Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Mattiolo P, Luchini C. Pseudocysts. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/pancreaspseudocyst.html. Accessed April 2nd, 2025.
Definition / general
- Encapsulated collection of homogenous fluid with a well defined fibrous / inflammatory wall
- Localized collections of pancreatic secretions that usually develop after pancreatitis (acute or chronic), trauma, ductal calculi or obstructive neoplasms
Essential features
- There is no epithelial lining
- Fluid content
- Nonneoplastic nature
Terminology
- Pseudocyst is the only standard term to indicate this condition
- Older terms (e.g., false cyst) are discouraged
ICD coding
- ICD-10: K86.3 - pseudocyst of pancreas
Epidemiology
- Male prevalence (M:F = 4:1)
- Peak of incidence in fourth to fifth decade
- Virtually all patients with pancreatic pseudocyst have symptoms and show an history of acute or chronic pancreatitis (Am J Gastroenterol 2018;113:464)
- Typically, pancreatic pseudocyst is unilocular and does not display calcification
- Involvement of main pancreatic duct is common and it might be irregularly dilated with stones (Pancreatology 2017;17:738)
Sites
- All parts of the pancreatic gland may be involved
- Distal location (body / tail) is more frequent than the cephalic presentation (65% of cases show body / tail involvement)
Etiology
- Thought to arise from disruption of the main pancreatic duct (or its intrapancreatic branches) and the consequent leakage of pancreatic juice that results in a persistent, localized fluid collection (Gut 2013;62:102)
- May also arise in the setting of acute necrotizing pancreatitis as a result of a disconnected duct syndrome, whereby pancreatic parenchymal necrosis of the neck or body of the gland isolates a still viable distal pancreatic remnant (Gastrointest Endosc 2008;68:91)
Clinical features
- Pseudocysts are detectable at least 4 weeks after the clinical presentation of acute pancreatitis, which requires at least 2 of the 3 following features:
- Abdominal pain consistent with acute pancreatitis (acute onset of a persistent, severe, epigastric pain, often radiating to the back)
- Serum lipase activity (or amylase activity) at least 3 times greater than the upper limit of normal
- Characteristic findings of acute pancreatitis on contrast enhanced CT; less commonly on MRI or transabdominal ultrasonography (Gut 2013;62:102)
Diagnosis
- First line of imaging to study pancreatic lesions is represented by CT / MRI
- Second line is represented by endoscopic ultrasound (EUS) with cyst fluid analysis
- Histopathology (Pathologica 2020;112:197)
Laboratory
- EUS could be easily associated with additional investigation, such as
- Cyst fluid analysis; in particular, in pseudocyst, there is usually a marked increase in amylase activity and a very low carcinoembryonic antigen (CEA) concentration in the cyst fluid (< 5 ng/mL) (Gut 2013;62:102, Gastrointest Endosc Clin N Am 2018;28:549)
- Pathology: see Cytology description and Microscopic (histologic) description
Radiology description
- In particular, CT is indicated for the detection of parenchymal, mural or central calcification, especially when differentiating pseudocysts associated with chronic pancreatitis from pancreatic cystic neoplasm (Gut 2018;67:789)
- If CT / MRI is not sufficient, EUS examination is recommended
- Presence of hyperenhancing solid components within cystic lesion rules out the diagnosis of pseudocyst
Case reports
- 7 year old boy with pancreatic pseudocyst caused by blunt abdominal trauma (J Med Case Rep 2017;11:217)
- 13 year old boy with pancreatic pseudocyst in a setting of incomplete pancreas divisum and recurrent acute pancreatitis (J Int Med Res 2021;49:3000605211014395)
- 40 year old woman with pseudocyst arising in gastric ectopic pancreas (Clin Res Hepatol Gastroenterol 2014;38:389)
- 41 year old woman with pancreatic pseudocyst treated with endoscopic management after stent displacement (World J Gastroenterol 2015;21:2249)
- 49 year old man with pancreatic pseudocyst that was suspected to be a cystic neoplasm, specifically a solid pseudopapillary neoplasm (Cureus 2020;12:e9883)
