Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Roden AC. Diffuse mesothelioma (pleura). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/pleuramesothelioma.Html. Accessed December 26th, 2024.
Definition / general
- Malignant neoplasm of mesothelial differentiation that arises from mesothelial lining cells of the pleura
- Can be of epithelioid or sarcomatoid cytology or a combination thereof
Essential features
- Aggressive neoplasm of mesothelial differentiation
- Epithelioid, biphasic or sarcomatoid subtype
- Overall survival, 4 - 27 months, depending on subtype
- Most commonly associated with remote (up to 40 years prior) asbestos exposure
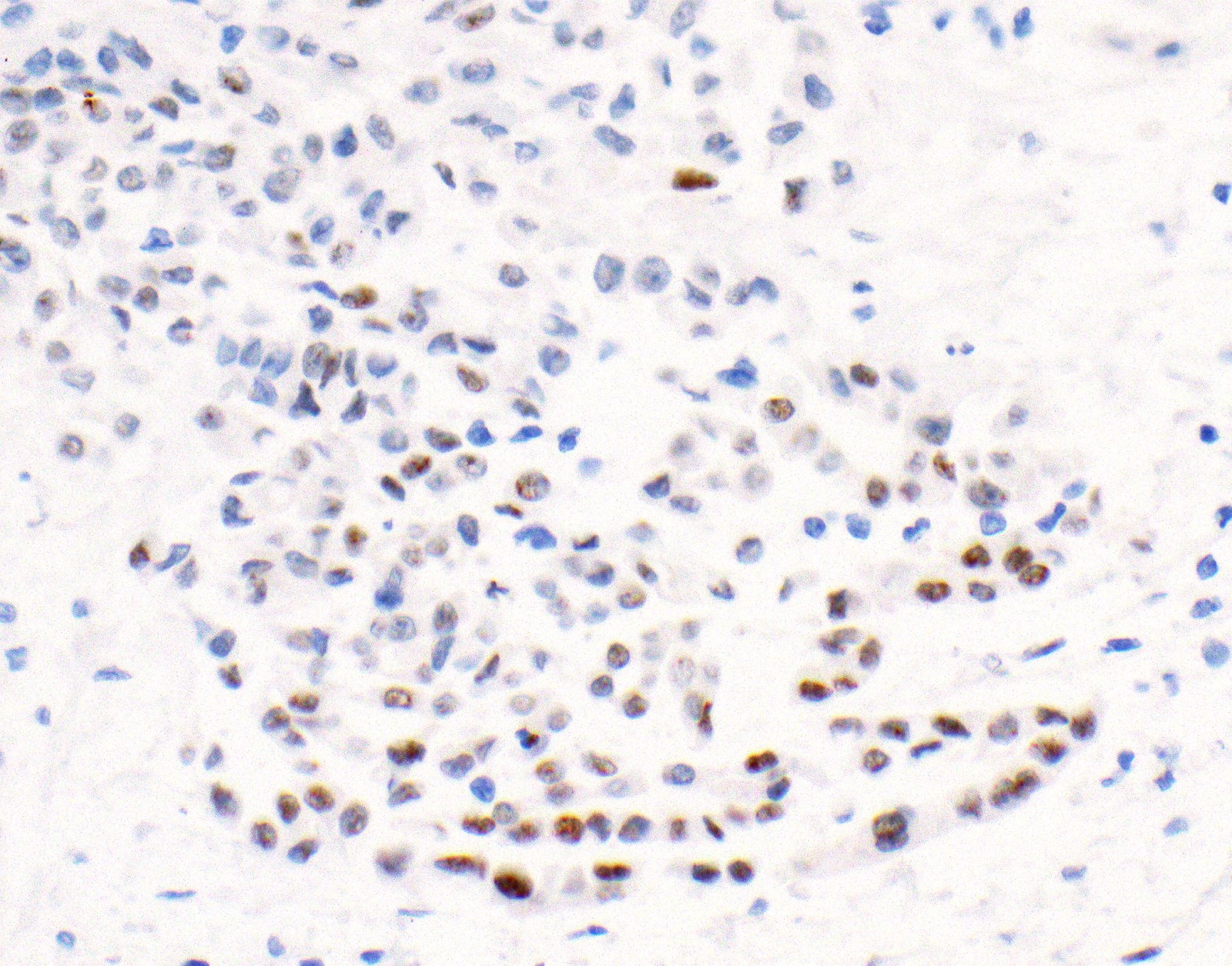
- Loss of expression of BRCA1 associated protein (BAP1) or methylthioadenosine phosphorylase (MTAP) or homozygous deletion of cyclin dependent kinase inhibitor 2A (CDKN2A) (p16) by FISH helps to distinguish reactive mesothelial proliferation from malignant pleural mesothelioma
Epidemiology
- Incidence varies by geographic location; up to 30 cases/million people/year in Australia, Great Britain; 2,000 - 3,000 new cases per year in the United States (Surg Pathol Clin 2020;13:73, Semin Respir Crit Care Med 2019;40:347)
- An estimated 28,000 - 43,000 people die from malignant pleural mesothelioma worldwide every year (Semin Respir Crit Care Med 2019;40:347)
- Elderly patients; median age, 63 - 70 years (range, 26 - 95 years) (Thorac Cancer 2019;10:1193, Ann Thorac Surg 2018;105:432, Surg Pathol Clin 2020;13:73)
- Male predominance; 75 - 87% male (Thorac Cancer 2019;10:1193, Ann Thorac Surg 2018;105:432, Surg Pathol Clin 2020;13:73)
- Latency period between asbestos exposure and development of disease of 20 to > 40 years, depending on severity and duration of exposure (Surg Pathol Clin 2020;13:73)
- Strong association with asbestos exposure either occupational (e.g., shipbuilding, construction, automotive industry) or residential (industrial contamination, environmental / domestic) (Br J Ind Med 1960;17:260, Lung Cancer 2004;45:S3)
- Rare in young patients (≤ 35 years old); in that population equal sex distribution, more commonly history of prior mantle radiation and family history of breast cancer, history of asbestos exposure less common than in older patients (Mod Pathol 2018;31:122)
Sites
- Parietal, visceral, mediastinal or diaphragmatic pleura
Pathophysiology
- Hypotheses for how asbestos causes malignant pleural mesothelioma include (Respirology 2005;10:2):
- Toxic oxygen radical generation
- Chronic pleural irritation
- Persistent kinase mediated signaling
- Asbestos fibers exert cytotoxic and genotoxic effects
- Long and thin fibers associated with higher mutagenesis (Transl Lung Cancer Res 2020;9:S39)
- Erionite and zeolite fibers are specifically linked to malignant pleural mesothelioma and likely more potent carcinogens than other asbestos fibers (Semin Oncol 2002;29:2)
- Common inactivated tumor suppressors in malignant pleural mesothelioma: BAP1, neurofibromin 2 (NF2), CDKN2A
- Malignant mesothelioma in situ has high risk of developing invasive mesothelioma (Mod Pathol 2020;33:297)
Etiology
- Asbestos exposure
- Erionite exposure in genetically predisposed people thought to be associated with unusual high prevalence of malignant mesothelioma in region of Cappadocia, Turkey (Cancer Res 2006;66:5063)
