Table of Contents
Definition / general | Essential features | Epidemiology | Sites | Pathophysiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Case reports | Radiology description | Radiology images | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Redding-Ochoa J, Chen L. Taenia solium (neurocysticercosis). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/parasitologytaeniasolium.html. Accessed April 1st, 2025.
Definition / general
- CNS infection caused by the ingestion of Taenia solium (pork tapeworm) eggs through fecal - oral route
- Cysticercus: the larval stage of the cestode (i.e., flatworm) Taenia solium
- Neurocysticercosis includes intraparenchymal, extraparenchymal and ocular forms of disease
Essential features
- Neurocysticercosis occurs when humans ingest the eggs of Taenia solium
- Taeniasis occurs when the larval cysts are ingested
- Clinical presentation ranges from asymtomatic to fatal, mimicking many other neurological disorders
- Neurocysticercosis is a common cause of seizure disorder in endemic areas (e.g., Latin America, India)
- Intraparenchymal disease is the most common form of neurocysticercosis
Epidemiology
- Endemic infection in Mexico, Central and South America, sub-Saharan Africa, India and southeast Asia (Clin Microbiol Rev 2020;33:e00085)
- Lack of hygiene and close human interaction with pigs are related to cysticerci infection
- Prevalence of asymptomatic neurocysticercosis assessed by computed tomography (CT) scan ranged from 9.1% to 18.8% in endemic rural communities (Neuroepidemiology 2003;22:139, Trans R Soc Trop Med Hyg 2011;105:531, PLoS Negl Trop Dis 2016;10:e0005130)
- Hispanic patients showed a 35 fold increased hospitalization risk (2.5/100,000), compared to non-Hispanic White patients (Emerg Infect Dis 2015;21:969)
- Neurocysticercosis in developed countries or in countries where pork is not commonly consumed occurs mostly in immigrants from endemic countries (J Travel Med 2023;30:taac102, Microorganisms 2021;9:1221, Am J Trop Med Hyg 2005;73:766)
Sites
- CNS infection
- Intraparenchymal (Clin Microbiol Rev 2020;33:e00085, Front Vet Sci 2021;8:615703)
- Cerebrum (most frequent site of involvement) (Arch Pathol Lab Med 2010;134:1560)
- Brainstem
- Cerebellum
- Spinal cord (uncommon)
- Extraparenchymal
- Intraventricular: the fourth ventricle is most affected
- Subarachnoid space
- Worse prognosis than intraparenchymal infection
- Intraparenchymal (Clin Microbiol Rev 2020;33:e00085, Front Vet Sci 2021;8:615703)
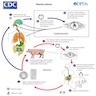
Pathophysiology
- Humans are the only definitive host
- Pigs and humans are intermediate hosts
- Life cycle (Clin Microbiol Rev 2020;33:e00085, ScientificWorldJournal 2012;2012:159821)
- Eggs pass into the environment in feces
- Intermediate hosts (e.g., pigs or humans) ingest the eggs in contaminated food or water
- Eggs hatch in the gastrointestinal (GI) tract when exposed to bile
- Oncospheres (i.e., the embryo inside the egg) penetrate the intestinal wall to gain access to the systemic circulation
- Dissemination to the skeletal muscle, subcutaneous tissue, eye or brain, where the oncospheres develop as cysticerci (i.e., larval cysts)
- If the cysticerci are ingested in raw or undercooked meat of infected pigs
- Cyst wall disintegrates
- Scolex attaches to the small bowel mucosa
- In months, the cysticercus grows as an adult hermaphrodite tapeworm
- Adults have ≥ 1,000 segments or proglottids
- If the cysticerci are ingested in raw or undercooked meat of infected pigs
- Proglottids of the adult tapeworm contain eggs; these are released into the GI tract and excreted in feces
Diagrams / tables
Clinical features
- 4 phases of infection are recognized
- Initial (viable): commonly asymptomatic
- Early inflammatory
- Degenerating
- Nonviable
- Location of the infestation determines the clinical manifestations
- Parenchymal
