Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Ojukwu K, Hutchings D. Heterotopic pancreas. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/pancreasheterotopic.html. Accessed March 31st, 2025.
Definition / general
- Pancreatic tissue that is anatomically separate from the main pancreatic gland and has no vascular or ductal connection to it
Essential features
- Pancreatic tissue that is anatomically separate from the main pancreatic gland and has no ductal or vascular connections to it the main pancreatic gland
- Composed of a variable mixture of pancreatic acini, ducts and islets
- Most common in stomach but may occur throughout GI tract; and rarely in extragastrointestinal sites
- Often incidental finding
- May develop the same diseases as the main pancreatic gland (e.g., pancreatitis or pancreatic neoplasms)
- May be asymptomatic or depending on size and location, may present with symptoms such as abdominal pain, GI bleed or obstruction
- Surgical management if symptomatic or if diagnosis is unknown
Terminology
- Ectopic pancreas, heterotopic pancreas, pancreatic rest, accessory pancreas, aberrant pancreas, pancreatic choristoma
ICD coding
- ICD-10: Q45.3 - other congenital malformations of pancreas and duct
Epidemiology
- M:F = 3:1
- Most commonly identified in fifth to sixth decades of life
- Incidence of 0.5 - 13% in autopsy studies
- Incidental finding in 0.2 - 0.9% of upper abdominal surgeries and gastrectomies, respectively
- Reference: Gastroenterology Res 2021;14:45
Sites
- Can occur anywhere in the gastrointestinal tract (GI)
- Most common in upper GI tract (stomach, duodenum and proximal jejunum) (Gastroenterology Res 2021;14:45)
- Less common in Meckel diverticulum, esophagus, ileum, colon, biliary tree, mesentery, spleen and lung (Gastroenterology Res 2021;14:45, Saudi Med J 2018;39:834)
Pathophysiology
- Exact embryologic mechanism unknown
- Misplacement theory: portion(s) of pancreas become separated during rotation of foregut and develop into mature pancreatic tissue (Radiographics 2017;37:484, Arch Pathol Lab Med 1999;123:707)
- Metaplasia theory: pancreatic metaplasia of endodermal tissue that has migrated to submucosa (Radiographics 2017;37:484, Arch Pathol Lab Med 1999;123:707)
- Totipotent cell theory: endodermal cells in GI tract differentiate into pancreatic tissue (Radiographics 2017;37:484)
Clinical features
- Often asymptomatic (Gastroenterology Res 2021;14:45, Radiographics 2017;37:484)
