Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Sharma A, Lastra RR. Serous borderline tumor. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/ovarytumorserousborderline.html. Accessed April 1st, 2025.
Definition / general
- Ovarian serous borderline tumor (SBT) is a low grade epithelial neoplasm of generally younger women with a favorable prognosis when diagnosed at an early stage
- Defined, nonobligate precursor to low grade serous carcinoma (LGSC)
- As a borderline tumor, can give rise to extra-ovarian abdominoperitoneal or lymph node implants
Essential features
- 2 morphologic subtypes:
- Conventional SBT shows hierarchically branching papillae lined by stratified, heterogenous epithelium with up to moderate atypia
- Micropapillary / cribriform SBT shows multiple nonbranching filiform structures without fibrovascular cores that are 5 times longer than they are wide, originating directly from bulbous central stalks
- When making distinction between SBT and LGSC in a properly sampled resection, numerous stipulations are in place regarding invasion (size and cytomorphology) and features of extra-ovarian implants (noninvasive versus invasive)
Terminology
- Serous borderline tumor is currently the sole recommended term for primary ovarian tumor (WHO female genital tumors, 2014 and 2020)
- Implant references extra-ovarian nonnodal disease rather than metastasis
- Involvement of lymph node by SBT is preferred over lymph node metastasis
- Micropapillary / cribriform SBT no longer considered definitionally synonymous with noninvasive LGSC per 2020 WHO
- SBT with microinvasion (as defined) is no longer, by definition, synonymous with LGSC per 2020 WHO
- Obsolete terminology no longer recommended:
- Atypical proliferative serous tumor
- Serous tumor of low malignant potential
- Semimalignant serous tumor
- Noninvasive LGSC / micropapillary SBT
ICD coding
- ICD-O: 8442/1 - serous cystadenoma, borderline malignancy (ICD-10: C56.9), serous tumor, NOS, of low malignant potential (C56.9), atypical proliferating serous tumor (C56.9)
- ICD-O: 8462/1 - serous papillary cystic tumor of borderline malignancy (C56.9), atypical proliferative papillary serous tumor (C56.9), papillary serous cystadenoma, borderline malignancy (C56.9), papillary serous tumor of low malignant potential (C56.9)
- ICD-O: 8463/1 - serous surface papillary tumor of borderline malignancy (C56.9)
- ICD-O: 9014/1 - serous adenofibroma of borderline malignancy, serous cystadenofibroma of borderline malignancy
- ICD-10: D39.1 - neoplasm of uncertain behavior of ovary
Epidemiology
- Up to 15% of ovarian serous neoplasms are of the borderline type (Hum Pathol 2000;31:539)
- Median age is fifth decade, with a range from the second to ninth decades; i.e. younger than high grade serous carcinoma (HGSC) demographic
- Nulliparity and unopposed estrogen therapy confer risk, which decreases with increasing parity (Gynecol Oncol 2017;144:571)
- Unlike most other ovarian borderline histotypes (endometrioid, clear cell, seromucinous), SBT is not associated with endometriosis
Sites
- Primary tumor:
- Ovaries, fallopian tube (lumen and paratubal serosa - extremely rare) and peritoneal surfaces