- Common: headache, seizures (Clin Microbiol Rev 2020;33:e00085, Pathogens 2023;12:1313, Am J Trop Med Hyg 2020;103:639)
- Other manifestations: visual alterations, focal signs
- Manifestation onset develops 3 - 5 years after ingestion of T. solium eggs
- May present decades after infestation
- Cysticercus degeneration or nonviable calcified organisms are associated with the clinical manifestations due to inflammation and edema
- Cysticercal encephalitis: marked immune host reaction and innumerable parenchymal cysticercal cysts
- Asymptomatic incidental disease is common (Pathogens 2023;12:1313)
- Spinal cord lesions are extremely rare (Neurosurg Focus 2002;12:e8)
- Extraparenchymal
- Intracranial hypertension and hydrocephalus: nausea, vomiting
- More common in adults than children (Clin Microbiol Rev 2020;33:e00085, Pediatr Infect Dis J 2006;25:801)
- Cranial nerve related manifestations: due to mass effect related compression or nerve entrapment in arachnoid exudates (Infect Dis Clin North Am 2019;33:153)
- Intracranial hypertension and hydrocephalus: nausea, vomiting
- Ocular / orbital
- Diplopia, eye pain, altered vision
- Funduscopic visualization of the cysticercus is an absolute criterion of neurocysticercosis
- Ocular cysticercosis should be ruled out before starting antiparasitic drug therapy (Clin Microbiol Rev 2020;33:e00085)
- Parenchymal
Diagnosis
- Definite neurocysticercosis diagnosis is based on neuroimaging and clinical / epidemiologic criteria (Clin Microbiol Rev 2020;33:e00085, Acta Neurol Scand 1997;96:76, J Neurol Sci 2017;372:202)
- 1 absolute criterion or
- 2 major neuroimaging criteria and epidemiological exposure criteria or
- 1 major imaging criterion and 2 epidemiological exposure criteria and exclusion of diseases with a similar radiologic presentation
- Probable neurocysticercosis
- 1 major neuroimaging criterion and 2 epidemiological exposure criteria
- 1 minor neuroimaging criterion and 1 epidemiological exposure criterion
- Absolute criteria
- Neuropathological assessment
- Subretinal cysticercus visualization
- Neuroimaging: cystic lesion with a scolex
- Neuroimaging criteria
- Major criteria
- Cystic lesion(s) with no discernible scolex
- Enhancing lesions
- Multilobulated subarachnoid cystic lesions
- Solid calcified lesion (usually < 1 cm)
- Minor criteria
- Obstructive hydrocephalus
- Abnormal enhancement of basal leptomeninges
- Major criteria
- Clinical / epidemiologic criteria
- Major criteria
- Serologic evidence of specific anticysticercal antibodies or cysticercal antigens
- Extraneural cysticercosis
- Household contact with taeniasis
- Minor criteria
- Suggestive clinical manifestations
- Residence in an endemic area
- Major criteria
Laboratory
- Serological tests are used for confirmation in individuals with clinically suspected disease (Clin Infect Dis 2018;66:e49)
- Enzyme linked immunoelectrotransfer blot (EITB) is the reference standard antibody test (J Neurol Sci 2017;372:202, J Infect Dis 1989;159:50)
- Sensitivity is higher in serological tests than cerebrospinal fluid (CSF) assays
- In single parenchymal lesions, the sensitivity is markedly decreased (J Clin Microbiol 2018;56:e00424)
Case reports
- 10 year old girl with massive neurocysticercosis (BMC Pediatr 2024;24:79)
- 31 year old man with new onset seizure disorder (Cureus 2021;13:e13897)
- 37 year old woman with isolated intraspinal neurocysticercosis (Front Neurol 2022;13:1030468)
Radiology description
- More commonly located in cerebral gray-white matter junction (Neuroimaging Clin N Am 2012;22:659)
- Early noninflammatory lesions (vesicular stage)
- Computed tomography (CT) and magnetic resonance imaging (MRI): small (5 - 20 mm), thin walled cyst without contrast enhancement
- Cyst fluid and CSF have similar imaging characteristics
- Little or no edema associated with cysts
