Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Ibad OA, Husain AN. Mesothelioma (pleura)-epithelioid. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/pleuramesotheliomaepithelioid.html. Accessed March 30th, 2025.
Definition / general
- Diffuse mesothelial malignancy with epithelioid histology
- Most frequent subtype of mesothelioma, accounting for 60 - 70% of cases (Arch Pathol Lab Med 2024 Apr 8 [Epub ahead of print])
- Can have multiple architectural, stromal and morphological patterns (Arch Pathol Lab Med 2024 Apr 8 [Epub ahead of print])
Essential features
- Diffuse mesothelial malignancy with epithelioid histology, accounting for 60 - 70% of cases in total
- Has the best overall prognosis of all histological subtypes
- Commonly diagnosed using a panel of immunohistochemistry stains (calretinin, WT1, D2-40 [podoplanin], cytokeratin 5 or 5/6 and HEG1)
- Other diagnostic modalities include cytological testing, molecular testing, next generation sequencing and electron microscopy
- Depending on the stage, treatment may include surgery, chemotherapy, radiation, immunotherapy or a combination thereof
Terminology
- Malignant epithelioid pleural mesothelioma
ICD coding
Epidemiology
- Represents 60 - 70% of all malignant pleural mesotheliomas (Arch Pathol Lab Med 2024 Apr 8 [Epub ahead of print])
- Of 5,989 histologically typed and diagnosed cases of pleural mesothelioma in the United States between 2000 - 2016, ~64.4% were of the epithelioid subtype (Cureus 2021;13:e14605)
- Of 11,539 patients diagnosed with pleural mesothelioma in the Netherlands between 1993 - 2018, 76% diagnosed were epithelioid (Thorax 2022;77:1260)
- Despite the overall decrease in prevalence of mesothelioma over time, the prevalence of epithelioid mesothelioma has increased due to better diagnostic accuracy (Cureus 2021;13:e14605)
Sites
- Pleura (parietal and visceral)
Pathophysiology
- Asbestos fibers induce tumorigenesis and mutagenesis through alterations in DNA repair genes (Thorac Surg Clin 2020;30:383)
- Pleural irritation and inflammation
- Mitotic interference
- Kinase mediated signaling
- Proto-oncogene activation
- Free radical formation
- Loss of tumor suppressor genes, including BAP1, CDKN2A / CDKN2B, NF2 and TP53 (Thorac Surg Clin 2020;30:383)
Etiology
- Asbestos exposure is seen in > 80% of mesotheliomas (Thorac Surg Clin 2020;30:383)
- Focal and larger chromosomal losses in 1p, 3p14-p21, whole chromosome 4, 6q, 9p and 22q with aneuploidy observed in 88% of epithelioid pleural mesotheliomas (Thorac Surg Clin 2020;30:383)
- Some chromosomal gain in chromosome 17q has been noted, although it is not as common (Thorac Surg Clin 2020;30:383)
- Associated with germline mutation of BRCA1 associated protein (BAP1) on chromosome 3p21 (Thorac Surg Clin 2020;30:383)
- Associated with somatic mutations in BAP1, CDKN2A / CDKN2B, NF2 and TP53 (Thorac Surg Clin 2020;30:383)
- Nonasbestos linked causes include erionite, fluoro-edenite and carbon nanotubes as well as radiation exposure (Thorac Surg Clin 2020;30:383)