- When ovarian, frequently bilateral (up to 33%) (Gynecol Oncol 2017;144:174)
- Rarely encountered in males; in the paratestis, is thought to derive from either multipotent mesodermal epithelium or Müllerian ductular remnants (Am J Surg Pathol 2001;25:373)
- Implants (all subtypes):
- Ipsilateral ovarian surface (autoimplant), contralateral ovarian surface, omentum, diaphragm, abdominal wall, serosal surface of other abdominopelvic organs
Pathophysiology
- There is data to suggest progression from serous cystadenoma / cystadenofibroma with BRAF / KRAS mutations → SBT → LGSC; however, this is still controversial (Ann Oncol 2016;27:i16)
- Alternative theory suggests that SBT directly arises from papillary hyperplasia of the fallopian tube, which exfoliates and travels through the lumen to implant on ovarian and peritoneal surfaces (Am J Surg Pathol 2011;35:1605)
Etiology
- High incidence of microinvasion in SBTs of pregnant patients
Clinical features
- About a third are asymptomatic; otherwise present with nonspecific pelvic / abdominal pain (Oncologist 2012;17:1515)
- Mass / compression effect on adjacent tissues (constipation, dysuria)
- Uncommonly abdominal bloating, ascites / distention, associated early satiety
Diagnosis
- Definitive diagnosis is made only after both adequate sampling of resection specimen and classification of any synchronous abdominoperitoneal implants (Pathology 2018;50:205)
- Frozen section diagnosis of SBT often dictates subsequent intraoperative staging protocols (ie, more extensive resection, omental and peritoneal biopsies / washings, extent of lymph node dissections)
Laboratory
- Preoperative serum levels of CA 125 are usually elevated (less frequently, CEA and CA19-9)
- Neither sensitive nor specific and cannot distinguish SBT from benign or frankly malignant neoplasms / conditions (Eur J Obstet Gynecol Reprod Biol 2014;183:5, Med Sci Monit 2020;26:e924497)
- Trended postoperatively as qualitative indicator of residual / recurrent disease (not evidence based)
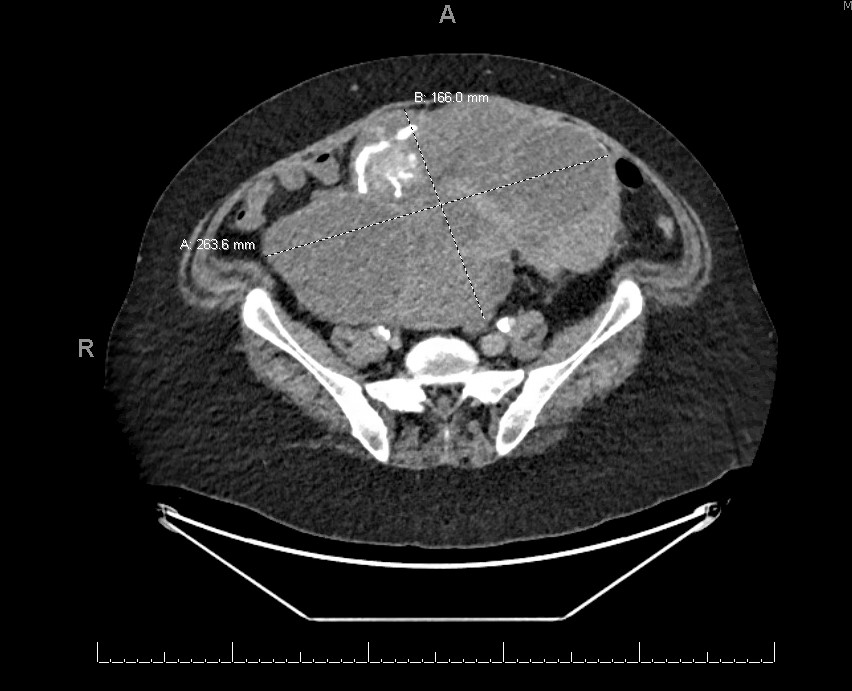
Radiology description
- Radiologically challenging (with any modality) to distinguish between SBT and LGSC
- Pelvic ultrasound (Abdom Radiol (NY) 2020 Aug 29 [Epub ahead of print])
- Primary modality of imaging evaluation - both transvaginal / abdominal components useful to characterize primary mass and appraise extrapelvic disease
- Spectrum of features from serous borderline to LGSC (former has fewer / smaller papillary projections, lower proportion of solid components, thinner septations / wall thicknesses and fewer microcalcifications)
- MRI
- Used to further characterize indeterminate masses or those too large to visualize on ultrasound (Jpn J Radiol 2020;38:782, J Magn Reson Imaging 2014;40:151)
- Variably multicystic to solid, bilateral irregularly enhancing masses with internal septations, fibrous branching and closely packed to loose papillations
- CT or PET / CT
- Used to further characterize enhancing solid components or qualities of extra-ovarian disease (Abdom Radiol (NY) 2020 Aug 29 [Epub ahead of print])
- Presence of nodularity and microcalcifications within peritoneal deposits more likely to indicate invasive (versus noninvasive) implants
