Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Pathophysiology | Etiology | Clinical features | Gross images | Microscopic (histologic) description | Positive stains | Negative stains | Molecular / cytogenetics description | Additional referencesCite this page: Kuhn E, Ayhan A. Epithelial tumors-overview / molecular. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/ovarytumorsurfacegeneral.html. Accessed April 1st, 2025.
Definition / general
- The ovary is a highly specialized organ physiologically dedicated to ovum production
- It is composed mainly of stromal cells that support maturing germ cells, covered by a monolayer modified mesothelium (so called ovarian surface epithelium)
- Most ovarian cancers are epithelial, but most testicular tumors derive from germ cells or sex cord stroma
Essential features
- Ovarian carcinoma (previously called ovarian surface epithelial malignant tumors) is not a single entity, but a group of neoplasms with different histotypes, immunohistochemical characteristics, origins and biology
- Although the WHO classification has not substantially changed from the first edition, recent molecular knowledge combined with epidemiological data has caused a considerable reevaluation of the pathogenesis, cell of origin and precursor lesions of ovarian carcinoma
Terminology
- Serous / Endometrioid / Clear cell / Seromucinous / Mucinous / Brenner / Undifferentiated
Epidemiology
- Epithelial cancers represent >90% of ovarian malignant tumors (Pathology 2011 Aug;43:420)
| Tumor type | Acronym | Prevalence |
| High grade serous carcinoma | HGSC | 70% |
| Low grade serous carcinoma | LGSC | 5 % |
| Endometrioid carcinoma | EMC | 10% |
| Clear cell carcinoma | CCC | 10% |
| Seromucinous carcinoma | SMT | rare |
| Mucinous carcinoma | MC | 3% |
| Malignant Brenner tumor | Malignant BT | rare |
| Undifferentiated carcinoma | UC | rare |
Pathophysiology
Traditional pathogenetic view:
Controversies with the traditional view
Realities to consider
Emerging pathogenetic views
- Cells of origin: ovarian surface epithelium (OSE)
- Primary site of origin: ovary
- Mechanism: OSE is a modified mesothelium which undergoes metaplasia, acquiring a Müllerian or non-Müllerian epithelial phenotype and successively undergoes neoplastic transformation
- In 1925, Sampson hypothesized that ovarian carcinoma may arise from malignant transformation of endometriosis, thought to derive from OSE metaplasia or embryonic residuals
- Fathalla’s theory of “incessant ovulation” identifies recurrent ovulation as a transforming event for OSE through direct damage, inflammation and exposure to sex hormone-rich follicular fluid (Lancet 1971;2:163)
- Therefore, incessant ovulation constitutes an index for individual ovarian cancer risk
- This theory assumes that all ovarian surface epithelial tumors have the same origin
