
- Documented metastases most often show adenocarcinomatous component
Diagnosis
- Biopsy, transurethral resection or prostatectomy specimens
Laboratory
- PSA elevated in some cases
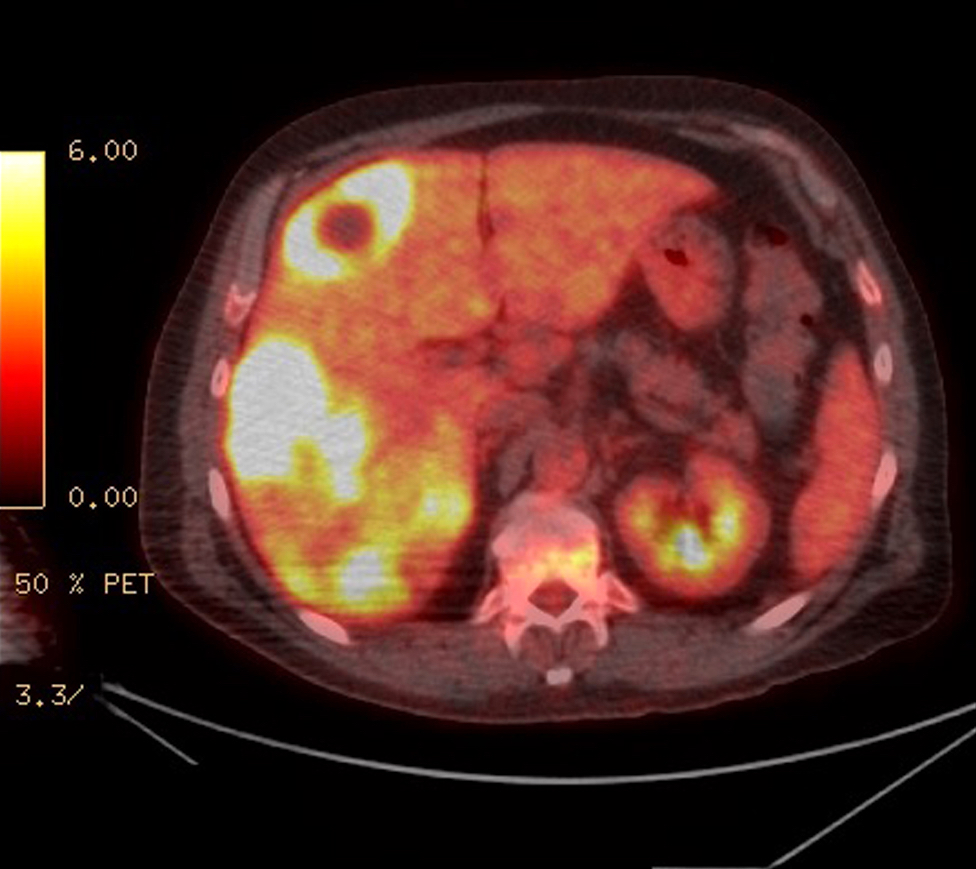
Radiology description
- Staging by MRI, CT and bone scan
Prognostic factors
- Median overall survival < 1 year
- Prognosis (overall survival) has been related to a modified staging system (Urology 2015;86:539)
- Organ confined: ≥ 5 years
- Locally advanced / bladder invasion: 9 months
- Metastatic disease: 7 months
Case reports
- 54 year old man with recurrence as sarcomatoid carcinoma (Case Rep Urol 2013;2013:631809)
- 76 year old man with urinary obstruction (Int J Clin Exp Pathol 2010;3:319)
- 80 year old man with large pelvic mass (Urol Case Rep 2020;34:101432)
- 80 and 72 year old men, each diagnosed less than 1 year from the original diagnosis of prostatic adenocarcinoma (World J Clin Cases 2021;9:1668)
