Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Sagan OA, Huber AR. Autoimmune pancreatitis type 1. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/pancreasaiptype1.html. Accessed April 2nd, 2025.
Definition / general
- 1 of 2 types of autoimmune pancreatitis
- Pancreatic manifestation of IgG4 related disease (IgG4 RD)
Essential features
- Autoimmune pancreatitis is the pancreatic manifestation of IgG4 RD so patients typically have increased IgG4 on serology and other organ involvement by the disease
- Diagnosis can be difficult due to rarity, vague presentation and being a mimic of pancreatic ductal adenocarcinoma
- International Consensus Diagnostic Criteria (ICDC) for autoimmune pancreatitis has set a standard diagnostic approach and criteria for diagnosis, which includes imaging, serology and histology
- Characteristic histologic findings are periductal lymphoplasmacytic infiltrate without granulocytic infiltration, obliterative phlebitis, storiform fibrosis and abundant IgG4 positive plasma cells
Terminology
- Autoimmune pancreatitis (AIP) type 1 or IgG4 related pancreatitis (IgG4 RP)
- Historically termed lymphoplasmacytic sclerosing pancreatitis (LPSP) or AIP without granulocyte epithelial lesions (GELs) (Pancreas 2011;40:352)
Epidemiology
- Patients are generally male and older than 50 years old (Pathologica 2020;112:197, World J Gastroenterol 2022;28:6867)
- Increased prevalence in Asia (Adv Med Sci 2020;65:403)
- Rare; prevalence is < 1% (World J Gastroenterol 2022;28:6867)
- Incidence has been increasing due to better description and recognition of the disease but overall numbers are most likely still underestimated due to the complexity of the disease (World J Gastroenterol 2022;28:6867)
Sites
- Pancreatic manifestation of IgG4 RD
- Extrapancreatic organ sites can be affected, including salivary and lacrimal glands, retroperitoneum, biliary tree, prostate and kidneys (Pathologica 2020;112:197, World J Gastroenterol 2022;28:6867, Adv Med Sci 2020;65:403)