- For many years, pathologists diagnosed and classified ovarian epithelial tumors based on histological similarity, size, clinical information and apparent cell of origin; fallopian tubes were inadequately sampled and ignored
Controversies with the traditional view
- No ovarian surface epithelial carcinomas (serous, endometrioid, clear cell, mucinous, seromucinous, transitional) resemble OSE
- Metaplasia of OSE is difficult to demonstrate and almost never observed
- There are no intermediate / hybrid phenotypes of OSE metaplasia tumors
- Real mesotheliomas are rare
- Most ovarian tumors do not express OSE molecular markers
- In the testis, analogous surface (tunica albuginea) tumors are rare
Realities to consider
- Recent advances in molecular genetics have found mutations in BRCA1 and BRCA2 tumor suppressor genes responsible for most hereditary ovarian cancer
- When patients with mutations underwent prophylactic salpingo-oophorectomy, dysplastic precursor lesions were found in fallopian tubes (later named Serous Tubal Intraepithelial Carcinoma, STIC), but not in ovaries
- Tubal ligation reduces the incidence of ovarian endometrioid, clear cell carcinoma and high grade serous carcinoma (Cancer Epidemiol Biomarkers Prev 1996;5:933, Int J Cancer 2016;138:1076)
- Thorough examination of fallopian tubes using the SEE-FIM protocol (Sectioning and Extensively Examining the FIMbriated end), revealed early fallopian tube cancer in sporadic high grade serous carcinomas