Treatment
- Standard treatments not established
- Localized sarcomatoid carcinoma treated by radical prostatectomy or external beam radiotherapy
Gross description
- Usually large transurethral resection samples
- Gray / yellow / white fibrous tissue
- Hemorrhage and necrosis prominent
- Reference: Journal of Case Reports 2014;4:33
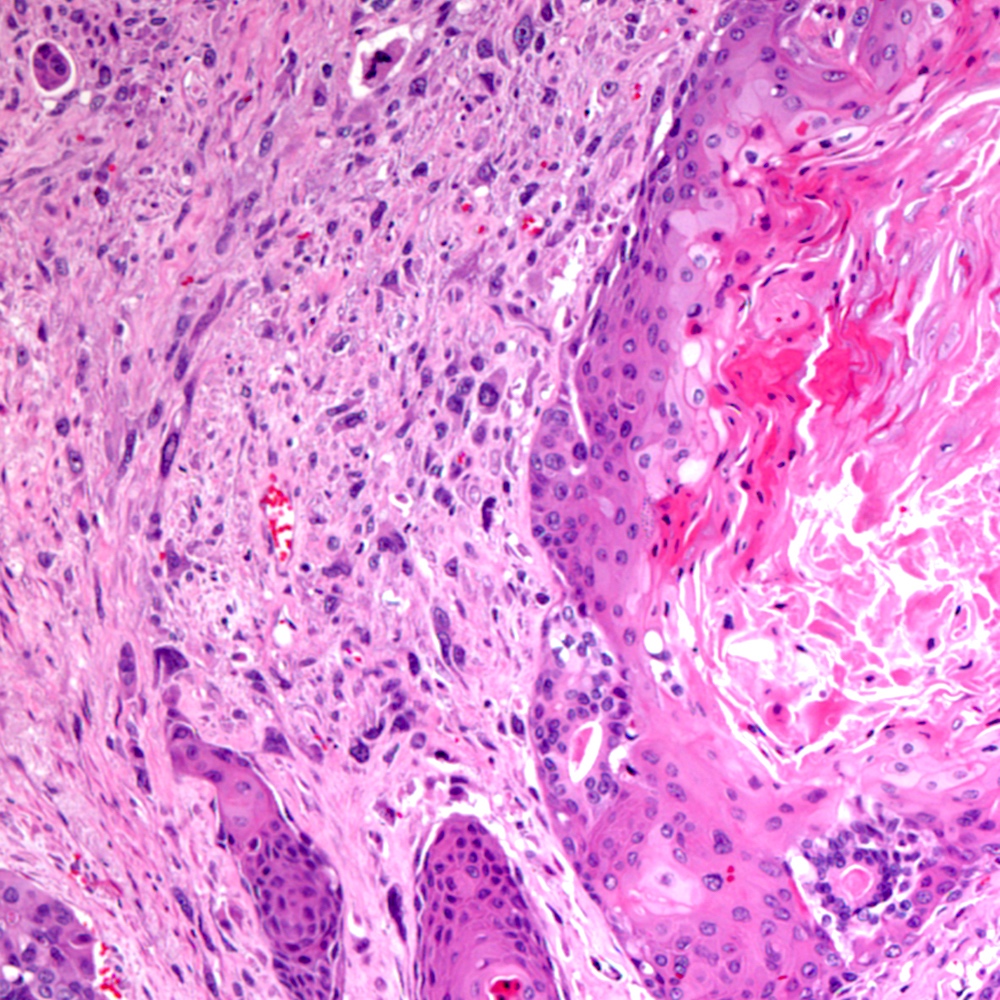
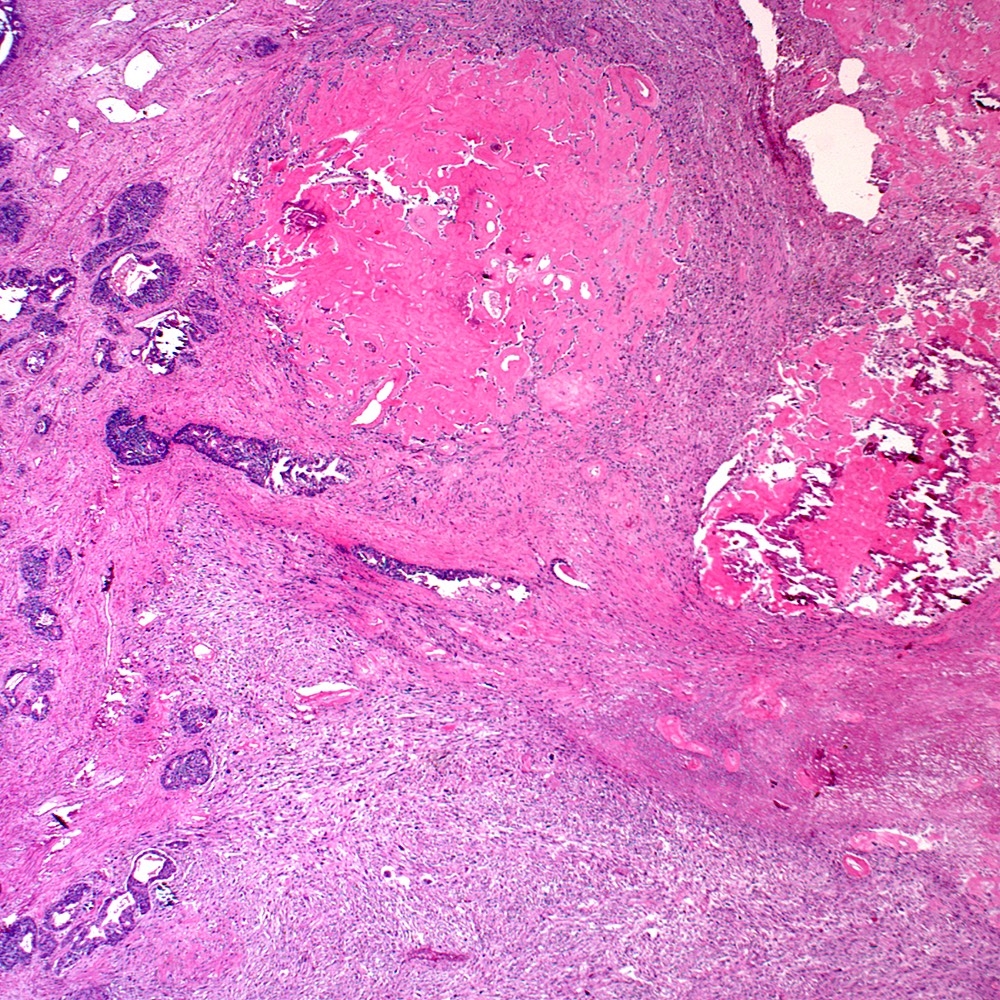
Microscopic (histologic) description
- Overall criteria, a tumor showing either:
- Biphasic admixture of malignant sarcomatoid component with recognizable conventional prostatic adenocarcinoma
- Monophasic sarcomatoid tumor with historical, immunohistochemical or molecular evidence relating it to prostatic adenocarcinoma
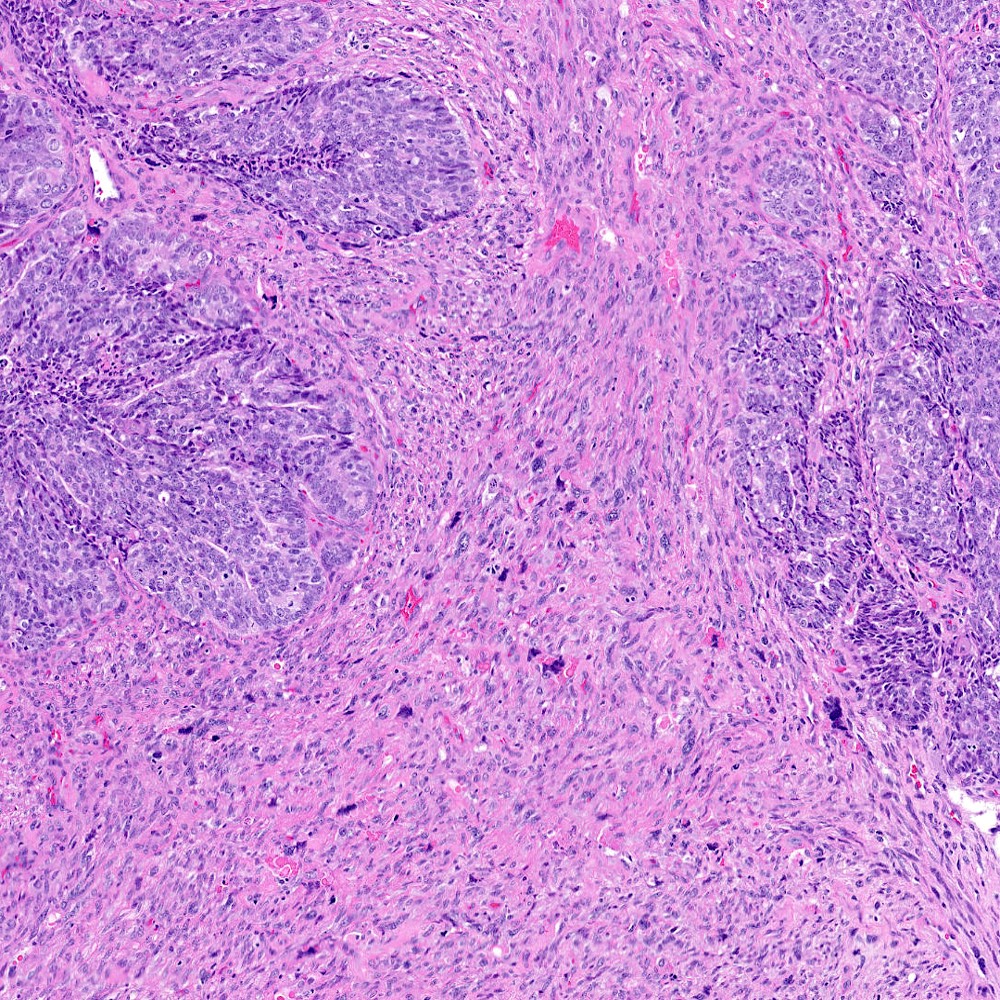
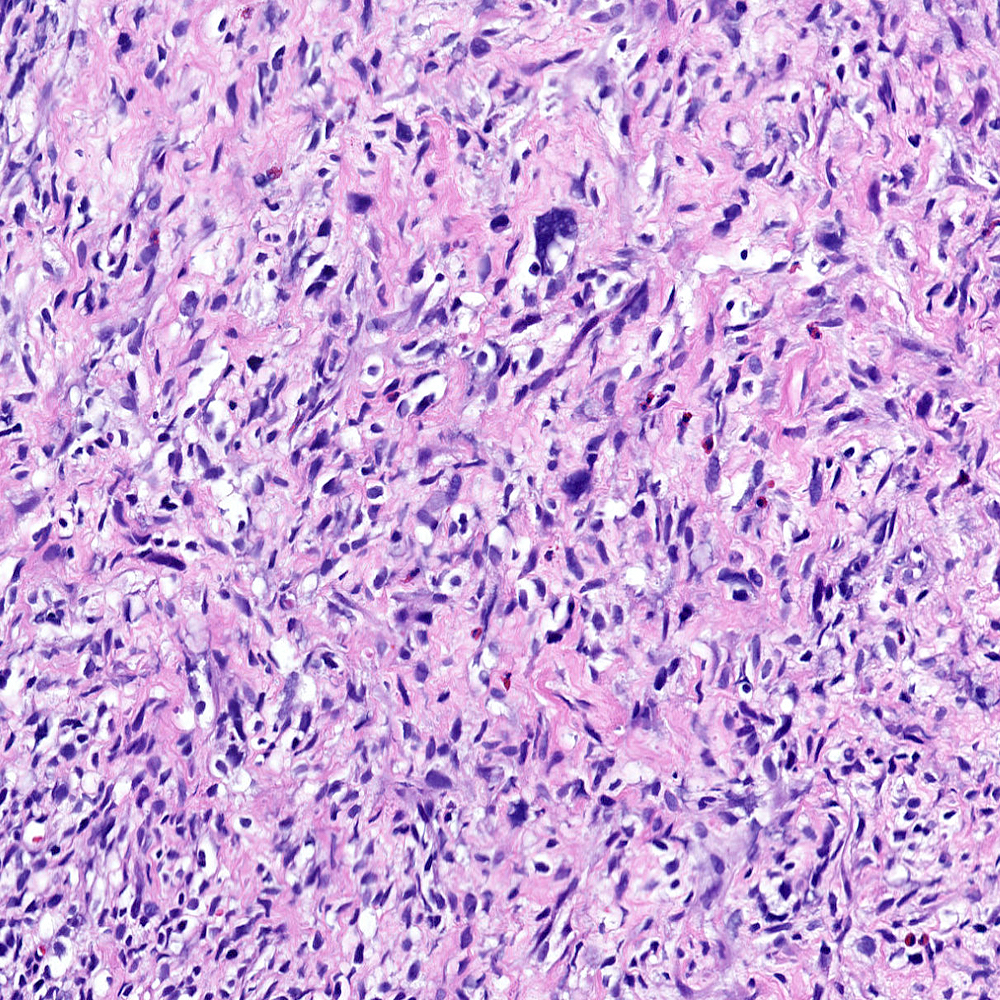