- Other organ involvement is defined as the manifestation of IgG4 related systemic disease and may be diagnosed through imaging, clinical exam or histology (Pancreas 2011;40:352)
Pathophysiology
- Currently unknown
- Still unclear whether the IgG4 antibodies are a reflection of overexpression caused by an unknown inflammatory stimulus or whether they are self reactive autoantibodies
- Has been linked to an increase in interferon type I (IFN I) produced by plasmacytoid dendritic cells but this is nonspecific to autoimmune pancreatitis
- H. pylori infection may be the trigger mechanism for an autoimmune response against pancreatic acini via molecular mimicry but this hypothesis is not supported at this time
- Reference: Adv Med Sci 2020;65:403
Etiology
- Multifactorial, including genetic, environmental and immunological factors
- HLA region genes, HLA DRB1 and ABCF1, are associated with disease susceptibility
- Repeated exposure to certain antigens, including mineral oils, solvents and industrial or metal dusts, may contribute
- Reference: Adv Med Sci 2020;65:403
Diagrams / tables
Clinical features
- Patients most commonly present with obstructive jaundice (Pathologica 2020;112:197, World J Gastroenterol 2022;28:6867)
- Patients can also present with a pancreatic mass, weight loss or abdominal pain / discomfort but do not present with acute pancreatitis symptoms (Adv Med Sci 2020;65:403)
- Diabetes commonly occurs with autoimmune pancreatitis (World J Gastroenterol 2022;28:6867)
- Other manifestations and symptoms of IgG4 RD can be present, including enlargement of the lacrimal and salivary glands as well as retroperitoneal fibrosis and inflammation of the biliary tree (Adv Med Sci 2020;65:403)
Diagnosis
- ICDC for autoimmune pancreatitis was published in 2011, which uses the following combination of categories for diagnosis (Pancreas 2011;40:352)
