Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Diagrams / tables | Clinical features | Prognostic factors | Case reports | Gross description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Armutlu A, Adsay NV. PanIN. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/pancreaspanin.html. Accessed March 28th, 2025.
Definition / general
- Pancreatic intraepithelial neoplasia (PanIN) is a microscopic neoplastic lesion of the pancreas that can progress to invasive ductal adenocarcinoma (Arch Pathol Lab Med 2017;141:1606)
- Regarded as the main precursor lesion to invasive pancreatic carcinoma
- Small, papillary or flat, noninvasive epithelial neoplasm characterized by variable mucin production and a spectrum / variable degrees of cytologic and architectural atypia (Am J Surg Pathol 2015;39:1730)
- By definition, it is a nontumoral form of dysplasia (intraepithelial neoplasia) (see intraductal papillary mucinous neoplasm for the tumoral version of intraepithelial neoplasia)
- As such, almost by default, it doesn't have any clinical manifestation
- Other noninvasive precursors of pancreatic ductal adenocarcinoma such as intraductal papillary mucinous neoplasms (and similar intraductal neoplams) and mucinous cystic neoplasms, all of which are defined as grossly visible (tumoral) forms of intraepithelial neoplasia, are classified separately (Am J Surg Pathol 2015;39:1730, Am J Surg Pathol 2004;28:977)
