Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Diagnosis | Laboratory | Prognostic factors | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Cite this page: Iczkowski KA. Atypical intraductal proliferation. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/prostateatypicalintraductal.html. Accessed April 2nd, 2025.
Definition / general
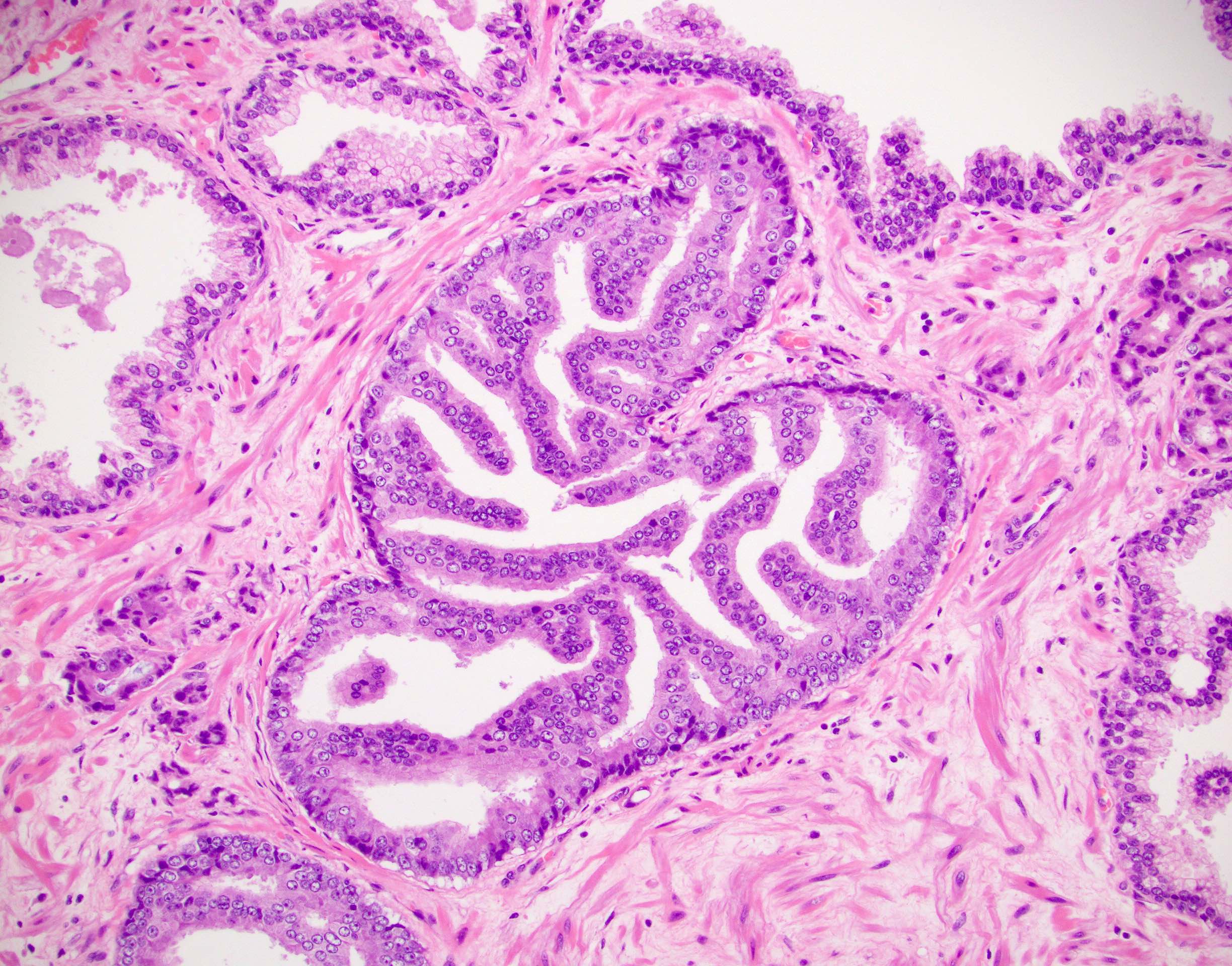
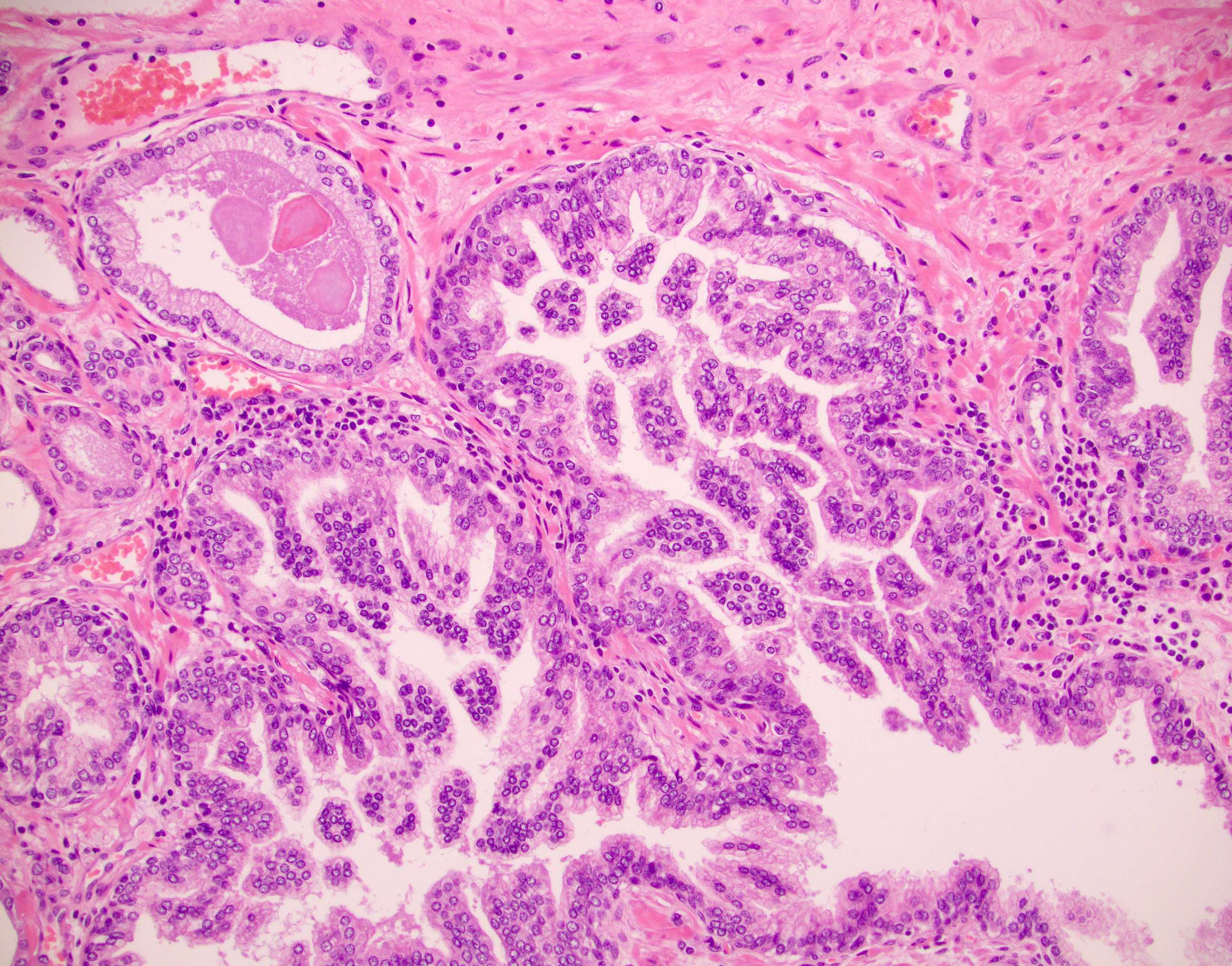
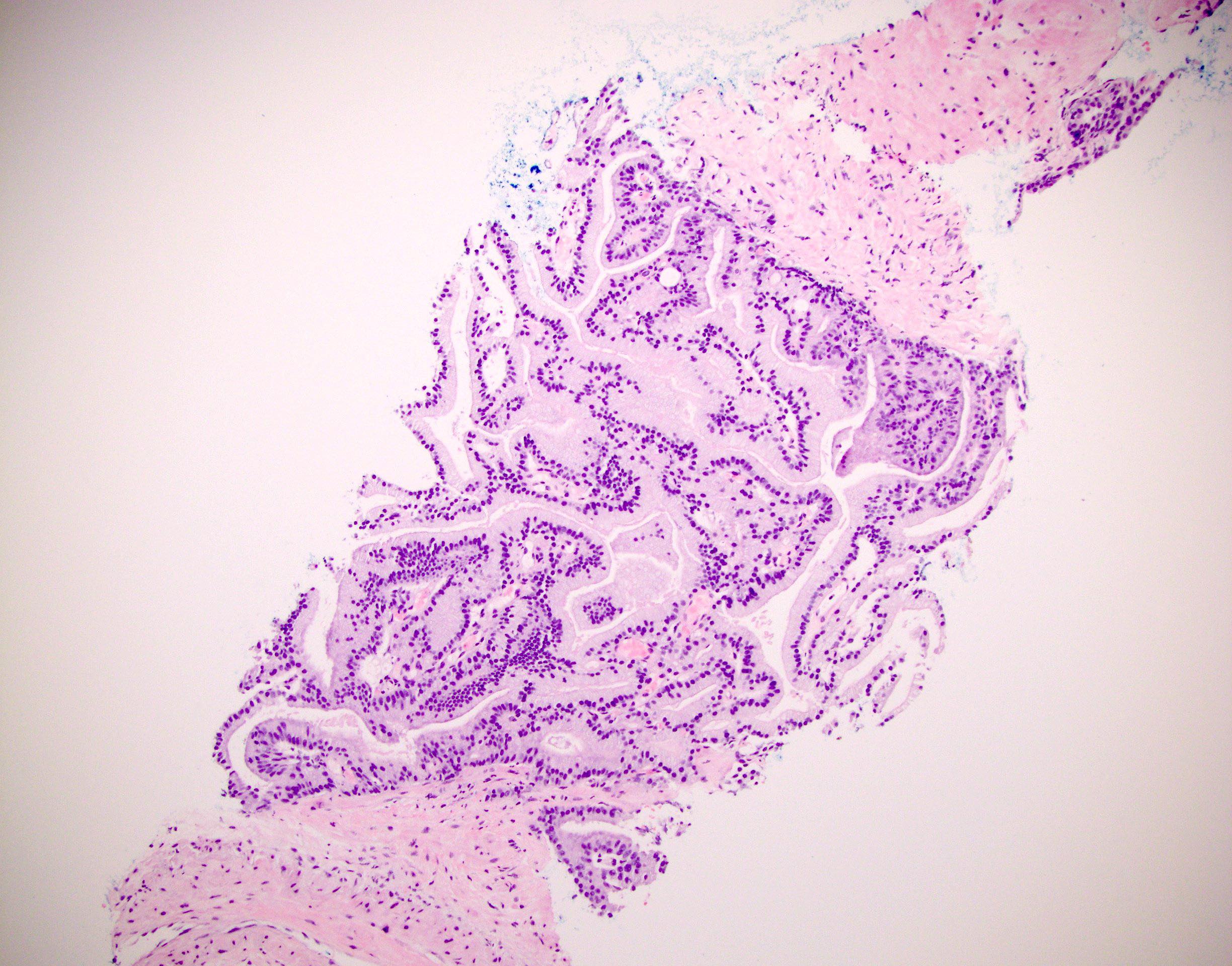
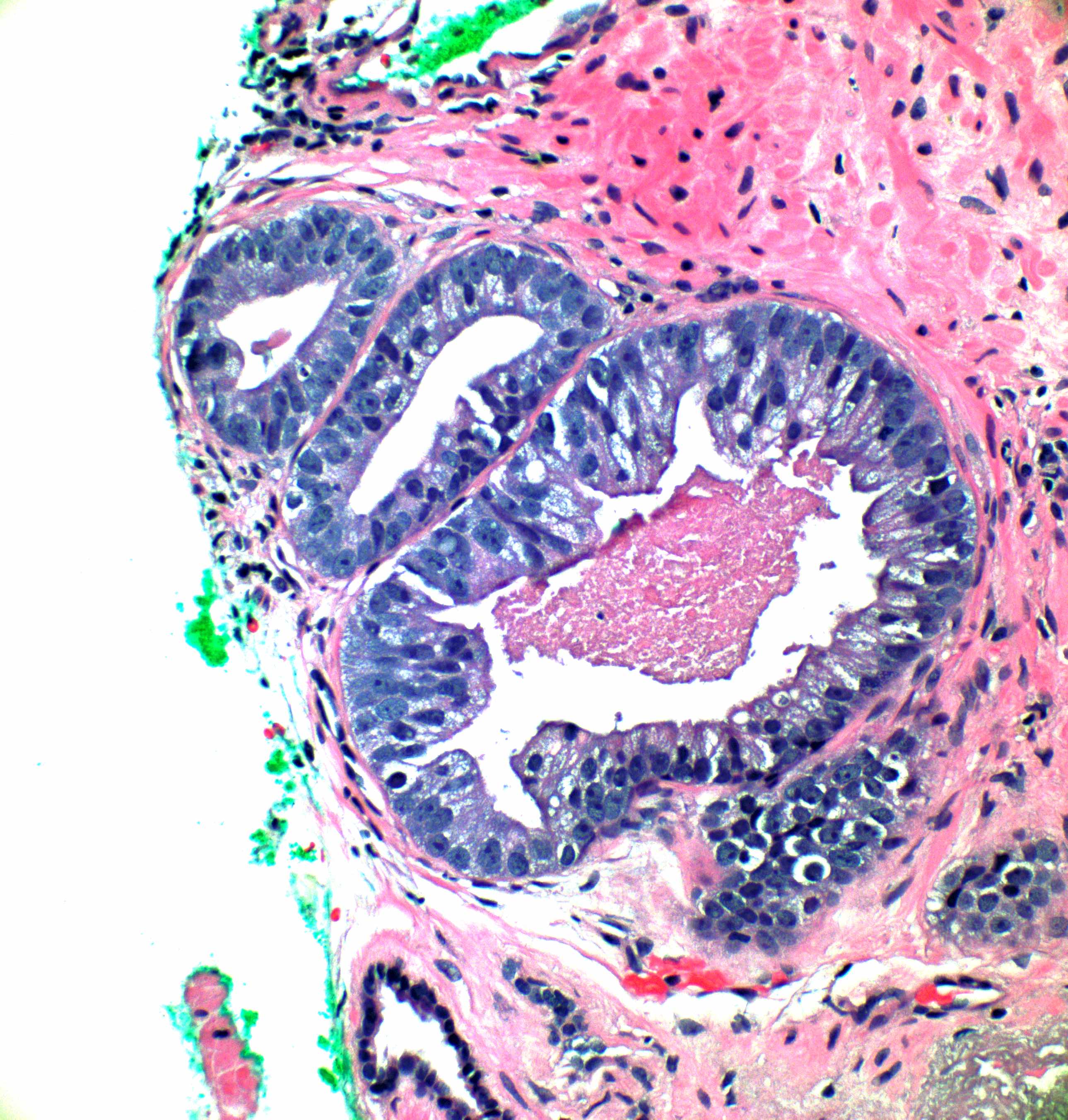
- Atypical intraductal proliferation (AIP) of the prostate is a gland space containing a usually cribriform arrangement of cells that have mild to moderate atypia, bounded by at least a partial basal cell layer
- It is a diagnosis of uncertainty on the continuum between high grade prostatic intraepithelial neoplasia and intraductal carcinoma, which is most applicable if tissue sampling is limited, as with biopsies
Essential features
