Table of Contents
Definition / general | Essential features | Techniques for cytologic sampling of pancreatic and bile duct lesions | Metrics | Papanicolaou system for reporting pancreaticobiliary cytology | Diagrams / tables | Laboratory | Case reports | Cytology description | Cytology images | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Jones V, Allison D. WHO reporting system for pancreaticobiliary cytopathology. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/pancreaspapanicolaousystem.html. Accessed March 31st, 2025.
Definition / general
- The Papanicolaou Society of Cytopathology (PSC) developed guidelines in 2014 for interpreting and reporting pancreaticobiliary cytology
- PSC was comprised of international, multidisciplinary pancreaticobiliary experts who met over an 18 month period to provide recommendations for:
- Clinical and imaging workup (Diagn Cytopathol 2014;42:325)
- Techniques for sampling lesions (Diagn Cytopathol 2014;42:333)
- Standardized terminology and nomenclature (Diagn Cytopathol 2014;42:338)
- Ancillary studies (Diagn Cytopathol 2014;42:351)
- Patient follow up and treatment (Diagn Cytopathol 2014;42:363)
- PSC was comprised of international, multidisciplinary pancreaticobiliary experts who met over an 18 month period to provide recommendations for:
- Standardization provides uniform diagnostic reporting, reproducibility and clinically relevant risk stratification
- Resulted in a 6 tiered reporting system: nondiagnostic, negative (for malignancy), atypical, neoplastic (benign or other), suspicious (for malignancy) and positive or malignant
- Second edition may consider the 2019 World Health Organization proposed updates (Diagn Cytopathol 2020;48:494, Cancer Cytopathol 2022;130:195)
Essential features
- Internationally adopted recommendations for sampling, diagnosing and managing pancreaticobiliary cytology lesions
- 6 tiered categorical system resulting in overall improved sensitivity / specificity and risk stratification
- Risks of malignancy inform multidisciplinary patient care
Techniques for cytologic sampling of pancreatic and bile duct lesions
- Primary method for sampling solid mass and cysts: endoscopic ultrasound fine needle aspiration (EUS FNA)
- Different biopsy devices may improve diagnostic yield of pancreatic cyst wall components (Cancer Cytopathol 2018;126:414, Endosc Ultrasound 2018;7:323, Gastrointest Endosc 2018;87:495)