- Scolex: eccentrically located iso or hyperintense nodule on T1 and T2 weighed MRI (N Engl J Med 1984;311:1492, AJNR Am J Neuroradiol 1989;10:1011)
- Swiss cheese appearance: multiple parenchymal cysts present
- Computed tomography (CT) and magnetic resonance imaging (MRI): small (5 - 20 mm), thin walled cyst without contrast enhancement
- Degenerative phase
- Thicker and irregular cyst walls with contrast enhancement
- Single lesions with ring-like enhancement raise questions about neoplastic or other infectious differential diagnoses
- Cystic fluid becomes hyperdense (CT) and hyperintense (T1 → mild hyperintensity; T2 or FLAIR → marked hyperintensity)
- Scolex may be absent
- Thicker and irregular cyst walls with contrast enhancement
- Calcified / inactive phase
- Nodular mineralized lesions
- No perilesional edema
- Early noninflammatory lesions (vesicular stage)
- Intraventricular and meningeal / subarachnoid forms
- Hard to detect on CT and MRI due to similar density (CT) or intensity (MRI) between the lesions and CSF
- Distortion of the ventricular system due to CSF flow obstruction may be the only neuroimaging indicator of neurocysticercosis (Neuroimaging Clin N Am 2012;22:659)
Treatment
- Acute symptom management (Am J Trop Med Hyg 2018;98:945)
- Intracranial hypertension
- Steroid therapy to reduce cerebral edema
- Obstructive hydrocephalus: surgical removal of cysticerci or placement of extraventricular drain
- Communicating hydrocephalus: third ventriculostomy or ventriculoperitoneal (VP) shunt (CSF diversion techniques)
- Antiparasitic therapy is contraindicated in individuals with intracranial hypertension
- Antiseizure therapy
- Commonly used antiepileptic drugs: phenytoin, carbamazepine, levetiracetam, clobazam (Neurol India 2006;54:157)
- Intracranial hypertension
- Parenchymal disease
- Antiparasitic therapy
- Praziquantel
- Mebendazole
- Albendazole
- Antiparasitic drugs are contraindicated in severe cysticercal encephalitis due to risk of inflammatory response triggering cerebral edema and herniation (PLoS Negl Trop Dis 2021;15:e0009883)
- Antiparasitic therapy
Gross description
- Cysts range in size from 0.5 to 2 cm
- Cysts are round or oval and contain semitranslucent or white more dense fluid (Arch Pathol Lab Med 2010;134:1560)
- Number of cysts varies between a single lesion to hundreds
- Protoscolex is visualized grossly as the solid central portion of the cysticercus
- Exudate may be observed in leptomeningeal cysticercosis
- In subarachnoid neurocysticercosis, the exudate turns into a fibrous plastron that obliterates the subarachnoid space
- CSF blockage by the cysts or the exudate results in ventricular expansion
Gross images
Microscopic (histologic) description
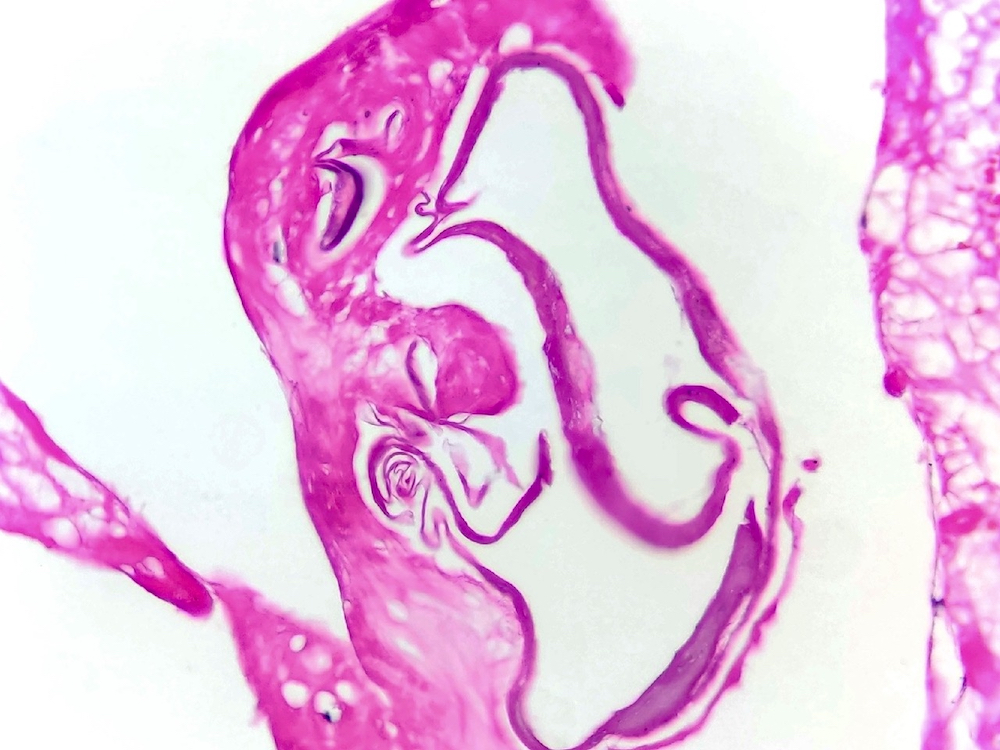
- Cysticercus consists of
- Bladder wall
- Fluid
- Invaginated scolex (i.e., protoscolex) surrounded by a spiral canal; the scolex contains suckers and rostellum with hooklets
- Bladder wall measures 100 - 200 micrometers in thickness and consists of
- Outer tegument with microvilli
- Haphazardly arranged smooth muscle cells
- Loose stroma
- Excretory channels
- Calcareous bodies (calcium concretions) (Rev Latinoam Microbiol 1999;41:303)
- 4 cysticercal stages are recognized (Lancet Infect Dis 2002;2:751)
- Vesicular stage: vesicles with viable organisms (Arch Pathol Lab Med 2010;134:1560)
- Viable organism → larva with an invaginated scolex surrounded by fluid and a thin wall
- Limited host tissue reaction is seen associated with viable cysts
- Early inflammatory phase
- Initial neutrophilic response with scattered eosinophils and granulation tissue
- Organism may survive for years if a weak inflammatory response is mounted (Epilepsy Curr 2004;4:107)
- Colloidal stage (Arch Pathol Lab Med 2010;134:1560)
- Cysticercus hyalinization
- Cystic fluid becomes more turbid
- More severe inflammatory response with lymphocytes, neutrophils, eosinophils and multinucleated giant cells is elicited