- Ionizing radiation for treatment of malignancy (Hodgkin lymphoma, non-Hodgkin lymphoma, testicular cancer) might be a risk factor (Cancer 2006;107:108, Cancer 2007;109:1432, J Natl Cancer Inst 2005;97:1354)
- Close family members of patients with malignant pleural mesothelioma might have increased risk (Eur Respir J 2016;48:873)
- Molecular aberrations / familial - germline pathogenic variants identified in 12% of patients including mutations in BAP1 (1 - 4%), MutS homolog 3 (MSH3), breast cancer gene 1 associated ring domain 1 (BARD1), RecQ-like helicase 4 (RECQL4), breast cancer gene 2 (BRCA2), MRE11 homolog, double strand break repair nuclease (MRE11A), SHQ1, H/ACA ribonucleoprotein assembly factor (SHQ1) (1% each) (J Thorac Oncol 2020;15:655)
- Association with SV-40 is controversial (Respirology 2005;10:2)
Diagrams / tables
Images hosted on other servers:
Table 1. Commonly used immunohistochemical stains for the diagnosis of malignant pleural mesothelioma
| Immunohistochemical stain | in malignant mesothelioma, % | Relevant other neoplasms that might express that marker at least in a subset of cases |
| Keratin AE1 / AE3a | Carcinomas | |
| CAM 5.2 | Carcinomas | |
| WT1 (nuclear)a | Carcinomas of the gynecologic tract (e.g., serous carcinomas), Wilms tumor | |
| Calretinin (nuclear and cytoplasmic)a | Granulosa cell tumor, squamous cell carcinoma | |
| CK5, CK5/6a | Squamous cell carcinoma, NUT carcinoma, breast carcinoma, urothelial carcinoma | |
| Podoplanin (D2-40)a | Squamous cell carcinoma, follicular dendritic cell tumor, angiosarcoma, seminoma, serous carcinoma | |
| GATA3 (nuclear)b | Breast carcinoma, urothelial carcinoma | |
| pCEA | Adenocarcinoma, squamous cell carcinoma, neuroendocrine tumors | |
| MOC31 | Adenocarcinoma, squamous cell carcinoma | |
| BerEP4 | Adenocarcinoma, squamous cell carcinoma | |
| B72.3 | Adenocarcinoma, squamous cell carcinoma | |
| Claudin 4 | Adenocarcinoma, squamous cell carcinoma, small cell carcinoma, sarcomatoid carcinoma | |
| MUC4 | Adenocarcinoma, squamous cell carcinoma | |
| TTF1 (8G7G3/1) | Lung adenocarcinoma, thyroid carcinoma | |
| TTF1 (SP141) | Lung adenocarcinoma, thyroid carcinoma | |
| Napsin A | Lung adenocarcinoma, renal cell carcinoma | |
| p40 | Squamous cell carcinoma, thymic carcinoma, NUT carcinoma, urothelial carcinoma | |
| CDX2 | Pancreatobiliary adenocarcinoma, intestinal type adenocarcinoma colorectal adenocarcinoma | |
| PAX8 | Renal cell carcinoma, thyroid carcinoma, thymic carcinoma (polyclonal antibody), carcinoma of gynecologic tract | |
| Estrogen receptor | Breast carcinoma, carcinomas of gynecologic tract |
Table 2. Markers that aid in the distinction between reactive and malignant mesothelial proliferation
Mod Pathol 2015;28:1043,
Am J Surg Pathol 2015;39:977,
Am J Surg Pathol 2016;40:714,
Arch Pathol Lab Med 2018;142:1549,
Hum Pathol 2017;60:86,
Lung Cancer 2017;104:98,
J Thorac Oncol 2015;10:565,
Lung Cancer 2018;125:198,
Mod Pathol 2020;33:245,
Ann Diagn Pathol 2017;26:31
| Markers | Sensitivity | Specificity |
| Loss of expression of nuclear BAP1 All mesothelioma Epithelioid mesothelioma Sarcomatoid mesothelioma | 56 - 81 0 - 63 | |
| Loss of expression of cytoplasmic MTAP Epithelioid mesothelioma Sarcomatoid mesothelioma | 80 | |
| Homozygous deletion of CDKN2A (p16) by FISH All mesothelioma Epithelioid mesothelioma Sarcomatoid mesothelioma | 48 - 78 67 | |
| Loss of BAP expression or homozygous deletion of CDKN2A All mesothelioma Epithelioid mesothelioma Sarcomatoid mesothelioma | 92 67 - 100 | |