- Imaging
- Serology
- Other organ involvement (OOI)
- Histology
- Response to steroid therapy
- Can be diagnosed without histology (Pancreas 2011;40:352)
- Other diagnostic criteria can be used, including the HISORt criteria published by Mayo Clinic; however, the ICDC is the most widely used (Adv Med Sci 2020;65:403)
- See Diagrams / tables
Laboratory
- IgG4 > 2 times the upper limit of normal on serology (Pancreas 2011;40:352)
- Serum IgG4 elevation is the single best marker of autoimmune pancreatitis (Pancreas 2011;40:352)
- Elevated IgG4 can also be seen in pancreatic carcinoma patients (10%) and so elevation is not sufficient to make a diagnosis unless seen in the setting of typical imaging or histologic findings (Adv Med Sci 2020;65:403, Pancreas 2011;40:352)
- Positive autoimmune markers are common, including antinuclear antibodies (ANA) (40%), anticarbonic anhydrase II antibodies (55%), antipancreatic secretory trypsin inhibitor (PSTI) antibodies (55%) and antilactoferrin antibodies (75%) (Adv Med Sci 2020;65:403)
- May have elevated CA 19-9, which can raise suspicion of malignancy (Pathologica 2020;112:197)
Radiology description
- Parenchymal imaging
- Typical: diffuse enlargement with delayed enhancement (sometimes associated with rim-like enhancement)
- Indeterminate (including atypical): segmental / focal enlargement with delayed enhancement
- Ductal imaging (endoscopic retrograde cholangiopancreatography [ERCP])
- Level 1: long (> one - third length of the main pancreatic duct) or multiple strictures without marked upstream dilatation
- Level 2: segmental / focal narrowing without marked upstream dilatation (duct size < 5 mm)
- Diffuse / non-mass forming autoimmune pancreatitis
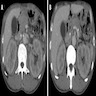
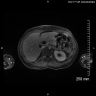
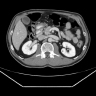
- Diffuse pancreas enlargement ("sausage" pancreas), often with low density edge (halo ring sign or capsule sign) on computed tomography (CT) (World J Clin Cases 2022;10:12458)