- AIP is a continuum of proliferations, mostly (90%) cribriform (but 10% may be noncribriform) in which a distinction is not possible between high grade prostatic intraepithelial neoplasia (HGPIN) and intraductal carcinoma of prostate (IDC) (Histopathology 2019;75:346)
- AIP architectural complexity or cytologic atypia exceed that of HGPIN but fall short of the criteria for IDC
- At least a partial basal cell layer is retained by H&E or immunostain
- Location of AIP / atypical cribriform lesion (ACL) within 3 mm from infiltrating cancer is considered to favor IDC, while > 3 mm from infiltrating cancer favors HGPIN
- Exclusion criteria: comedonecrosis or extreme nuclear atypia; total loss of a basal cell layer
- Most AIP occurrence on needle biopsy is accompanied by invasive carcinoma; whereas if there is only isolated AIP, repeat biopsy may show invasive carcinoma (Histopathology 2019;75:346)
Terminology
- Atypical cribriform lesion (ACL) (Hum Pathol 2014;45:1572)
- Atypical cribriform intraductal proliferation
- Atypical proliferation suspicious for intraductal carcinoma (ASID) (Pathology 2018;50:60)
ICD coding
- ICD-9: 602.3 - dysplasia of prostate
Epidemiology
- Mean age: 65; mean serum PSA: 8 (Histopathology 2019;75:346)
- Incidence in radical prostatectomy cases: incidence was 17.9% of cases for all lesions, among which 12.8% were isolated lesions; that is, not associated with infiltrating cancer (Am J Surg Pathol 2010;34:470)
- Incidence in needle biopsies: incidence was estimated at 1% (8/796) (Histopathology 2019;75:346)
- AIPs unaccompanied by invasive carcinoma comprised only 10% of the above, for a 0.1% incidence
Sites
- Prostate
Diagnosis
- Histologic finding of prostate biopsy or prostatectomy; rarely sampled in transurethral resection
- No radiologic correlates are known
Laboratory
- Elevated serum PSA
Prognostic factors
- Histologic findings in prostatectomy specimens
- AIP was associated with higher Gleason score and larger tumor volume, than in prostates without it (Am J Surg Pathol 2010;34:470)
- Compared to prostatectomy with high grade PIN, AIP was associated by multivariate analysis with higher Gleason score, larger tumor volume and more advanced stage than in cases with only high grade PIN (Hum Pathol 2014;45:1572)