- Primary method for sampling strictures: endoscopic retrograde cholangiopancreatography (ERCP) guided brush cytology
- Risk of pancreatitis
- Endoscopic forceps biopsy may improve poor sensitivity (Diagn Cytopathol 2014;42:333)
Metrics
- Risk of malignancy (ROM) = total number FNA specimens that proved malignant on follow up / total number of surgical resections and clinical follow ups (Cancer Cytopathol 2020;128:29)
- Average ROM for each diagnostic category (next section) is provided by the Saieg and Pitman review of several studies (Diagn Cytopathol 2020;48:494)
- Diagnostic variation due to:
- Operator skill, experience and medical device
- Variation in prevalence of pathology and patient populations
- Limited availability of ancillary tests (Cytopathology 2020;31:564)
- Absence of rapid on site assessment (ROSE) (Oncotarget 2017;8:8154)
Papanicolaou system for reporting pancreaticobiliary cytology
- Category I: nondiagnostic
- Provides no useful diagnostic or useful information about the solid or cystic lesion sampled
- Entities include:
- Cells obscured by artifacts such as hemorrhage or necrosis
- Pure gastrointestinal contamination indicates missed lesion
- Acellular specimens obtained from cyst with no evidence of mucinous etiology
- Benign pancreatic parenchyma with well defined mass on imaging
- Ancillary testing may result in reduced utilization of this category for cystic lesions (Diagn Cytopathol 2017;45:303)
- Average ROM: 23.1%
- Management: reassess or repeat sampling
- Category II: negative (for malignancy)
- Contains adequate cellular or extracellular tissue to evaluate or define a lesion that is identified on imaging
- Minor degree of loss of cell or nuclear polarity
- No cytologic atypia observed
- Entities include:
- Acute, chronic or autoimmune pancreatitis
- Pseudocyst
- Lymphoepithelial cyst
- Splenule or accessory spleen
- Benign pancreatic parenchyma with no discrete mass on imaging
- Ancillary tests such as amylase and carcinoembryonic antigen cyst fluid analysis can be helpful for cystic lesions (Diagn Cytopathol 2017;45:303)
- Increased false negative rate in biliary brushings due to underlying inflammatory lesions and subepithelial tissue entrapped within desmoplasia
- Average ROM: 8.2%
- Management: respective conservative care unless patients have irretractable symptoms or high risk of malignancy