- Contrast can highlight the capsule and shows mild enhancement during the venous or delayed phase (World J Clin Cases 2022;10:12458)
- Mass forming autoimmune pancreatitis
- Often shows a low density or isodense mass in the head of the pancreas on CT (World J Clin Cases 2022;10:12458)
- Contrast enhanced CT shows slight enhancement of the lesion in the arterial phase and obvious uniformity and delayed enhancement in the venous phase (World J Clin Cases 2022;10:12458)
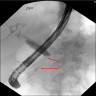
- Ducts
- Smooth stenosis at the lower end of the common bile duct can be shown in autoimmune pancreatitis patients with obstructive jaundice (World J Clin Cases 2022;10:12458)
- Pancreatic duct is usually irregularly narrow and interrupted and often exceeds one - third of the total length of the main pancreatic duct or appears as a jumping stenosis (World J Clin Cases 2022;10:12458)
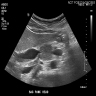
- Contrast enhanced CT and magnetic resonance imaging (MRI) show similar results; ultrasound can be variable in diagnostic quality (Adv Med Sci 2020;65:403)
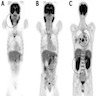
- Other organ involvement can also be seen on imaging and help support the diagnosis of autoimmune pancreatitis
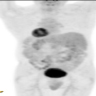
Radiology images
Prognostic factors
- Patients commonly relapse (40%) despite remarkable responses to treatment (Pathologica 2020;112:197, Adv Med Sci 2020;65:403)
Case reports
- 49 year old woman with weight loss, abdominal pain and jaundice (Cureus 2023;15:e47471)
- 54 year old man with weight loss and abdominal pain status post-COVID vaccine (ACG Case Rep J 2023;10:e00950)
- 55 year old man with weight loss, jaundice and pruritus (Cureus 2023;15:e45970)
- 64 year old Japanese woman with new onset bleeding due to acquired hemophilia A with known longstanding disease (Int J Hematol 2018;108:335)