- Depending on location and size, can present with symptoms of abdominal pain, melena, obstruction or intussusception (Gastroenterology Res 2021;14:45, Radiographics 2017;37:484)
- May develop the same diseases as the main pancreas, including acute or chronic pancreatitis and neoplasms (Radiographics 2017;37:484)
Diagnosis
- Frequently is an incidental finding
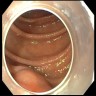
- Radiographically, typically appears as ovoid intramural mass (Radiographics 2017;37:484)
- Endoscopically presents as a solid submucosal mass (Radiographics 2017;37:484)
- Definitive diagnosis requires histologic examination
Laboratory
- Most cases lack specific lab abnormalities
- Rare cases may show lab abnormalities associated with disease of the heterotopic pancreatic tissue:
- Microcytic anemia related to GI bleed caused by heterotopic tissue (BMC Gastroenterol 2019;19:57)
- Elevated serum amylase in heterotopic pancreas with acute pancreatitis (Case Rep Gastrointest Med 2018;2018:5640379)
- Elevated CA 19-9 in adenocarcinoma arising in heterotopic pancreas (Int Surg 2012;97:351)
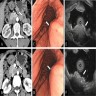
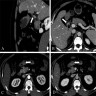
Radiology description
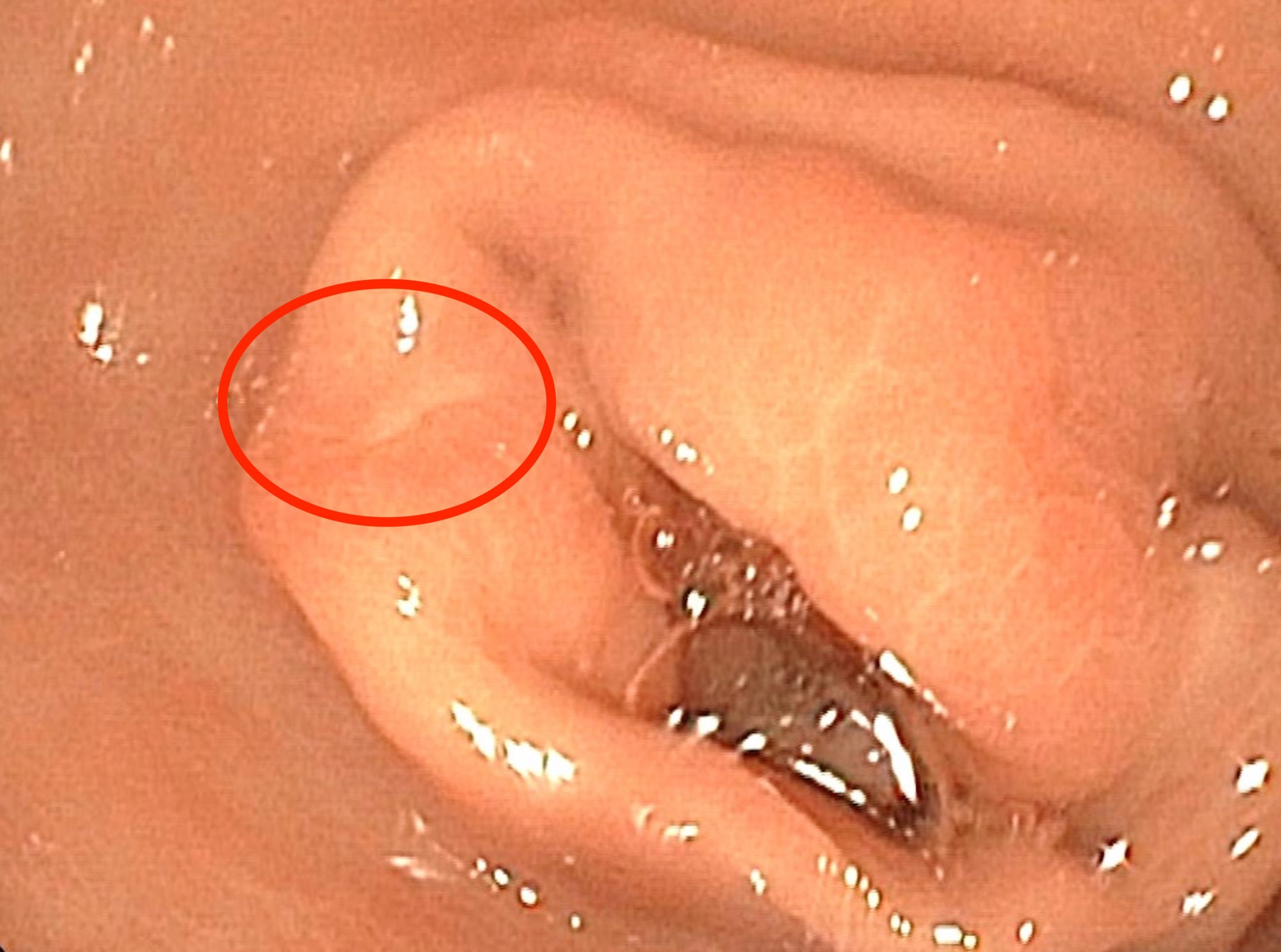
- Endoscopically appears as a round, subepithelial lesion, often with a central depression that represents the opening of the duct (Insights Imaging 2021;12:144, Gastroenterol Rep (Oxf) 2017;5:237)
- Endoscopic ultrasound (EUS) shows solid, submucosal mass that is hypoechoic relative to the hyperechoic submucosa and isoechoic relative to the muscularis propria (Insights Imaging 2021;12:144)
- Typical computed tomography (CT) findings include small (< 3 cm), oval, intramural mass with microlobulated margins and an endoluminal growth pattern (Insights Imaging 2021;12:144, Radiographics 2017;37:484)
- Acinus dominant lesions show avid homogenous enhancement with intravenous contrast
- Duct dominant lesions are hypovascular and heterogenous
- By magnetic resonance imaging (MRI), heterotopic pancreatic tissue will show similar characteristics to the native gland (Insights Imaging 2021;12:144, Radiographics 2017;37:484)
- Magnetic resonance cholangiopancreatography (MRCP) can show ectopic duct sign (i.e., a dilated ectopic pancreatic duct) (Clin Radiol 2010;65:403)
Radiology images
Prognostic factors
- Overall good prognosis but with potential to vary, depending on disease state of heterotopic tissue