- There are overlapping molecular alterations with STIC and high grade serous carcinoma regarding p53, p16, FAS, RSF1, CCNE1 and centrosome amplification, suggesting a clonal relationship (Mod Pathol 2016;29:1254)
- Endometrioid (40%) and clear cell carcinoma (50-90%) are commonly associated with endometriosis, and patients with endometriosis have a 3-10x increased risk of developing ovarian endometrioid and clear cell carcinomas
- Molecular genetic alterations reveal a clonal relationship between endometriosis and endometrioid and clear cell carcinomas (ARID1A, KRAS, MET) (Int J Gynecol Cancer 2012;22:1310)
- Primary mucinous ovarian carcinomas are uncommon: the real incidence of mucinous carcinomas is less than previously thought
Emerging pathogenetic views
- Ovarian carcinoma (so called ovarian surface epithelial tumors) is not a single entity
- Cells of origin: extra-ovarian Müllerian epithelia (endometrium or fallopian tube epithelium)
- Primary site of origin: extra-ovarian Müllerian epithelia (endometrium or fallopian tube epithelium) or ovary (endometriosis or fallopian tube epithelium seeded on the ovary as inclusion cysts)
- Mechanism: genetically normal or initially transformed epithelial cells from fallopian tube or endometrium seed the ovary, whose microenvironment promotes their neoplastic transformation
- Mucinous carcinomas are composed of tumors with different genesis, including mature cystic teratoma and extra-ovarian Müllerian epithelium (Brenner tumors or endometriosis)
Etiology