Essential features
- Usually < 0.5 cm (very small to be seen grossly or by radiologic imaging)
- 2 tiered classification, low grade versus high grade, was recommended to replace the former 3 tiered classification for PanIN, intraductal papillary mucinous neoplasm and mucinous cystic neoplasm at the Baltimore consensus meeting (2014) (Am J Surg Pathol 2015;39:1730)
- Only carcinoma in situ type lesions are regarded high grade and warrant clinical attention
ICD coding
- ICD-O: 8148/2 (for high grade only)
Epidemiology
- Increase in frequency by age
- Low grade lesion is a very common incidental finding (found in over half of the population > 50 years) (Ann Surg Oncol 2013;20:3643, Mod Pathol 2003;16:996)
- In contrast, high grade is rarely seen without invasive ductal adenocarcinoma (Mod Pathol 2003;16:996)
- It can occasionally be seen incidentally in pancreatectomy performed for other lesions
Sites
- Anywhere in the pancreas, mostly in cases with resected pancreatic ductal adenocarcinoma
- It is more common in branch ducts rather than in main pancreatic duct
- Can be present in heterotopic pancreas (Am J Surg Pathol 2017;41:833)
Clinical features
- Does not manifest clinically
- PanINs are very common adjacent to resected pancreatic ductal adenocarcinoma (Ann Surg Treat Res 2018;94:247)
Prognostic factors
- Low grade is inconsequential