- Clinical findings in prostatectomy specimens
- AIP / ACL showed a risk for biochemical recurrence that was intermediate between cases with high grade PIN and intraductal carcinoma (Hum Pathol 2014;45:1572)
- High grade PIN only, without AIP, by multivariate analysis indicated a less frequent biochemical recurrence
Treatment
- On biopsies if there is concomitant invasive carcinoma, definitive treatment or active surveillance is needed
- On biopsies if the AIP finding is isolated, repeat biopsy is advisable; this may show cancer (4 of 6 cases) or benign prostate (2 of 6) (Histopathology 2019;75:346)
Microscopic (histologic) description
- Concept of AIP / ACL was introduced in a pair of articles in 2010 (Am J Surg Pathol 2010;34:470, Am J Surg Pathol 2010;34:478)
- One definition of AIP was a loose cribriform intraductal proliferation with greater architectural complexity when compared to HGPIN but lacking significant nuclear pleomorphism or necrosis (Hum Pathol 2014;45:1572)
- In another study, cases were divided into isolated ACL and ACL associated with invasive cancer (Am J Surg Pathol 2010;34:470)
- Isolated ACL almost never (1/23 cases) had a branching contour, none had comedonecrosis and none had 6x nuclear enlargement, standing in contrast to 84%, 33% and 28%, respectively of cases associated with invasive cancer
- Cases with associated cancer were considered intraductal carcinoma
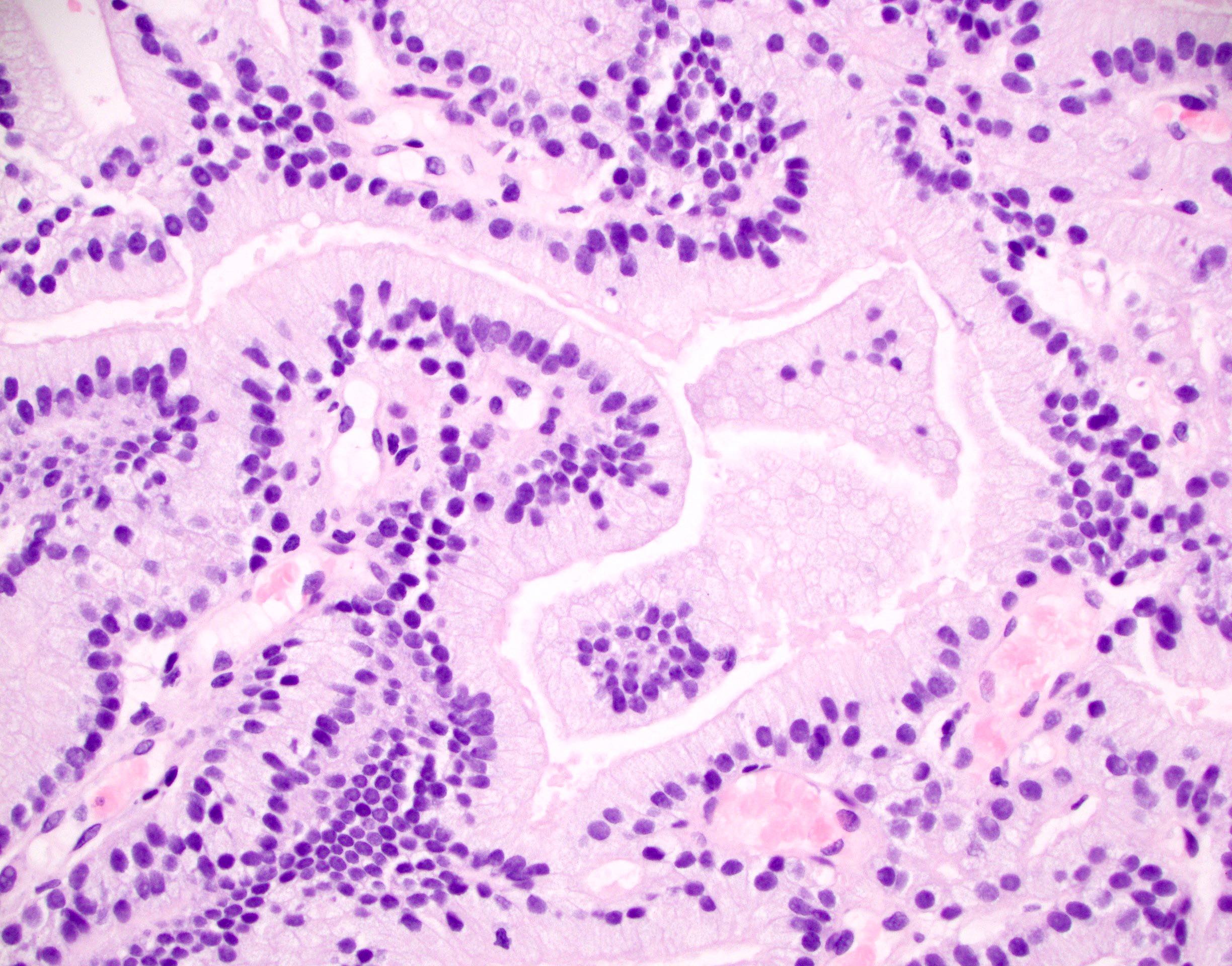
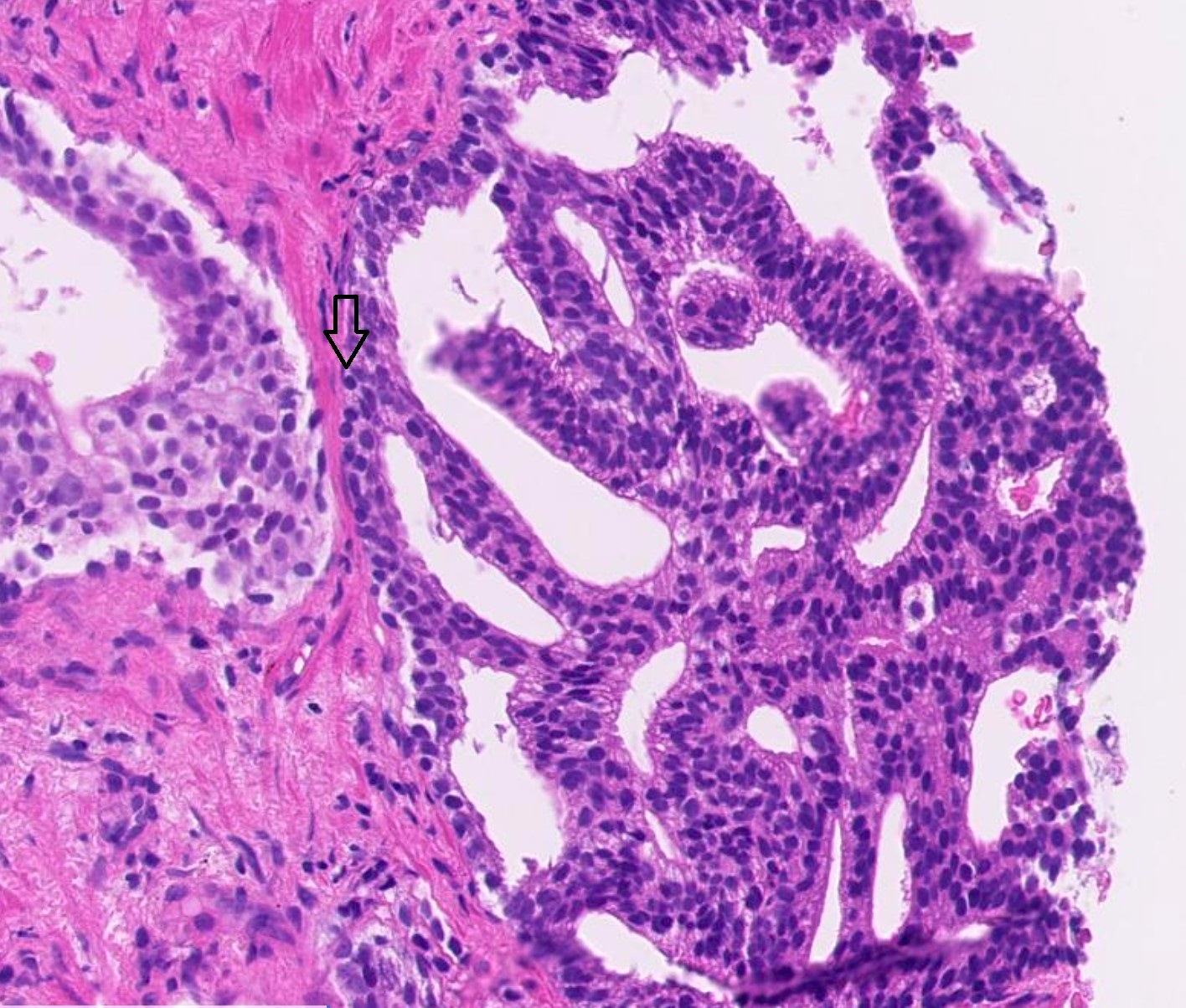
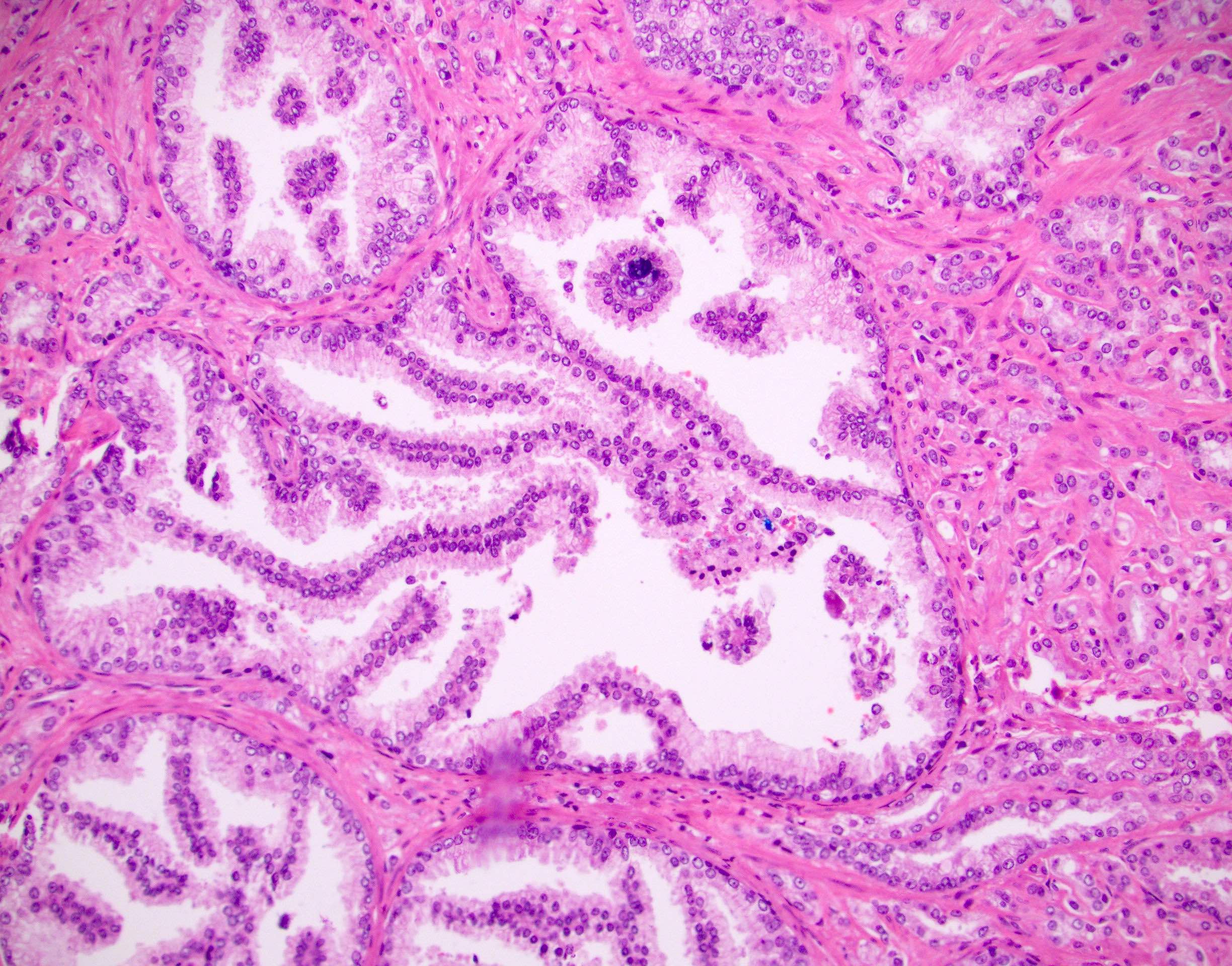
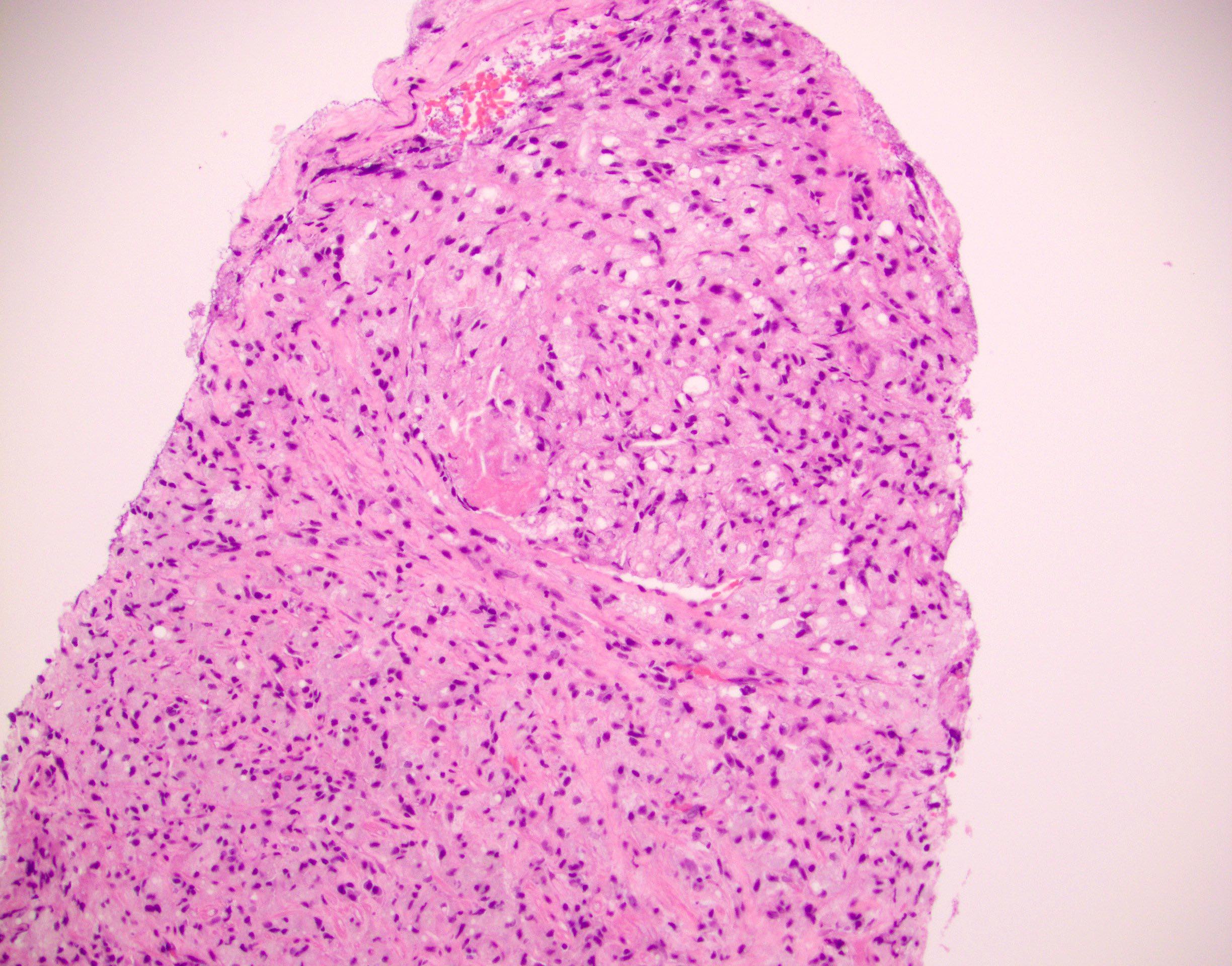
Microscopic (histologic) images
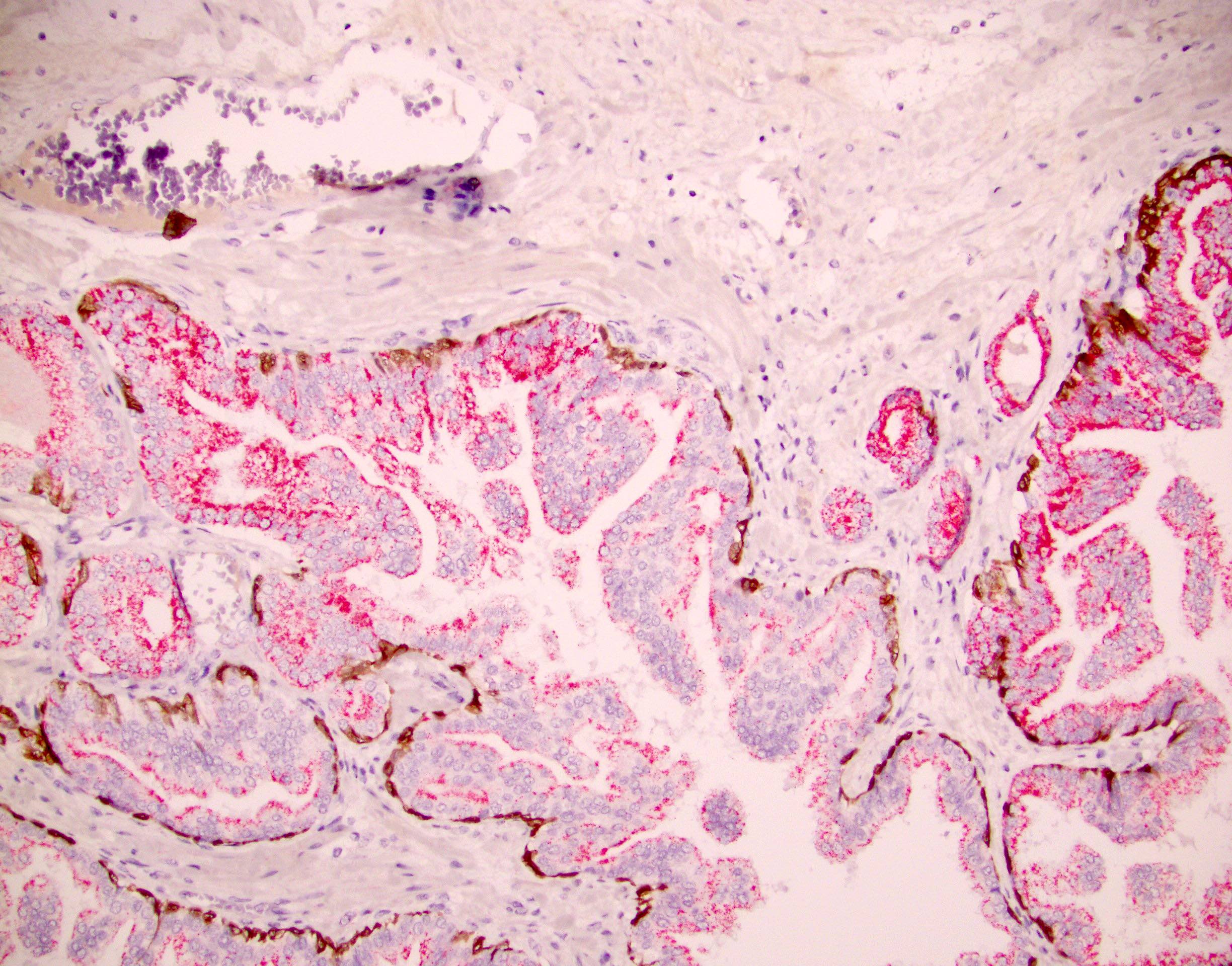
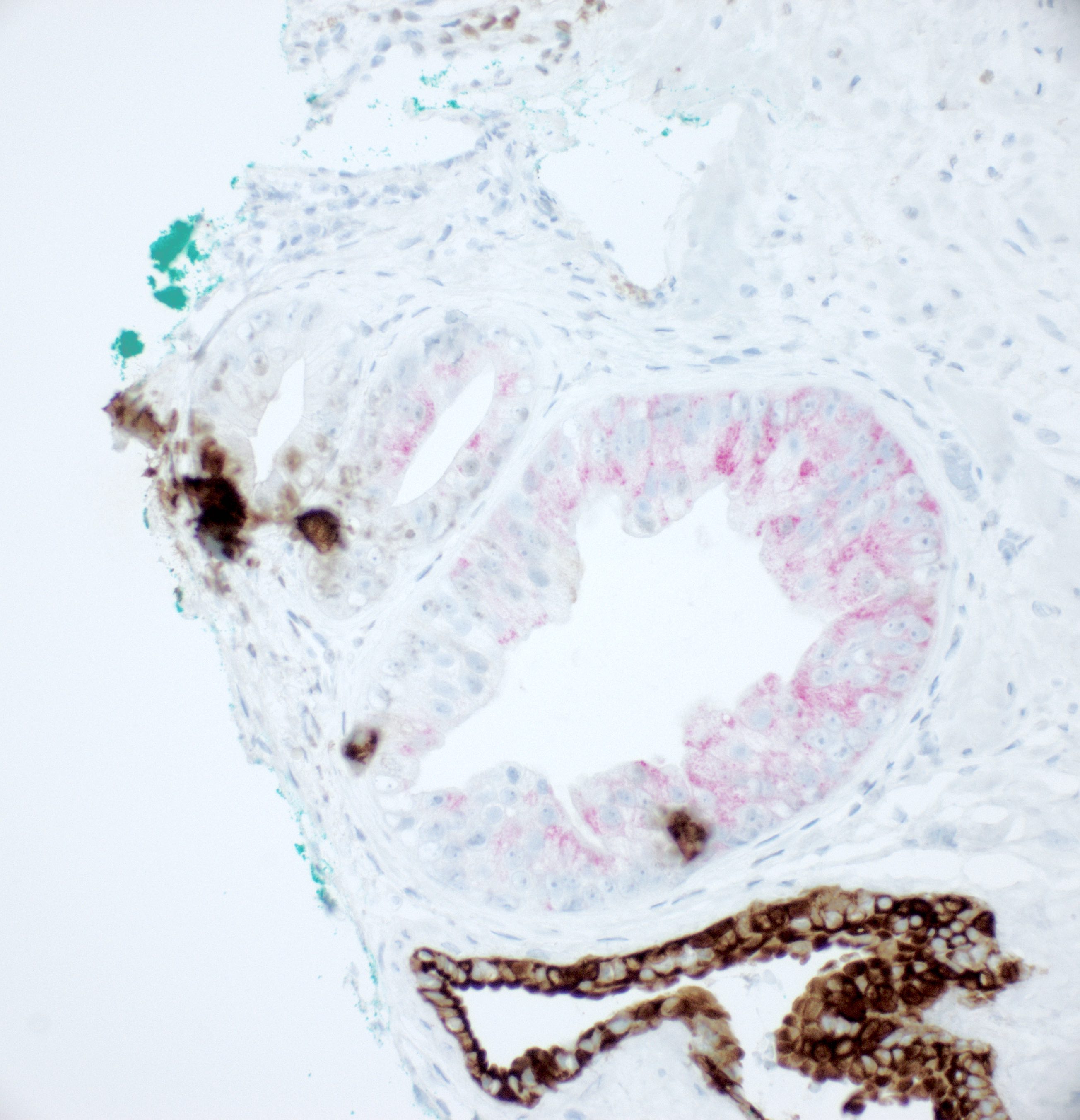
Positive stains
- NKX3.1, prostate specific antigen, prostatic acid phosphatase, prostein (P501S), AMACR
- Basal cell reactivity at least focally retained as shown by CK 34 beta E12, p63 or CK5/6
- ERG reactivity in some cases (Am J Surg Pathol 2010;34:470)
Molecular / cytogenetics description
- Break apart FISH probe was used to investigate AIP