- The most significant risk factor is family history (Bethesda (MD): National Cancer Institute (US); 2002)
- Contributing factors: excessive gonadotropins, androgen stimulation, pelvic inflammation, ovarian exposure to contaminants and carcinogens (talc and asbestos), nulliparity, refractory infertility, obesity
- Protective factors: multiparity, tubal ligation, hysterectomy, oral contraceptives (use for 5+ years reduces risk 50% even for those with a family history, Cancer Epidemiol Biomarkers Prev. 2009 Feb;18:601)
Clinical features
Genetic syndromes and germline alterations
- Hereditary Breast and Ovary Cancer syndrome from germline mutations in BRCA1 and BRCA2
- Lynch syndrome from germline mutations in DNA mismatch repair genes (MLH1, MSH2, MSH6, PMS2)
- Peutz-Jeghers syndrome from germline mutations in STK11
- Cowden syndrome from germline mutations in PTEN
- Coffin-Siris Syndrome from germline mutations in ARID1A
- Li-Fraumeni syndrome from germline mutations in TP53 / p53
- Other germline mutations that are correlated to ovarian carcinoma affect BRIP1, CHEK2, RAD51
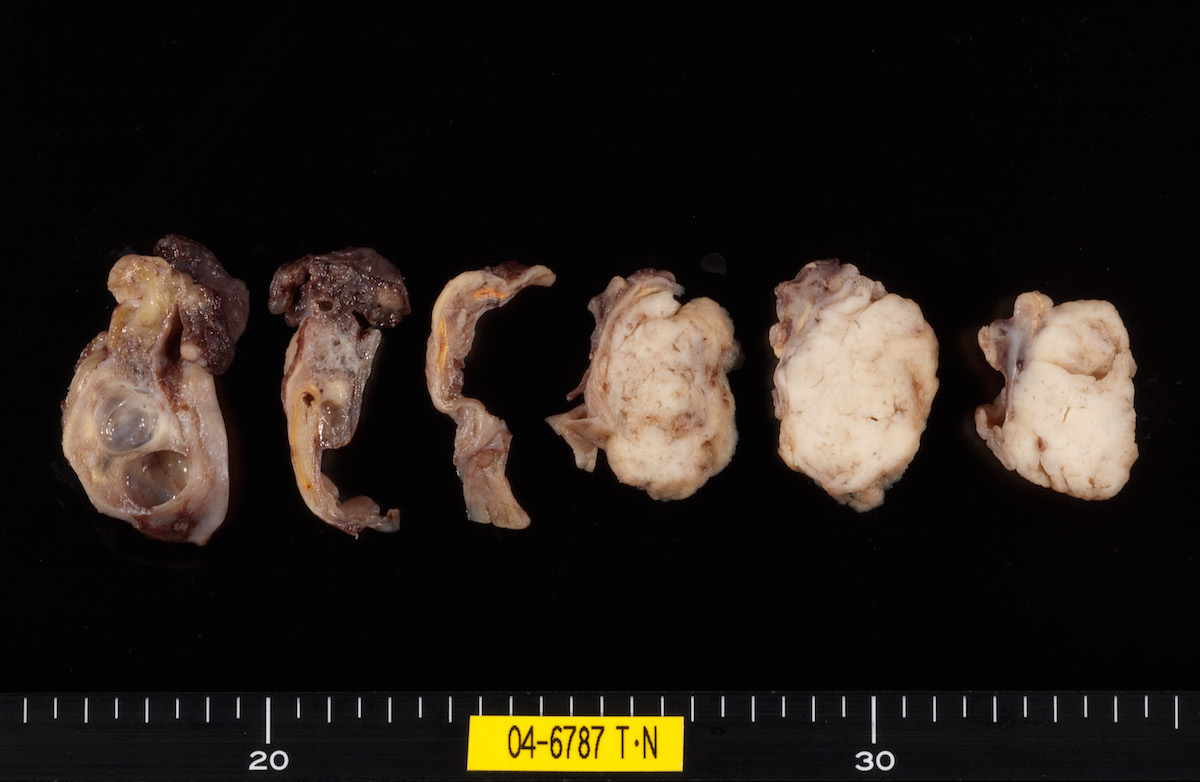
Gross images
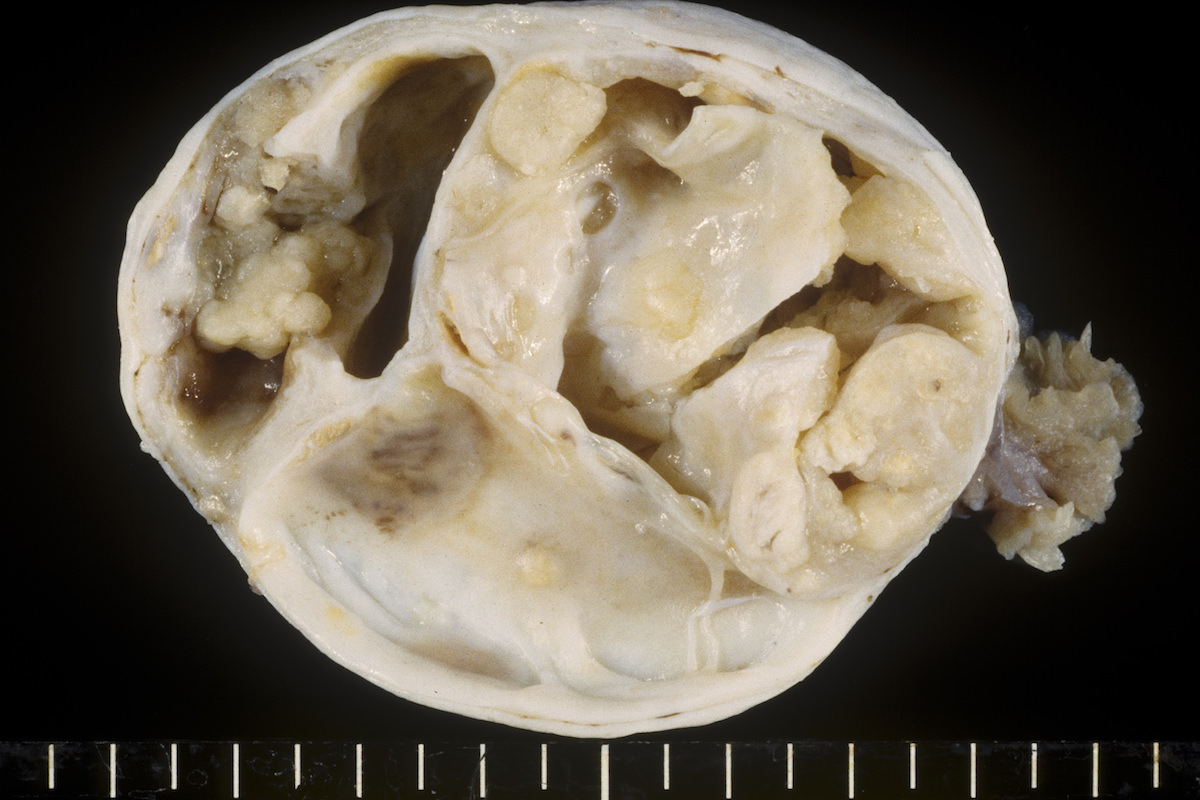
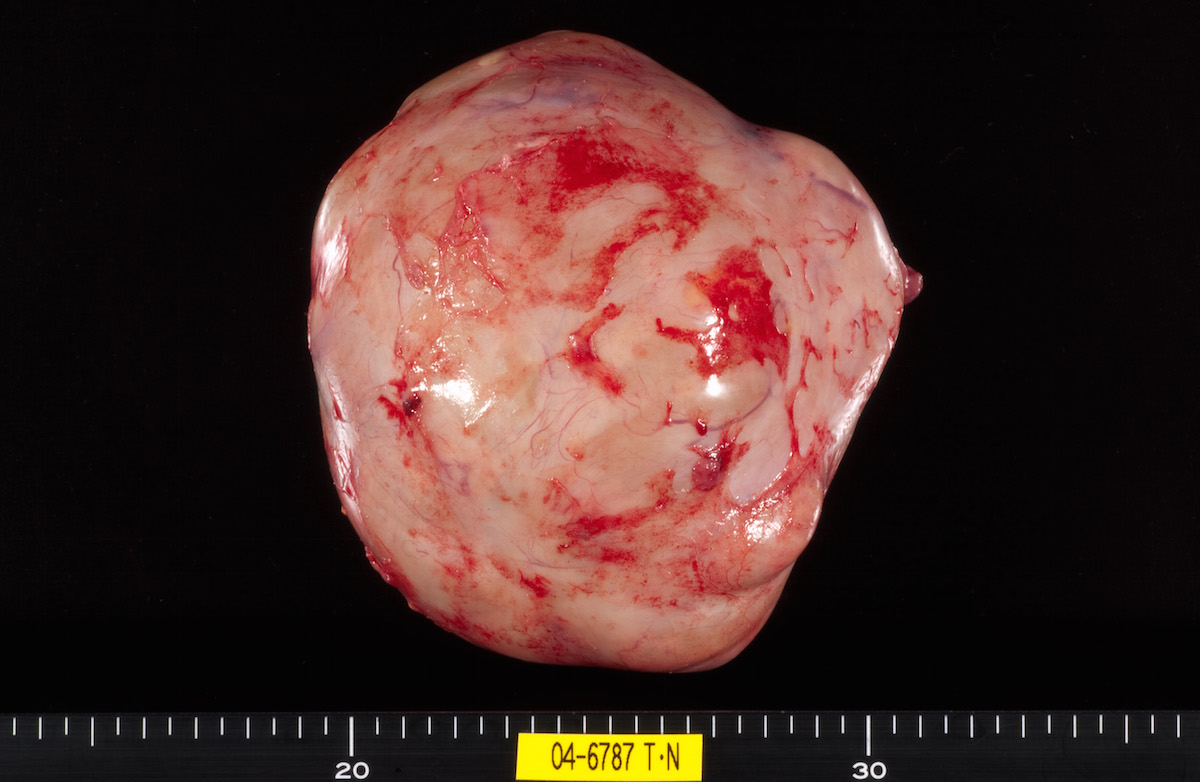
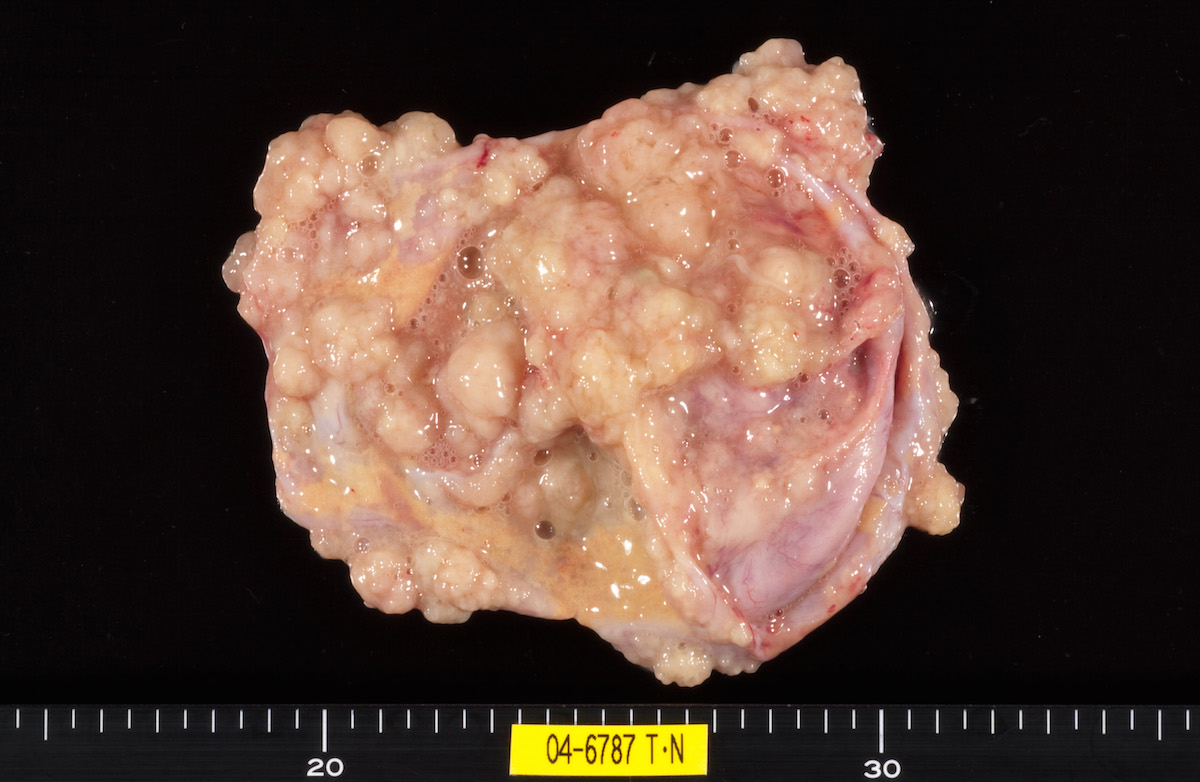
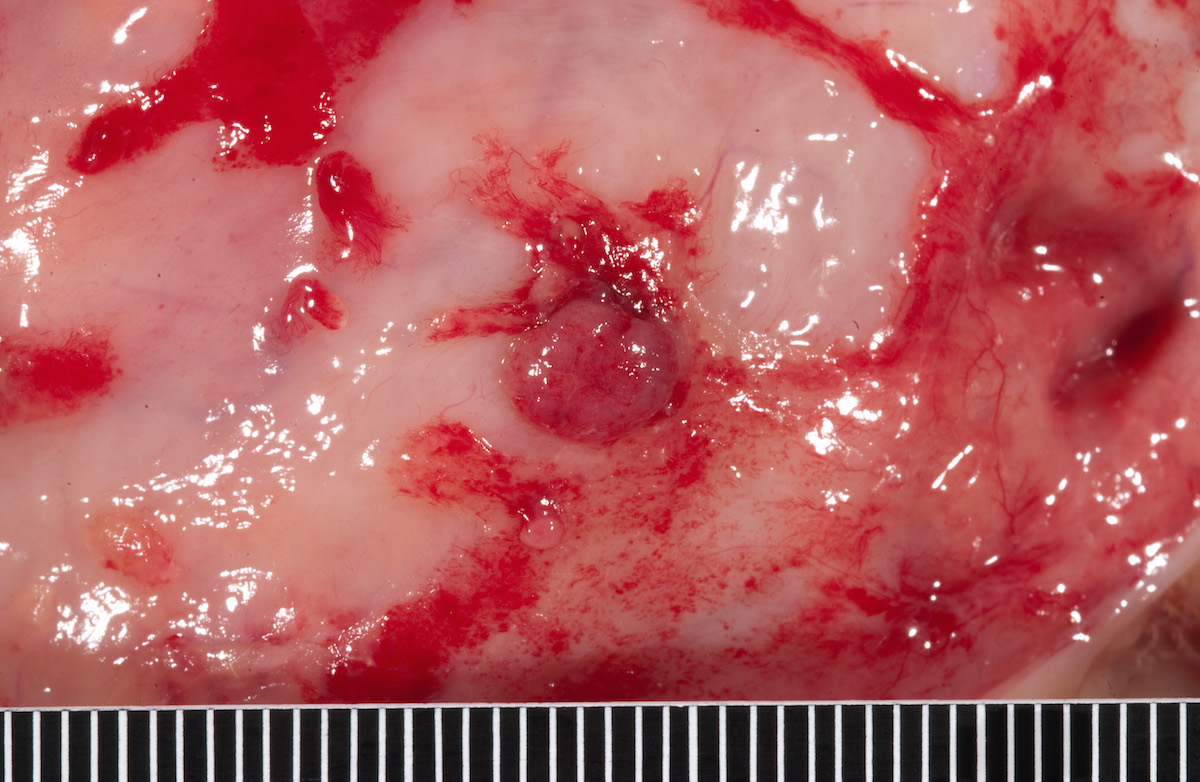
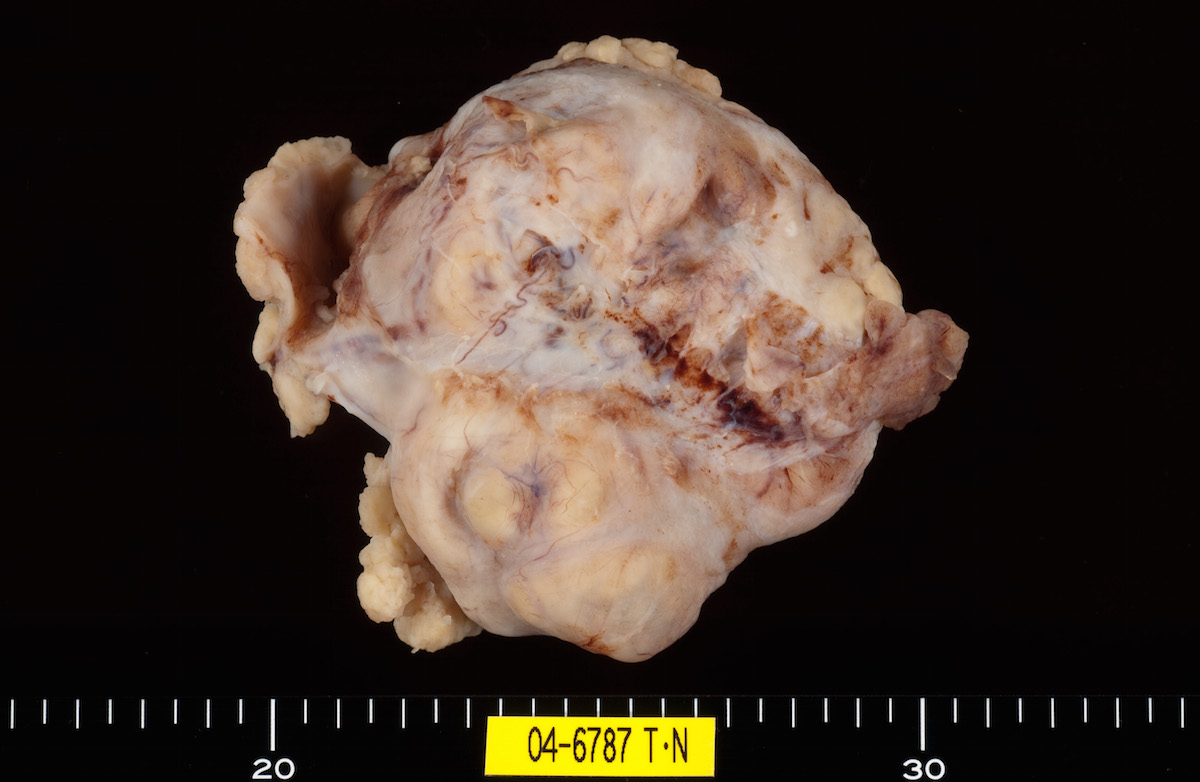
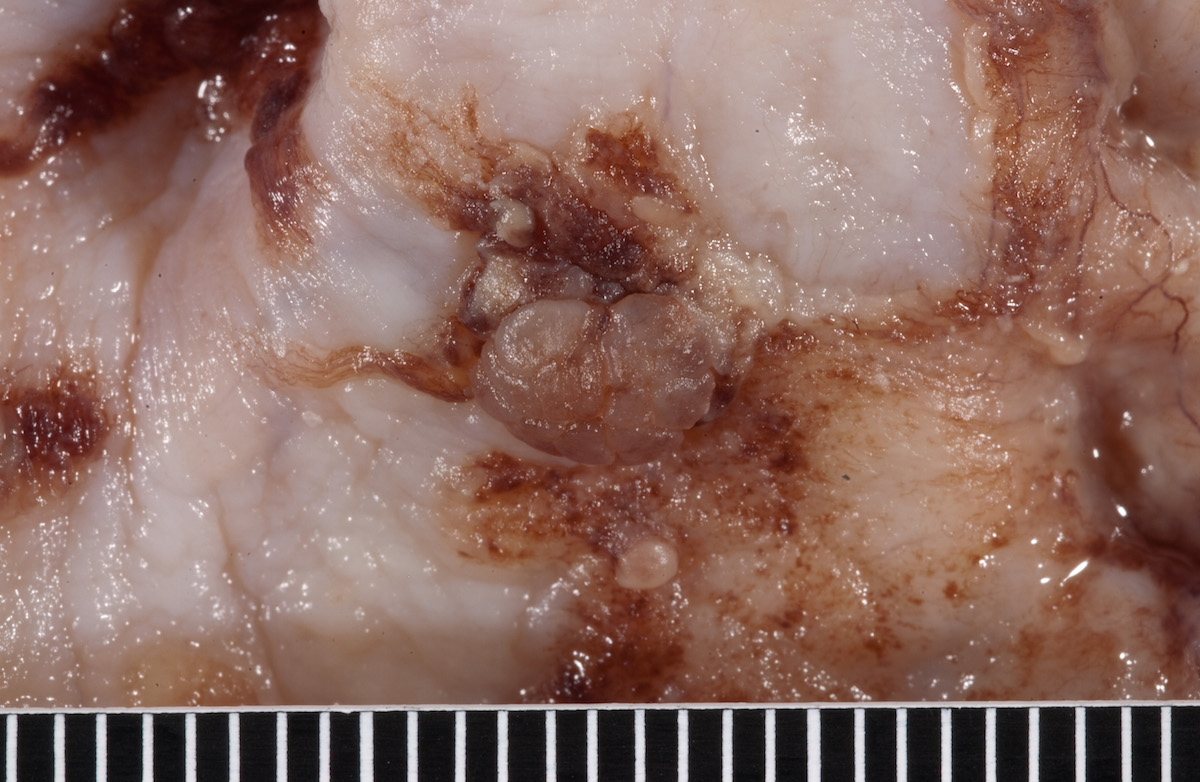
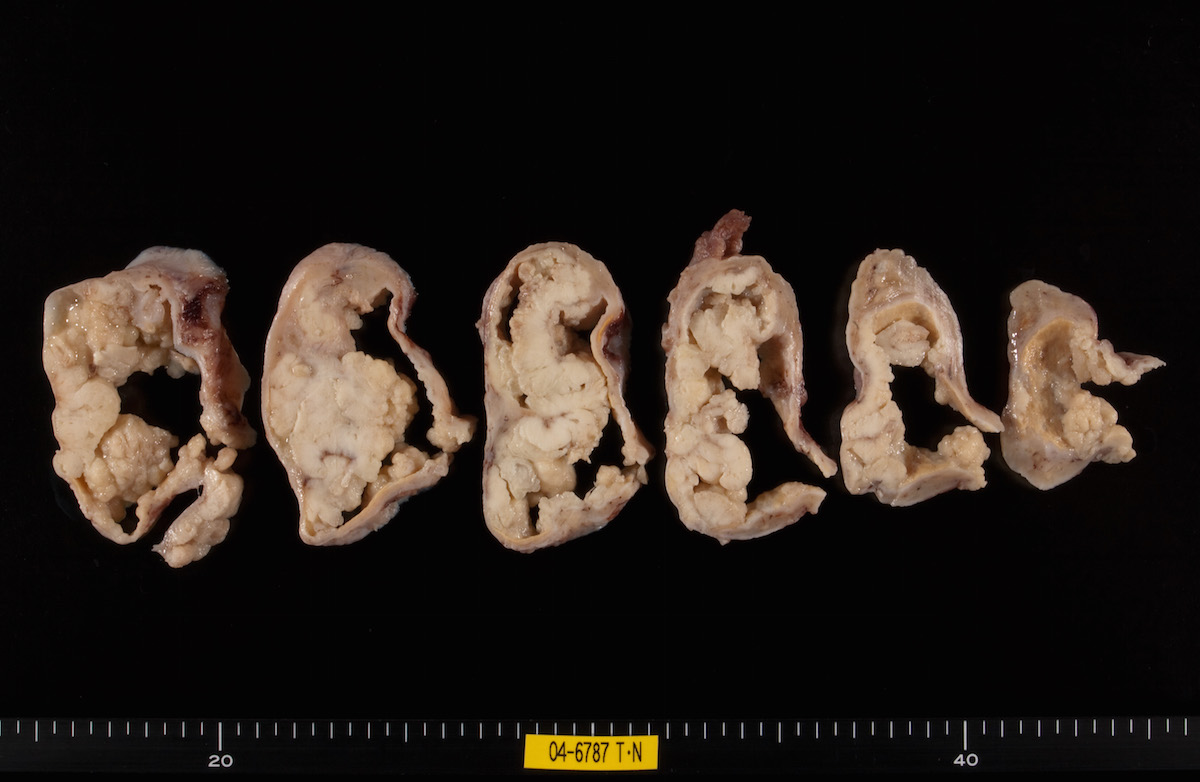
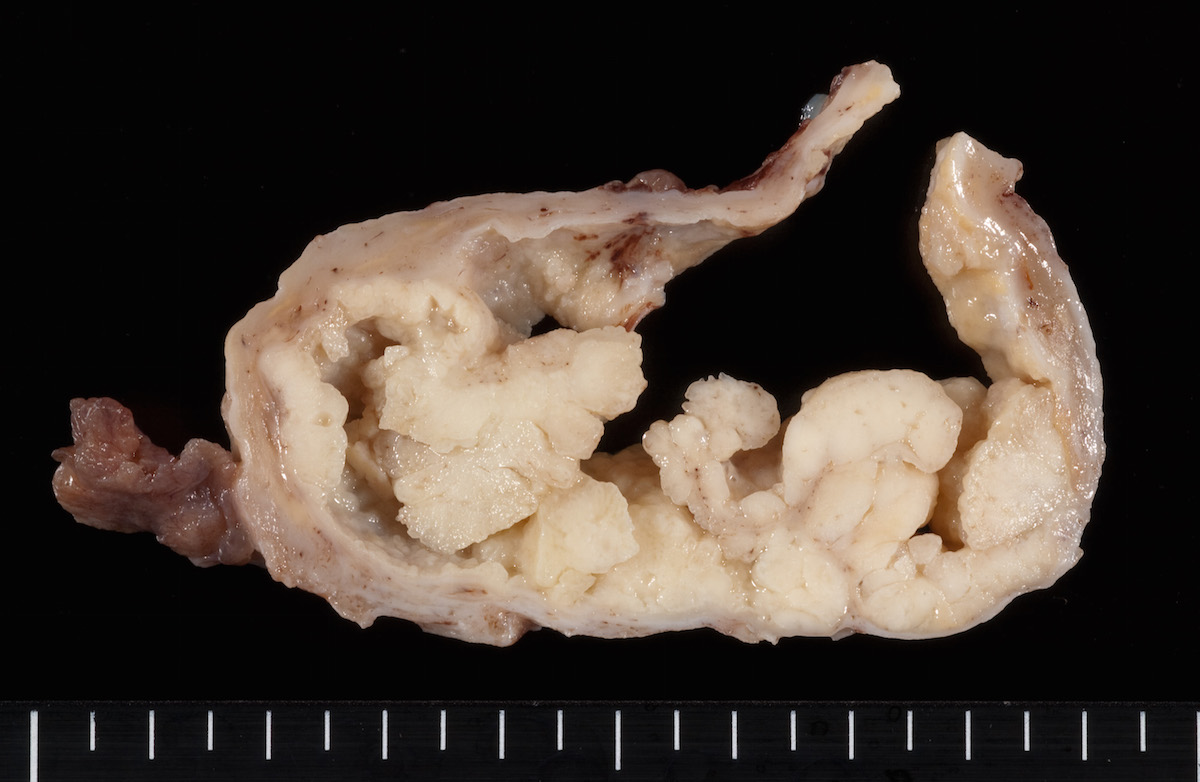
Contributed by Ayse Ayhan, M.D.