- Contains adequate cellular or extracellular tissue to evaluate or define a lesion that is identified on imaging
- Category III: atypical
- Cells present with cytoplasmic, nuclear or architectural features that are not consistent with normal or reactive changes of the pancreas and bile ducts and insufficient to classify as neoplasm or suspicious for high grade malignancy
- Slight nuclear membrane irregularity, nuclear crowding with minor degree of overlap, nuclei haphazardly occupy lower 66% of the cell at the base, minimal degree of anisonucleosis (2:1), near normal N:C ratio, parachromatin clearing, background smear is clean or contains red blood cells
- Also includes entities with abundant intracytoplasmic mucin in the epithelium without overt cytologic atypia
- Absent features: necrosis, true nuclear molding, macronucleoli and features of malignancy
- Category with the widest range of reported ROM: 28 - 100%, average of 46.8%
- Management: repeat sampling, additional ancillary studies, continued conservative care or surgery referral as determined by team
- Cells present with cytoplasmic, nuclear or architectural features that are not consistent with normal or reactive changes of the pancreas and bile ducts and insufficient to classify as neoplasm or suspicious for high grade malignancy
- Category IV: neoplastic
- Neoplastic: benign
- Cytological specimen sufficiently cellular and representative (with or without the context of clinical, imaging and ancillary studies) to be diagnostic of a benign neoplasm
- Entities include:
- Serous cystadenoma (most common)
- Lymphangioma
- Cystic teratoma
- Schwannoma
- Average ROM: 20.2%
- Management: conservative unless irretractable symptoms, large sized lesions at risk for rupture (> 4 cm serous cystadenomas) or increased risk of recurrence (lymphangiomas)
- Neoplastic: other
- Neoplasm that is either premalignant or low grade malignant
- Does not define the neoplasm as benign or malignant
- Entities include:
- Premalignant
- Intraductal papillary mucinous neoplasm (IPMN) with low and high grade dysplasia
- Intraductal papillary neoplasm of the bile duct (IPNB) with low and high grade dysplasia
- Mucinous cystic neoplasm (MCN) with low grade dysplasia
- Note that cases with prominent high grade dysplasia may often end up in the suspicious for malignancy category
- Low grade malignant
- Pancreatic neuroendocrine tumor (PanNET)
- Infers well differentiated neoplasm > 0.5 cm unless otherwise classified