- High grade believed to have a high frequency of progression into pancreatic ductal adenocarcinoma and therefore needs careful evaluation
- PanIN of any grade at a margin of a resected pancreas that already has invasive carcinoma doesn't seem to have any prognostic implications; however, if PanIN 3 (carcinoma in situ) is encountered at a margin in a pancreas without carcinoma, it needs to be carefully evaluated (Am J Surg Pathol 2015;39:1730)
Case reports
- 47 year old woman with PanIN in heterotopic pancreas that was incidentally diagnosed on endoscopic mucosal resection of a duodenal polyp (BMJ Case Rep 2018 Jun 23;2018)
- 58 year old man with PanIN 2 in heterotopic pancreas (J Gastrointestin Liver Dis 2017;26:199)
- 63 year old man and 77 year old man with high grade PanIN over long time periods (Oncology 2017;93:81)
- 82 year old man with concurrent PanIN and type 1 autoimmune pancreatitis with marked pancreatic duct dilatation (Clin J Gastroenterol 2016;9:266)
Gross description
- Not detectable grossly
- Occasionally obstructs a duct and lead to subtle fibrosis of upstream pancreatic tissue
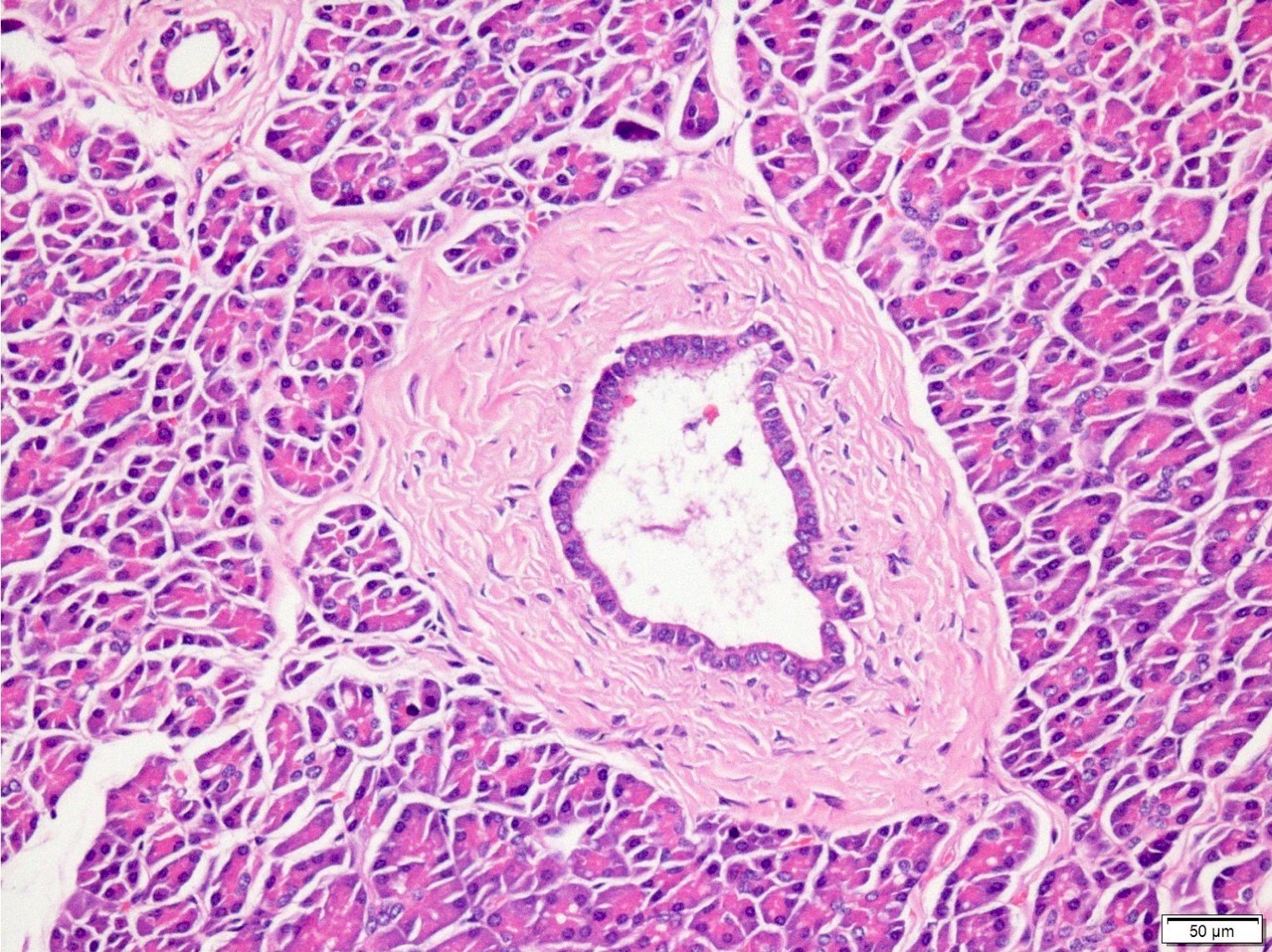
Microscopic (histologic) description
- Usually < 0.5 cm
- 2 tiered classification, low grade versus high grade, was recommended to replace the former 3 tiered classification for PanIN, intraductal papillary mucinous neoplasm and mucinous cystic neoplasm at the Baltimore consensus meeting (2014) (Am J Surg Pathol 2015;39:1730)