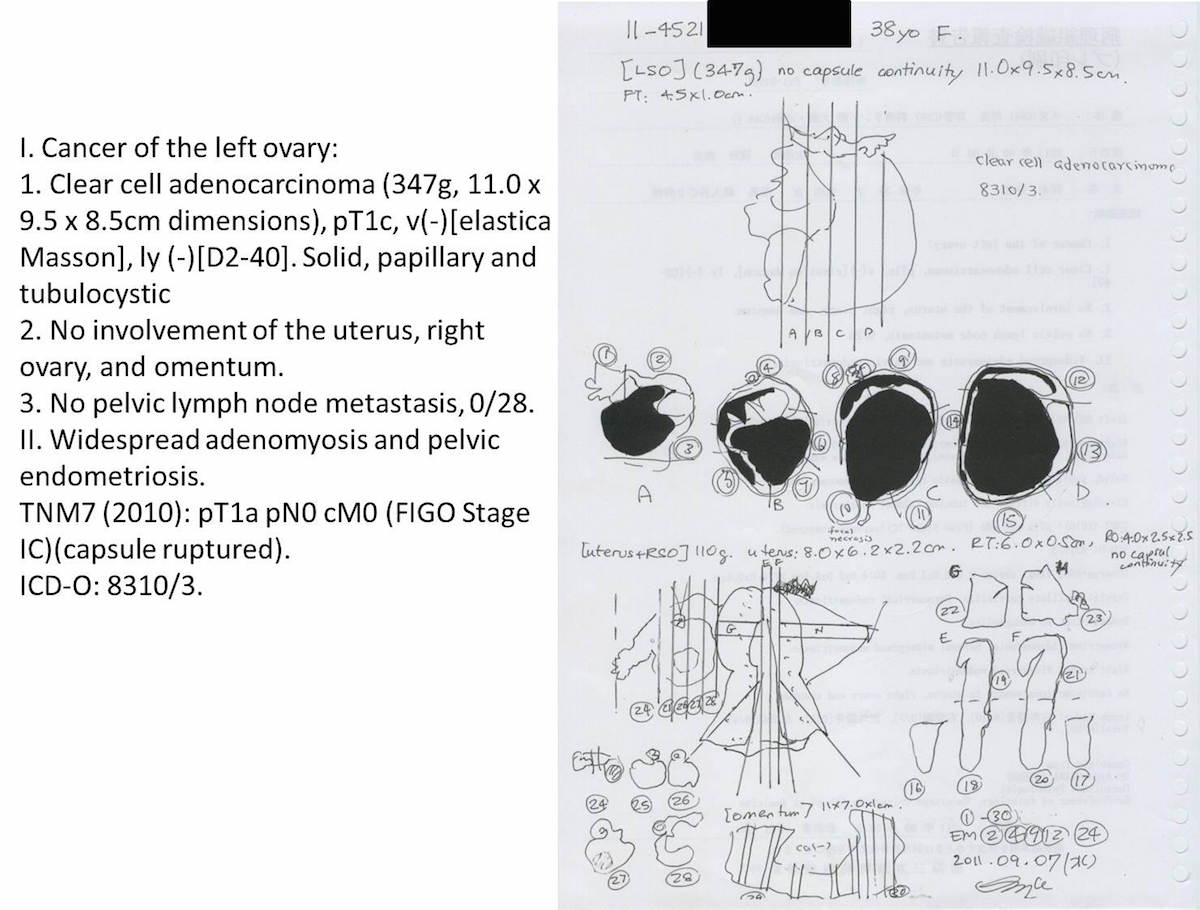
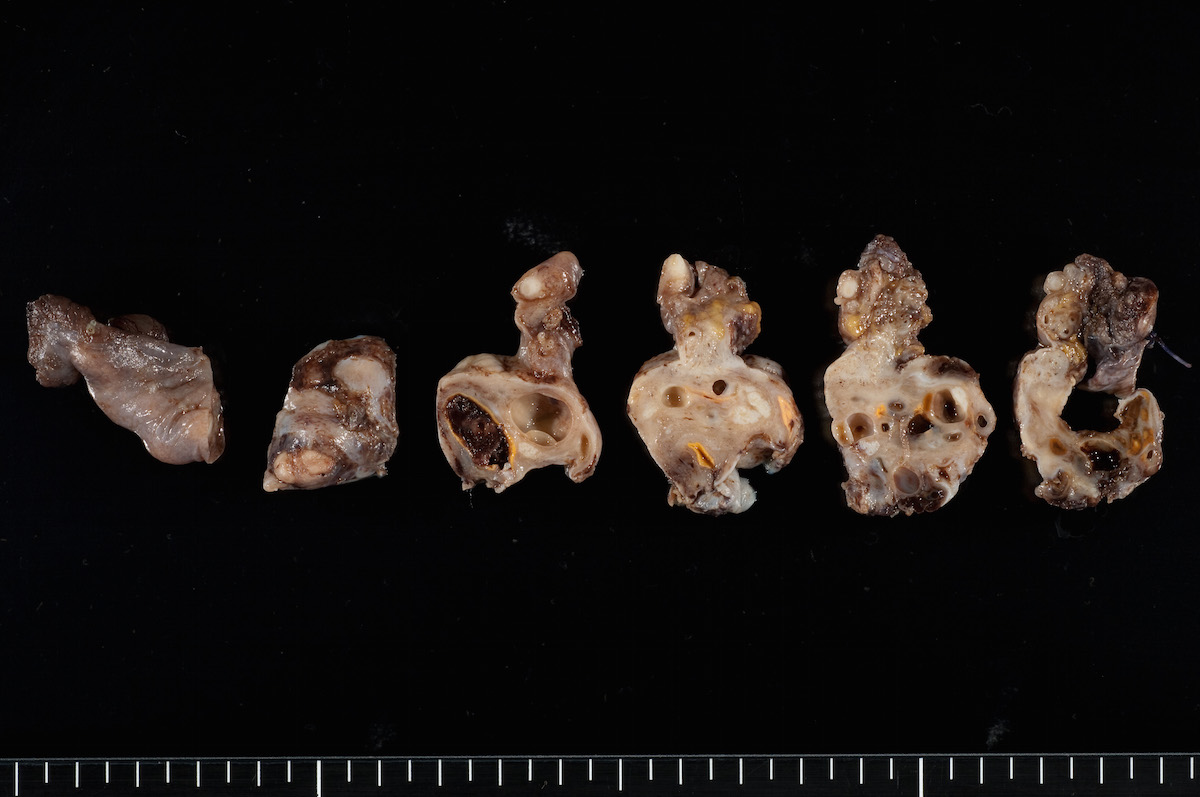
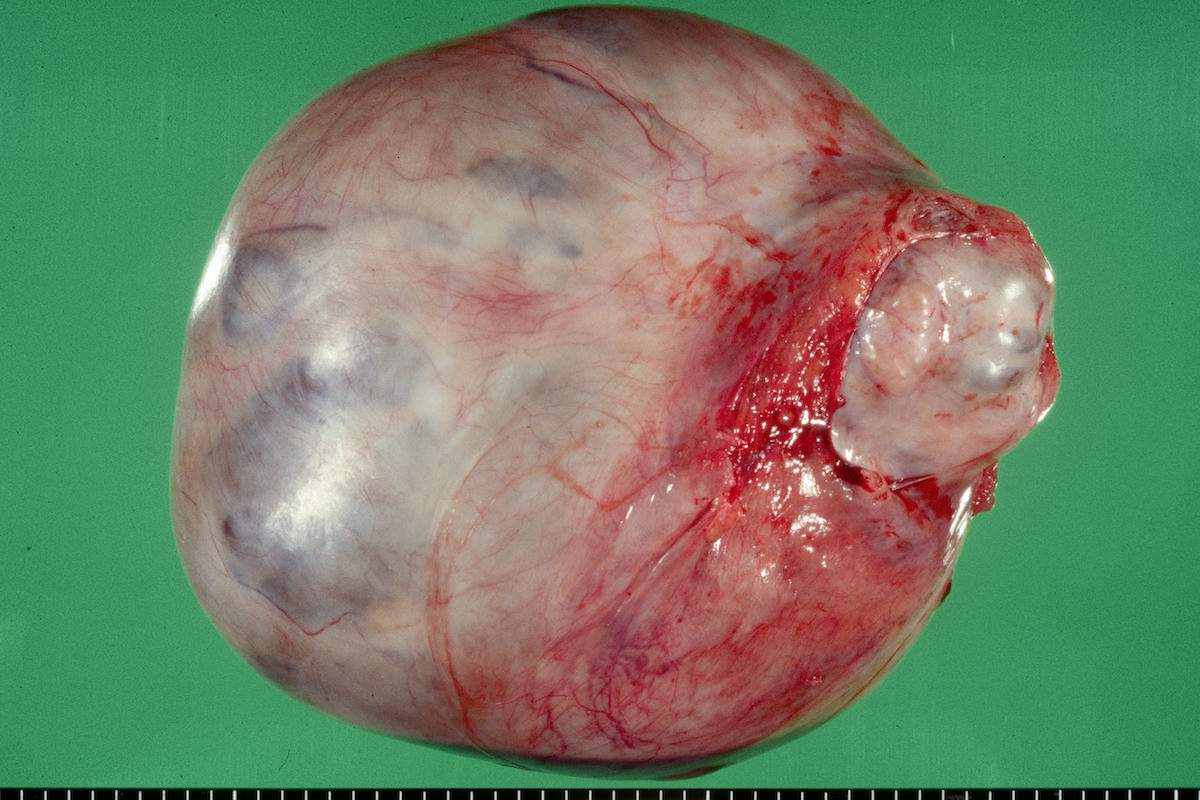