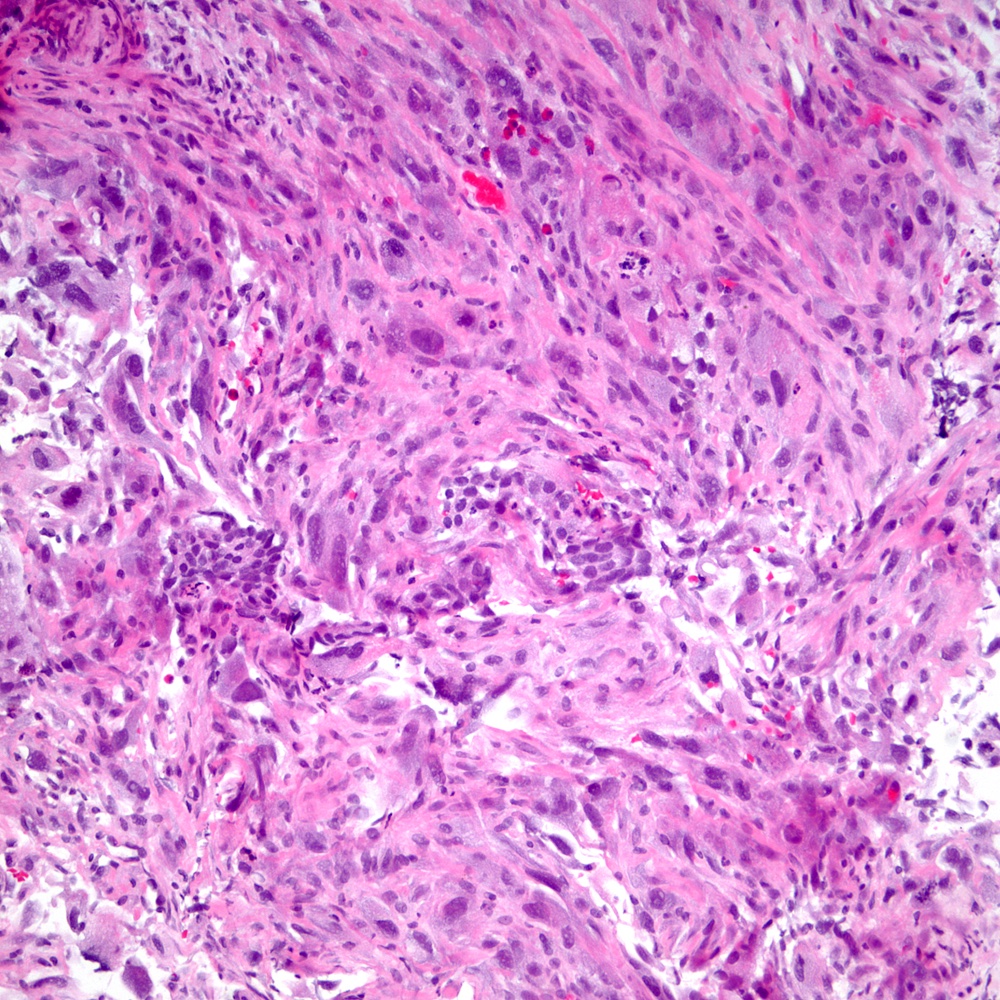
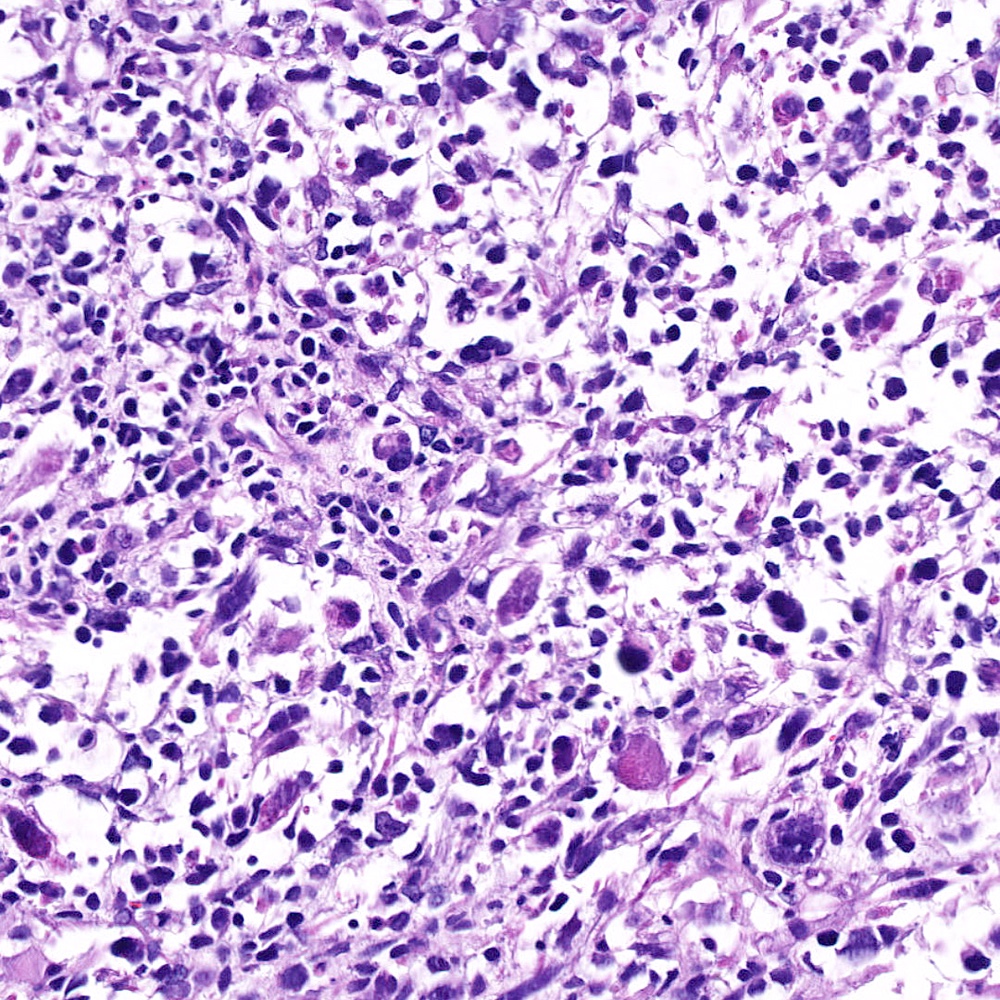
- Sarcomatoid component
- Most commonly a nondescript spindle cell appearance
- Hypercellular fusiform cells
- Storiform, fascicular or patternless architecture
- High grade with large hyperchromatic nuclei
- Frequent mitotic activity and necrosis
- May resemble high grade pleomorphic sarcoma with giant cells and bizarre nuclei
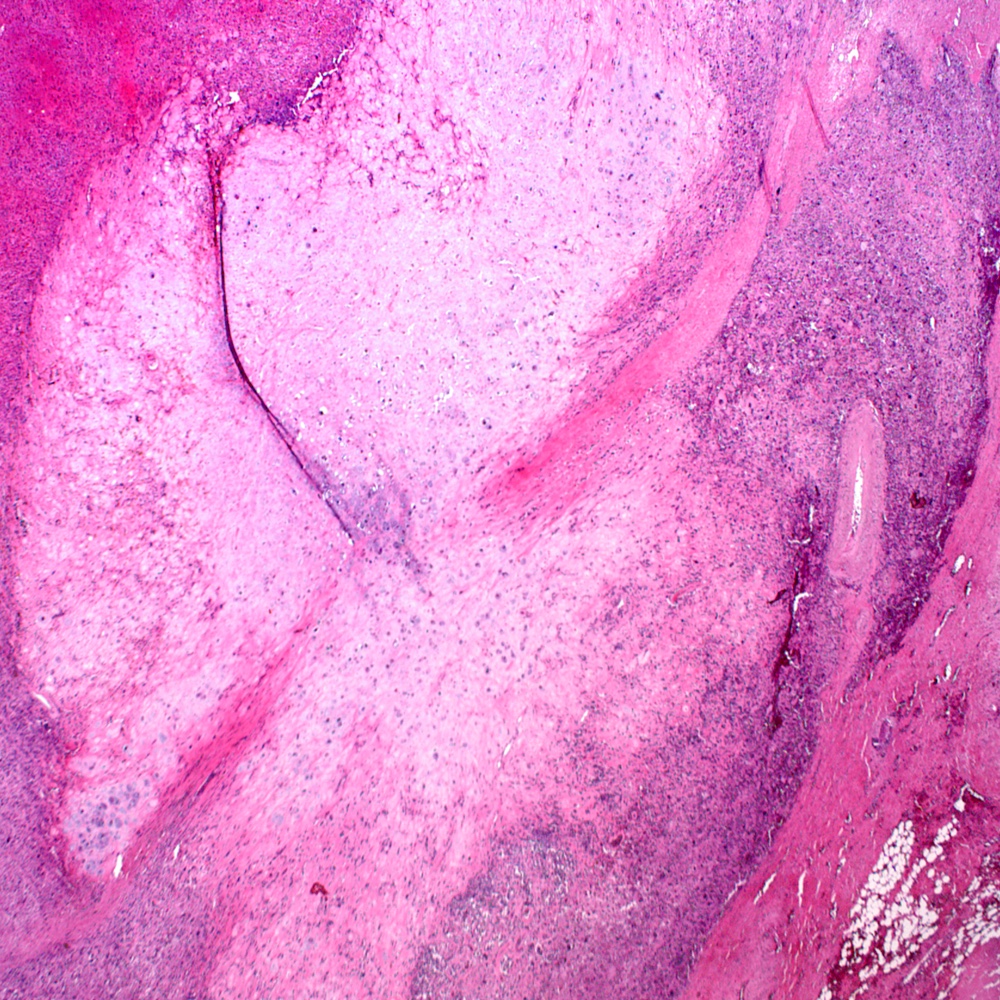
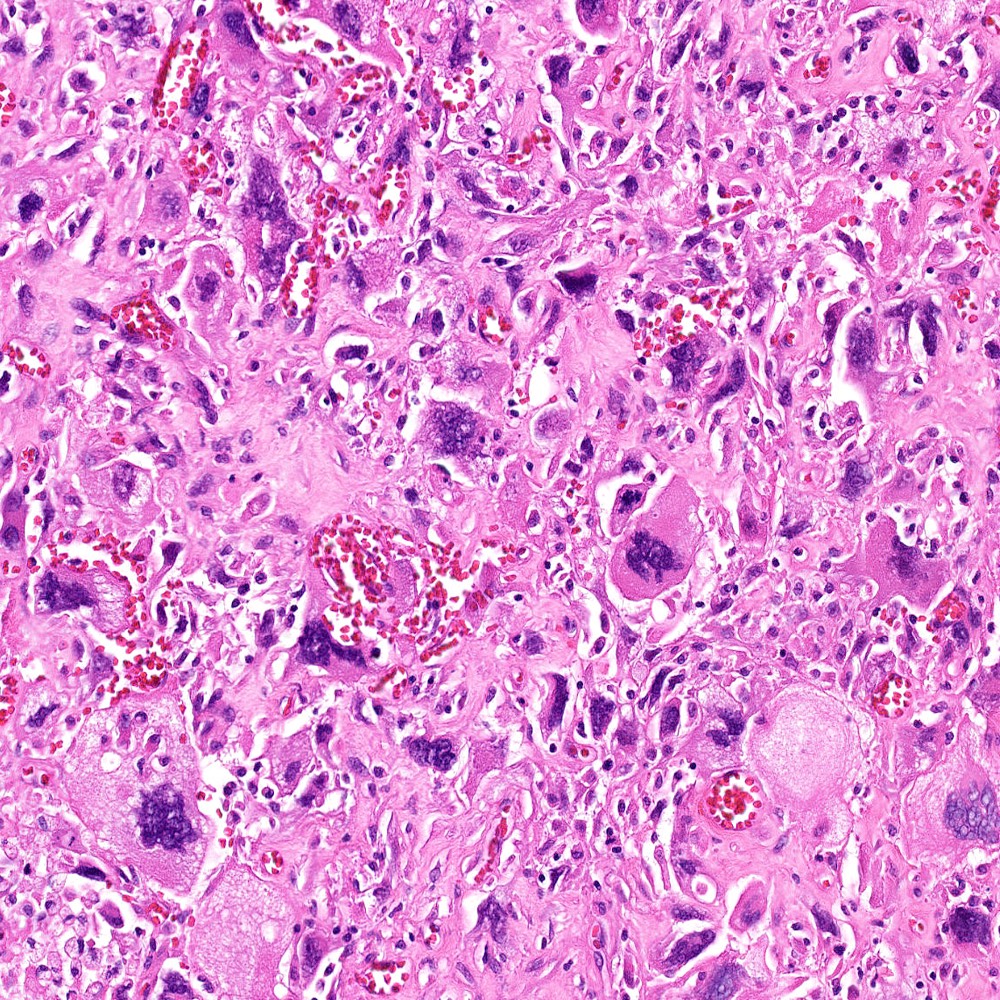
- Subset with heterologous sarcoma patterns with differentiation toward other mesenchymal lineages
- Osteosarcoma (most common)
- Rhabdomyosarcoma
- Chondrosarcoma
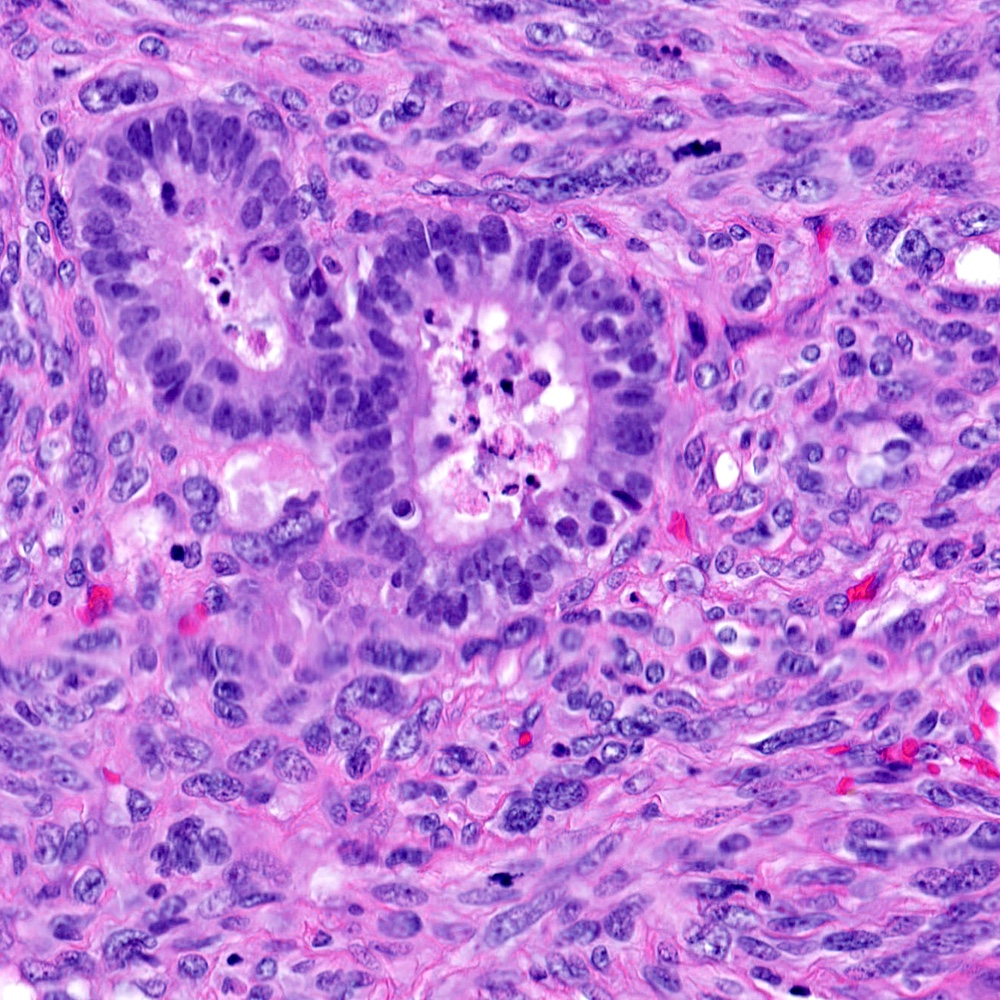
- Carcinomatous component
- Most commonly high grade acinar adenocarcinoma
- Uncommonly: foamy gland, ductal, adenosquamous, signet cell presentations