- PanIN 1A / low grade:
- Earliest precursor lesion
- Flat epithelium composed of tall columnar mucin producing cells with basally located small round to oval nuclei
- Cells show essentially no cytologic atypia
- PanIN 1B / low grade:
- Identical to PanIN 1A but epithelium shows papillary, micropapillary or basally pseudostratified architecture
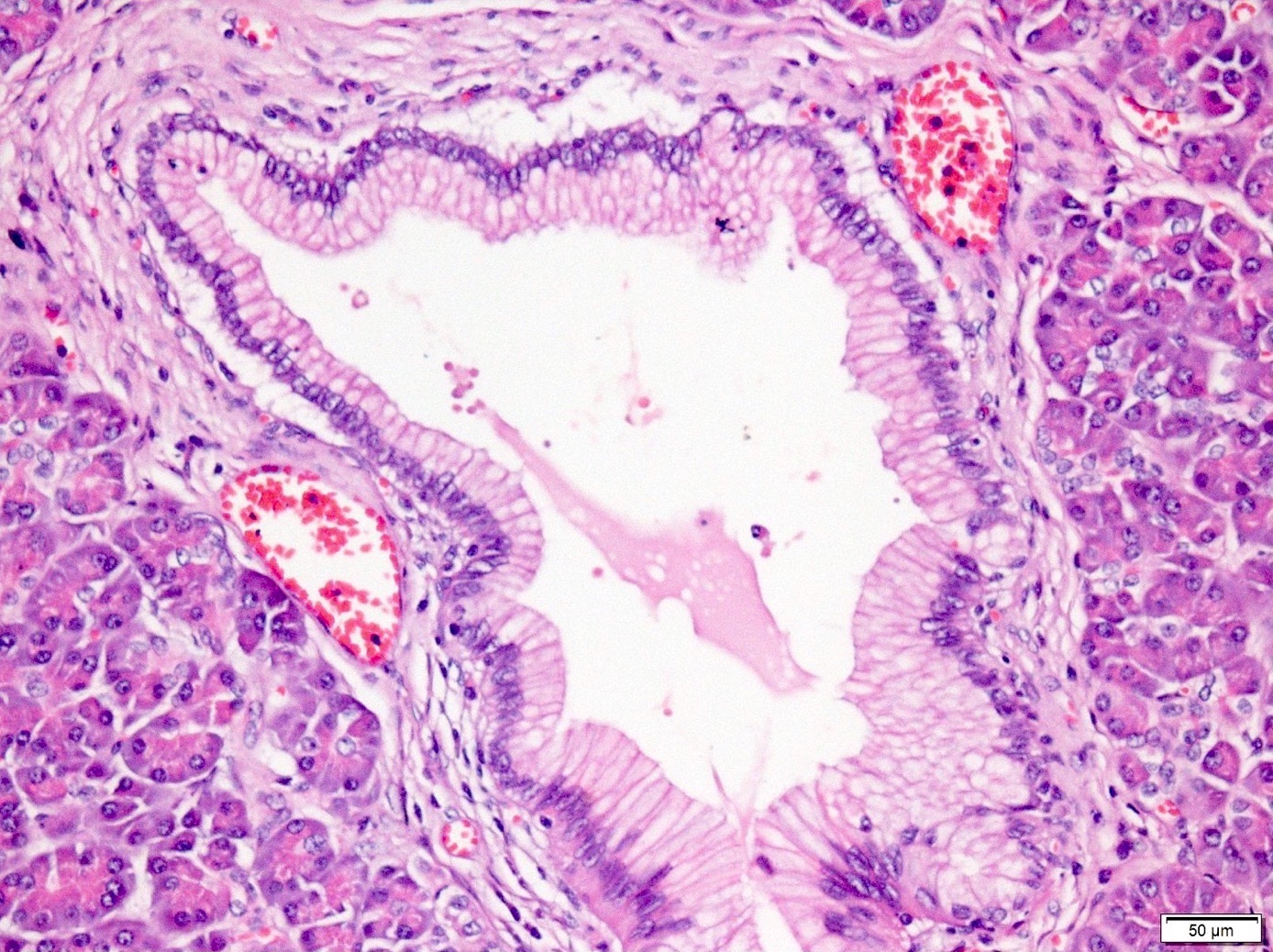
- PanIN 2 / low grade:
- Flat to papillary mucinous epithelial proliferations with focal or mild nuclear abnormalities, such as loss of polarity, nuclear enlargement, nuclear crowding, hyperchromatism and pseudostratification
- Rare mitoses but not apical or atypical
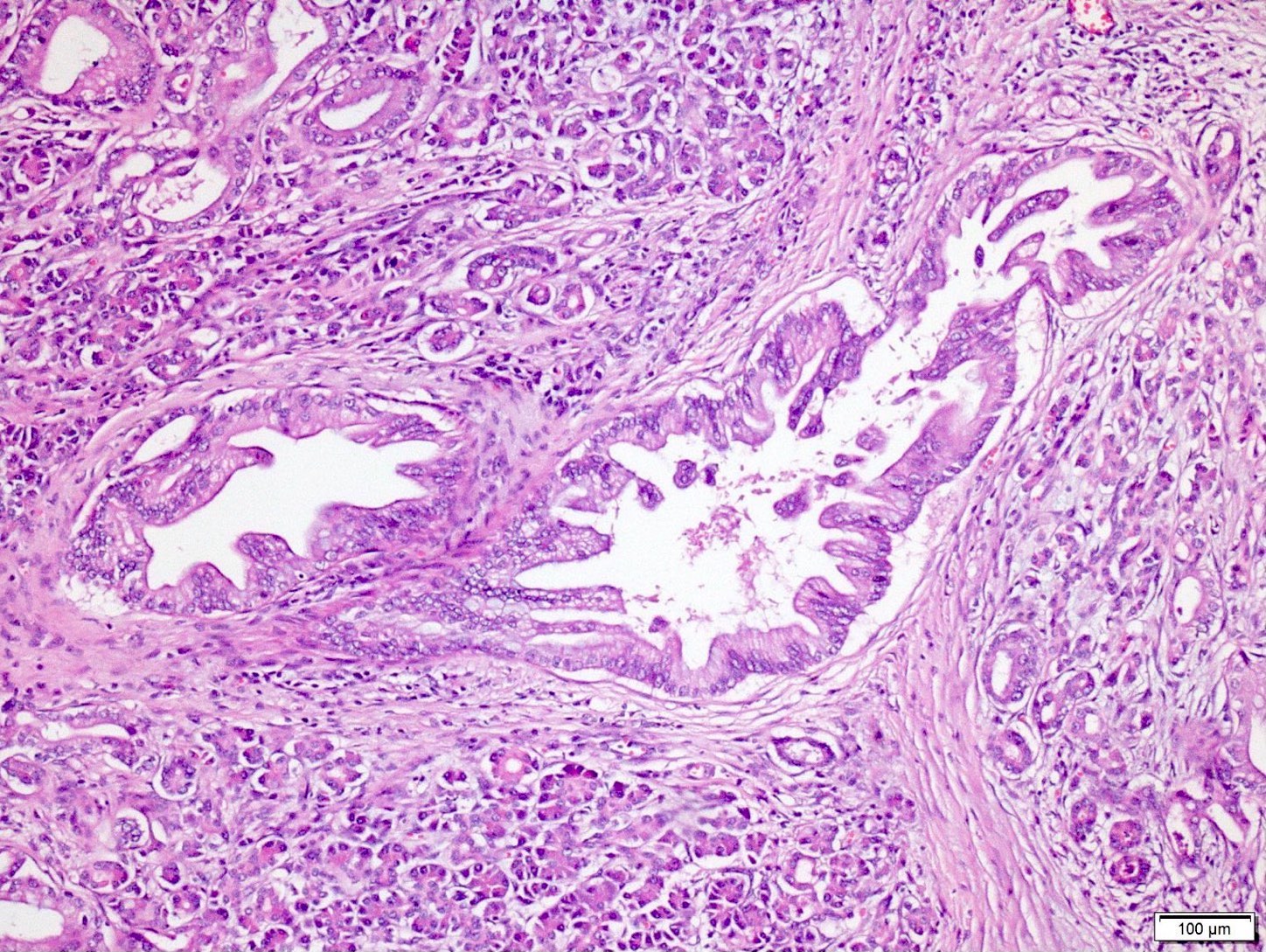
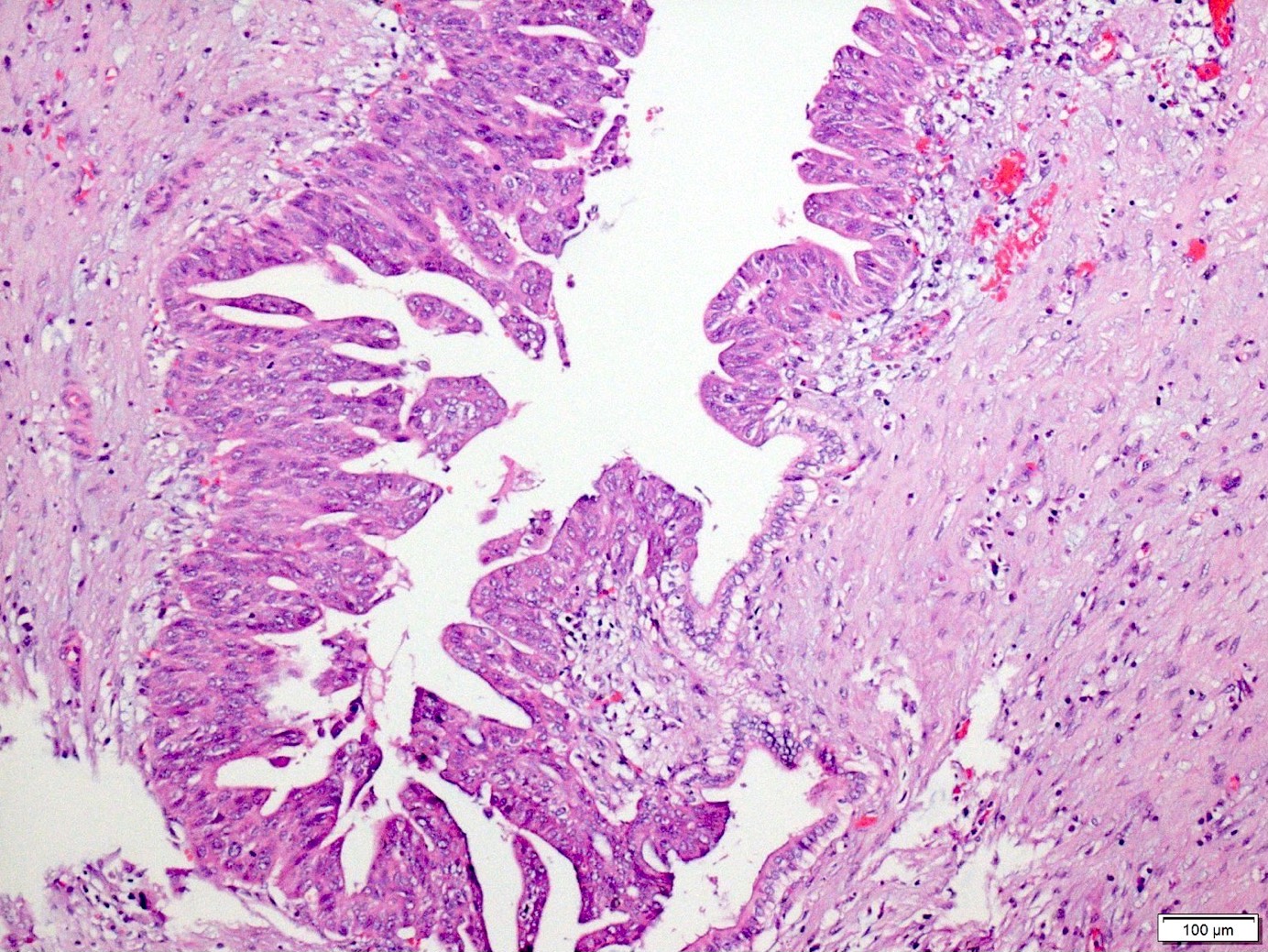
- PanIN 3 / high grade / carcinoma in situ:
- Predominantly papillary or micropapillary architecture, rarely flat
- Cribriforming, tufting pattern and luminal necrosis suggests PanIN 3
- Cytologically, these lesions have loss of polarity, dystrophic mucinous cells, enlarged irregular nuclei and prominent (macro) nucleoli
- Increased mitotic figures, including atypical forms
- Only carcinoma in situ type lesions are regarded as PanIN 3 / high grade (Am J Surg Pathol 2015;39:1730)
| Pancreatic intraepithelial neoplasia classification | ||