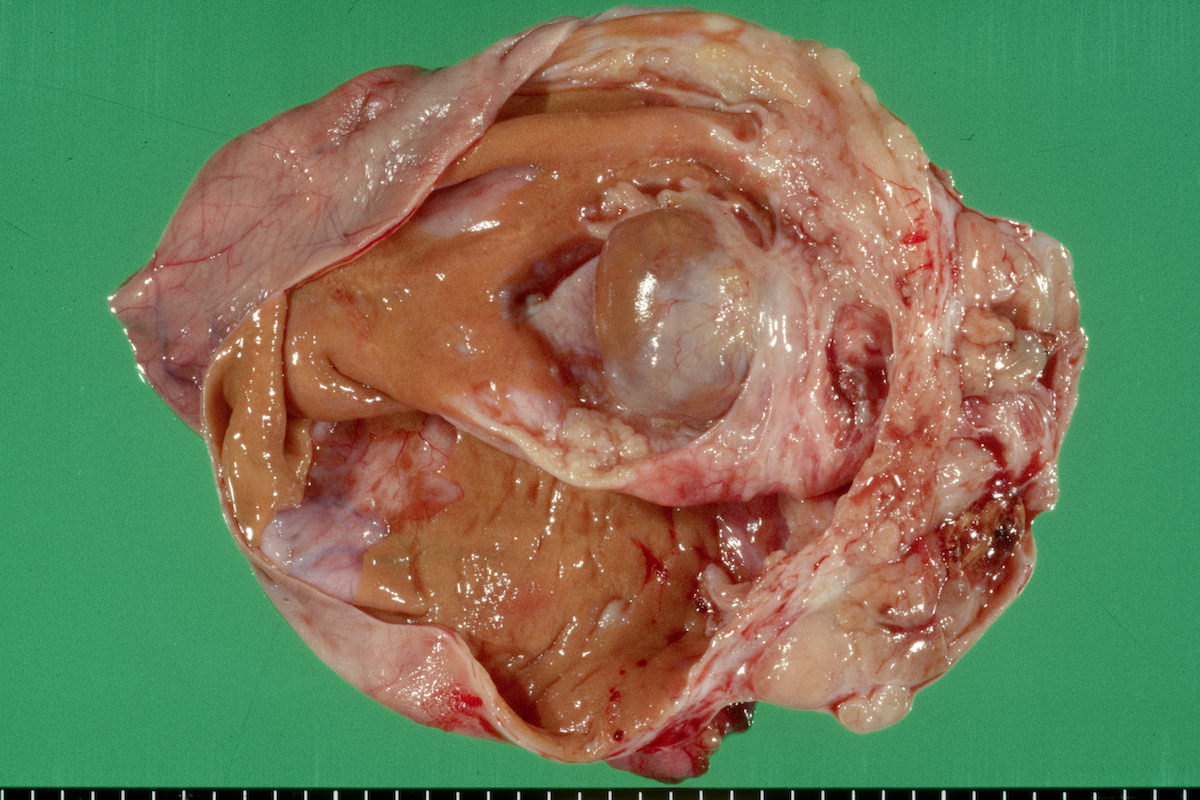
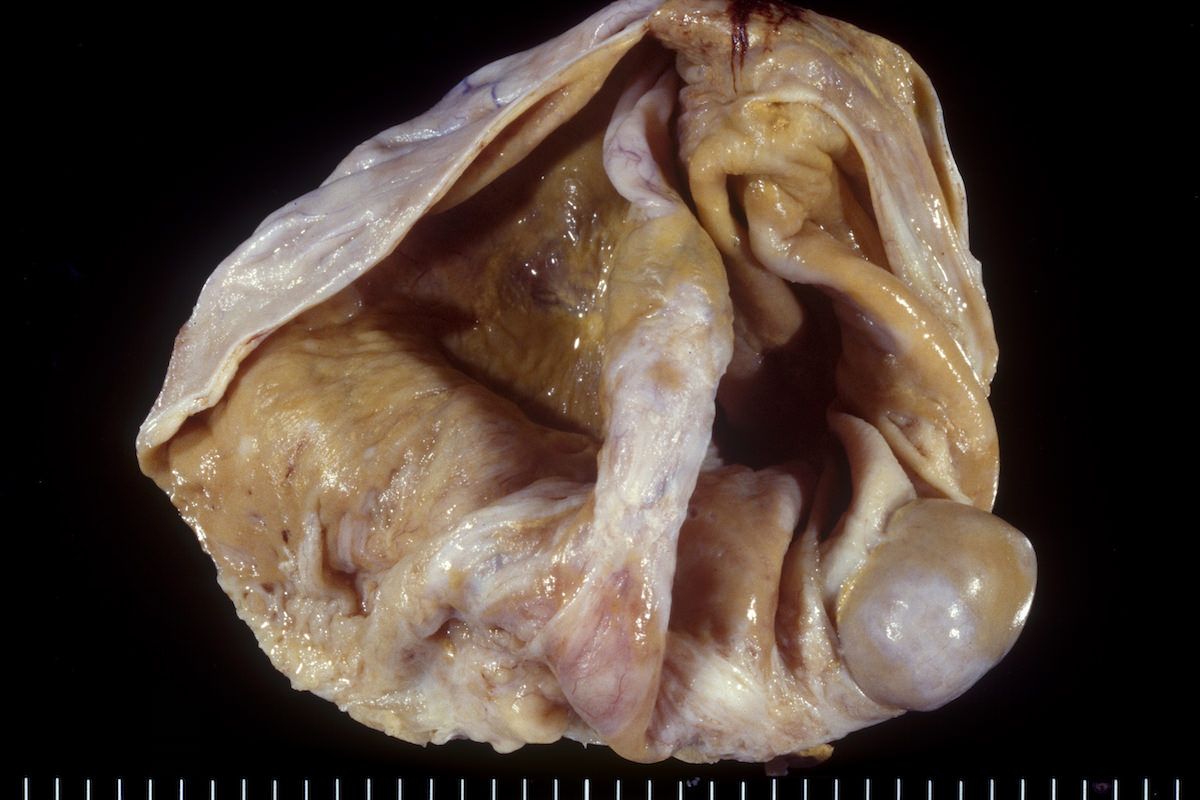