Prognostic factors
- Prognosis largely stage dependent (FIGO / TNM):
- Early stage disease (ovary confined, constituting a majority of patients) has survival comparable to that of general population (Gynecol Oncol 2014;134:267)
- Advanced stage at presentation (i.e. extra-ovarian disease) associated with decreased survival; however, prognosis of advanced stage SBT hinges on presence of established poor prognosticators (invasive versus noninvasive implants, micropapillary / cribriform histotype) not reflected in conventional TNM staging
Case reports
- 50 and 61 year old women with early recurrence of ovarian serous borderline tumor as high grade carcinoma (Int J Gynecol Pathol 2004;23:265)
- 61 year old woman with ovarian mesonephric-like adenocarcinoma arising in serous borderline tumor (Diagn Pathol 2020;15:91)
Treatment
- Primary treatment is surgical and subsequently stage dependent:
- Hysterectomy and bilateral salpingo-oophorectomy with complete staging; if fertility desired, then unilateral salpingo-oophorectomy with complete staging (both only if early stage preoperatively)
- With bulky disease or advanced preoperative stage: neoadjuvant chemotherapy followed by cytoreductive surgery
- Advanced postoperative stage, incomplete staging, quality of implants (invasive or noninvasive) and desire for fertility dictates postoperative management
- Observation, completion surgery, adjuvant platinum / taxane based or hormonal chemotherapy (or combination thereof)
- In contrast to high grade serous carcinoma, SBT / LGSC is suboptimally sensitive to platinum based chemotherapy (NCCN: Clinical Practice Guidelines in Oncology (NCCN Guidelines®) [Accessed 11 February 2021])
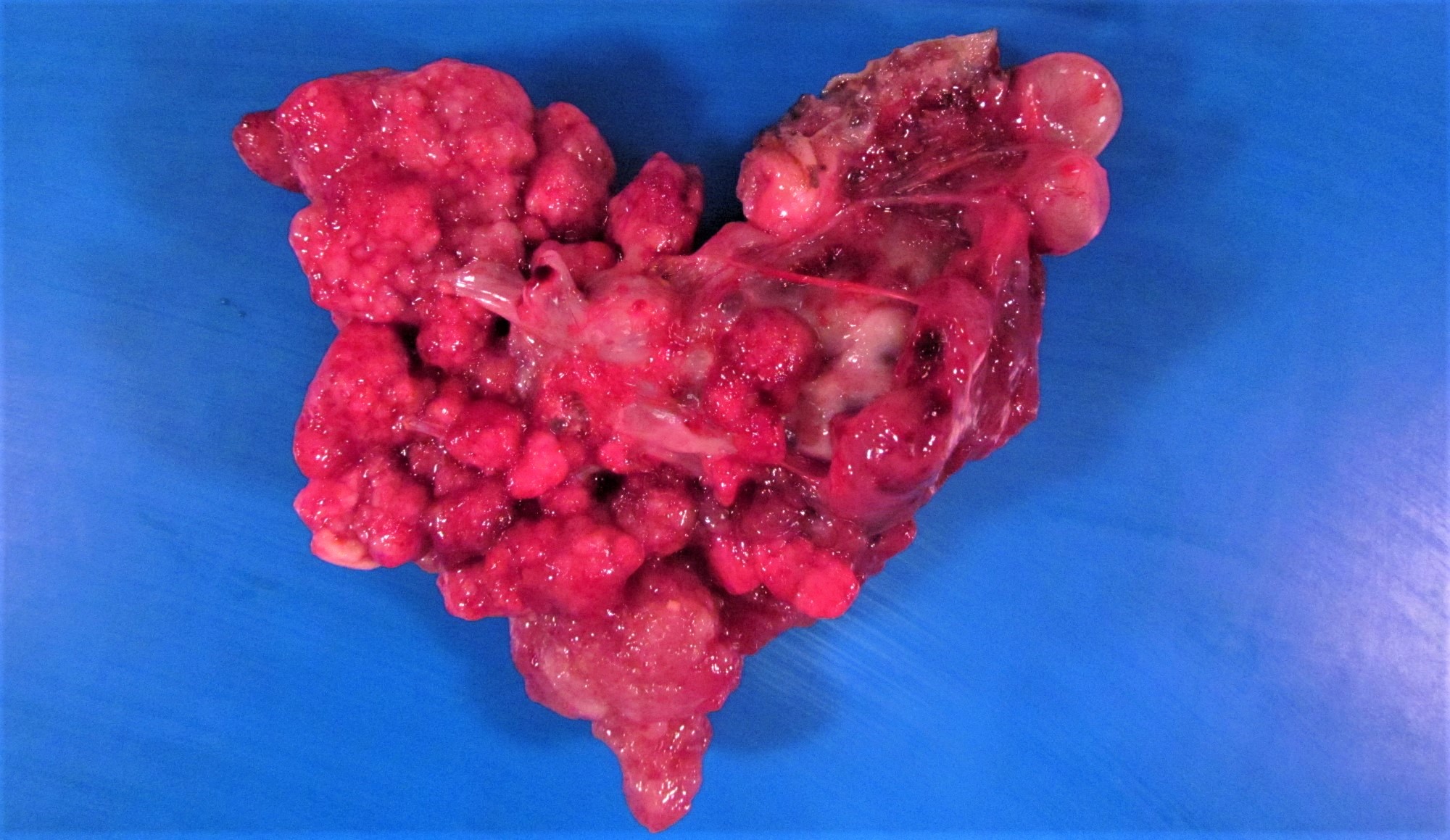
Gross description
- Received from surgery (often for frozen section) as distended, lobulated bulbous ovarian, adnexal or pelvic mass
- Upon receipt and prior to inking, crucial to document:
- Presence of surface autoimplants (plaque-like deposits, suspicious adhesions)
- Integrity of surface / capsule - intact or focally / diffusely ruptured
- Cut surface:
- Multiloculated cystic to solid mass with variably thick dissecting septations
- Firm to friable tan-yellow edematous papillary outgrowths from inner wall - resembling clusters of small berries (Am J Surg Pathol 2002;26:1111)
- Can be either subtle, inconspicuous foci in an overall smooth walled cyst or solid proliferations overtaking entire cavity
- Serosanguinous fluid contents
- Gross coagulative necrosis rare
- Proper sampling for permanent sections to exclude areas of destructive invasion, atypia or solid growth that would merit promotion to LGSC
- At least 2 representative sections per centimeter of greatest gross tumor dimension (i.e. solid or papillary areas, not the overall simple cyst)
- Grossly normal omentum: 5 - 10 sections total
Gross images
Frozen section description
- Request for frozen section anticipated preoperatively based on imaging and results of peritoneal cytopathology
- Gross intraoperative evaluation:
- Representative sections of any papillary excrescences or solid areas should be evaluated histologically