| Oldest terminology | WHO 2010 classification | Baltimore consensus meeting 2014 |
| Mucinous (goblet cell) metaplasia, flat duct lesion without atypia, mucinous ductal hyperplasia, simple hyperplasia, mucinous cell hyperplasia, flat duct hyperplasia, nonpapillary epithelial hypertrophy | PanIN 1A | Low grade PanIN |
| Papillary hyperplasia, papillary duct lesion without atypia, ductal hyperplasia, adenomatoid hyperplasia | PanIN 1B | |
| Atypical hyperplasia, papillary duct lesion with atypia, low grade dysplasia, moderate dysplasia | PanIN 2 | |
| Carcinoma in situ, intraductal carcinoma, high grade dysplasia, severe dysplasia | PanIN 3 | High grade PanIN |
Microscopic (histologic) images
Cytology description
- PanINs are, by definition, microscopic incidental lesions, not visible clinically or radiologically and therefore are not amenable to targeting by fine needle aspiration biopsy
- On rare occasions that they come to aspiration material, they would appear as mucinous glandular epithelium with atypical changes but their nature as PanIN cannot be affirmed
- The diagnosis of a PanIN should not be rendered on cytologic preparations
Positive stains
- MUC5AC, MUC6, HER2 nonbreast and fascin
- MUC1 (luminal membranous; cytoplasmic in high grade) and cyclin D1
- Ki67 labeling increases with PanIN grade
- In high grade lesions, a subset with p53 overexpression or SMAD4 / DPC4 loss