| Loss of expression of BAP1 or MTAP All mesothelioma Sarcomatoid mesothelioma | 90 | |
Clinical features
- Shortness of breath
- Chest wall pain, pleurisy
- Cough
- Weight loss
- Recurrent unilateral pleural effusion; might be hemorrhagic
- Occasionally asymptomatic when discovered at early stage
Diagnosis
- Pleural thickening or recurrent pleural effusion on chest Xray followed up with contrast enhanced chest CT scan
- Thoracocentesis acquiring pleural fluid for cytology
- In the past, was often considered not sufficient for definite diagnosis
- With BAP1 and MTAP immunostaining and FISH for homozygous deletion of CDKN2A, diagnosis of malignant pleural mesothelioma possible on at least a subset of fluids) (Cancer Cytopathol 2018;126:54)
- Pleural biopsy (e.g., video assisted thoracoscopic surgery [preferred], CT guided core biopsy, open biopsy
- Mediastinoscopy with lymph node sampling
Laboratory
- Serology for mesothelin and fibulin-3 might be useful as screening markers for malignant pleural mesothelioma, osteopontin lacks specificity (Curr Opin Pulm Med 2015;21:352, Carcinogenesis 2019;40:1320)
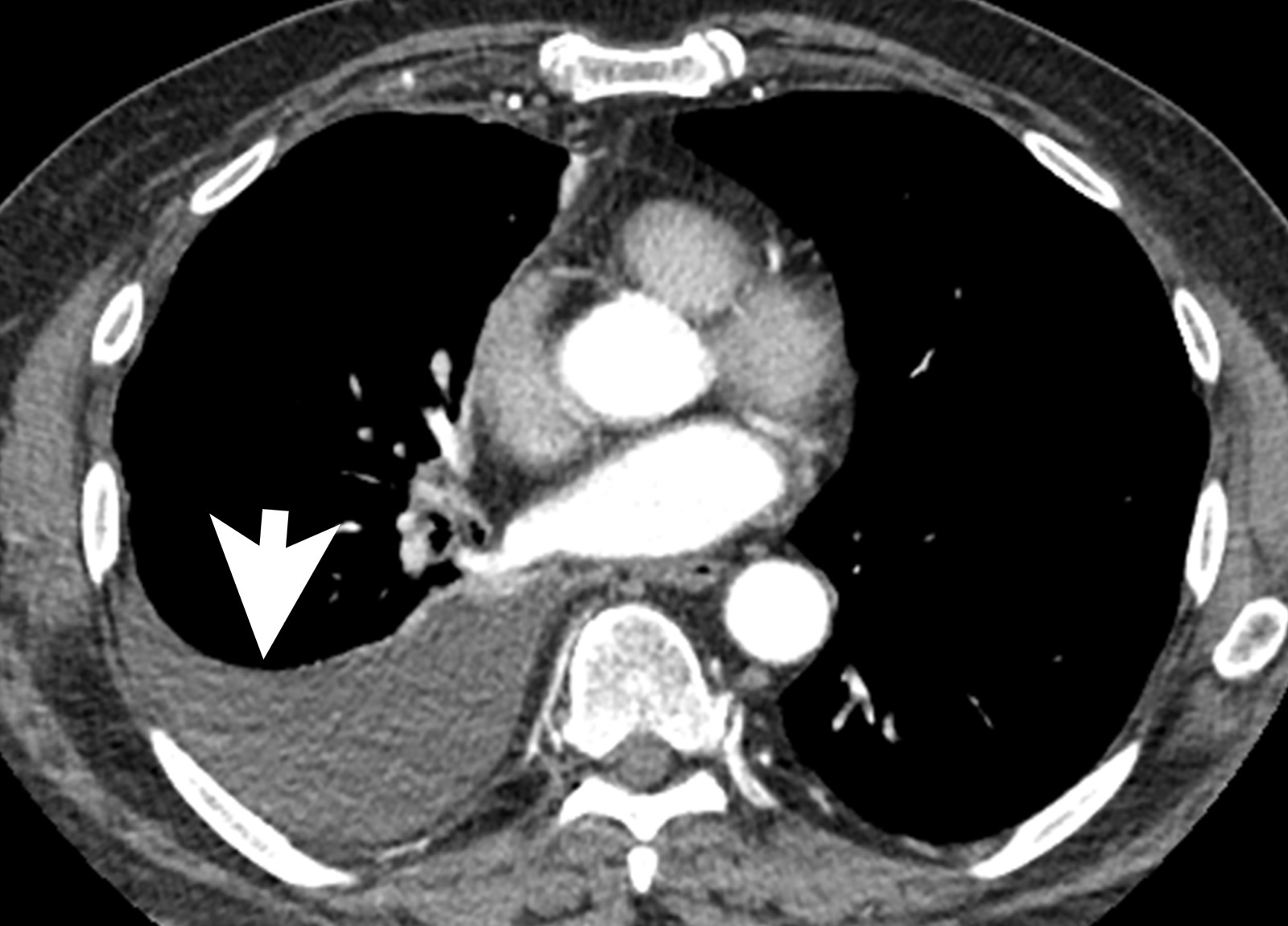
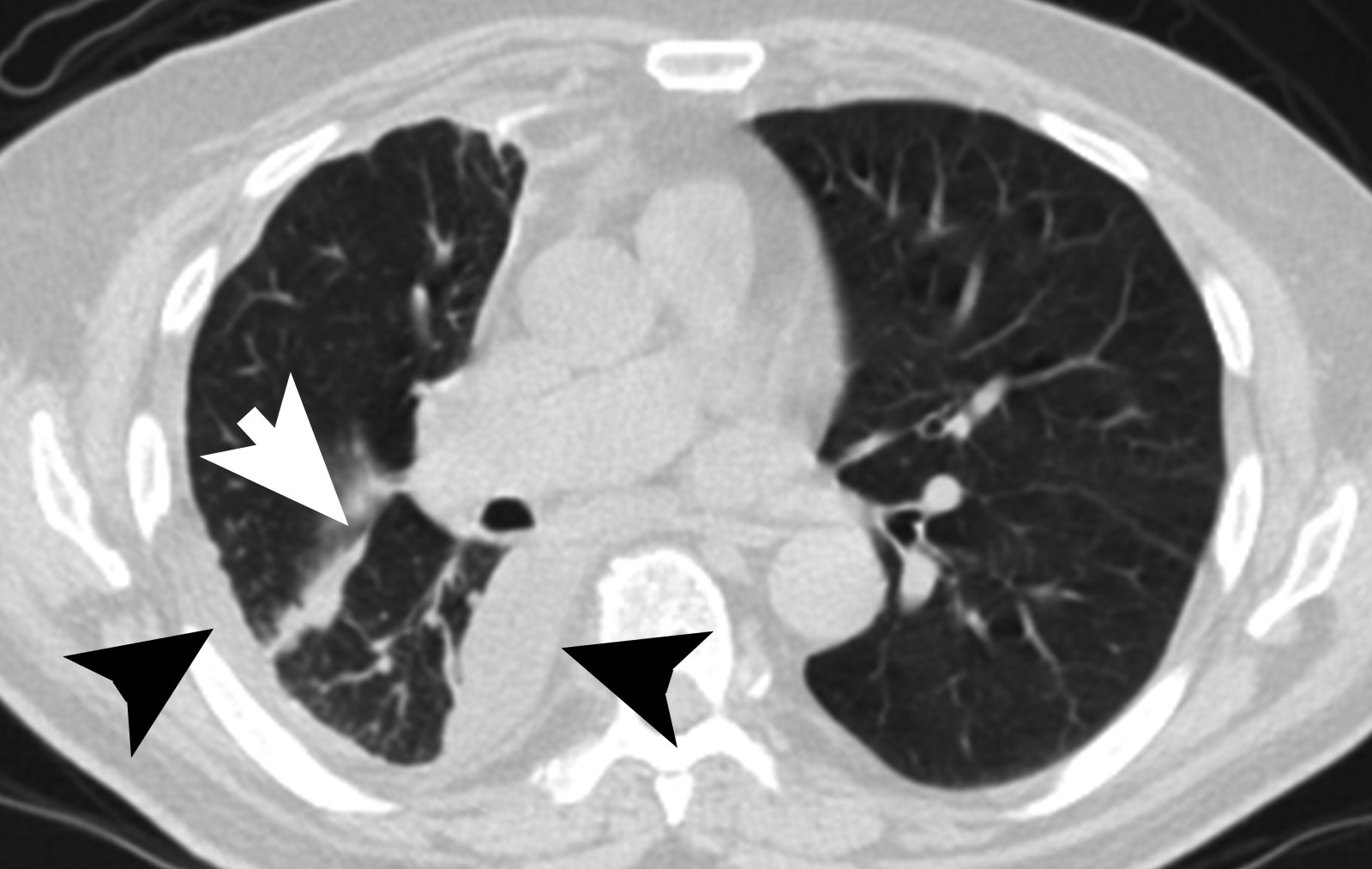
Radiology description
- Chest Xray:
- Unilateral effusion
- CT scan ideally with contrast enhancement (Surg Pathol Clin 2020;13:73):
- Unilateral pleural effusion
- Loculated, nodular or diffuse pleural thickening
- Multifocal nodules studding pleural surfaces including visceral, parietal and diaphragmatic pleura and possibly extending into fissures
- Thick rind of pleura
- Localized pleural or subpleural mass, rare, might measure up to 15 cm (Surg Pathol Clin 2020;13:73)
- Anterior mediastinal mass, rare, might invade into abdomen including liver
- Benign pleural plaques of parietal pleura, usually bilateral, might be seen in addition to malignant pleural mesothelioma; indicate exposure to asbestos; they are not a sign of malignant pleural mesothelioma
- MRI and PET / CT: complementary to contrast enhanced CT
- MRI might help to better delineate relationship of malignant pleural mesothelioma to adjacent structures and organs
- PET / CT might help to identify metastatic disease
Prognostic factors
- Morphologic subtype: epithelioid subtype (best prognosis) > biphasic > sarcomatoid / desmoplastic subtype (worst prognosis) (Ann Thorac Surg 2018;105:432)
- Epithelioid: overall survival, 12 - 27 months, lowest T stage (23% T1), 12% M1 (Ann Thorac Surg 2018;105:432, J Surg Res 2015;196:23, Thorac Cancer 2019;10:1193, Semin Respir Crit Care Med 2019;40:347)
- Biphasic: overall survival, 8 - 21 months; % epithelioid component might be associated with outcome (Semin Respir Crit Care Med 2019;40:347)
- Sarcomatoid: overall survival, 4 - 18 months, highest T stage (29% T4), 17% M1
- Overall survival is independently associated with (Thorac Cancer 2019;10:1193, Mod Pathol 2012;25:260):
- Male gender
- Histology (biphasic and sarcomatoid)
- Lymph node metastasis
- Treatment without chemotherapy
- Lymph node dissection: independently associated with worse overall survival
- Proposed nuclear grading of epithelioid malignant pleural mesothelioma
- Advanced age: associated with worse overall survival (Thorac Cancer 2019;10:1193)
- Staging: prognostic significance is controversial (Thorac Cancer 2019;10:1193)
Case reports