- Unless focally thickened, uniform smooth walled areas are not grossly concerning and do not merit freezing
- Histologic intraoperative evaluation:
- After concerning histology is judiciously followed up with additional representative sections, report concerning features that would impact subsequent intraoperative course:
- Ambiguous volume of epithelial proliferation
- Micropapillary or cribriform / foci
- True invasion (microinvasion, as defined below, is not an adverse prognosticator and does not influence management)
- After concerning histology is judiciously followed up with additional representative sections, report concerning features that would impact subsequent intraoperative course:
- Correlation between frozen and permanent sections in SBT (up to 93% consistent) better than other borderline histotypes, e.g. endometrioid or mucinous (Gynecol Oncol 2011;123:517, Gynecol Oncol 2007;107:248)
- Risk of underdiagnosis of SBT (frozen) and subsequently LGSC (permanents) increases with larger tumor size (≥ 8 cm)
Frozen section images
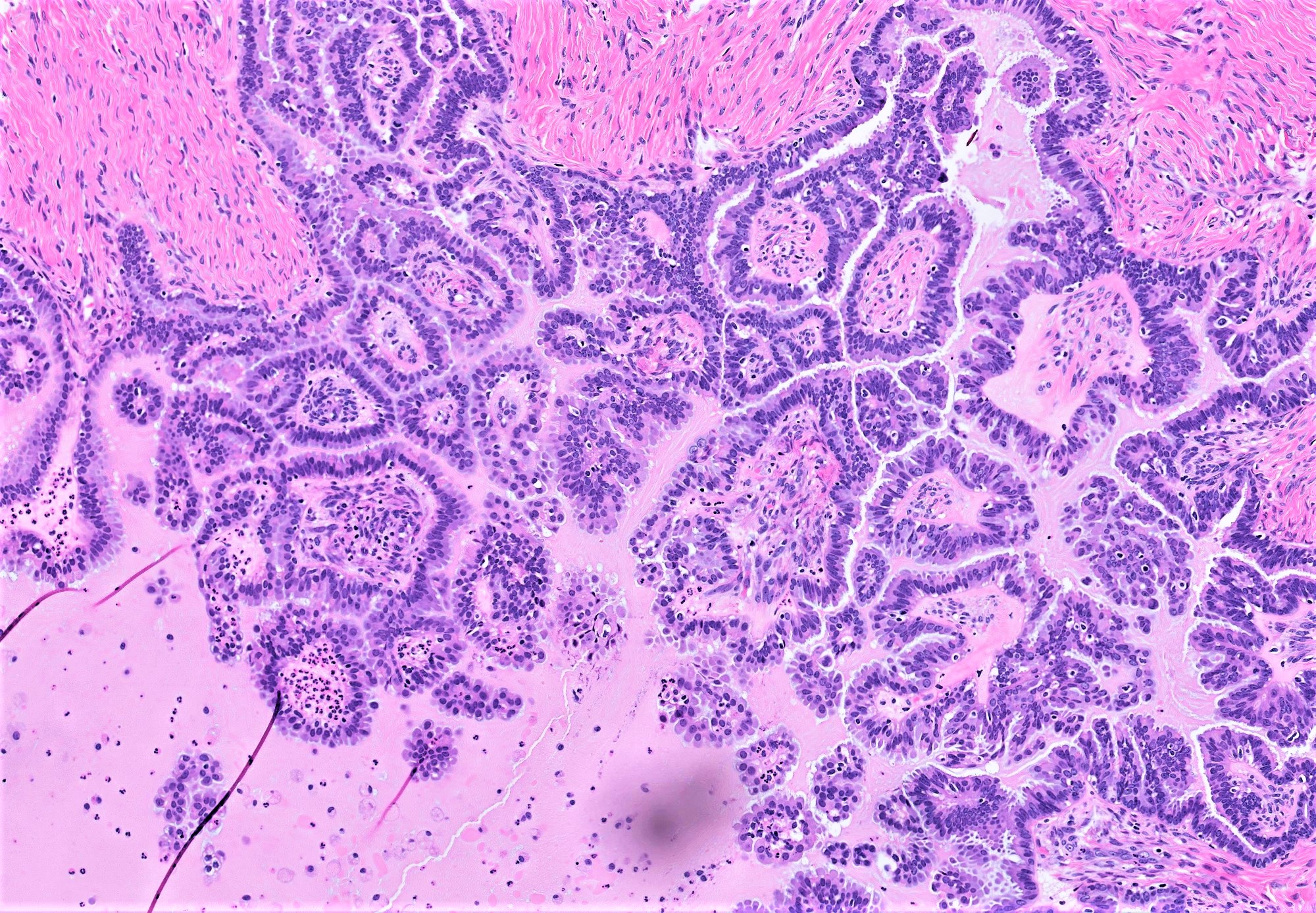
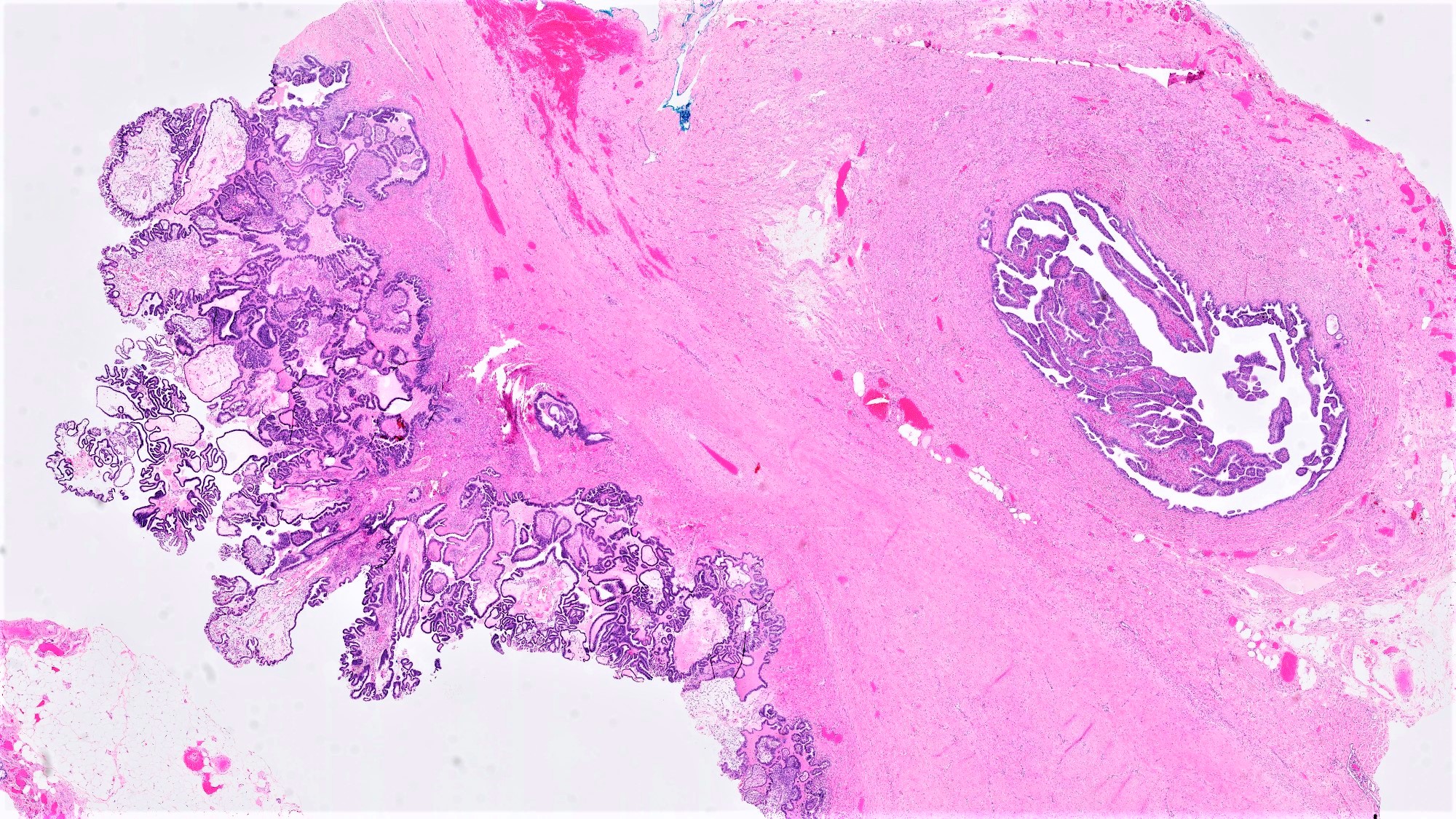
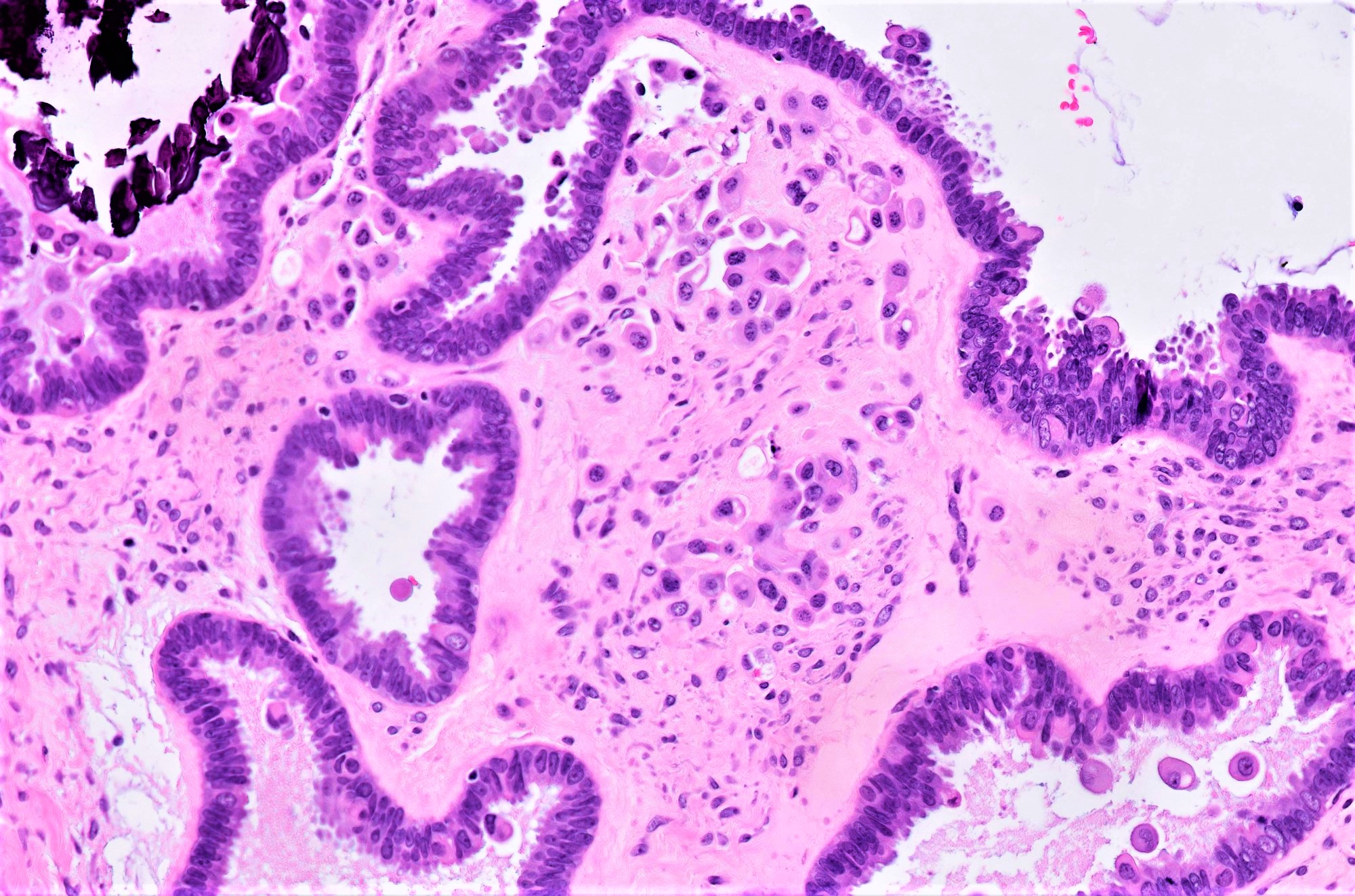
Microscopic (histologic) description
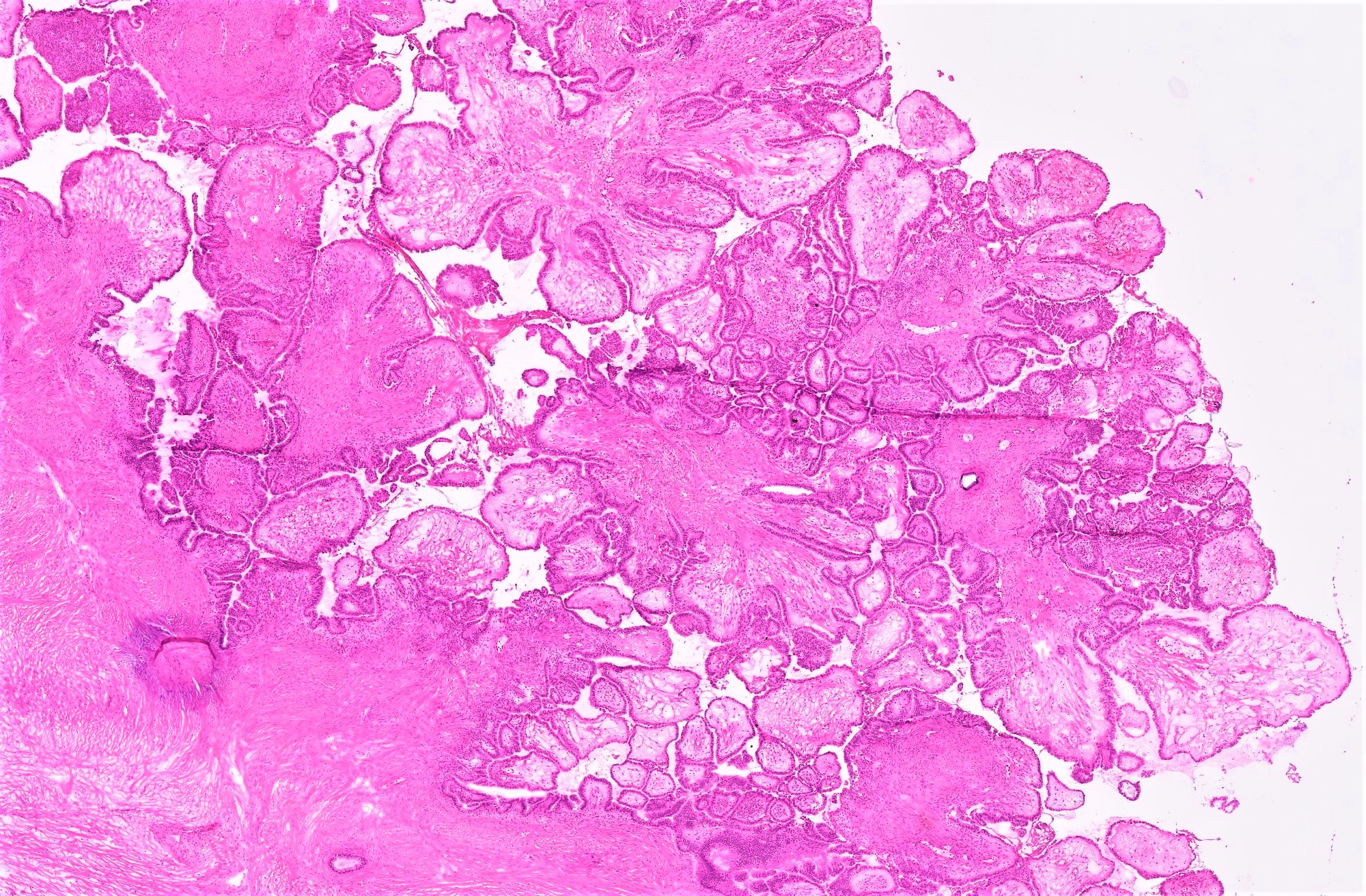
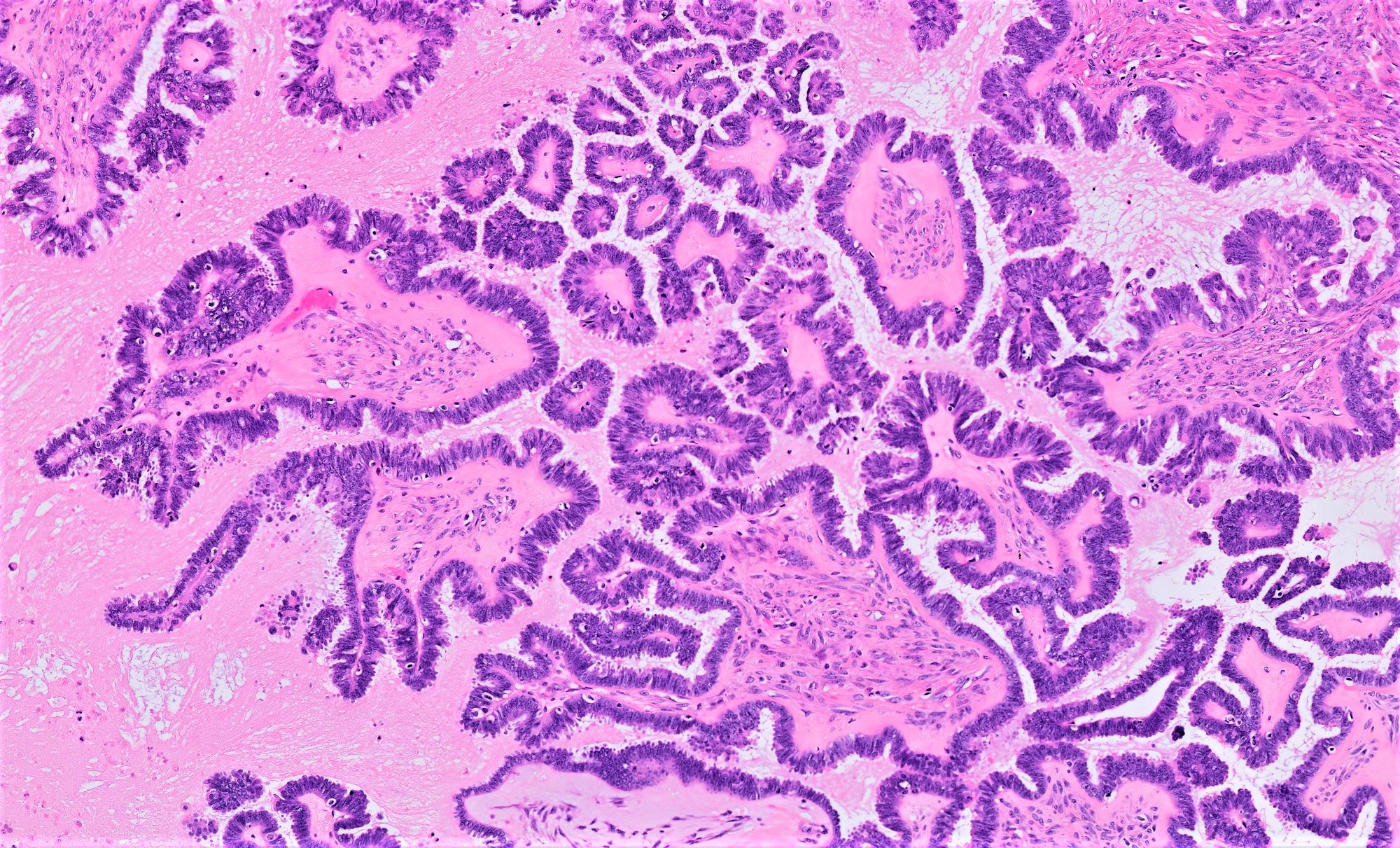
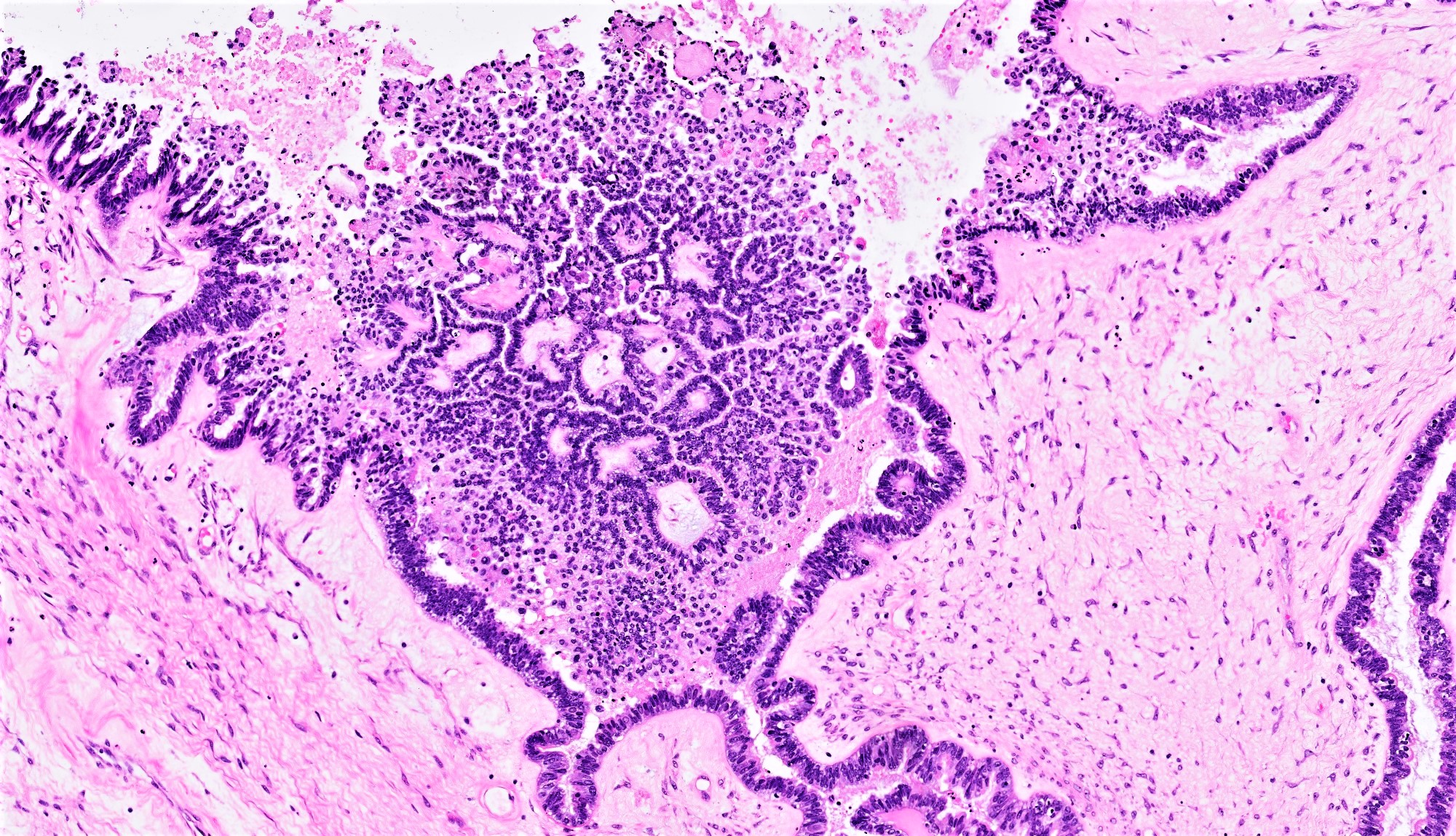
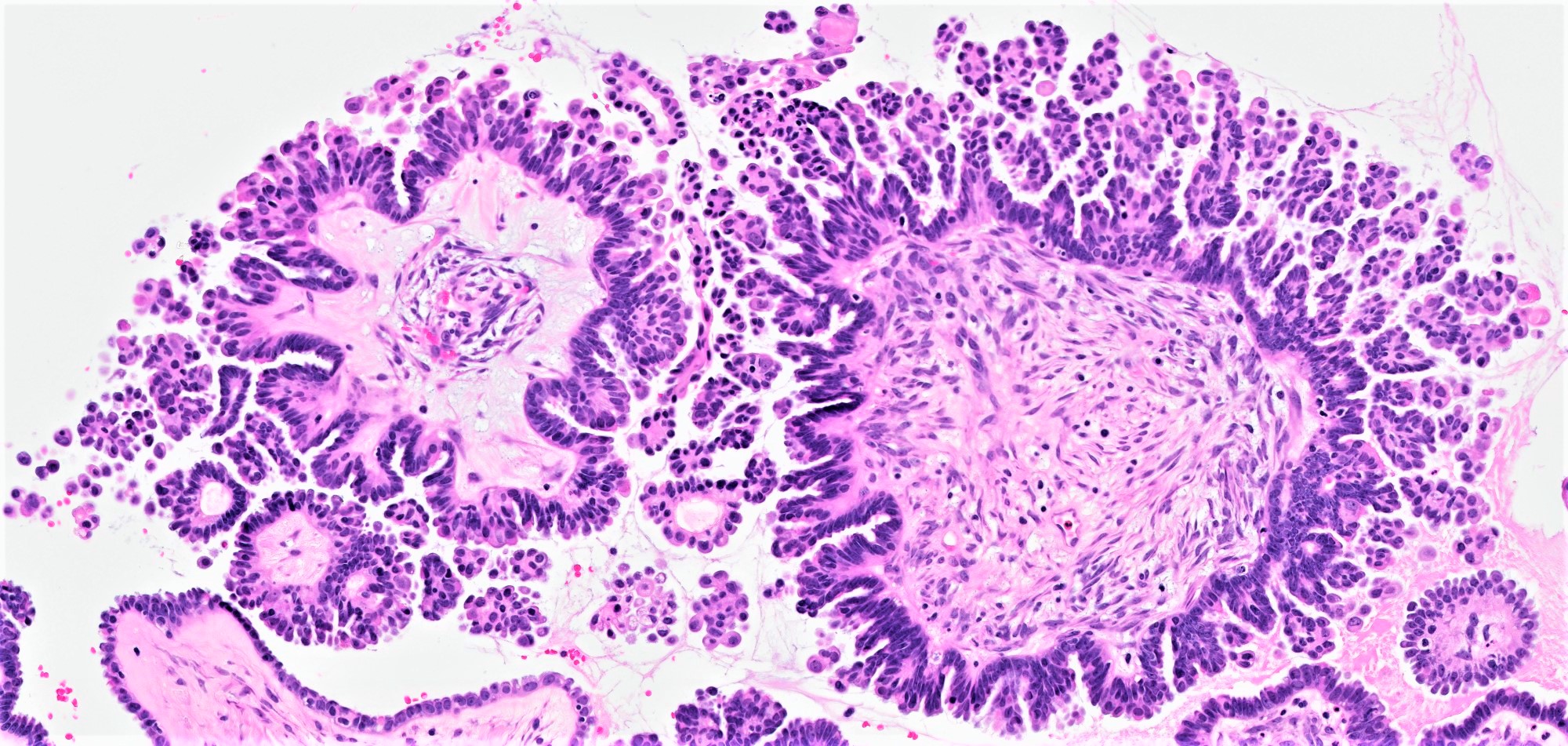
- Conventional SBT
- Architecture:
- Numerous slender to bulbous, irregularly contoured papillae with fibrous, hyaline or myxoid cores
- Hierarchical branching pattern - larger papillae arborize to sequentially smaller papillae terminating in epithelial clusters or single cells
- Pseudostratified, crowded lining with hobnailing or epithelial tufting, which exfoliate from papillae
- Psammomatous (concentrically lamellated) or dystrophic calcifications
- Cytologic features:
- Heterogenous cellular population - cuboidal to columnar ciliated, secretory or eosinophilic cells (latter can have baseline atypia, are more numerous during pregnancy)
- Up to moderate atypia - mild cellular enlargement, chromatin coarsening, small nucleoli
- Minimal, nonatypical mitotic activity
- Architecture:
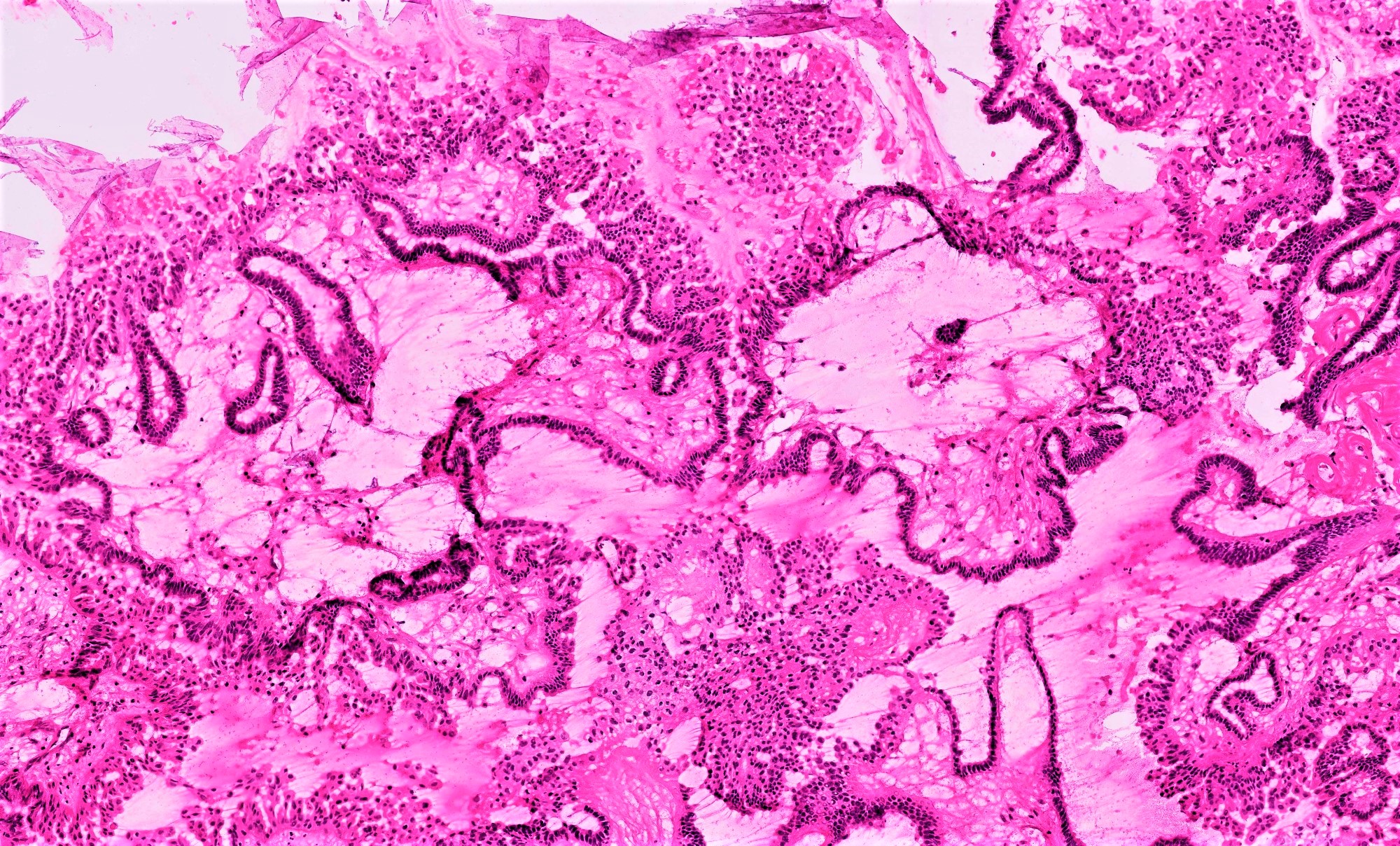
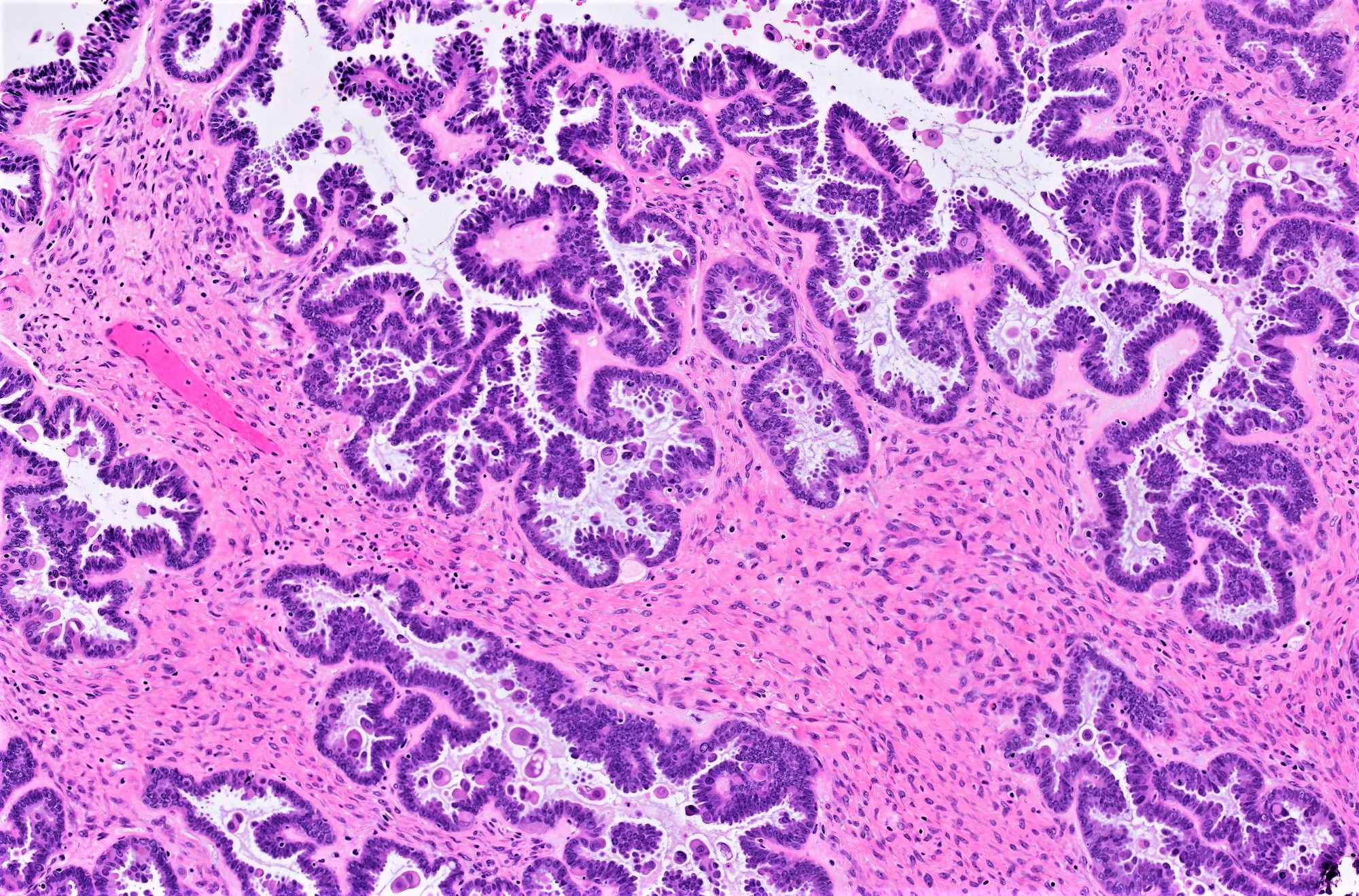
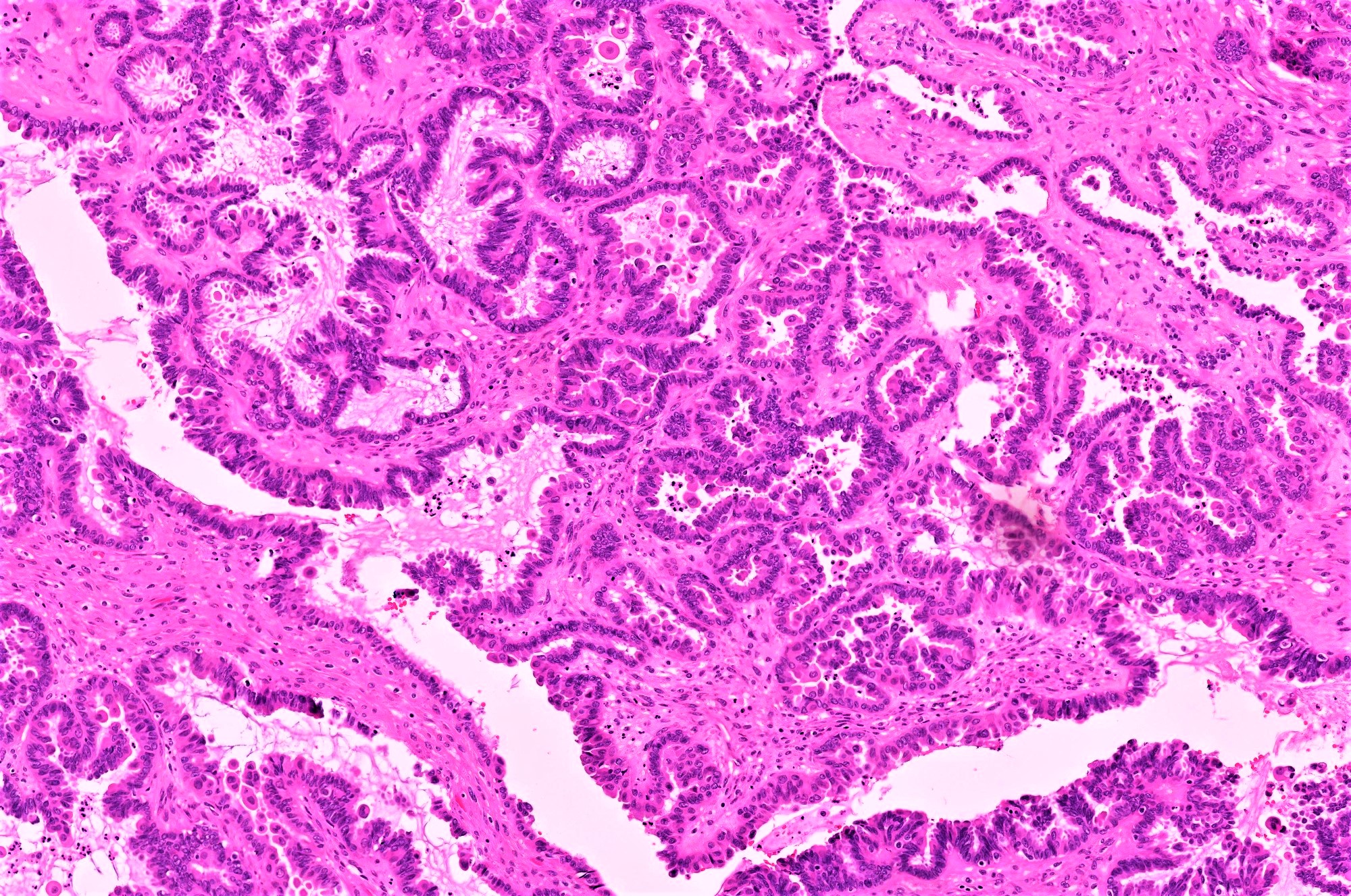
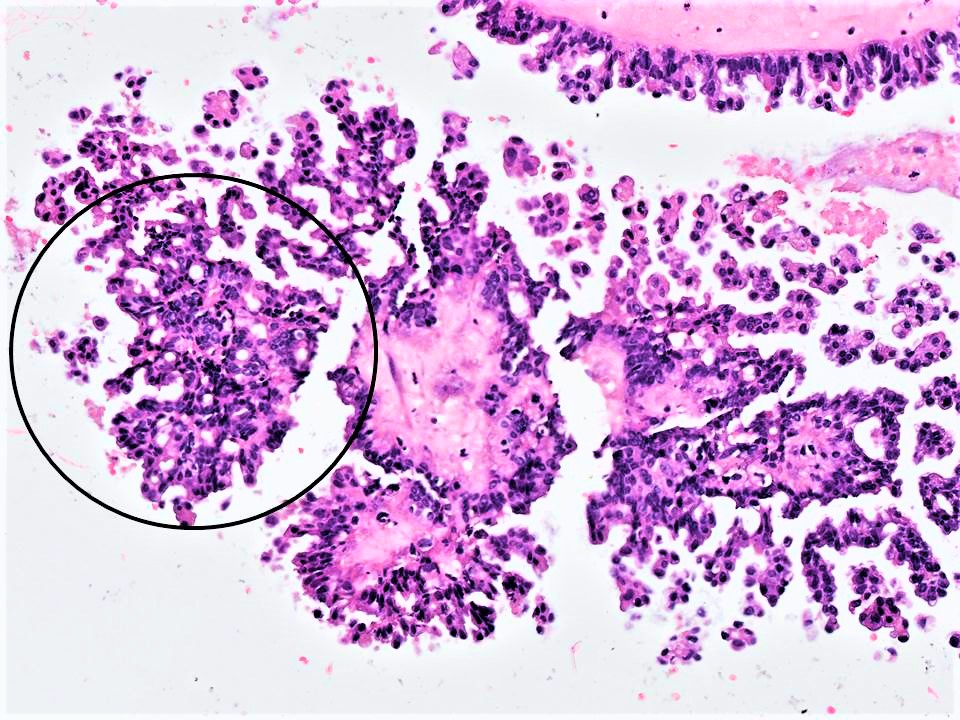
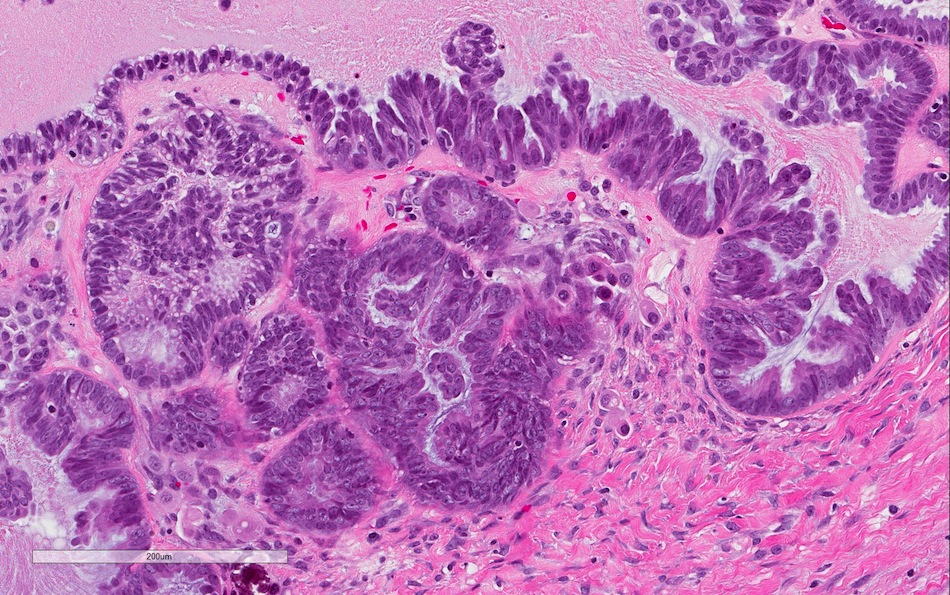
- Micropapillary / cribriform SBT
- Often admixed with areas of conventional SBT
- Micropapillary / cribriform subtype defined as SBT harboring a focus of ≥ 5 mm (confluent linear extent) of pure micropapillary / cribriform growth
- Some experts also cite 10 mm2 (2 dimensional area) towards this definition but this is not recognized in 2020 WHO (Am J Surg Pathol 1999;23:397, Am J Surg Pathol 2002;26:1111)
- Architecture - often combination of both patterns:
- Micropapillary:
- Thread-like extensions lacking true fibrovascular cores, radiating directly from cyst walls or central bulbous and smooth contoured prominences with fibromatous / myxoid stroma
- Micropapillae appear 5 times longer than they are wide, resembling mythical snakes of Medusa’s head
- Low power appearance of labyrinthine spaces created by complex micropapillary interweaving