- Solid pseudopapillary neoplasm (SPN)
- If FNA is suggestive but not diagnostic, categorize as atypical rather than suspicious
- Gastrointestinal stromal tumor (GIST)
- Extra-adrenal paraganglioma
- Novel reports of preoperatively diagnosing pancreatic solitary fibrous tumor by FNA (Cytopathology 2022;33:222, J Am Soc Cytopathol 2020;9:272)
- Pancreatic neuroendocrine tumor (PanNET)
- Average ROM: 37.5%
- Management: conservative, especially if lesion is small (PanNET < 2 cm)
- Premalignant
- Neoplastic: benign
- Category V: suspicious for malignancy
- Some (but an insufficient number) of the typical features of a specific neoplasm are present, mainly pancreatic adenocarcinoma
- Significant cellular or architectural atypia but insufficient qualitative / quantitative findings for conclusive diagnosis, insufficient tissue for confirmative ancillary testing or rare cells with multiple major criteria, as listed below:
- Significant nuclear enlargement, nuclear membrane irregularities, course chromatin pattern, distinct nucleoli, increased N:C ratio, anisonucleosis (3:1 to 4:1), sometimes mucinous metaplasia
- Loss of cellular and architectural polarity
- Entities include:
- Pancreatic adenocarcinoma (most common)
- Solid cellular smear patterns suggestive of:
- PanNET, acinar cell carcinoma, pancreatoblastoma, solid pseudopapillary neoplasm (SPN)
- Cyst aspirates with solid mural nodule or cyst wall mass
- Lymphoma
- Metastases
- High grade biliary intraepithelial neoplasia (BilIN) sampled by brushing (not high grade PanIN)
- Average ROM: 92.1%
- Management: reassess or repeat sampling; perform ancillary testing (may refer for surgery if positive FISH or loss of SMAD4 staining); consult experts
- Category VI: positive for malignancy
- Neoplasms that unequivocally display malignant cytologic characteristics
- Pancreatic ductal adenocarcinoma (PDAC) (most common):
- Well differentiated
- Difficult to diagnose; often classified as suspicious unless clearly malignant
- Moderately and poorly differentiated:
- Nuclear overlap and molding, marked loss of nuclear polarity, anisonucleosis > 4:1, disorganization (drunken honeycomb), macronucleoli, bizarre nuclei, nuclear hyperchromasia, elevated N:C ratio > 0.6, irregular chromatin distribution, lack of glandular differentiation, presence of cell balls, papillary fragments, cell in cell arrangements, tumor diasthesis, 3 dimensional fragments, cytoplasmic mucin vacuoles, background necrosis and mitoses