- 45 year old woman with epithelioid malignant pleural mesothelioma with brain metastasis and peritoneal carcinomatosis with response to pembrolizumab (Lung Cancer 2020;142:47)
- 65 year old man with metastatic epithelioid malignant pleural mesothelioma harboring a BRAF V600E mutation (Lung Cancer 2018;116:96)
- 68 year old man with epithelioid malignant pleural mesothelioma diffusely expressing CK20 (Appl Immunohistochem Mol Morphol 2019;27:e93)
- 73 year old man with malignant pleural mesothelioma in situ (Virchows Arch 2020;476:469)
- 77 year old man with epithelioid malignant pleural mesothelioma colliding with lung adenocarcinoma (Diagn Pathol 2016;11:38)
Treatment
- Management by multidisciplinary team
- Most patients (40%): no specific modality of treatment (Ann Thorac Surg 2018;105:432)
- Chemotherapy alone: most common treatment (Ann Thorac Surg 2018;105:432)
- Trimodality treatment (chemotherapy, surgical resection, radiation therapy): best survival > combination chemotherapy and resection (Ann Thorac Surg 2018;105:432)
- Surgery within multimodal therapy suggested only to suitable patients (i.e., young patients with early stage epithelioid histology; good performance status, epithelioid or possibly biphasic morphology) (Thorac Cancer 2019;10:1193)
- NCCN guidelines (Version 1.2020):
- Clinical stage I - IIIA and epithelioid or biphasic morphology (surgery should be considered for biphasic if early stage disease)
- Induction chemotherapy followed by surgical exploration (pleurectomy / decortication or extrapleural pneumonectomy; mediastinal lymph node sampling)
- If pleurectomy / decortication: followed by observation or radiation
- If extrapleural pneumonectomy: followed by hemithoracic radiation; if found unresectable, chemotherapy
- Surgical exploration (pleurectomy / decortication or extrapleural pneumonectomy; mediastinal lymph node sampling)
- If pleurectomy / decortication: followed by chemotherapy followed by observation or radiation
- If extrapleural pneumonectomy: followed by sequential chemotherapy and hemithoracic radiation; if found unresectable chemotherapy
- Surgery associated with morbidity and mortality; extrapleural pneumonectomy and extended pleurectomy / decortication: perioperative mortality of 6.8% and 2.9%; morbidity of 62% and 27.9%, respectively (Lung Cancer 2014;83:240)
- Sarcomatoid and desmoplastic mesothelioma or patients with poor performance status: chemotherapy or supportive care only
- Supportive care: pleurodesis or pleural catheter if recurrent pleural effusion
- Anti-PD1 or anti-PDL1 treatment on occasion, not standardized
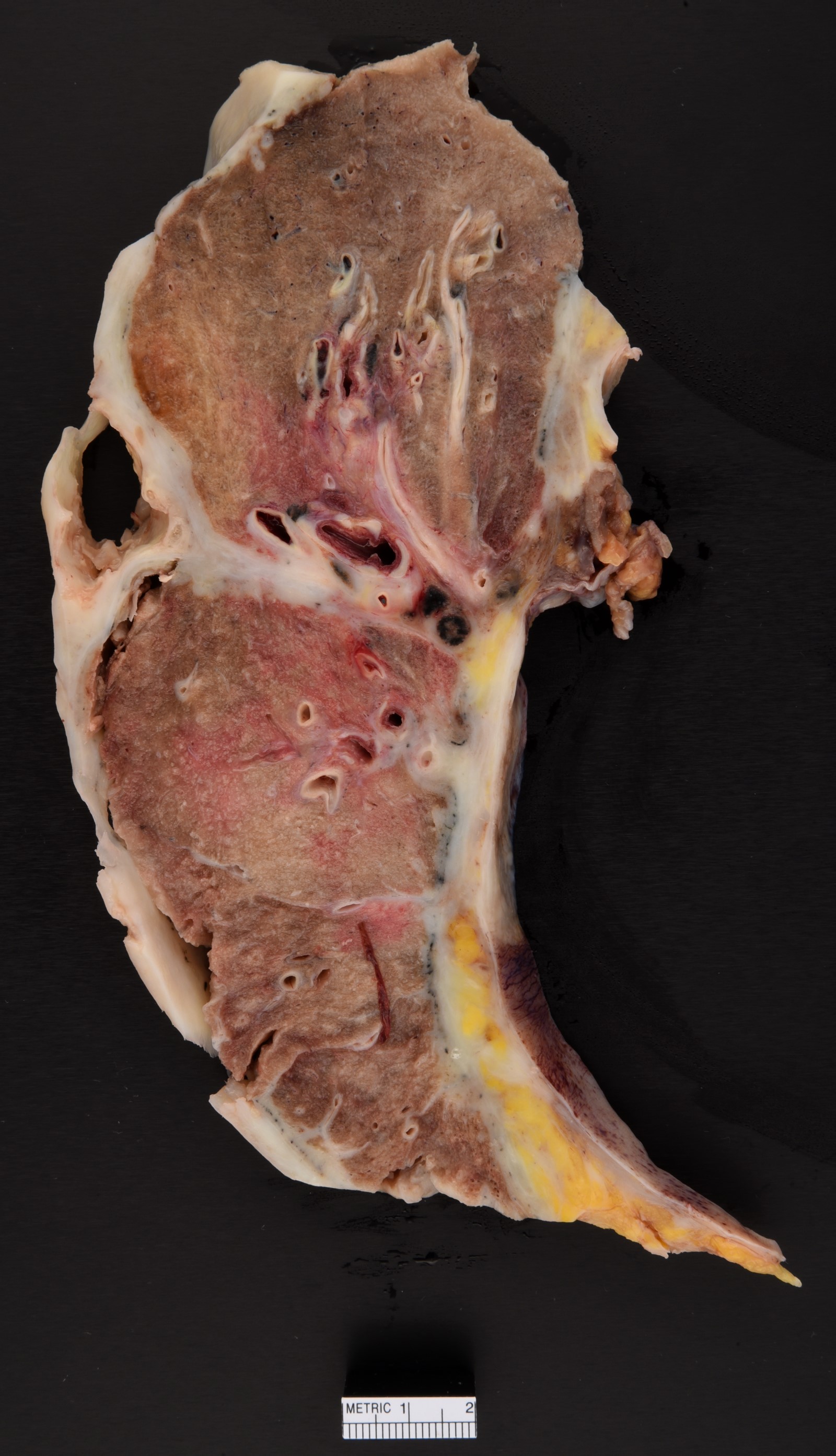
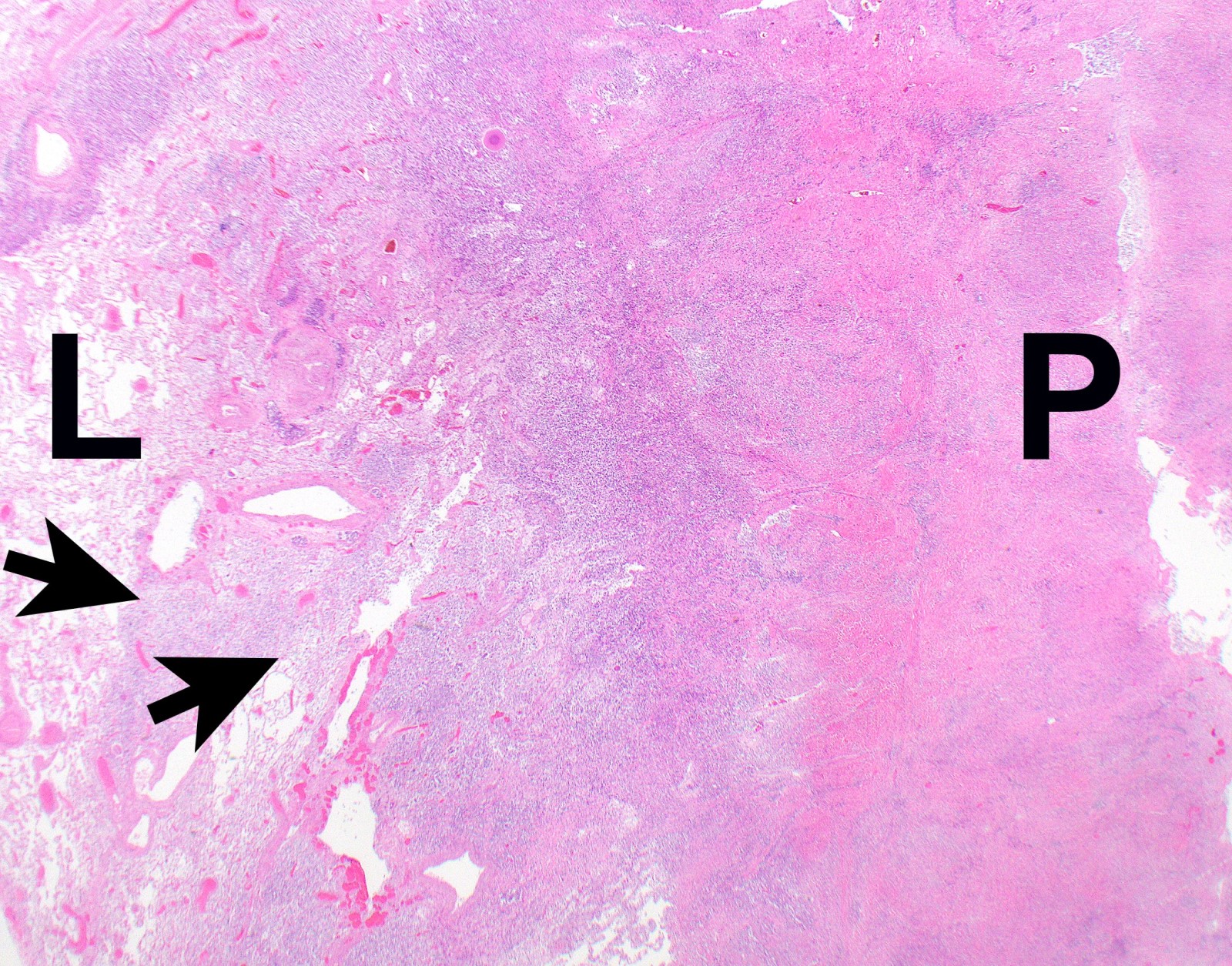
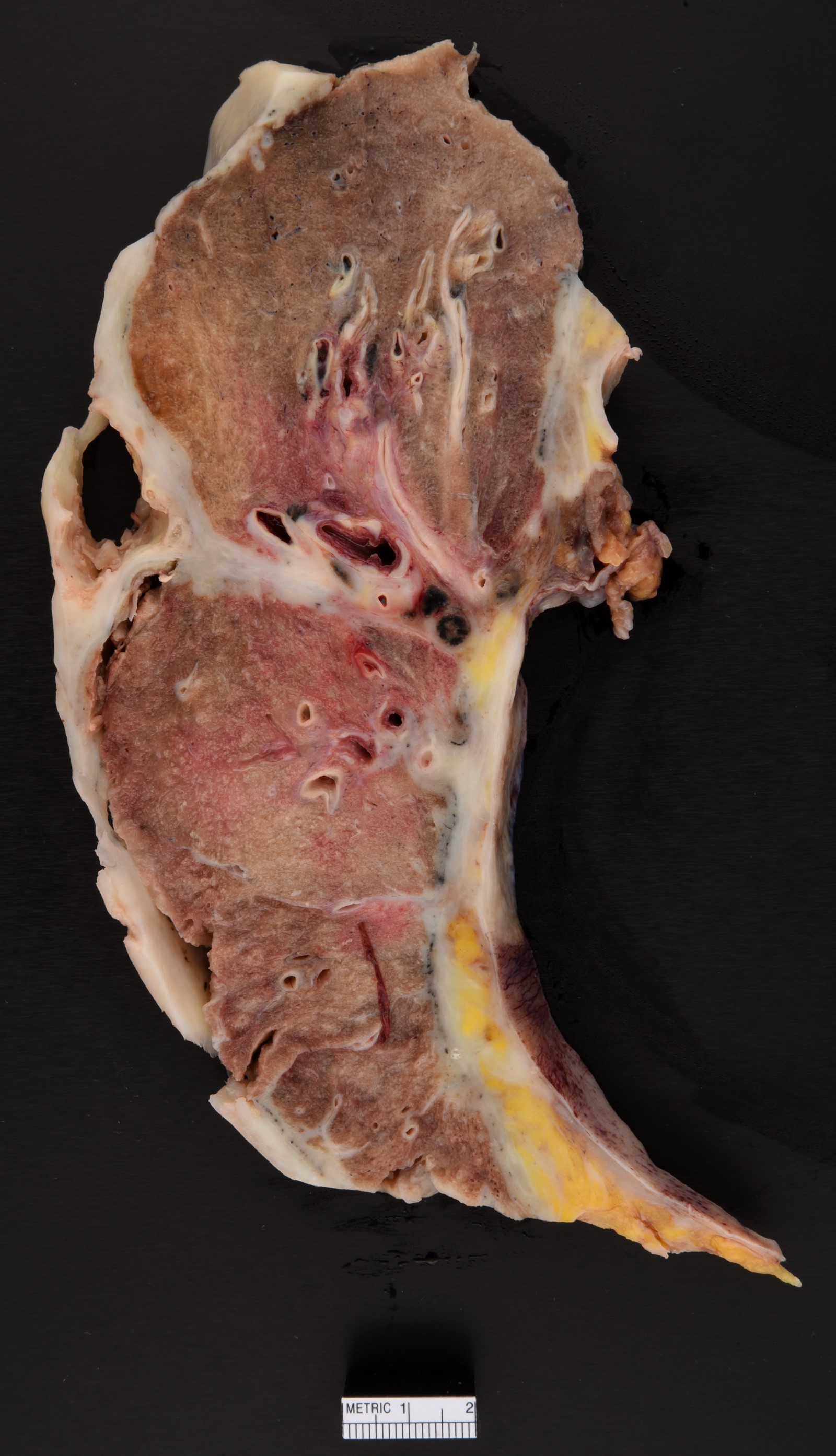
Gross description
- Thick rind of pleura possibly extending into fissures
- Studding of pleura
- Multiple pleural nodules
- Single pleural based mass rare (localized malignant pleural mesothelioma) (Mod Pathol 2020;33:281)
- Optimal orientation of specimen important for assessment of invasion: pleural sections submitted perpendicular to surface; should contain entire pleural thickness together with adjacent structures such as lung, adipose tissue or skeletal muscle
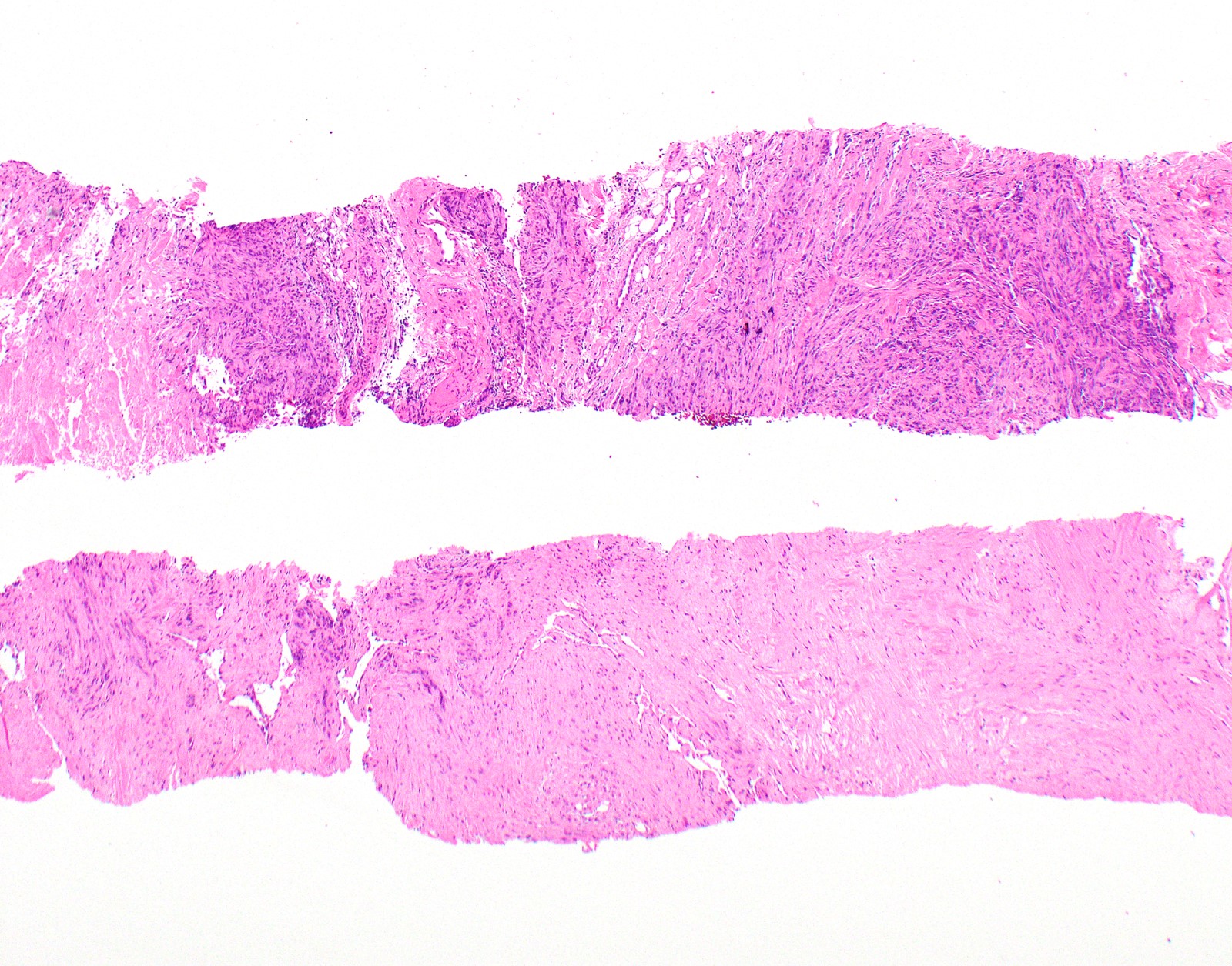
Frozen section description
- Assess adequacy of sampled tissue that includes sufficient tissue to assess growth pattern and invasion