- Patients with adenocarcinoma arising in pancreatic heterotopia may do better, compared to those with tumors arising in the main gland (BMC Surg 2017;17:53)
Case reports
- 9 month old girl with pancreatic heterotopia in omphalomesenteric duct remnant (Diagn Pathol 2017;12:49)
- 9 year old boy with inflamed Meckel diverticulum with elevated serum amylase mimicking acute pancreatitis (Indian J Crit Care Med 2017;21:789)
- 27 year old woman with large pancreatic heterotopia presenting with gastric outlet obstruction (Gastroenterol Rep (Oxf) 2017;5:237)
- 48 year old man with pancreatic heterotopia in jejunum and elevated serum chromogranin A (Cureus 2021;13:e15409)
- 71 year old woman with gastric pancreatic heterotopia with intraductal papillary mucinous neoplasm (IPMN) with GNAS mutation (World J Surg Oncol 2021;19:309)
Treatment
- Surgery, if symptomatic
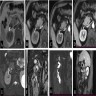
Clinical images
Gross description
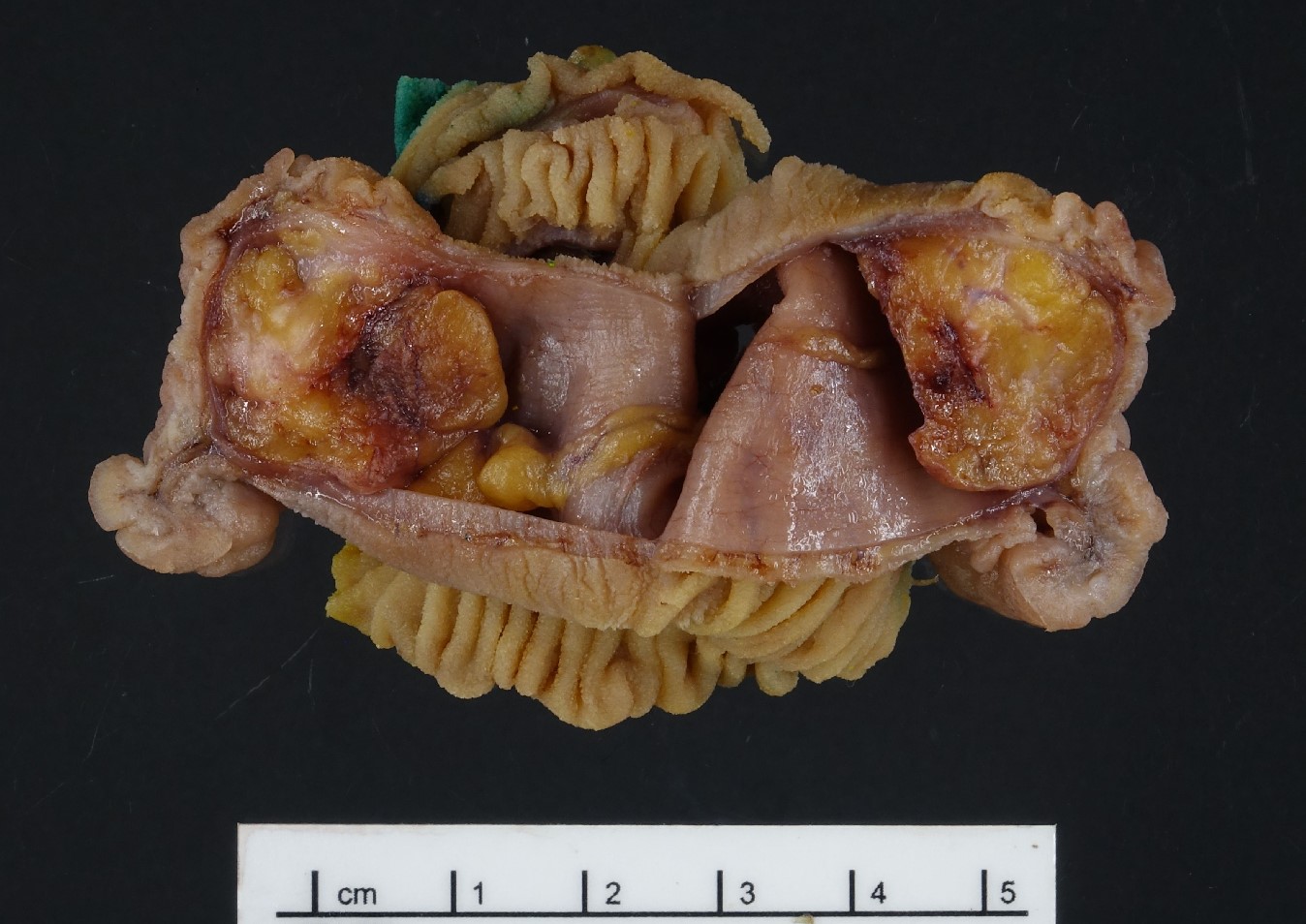
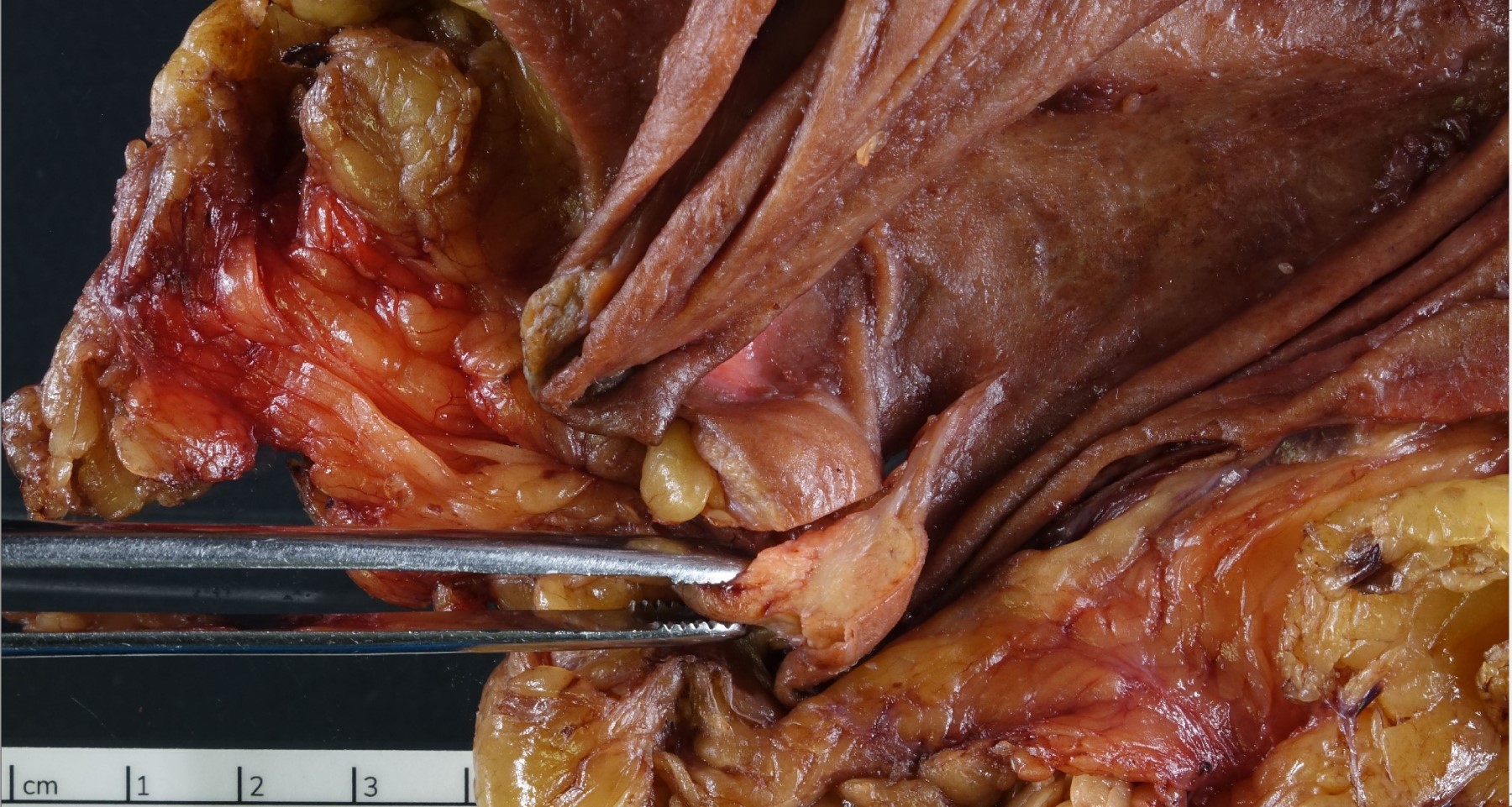
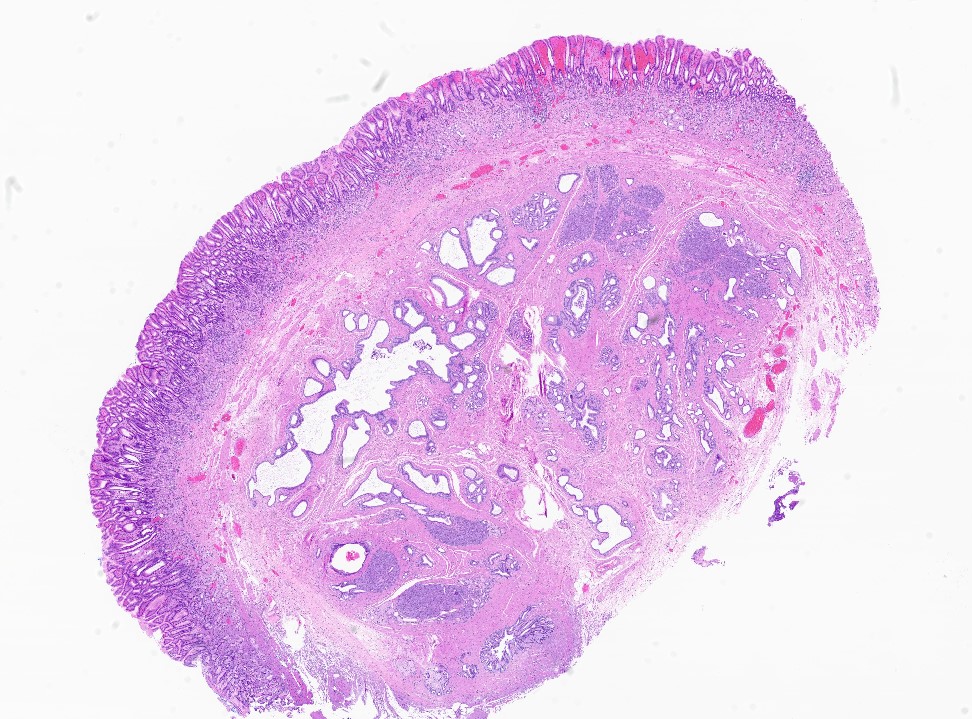
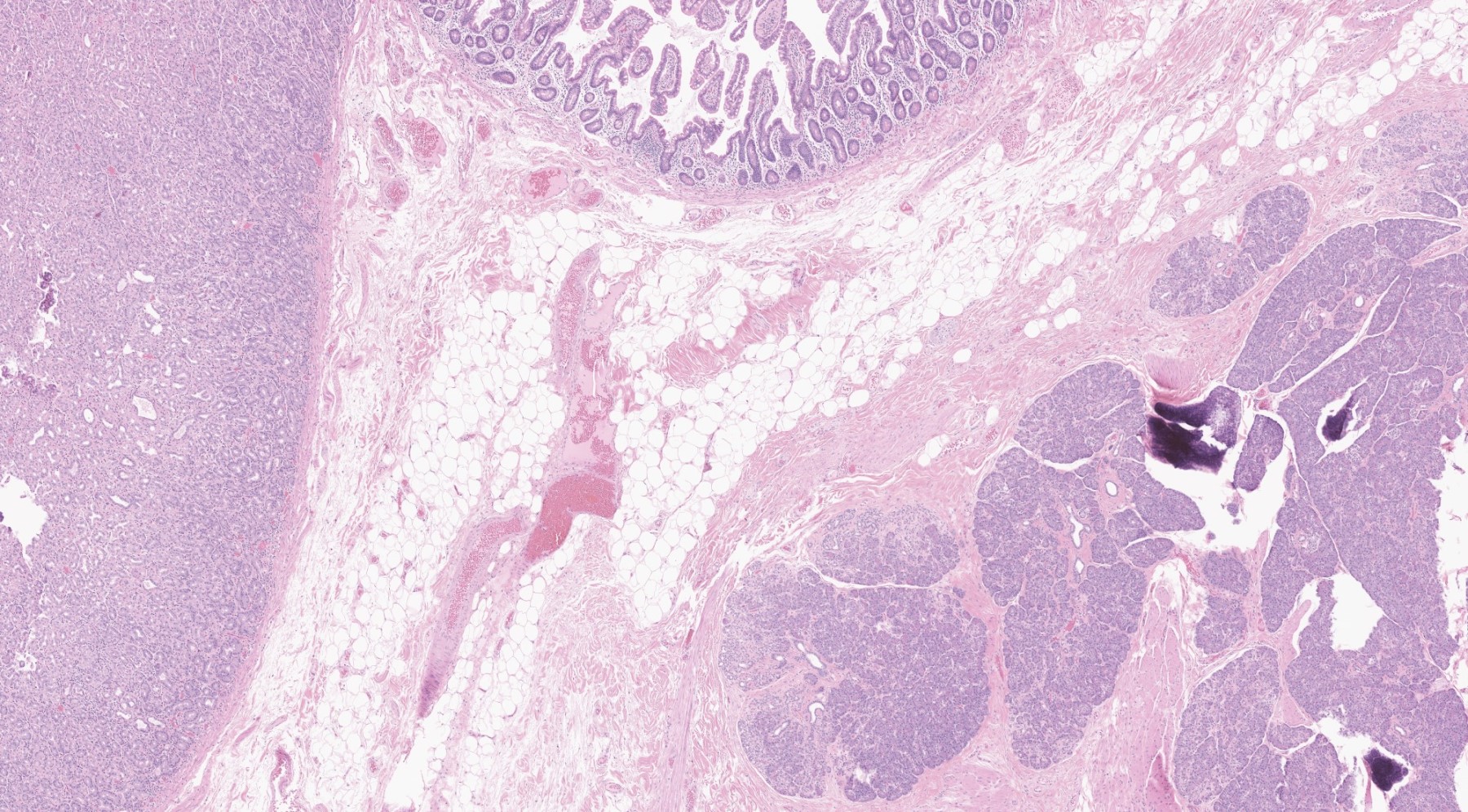
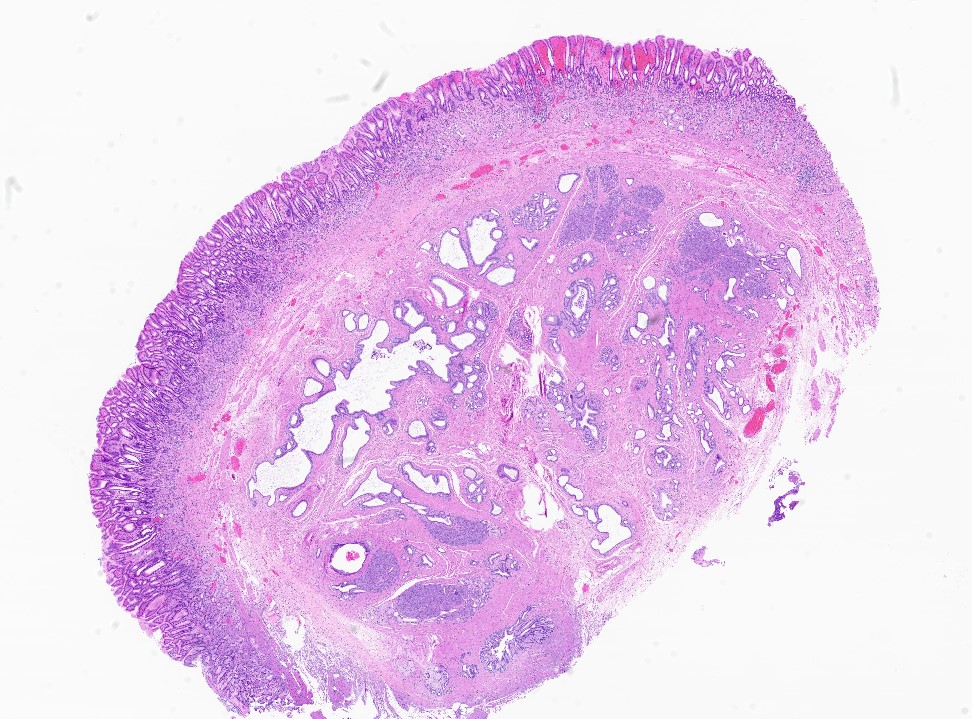
- Usually is a submucosal based lesion but may involve other layers of GI tract (Hum Pathol 2016;55:135)