- Granular nodular stage
- Scolex degeneration and vesicle involution
- Cyst wall thickening
- Calcification: indicates the nonviable phase
- Nodular calcified stage
- Cysticercus is replaced by collagen and is calcified
- Vesicular stage: vesicles with viable organisms (Arch Pathol Lab Med 2010;134:1560)
- Racemose form: distinct multilobulated grape-like cysts without a scolex
- Common in subarachnoid neurocysticercosis
- Aggressive disease which may present as hydrocephalus, intracranial hypertension, meningitis and neurologic deficit
Microscopic (histologic) images
Differential diagnosis
- Abscess:
- Central suppurative area is surrounded by vascular congestion and fibroblastic proliferation
- Tegument and scolex are absent
- Tuberculoma:
- Central caseous necrosis, Langhans type giant cells, acid fast mycobacteria (Ziehl-Neelsen positive bacilli) (BMJ 2013;347:f6604)
- Paragonimiasis:
- Eggs contain an operculum (i.e., a brown shell) and measure 80 - 120 micrometers
- Hydatid disease:
- Cysts are usually much larger
- Its thick outer wall measures 2 - 3 mm
- Scolices show 4 suckers with 2 rows of hooklets
- Pilocytic astrocytoma:
- Rosenthal fibers and eosinophilic granular bodies
- Compact fibrillar background
- Vascular proliferation
- Activation of mitogen activated protein kinase (MAPK) pathway
Board review style question #1
A 35 year old man without prior disease presented with focal seizures with secondary generalization. There is no prior history of head trauma. A computed tomography (CT) scan reveals a solid / cystic, 0.5 cm lesion with focal calcification in the middle temporal lobe. Due to concerns of neoplastic origin, a surgical resection is scheduled. The microscopic image of the surgical specimen is shown above. What is the intermediate host in the life cycle of this parasite?
- Cows
- Pigs
- Rabbits
- Rats
Board review style answer #1
B. Pigs. Although humans may act as intermediate hosts in the life cycle of Taenia solium, pigs are more commonly involved as intermediate hosts after ingesting contaminated food or water by gravid proglottids or eggs. Answer A is incorrect because only cows are intermediate hosts of Taenia saginata. Note that the eggs of T. saginata and T. solium are identical. Answer D is incorrect because rats are intermediate hosts of Taenia taeniaeformis. Answer C is incorrect because rabbits are intermediate hosts of Taenia pisiformis.
Comment Here
Reference: Taenia solium (neurocysticercosis)
Comment Here
Reference: Taenia solium (neurocysticercosis)
Board review style question #2
A 45 year old man without relevant medical history is brought to the ER with a stupor and a recent onset of vomit, headache and seizures. CT and MRI show marked dilation of the ventricular system and partial compression of the right lateral ventricle. There is a rapid worsening of the clinical status and the patient dies. A brain autopsy is performed, which is remarkable for a 3.5 x 1.8 x 1.2 cm, grape-like lesion in the subarachnoid space of the right Sylvian fissure. Which parasitic structure is absent in this form of cysticercosis?
- Bladder wall
- Outer tegument with microvilli
- Scolex
- Smooth muscle
Board review style answer #2
C. Scolex. The grape-like subarachnoid lesion represents an example of the racemose form of cysticercosis, which is remarkable for the absence of the scolex, in addition to the grape-like morphology. Also, these forms of cysticercosis are difficult to detect by CT and MRI due to the similarity in density (CT) or intensity (MRI) with the surrounding CSF. Answers A, B and D are incorrect because the outer tegument with microvilli (which contain smooth muscle fibers) and a bladder wall are present in this form of cysticercosis.
Comment Here
Reference: Taenia solium (neurocysticercosis)
Comment Here
Reference: Taenia solium (neurocysticercosis)