Treatment
- Steroids are the main treatment strategy
- Steroid trial of prednisone 0.6 - 1 mg/kg for 2 weeks with improvement on repeat imaging and serology can be confirmatory in the appropriate context (Pancreas 2011;40:352)
- Steroid trial should not be done in isolation for diagnosis of autoimmune pancreatitis (Pancreas 2011;40:352)
- Immunosuppressants or rituximab can be started if corticosteroids are not effective or cannot be tolerated
- Patients with asymptomatic disease should be treated to prevent the progression of fibrotic changes and decrease the risk of developing pancreatic insufficiency or relapse
- Follow up should consist of a combination of methods (symptoms, serology, imaging) and not IgG4 levels alone
- Endoscopic procedures may be needed in cases of insufficient drug effect or obstructive jaundice
- Surgery should be reserved for cases where malignancy cannot be excluded, extensive fibrosis is present or endoscopic biliary drainage cannot be performed
- Reference: Adv Med Sci 2020;65:403
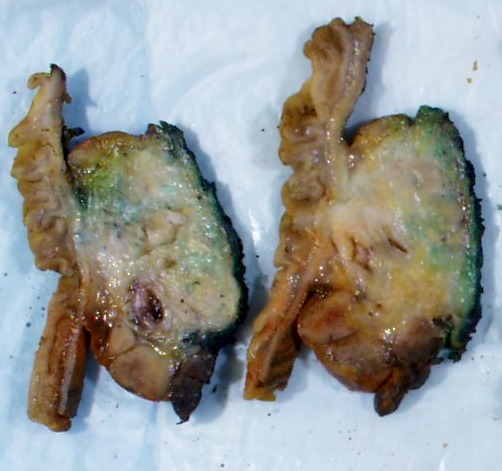
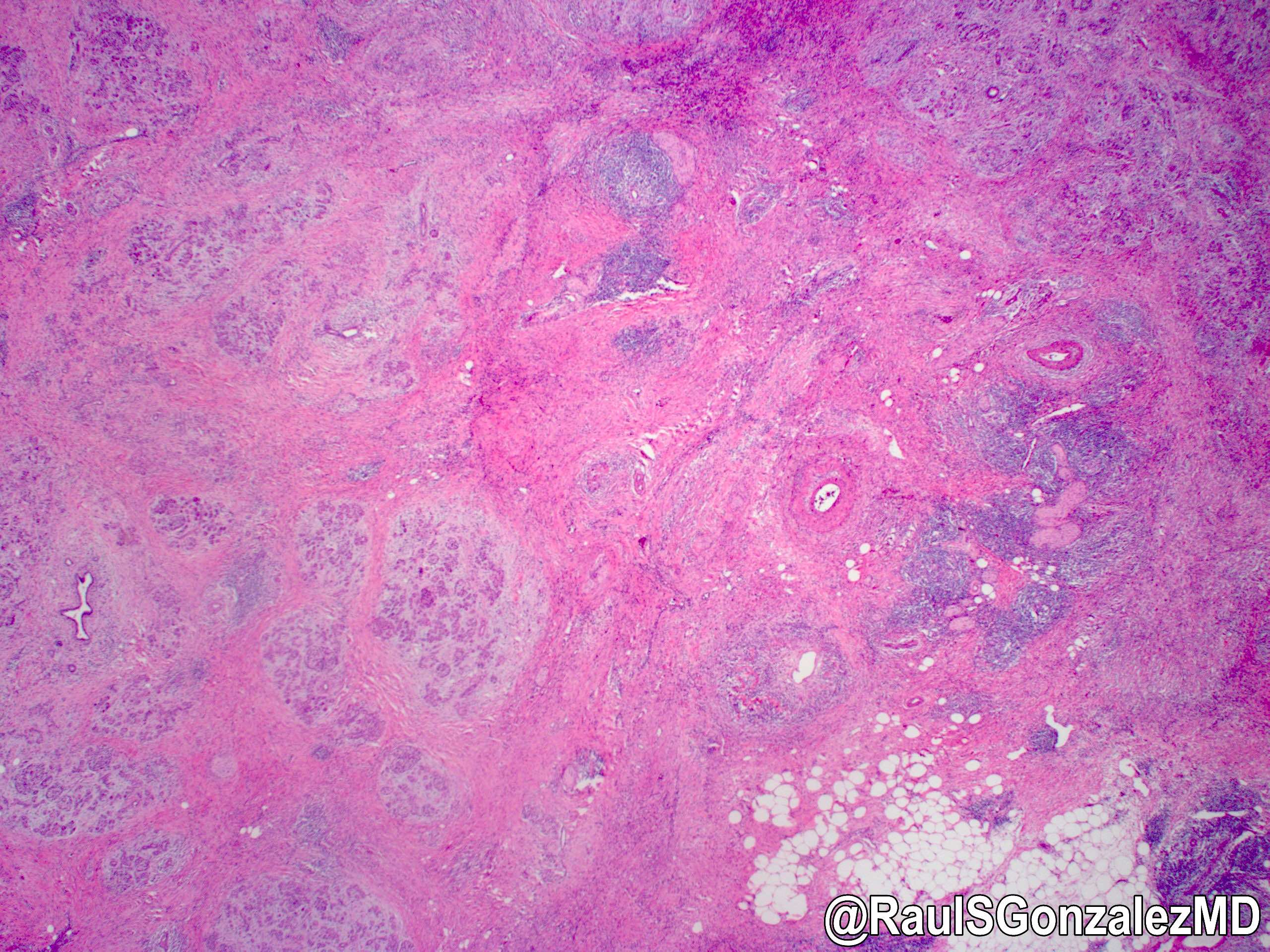
Gross description
- Disease is localized mainly in the pancreatic head, less frequently in the body / tail
- Diffuse enlargement or "sausage" pancreas may be observed without a discrete mass
- Segmental stenosis of the main duct may be seen
- References: Pathologica 2020;112:197, Abdom Radiol (NY) 2016;41:1003, World J Gastroenterol 2022;28:6867
Gross images
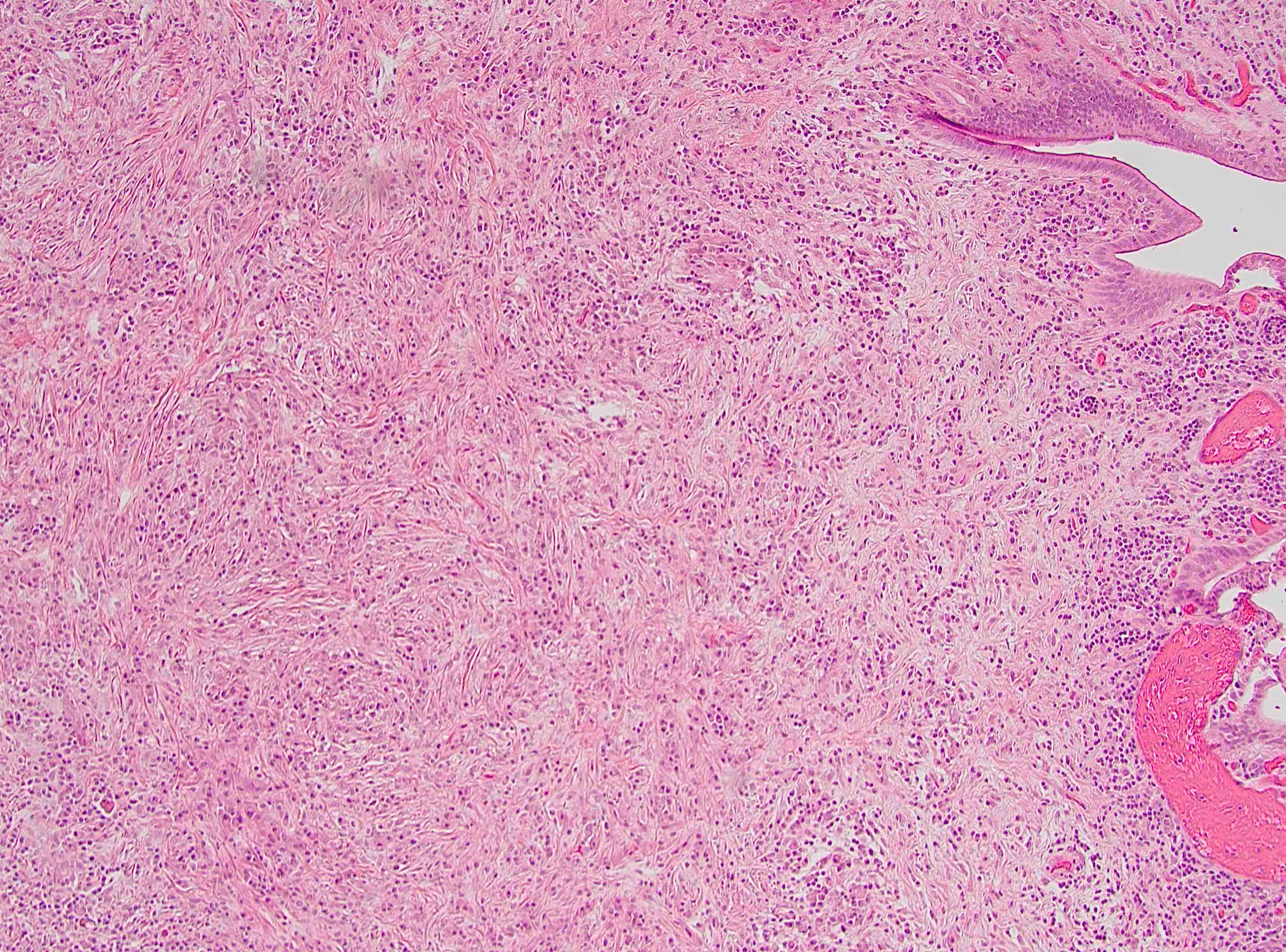
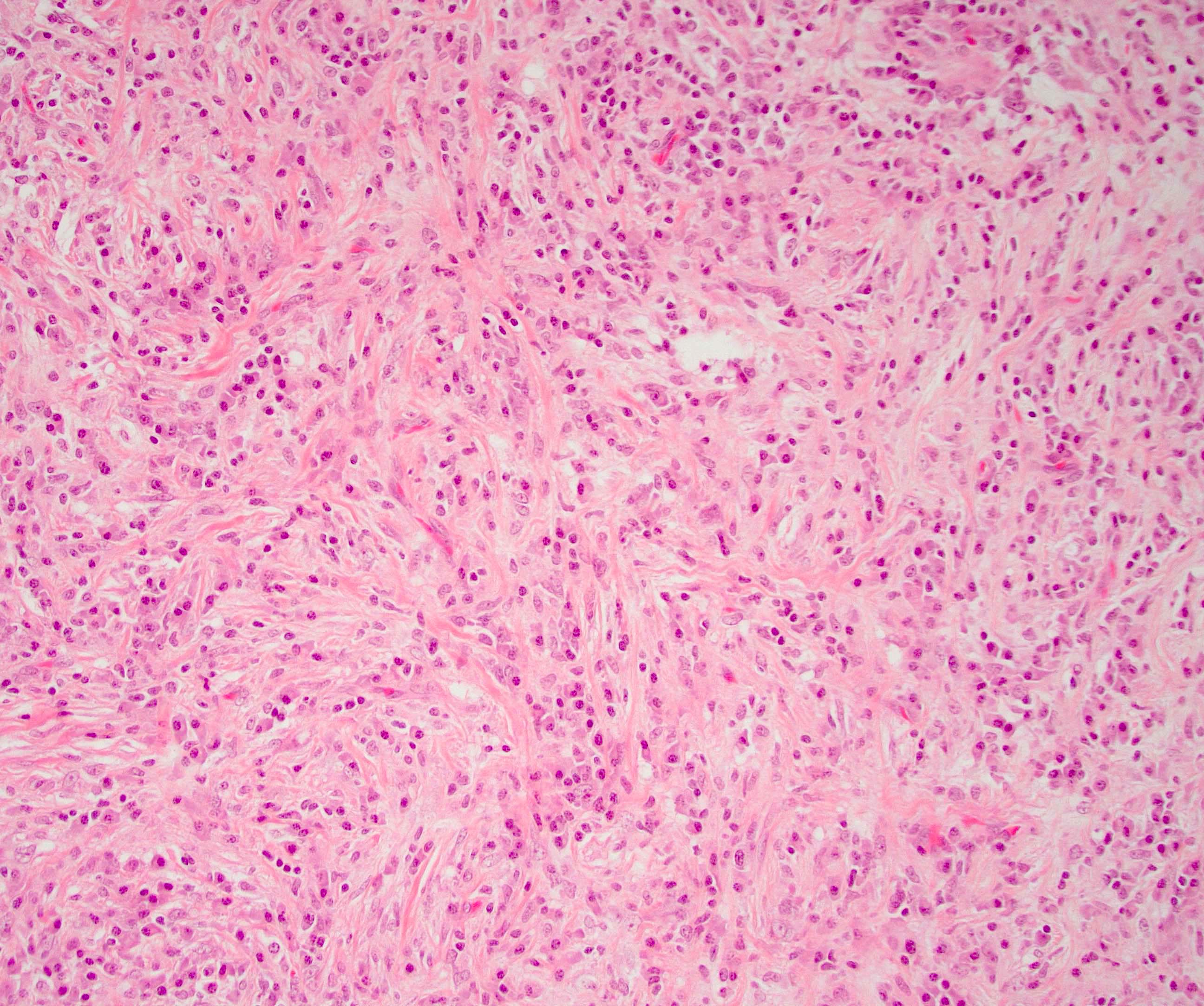
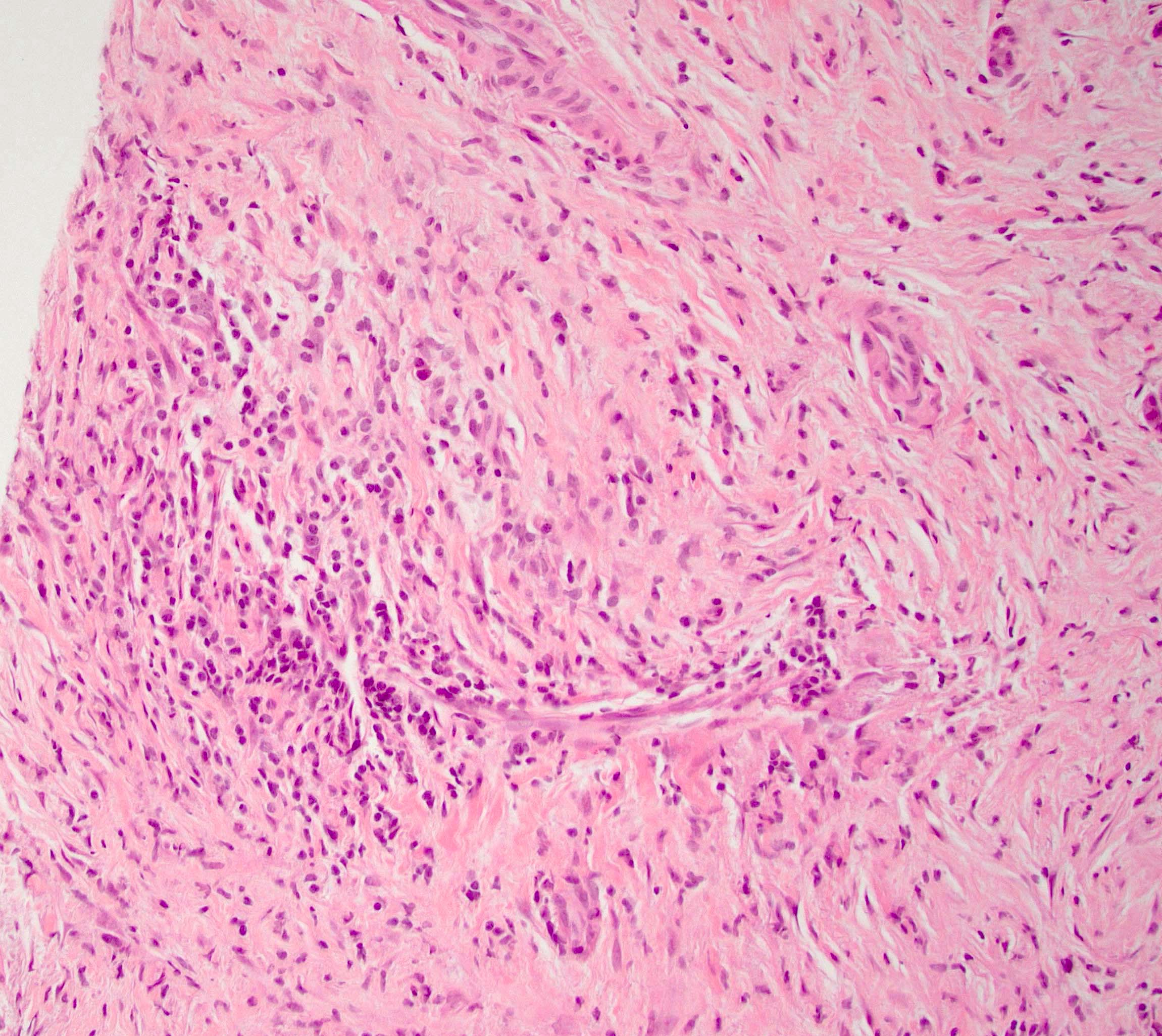
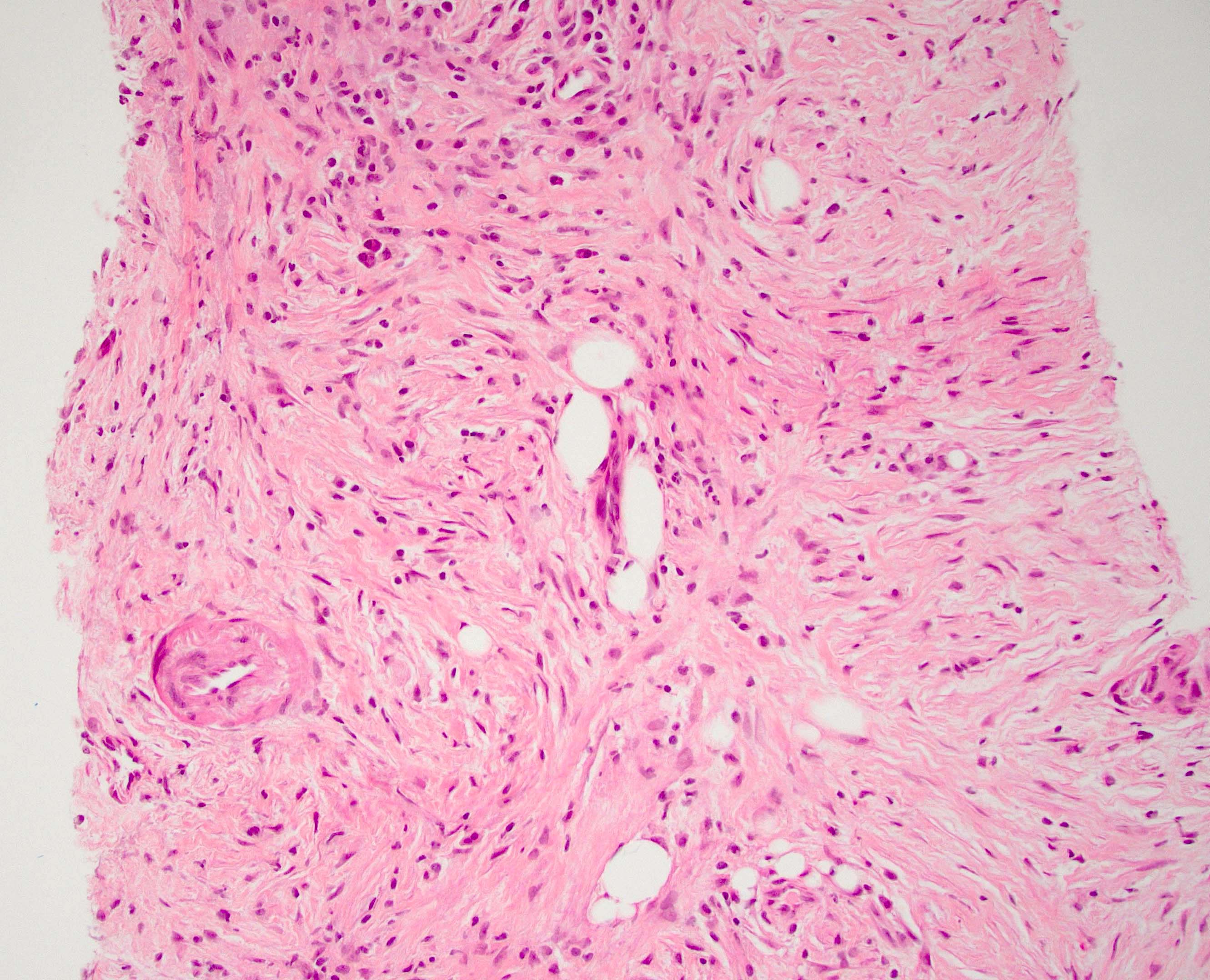
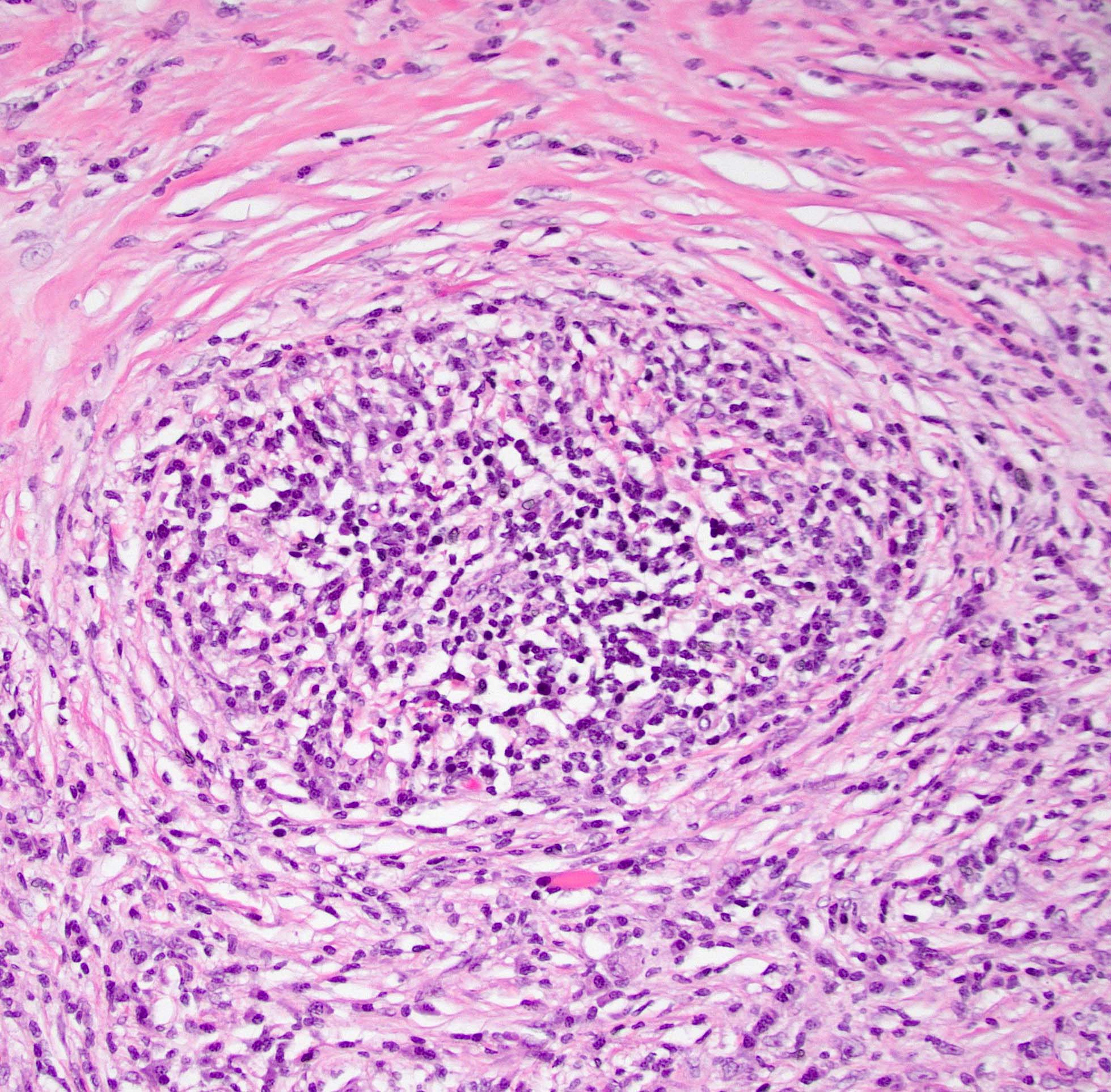
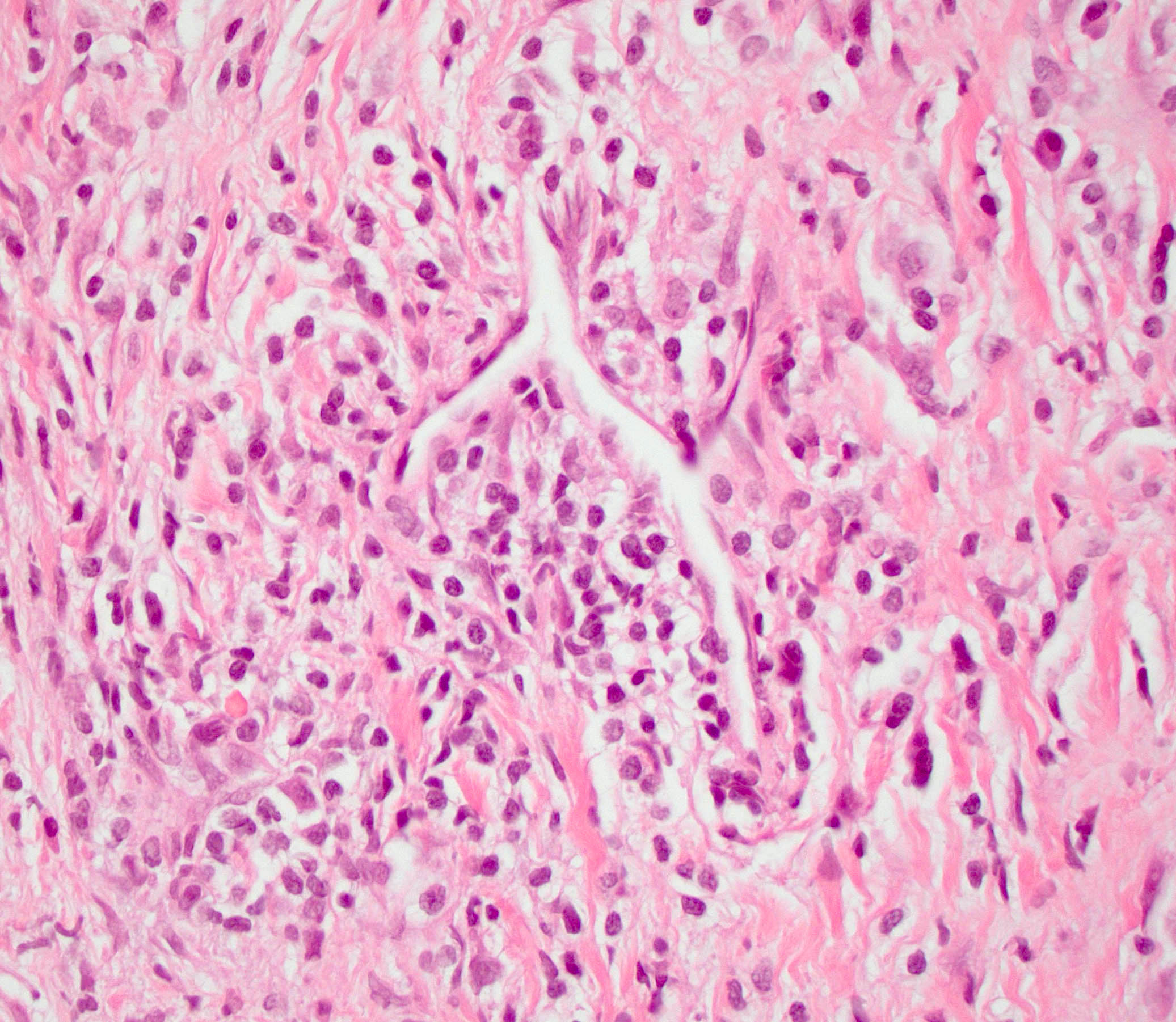
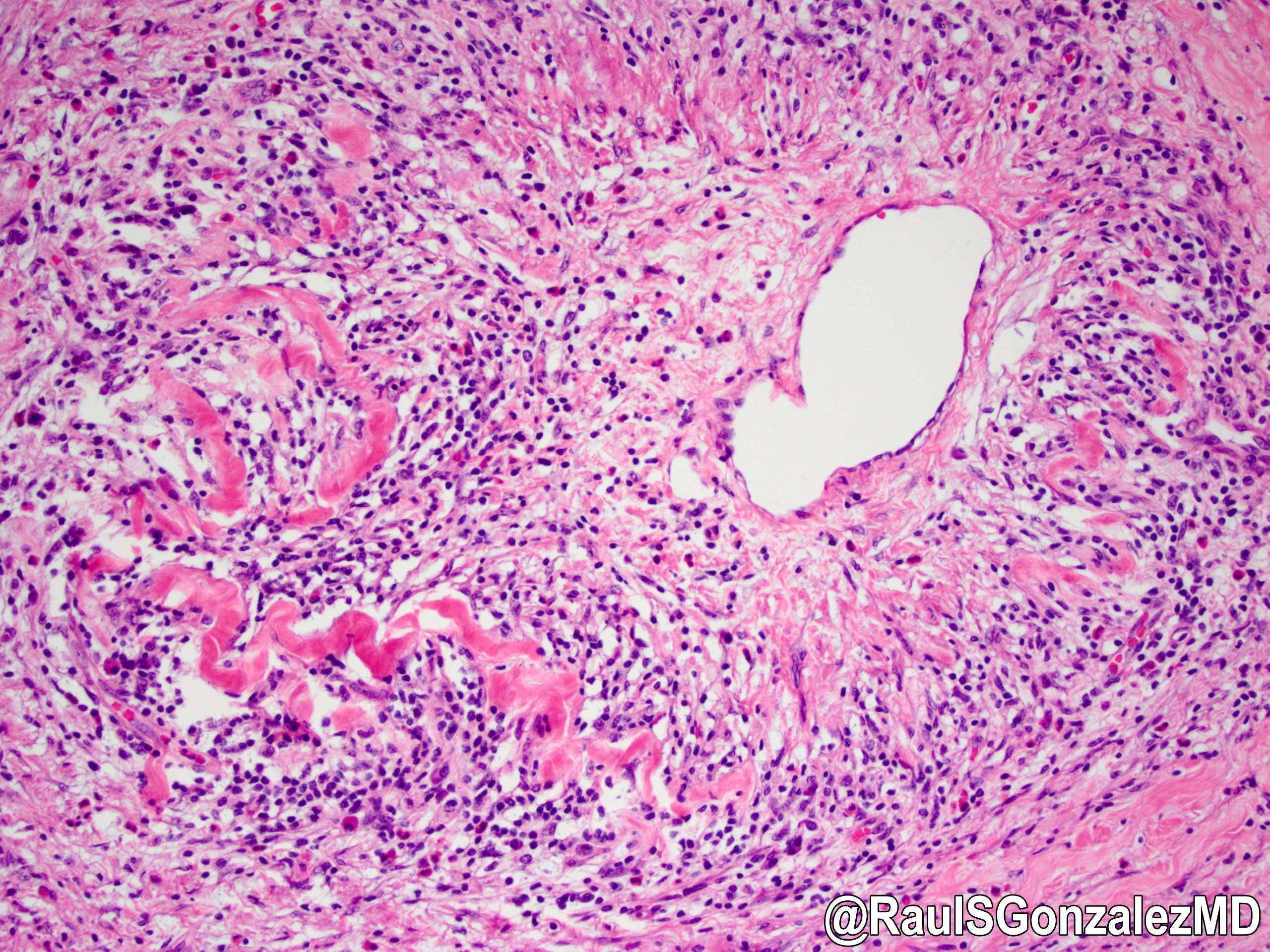
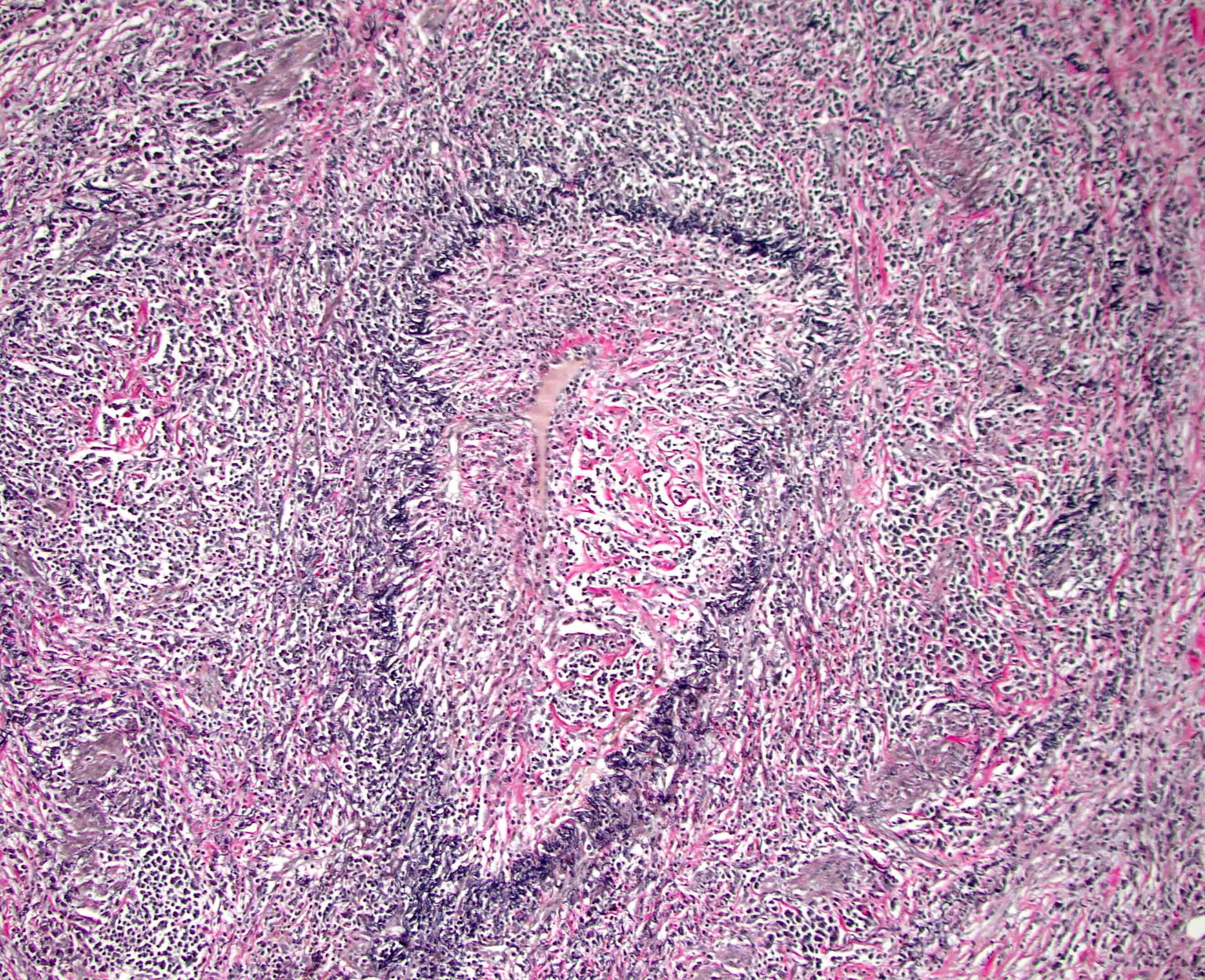
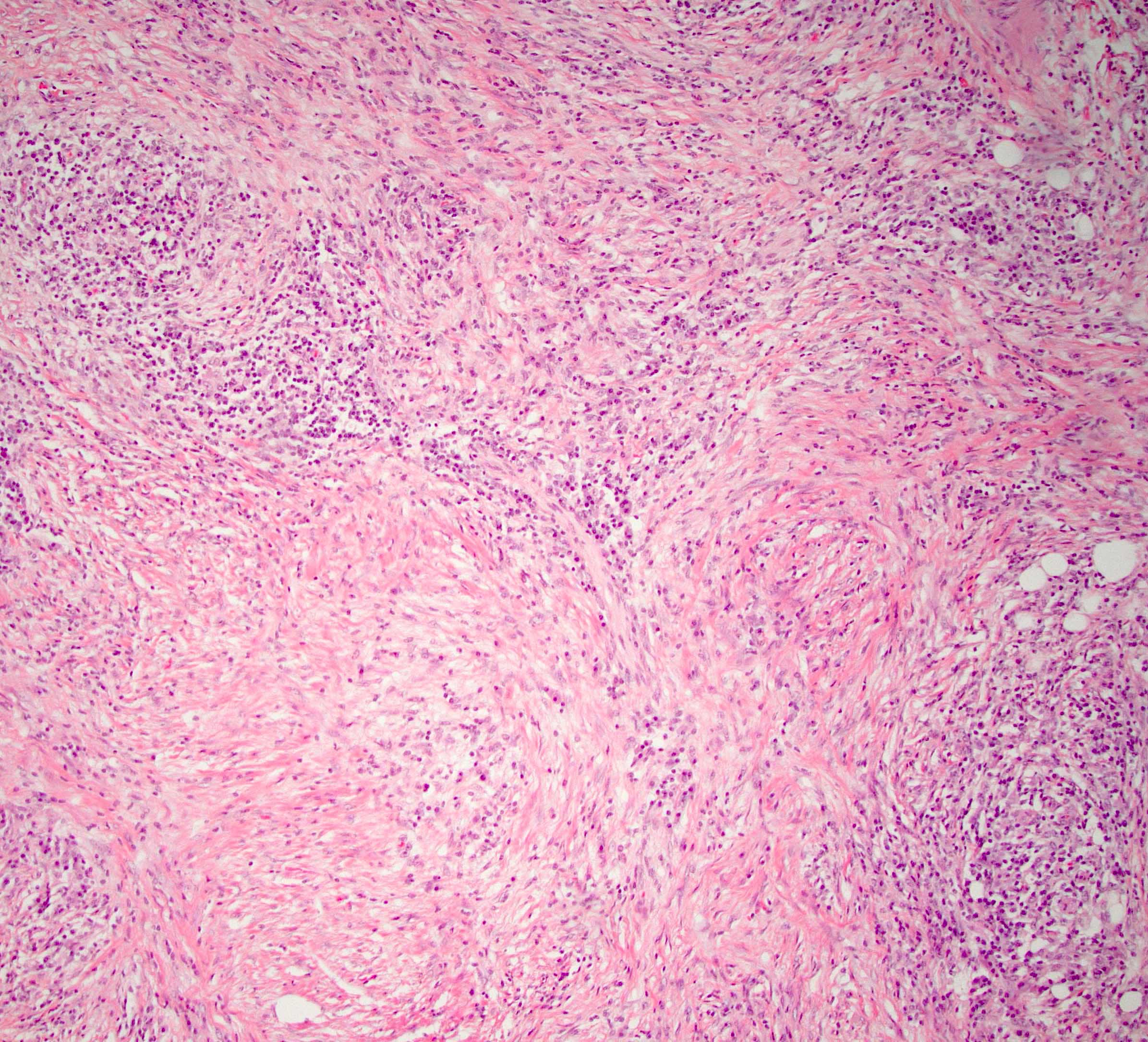
Microscopic (histologic) description