Molecular / cytogenetics description
- Early molecular changes: telomere shortening, KRAS oncogene activation (can be seen in PanIN 1 / low grade), p21 upregulation
- Intermediate steps: inactivation of tumor suppressor gene p16 CDKN2A
- Late stages: BRCA2, SMAD4 (DPC4) and TP53 tumor suppressor gene inactivation (Am J Clin Pathol 2014;141:168, Arch Pathol Lab Med 2017;141:366)
Sample pathology report
- Pancreas, tail, distal pancreatectomy:
- Incidental low grade pancreatic intraepithelial neoplasm (see comment)
- Comment: Incidental pancreatic intraepithelial neoplasms that are low grade are of no known clinical significance to an extent that consensus is that it may not even have to be reported. They are very common in general population.
- Pancreas, tail, distal pancreatectomy:
- High grade pancreatic intraepithelial neoplasm (carcinoma in situ) (see comment)
- Comment: High grade pancreatic intraepithelial neoplasms are seldom detected in isolation. Invariably they represent carcinoma elsewhere in the gland. In fact, this finding may represent pagetoid spread of invasive carcinoma from somewhere else in the pancreas which is now showing intraductal spread ("cancerization") and creating the image of high grade pancreatic intraepithelial neoplasm. This possibility needs to be excluded with careful evaluation of the patient before this is accepted as a mere precursor lesion.