Treatment
- For evacuation of the material that sustains the pseudocyst, 2 major approaches (endoscopic or laparoscopic) are used
- Endoscopic drainage consists of stent implantation by endoscopic retrograde cholangiopancreatography or by EUS
- Advantages: short operation time, low intraoperative blood loss, low to null intra-abdominal sewing, low chances of injury to blood vessels and nerves, short hospital stay, low opioid demand
- Disadvantages: complications, such as hemorrhage, recurrence and stent exchange, occur in 5 - 19% of procedures
- Laparoscopic drainage
- Advantages: removal of more consistent quantities of necrotic tissue and better exploration of cyst wall structure than with the endoscopic approach
- Disadvantages: complications such as abdominal contamination, incomplete anastomosis, gastric perforation (Pancreas 2021;50:788)
- Endoscopic drainage consists of stent implantation by endoscopic retrograde cholangiopancreatography or by EUS
- Current guidelines for treatment of cystic pancreatic lesions discourage surgical treatment of pancreatic cystic lesions, unless there is a high probability of neoplastic degeneration or in order to relieve symptoms of clinical pancreatitis (Pancreatology 2017;17:738)
- Patients with asymptomatic cysts do not require treatment or surveillance if they are diagnosed as pseudocysts on initial imaging and clinical history or if they have a very low risk of malignant transformation (e.g., serous cystadenoma of the pancreas) (Am J Gastroenterol 2018;113:464)
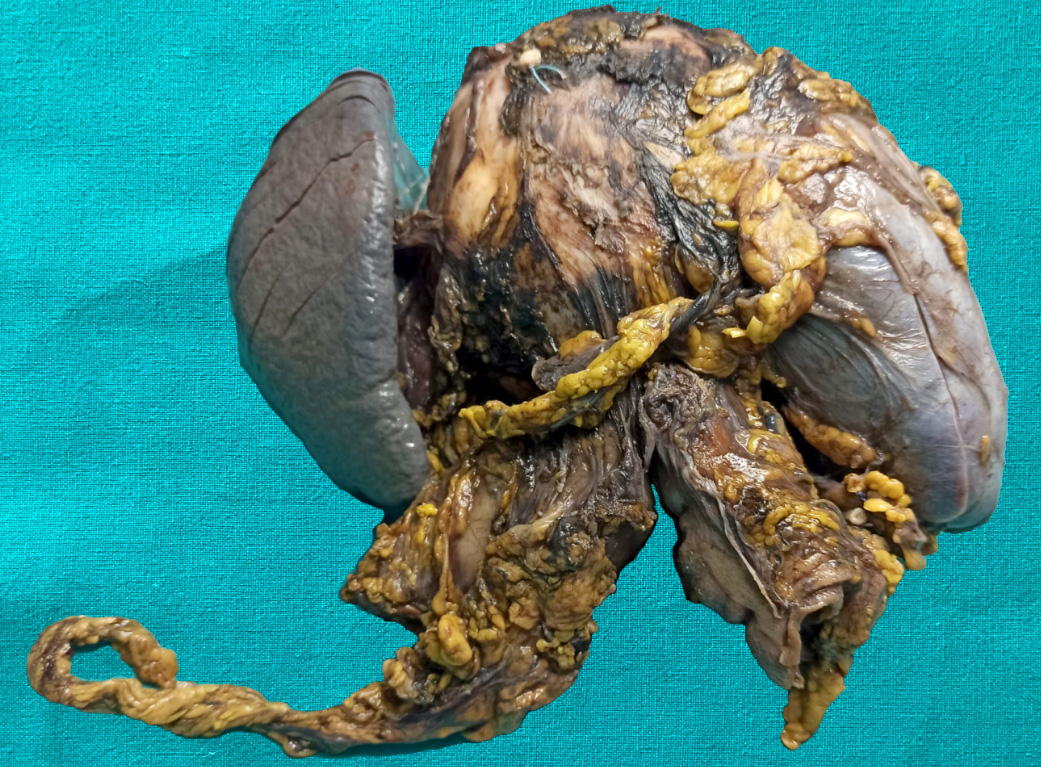
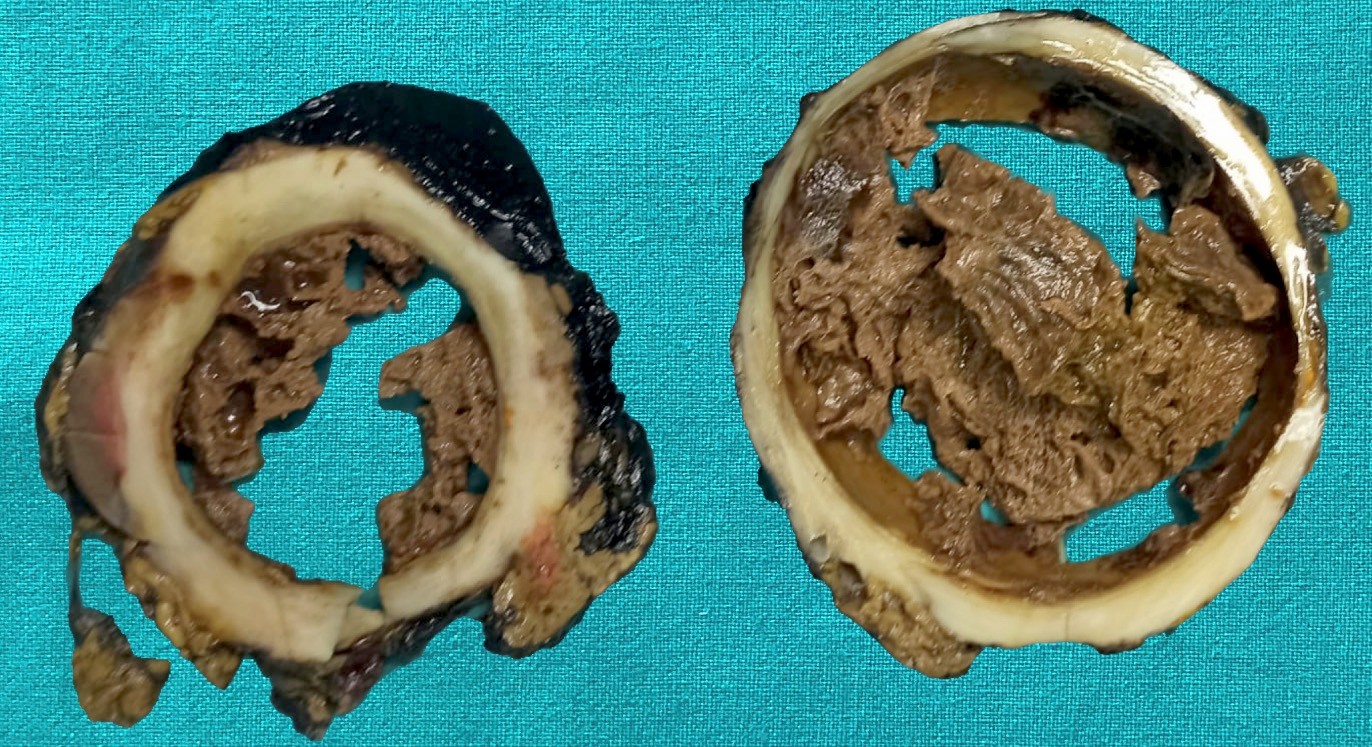
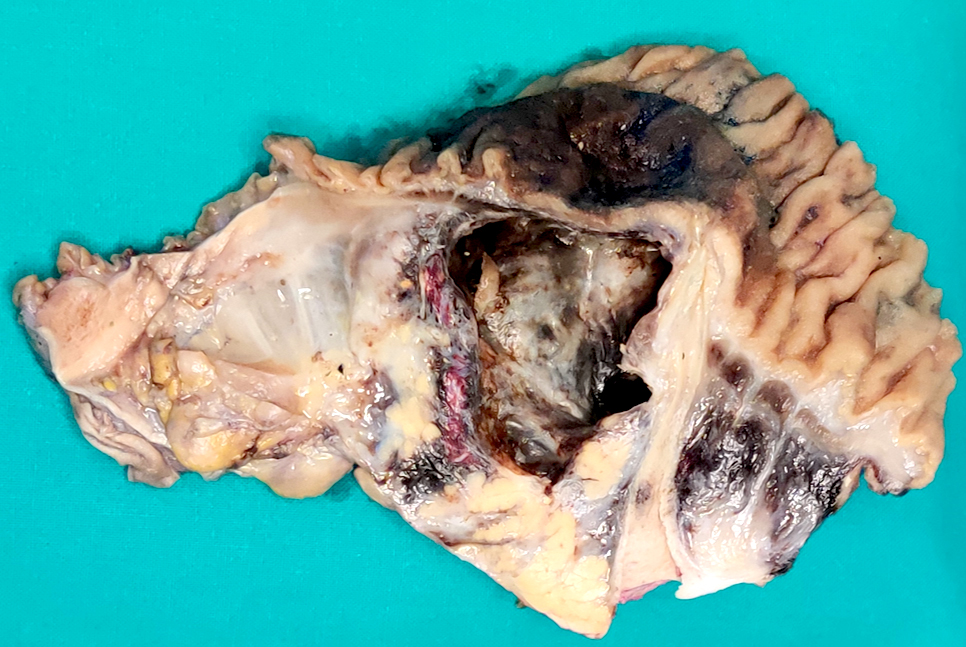
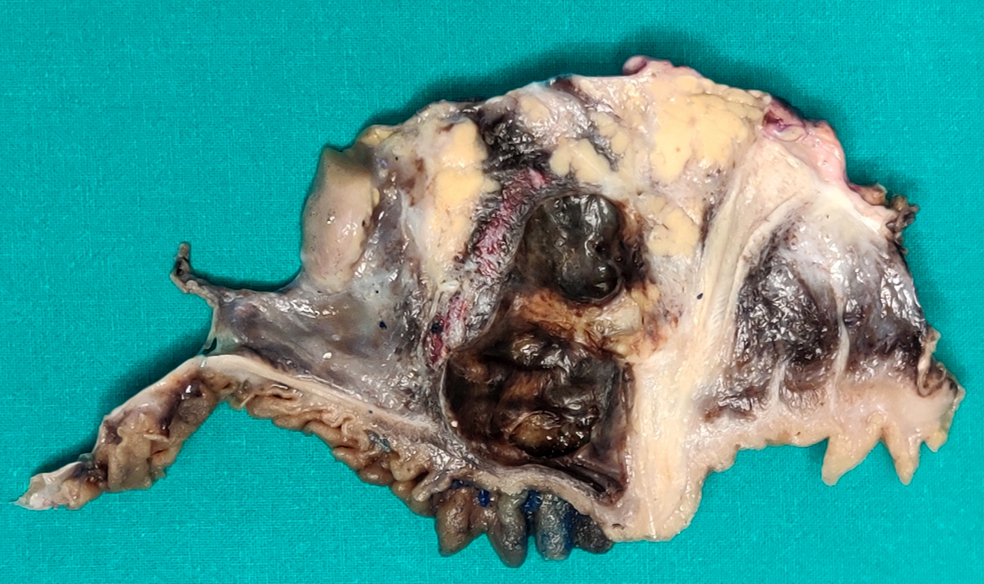
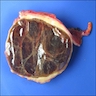
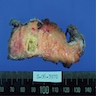
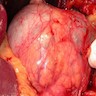
Gross description
- Typically unilocular
- Diameter is variable; can be > 20 cm in size
- Thick, irregular wall that contains turbid, sometimes blood tinged fluid (Pathologica 2020;112:197)
Gross images
Contributed by Paola Mattiolo, M.D. and Claudio Luchini, M.D., Ph.D.