- Individual polygonal or epithelioid cells may be present within the sarcomatoid component
- Adenosquamous small cell components may be present
- Monophasic spindle cell morphology
- History documents a prior acinar adenocarcinoma
- Immunohistochemical evidence of epithelial differentiation
- Expression of keratins, prostate lineage markers
- Molecular evidence of prostatic origin
- Rearrangements of ERG of other ETS family proto-oncogenic transcription factors
- Reference: Am J Surg Pathol 2006;30:1316
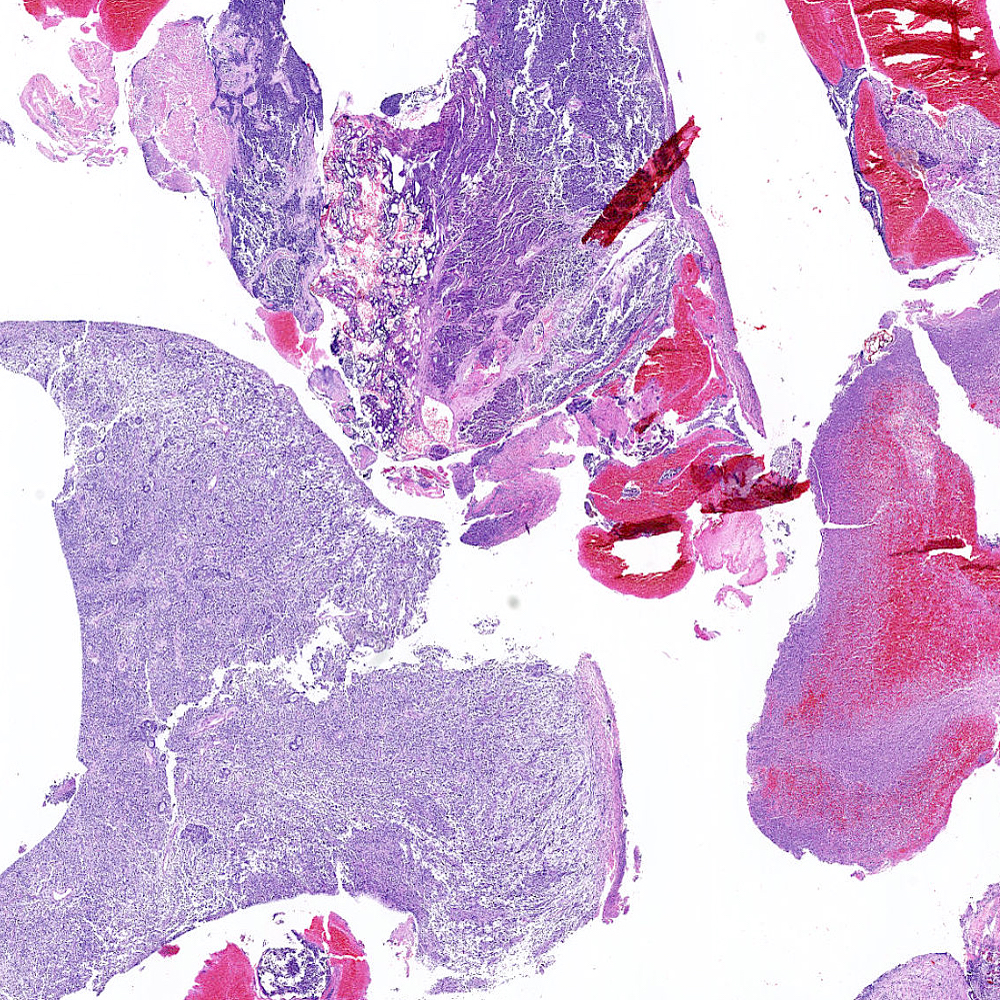
Microscopic (histologic) images
Contributed by Steven Christopher Smith, M.D., Ph.D.
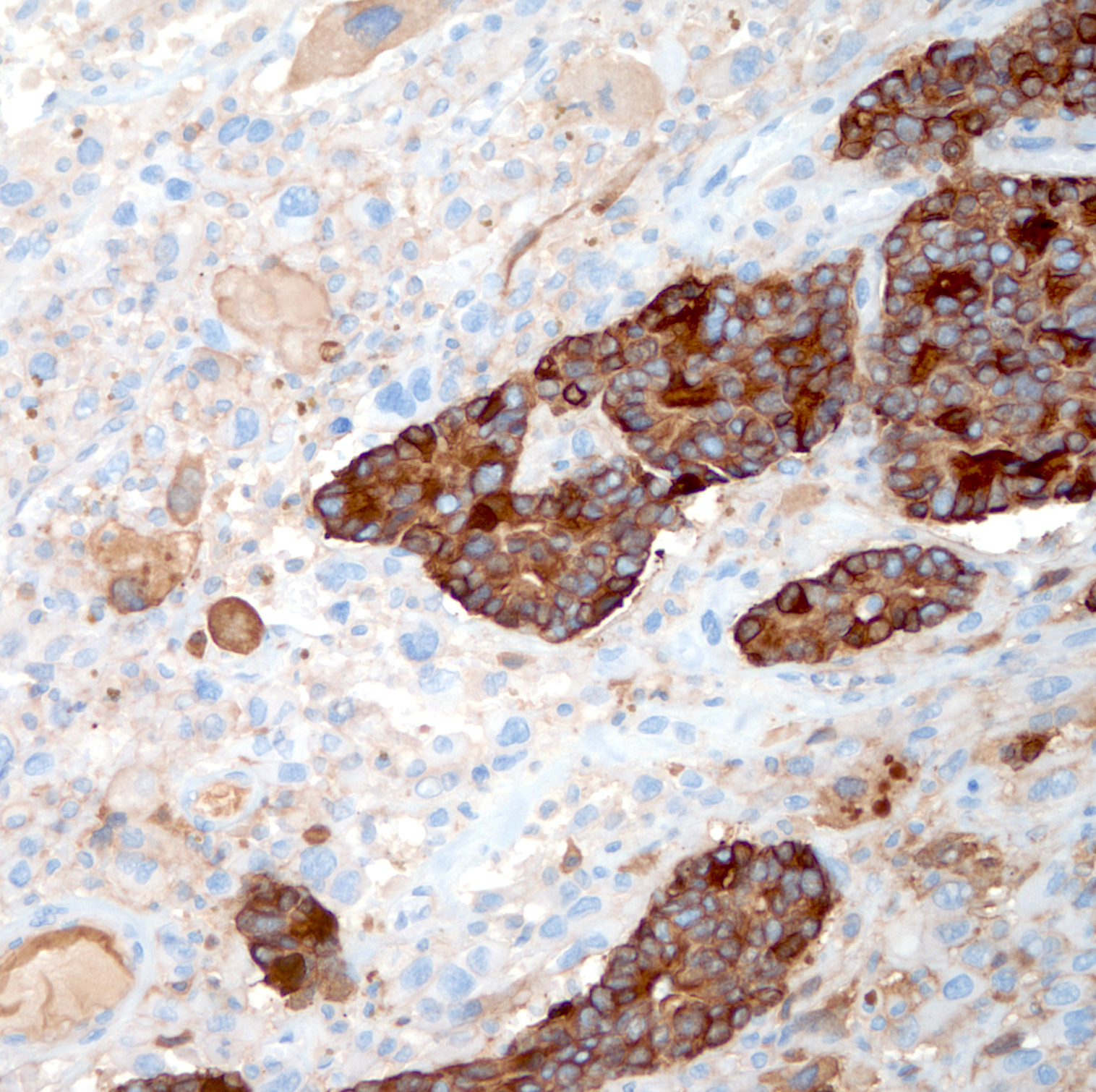
Immunohistochemistry & special stains
- Carcinomatous component
- Sarcomatoid component
- Pancytokeratins: AE1 / AE3, CAM5.2, OSCAR
- Usually focally positive in sarcomatoid spindle cell component