Differential diagnosis
- Normal mucosal elements and peribiliary glands in ampulla and bile ducts:
- In the vicinity of the ampulla and bile ducts, normal mucosal elements often show cytologic findings that would otherwise qualify as low grade PanIN if they occur in the pancreatic ducts
- Transitional / squamous metaplasia:
- Shows stratified cells with high N/C ratio and this can closely mimic high grade PanIN
- Cancerization of ducts:
- Invasive carcinomas may re-enter and grow within the pancreatic ducts in a pagetoid spreading pattern, mimicking PanIN 3
- Invasive carcinoma near a duct lesion transitioning from high grade atypia to normal duct epithelium suggests cancerization but it can sometimes be impossible to make this determination
- In a case with invasive carcinoma, high grade lesion in a duct may have to be considered cancerization of ducts, especially if it is spatially close to invasive glands (Am J Surg Pathol 2015;39:1730)
- Tumoral intraepithelial neoplasia:
- Defined as clinically and grossly visible intramucosal / intraepithelial tumors that are generally > 1 cm
- These include the branch duct spread of tumors like intraductal papillary mucinous neoplasm
- If taken in isolation at microscopic level, especially when they have more gastric / endocervical-like morphology, these tumors can be indistinguishable from PanINs
- Gastric type intraductal papillary mucinous neoplasms have significant morphologic overlap with PanINs at a microscopic level (Ann Surg 2016;263:162)
- Though not widely employed, for cysts > 0.5 cm and < 1 cm lined by gastric-like mucinous epithelium, the term incipient intraductal papillary mucinous neoplasm has been proposed (Am J Surg Pathol 2014;38:360)
- In indeterminate cases, should be classified as PanIN, not intraductal papillary mucinous neoplasm
- Simple mucinous cysts with flat mucinous lining:
- Cysts with usually flat, gastric phenotype, lack ovarian stroma and are > 1 cm (Am J Surg Pathol 2017;41:121)
- Can show dysplastic changes of variable degrees and sometimes are diagnosed as simple mucinous cyst with PanIN
- Vascular invasion in pancreatic ductal adenocarcinoma:
- If the invasive neoplastic cells form complete luminal structures inside of the blood vessels, classify the case as having PanIN-like vascular invasion (Am J Surg Pathol 2012;36:235)
Board review style question #1
A 60 year old man, who is an alcoholic, presented with back pain, weight loss and jaundice. Gross sections showed firm, white-beige appearance in the head of the pancreas. Initial histologic sections demonstrated chronic pancreatitis and this lesion in a focal area. Which of the following statements concerning this case and this pancreatic lesion is true?
- p63 / p40 often labels this lesion
- This does not need to be reported
- This is low grade pancreatic intraepithelial neoplasia (PanIN 1)
- This is probably accompanied by carcinoma and warrants a thorough examination of the remaining pancreas
- This lesion is a very common incidental lesion in the general population
Board review style answer #1
D. This is probably accompanied by carcinoma and warrants a thorough examination of the remaining pancreas
Comment Here
Reference: PanIN (pancreatic intraepithelial neoplasia)
Comment Here
Reference: PanIN (pancreatic intraepithelial neoplasia)
Board review style question #2
Which of the following statements concerning pancreatic intraepithelial neoplasia is true?
- High grade PanIN (PanIN 3) is usually seen in pancreas without invasive ductal adenocarcinoma
- PanIN 2 is a high grade pancreatic intraepithelial neoplasia by new classification
- PanIN of any grade (including low grade) at a margin of a resected pancreas has very important prognostic implications
- PanINs are large lesions that can be seen grossly or by radiologic imaging
- Recommended latest terminology from Baltimore consensus meeting is a 2 tiered system: low grade and high grade PanIN
Board review style answer #2
E. Recommended latest terminology from Baltimore consensus meeting is a 2 tiered system: low grade and high grade PanIN
Comment Here
Reference: PanIN (pancreatic intraepithelial neoplasia)
Comment Here
Reference: PanIN (pancreatic intraepithelial neoplasia)