- Cribriform: confluent sieve-like microacini (occasionally appearing solid), formed by fusion of micropapillae
- Micropapillary:
- Cytologic features distinct from those of conventional SBT:
- Epithelial population is uniform rather than heterogenous - low cuboidal monotonous cells with high nuclear to cytoplasmic (N/C) ratio and small, prominent nucleoli
- Rare to absent ciliation or cytoplasmic eosinophilia
- Minimal, nonatypical mitotic activity and up to moderate atypia
- Often admixed with areas of conventional SBT
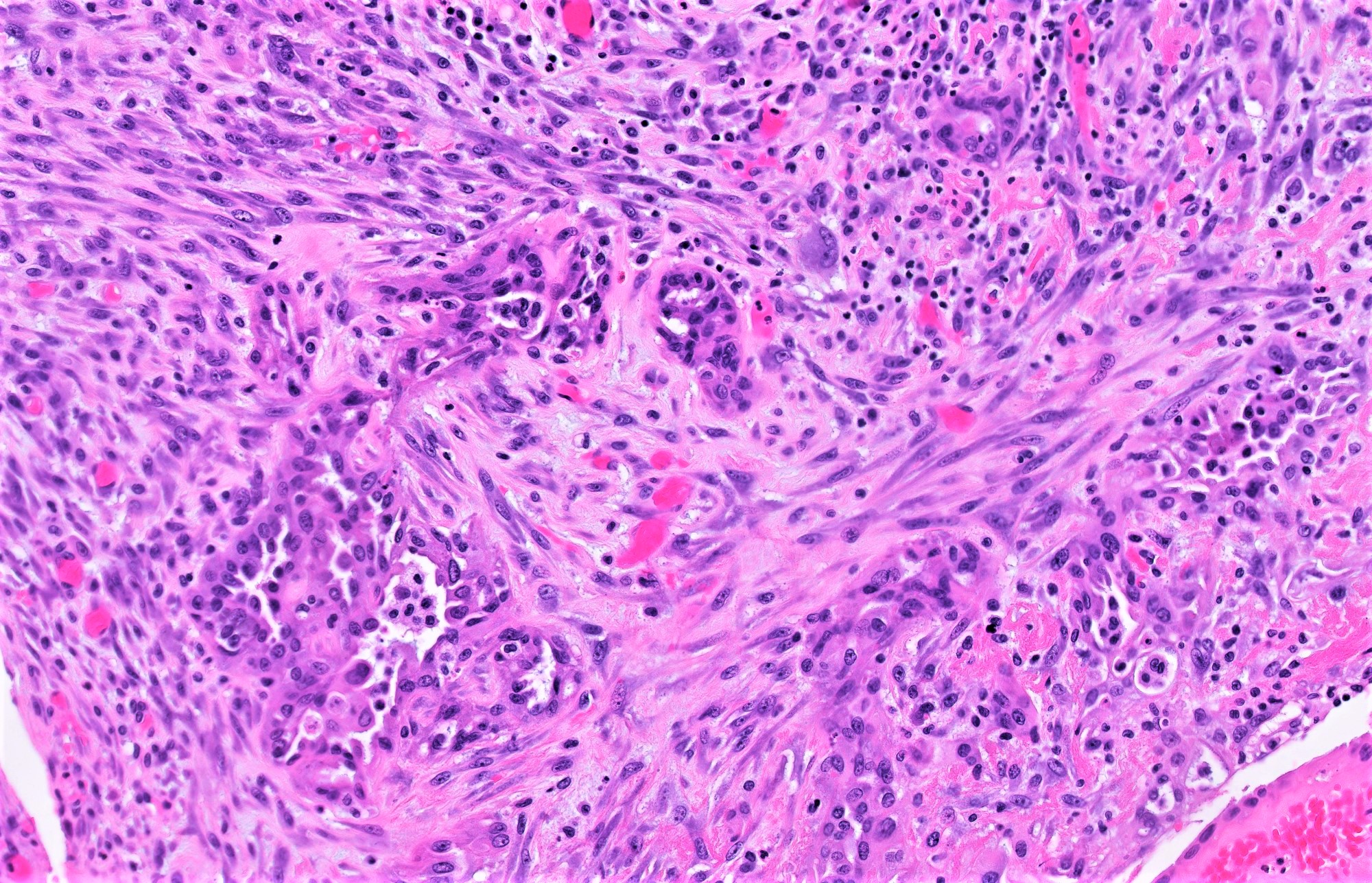
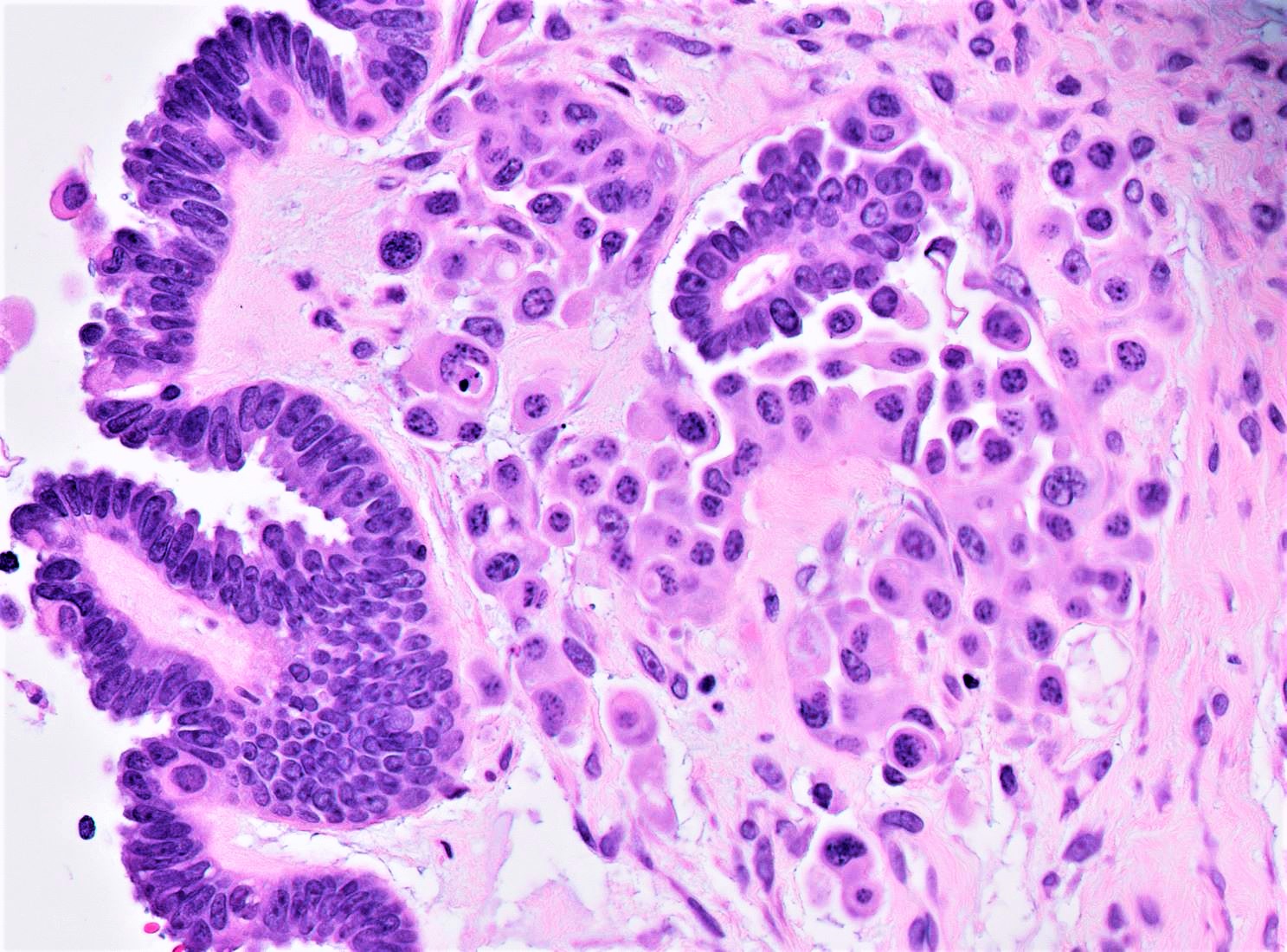
- SBT with microinvasion
- Distinction from LGSC in this context is made on size and cytoarchitectural features of the area of concern:
- Foci of microinvasion with cytoarchitecture of SBT actually represent senescent cells and confer no prognostic detriment (Am J Surg Pathol 2014;38:743)
- Individual cells to rounded clusters invading papillary or intracystic stroma with retraction clefting
- Minimally atypical cells with cytoplasmic hypereosinophilia, scant nonatypical mitoses (< 3 - 4/10 high power fields) and lower Ki67 index than surrounding tumor
- Surrounding stroma is unremarkable to minimally reactive (fibroblastic reaction, edema)
- Often multiple foci within same tumor (cutoff for progression to LGSC not defined) but any individual one must be < 5 mm in greatest dimension (otherwise defined as invasive LGSC arising in background of SBT)
- Foci of microinvasion with cytoarchitecture of LGSC are defined as microinvasive LGSC or SBT with microinvasive LGSC only after proper sampling to exclude frankly invasive LGSC
- Micropapillae, inverted micropapillae or confluent cribriform structures haphazardly infiltrating papillary or intracystic stroma in a fashion similar to conventional invasive LGSC (Am J Surg Pathol 2006;30:1209)
- Monotonous hyperchromatic cells with scant cytoplasm and prominent nucleoli
- Must measure < 5 mm (confluent linear growth) in greatest dimension in any single focus, otherwise defined as frankly invasive LGSC
- Foci of microinvasion with cytoarchitecture of SBT actually represent senescent cells and confer no prognostic detriment (Am J Surg Pathol 2014;38:743)
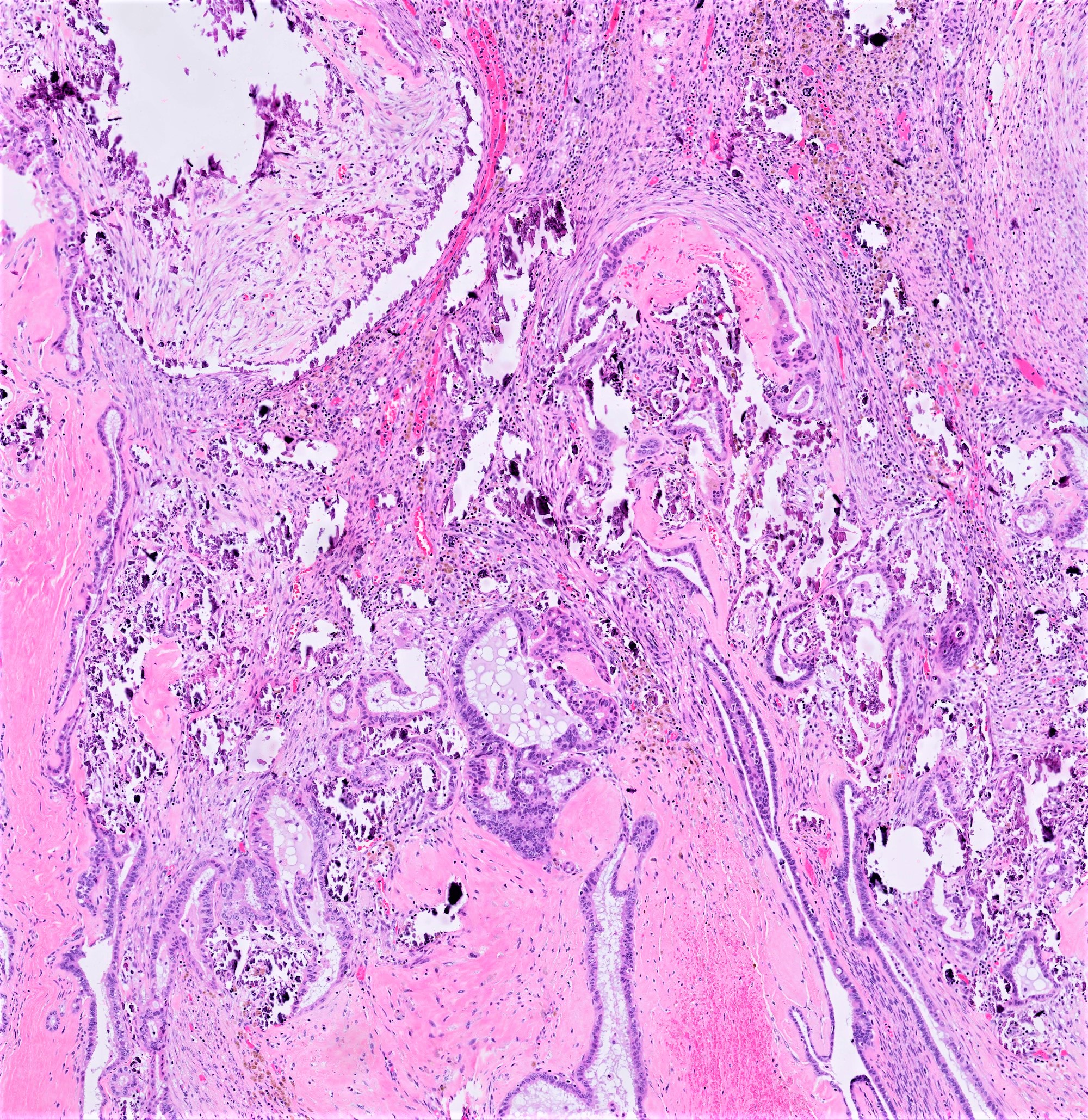
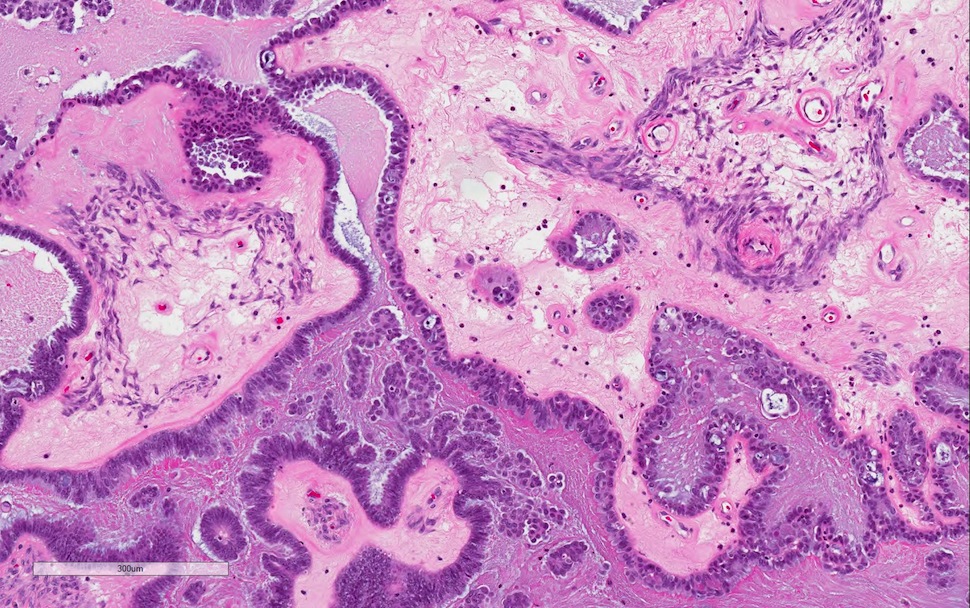
- Implants
- 2 types of implants: noninvasive and invasive, latter now classified as extra-ovarian LGSC
- Noninvasive implant:
- Grossly, both epithelial and desmoplastic noninvasive implants appear plastered onto peritoneal surface
- 2 histologic subtypes of no clinical bearing but important to recognize each in the differential of invasive implants
- Epithelial:
- Hierarchical papillary proliferations or cellular tufts without associated underlying stroma
- Planted on mesothelial surface or within cystic mesothelial invaginations, with overall smooth epithelial stromal interface
- Cytologic features similar to conventional SBT
- Desmoplastic:
- True papillae with clusters of or single hypereosinophilic cells blending into (compressed by) inflamed fibroblastic, granulation tissue-like stroma
- Despite reactive stroma, neoplastic groups remain serosa based, retain smooth and sharply delineated contours at stromal interface and invade without underlying stromal destruction