Images hosted on other servers:
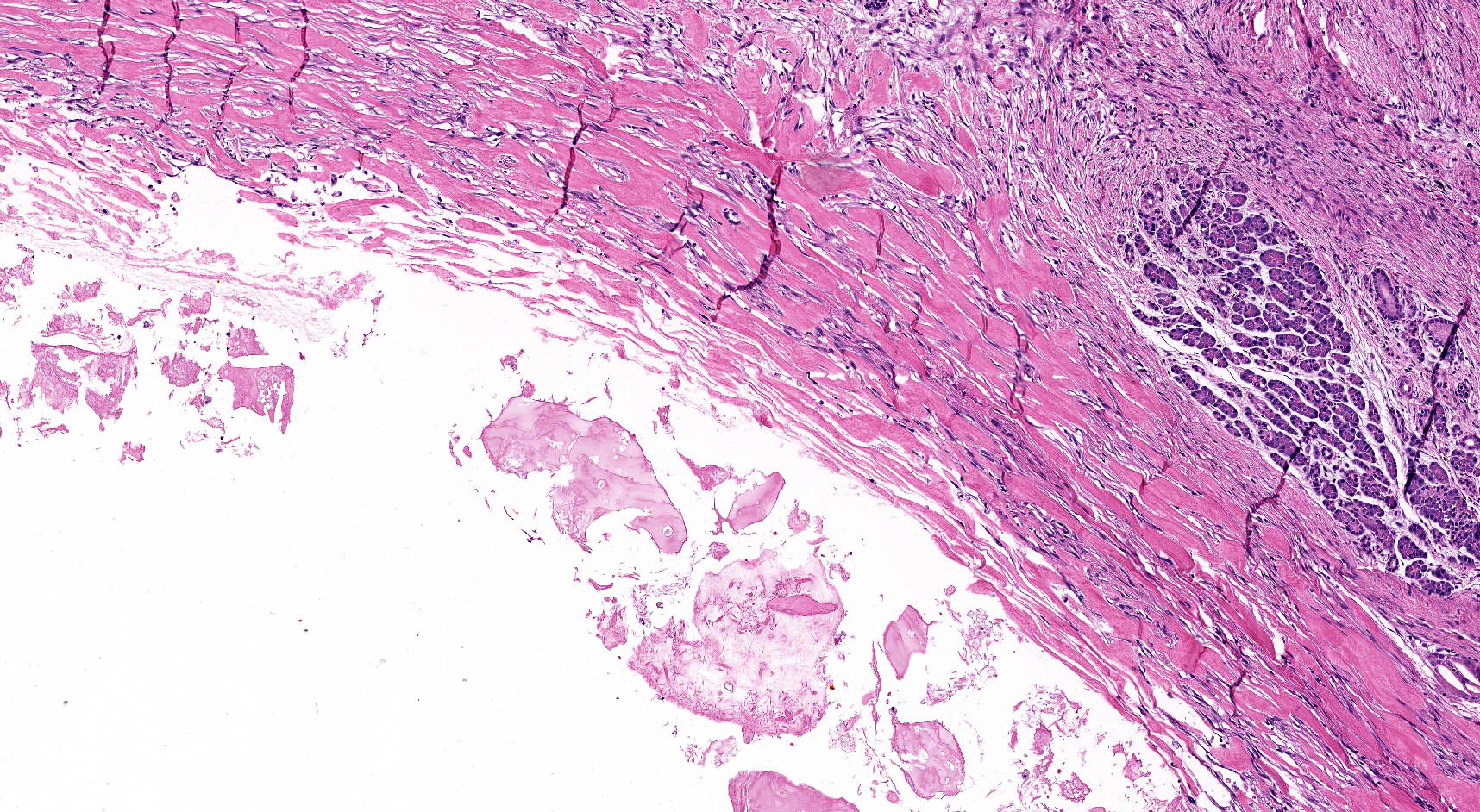
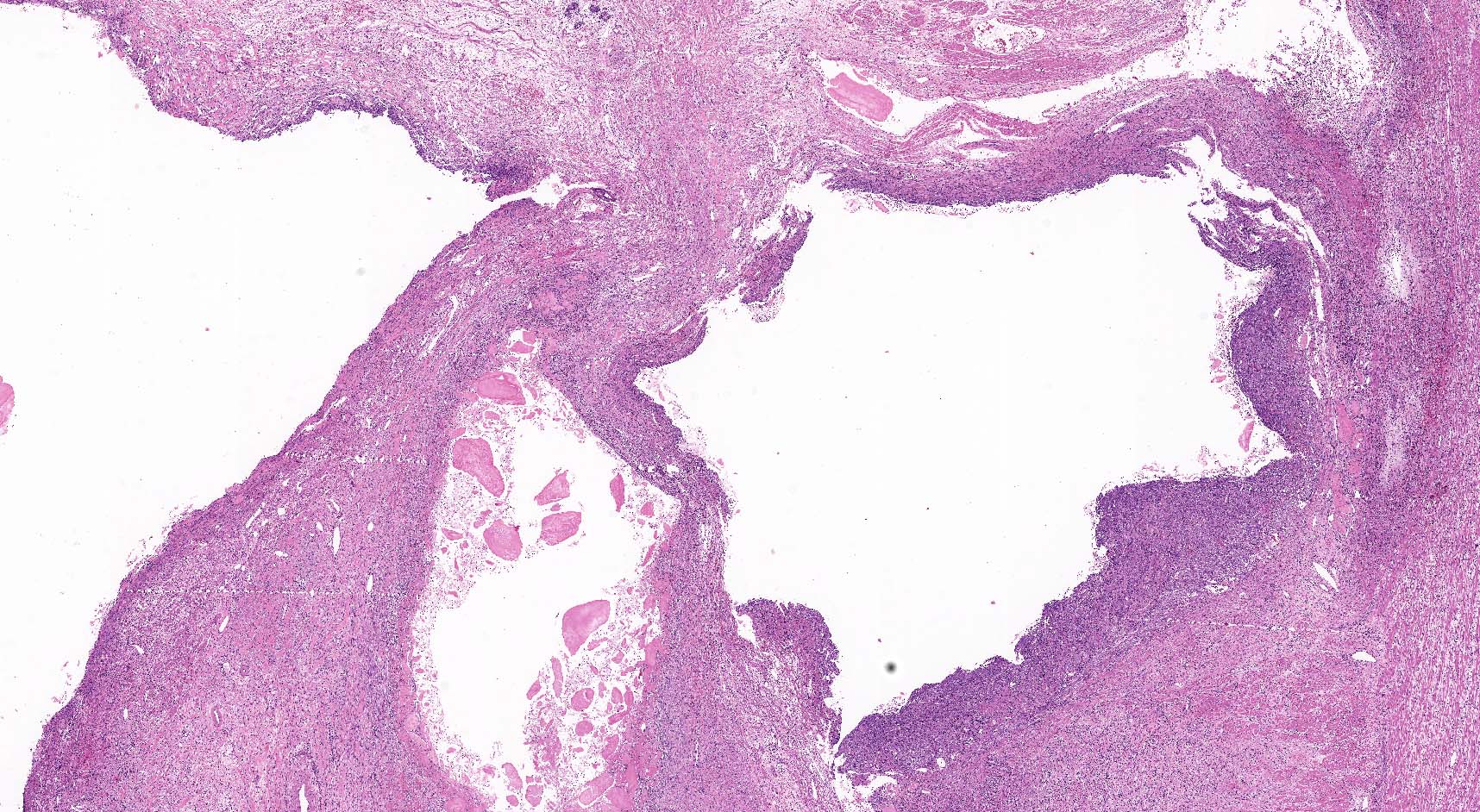
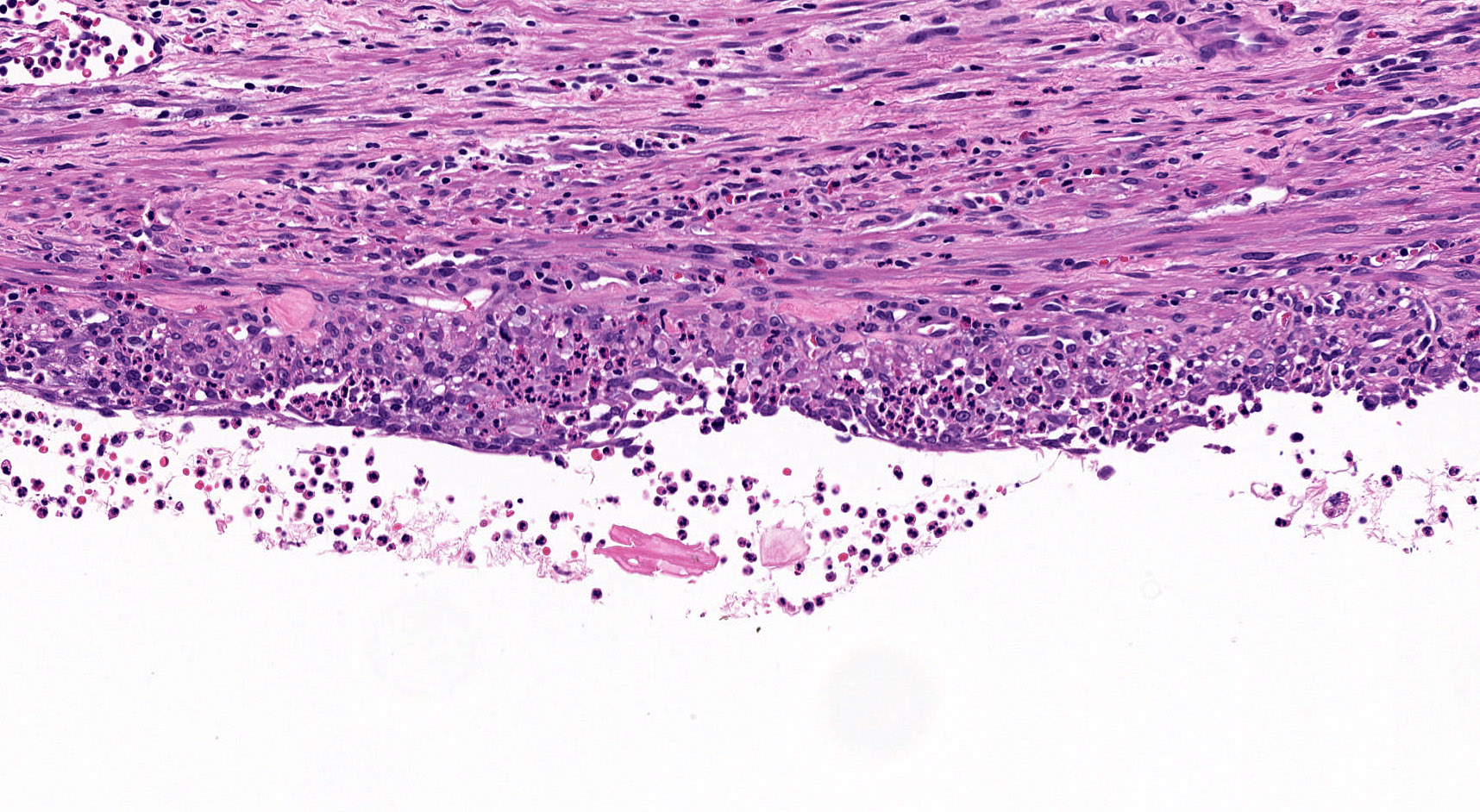
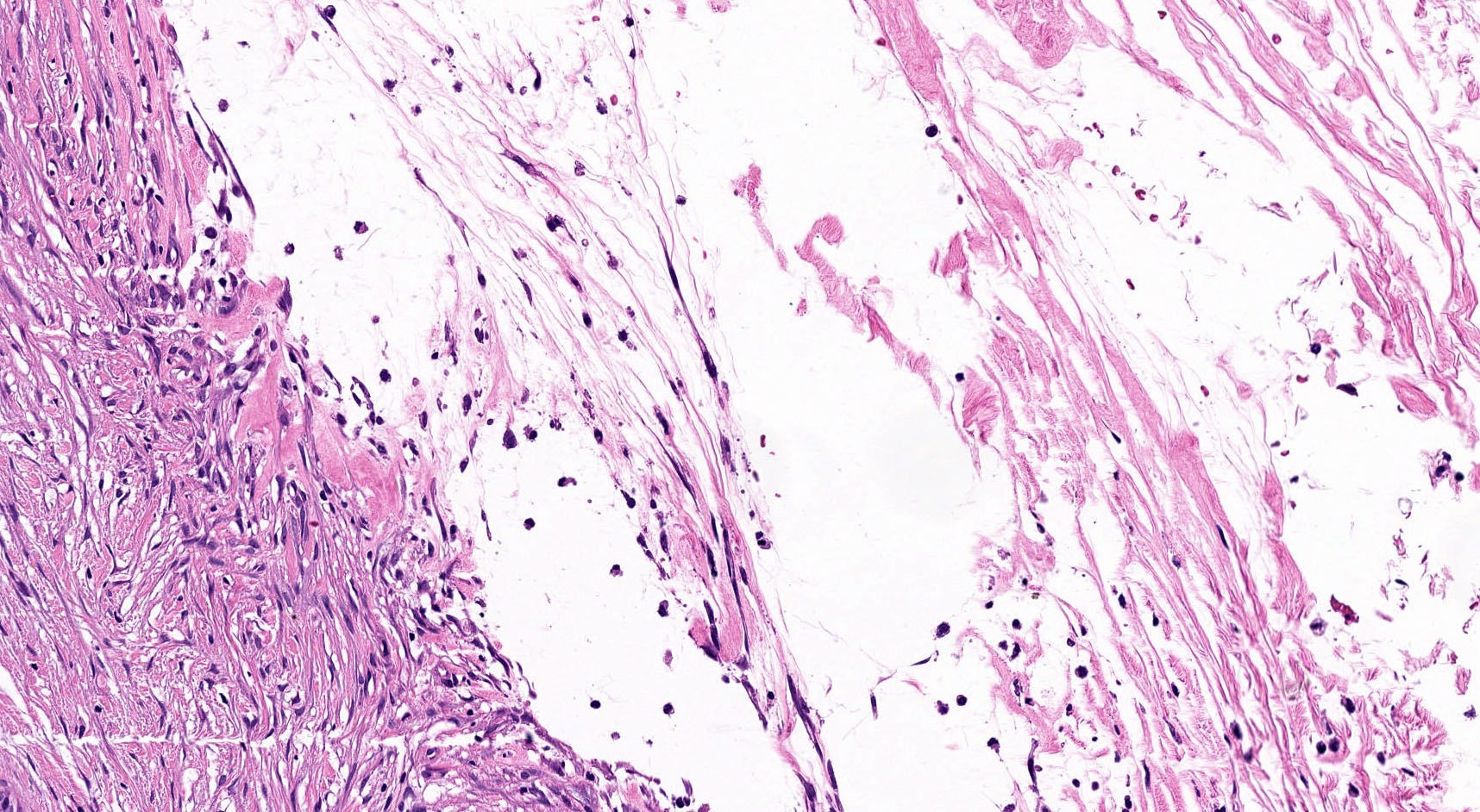
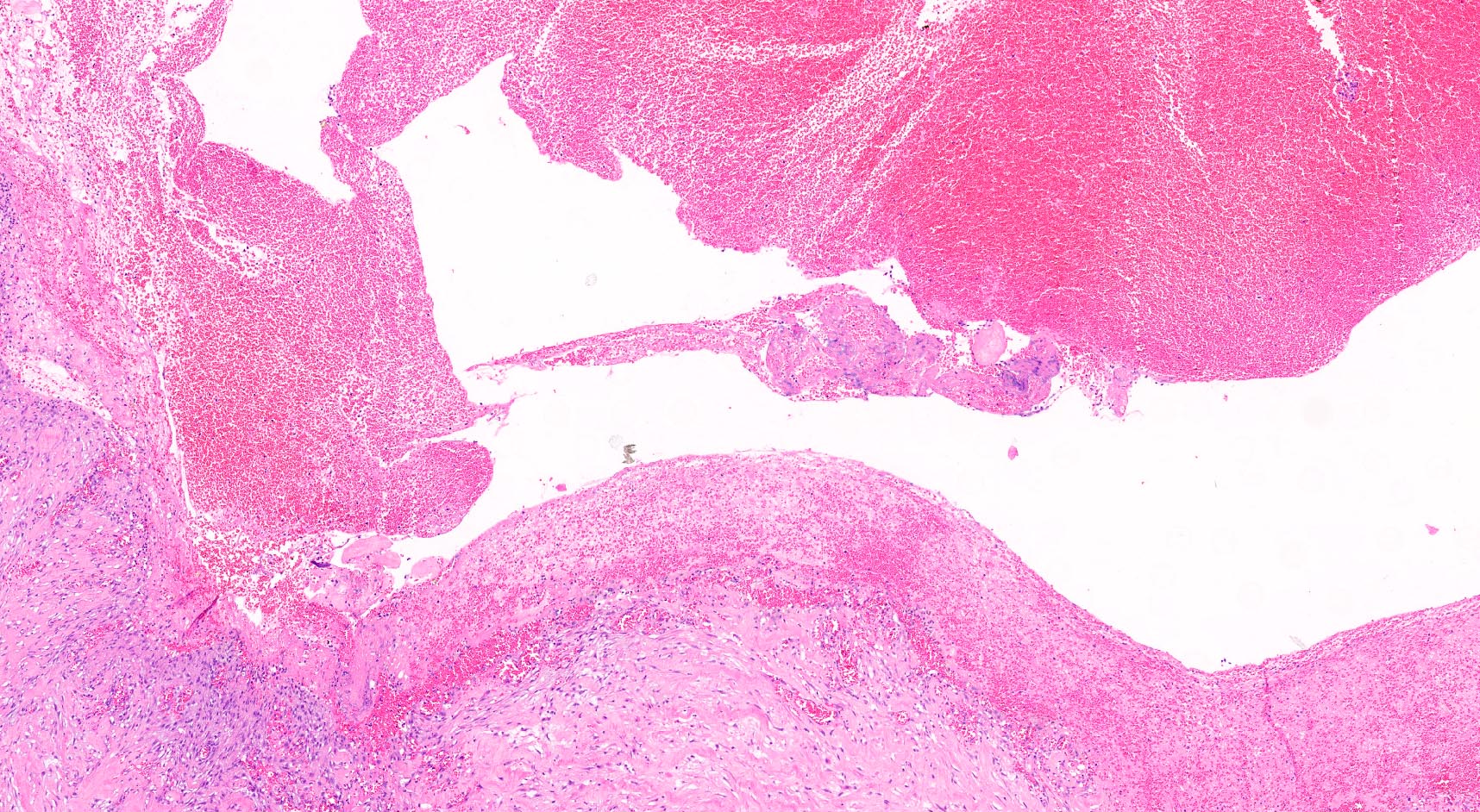
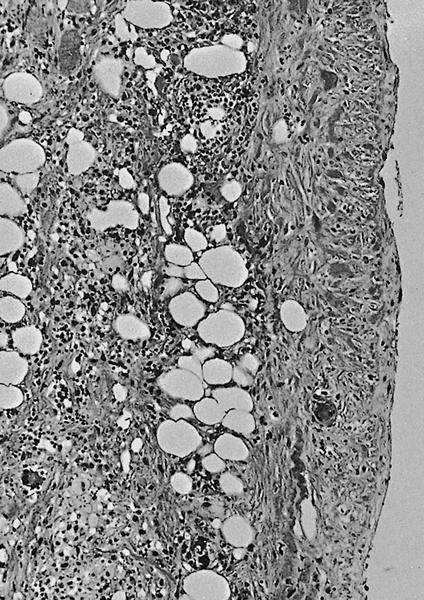
Microscopic (histologic) description
- By definition, there is no epithelial lining; please note that large pancreatic ducts may interface between pseudocyst and adjacent pancreas
- Cyst wall may contain histiocytes, giant cells, granulation tissue, rarely eosinophils