- Well demarcated borders with vaguely lobular appearance (Hum Pathol 2016;55:135)
- On cut surface, typically appears pale yellowish white in color with solid and firm consistency (Hum Pathol 2016;55:135)
- Central mucosal dimple (opening of pancreatic ducts) may be visible
Gross images
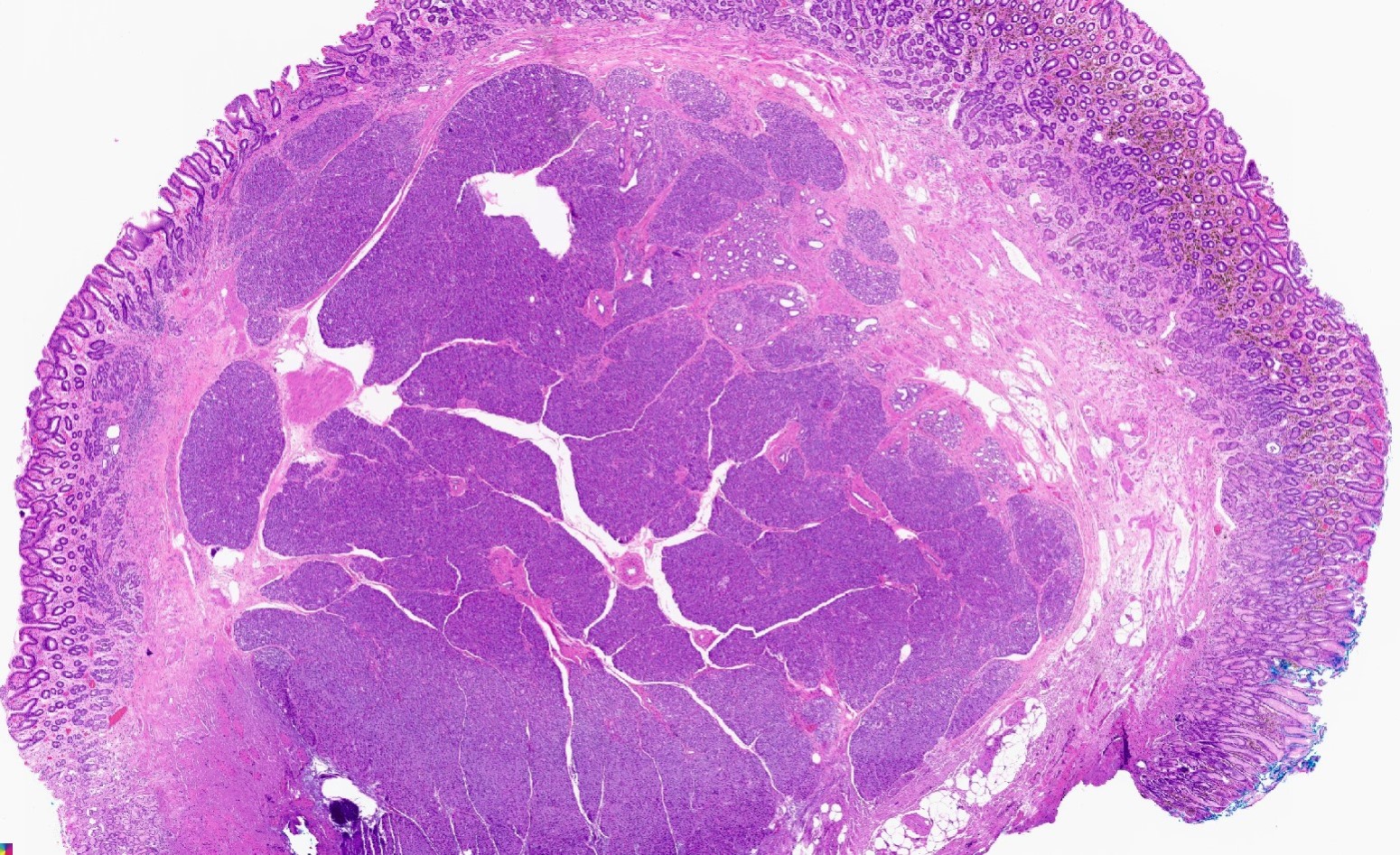
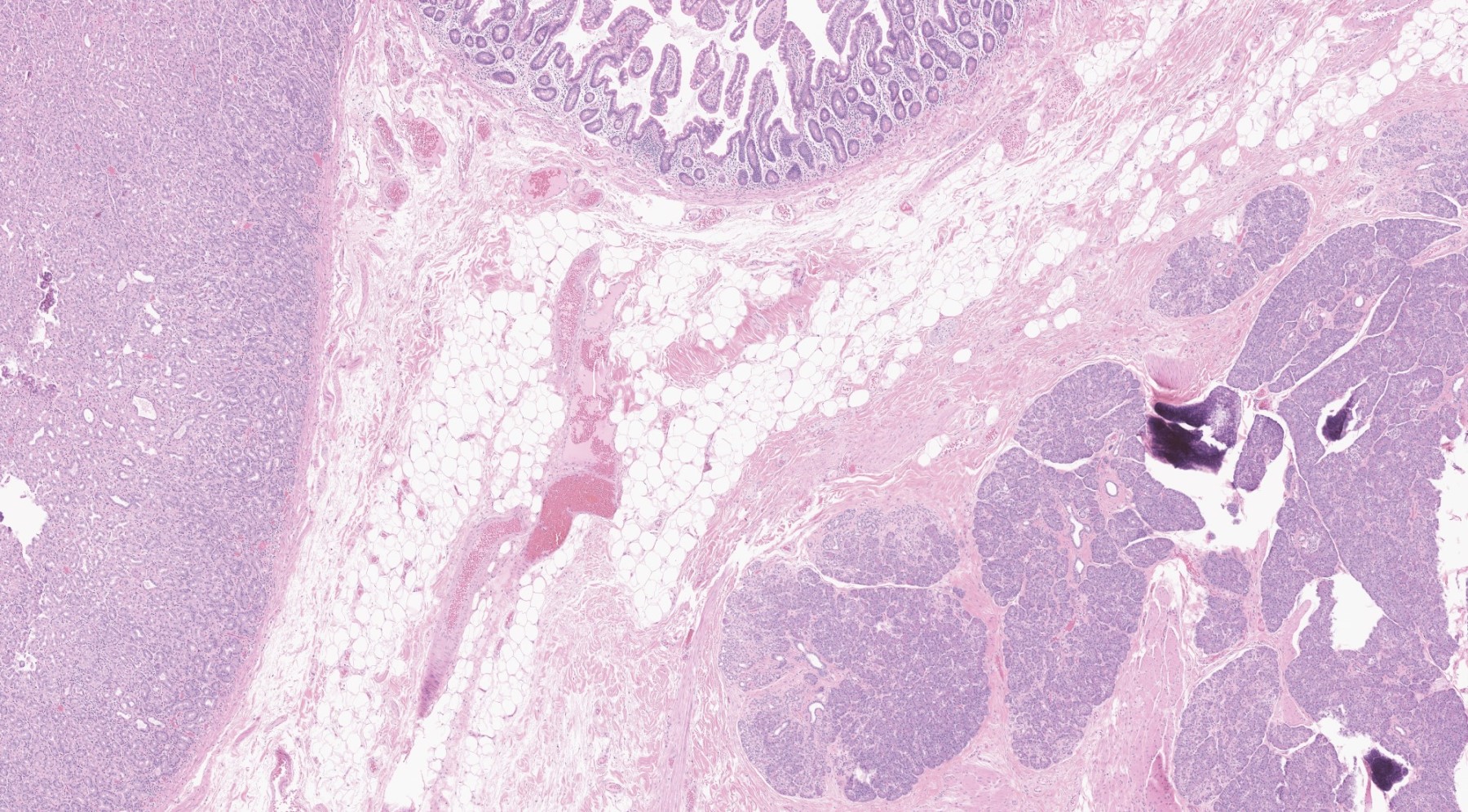
Microscopic (histologic) description
- In GI tract, typically centered in submucosa but may involve other layers
- Rarely may involve extragastrointestinal sites
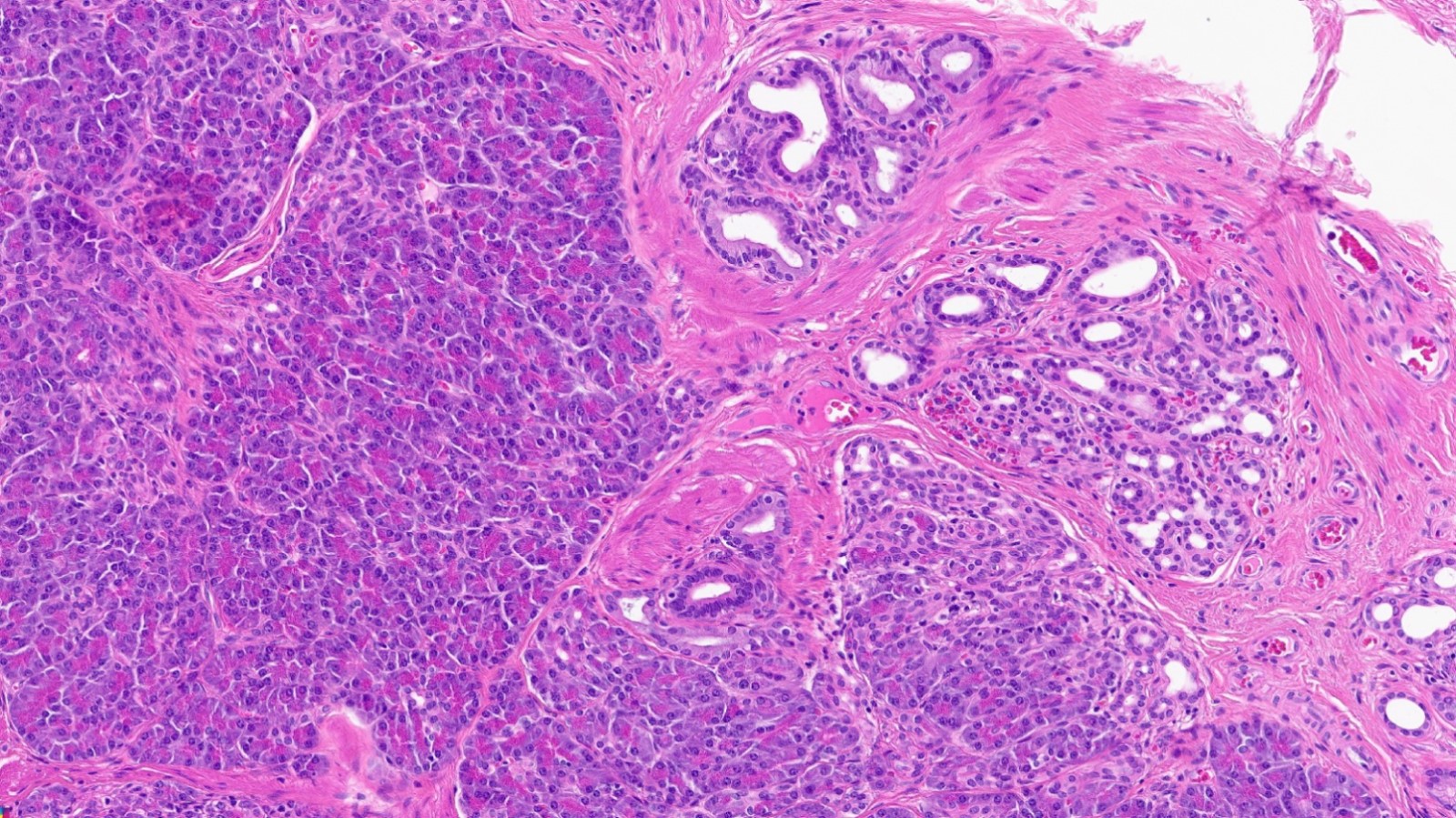
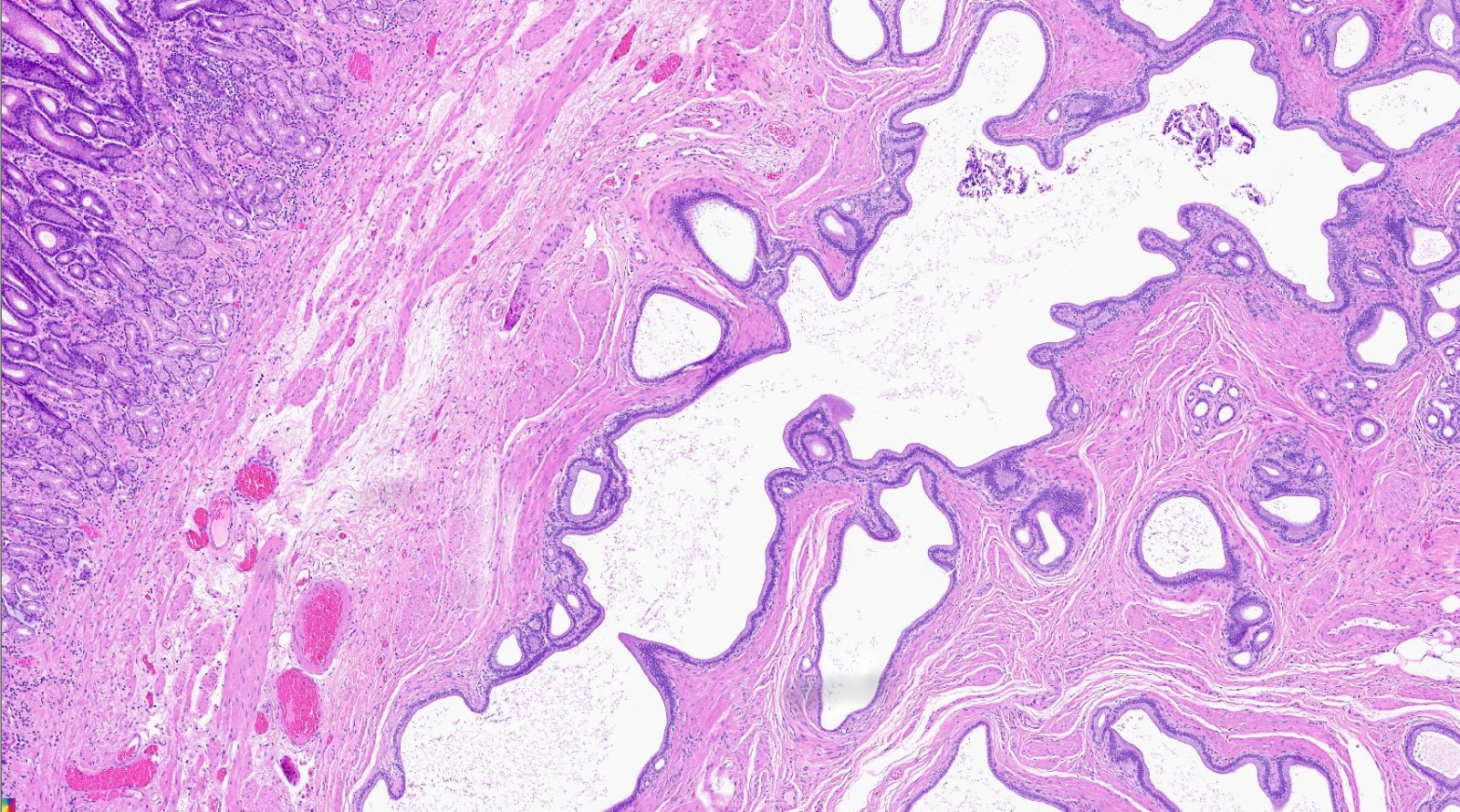
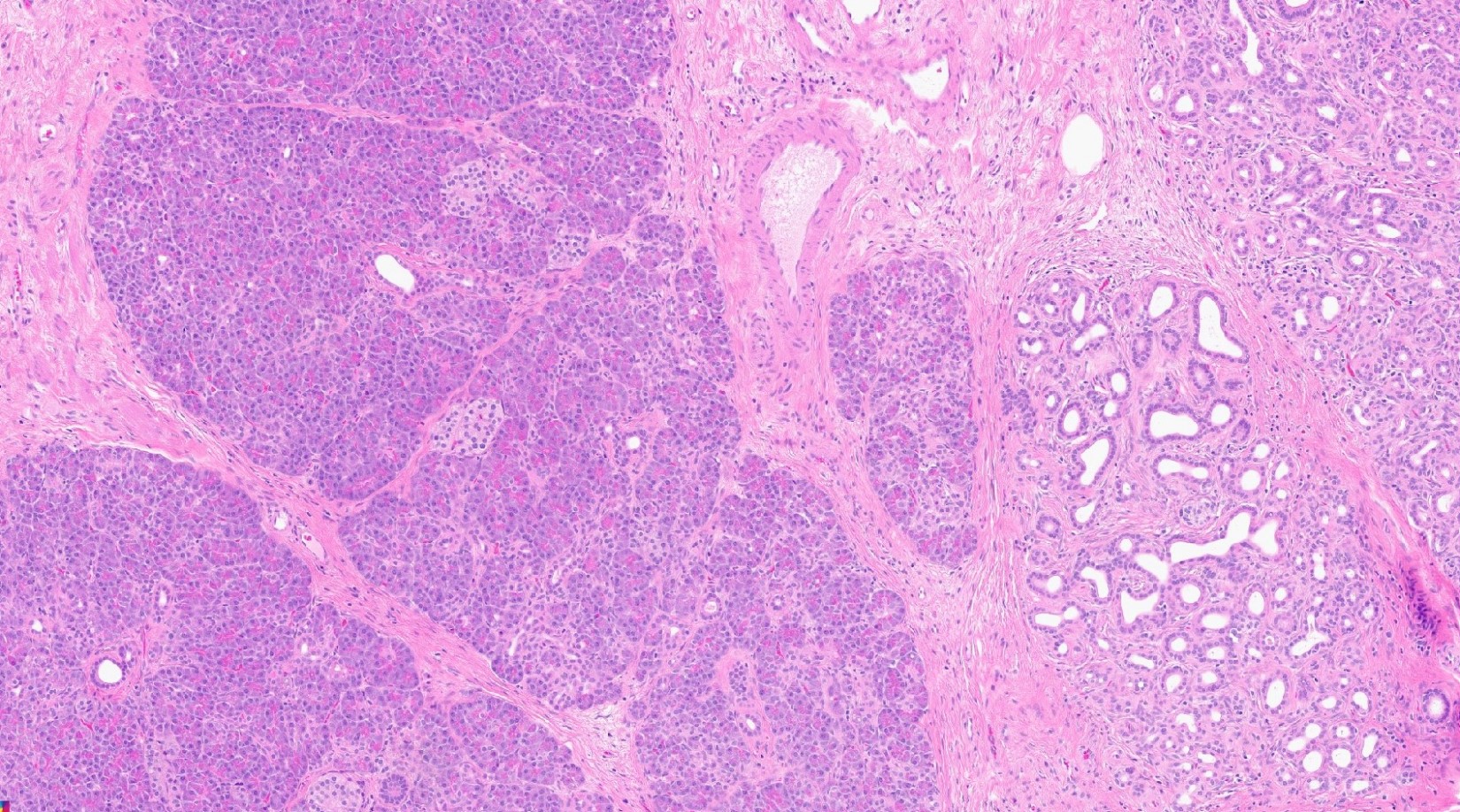
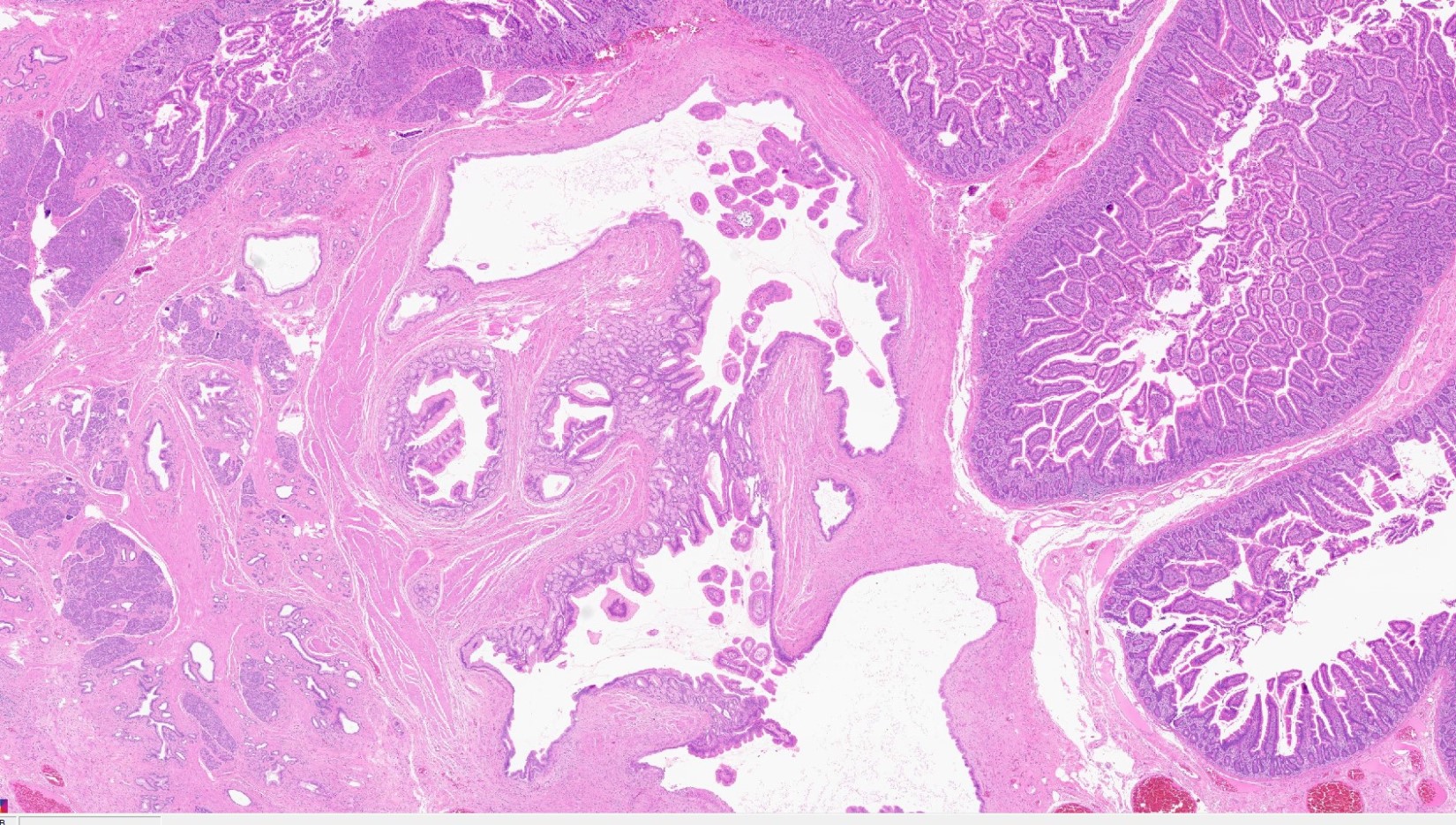
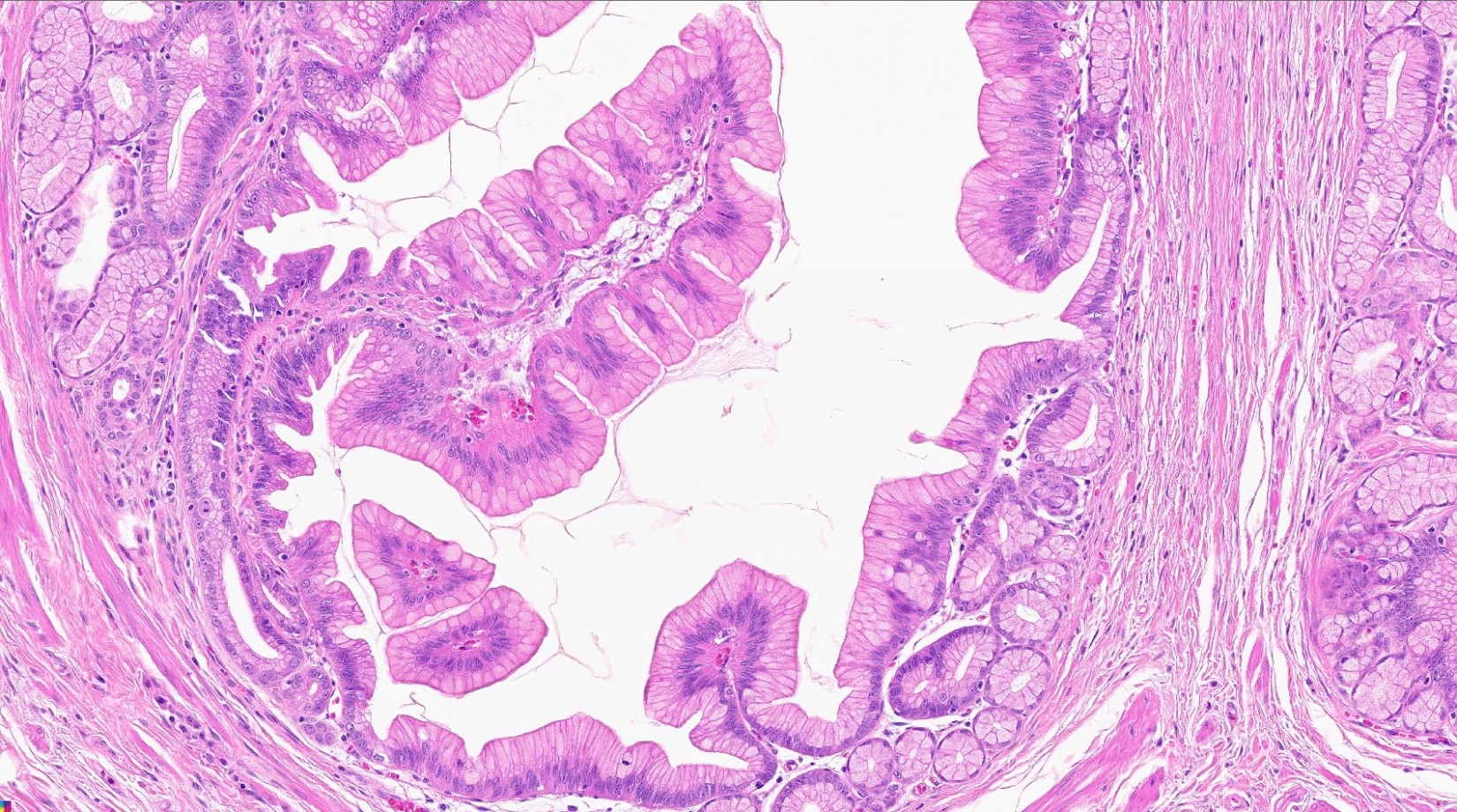
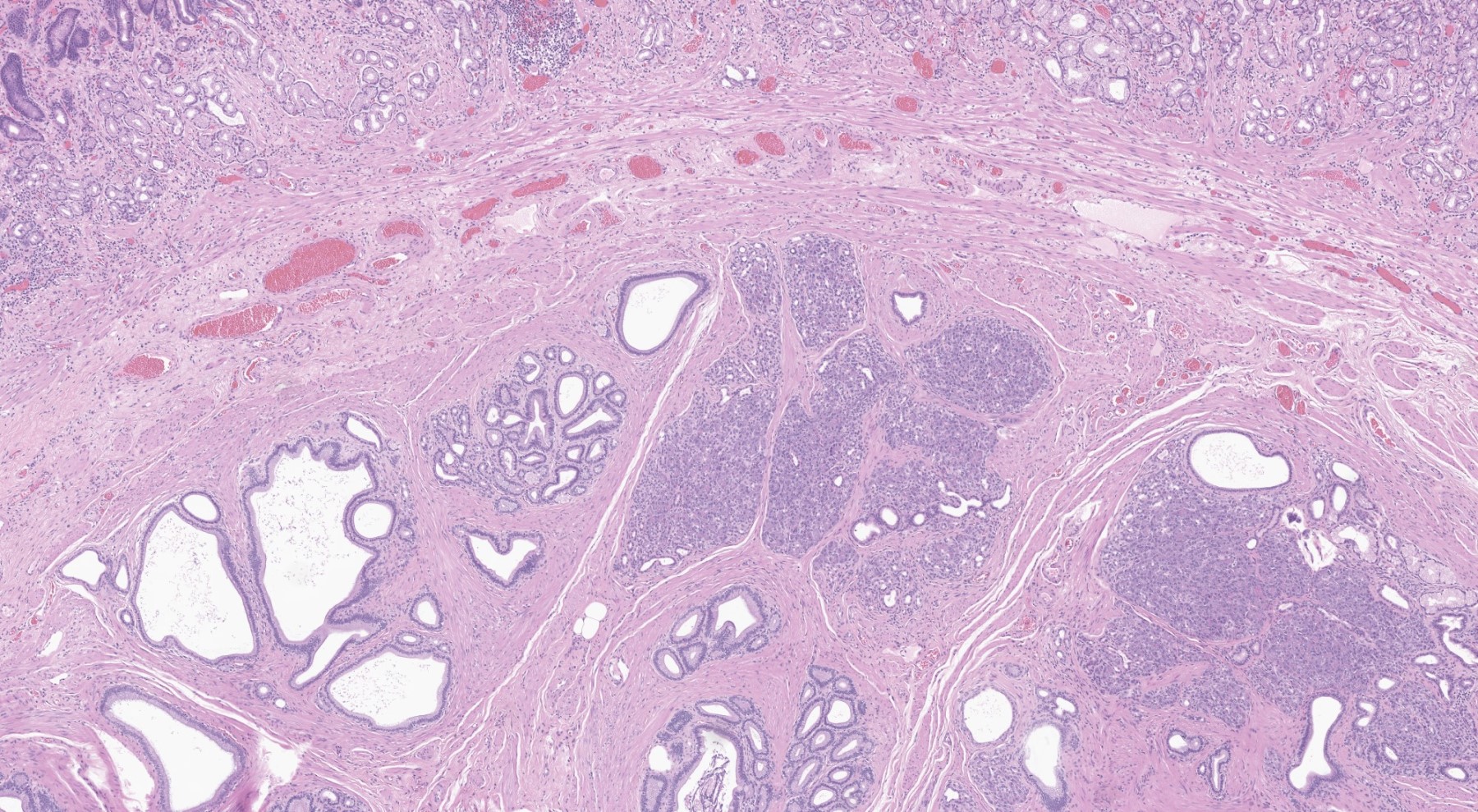
- Variable mixture of pancreatic acini, ducts, islets
- May contain hypertrophic smooth muscle bundles
- 4 types of pancreatic heterotopia, originally described by Heinrich in 1909 and modified in 1973 by Gasper-Fuentes (Radiographics 2017;37:484):
- Type 1:
- Contains all elements of normal pancreatic tissue (acini, ducts and islets)
- Type 2:
- Acini and ducts (no islets) in Heinrich classification