- Characteristic findings (at least 3 for level 1 criteria) (Pancreas 2011;40:352)
- Periductal lymphoplasmacytic infiltrate without granulocytic infiltration
- Obliterative phlebitis
- Storiform fibrosis
- Abundant (> 10 cells/high power field) IgG4 positive plasma cells
- Biopsy showing some but not all of the above features can be used as supportive evidence for the diagnosis of autoimmune pancreatitis (Pancreas 2011;40:352)
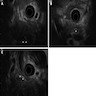
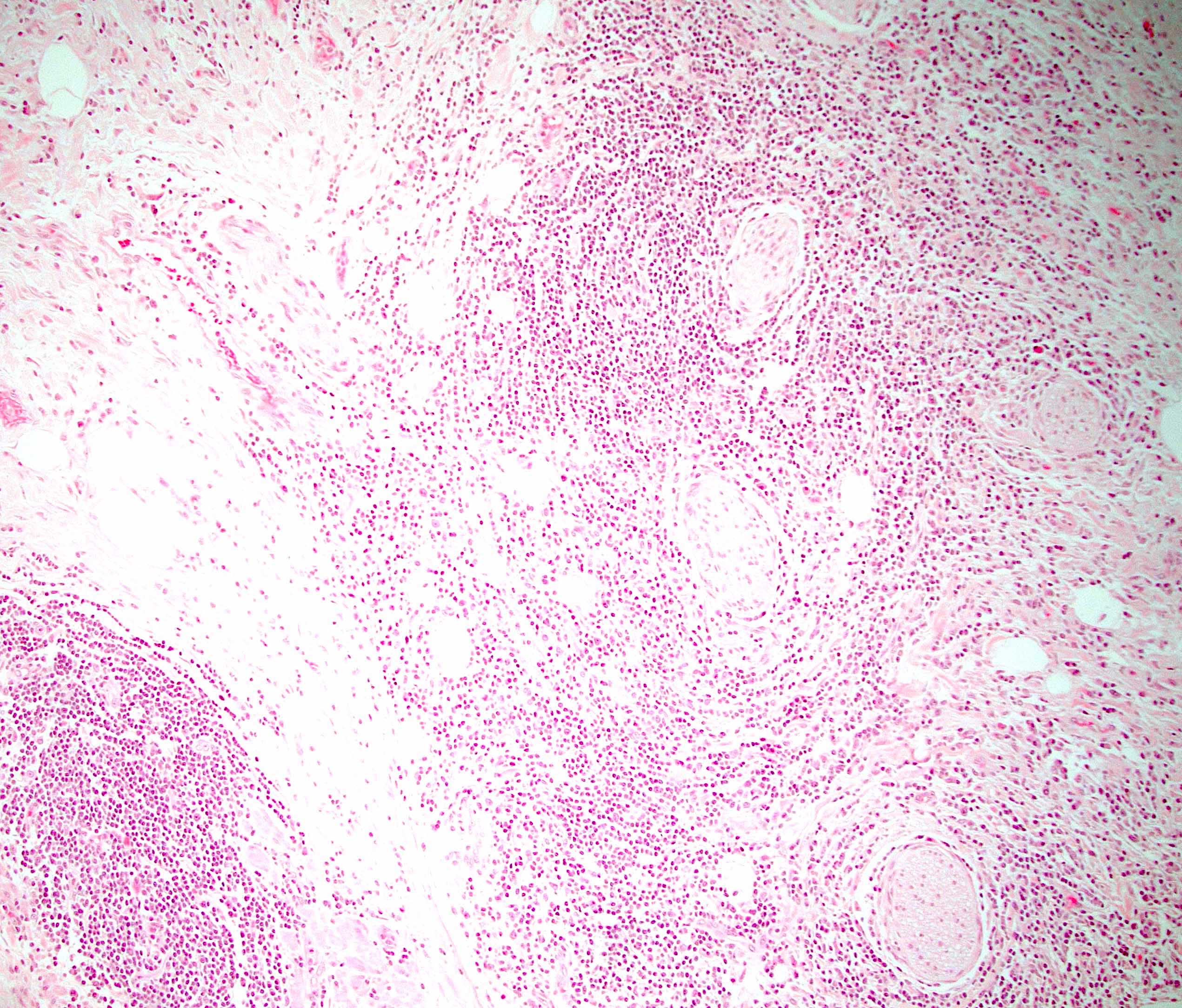
- Inflammation is localized within the pancreatic parenchyma and is centered around / within medium to large interlobular ducts, which causes shrinkage of the ductal lumen (Pathologica 2020;112:197)
- Inflammation can also be seen between the pancreatic parenchyma and peripancreatic adipose tissue (Pathologica 2020;112:197)
- Inflammation of the venous wall can progress to obliterative phlebitis with fibrosis of the lumen (Pathologica 2020;112:197)
- As the inflammation progresses, fibrosis becomes more diffuse, assuming a whorled or storiform pattern (Pathologica 2020;112:197)
- Perineural inflammation can also be present (Pathologica 2020;112:197)
- Involvement of the pancreatic neck margin or biliary resection margin should be clearly stated in the pathology report for therapeutic purposes (Pathologica 2020;112:197)
Microscopic (histologic) images
Cytology description
- FNA has variable results with diagnosing autoimmune pancreatitis type 1 but can be used to rule out malignancy in mass forming lesions (Diagnostics (Basel) 2021;11:1653)
- Lymphoplasmacytic infiltration, storiform fibrosis and obliterative phlebitis can be seen alone or in combination (Diagnostics (Basel) 2021;11:1653, World J Gastroenterol 2012;18:3883)
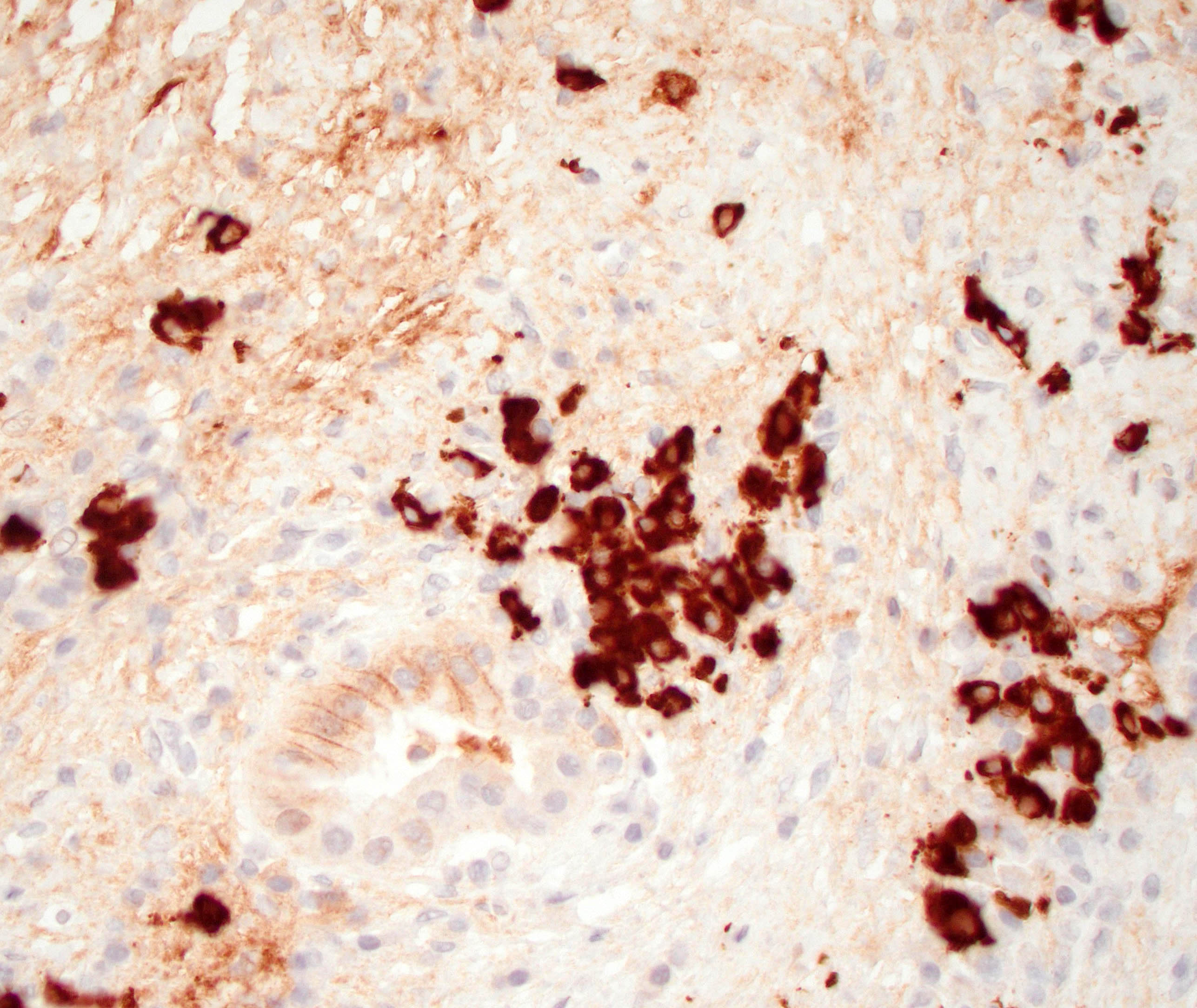
Positive stains
- IgG and IgG4
- > 10 IgG4 positive cells per high power field in biopsy specimens (Pancreas 2011;40:352)
- > 50 IgG4 positive cells per high power field in resection specimens (Virchows Arch 2018;472:545)