- High molecular weight cytokeratins: CK903 / 34 beta E12 and CK5/6
- May show greater positivity in spindle cell component than pancytokeratins in some cases
- p63 (focally) in sarcomatoid component
- Other mesenchymal makers:
- Pancytokeratins: AE1 / AE3, CAM5.2, OSCAR
- References: Am J Surg Pathol 2006;30:1316, Mod Pathol 2018;31:S133
Molecular / cytogenetics description
- ERG rearrangements detectable by FISH or sequencing in both carcinomatous and sarcomatoid components in cases with rearrangements (Histopathology 2015;66:898, Histopathology 2020;77:890)
Sample pathology report
- Prostate gland, transurethral resection:
- Sarcomatoid carcinoma of the prostate, involving 10 of 15 tissue fragments resected and ~75% of the tissue sampled (see comment)
- Focal heterologous osteosarcomatous differentiation is present
- Associated prostatic adenocarcinoma, grade group 5 (Gleason score 4+5=9)
- Comment: Sarcomatoid carcinoma of the prostate is an aggressive malignancy where carcinoma undergoes dedifferentiation to a sarcomatous component demonstrating variable but usually spindle cell or pleomorphic morphology. Many cases are associated with previous hormone or radiotherapy for prostatic adenocarcinoma.
Differential diagnosis
- Prostatic stromal sarcoma:
- Prostatic leiomyosarcoma:
- Prostatic solitary fibrous tumor:
- No admixed (or prior) adenocarcinomas component
- More cytologically uniform tumor with small spindled to polygonal cells
- Uniform, angulated or carrot shaped nuclei
- Patternless architecture, staghorn thin walled vasculature
- Diffuse expression of CD34 and STAT6
- Lacks keratin, p63, prostate marker expression
Additional references
Board review style question #1