- Well differentiated
- Pancreatic ductal adenocarcinoma (PDAC) (most common):
- Entities include:
- Pancreatic ductal adenocarcinoma and its variants
- Cholangiocarcinoma
- Acinar cell carcinoma
- High grade neuroendocrine carcinoma (small cell and large cell)
- Pancreatoblastoma
- Lymphomas
- Sarcomas
- Metastases to the pancreas
- Average ROM: 99.6%
- Management: surgical resection; may also consider neoadjuvant therapy or clinical trials if lesions are unresectable
- Neoplasms that unequivocally display malignant cytologic characteristics
Diagrams / tables
Table 1: Papanicolaou reporting system for pancreaticobiliary cytology
| Diagnostic categories | Average risk of malignancy | Examples of entities | Selected ancillary testing | Management |
I. Nondiagnostic | 23.1% | • Cellular artifact, obscuring hemorrhage or necrosis • Pure GI contamination • Acellular cyst aspirate with no evidence of mucinous etiology • Benign pancreatic parenchyma with clearly defined mass on imaging | Cyst fluid analysis: CEA and amylase (no mucinous etiology = lack of mucin, lack of elevated CEA, lack of KRAS or GNAS mutations) | Reassess / repeat |
II. Negative | 8.2% | • Pancreatitis • Pseudocyst • Lymphoepithelial cyst • Splenule / accessory spleen • Benign pancreatic parenchyma with no mass on imaging | Pseudocyst: high amylase + low CEA | Conservative |
III. Atypical | 46.8% (range: 28 - 100) |
• Ductal cells with indeterminate atypia (reactive versus low grade dysplasia, versus scant lesional tissue) • Abundant intracytoplasmic mucin in the epithelium | Cyst fluid analysis: CEA and amylase | Conservative, repeat or surgery |
IV. Neoplastic: benign | 20.2% |
• Serous cystadenoma (SCA) • Lymphangioma • Cystic teratoma • Schwannoma | SCA: low amylase + low CEA, VHL mutation, positive inhibin staining | Conservative |
IV. Neoplastic: other | 37.5% |
• GIST • Extra-adrenal paraganglioma • Pancreatic solitary fibrous tumor Premalignant • IPMN with dysplasia • IPNB • MCN with dysplasia Low grade malignant • PanNET • Solid pseudopapillary neoplasm (SPN) |
Solitary fibrous tumor: STAT6 mutation Intraductal papillary mucinous neoplasm: KRAS, RNF43, GNAS (specific), TP53, SMAD4 mutations Solid pseudopapillary neoplasm: CTNNB1 (beta catenin) mutation | Surgery with an option for conservative management in select scenarios |
V. Suspicious | 92.1% |