- Ducts only in Gasper-Fuentes classification
- Type 3:
- Ducts only in Heinrich classification
- Acini only in Gasper-Fuentes classification
- Type 4:
- Does not exist in Heinrich classification
- Islet cells only in Gasper-Fuentes classification (very rare) (Arch Pathol Lab Med 2002;126:464)
- Type 1:
- May show the same pathologic changes as the main gland, including acute or chronic pancreatitis, pseudocyst formation or neoplasia
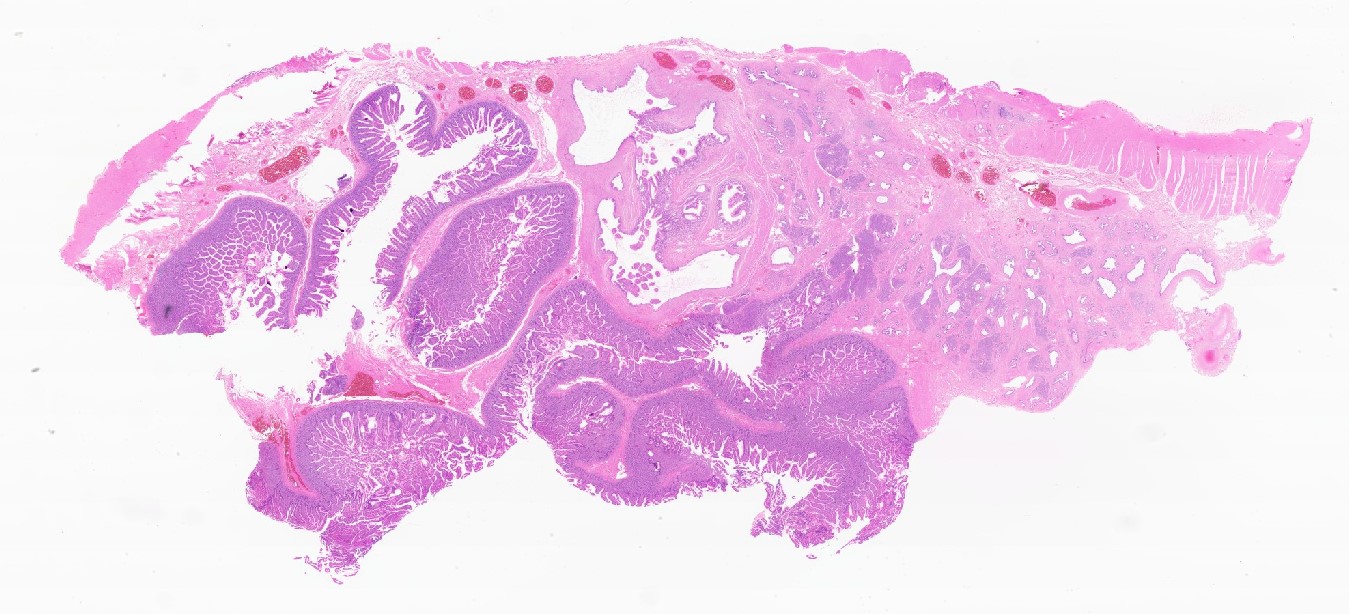
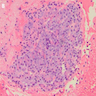
Microscopic (histologic) images
Contributed by Kenechukwu Ojukwu, M.D., M.P.P. and Danielle Hutchings, M.D.
Pancreatic heterotopia in the stomach
Cytology description
- Predominantly pancreatic acini: polygonal cells with abundant granular cytoplasm and eccentric nuclei
- Ductal structures: cuboidal to columnar cells, honeycomb sheets
- Islet cells usually not seen
- Reference: World J Gastroenterol 2015;21:2367
Cytology images
Positive stains
- Heterotopic pancreas shows staining similar to the native gland
- Acinar cells: positive for trypsin, chymotrypsin, PAS and BCL10 (Virchows Arch 2009;454:133)
- Ductal epithelial cells: positive for CK7, CK8 / CK18