An 81 year old man presents with an obstructing urethral mass and on CT scan imaging is shown to have recent development of a 6 cm obstructing mass arising from the prostate, compressing and displacing the urethra. Transurethral resection demonstrates a spindle cell tumor with brisk mitotic activity and nuclear pleomorphism but no prostatic or urothelial carcinoma is visible. What additional findings would argue most strongly for diagnosis of sarcomatoid carcinoma of the prostate?
- Dense, fascicular growth, diffuse expression of desmin and SMA
- Focal desmin positivity, diffuse vimentin positivity
- Patchy high molecular weight keratin expression in subsets of the spindle cells, history of high grade prostatic adenocarcinoma treated by androgen deprivation therapy and pelvic radiotherapy 5 years previously
- Staghorn vasculature, CD34 and STAT6 expression
Board review style answer #1
C. Patchy high molecular weight keratin expression in subsets of the spindle cells, history of high grade prostatic adenocarcinoma treated by androgen deprivation therapy and pelvic radiotherapy 5 years previously
Comment Here
Reference: Sarcomatoid carcinoma
Comment Here
Reference: Sarcomatoid carcinoma
Board review style question #2
A 48 year old man presents with an obstructing mass arising in the prostate and bladder neck. It demonstrates dense, organized fascicular growth of fusiform cells with dense eosinophilic cytoplasm and elongated nuclei. What features would support consideration of a prostatic leiomyosarcoma?
- Positive rearrangement of the ERG locus by FISH
- Scattered admixed glands with cytoplasmic atypia; focal p63 and CK903 / 34 beta E12 expression in scattered spindle cells
- Strong, diffuse expression of SMA and desmin
- Thin walled ramifying vasculature, nuclear STAT6 positivity
Board review style answer #2