• Pancreatic ductal adenocarcinoma (PDAC) • PanNET • Acinar cell carcinoma • Pancreatoblastoma • Solid pseudopapillary neoplasm • Cyst aspirate with solid mural nodule or cyst wall mass • Lymphoma • Metastases • High grade BilIN | Immunocytochemistry, FISH molecular analysis Cyst fluid analysis: CEA and amylase | Surgery |
VI. Positive | 99.6% | • PDAC and variants • Cholangiocarcinoma • Acinar cell carcinoma • Small and large cell neuroendocrine carcinoma • Pancreatoblastoma • Lymphomas • Sarcomas • Metastases to the pancreas | Immunocytochemistry patterns in malignancy: • Positive: S100P, IMP3, MUC4, mesothelin • Negative: pVHL, CD10, clusterin beta, SMAD4 FISH significantly improves diagnostic sensitivity of adenocarcinoma (copy number abnormalities in CEP3, CEP7, CEP17 and of band 9p21) | Surgery or neoadjuvant therapy / clinical trials |
- Reference: Diagn Cytopathol 2020;48:494
Laboratory
- Pancreaticobiliary strictures (Diagn Cytopathol 2014;42:351)
- Fluorescence in situ hybridization (FISH)
- DNA probe set for biliary brush specimens; outperforms routine cytology in sensitivity, thus is the preferred complement
- Malignancy: chromosomal copy number abnormalities in CEP3, CEP7, CEP17 and of band 9p21 (CEP = centromere enumeration probe)
- Successful for evaluating negative, inconclusive and malignant specimens
- DNA probe set for biliary brush specimens; outperforms routine cytology in sensitivity, thus is the preferred complement
- Immunocytochemistry
- Marker patterns frequently observed in malignancy: S100P+, pVHL-, IMP3+, CD10-, MUC4+, mesothelin+, clusterin beta-
- KRAS mutational analysis
- Sensitive for invasive carcinomas and PDAC but not specific
- Abnormalities also observed in low grade dysplasias and chronic pancreatitis
- Shown utility in distinguishing benign from malignant in atypical specimens
- Not supported as useful in diagnosing solid pancreatic masses and bile duct strictures
- Sensitive for invasive carcinomas and PDAC but not specific
- Fluorescence in situ hybridization (FISH)
- Pancreatic cystic lesions
- Histochemical stains
- Positive staining of mucin (mucicarmine and Alcian blue / PAS)
- Diagnostically useful to identify mucinous lesions
- Biochemical tests
- Amylase and carcinoembryonic antigen (CEA) levels help diagnose:
- Pseudocysts (high amylase in the 1,000s U/L, low CEA)
- Serous cystadenomas (low amylase < 1,000 U/L, low CEA)