- Islet cells: positive for synaptophysin, chromogranin A and INSM1 (Am J Clin Pathol 2015;144:579)
Sample pathology report
- Gastric antrum, endoscopic mucosal resection:
- Pancreatic heterotopia involving submucosa and focally mucosa
- Overlying unremarkable antral mucosa
Differential diagnosis
- Pancreatic metaplasia:
- Involves mucosa
- Acinar cells only, no ducts or islet cells present
- Adenocarcinoma:
- Duct predominant heterotopia may show branching pattern and be mistaken at low power for adenocarcinoma
- Adenocarcinoma shows infiltrative growth, irregular glands, desmoplasia and cytologic atypia
Additional references
Board review style question #1
Board review style answer #1
C. Meckel diverticulum with pancreatic and gastric heterotopia
Comment Here
Reference: Heterotopic pancreas
Comment Here
Reference: Heterotopic pancreas
Board review style question #2
A 55 year old man presents with persistent anemia, abdominal pain and intermittent melena. Endoscopy reveals a subepithelial gastric antral mass with overlying umbilicated mucosa. Endoscopic mucosal resection shows the images above. What is the most likely diagnosis?
- Gastric adenocarcinoma
- Heterotopic pancreas
- Metastatic adenocarcinoma
- Pancreatic metaplasia
Board review style answer #2