Clinical features
- Breathlessness
- Chest pain
- Cough
- Weight loss and fatigue
- Fevers and night sweats
- Thoracic bone pain and neuropathic pain when tumor invades surrounding structures
- Recurrent unilateral pleural effusion
- Reference: J Thorac Dis 2018;10:S253
Diagnosis
- Chest computed tomography (CT) scan with intravenous (IV) contrast is the preferred initial evaluation for suspected cases and can be paired with PET CT (Eur Respir J 2020;55:1900953)
- Thoracocentesis for patients with pleural effusion (Eur Respir J 2020;55:1900953)
- Thoracoscopic biopsies are the standard for biopsy (Eur Respir J 2020;55:1900953)
- Other methods for biopsy include (Eur Respir J 2020;55:1900953)
- CT guided or ultrasound guided pleural biopsy
- Video assisted thoracic surgery
- Local anesthetic thoracoscopy
- Open pleural biopsy
- Algorithmic approach can be taken to diagnose epithelioid pleural mesothelioma (Arch Pathol Lab Med 2024 Apr 8 [Epub ahead of print])
- Broad spectrum cytokeratin stain is used to establish mesothelial / epithelial tissue origin
- 2 mesothelium specific stains (calretinin, WT1, D2-40 [podoplanin] and CK5/6) and 2 epithelium specific stains (claudin4, MOC31, BerEP4, CEA, BG8, B72.3) to further determine the origin
- Recent literature suggests only 2 markers (HEG1 and claudin4) can be used for diagnosis but this is not practiced in widespread clinical use
- If morphologically malignant, then it is considered an epithelioid mesothelioma
- If the morphology is diagnostically challenging from a benign mesothelial proliferation, it is proven to be malignant with loss of BAP1 / MTAP on immunohistochemistry
- If BAP1 / MTAP are still retained, molecular studies exhibiting homozygous loss of CDKN2A or next generation sequencing can be used to prove malignant nature
- Further immunohistochemistry stains may be used to rule out common differential diagnoses (see Stains and Differential diagnosis)
- Cytologic analysis on pleural fluid specimens when paired with immunohistochemistry can be used much like tissue specimens (Cytopathology 2015;26:142)
- Given the diagnostic accuracy of immunohistochemistry, electron microscopy is now rarely used for diagnosis (Arch Pathol Lab Med 2024 Apr 8 [Epub ahead of print])
- Next generation sequencing panels may be used but are not routinely performed (Arch Pathol Lab Med 2024 Apr 8 [Epub ahead of print])
Laboratory
- Serum mesothelin may be used as a monitoring biomarker but due to great variability, is not generally used (Chest 2017;152:143)
- Other studied markers (fibulin3, ENOX2, osteopontin) either lack specificity or require additional studies for validation (Eur Respir J 2020;55:1900953)
Radiology description
- Xray (J Thorac Dis 2018;10:S253)
- Pleural effusion
- Nodular pleural thickening
- Loss of volume in ipsilateral hemithorax
- Low sensitivity
- Further imaging is usually required
- Thoracic ultrasound (J Thorac Dis 2018;10:S253)
- Pleural thickening > 1 cm