- Mucinous cysts (CEA > 192 ng/mL)
- Cyst fluid CEA alone reliably distinguishes mucinous from nonmucinous lesions (Diagn Cytopathol 2014;42:351)
- Not useful for predicting malignancy
- Optimal CEA cutoff of 192 is supported by meta analysis and is the most widely accepted and utilized (Dig Dis Sci 2021 Nov 16 [Epub ahead of print])
- CA125 and CA19-9 generally not useful for diagnosis
- MUC2, MUC4 and MUC7 useful in recognizing dysplasia and malignancy in cystic neoplasms
- Amylase and carcinoembryonic antigen (CEA) levels help diagnose:
- DNA analysis
- Aneuploid and tetraploid results support malignancy
- Elevated cyst fluid DNA supports malignancy
- No significantly improved diagnostic accuracy over routine cytology
- Molecular tests
- Mutations: supported diagnosis
- KRAS, GNAS, RNF43: IPMN and MCN (GNAS absent in MCN)
- TP53, SMAD4, CTTNB1, mTOR genes: advanced neoplasia (high grade dysplasia or carcinoma)
- VHL: serous cystadenomas
- CTNNB1 (beta catenin): solid pseudopapillary neoplasms
- Mutations: supported diagnosis
- Histochemical stains
- Solid pancreatic neoplasms
- Immunohistochemical tests
- Adenocarcinoma: DPC4 / SMAD4-
- PanNET: chromogranin+, synaptophysin+, INSM1+, CD56+, neuron specific enolase+, CK8/18+
- Acinar cell carcinoma: BCL10+, amylase+, trypsin+, chymotrypsin+, lipase+, CK7+
- Solid pseudopapillary neoplasm: nuclear beta catenin+, alpha-1 antitrypsin+, CD10+, SOX11+, synaptophysin+, chromogranin-, trypsin- (Cancer Cytopathol 2017;125:831)
- Pancreatic and biliary tract lymphoma: will be dependent on the type of lymphoma
- Molecular analysis
- Adenocarcinoma
- KRAS mutation not used due to low specificity for malignancy
- p16 / CDKN2A, TP53 and SMAD4 inactivation
- FISH significantly improves diagnostic sensitivity; the most reliable adjunct test for confirmation for biliary tract brushing
- Copy number abnormalities in CEP3, CEP7, CEP17 and of 9p21
- MicroRNA and loss of heterozygosity: clinical use and importance to be determined
- PanNET
- Loss of heterozygosity: malignant associations but clinical importance to be determined
- ATRX FISH performed by some labs along with DAXX IHC to identify patients with a poor prognosis (Cancer Cytopathol 2017;125:544)
- Acinar cell carcinoma
- Associated with losses of 11p; diagnostic significance unclear
- Solid pseudopapillary neoplasm
- Somatic point mutation in exon 3 of beta catenin
- Adenocarcinoma