- In omentum, desmoplastic implants can create infiltrative inflammatory fibrous septation but do not engulf or extend into adipose tissue
- Autoimplant: deposit of tumor on ovarian surface, often of desmoplastic noninvasive histomorphology
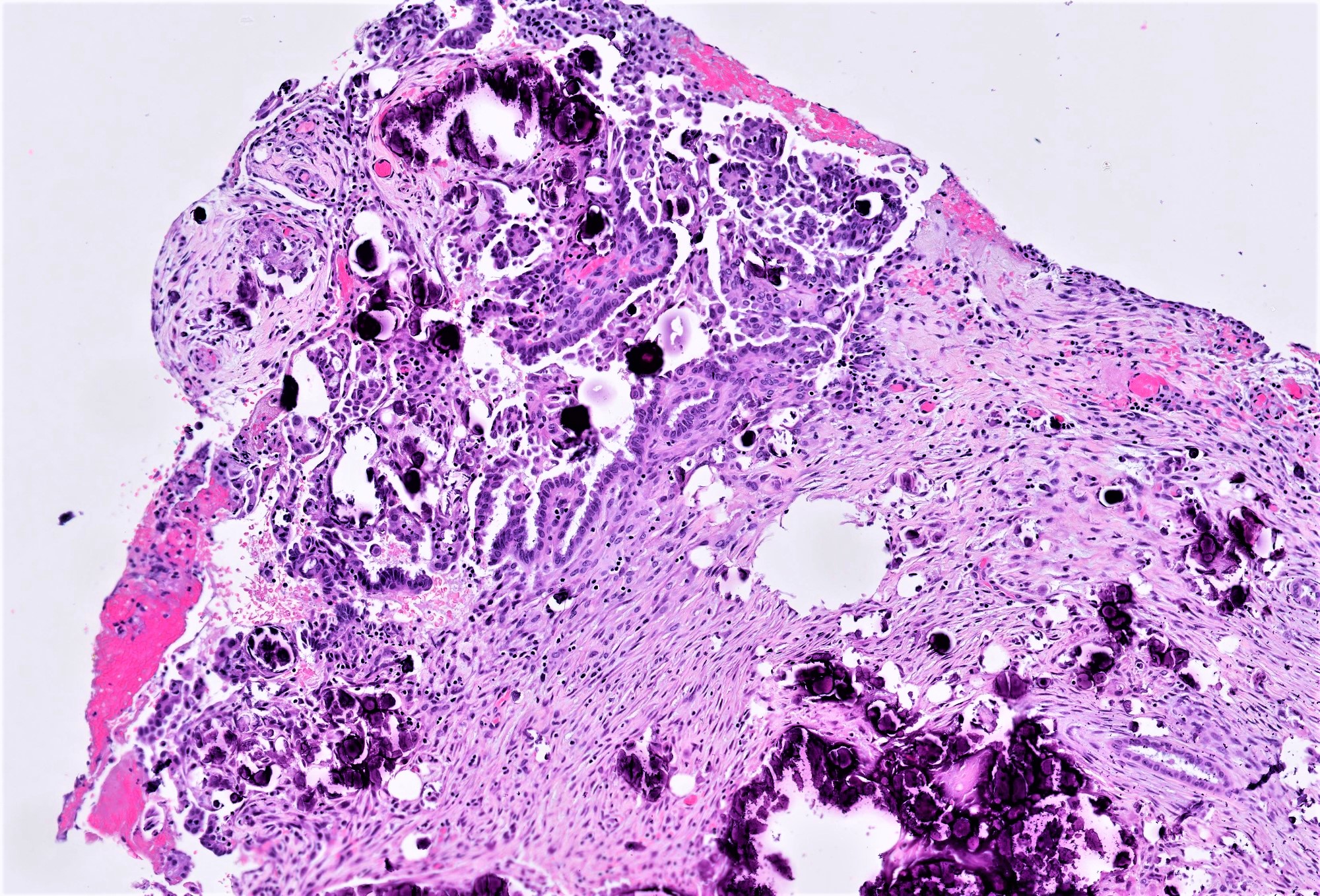
- Peritoneal extra-ovarian LGSC (formerly known as invasive implant) - epithelial predominant lesions:
- Invasive implant defines said focus as extra-ovarian LGSC (even if primary tumor remains as borderline - uncommon situation in which sufficient sampling must be ensured, to exclude histologic features meriting upgrade of the primary)
- Most critical histologic feature of invasion in the context of an SBT implant is presence of destructive stromal growth evident at low magnification (should not be present in desmoplastic noninvasive implants)
- Haphazard infiltration of solid epithelial nests with peripheral retraction clefting / halos resembling lymphatic channels
- In omentum, invasive implants have expansile to infiltrative borders with interruption of normal lobulated fibroadipose architecture
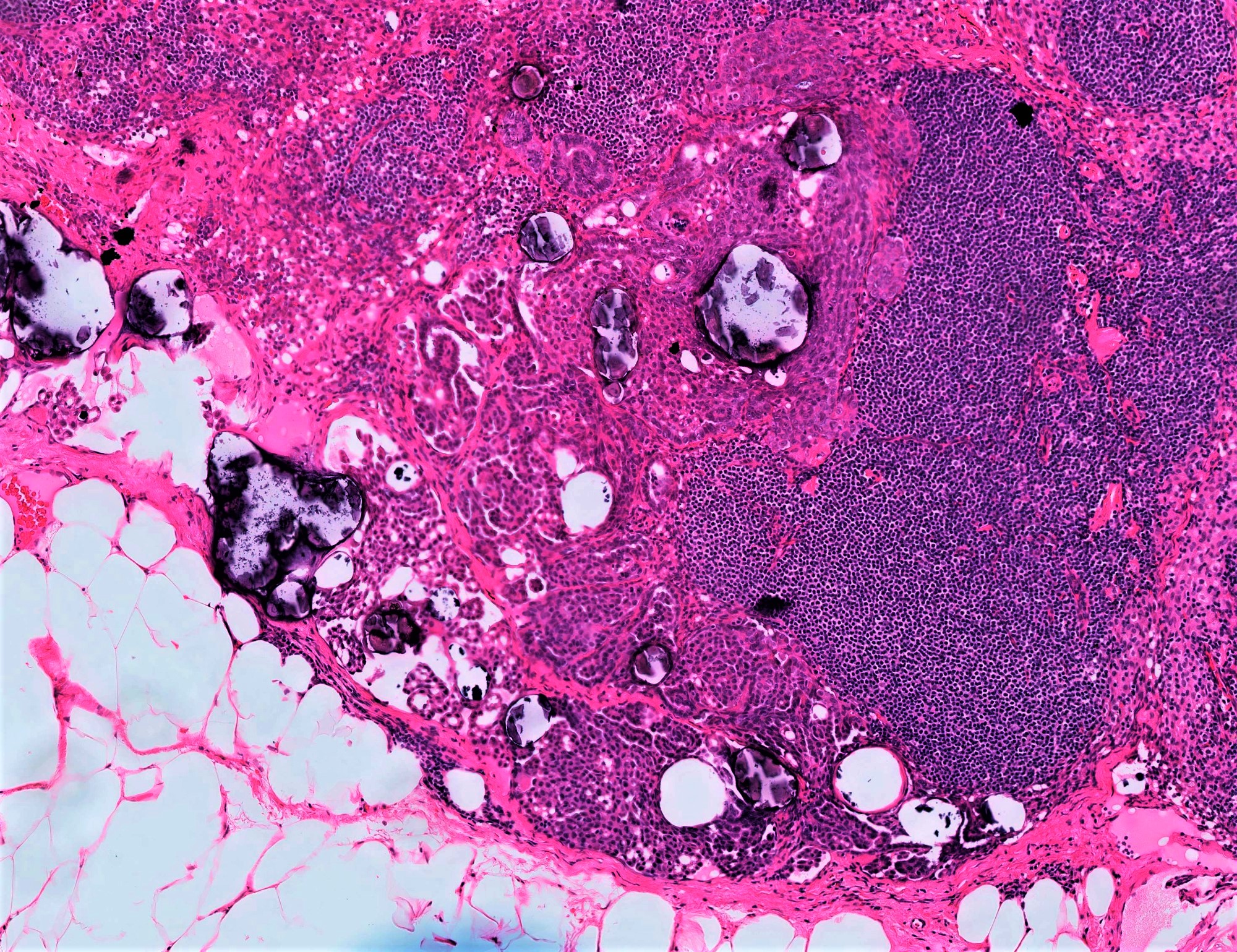
- Lymph node involvement
- Does not portend worse prognosis but retroperitoneal nodes with involvement of SBT still staged per usual (as N1), not as an extra-ovarian implant
- Composed of single cells, small clusters, micropapillae or macropapillae and cribriform glands located in nodal subcapsular sinuses or parenchyma
- Cytologically similar to conventional SBT
- Adjacent endosalpingiosis is common
- Distinction from LGSC in this context is made on size and cytoarchitectural features of the area of concern:
Microscopic (histologic) images
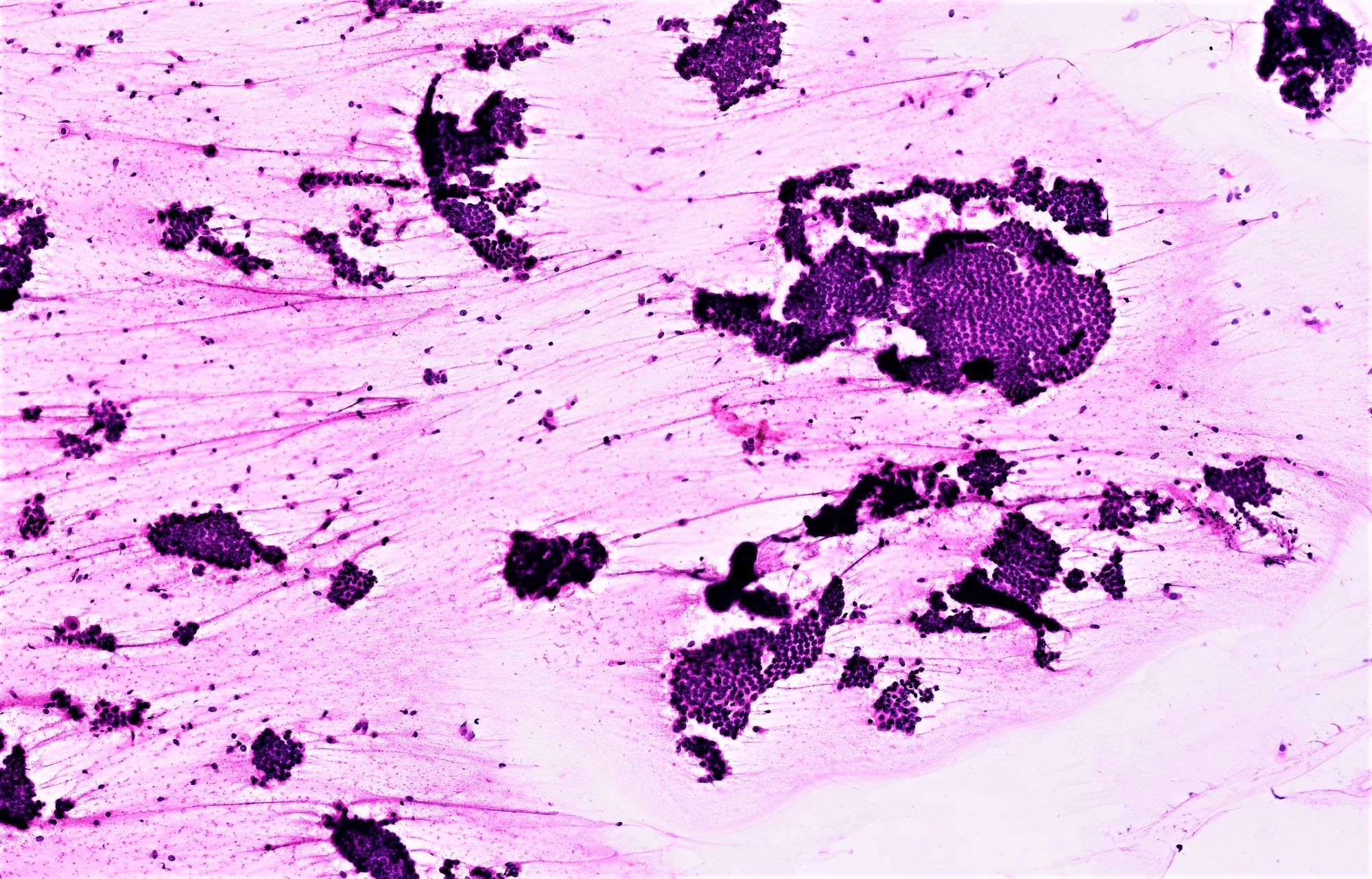
Cytology description
- Peritoneal / pelvic washings for ovarian or pelvic masses are taken immediately post entry into the abdominal cavity (during primary resection operation) to minimize contamination from potential intraoperative tumor rupture
- Rarely can be taken along with preoperative peritoneal or omental biopsies for advanced stage disease
- Positive washings upgrade FIGO / TNM stage of both SBT and LGSC:
- Implies presence of any of the following: ovarian surface involvement, occult extra-ovarian disease (i.e. microscopic invasive / noninvasive implants) or pre/intraoperative tumor rupture
- No reliable cytologic features to distinguish between the above scenarios or between involvement by SBT versus LGSC
- Cytologic features of SBT in washings (Diagn Cytopathol 2004;30:313, Cancer Cytopathol 2012;120:238):
- 3 dimensional clusters with smooth or irregular contours, papillations (more prominent on cell block) or other architecturally complex structures
- 2 cell population of minimally atypical cuboidal nonciliated cells with high N/C ratio and frequent cytoplasmic vacuolization
- Psammomatous calcifications can be seen in any of the following: endosalpingiosis, SBT and serous carcinoma (low or high grade)
Cytology images
Negative stains
- p53 wild type (patchy and weak expression in scattered nuclei)