- Nodular thickening
- Diaphragmatic nodularity
- Low sensitivity
- Further imaging is usually required
- CT scan with intravenous contrast is preferred (allows visualization of entire pleura and diaphragm)
- Pleural rind (Orphanet J Rare Dis 2008;3:34)
- Nodular thickening of the pleura (Orphanet J Rare Dis 2008;3:34)
- Pleura thicker than 1 cm (Orphanet J Rare Dis 2008;3:34)
- Involvement of the mediastinal pleura (J Thorac Dis 2018;10:S253)
- Magnetic resonance imaging (MRI)
- Not routinely used unless CT with intravenous contrast is contraindicated (Orphanet J Rare Dis 2008;3:34)
- May be used with gadolinium contrast in addition to CT for additional staging information through identification of tumor extension into the diaphragm, chest wall or pericardium (J Thorac Dis 2018;10:S253)
- Standardized uptake value PET (J Thorac Dis 2018;10:S253)
- Used to identify mediastinal nodal metastasis, stage and preoperatively evaluate mesothelioma by evaluating uptake
- Adjunct to CT in the setting of CT guided pleural biopsy
Radiology images
Prognostic factors
- Compared to other histological subtypes, epithelioid histological pattern has a better overall prognosis and a higher median survival rate (J Surg Res 2015;196:23)
- Favorable prognostic features (J Thorac Oncol 2022;17:608)
- Architectural patterns: tubulopapillary, trabecular, adenomatoid, solid, micropapillary
- Cytologic patterns: lymphohistiocytiocytoid
- Stromal features: myxoid (if predominant [i.e., ≥ 50% of tumor is myxoid] with < 50% solid pattern)
- Low nuclear grade
- Loss of BAP1 by immunohistochemistry
- Unfavorable prognostic features (J Thorac Oncol 2022;17:608)
- Architectural patterns: solid > 50% and micropapillary
- Cytologic features: rhabdoid, pleomorphic
- High nuclear grade
- Necrosis
- Homozygous deletion of CDKN2A and MTAP loss
Case reports
- 65 year old woman presented with complete dysphagia and developed chylous bilateral pleural effusion after initial treatment (BMJ Case Rep 2021;14:e243803)
- 66 year old man diagnosed with epithelioid pleural mesothelioma treated with pembrolizumab monotherapy, showing no evidence of disease 33 months after diagnosis (Case Rep Oncol 2020;13:1483)
- 70 year old man with known malignant pleural mesothelioma and mass on left lateral tongue discovered to be metastases (BMJ Case Rep 2021;14:e242510)
- 70 year old man presented with a left pleural effusion that had features of both adenocarcinoma and epithelioid pleural mesothelioma (Respir Med Case Rep 2018;26:45)
- 82 year old woman who was diagnosed with epithelioid mesothelioma and survived 3.5 years without any treatment measures (Respir Med Case Rep 2021;33:101381)
Treatment
- Clinical stage I - IIIA with epithelioid histology (J Natl Compr Canc Netw 2024;22:72)
- Option 1: induction chemotherapy with pemetrexed and cisplatin followed by surgical exploration to determine if disease is resectable
- Resectable: pleurectomy / decortication or extrapleural pneumonectomy followed by radiotherapy