- In prostatectomy lesions that were cribriform HGPIN, 0/16 (0%) had ERG rearrangement
- ACL lesions with associated invasive cancer were presumed to be intraductal carcinoma and 75% (36/48) had ERG rearrangement
- Among these, 65% were through deletion and 35% through insertion (Am J Surg Pathol 2010;34:478)
- Those ACL with marked atypia (comedonecrosis or 6x nuclear enlargement) had a similar incidence of ERG rearrangement to those that did not (78% versus 72%)
- PTEN loss status was similar to adjacent invasive cancer in 88% of biopsy cases designated AIP and in 91% of cases designated IDC, suggesting 2 different morphologic spectra of IDC (Histopathology 2017;71:693)
Sample pathology report
- Prostate, right apex, biopsy:
- Isolated atypical intraductal proliferation, cannot distinguish between prostatic intraepithelial neoplasia and intraductal carcinoma (see comment)
- Comment: This lesion may be associated with invasive cancer. Repeat biopsy may be advisable to rule out unsampled prostate cancer.
Differential diagnosis
- Clear cell cribriform hyperplasia:
- Located in central zone / base of prostatic biopsies
- Typically clear cytoplasm
- No cytologic atypia; nucleoli not visible in central cells but may be visible in basal cells
- Basal cell layer is retained
- No comedonecrosis
- High grade prostatic intraepithelial neoplasia (HGPIN):
- The cribriform pattern is 1 of 4 HGPIN patterns
- Cytologic atypia is not extreme
- Usually stratified vesicular nuclei
- At least a patchy basal cell layer is retained
- No comedonecrosis
- Intraductal carcinoma of prostate:
- Defined as a confluent sheet of contiguous malignant epithelial cells with multiple glandular lumina that are easily visible at low power (objective magnification 10x); there should be no intervening stroma or mucin separating individual or fused glandular structures (Am J Surg Pathol 2020;44:e87)
- More extreme cytologic atypia and nuclear enlargement (up to 6x)
- Branching of duct contours is common
- Possibly comedonecrosis
- Ductal carcinoma of prostate:
- Lacks a basal cell layer
- Pseudostratified columnar epithelium with cytoplasmic tufting
- May have pagetoid spread
- Invasive Gleason 4 (or 5) cribriform prostatic adenocarcinoma:
- Lacks a basal cell layer, unlike AIP or intraductal carcinoma
- May have comedonecrosis; if so, the grade is elevated to Gleason 5
Board review style question #1
Board review style answer #1
C. Loose cribriform arrangement of cells. Most atypical intraductal proliferations (or atypical cribriform lesion [ACL]), like this one, do contain a loose cribriform pattern. We do not see 6x nuclear enlargement in this example and if present, it would favor intraductal carcinoma (IDC) (answer A). We do not see comedonecrosis here and again this would favor IDC (answer B). Atypical intraductal proliferation (AIP) should retain at least some basal cells and there probably are some by H&E on the left edge of the proliferation. Absence of basal cells would favor IDC (answer D).
Comment Here
Reference: Atypical intraductal proliferation
Comment Here
Reference: Atypical intraductal proliferation