- To be considered aberrant (aka mutation type), p53 must be either diffusely positive in > 80% of nuclei, completely negative (null type mutant pattern) or any amount of unequivocal cytoplasmic staining (blush does not qualify)
- p16 mosaic (patchy and weak nuclear / cytoplasmic expression)
- To be considered positive, p16 must have strong, diffuse, block-like nuclear and cytoplasmic positivity throughout the area of interest
- CK20, HNF-1B, Napsin A negative
Molecular / cytogenetics description
- KRAS mutations implicated in the progression from SBT to LGSC and are associated with higher risk of recurrence as LGSC (Cancer Res 2004;64:6915, J Pathol 2013;231:449)
- BRAF mutations implicated in the progression from serous cystadenoma to SBT but these are rarely associated with advanced stage SBT or progression to LGSC
- Molecular studies (X inactivation, loss of heterozygosity, KRAS / BRAF sequencing) conflict in regards to clonal versus multifocal versus metaplastic origin of SBT implants (Ann Oncol 2016;27:i16)
Sample pathology report
- Sample report 1:
- Right adnexal mass, excision:
- Ovarian serous borderline tumor (7.5 cm) with surface involvement (see synoptic report)
- Cecal nodule, excision:
- Low grade serous carcinoma (invasive implant)
- Right adnexal mass, excision:
- Sample report 2:
- Left fallopian tube and ovary, left salpingo-oophorectomy:
- Serous ovarian borderline tumor, without ovarian surface involvement (see comment and synoptic report)
- Fallopian tube without diagnostic abnormality
- Comment: Immunohistochemical stains performed with adequate controls on sections of the right ovarian tumor show the tumor cells to be diffusely positive for PAX8, WT1, ER and PR, with focal / weak expression of p53 (wild type expression) and patchy positivity for p16. The combined morphologic and immunohistochemical findings support the above diagnosis.
- Left fallopian tube and ovary, left salpingo-oophorectomy:
Differential diagnosis
- With ovarian SBT
- Serous cystadenoma / cystadenofibroma:
- Should not harbor architectural complexity in the form of papillae, micropapillae, cribriforming or nesting - otherwise:
- If proliferation constitutes < 10% of overall tumor: serous cystadenoma / cystadenofibroma with focal epithelial proliferation
- If > 10% of overall tumor: serous borderline tumor
- Similar heterogenous population of secretory / ciliated cells but without atypia or mitoses
- Should not harbor architectural complexity in the form of papillae, micropapillae, cribriforming or nesting - otherwise:
- Low grade serous carcinoma:
- Any single focus of invasion with nuclear features of borderline tumor measuring ≥ 5 mm in greatest linear extent (invasive LGSC)
- Any single focus of invasion with nuclear features of low grade serous carcinoma:
- < 5 mm in greatest linear extent - microinvasive LGSC
- > 5 mm - invasive LGSC
- Noninvasive LGSC and SBT often coexist and can be challenging to distinguish in the absence of invasion
- SBT should not have architecturally confluent cribriform or micropapillary growth - i.e. solid, florid proliferation of low grade cells (amount required to define carcinoma over borderline not defined)
- Endometrioid borderline tumor:
- Arises from / associated with endometriosis (unlike SBT)