- Unresectable: radiotherapy
- Option 2: surgical exploration
- Resectable: pleurectomy / decortication or extrapleural pneumonectomy followed by chemotherapy and radiotherapy
- Unresectable: systemic therapy and consider radiotherapy
- Option 3: systemic therapy composed of chemotherapy and radiation therapy
- Option 4: observation, if patient is asymptomatic with minimal burden of disease or if systemic therapy is planned on radiologic progression or symptomatic disease
- Option 1: induction chemotherapy with pemetrexed and cisplatin followed by surgical exploration to determine if disease is resectable
- Clinical stage IIIB - IV
- Performance status 0 - 2: systemic therapy or observation
- Performance status 3 - 4: best supportive care
- Systemic therapy
- Preferred regimens include pemetrexed / cisplatin or nivolumab / ipilimumab
- Bevacizumab is only added in healthy patients on the pemetrexed / cisplatin regimen
- ERS / ESTS / EACTS / ESTRO guidelines
- Combination chemotherapy of platinum and pemetrexed with folic acid and B12 supplementation is first line in patients fit enough (Eur Respir J 2020;55:1900953)
- Combined with bevacizumab in patients fit enough
- Thoracoscopic talc poudrage via thoracoscopy is used for recurrent effusion (Eur Respir J 2020;55:1900953)
- Video assisted thoracoscopic surgery - partial pleurectomy in those who fail chemical pleurodesis or catheter placement for recurrent effusion (Eur Respir J 2020;55:1900953)
- Radiotherapy may be used for palliative pain relief (Eur Respir J 2020;55:1900953)
- Combination chemotherapy of platinum and pemetrexed with folic acid and B12 supplementation is first line in patients fit enough (Eur Respir J 2020;55:1900953)
Gross description
- Small nodules in the parietal pleura
- Can extend and involve visceral and parietal pleura to form adhesions
- Encases the lung parenchyma in a rind, extending into the fissures
- Reference: Environ Health Prev Med 2008;13:60
Frozen section description
- Typically done to determine presence of malignancy before resection
- In patients undergoing surgical biopsy for suspected mesothelioma, tissue may be submitted to determine if lesional tissue is present
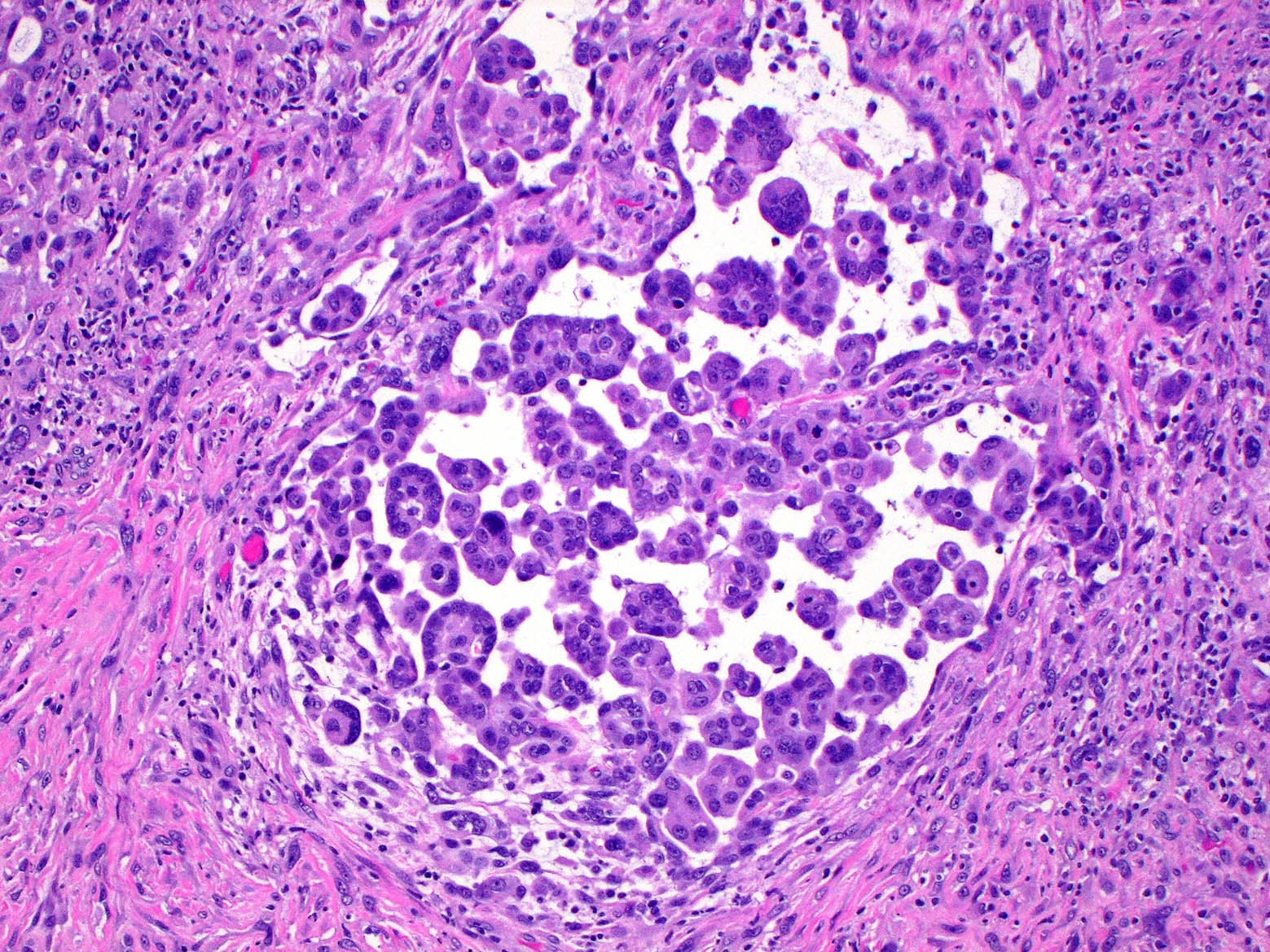
Microscopic (histologic) description
- Polygonal, oval or cuboidal cells
- Round nuclei with prominent nucleoli
- Moderately eosinophilic cytoplasm
- May have necrosis, atypical mitotic figures and psammoma bodies
- Stroma can be myxoid with hyaluronic acid or desmoplastic
- Common architectural patterns: tubulopapillary, acinar (glandular), adenomatoid (microglandular), sheets (solid) or mixed
- Less common architectural patterns: rhabdoid, small cell, signet ring, deciduoid and clear cell
- Lymphohistiocytoid pattern
- Nuclear grading (J Thorac Oncol 2022;17:608)
- See Diagrams / tables
- Low grade = grade I and II without necrosis
- High grade = grade II with necrosis and grade III with or without necrosis
Microscopic (histologic) images
Contributed by Aliya N. Husain, M.D.