- Distinction between metastatic carcinoma, sarcoma, melanoma and mesothelioma is in general not possible; neither is it important at time of frozen section evaluation
Frozen section images
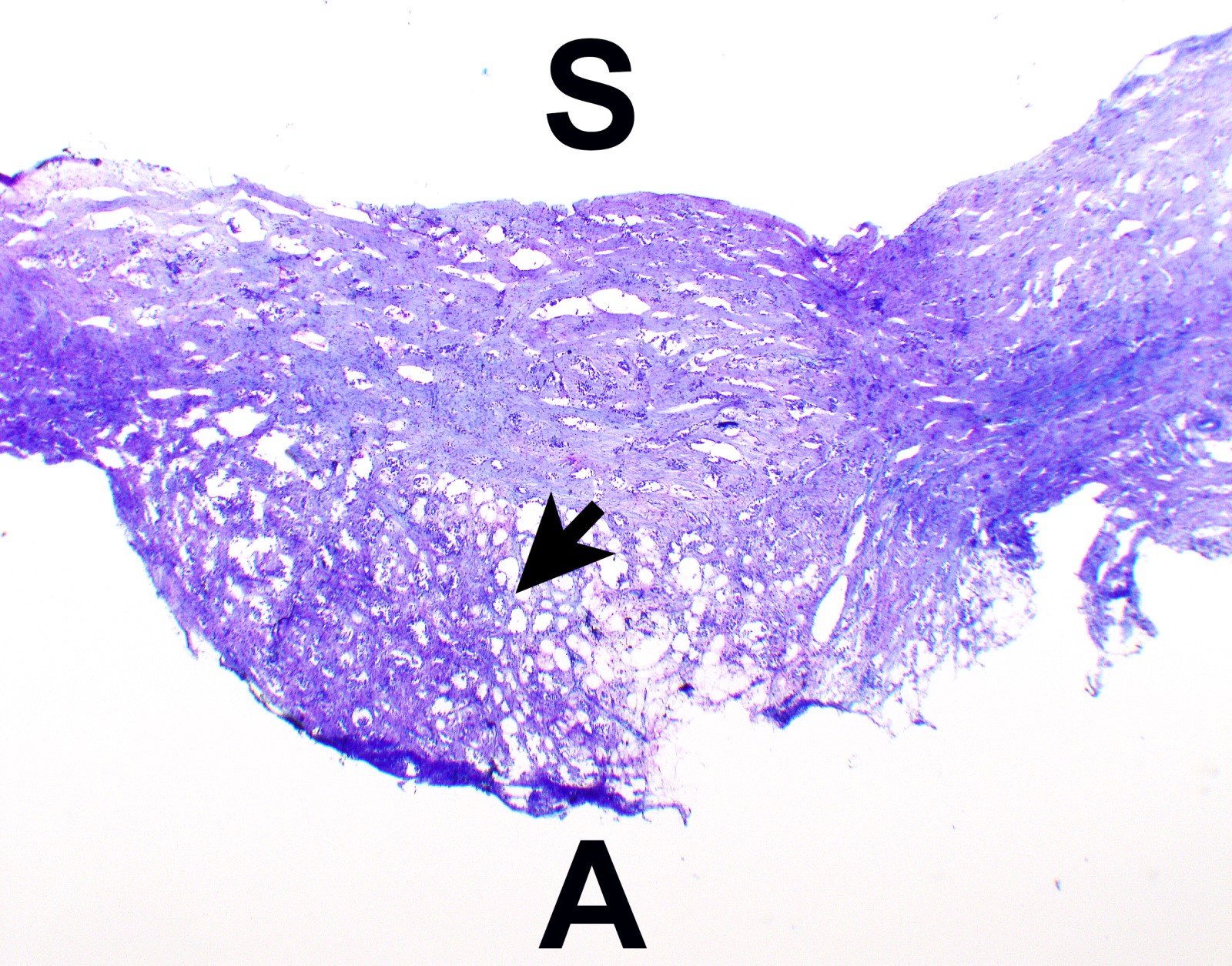
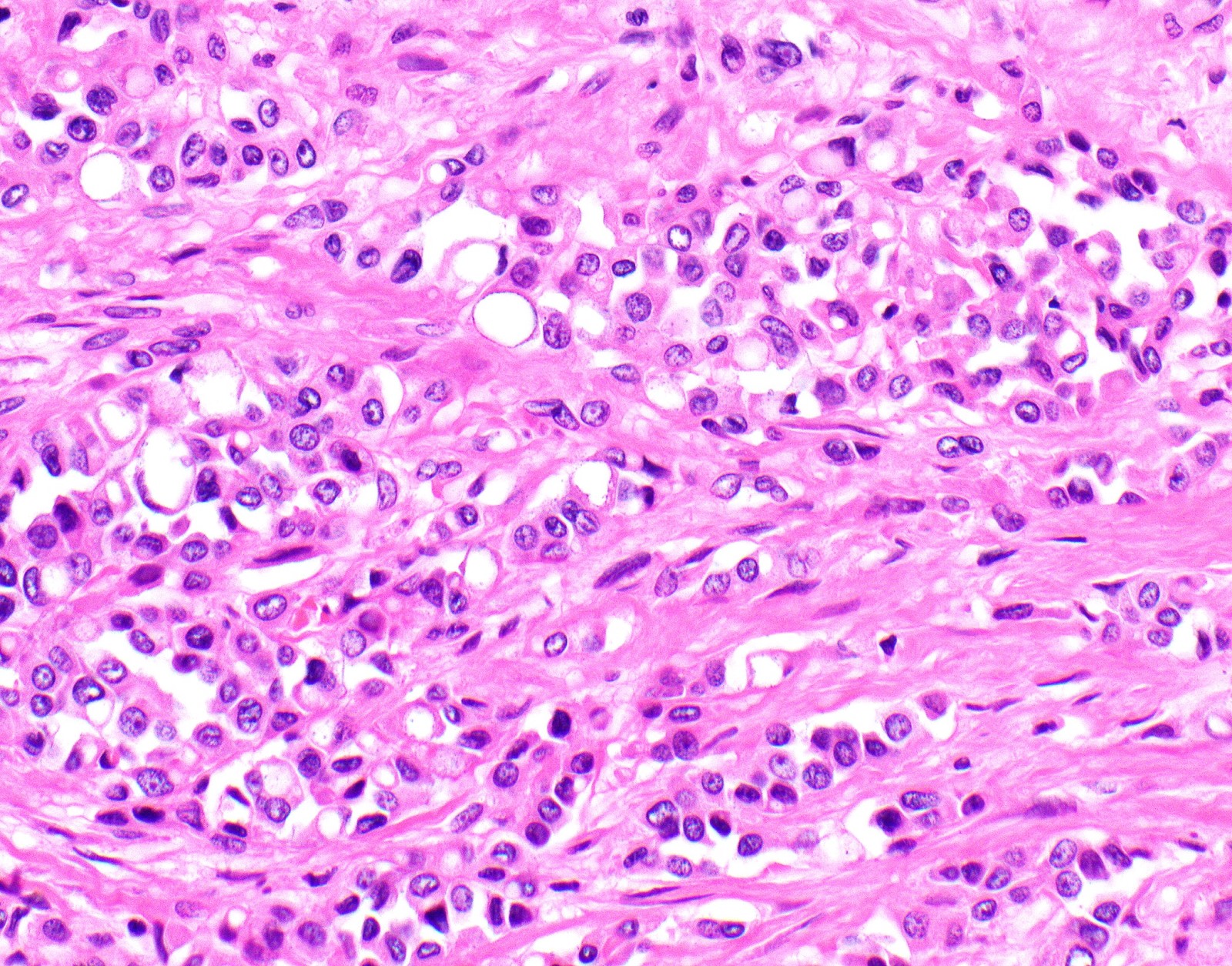
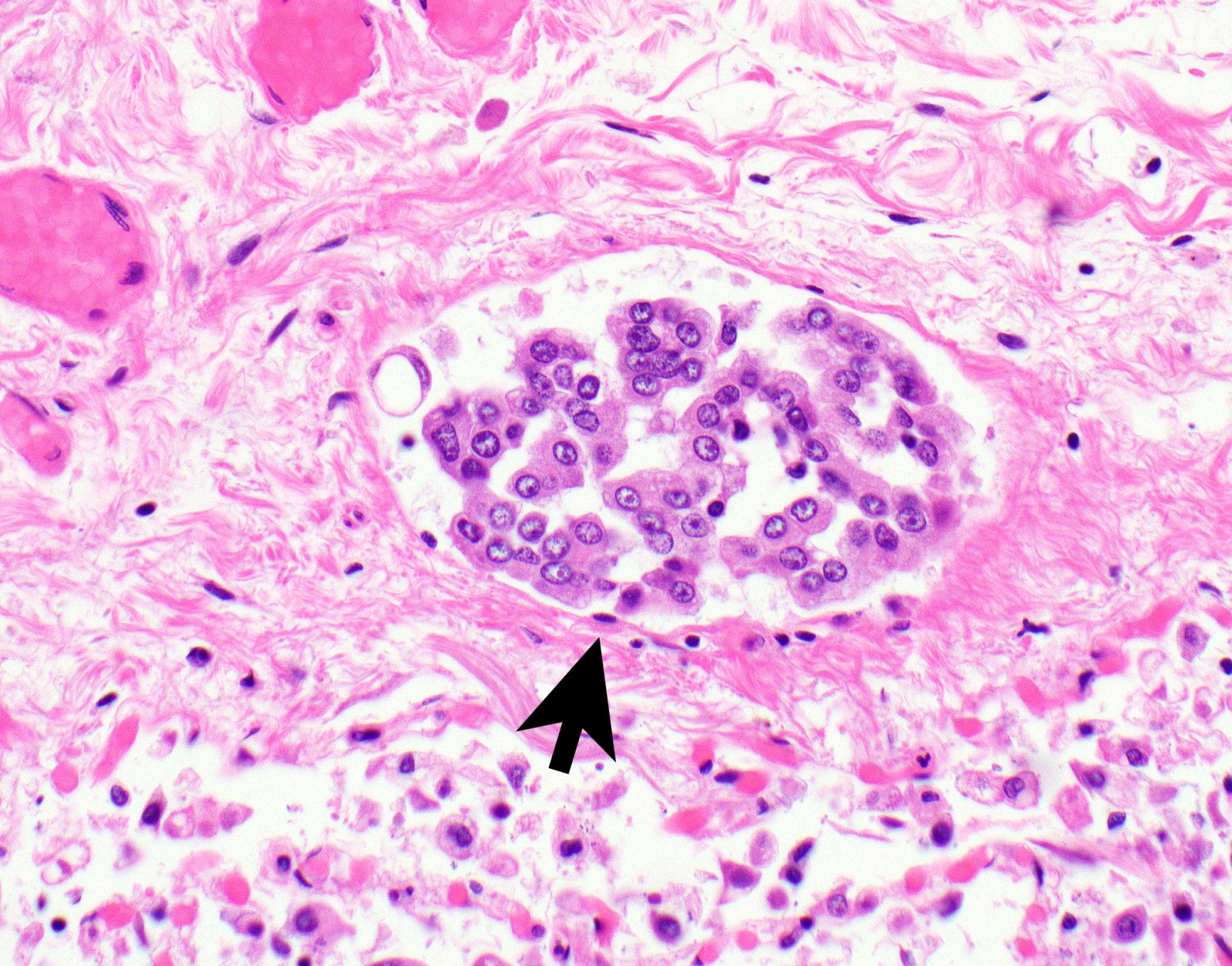
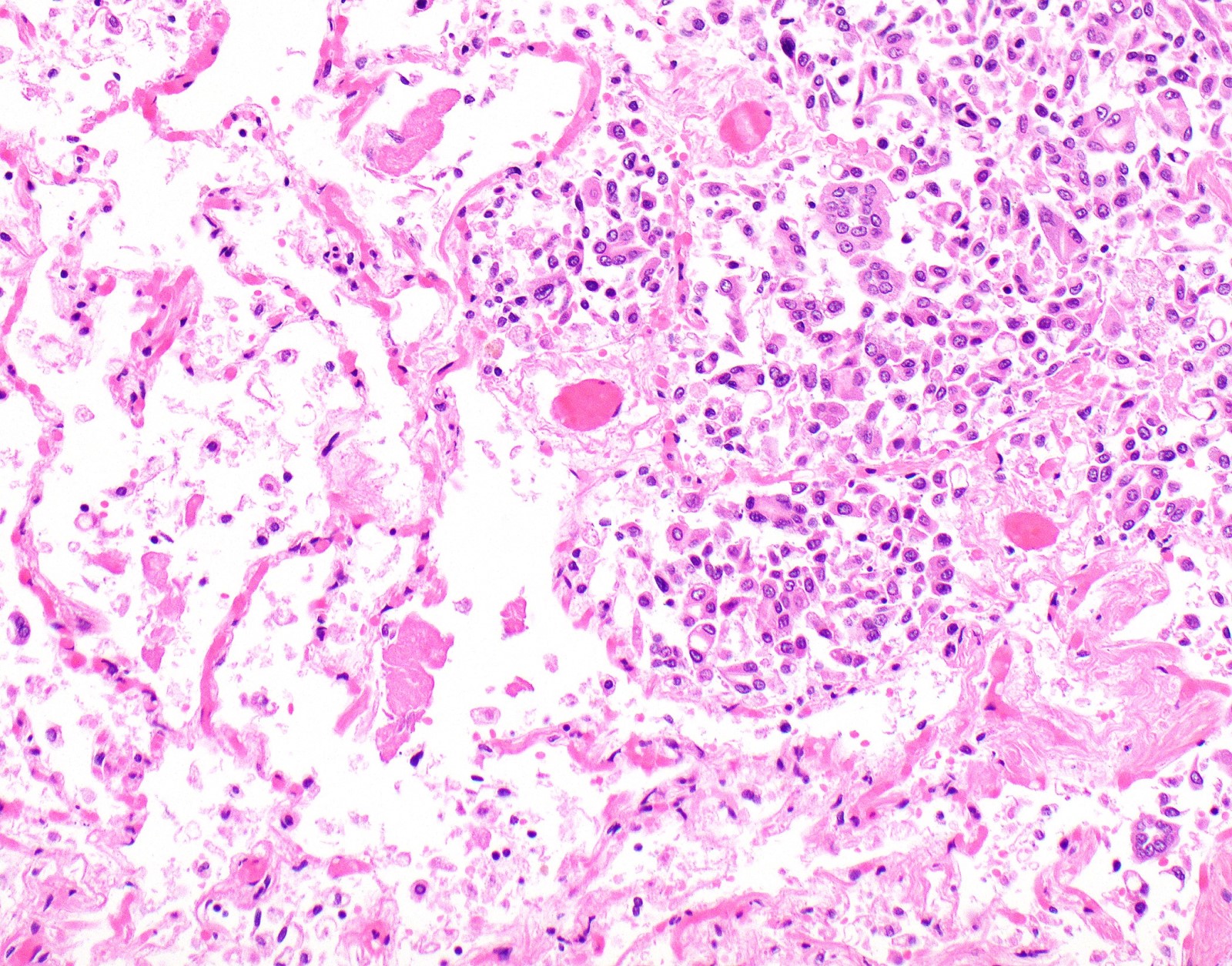
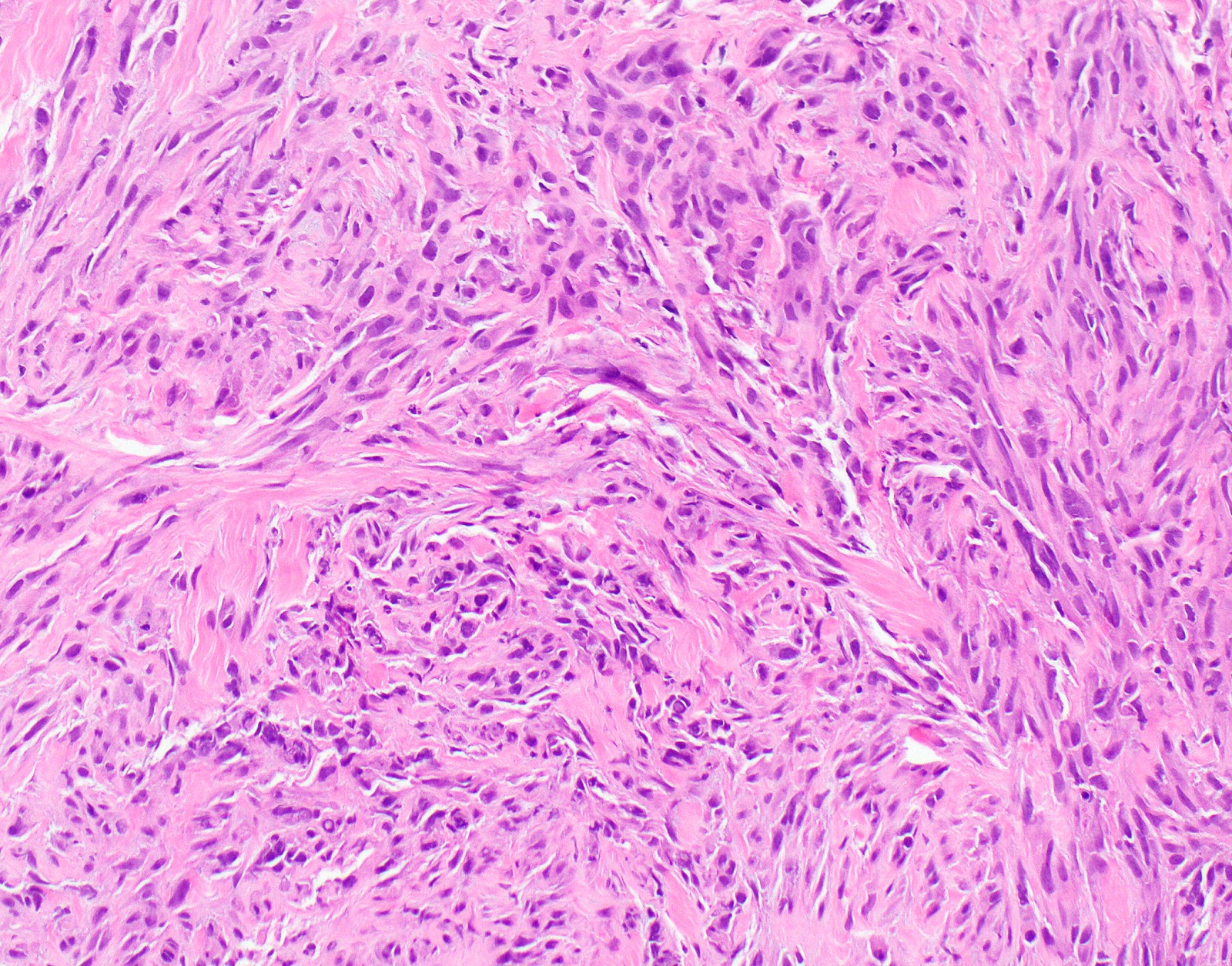
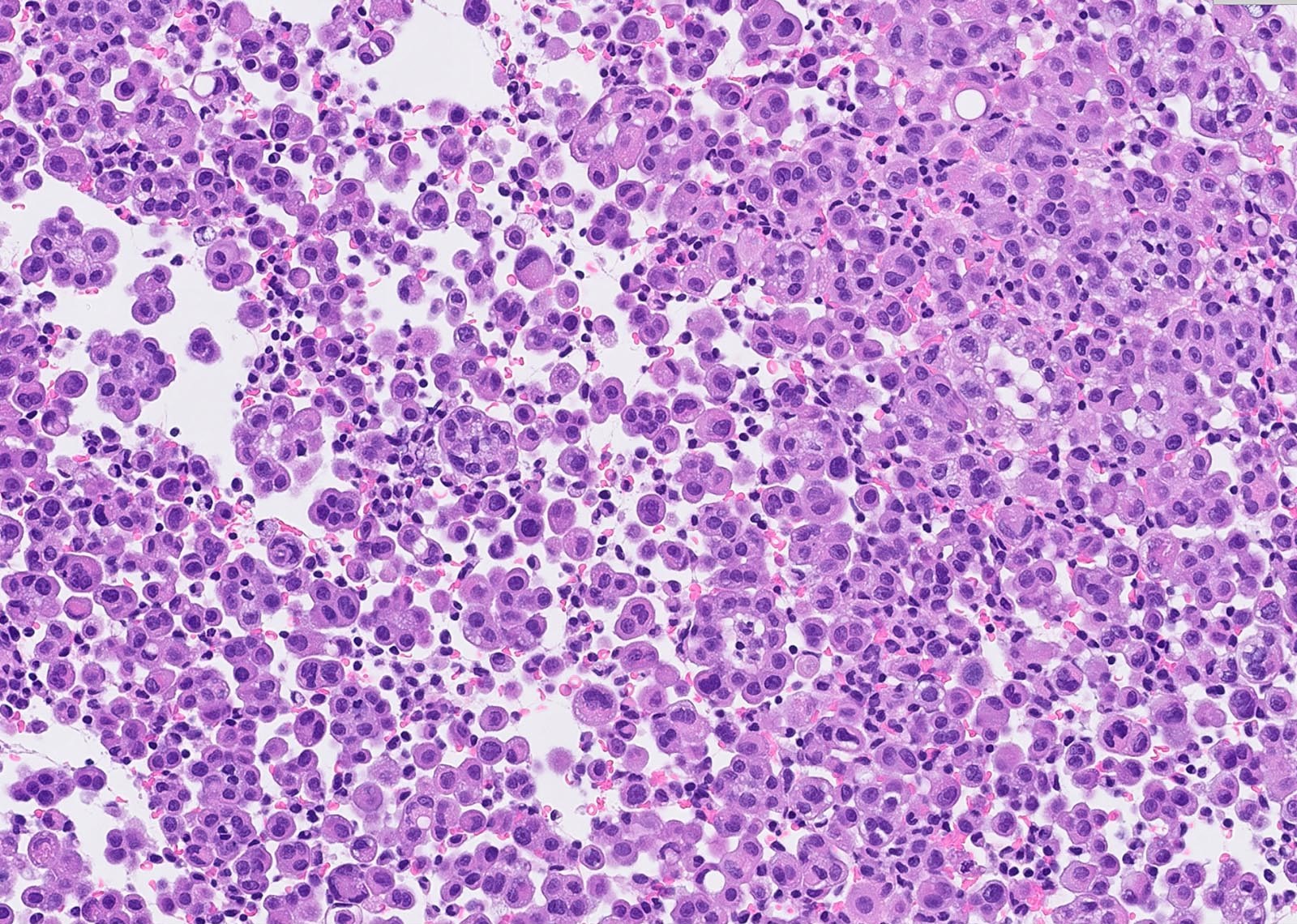
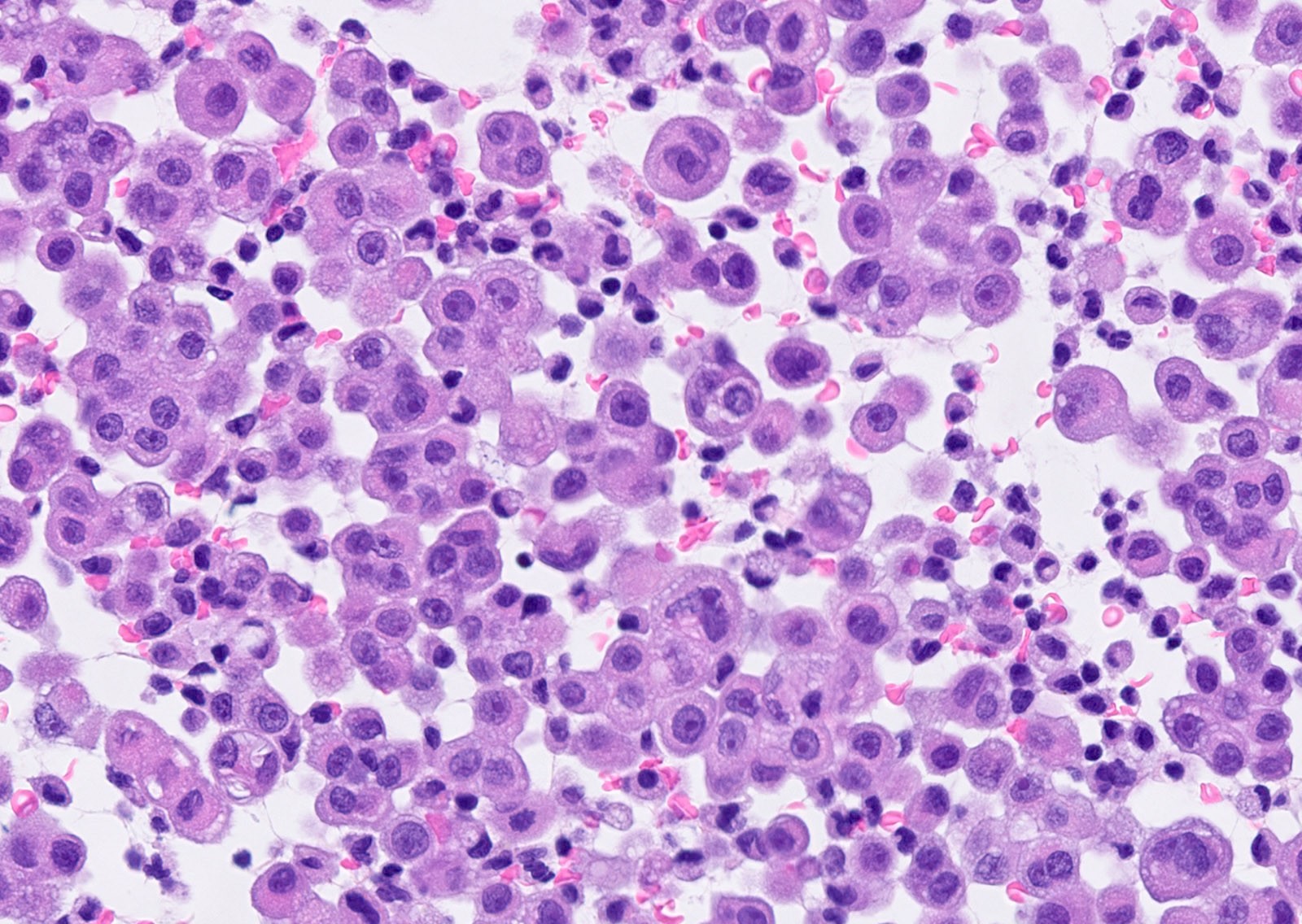
Microscopic (histologic) description
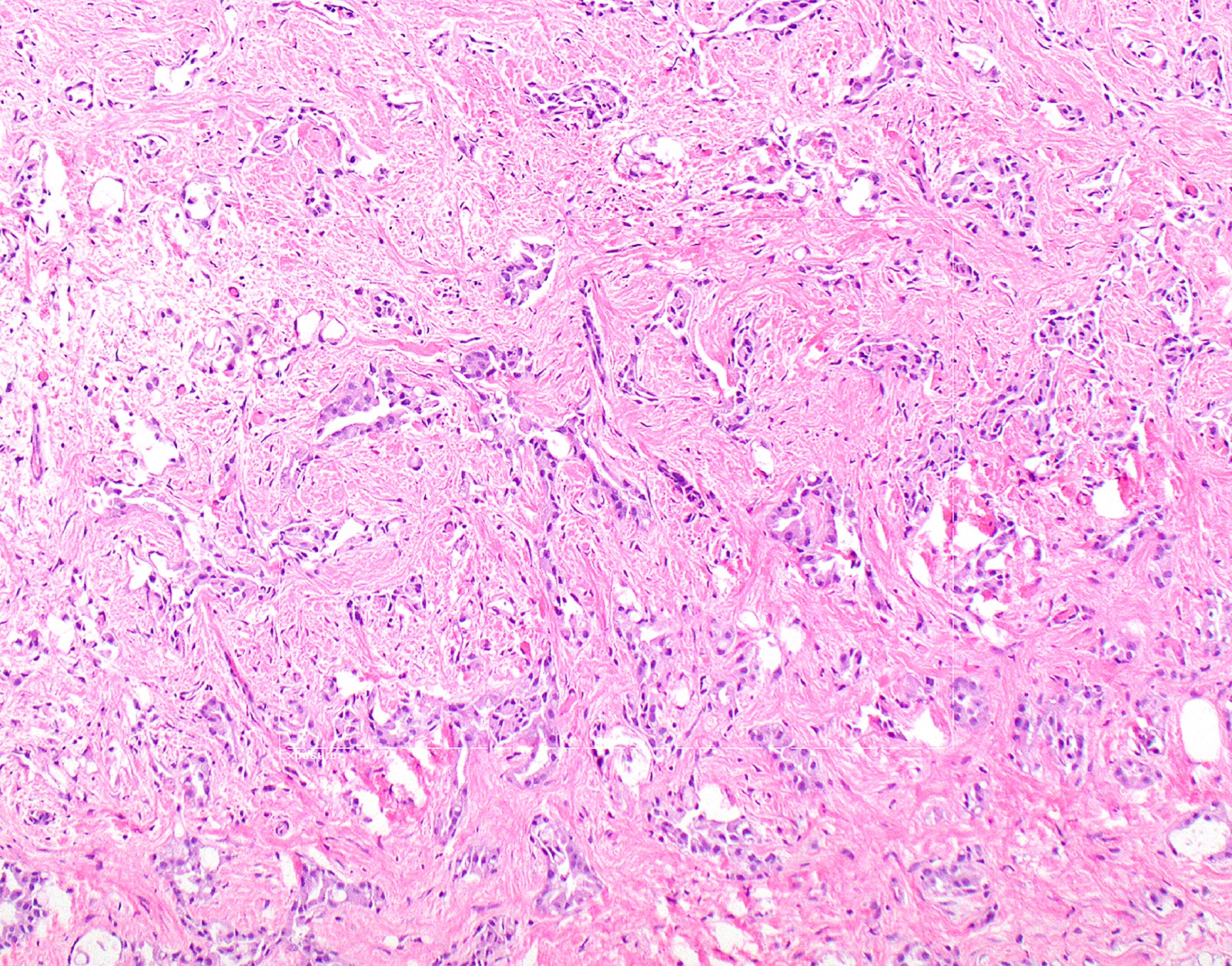
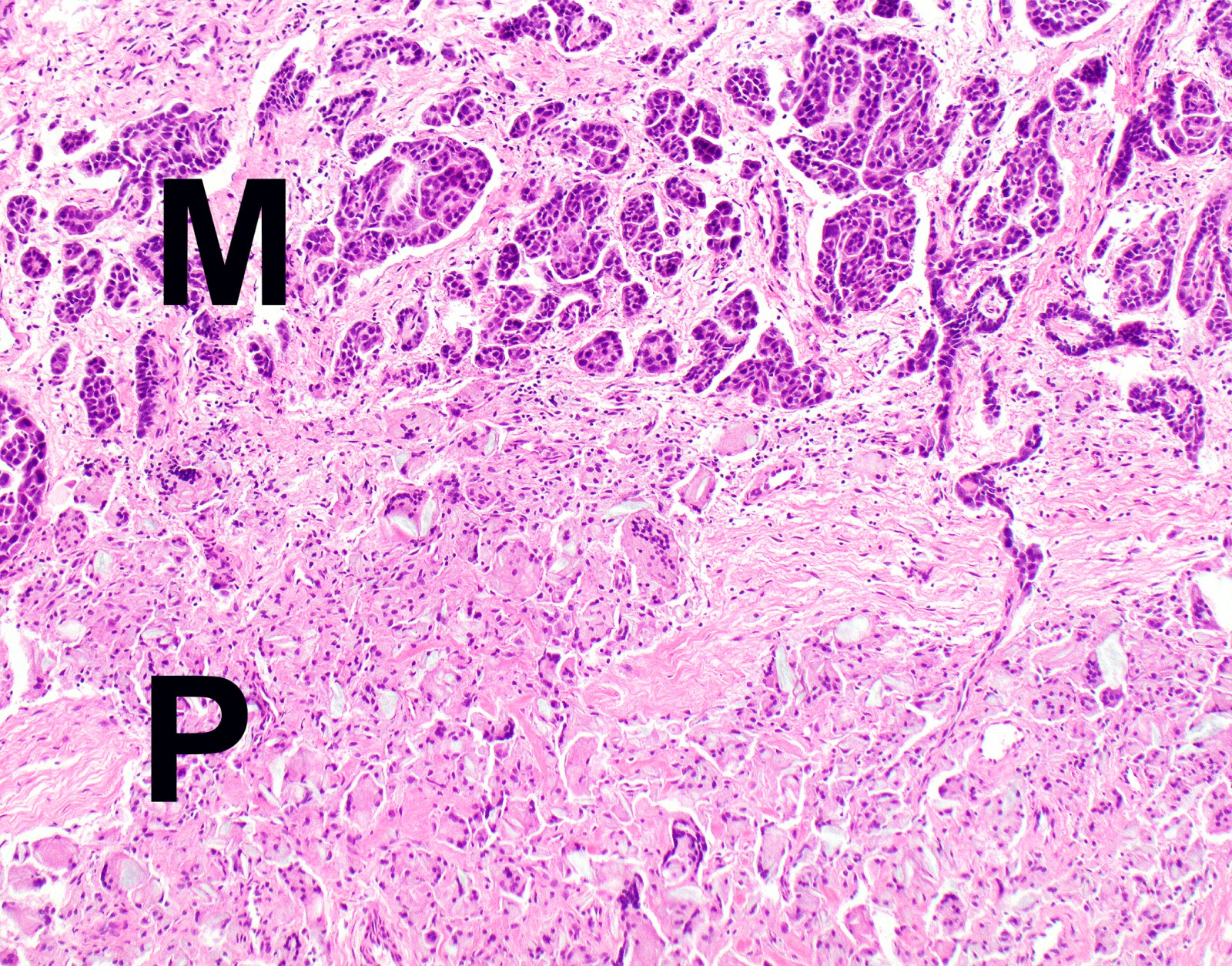
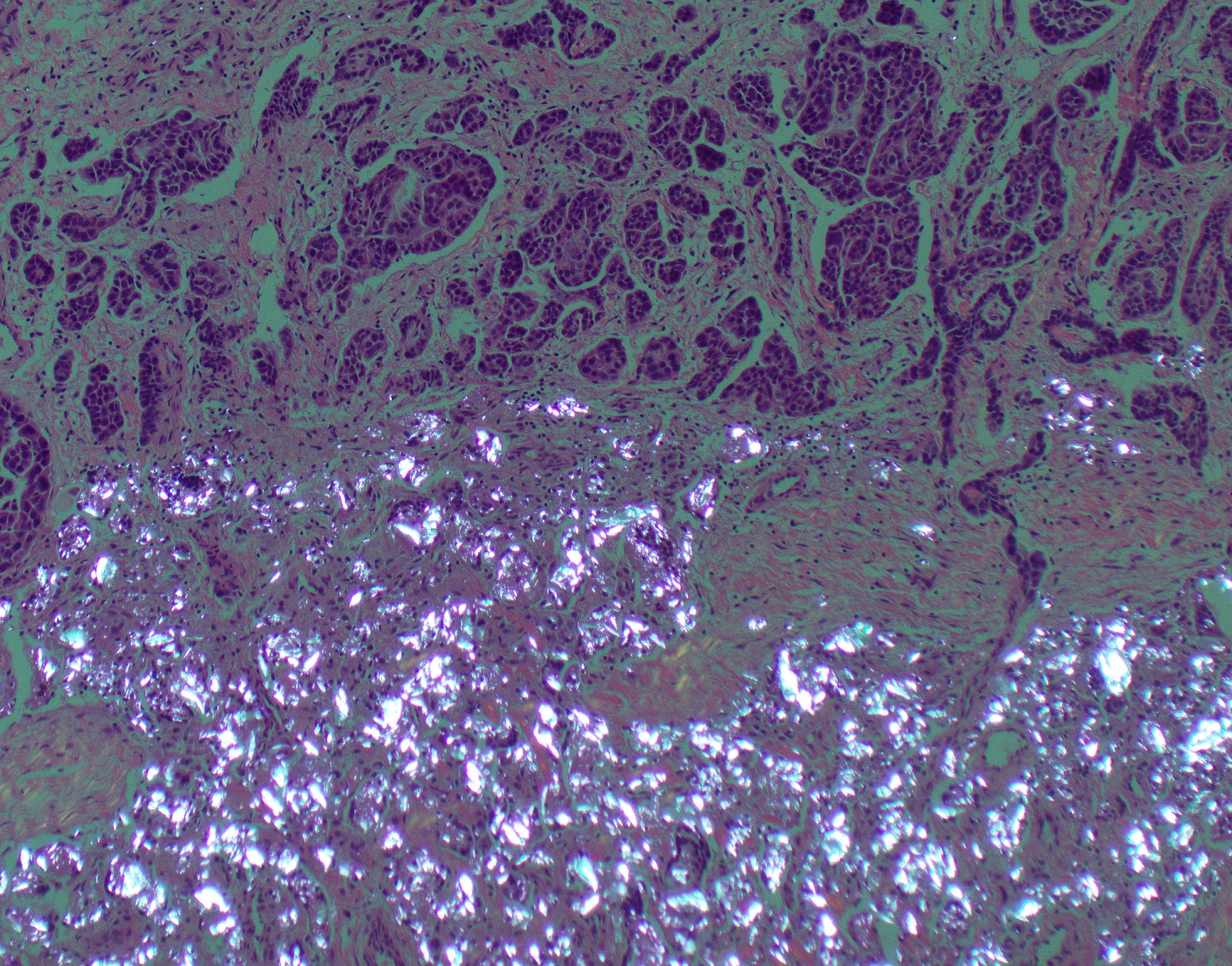
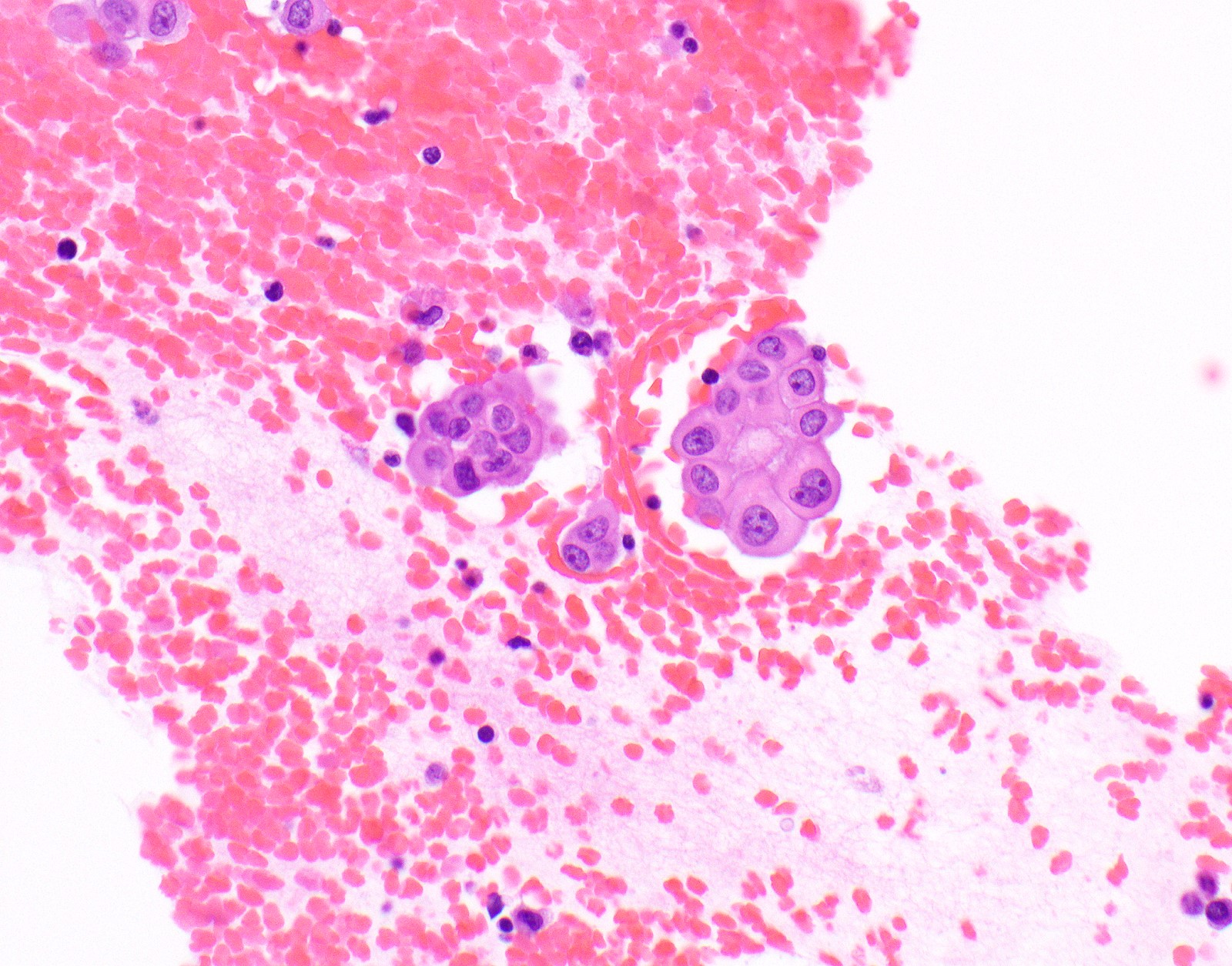
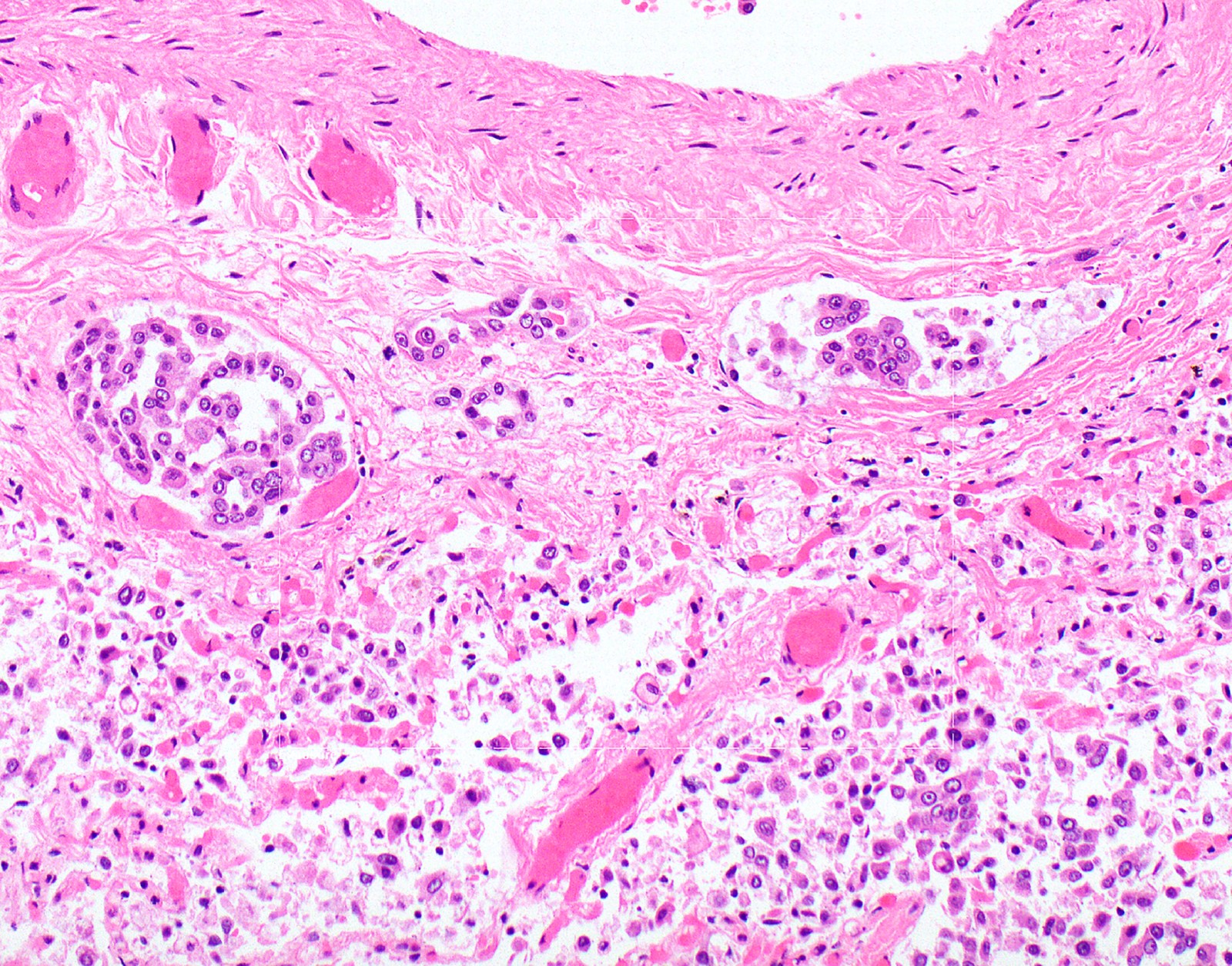
- Thickened pleura due to neoplastic cells and desmoplastic stromal reaction with or without necrosis
- In contrast, benign pleura: mesothelial cells are horizontally oriented, venules are perpendicular oriented, zonation of mesothelial cells [highest cellularity near serosal surface, trailing off toward chest wall], no invasion, no tumefactive growth (J Clin Pathol 2006;59:564)
- Invasion of neoplastic cells into adipose tissue, skeletal muscle or lung
- Tumefactive growth of malignant cells
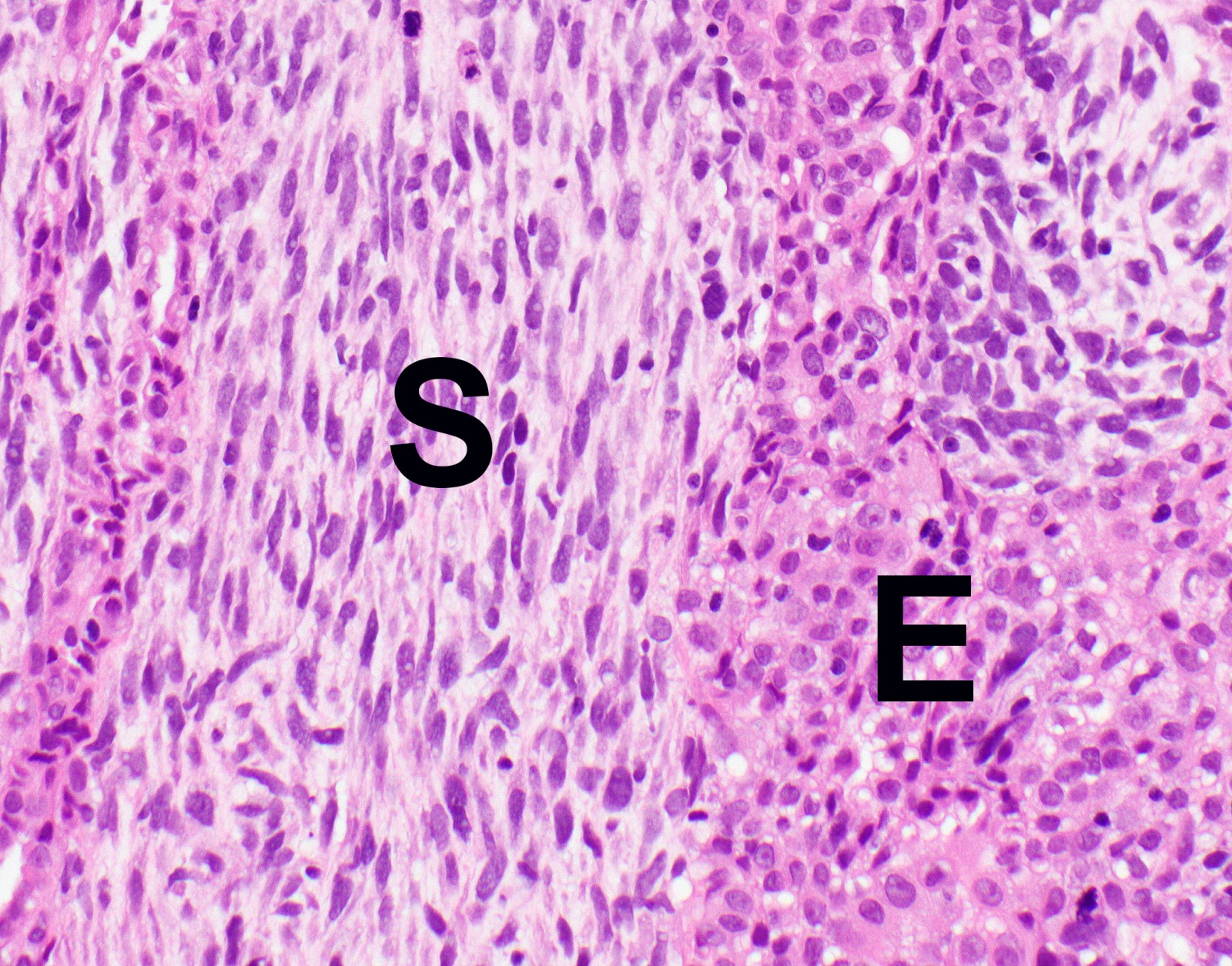
- Malignant mesothelial cells are either of epithelioid (epithelioid subtype) or spindled (sarcomatoid / desmoplastic subtype) cytology or a combination thereof (biphasic)
- Epithelioid subtype most common, 36 - 53%; sarcomatoid subtype, 12 - 27%, biphasic 11 - 19% (Thorac Cancer 2019;10:1193, Ann Thorac Surg 2018;105:432)
- If biphasic: provide percentage of sarcomatoid component
- Epithelioid:
- Cytology usually bland
- Patterns: solid, acinar (glandular), tubulopapillary, trabecular, micropapillary, microcystic (adenomatoid), clear cell, deciduoid, small cell
- Psammoma bodies might be present in any pattern
- Be aware of reactive spindle cells that might mimic sarcomatoid component
- 2 - 5% show cytoplasmic mucin (Ultrastruct Pathol 2006;30:3)
- Proposed nuclear grading (Mod Pathol 2012;25:260):
- Grade I - III
- Scoring:
- Mitotic count: score 1 (0 - 1 mitoses/10 HPF), score 2 (2 - 4/10 HPF), score 3 (≥ 5/10 HPF)
- Nuclear atypia: score 1, mild atypia, score 2, moderate atypia, score 3, severe atypia
- Composite score: 2 - 3 (grade I), 4 - 5 (grade II), 6 (grade III)
- Sarcomatoid:
- Haphazard growth of malignant cells
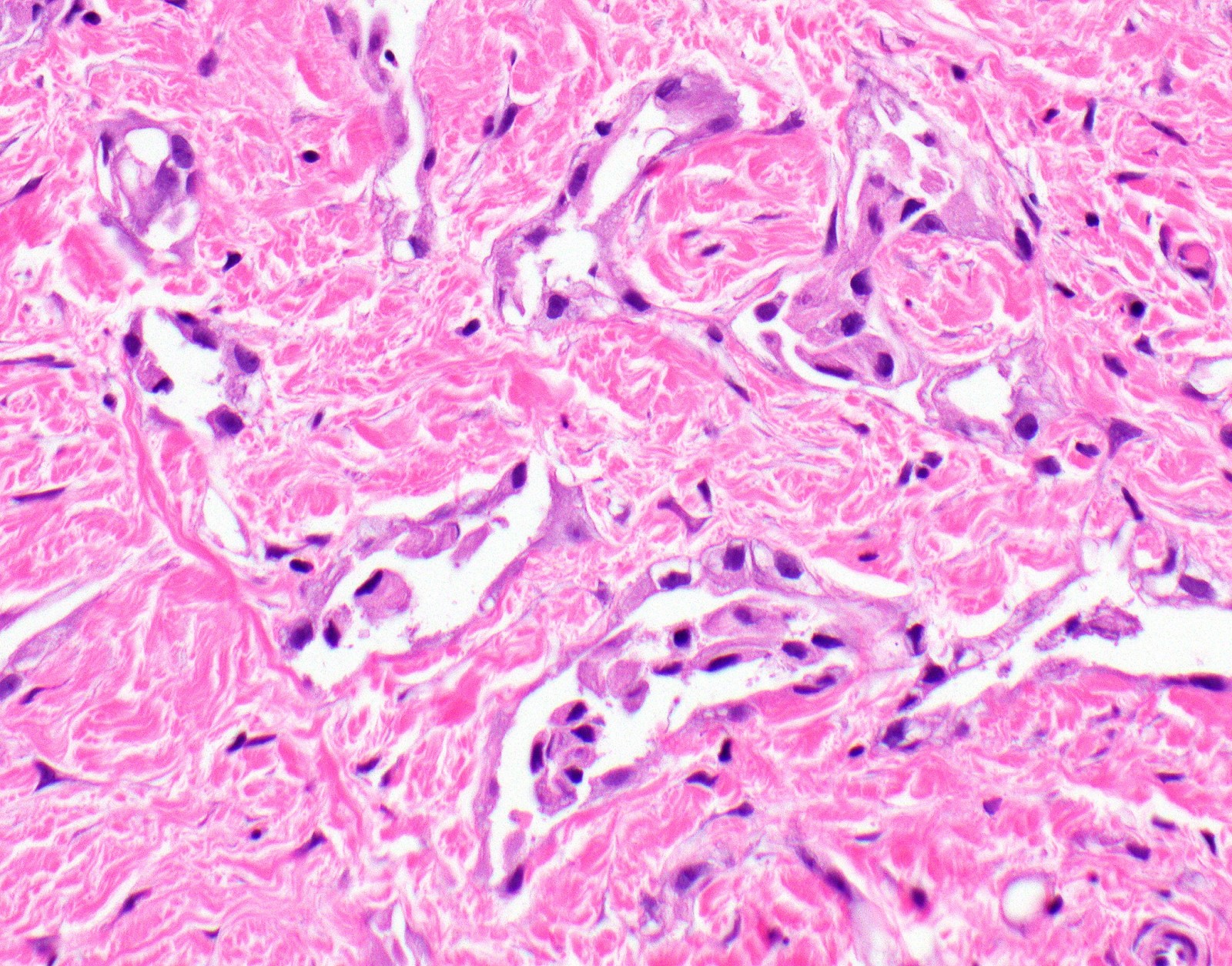
- Desmoplastic variant: paucicellular, usually bland appearing spindle cells growing in a storiform pattern in a collagenized background with stromal invasion and possible bland necrosis (Arch Pathol Lab Med 2018;142:89)
- Transitional pattern appears to group closer together with sarcomatoid than epithelioid subtype (J Thorac Oncol 2020;15:1037)
- Malignant mesothelioma in situ (Histopathology 2018;72:1033):
- Defined by
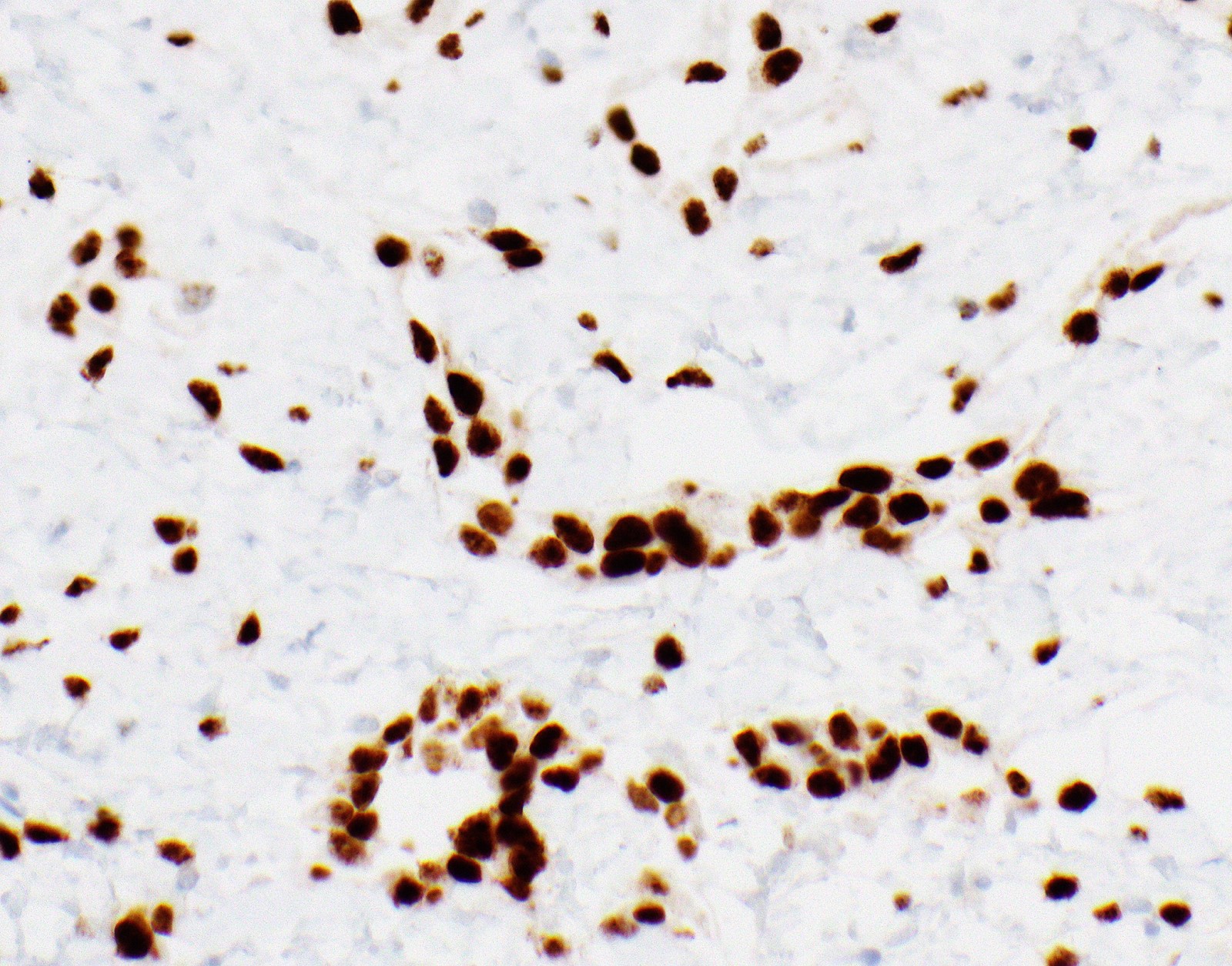
- Single layer of surface mesothelial cells that lost BAP1 expression
- Usually presenting as unilateral pleural effusion
- No evidence of tumor by imaging or by direct examination of pleura
- No invasive mesothelioma developing for at least 1 year
- CDKN2A homozygous deletion is rare in these tumors
- Defined by
- Diffuse intrapulmonary malignant mesothelioma; rare, predominantly intrapulmonary growth and minimal pleural involvement clinically simulating interstitial lung disease (Am J Surg Pathol 2019;43:147)