- Immunohistochemical tests
Case reports
- 31 year old man with pancreatoblastoma (positive) (Clin J Gastroenterol 2018;11:161)
- 58 year old man with intraductal papillary neoplasm of the biliary tract (atypical) (Diagn Cytopathol 2019;47:922)
- 60 year old man with paraduodenal pancreatitis found after an initially nondiagnostic biopsy (nondiagnostic) (Case Rep Surg 2020;2020:5021578)
- 62 year old man with pancreatic lymphoepithelial cyst (negative) (Case Rep Med 2020;2020:4590758)
- 67 year old woman with cystic lymphangioma of the pancreas (neoplastic) (Gland Surg 2018;7:487)
- 84 year old man with pancreatobiliary neoplasm of the duodenum (suspicious) (Diagn Cytopathol 2019;47:1059)
Cytology description
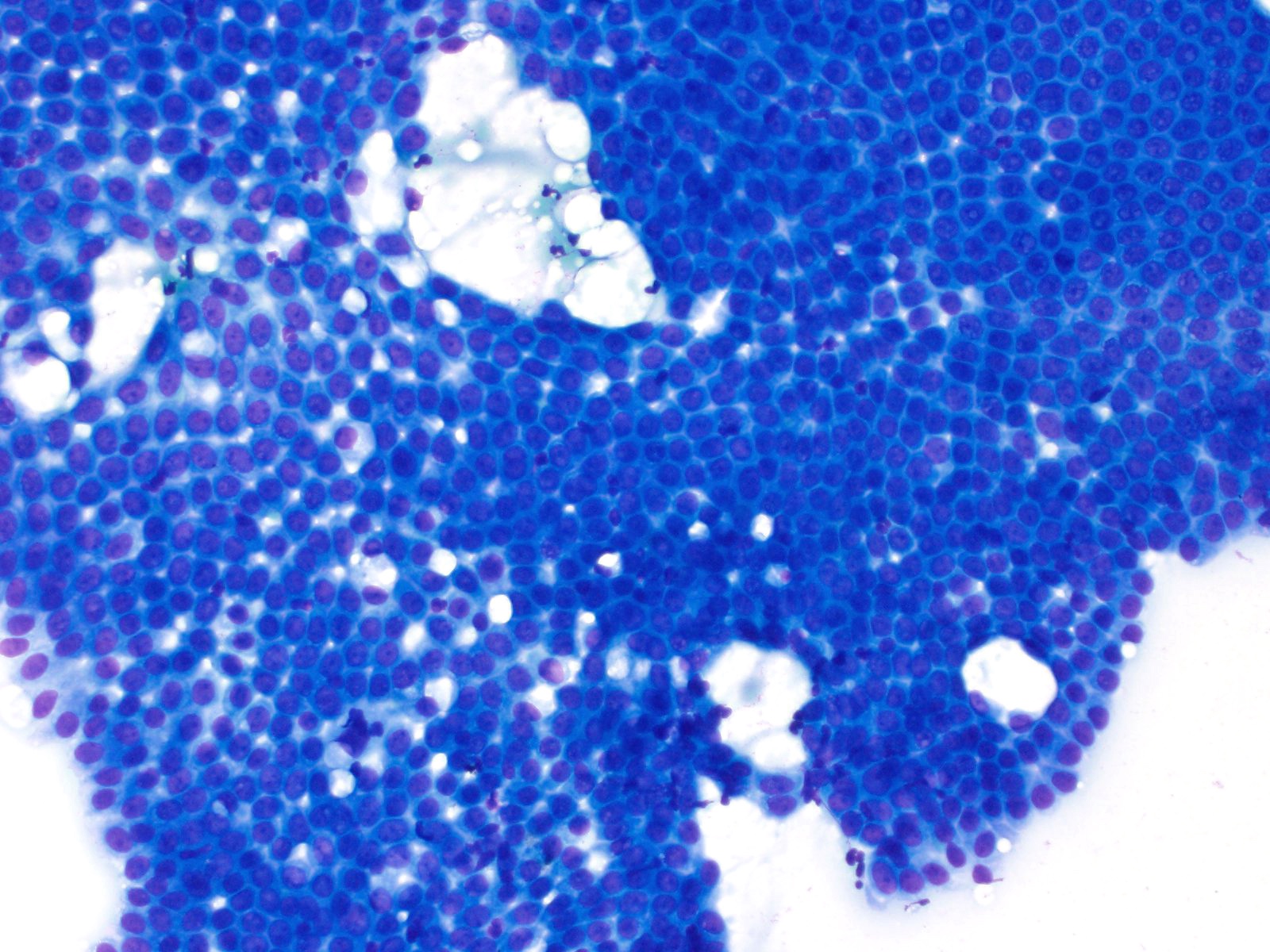
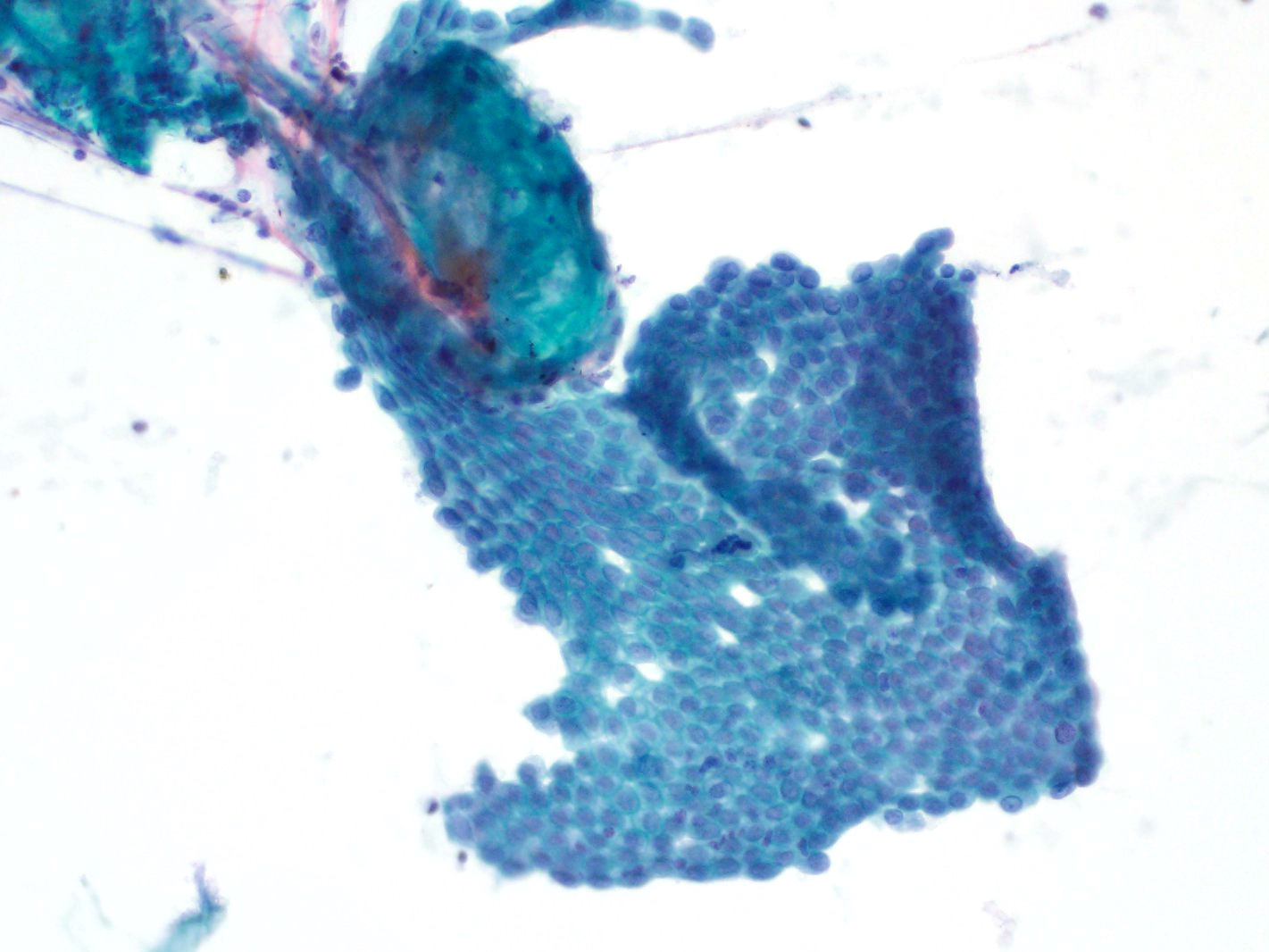
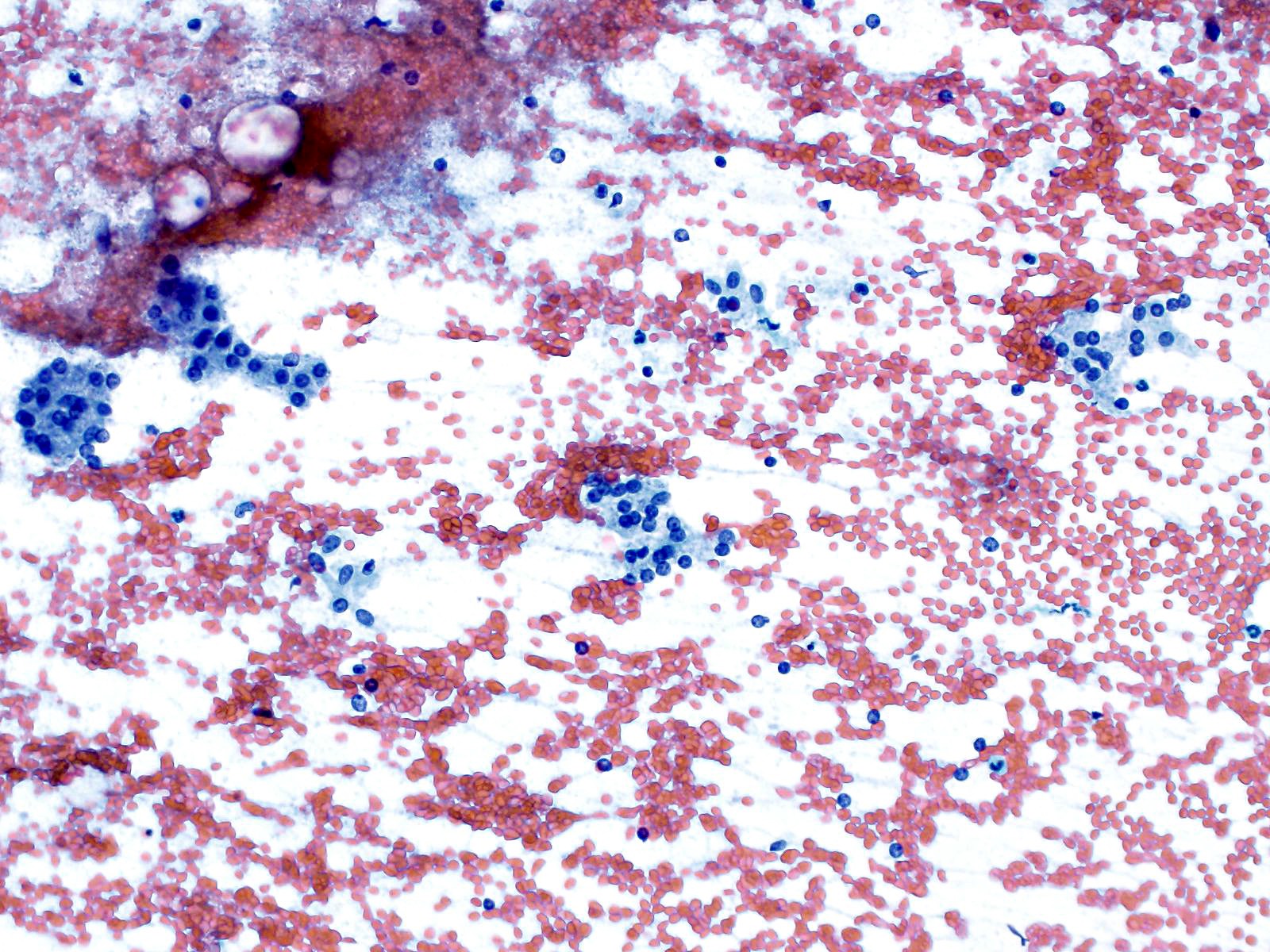
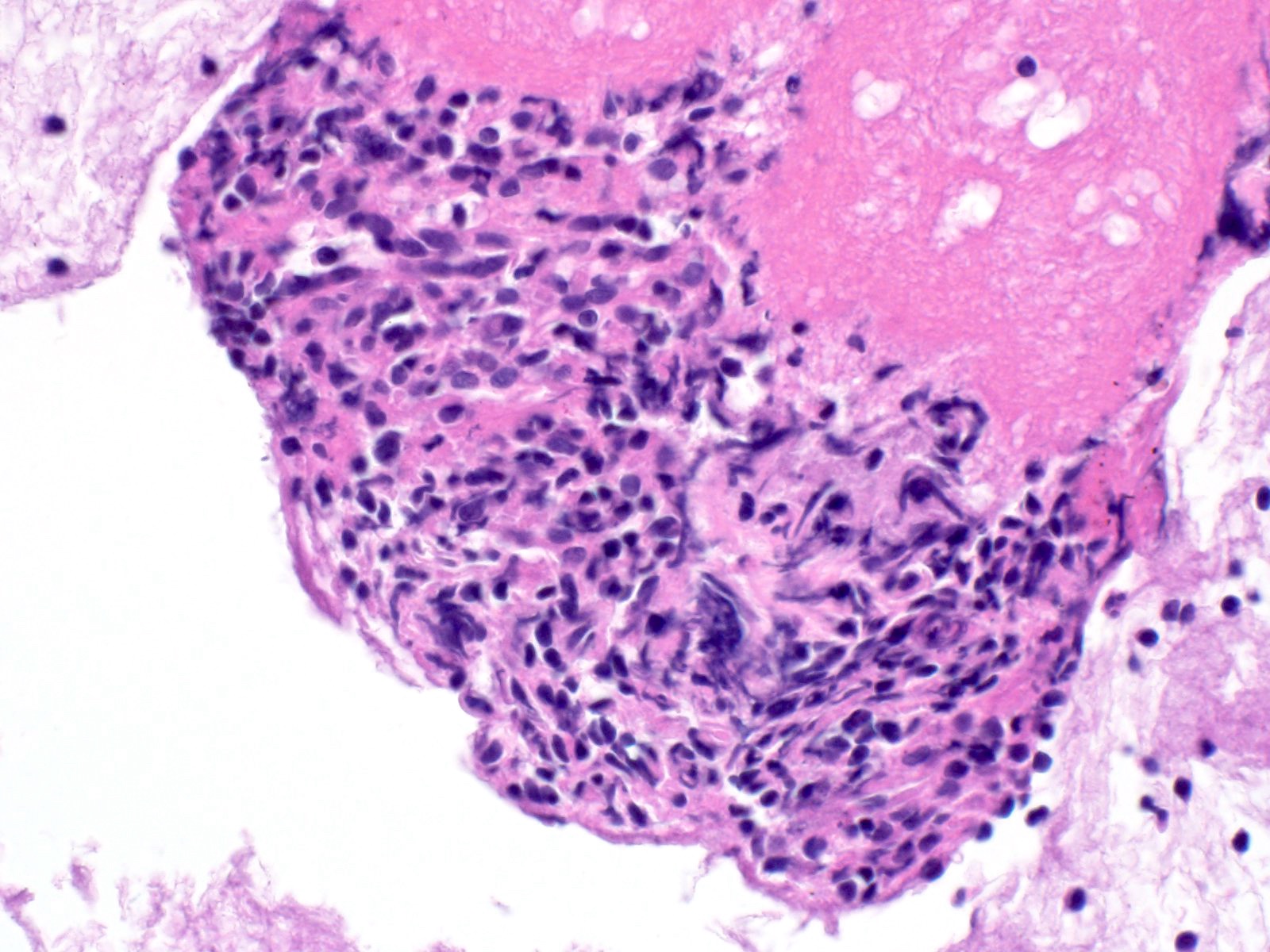
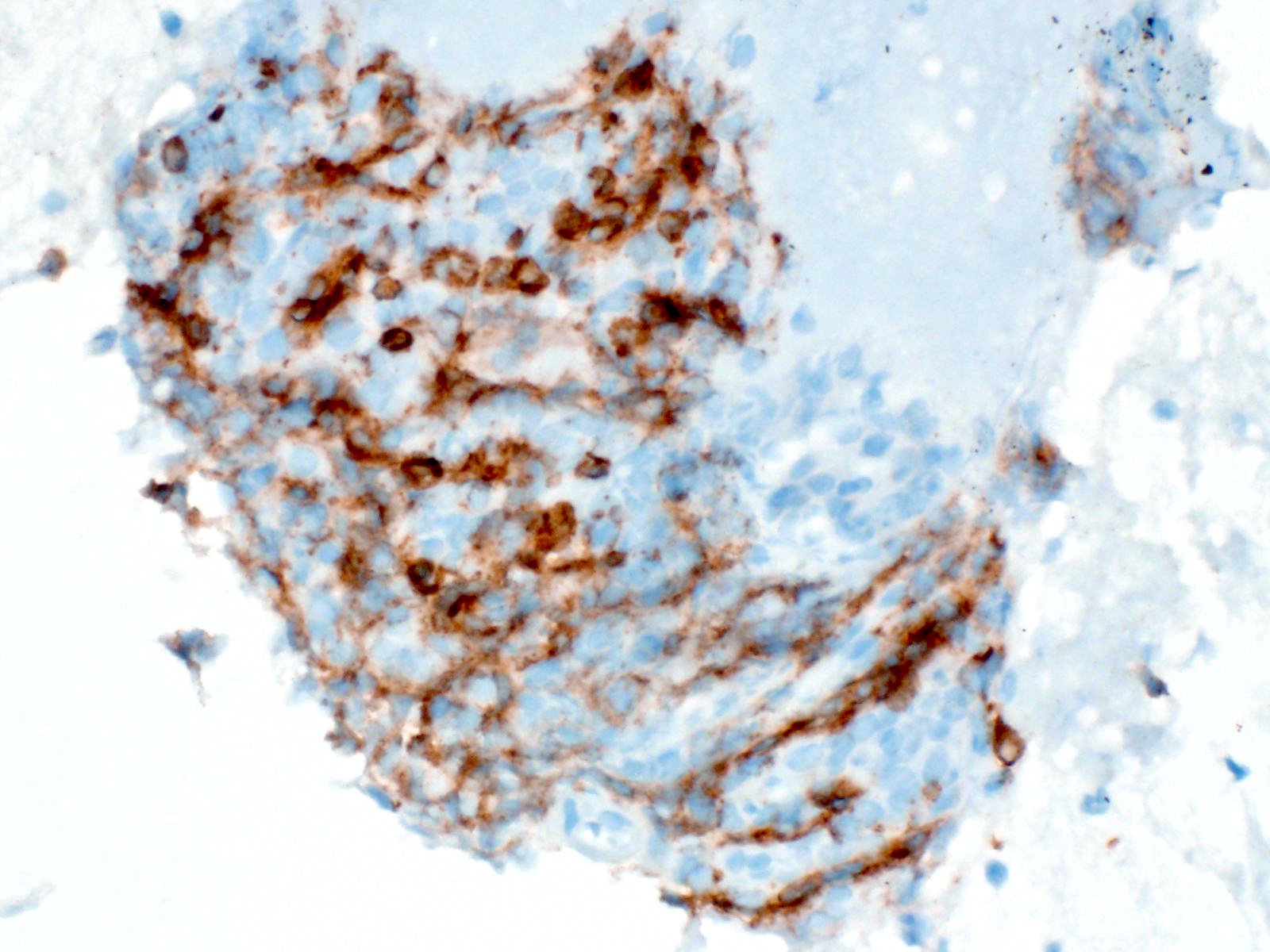
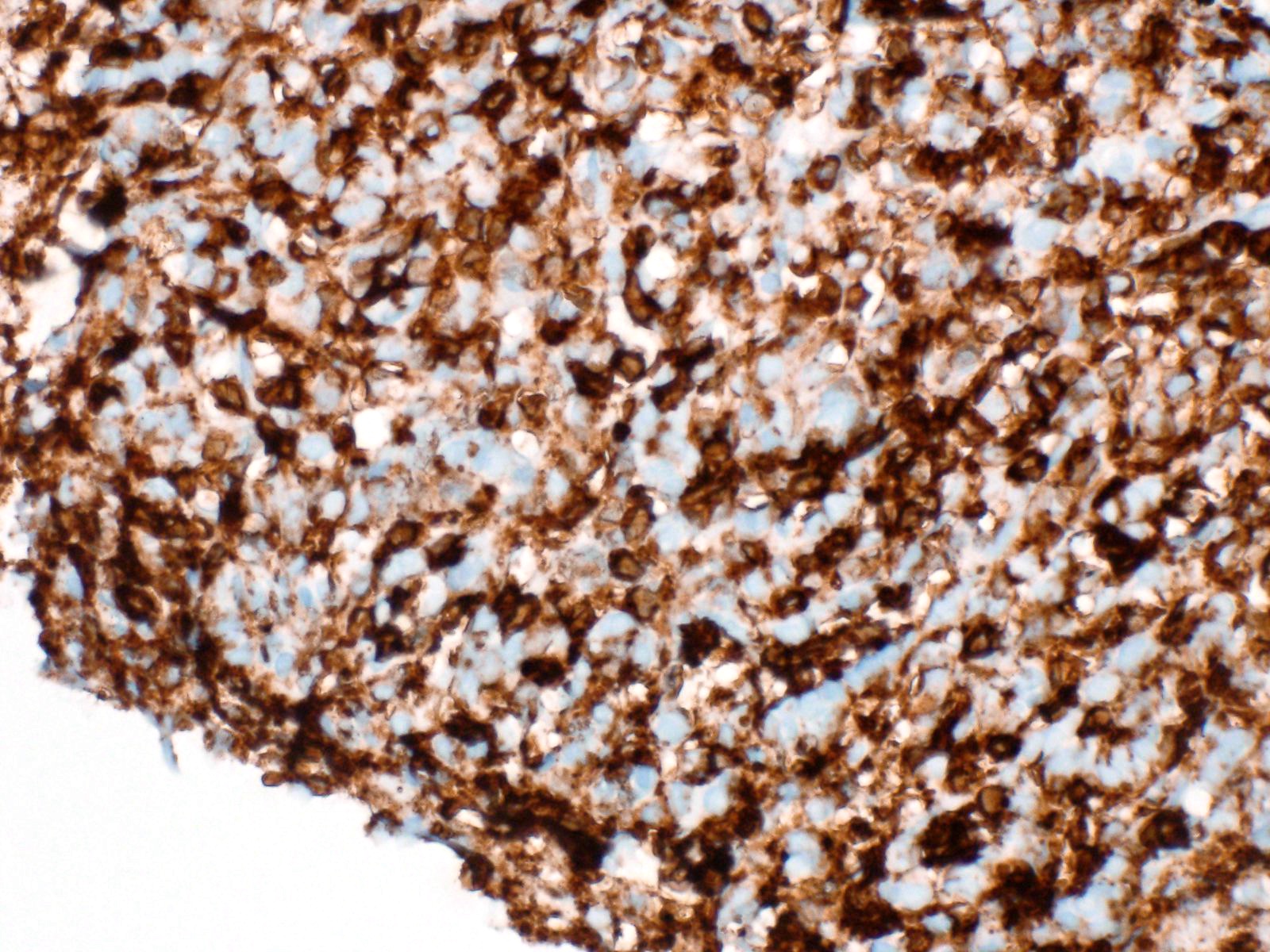
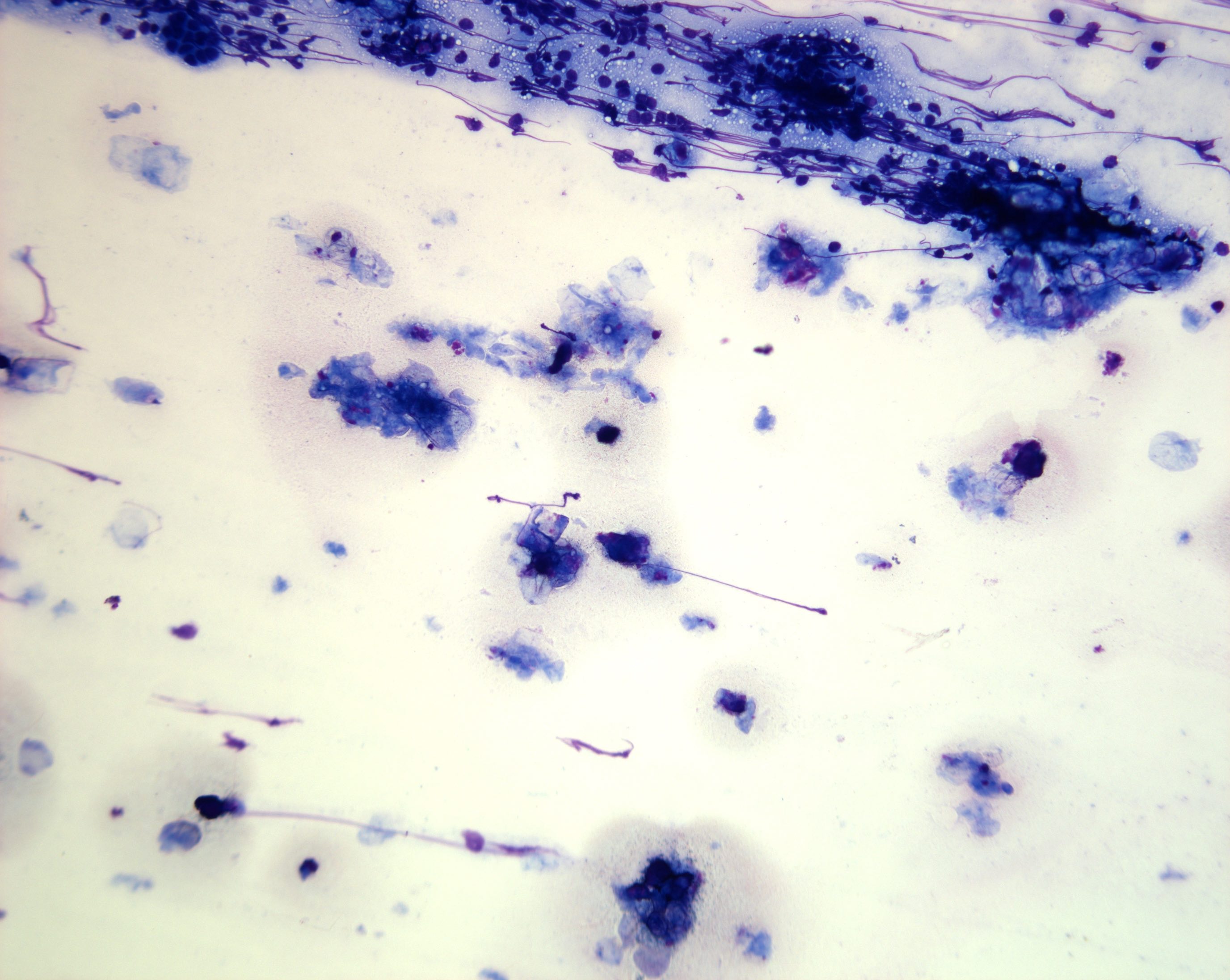
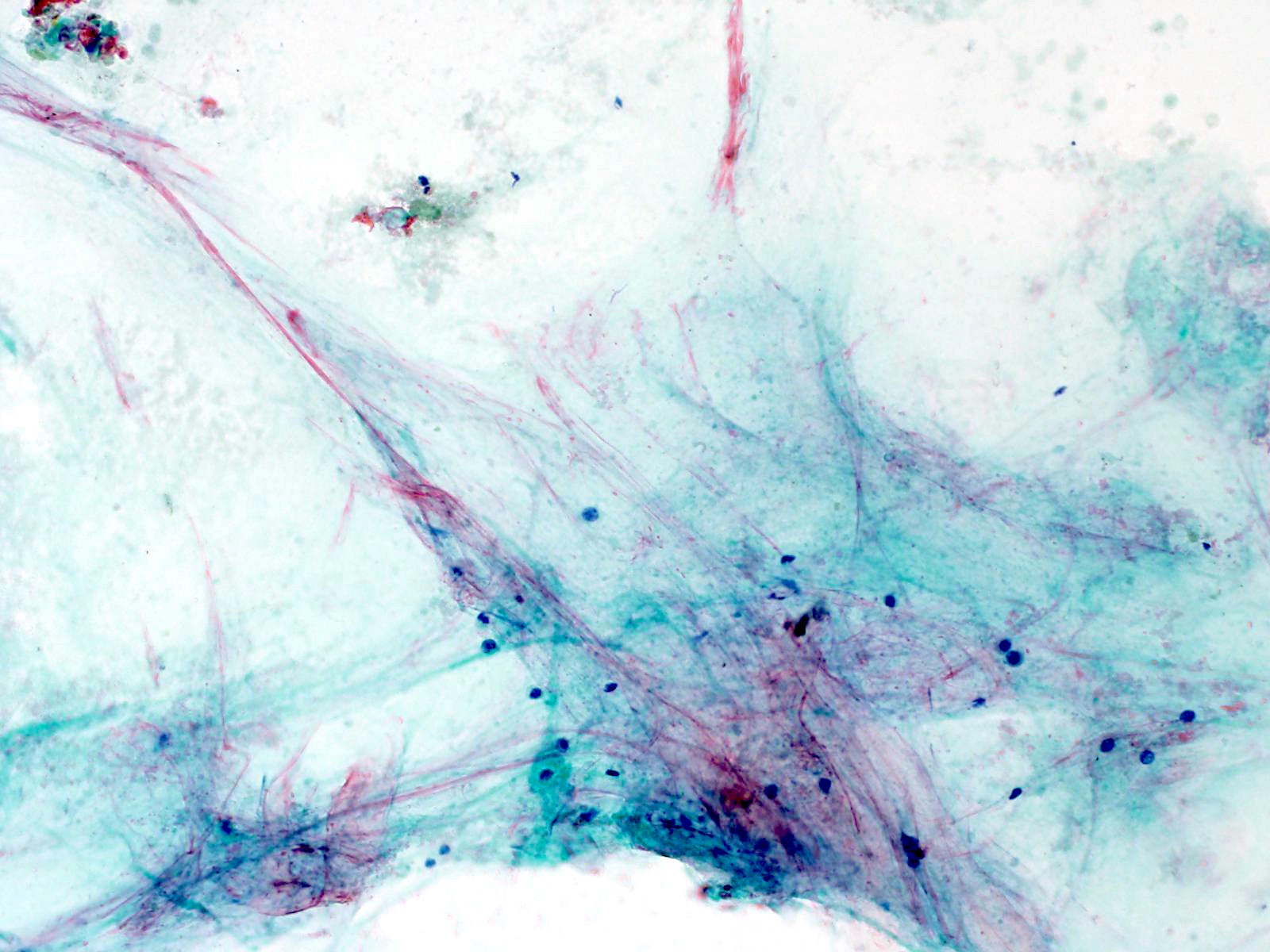
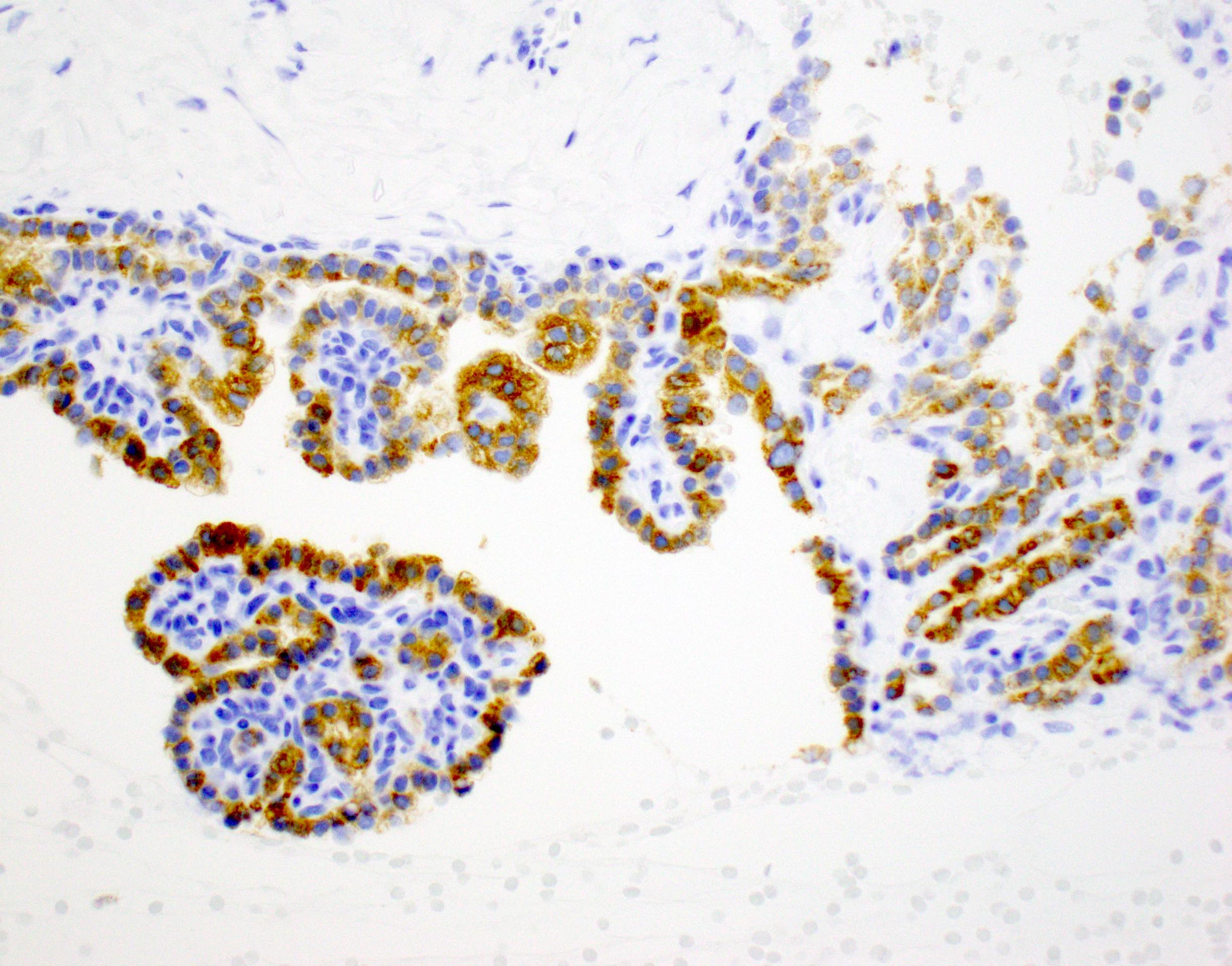
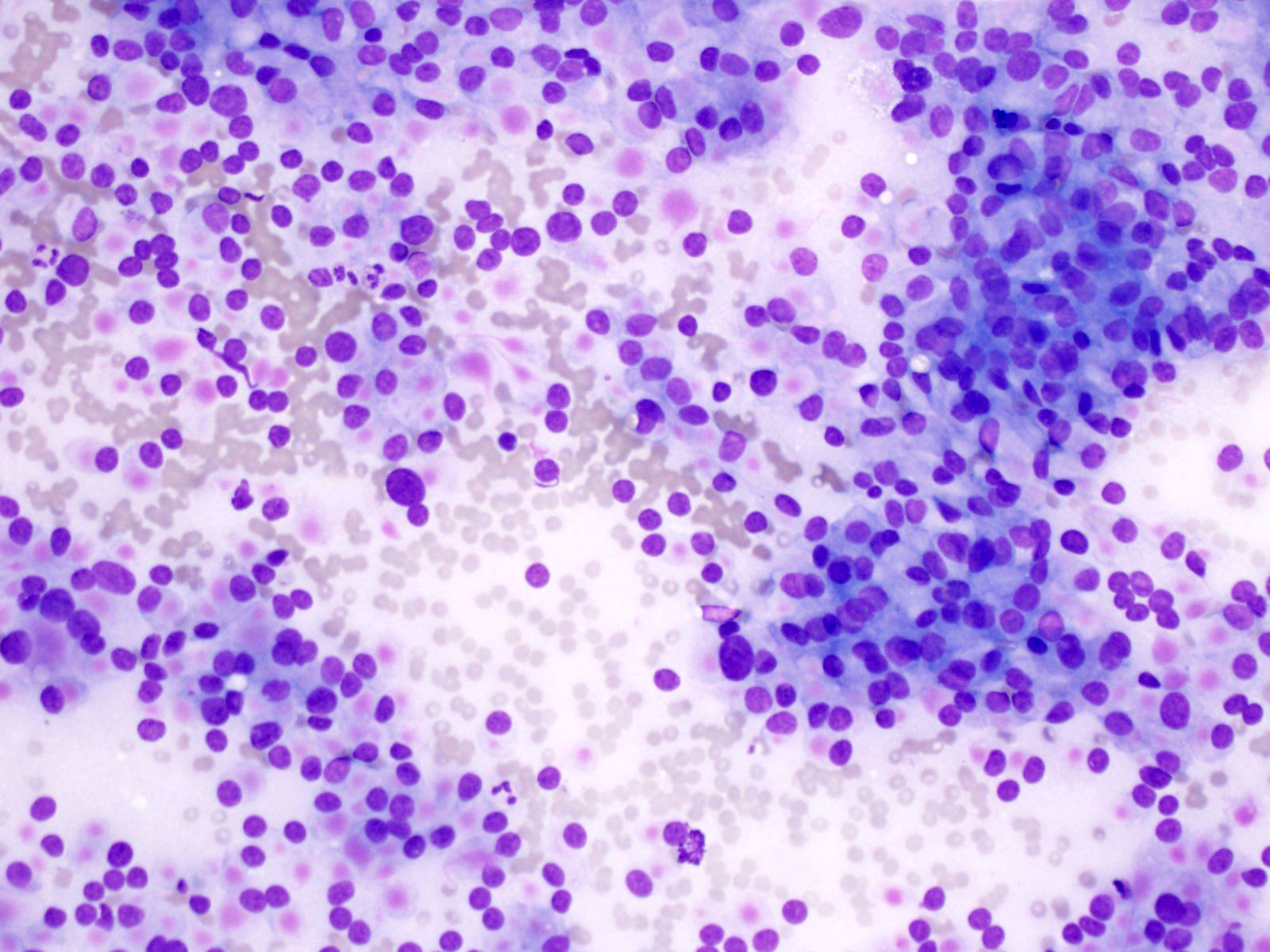
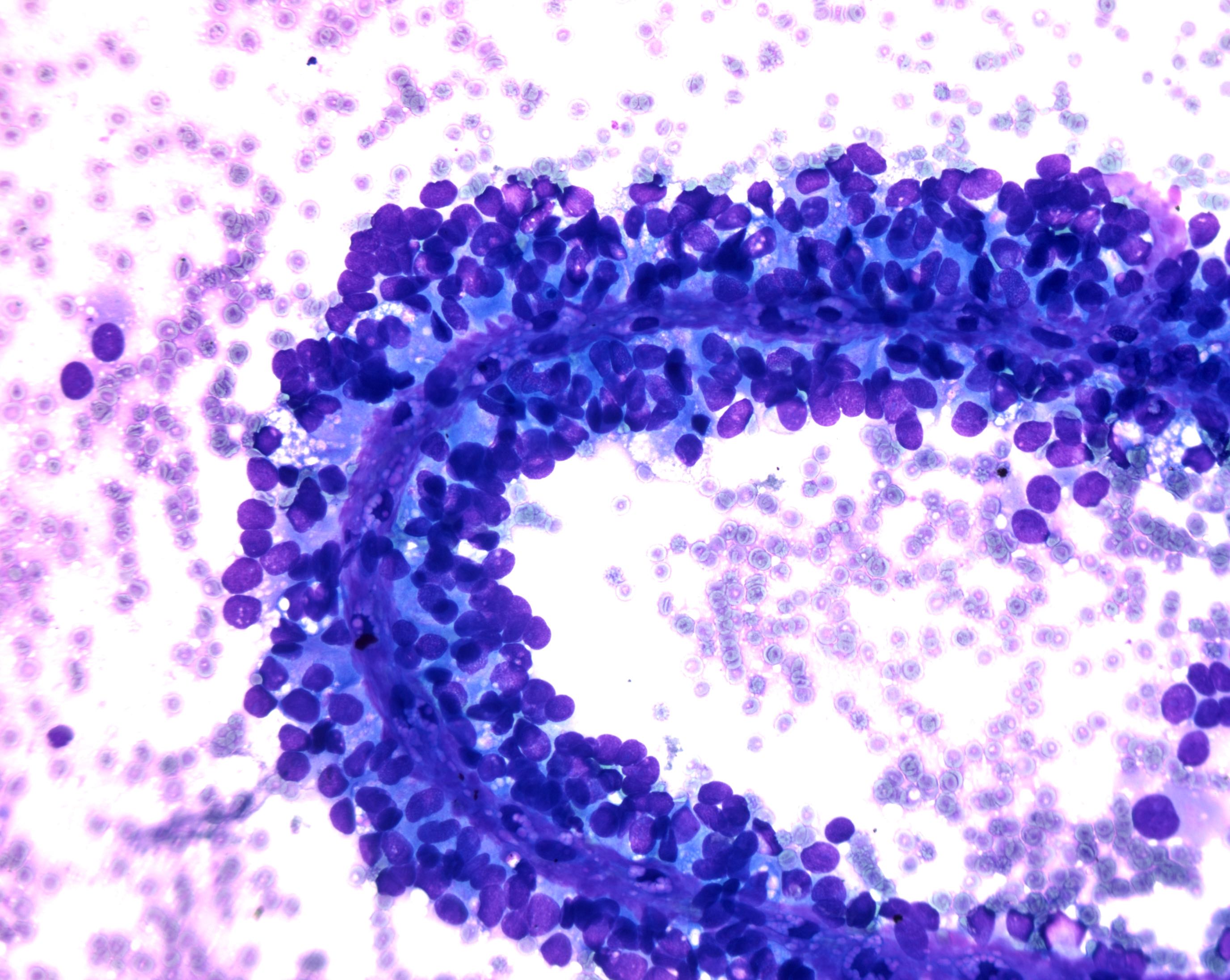
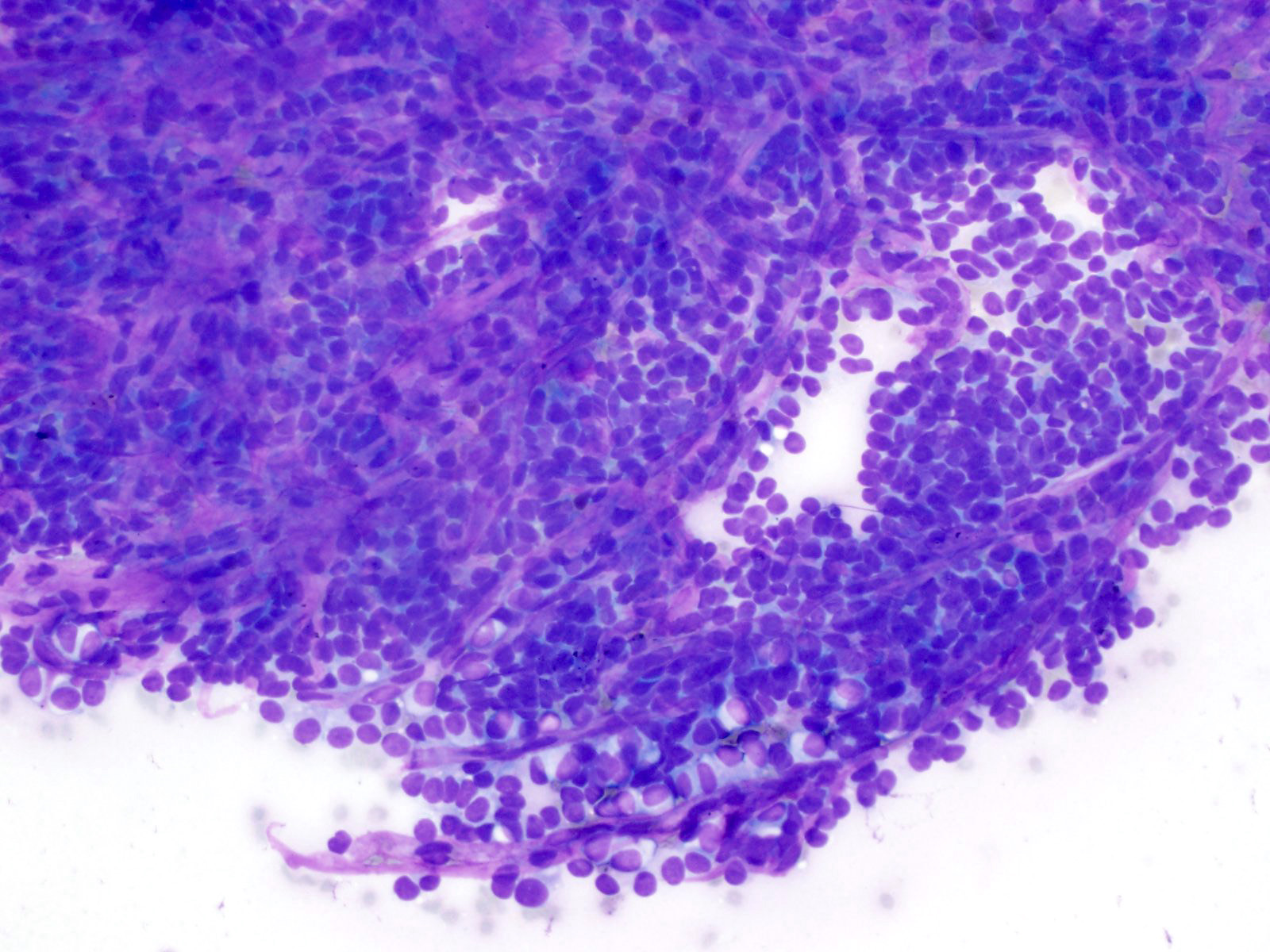
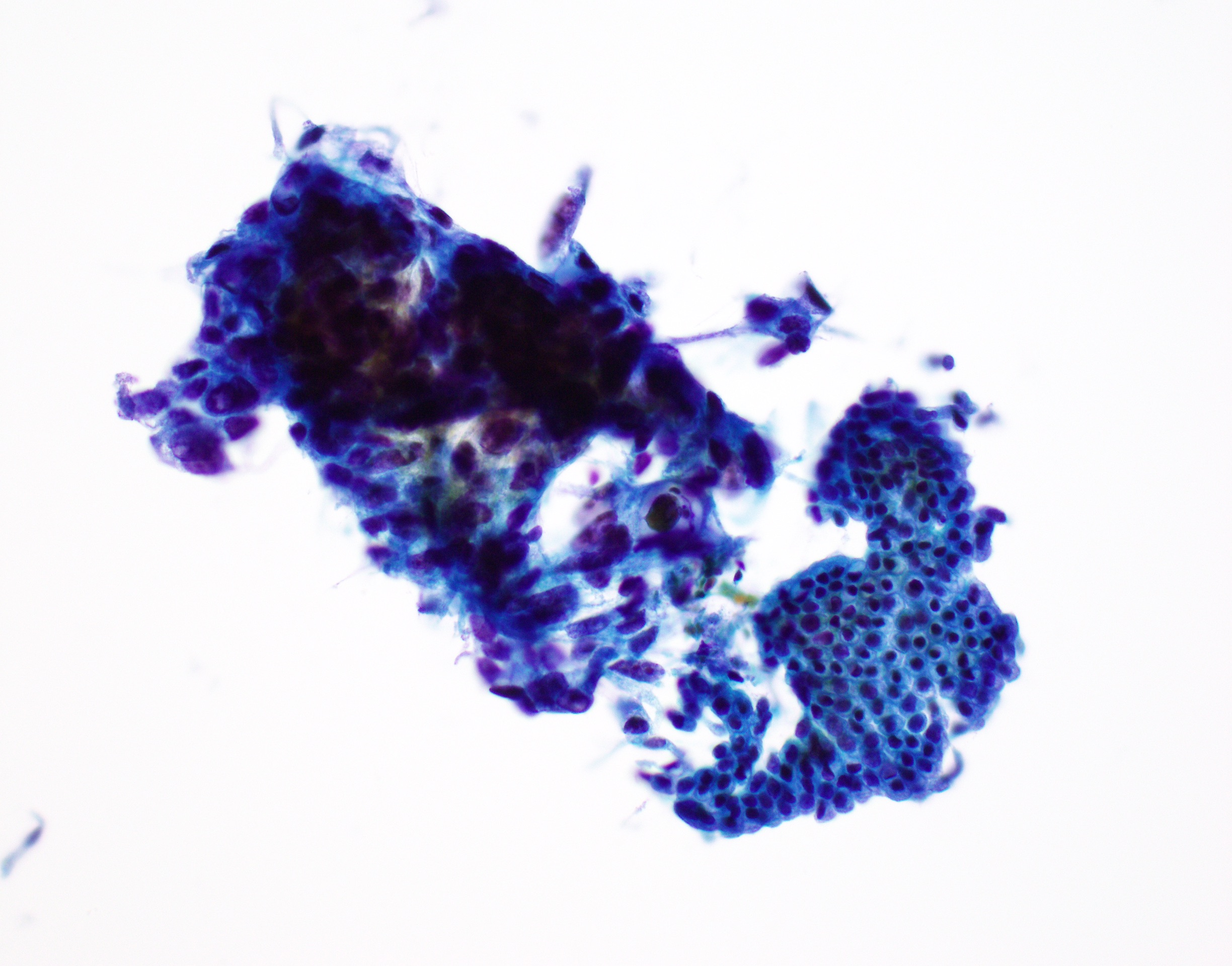
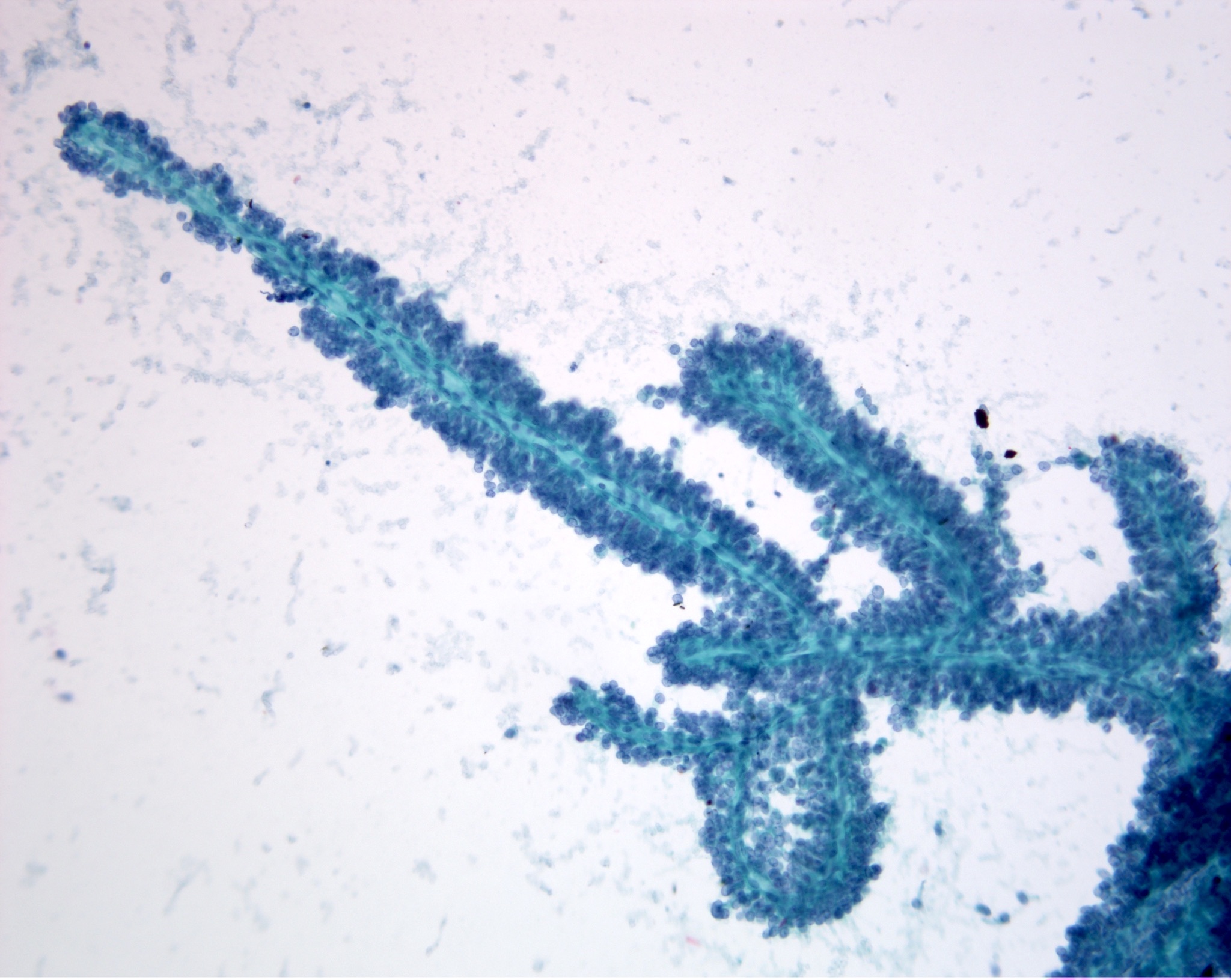
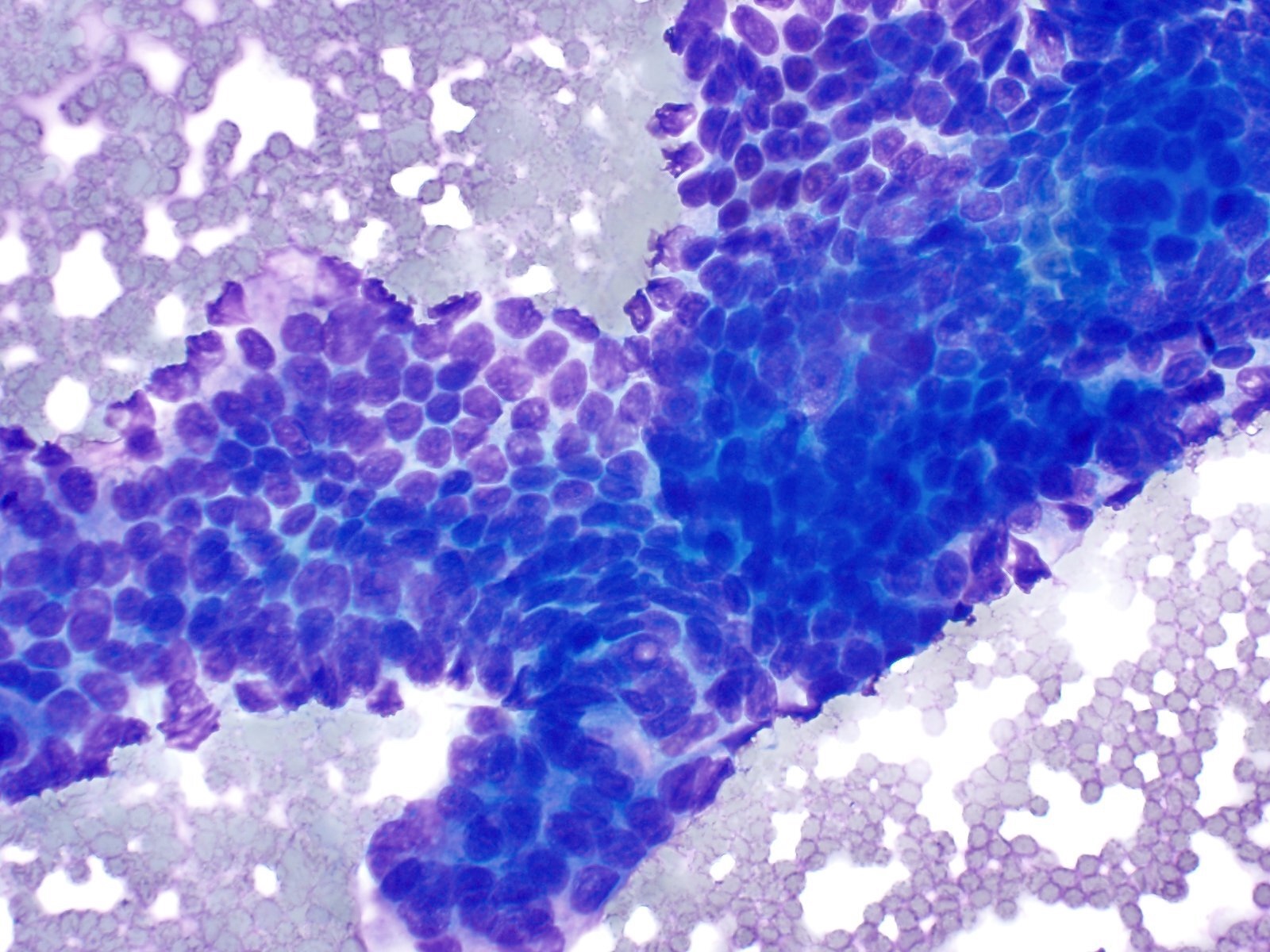
Cytology images
Contributed by Derek Allison, M.D.
Additional references
Board review style question #1
Board review style answer #1
C. Neoplastic: other. Solid pseudopapillary neoplasm of the pancreas, characterized by small, discohesive cells surrounding branching capillaries, is considered a low grade malignant entity. The neoplastic: other category includes neoplasms that are either premalignant or low grade malignant.
Comment Here
Reference: Pancreas - Papanicolaou system
Comment Here
Reference: Pancreas - Papanicolaou system
Board review style question #2
Board review style answer #2
B. Negative DPC4 / SMAD4 by IHC. Pancreatic adenocarcinoma is supported by DPC4 / SMAD4 loss. The presence of copy number abnormalities in CEP3, CEP7, CEP17 (note the correct chromosomes) and of band 9p21 are characteristic of pancreatic and biliary malignancies. Serous cystadenoma is associated with mutated VHL. Nuclear beta catenin supports a diagnosis of solid pseudopapillary neoplasm.
Comment Here
Reference: Pancreas - Papanicolaou system
Comment Here
Reference: Pancreas - Papanicolaou system