Cytology description
- Highly cellular effusion containing epithelioid cells with nuclear atypia
- Both large and small tissue fragments
- Berry-like tissue fragments with clear spaces / windows between cells and scalloped surface
- Cell in cell arrangements
- Multinucleated cells
- Eosinophilic and orangeophilic cells
- Reference: Cytopathology 2015;26:142
Positive stains
- Broad spectrum cytokeratin stains (AE1, CAM5.2, CK OSCAR) are 100% sensitive for epithelioid mesothelioma and can be used initially to establish mesothelial lineage
- Positive epithelioid mesothelioma markers (calretinin, CK5/6, WT1, D2-40 [podoplanin], HEG1) should be used to rule in epithelioid mesothelioma
- Reference: Arch Pathol Lab Med 2024 Apr 8 [Epub ahead of print]
Negative stains
- Positive epithelial markers (claudin4, MOC31, BerEP4, CEA, BG8, B72.3, CD15) can be used to rule out epithelial lineage
- Positive lung squamous cell carcinoma markers (claudin4, CEA, p40, p63, BG8, MOC41, BerEP4) can be used to rule out lung squamous cell carcinoma
- Positive lung adenocarcinoma markers (TTF1, Napsin A) can be used to rule out lung adenocarcinoma
- Loss of BAP1 is seen in 60 - 70% of epithelioid mesotheliomas and can support diagnosis in differential with certain carcinomas (seen in < 1% of lung cancers and serous carcinomas)
- Loss of MTAP is seen in 40% of epithelioid mesotheliomas; it helps to establish malignancy but does not aid in formulating a differential diagnosis
- Reference: Arch Pathol Lab Med 2024 Apr 8 [Epub ahead of print]
Electron microscopy description
- Apical cell membranes with microvilli at basolateral surfaces forming extracellular neolumina or intracellular vesicle / intracellular neolumina
- Long, branching microvilli without any core rootlets with the absence of a glycocalyx
- Enlarged desmosomes
- Reference: Cytopathology 2015;26:142
Molecular / cytogenetics description
- Homozygous deletion of CDKN2A, detectable by FISH
- Other genes implicated include BAP1, MTAP, NF2, TP53, LATS1 / LATS2 and SETD2
- Reference: Thorac Surg Clin 2020;30:383
Videos
Pleura: mesothelioma (epithelioid) microscopy - talking slide
Sample pathology report
- Left pleura, core needle biopsy:
- Epithelioid mesothelioma (see comment)
- Mesothelioma pathologic parameters
- Macroscopic
- Specimen type: surgical biopsy
- Tumor site: right pleura
- Microscopic
- Histologic type: epithelioid mesothelioma (80% solid, 20% papillary)
- Nuclear grade (for epithelioid only): II of III
- Nuclear atypia score: 2 (1 for mild, 2 for moderate, 3 for severe)
- Mitotic count: 2 (1 for low [1/10], 2 for intermediate [2 - 4/10], 3 for high [5+/10])
- Sum: 4 (2 or 3 = grade I, 4 or 5 = grade II, 6 = grade III)
- Necrosis (for epithelioid only): absent
- 2 tier grade: low grade
- Extent of invasion: into adipose tissue
- Block(s) for molecular markers: A1
- Additional pathologic findings
- Macroscopic
- Comment: The neoplastic cells are positive for calretinin and CK5/6 while they are negative for CK7, CK20 and TTF1. BAP1 is lost in tumor nuclei. PDL1 and molecular studies are requested.