- Recommendations by European Network for Rare Adult Solid Cancers (EURACAN) and International Association for the Study of Lung Cancer (IASLC) to update histologic classification of malignant pleural mesothelioma using a multidisciplinary approach include (J Thorac Oncol 2020;15:29):
- To update classification to include architectural patterns and stromal and cytologic features that refine prognostication
- Malignant mesothelioma in situ could be an additional category
- Routinely grade epithelioid malignant pleural mesothelioma
- Routinely stage resection specimens; amongst others
- Many of these recommendations will likely be included in the upcoming WHO classification
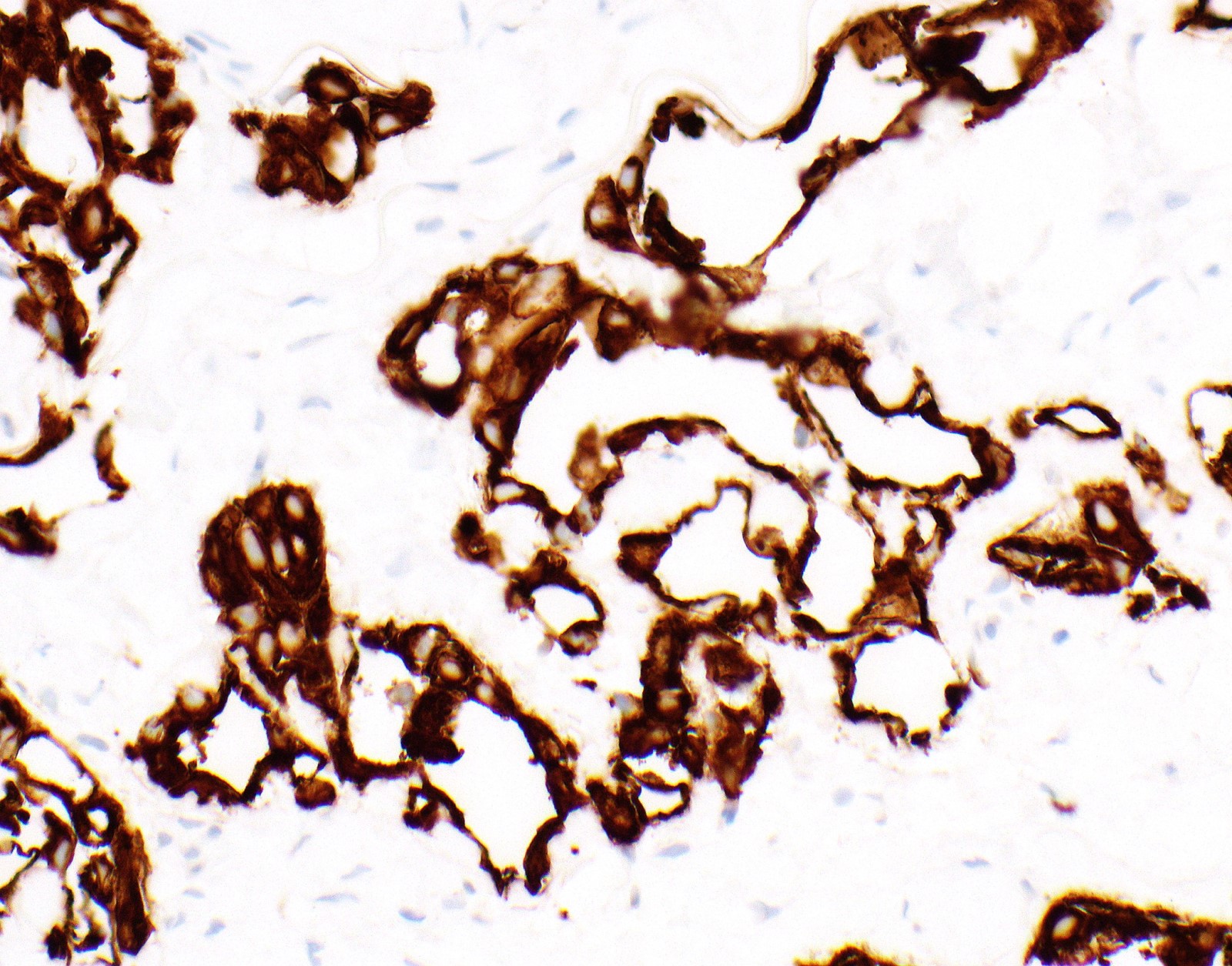
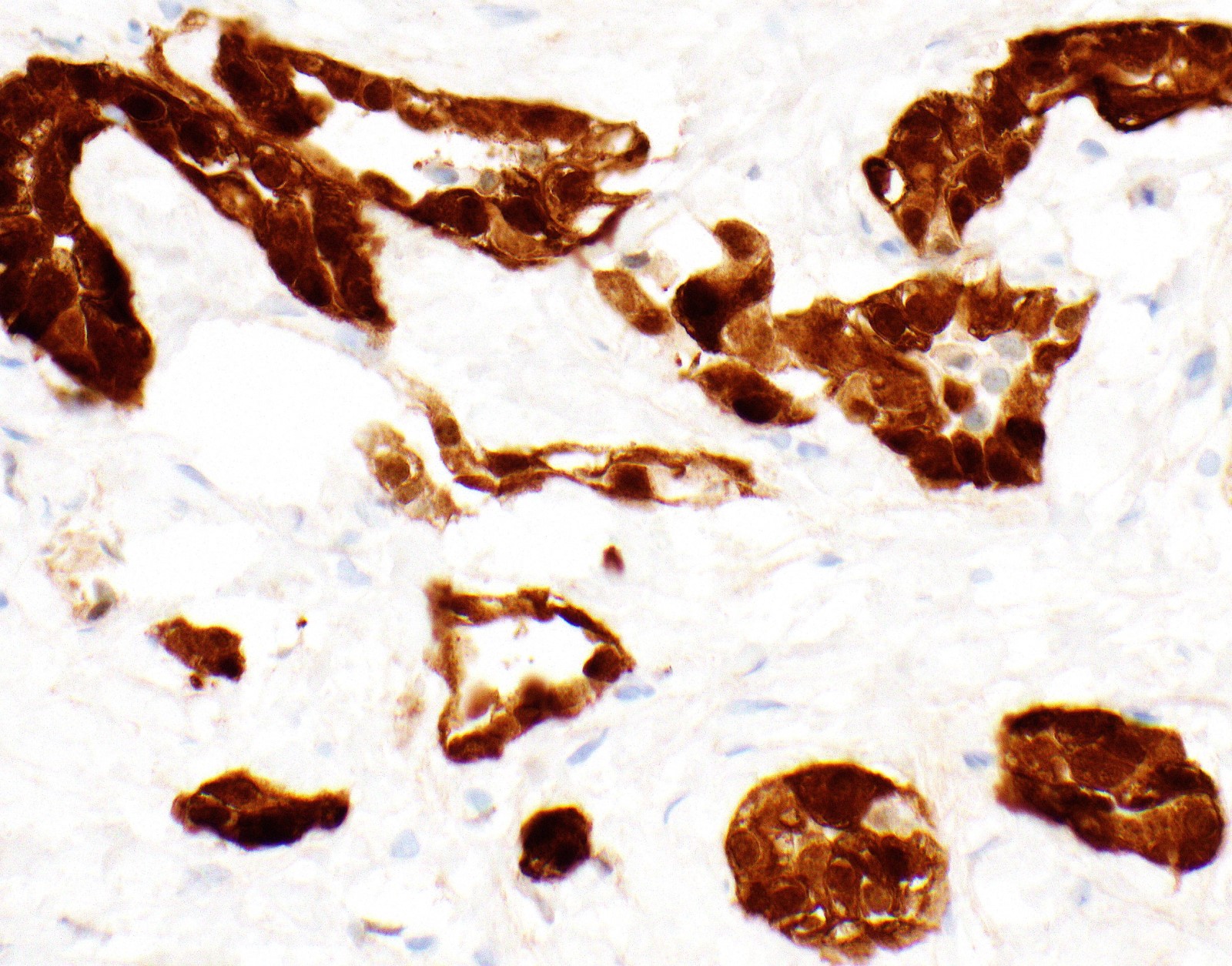
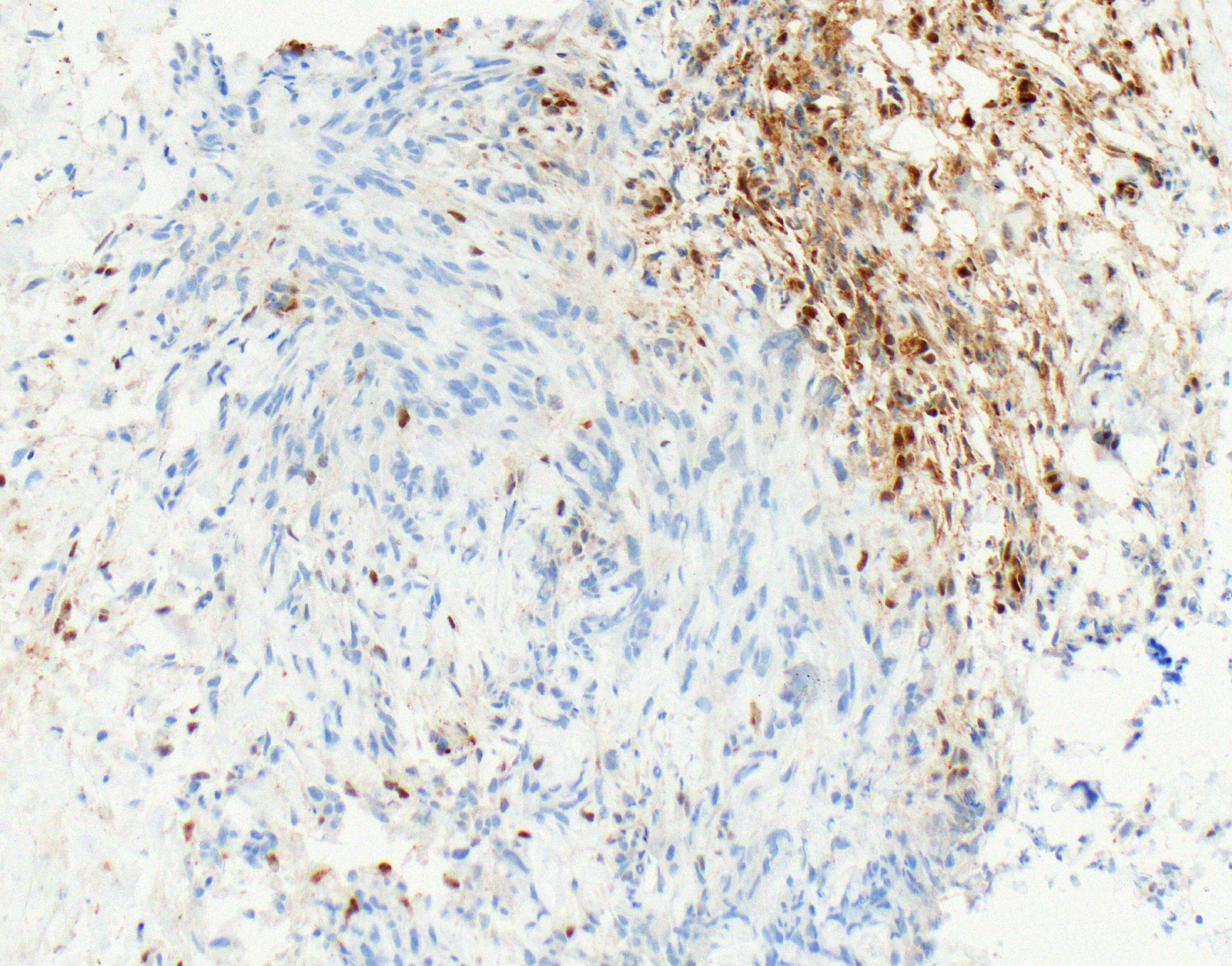
Microscopic (histologic) images
Contributed by Anja C. Roden, M.D.
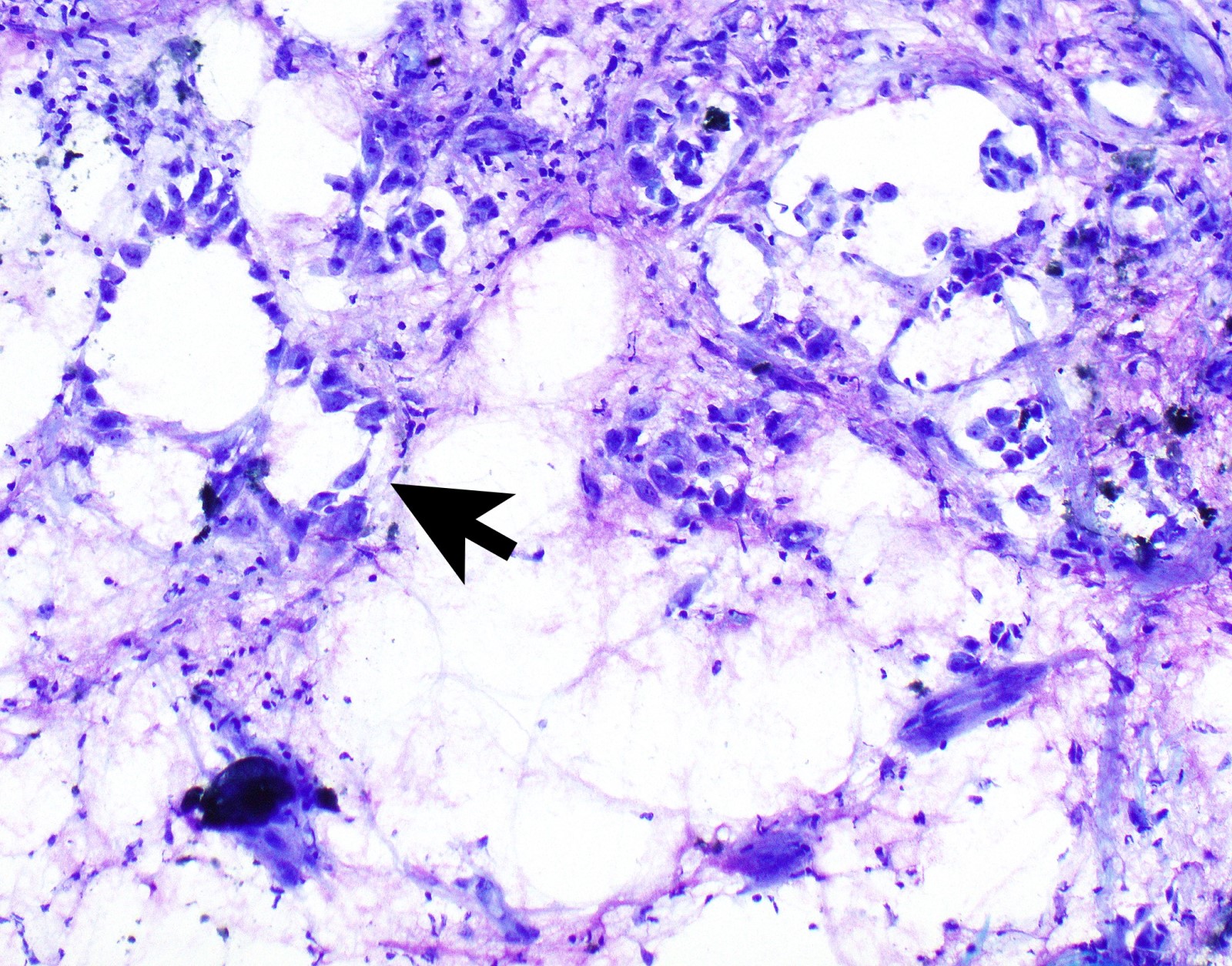
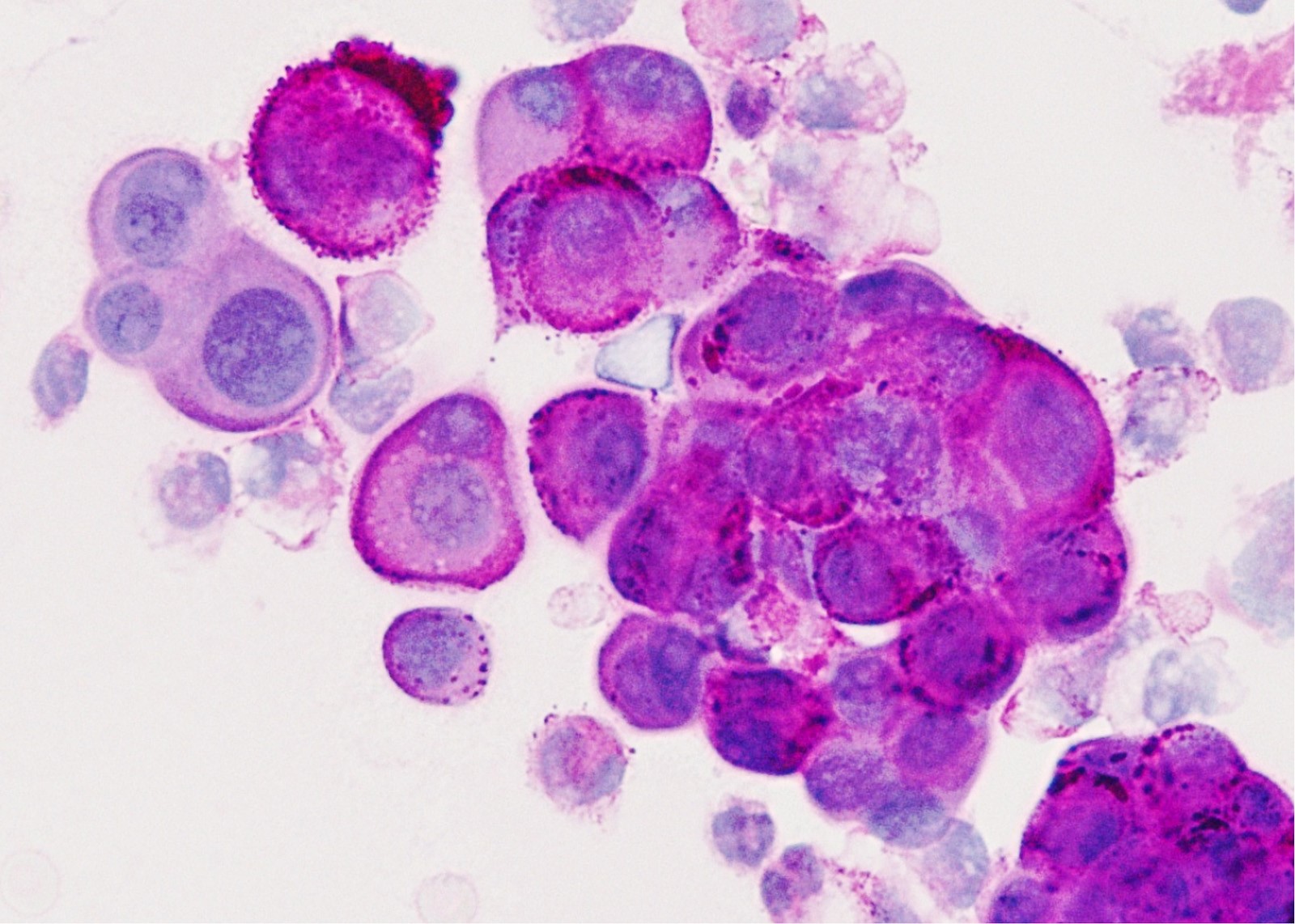
Cytology description
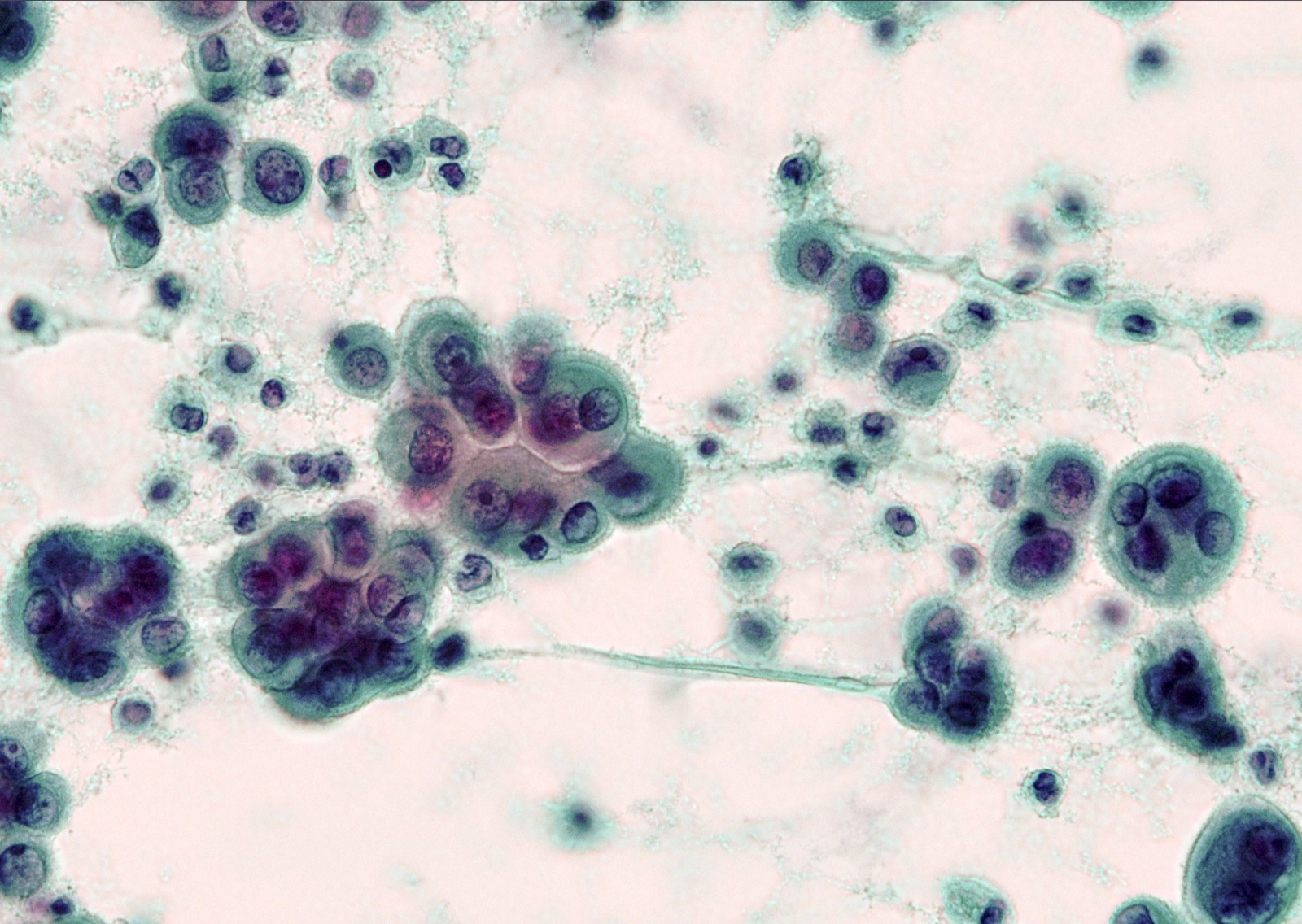
- In general, only epithelioid malignant pleural mesothelioma shed into pleural effusion
- Epithelioid cells in sheets, clusters, morules, papillae
- Usually bland cytology, can be pleomorphic
- Psammoma bodies possible
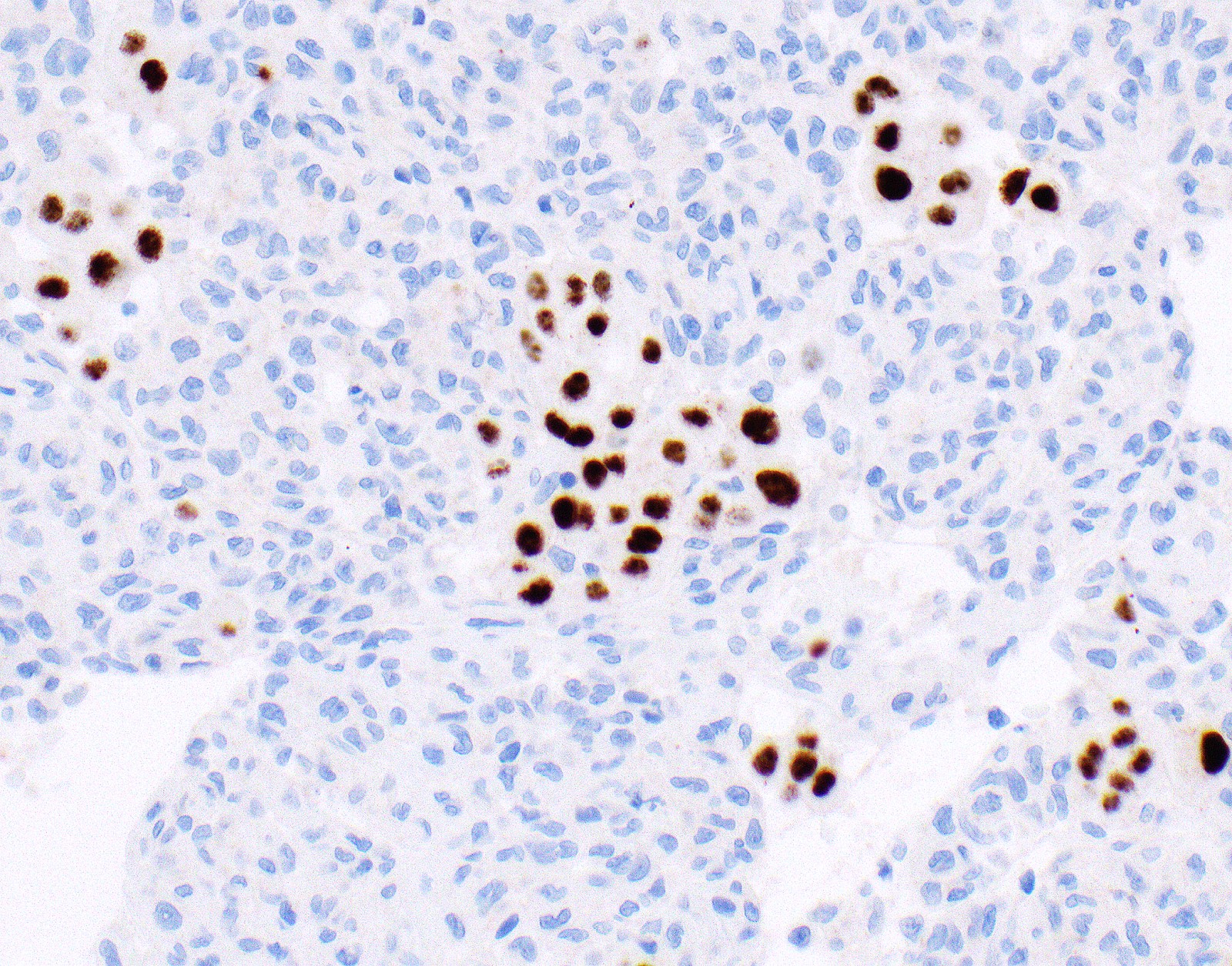
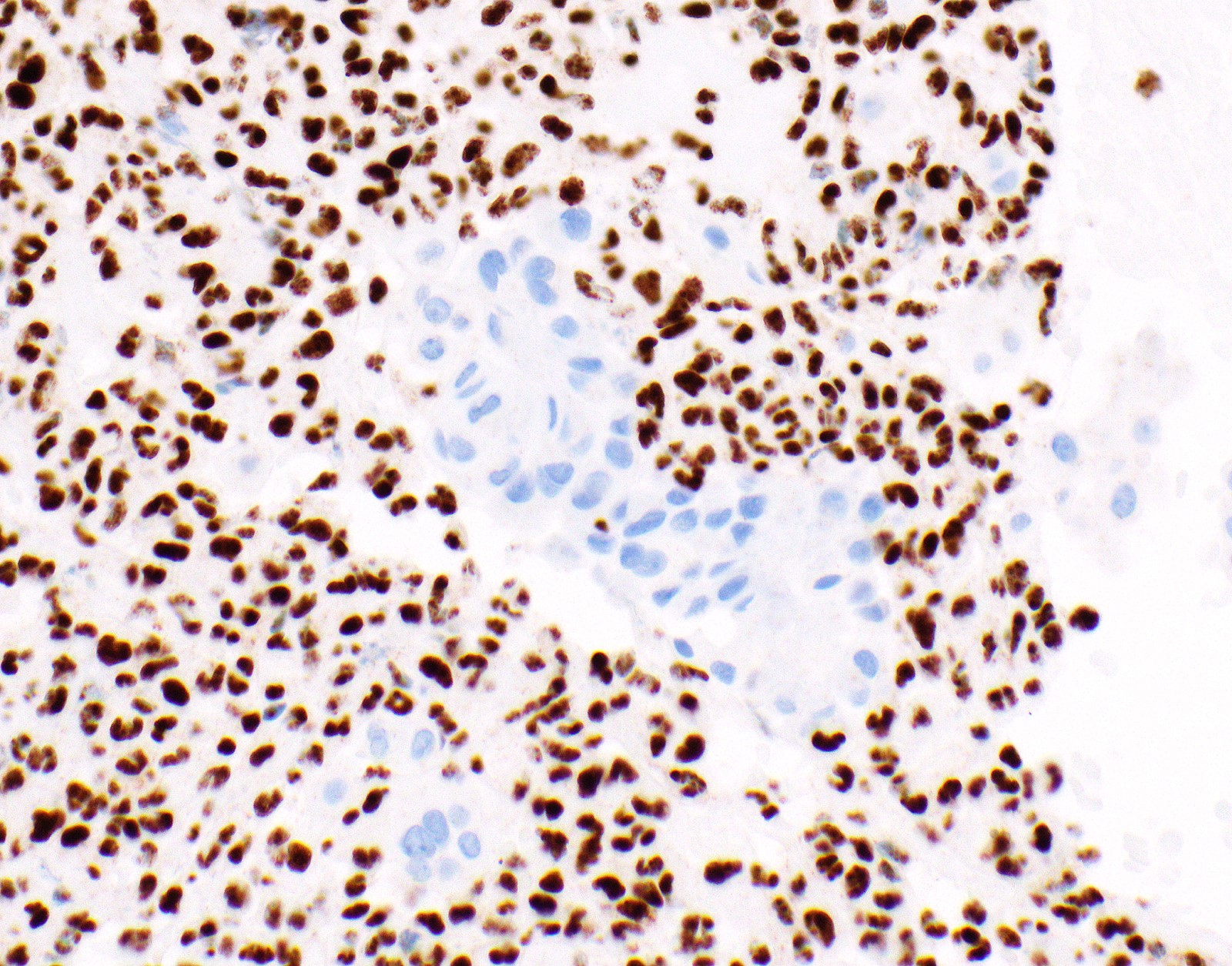
- Loss of BAP1 or MTAP expression or homozygous deletion of CDKN2A helpful in establishing the diagnosis of malignant pleural mesothelioma (see below) (Cancer 2003;99:51)
- On cytology specimens (Klin Khir 1995;2:53):
- Lack of MTAP and BAP1 expression by immunohistochemistry: 100% specific for malignant pleural mesothelioma (versus reactive mesothelial proliferation); 42 and 60% sensitive, respectively
- Loss of MTAP or BAP1 expression: 78% sensitive for malignant pleural mesothelioma
- Homozygous deletion of CDKN2A by FISH: 62% sensitive for malignant pleural mesothelioma, 100% specific
- Combination of loss of BAP1 expression or homozygous deletion of CDKN2A by FISH 84% sensitive for malignant pleural mesothelioma
- Loss of MTAP expression: sensitivity and specificity for homozygous deletion of CDKN2A by FISH; 68 and 100%, respectively
- Exclude metastatic carcinoma using immunostains (see below)
Cytology images
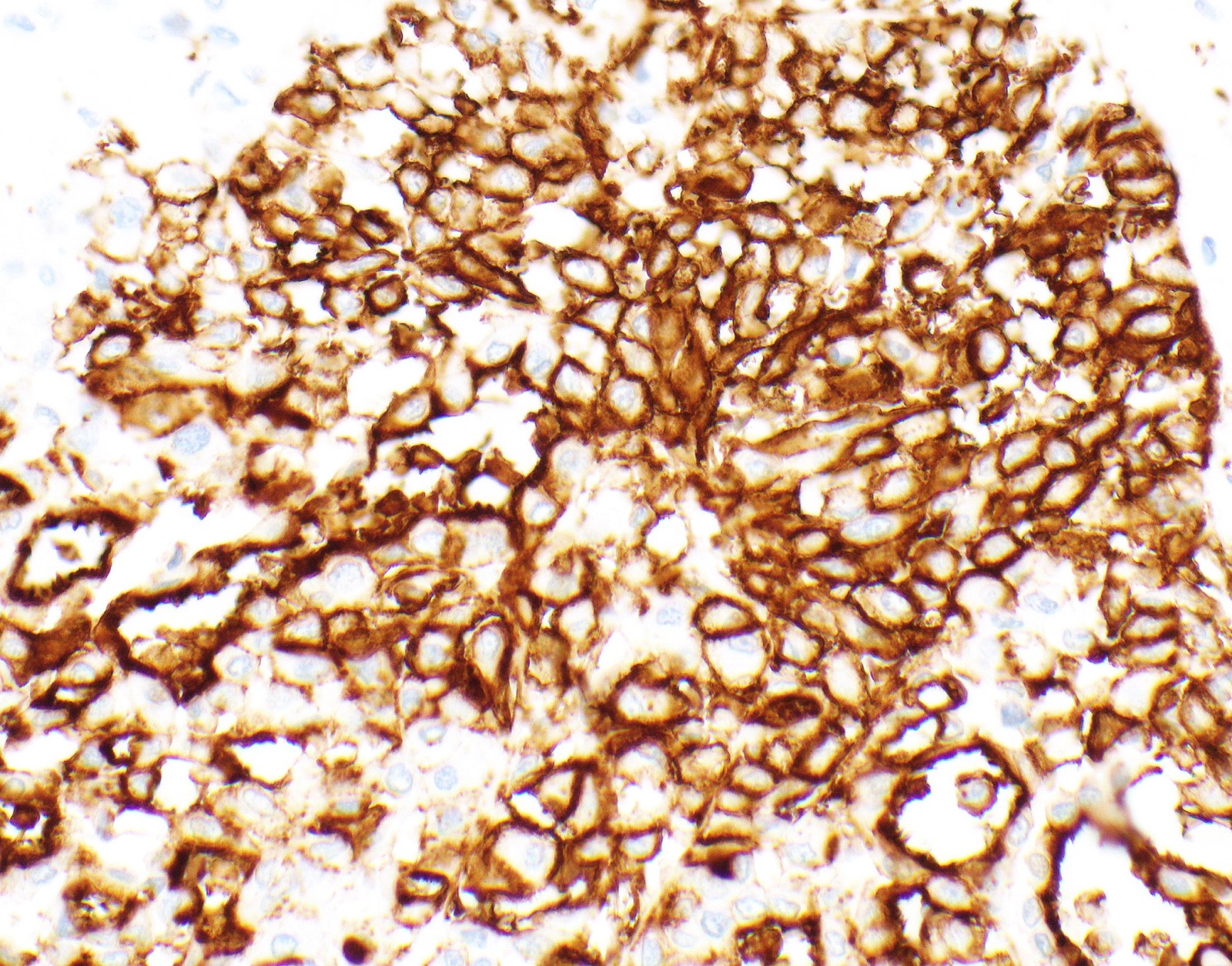
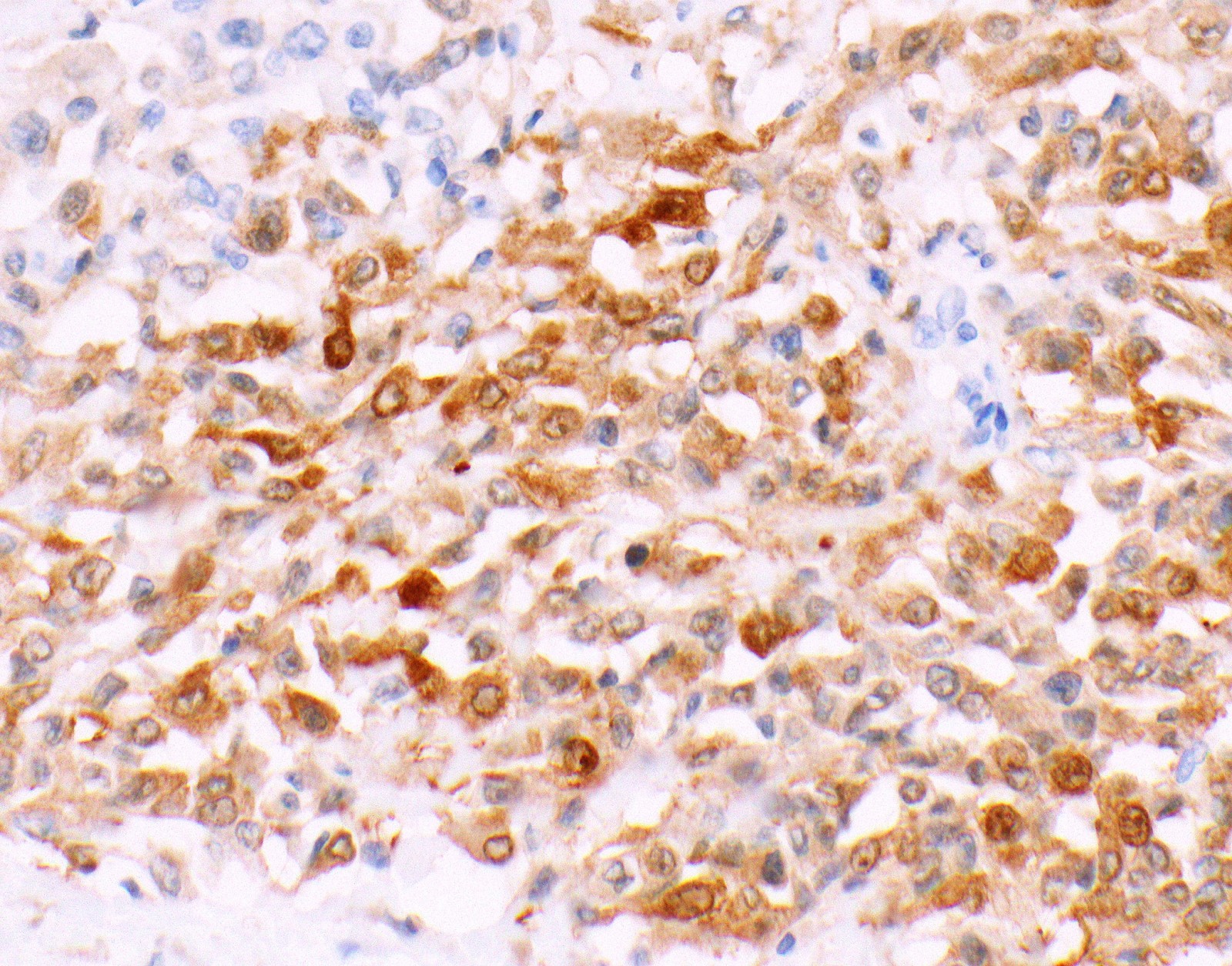
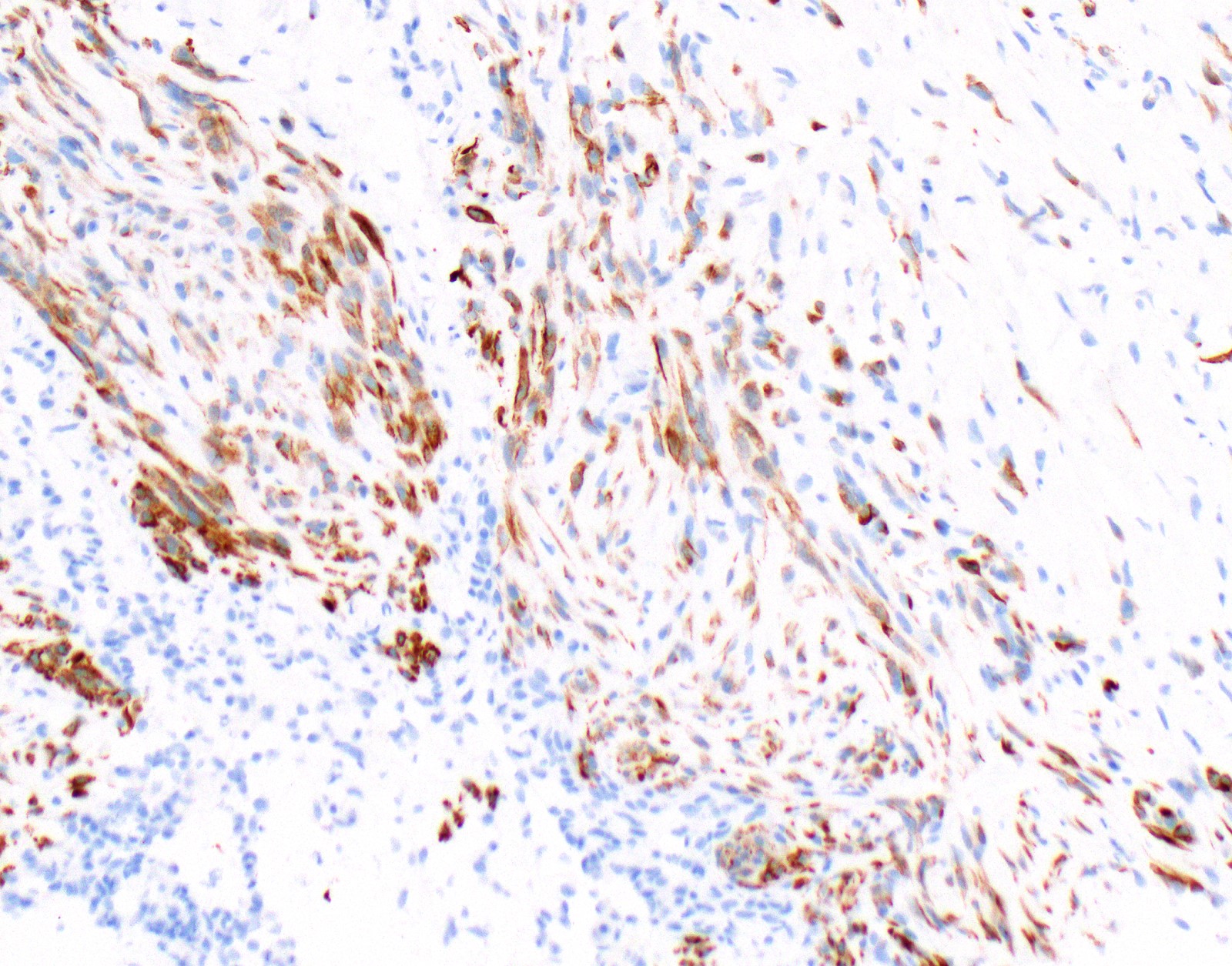
Positive stains
- See Table 1 and Table 2
- Keratin (e.g., AE1 / AE3):
- Expressed in virtually all epithelioid malignant pleural mesothelioma and almost all sarcomatoid malignant pleural mesothelioma (if multiple keratins are negative consider other diagnosis)
- Useful to:
- Distinguish from sarcoma, malignant melanoma and other differential diagnoses
- Identify or confirm invasion
- Markers for mesothelial differentiation:
- No single marker sufficiently sensitive or specific for mesothelial differentiation → panel of at least two carcinoma markers (e.g., pCEA, TTF1, among others) and two mesothelial markers (i.e., WT1, calretinin, CK5, CK5/6, D2-40) recommended (Table 1)
- MOC31 and BerEP4, although considered carcinoma markers, often at least focally expressed in malignant pleural mesothelioma
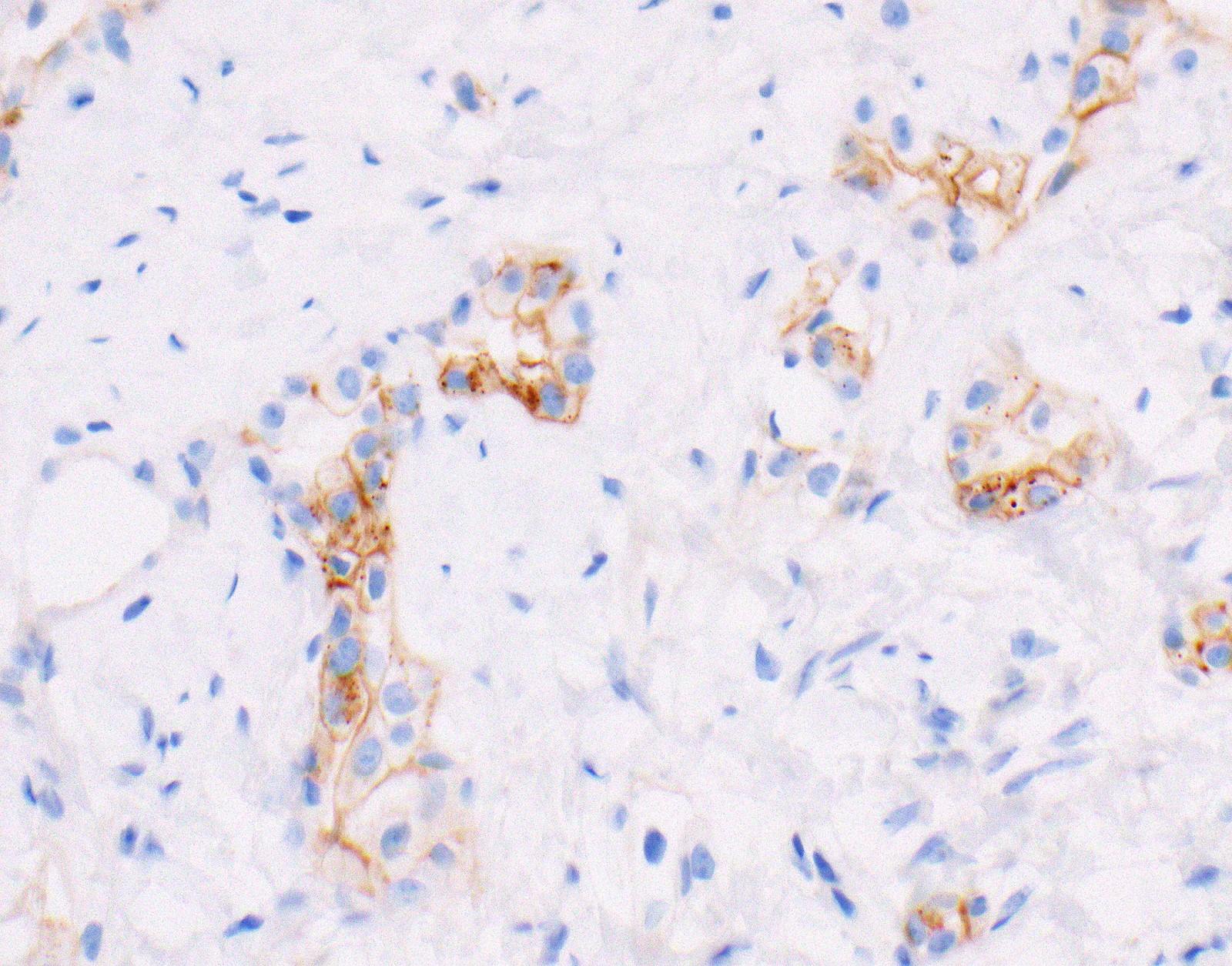
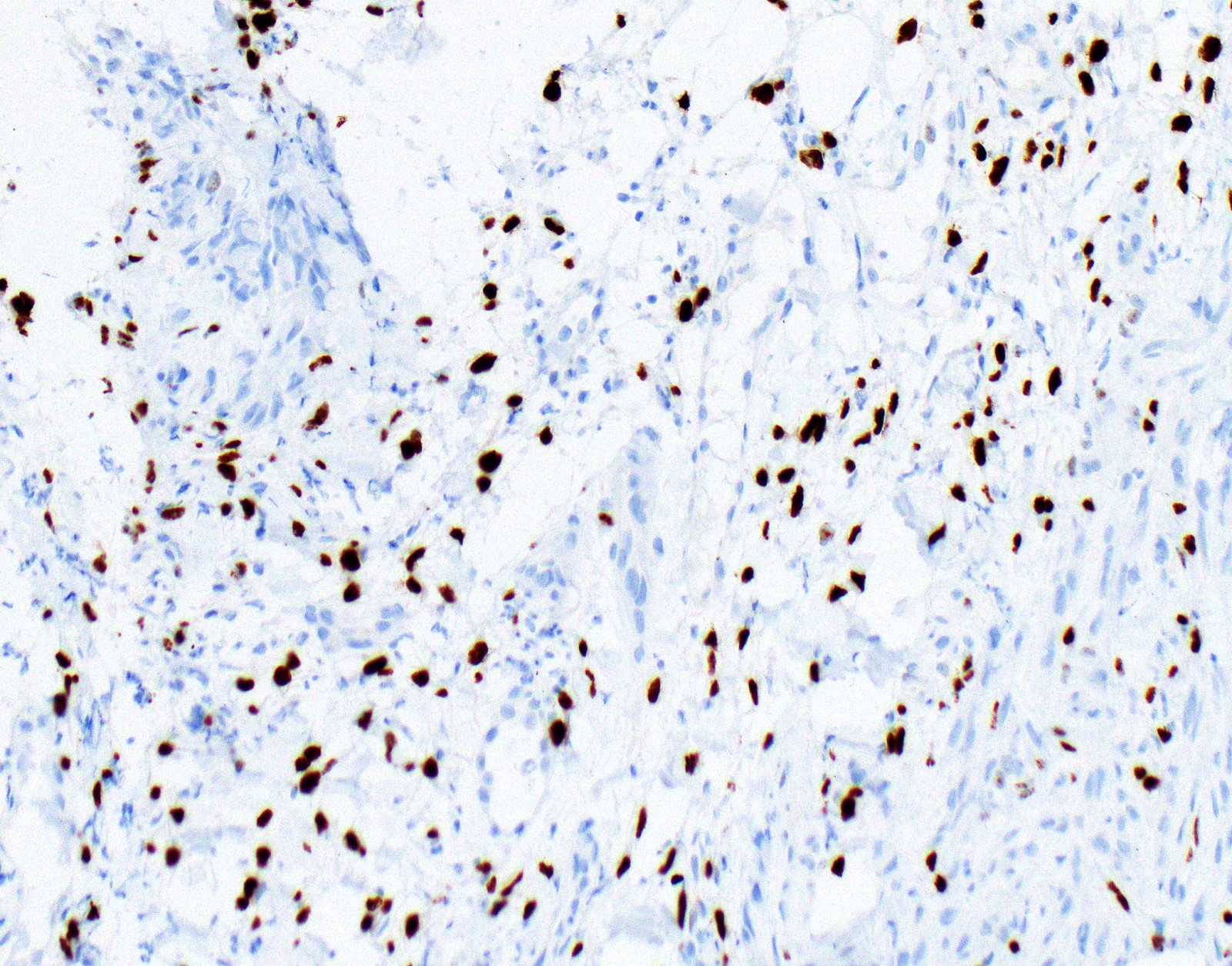
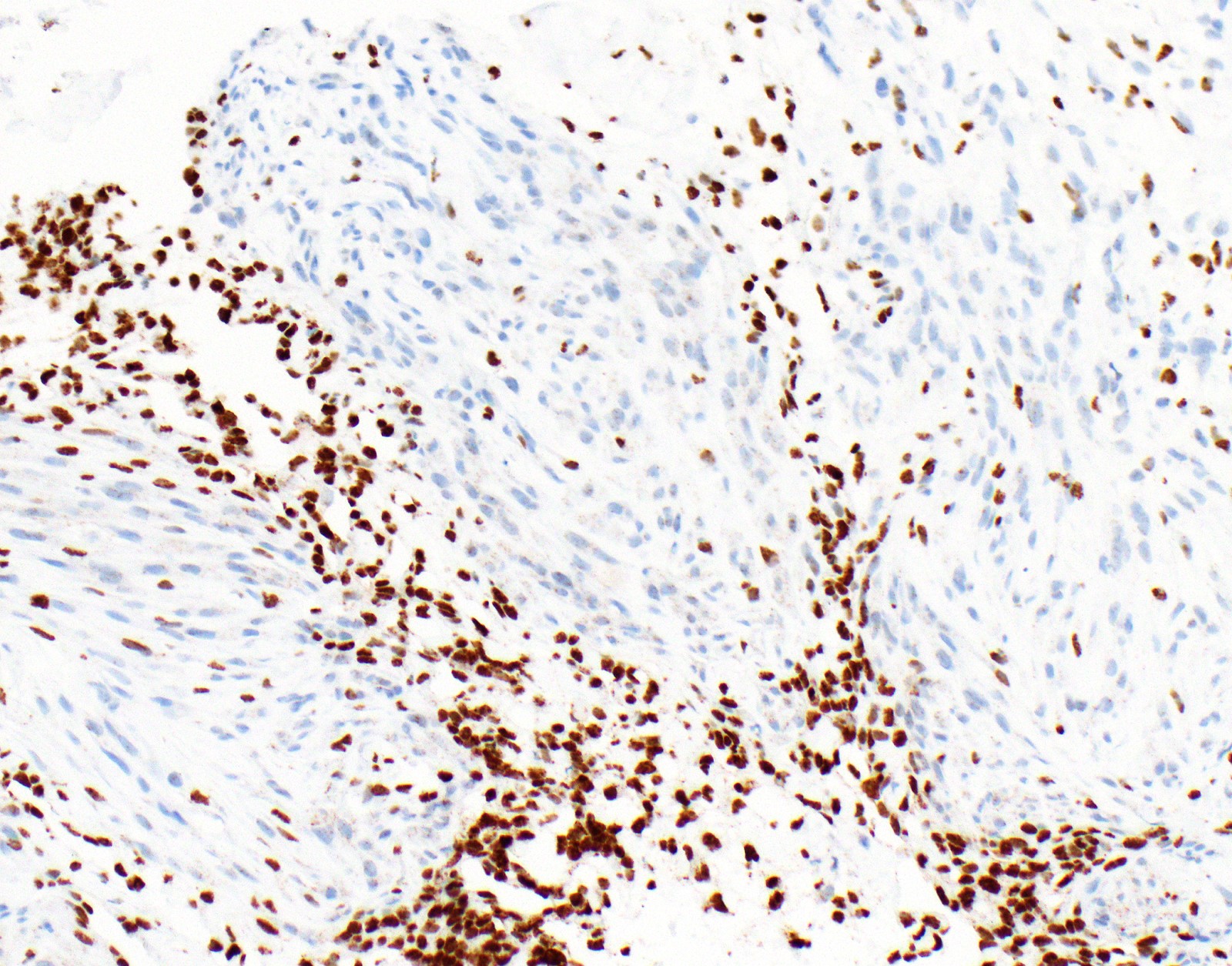
Negative stains
- See Positive stains, Table 1 and Table 2
- Loss of nuclear BAP1 or cytoplasmic (or substantially weaker than positive internal control) MTAP expression confirm malignancy but are not specific for malignant pleural mesothelioma (see Table 2 above)