- Can have papillary structures but not in hierarchically branching configuration
- Luminal surface of endometrioid glands is nonciliated and crisp / linear, not exfoliative, hobnailing or tufting like SBT
- Squamous, squamous morular or mucinous metaplasia (extremely rare in both low and high grade serous neoplasms)
- Seromucinous borderline tumor (formerly endocervical type mucinous borderline tumor):
- Arises from / associated with endometriosis (unlike SBT)
- Has hierarchically branching edematous papillae but in contrast to SBT, these have a prominent stromal acute inflammatory infiltrate
- Lined by endocervical type mucinous but occasionally ciliated to indifferent cells (unclassifiable cells unique to seromucinous tumors - abundant eosinophilic cytoplasm and prominent nucleoli)
- Serous cystadenoma / cystadenofibroma:
- With SBT autoimplant
- Invasive LGSC arising in SBT (Am J Surg Pathol 2006;30:457):
- As autoimplants are usually identical in morphology to peritoneal noninvasive desmoplastic implants, can mimic invasive LGSC arising in the borderline tumor if they are situated between papillae of exophytic or intracystic tumor
- Autoimplants are more stromal dominant with a more prominent inflammatory infiltrate
- Epithelial component of autoimplants appears compressed by desmoplastic reaction, rigid structural retention like the nests of microinvasive LGSC
- Invasive LGSC arising in SBT (Am J Surg Pathol 2006;30:457):
- With noninvasive peritoneal implant of SBT
- Mesothelial hyperplasia:
- Diffuse sheets to papillary proliferations of monotonous mesothelial cells restricted to serosa and demarcated from underlying stroma
- Variable cellular atypia with rare mitoses
- Both can be PAX8, WT1 positive but SBT is negative for calretinin, D2-40
- Well differentiated papillary mesothelioma:
- Tubulopapillary architecture (uniformly short / blunted rather than hierarchically branching papillae) with bland cuboidal monolayered mesothelial lining
- Psammoma bodies associated with both
- Immunoprofile similar to mesothelial hyperplasia (above)
- Endosalpingiosis:
- Endosalpingiosis is limited to simple glandular structures lined by a monolayer of cells similar to fallopian tube epithelium with neither atypia nor cellular crowding / tufting
- Primary peritoneal SBT:
- Can be multifocal and is histomorphologically identical to either epithelial or desmoplastic noninvasive implants of ovarian SBT
- Defined as primary peritoneal SBT only when a primary ovarian SBT is excluded
- Can be multifocal and is histomorphologically identical to either epithelial or desmoplastic noninvasive implants of ovarian SBT
- Mesothelial hyperplasia:
Additional references
Board review style question #1
Board review style answer #1
B. Increased risk of extra-ovarian peritoneal implants
Comment Here
Reference: Serous borderline tumor
Comment Here
Reference: Serous borderline tumor
Board review style question #2
Which of the following features, if present, increases the TNM stage of a serous borderline tumor?
- Lymphovascular invasion
- Positive pelvic washings
- Presence of frank destructive invasion
- Presence of microinvasion
- Tumor size ≥ 10 cm
Board review style answer #2