- To rule out the presence of neoplastic epithelium or dysplastic changes, examination of the entire cyst wall is strongly suggested (Pathologica 2020;112:197, Am J Gastroenterol 2018;113:464)
Microscopic (histologic) images
Cytology description
- Cyst fluid cytology typically demonstrates hemosiderin laden macrophages, acute inflammatory cells or lymphocytes, fibrin, debris, bile pigment and lack of neoplastic cells
- Through the needle microforceps biopsy: modality that allows the collection of samples from pancreatic cystic wall for microscopic histological examination through a 19 gauge needle
- Advantages: diagnostic accuracy is high (up to around 80%) (Gastrointest Endosc 2019;90:784)
- Disadvantages: the procedure is burdened by the occurrence of adverse effect in ~9% of cases, 4% of cases with severe adverse events and 1% of cases with fatality, while it changes the clinical management in ~11% of cases; thus, patient selection should be strictly managed and limited to those in whom sampling has a high probability of changing the clinical management (Endoscopy 2021;53:44)
Negative stains
- Cytokeratin (all types) are negative: by definition, no epithelial cells are present in pseudocyst lining
Sample pathology report
- Duodenum and pancreatic head, pancreaticoduodenectomy:
- Pancreatic head with the presence of cyst without an epithelial lining (see comment)
- The pancreatic cyst has been entirely analyzed
- Regional lymph nodes with reactive changes; duodenum without pathological modifications
- Comment: The histological appearance is consistent with the diagnosis of pancreatic pseudocyst. Correlation of histology with clinical history and radiology is strongly suggested.