- Loss of cytoplasmic MTAP expression is a reasonable alternative for homozygous deletion of CDKN2A, k = 0.63 - 0.69; sensitivity and specificity of loss of cytoplasmic MTAP expression for homozygous deletion of CDKN2A, 78 - 93% and 96 - 100%, respectively; if MTAP expression is preserved but morphology and immunophenotype are highly suspicious for malignant pleural mesothelioma: CDKN2A by FISH might be performed (Lung Cancer 2018;125:198, Mod Pathol 2020;33:245, Lung Cancer 2018;125:198)
- Aberrant expression of CK20 rare (Appl Immunohistochem Mol Morphol 2019;27:e93)
Electron microscopy description
- In general not used anymore in the distinction from adenocarcinoma (Arch Pathol Lab Med 2018;142:89)
- Numerous long, thin, sinuous microvilli that are not covered by a glycocalyx (in contrast to adenocarcinoma in which microvilli are usually also shorter) (Ultrastruct Pathol 2006;30:3)
- Large desmosomes and prominent junctional complexes
- Tonofilaments frequently in a perinuclear distribution
Molecular / cytogenetics description
- See also Etiology and Table 2
- In a subset of malignant pleural mesothelioma
- Somatic inactivating mutations in BAP1 gene
- Germline mutations in BAP1 gene
- Homozygous deletion of CDKN2A
- Deletion of NF2 (53 - 76%) (Mod Pathol 2020;33:235, Mod Pathol 2018;31:122)
Sample pathology report
- Pleura, right, pleurectomy:
- Malignant mesothelioma, epithelioid type (see synoptic report)
- 1 of 2 lymph nodes involved by malignant mesothelioma
Differential diagnosis
- Metastatic disease:
- Immunohistochemistry (Table 1) and history required
- Metastatic adenocarcinoma
- Metastatic squamous cell carcinoma
- Keratinization or intercellular bridges in a subset
- Expression of p40
- Metastatic sarcomatoid carcinoma
- Expression of claudin 4 might be helpful
- Sarcoma (primary or metastatic)
- Lack of expression of keratin
- Expression of lineage specific markers
- Presence of disease defining molecular alterations such as t(X;18) and rearrangements of SS18 gene
- Metastatic carcinoma
- Expression of carcinoma markers in carcinoma component
- Possible expression of lineage specific markers in sarcoma component
- Malignant melanoma
- Epithelioid hemangioendothelioma (EHE)
- Reactive mesothelial proliferation:
- Lack of invasion, tumefactive or haphazard growth (see Microscopic (histologic) description)
- Expression of BAP1 and MTAP preserved (Table 2)
- Wild type of CDKN2A (Table 2)
- Lung adenocarcinoma directly extending into pleura:
Board review style question #1
- Which clinical history would be most likely for this patient?
- 30 year old man who underwent orchiectomy for immature teratoma as a child
- 55 year old man who is on dialysis for diabetic nephropathy
- 60 year old man who worked in a shipyard 35 years ago
- 65 year old man who worked in a coal mine for 30 years
- 65 year old woman with history of breast carcinoma
Board review style answer #1
C. This patient was likely exposed to asbestos 35 years ago. Unilateral pleural thickening is highly suspicious for malignant pleural mesothelioma.
Answer A is false; metastases from germ cell tumors would be usually bilateral. B is false; this patient would likely present with bilateral pleural effusion and possible bilateral pleural thickening. D is false; exposure to coal dust is not a risk factor for malignant pleural mesothelioma. E is false; metastases from breast carcinoma would be usually bilateral.