- IgG4/IgG ratio > 40% (Virchows Arch 2018;472:545)
- Elastic: highlights remnant veins in longstanding autoimmune pancreatitis
- Masson trichrome: highlights storiform fibrosis
- Normal / intact expression of DPC4 in entrapped ducts
Sample pathology report
- Pancreas, mass, core needle biopsy:
- Chronic pancreatitis with storiform fibrosis and increased IgG4 positive plasma cells (see comment)
- Negative for malignancy
- Comment: H&E sections demonstrate pancreatic parenchyma with dense storiform fibrosis and numerous chronic inflammatory cells including abundant plasma cells. A partially obliterated vein is seen. Immunohistochemical stain for IgG4 shows increased IgG4 positive plasma cells (up to 20 - 25 per high power field). The combined findings are most consistent with autoimmune pancreatitis type 1.
Differential diagnosis
- Pancreatic ductal adenocarcinoma:
- Can have similar presentation (vague abdominal symptoms, jaundice, pancreatic mass) with similar radiographic findings and serology (increased CA 19-9 with or without increased IgG4)
- Infiltrating well to poorly formed glandular / ductal structures surrounded by remarkably desmoplastic stroma
- Perineural or lymphovascular invasion
- Molecular alterations not present in AIP type 1 (World J Gastroenterol 2023;29:2241)
- Autoimmune pancreatitis type 2:
- Younger patients with no sex predilection
- Associated with chronic inflammatory bowel disease
- Serum IgG4 antibody levels are within normal ranges
- Requires histological specimen to make definitive diagnosis (Pancreas 2011;40:352)
- Duct centric granulocytic abscesses and lobular (but not storiform) fibrosis
- Follicular pancreatitis:
- Inflammation with numerous lymphoid follicles with or without prominent germinal centers
- Lacks storiform fibrosis, obliterative phlebitis and IgG4+ cells
- Obstructive chronic pancreatitis:
- Groups of acini and tubules scattered in fibrous tissue
Board review style question #1
Which of the following clinical and histologic features would support a diagnosis of autoimmune pancreatitis (AIP) type 1 over AIP type 2?
- 25 year old patient
- Abundant IgG4 positive plasma cells on histologic section
- Female patient
- Lobular fibrosis
Board review style answer #1
B. Abundant IgG4 positive plasma cells on histologic section is the most supportive of a diagnosis of AIP type 1. AIP type 1 is the pancreatic manifestation of IgG4 related disease (IgG4 RD) and so patients will typically have elevated IgG4 levels on serology as well as demonstrate abundant IgG4 positive plasma cells on histologic section. Answers A and C are incorrect because AIP type 1 patients are more commonly over 50 years old and male. Answer D is incorrect because AIP type 1 also shows characteristic storiform fibrosis, not lobular fibrosis, which can be seen in AIP type 2.
Comment Here
Reference: Autoimmune pancreatitis type 1
Comment Here
Reference: Autoimmune pancreatitis type 1
Board review style question #2
Board review style answer #2
C. Elevated IgG4 on serology. The histologic section shows the characteristic findings of autoimmune pancreatitis (AIP) type 1 (storiform fibrosis and a lymphoplasmacytic infiltrate). Most AIP type 1 patients have elevated IgG4 on serology. Answer A is incorrect because patients do not present with symptoms of acute pancreatitis (acute abdominal pain with elevated lipase on serology). Answer B is incorrect because AIP type 1 patients can present with elevated CA 19-9 on serology but this is not as common as elevated IgG4. Answer D is incorrect because inflammatory bowel disease is associated with AIP type 2, not type 1. Answer E is incorrect because a pancreas mass can be seen in AIP type 1 but this is variable.
Comment Here
Reference: Autoimmune pancreatitis type 1
Comment Here
Reference: Autoimmune pancreatitis type 1