Differential diagnosis
- Pancreatic cystic neoplasm:
- Particularly if multiloculated, may display significant denudation of the lining epithelium (Dig Liver Dis 2020;52:547)
- Intraductal papillary mucinous neoplasms (IPMNs)
- Serous cystic neoplasms:
- Even if completely denudated, it displays a subepithelial network of delicate capillary channels (Am J Surg Pathol 2012;36:726)
- Mucinous cystic neoplasms (MCNs):
- Even if completely denudated, it displays ovarian type stroma
- Cystic neuroendocrine neoplasms:
- Display a neuroendocrine epithelial lining, positive at immunohistochemistry for chromogranin A and synaptophysin
- Solid pseudopapillary neoplasms:
- Present neoplastic tissue, with necrotic / hemorrhagic cystic changes and tumor cells that are positive at immunohistochemistry for beta catenin (nuclear), LEF1 and CD10
- Cystic pancreatic ductal adenocarcinoma:
- Particularly if multiloculated, may display significant denudation of the lining epithelium (Dig Liver Dis 2020;52:547)
- Acute necrotic collection:
- Differs from pseudocyst because it is not well defined and it is filled by a commixture of fluid and necrosis, with different density
- Appears during the clinical manifestation of the necrotizing pancreatitis
- Walled off necrosis:
- Differs from pseudocyst because it is filled by a commixture of fluid and necrosis, with different density
- Infective cysts:
- Secondary bacterial infection may produce cyst
- Rarely, Echinococcus spp. may cause hydatid cyst in the pancreas and it should be suspected in endemic region (Diagn Cytopathol 1992;8:65, World J Gastrointest Surg 2014;6:190)
- Cystic lymphangiomas:
- Lesion associated with prominent lymphoid tissues that demonstrates ectatic spaces lined by endothelial type cells
- Lymphoepithelial cysts:
- Radiologically and sonographically indistinguishable from pseudocyst
- Smears show abundant anucleated squamous cells, keratinous debris, nucleated squamous cells and it is possible to recognize squamous cell block with preserved granular layer (Cancer 2006;108:501)
- Cysts associated with paraduodenal pancreatitis:
- Differential diagnosis is given by the clinicopathological context
- Squamoid cysts of the pancreas:
- Extremely uncommon cyst that is lined exclusively by squamous epithelium, lacking in solid areas
- Acinar cystic transformation of the pancreas:
- Cystic lesion lined by acinar epithelium
- Not excluded the presence of foci of mucinous or squamous epithelium
- Congenital cysts of the pancreas:
- Rare cysts that arise by developmental errors of pancreatic ducts, presumably related to localized obstruction of the duct in the utero
- Typically, these cysts are symptomatic before the second year of age and histologically, they present nonmucin producing cuboidal, columnar or flattened cells (Semin Diagn Pathol 2000;17:7)
- Enteric duplication cysts:
- Rare congenital malformations that are most commonly diagnosed in children
- These cysts display gastric or intestinal type lining epithelium and a well developed, bilayered muscular wall
- Ciliated foregut cysts:
- Cytology is characterized by amorphous debris, rare macrophages and ciliated columnar cells and detached ciliary tufts, which distinguish ciliated foregut cyst from pseudocyst
- Epidermoid cysts:
- Presence of squamous epithelium and keratin flakes
- Colliquated metastasis:
- Renal cell carcinoma is the most common source of metastasis to the pancreas
- Other malignancies that involve the pancreatic district are melanoma, pulmonary, mammary, gastric and colonic adenocarcinoma
Additional references
Board review style question #1
Is this histological image of pancreatic parenchyma sufficient to support the diagnosis of pseudocyst?
- No, it is necessary to examine the entire lesion in order to exclude the presence of epithelium
- Yes, the image proves the absence of dysplastic epithelium
- Yes, the image proves the presence of a fibrotic wall
- Yes, the image shows a fibrotic wall with bona fide disepithelization
- Yes, the image shows the presence of residual pancreatic parenchyma
Board review style answer #1
A. No, it is necessary to examine the entire lesion in order to exclude the presence of epithelium. For the diagnosis of pseudocysts, first it is mandatory to exclude the presence of an epithelial cell lining. A random sampling is not sufficient; the sampling should allow the examination of the entire lesion.
Comment Here
Reference: Pseudocysts
Comment Here
Reference: Pseudocysts
Board review style question #2
Which analysis of cystic fluid is most suspicious for pseudocyst?
- High carcinoembryonic antigen (CEA), high amylase
- High CEA, high lipase
- High CEA, low amylase
- Low CEA, high amylase
- Low CEA, low amylase
Board review style answer #2
D. Low CEA, high amylase. Typically, the cyst fluid analysis of a pseudocyst usually shows a very low CEA concentration in the cyst fluid (< 5 ng/mL); the amylase activity is usually markedly increased.
Comment Here
Reference: Pseudocysts
Comment Here
Reference: Pseudocysts