Comment Here
Reference: Diffuse malignant mesothelioma
Answer A is false; metastases from germ cell tumors would be usually bilateral. B is false; this patient would likely present with bilateral pleural effusion and possible bilateral pleural thickening. D is false; exposure to coal dust is not a risk factor for malignant pleural mesothelioma. E is false; metastases from breast carcinoma would be usually bilateral.
Comment Here
Reference: Diffuse malignant mesothelioma
Board review style question #2
- This patient has a history of ovarian carcinoma. Which would be the most useful panel of immunostains to rule out malignant mesothelioma in this patient?
- Calretinin, CK5, PAX8, pCEA
- CAM 5.2, WT1, MOC31, D2-40
- D2-40, WT1, pCEA, MOC31
- Keratin, AE1 / AE3, WT1, estrogen receptor, MOC31
- WT1, pCEA, MOC31, BerEP4
Board review style answer #2
A. Expression of calretinin and CK5 would indicate malignant pleural mesothelioma while PAX8 and pCEA indicate metastatic ovarian carcinoma. Be aware that PAX8 can be positive in a small subset of malignant pleural mesothelioma.
Answer B is false; CAM5.2 and WT1 will be positive in both, malignant pleural mesothelioma and metastatic ovarian carcinoma; MOC31 will be expressed in metastatic carcinoma but can be at least focally expressed in malignant pleural mesothelioma. D2-40 can be expressed in serous carcinomas. C is false; although two malignant pleural mesothelioma markers (D2-40, WT1) and two carcinoma markers (pCEA and MOC31), WT1 can be positive in ovarian carcinomas and MOC31 is sometimes at least focally expressed in malignant pleural mesothelioma. D is false; keratin AE1/AE3 and WT1 will be positive in both, malignant pleural mesothelioma and metastatic ovarian carcinoma; MOC31 will be expressed in metastatic carcinoma but can be at least focally expressed in malignant pleural mesothelioma. E is false; these are three carcinoma markers (pCEA, MOC31, BerEP4) and only one malignant pleural mesothelioma marker which also is frequently expressed in ovarian carcinomas. For workup of malignant pleural mesothelioma, at least two carcinoma markers and two mesothelial markers should be used.
Comment Here
Reference: Diffuse malignant mesothelioma
Answer B is false; CAM5.2 and WT1 will be positive in both, malignant pleural mesothelioma and metastatic ovarian carcinoma; MOC31 will be expressed in metastatic carcinoma but can be at least focally expressed in malignant pleural mesothelioma. D2-40 can be expressed in serous carcinomas. C is false; although two malignant pleural mesothelioma markers (D2-40, WT1) and two carcinoma markers (pCEA and MOC31), WT1 can be positive in ovarian carcinomas and MOC31 is sometimes at least focally expressed in malignant pleural mesothelioma. D is false; keratin AE1/AE3 and WT1 will be positive in both, malignant pleural mesothelioma and metastatic ovarian carcinoma; MOC31 will be expressed in metastatic carcinoma but can be at least focally expressed in malignant pleural mesothelioma. E is false; these are three carcinoma markers (pCEA, MOC31, BerEP4) and only one malignant pleural mesothelioma marker which also is frequently expressed in ovarian carcinomas. For workup of malignant pleural mesothelioma, at least two carcinoma markers and two mesothelial markers should be used.
Comment Here
Reference: Diffuse malignant mesothelioma