Differential diagnosis
- Reactive mesothelial hyperplasia:
- No loss of BAP1 / MTAP in reactive mesothelial hyperplasia
- Next generation sequencing
- Homozygous deletion of CDKN2A
- Adenocarcinoma:
- Squamous cell carcinoma of the lung:
- CK5/6 and D2-40 (podoplanin) are not useful in distinguishing (positive in 100% and 50% of squamous cell carcinomas, respectively)
- p63, MOC31, BG8 and BerEP4 should be used
- Malignant melanoma:
- Pancytokeratin negative
- Angiosarcoma:
- D2-40 (podoplanin) is not useful in distinguishing
- CD31 and CD34 should be used
- Lymphoma:
- Pancytokeratin negative
- Renal cell carcinoma:
Board review style question #1
Board review style answer #1
A. BAP1 loss. When differentiating malignant mesothelioma from a benign mesothelial proliferation, BAP1 loss proves malignancy because no benign lesion has been shown to lose BAP1. Answer E is incorrect because WT1, although 88% specific for epithelioid mesothelioma, does not possess the ability to determine if a tissue is benign or malignant. Answer C is incorrect because claudin4, in addition to being virtually always negative for tissue of mesothelial origin, does not possess the ability to determine if a tissue is benign or malignant. Answer B is incorrect because CK5/6, although positive in 91% of epithelioid mesotheliomas, does not differentiate between malignant and benign tissue. Answer D is incorrect because while pancytokeratin has near 100% sensitivity for detecting tissue of mesothelial origin, it does not differentiate between malignant and benign tissue.
Comment Here
Reference: Mesothelioma (pleura)-epithelioid
Comment Here
Reference: Mesothelioma (pleura)-epithelioid
Board review style question #2
When differentiating mesothelioma from carcinoma, which one of the following stain panels is in line with best practices?
- AE1, D2-40 (podoplanin), CK5/6, calretinin, WT1
- Pancytokeratin, BAP1, MTAP
- Pancytokeratin, calretinin, WT1, claudin4, MOC31
- Pancytokeratin, HEG1, claudin4, S100
- TTF1, Napsin A, claudin4, MOC31
Board review style answer #2
C. Pancytokeratin, calretinin, WT1, claudin4, MOC31. When determining mesothelial or carcinoma tissue of origin, current best practices suggest a panel of broad spectrum cytokeratin (pancytokeratin), 2 mesothelial markers (calretinin and WT1) and 2 carcinoma markers (claudin4 and MOC31). Answer D is incorrect because although recent literature suggests HEG1 and claudin4 may be used as the sole markers for tissue of mesothelial and epithelial origin respectively, S100 is neither specific nor sensitive for either. Answer B is incorrect because loss of the stains BAP1 and MTAP are used to prove malignant mesothelial tissue and have no role in differentiating mesothelial tissue from carcinoma tissue. Answer A is incorrect because all stains mentioned (except AE1) are sensitive / specific for tissue of mesothelial origin. Answer E is incorrect because no stain mentioned is sensitive / specific for tissue of mesothelial origin.
Comment Here
Reference: Mesothelioma (pleura)-epithelioid
Comment Here
Reference: Mesothelioma (pleura)-epithelioid