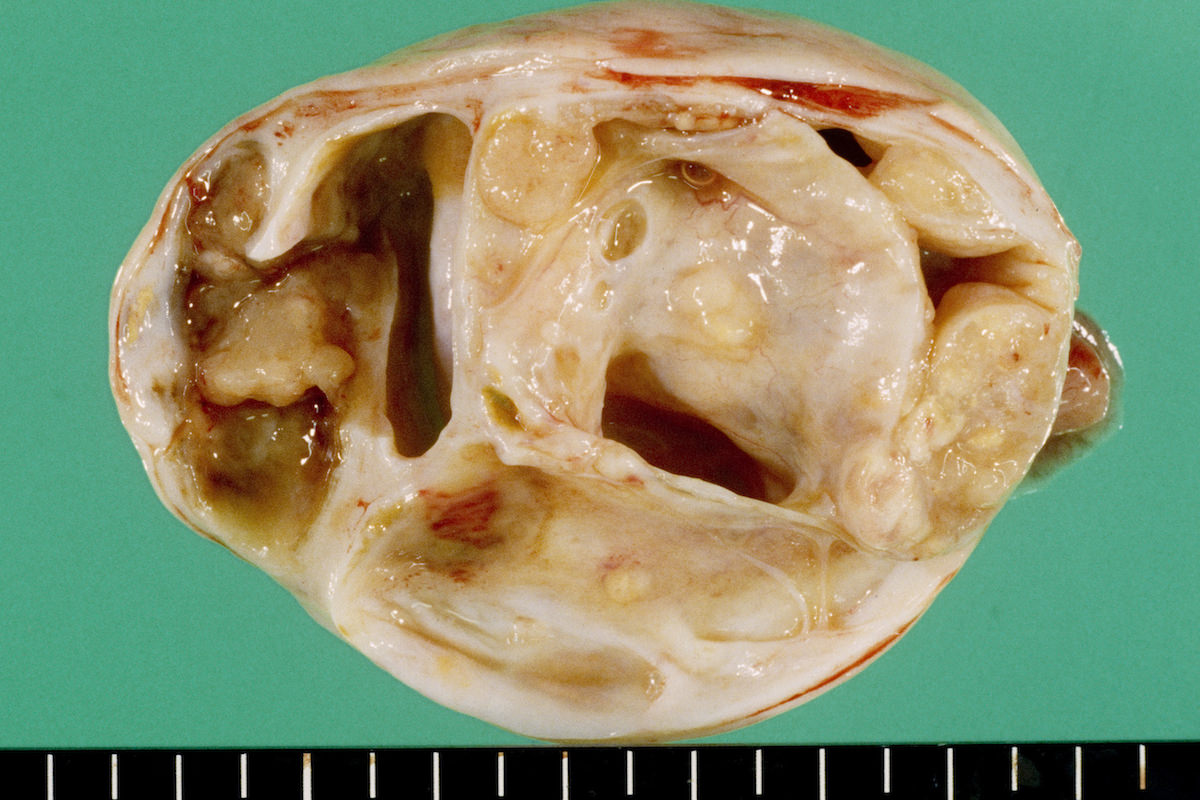
Clear cell carcinoma, case 1:
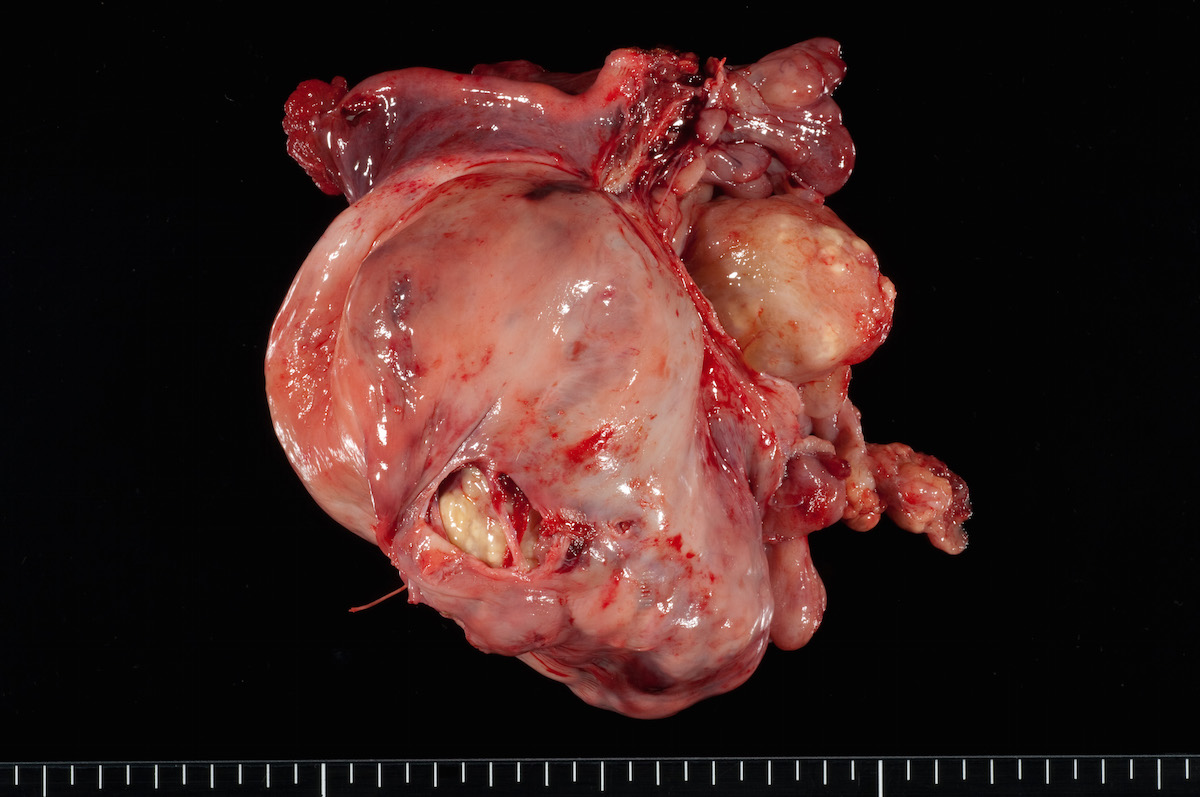
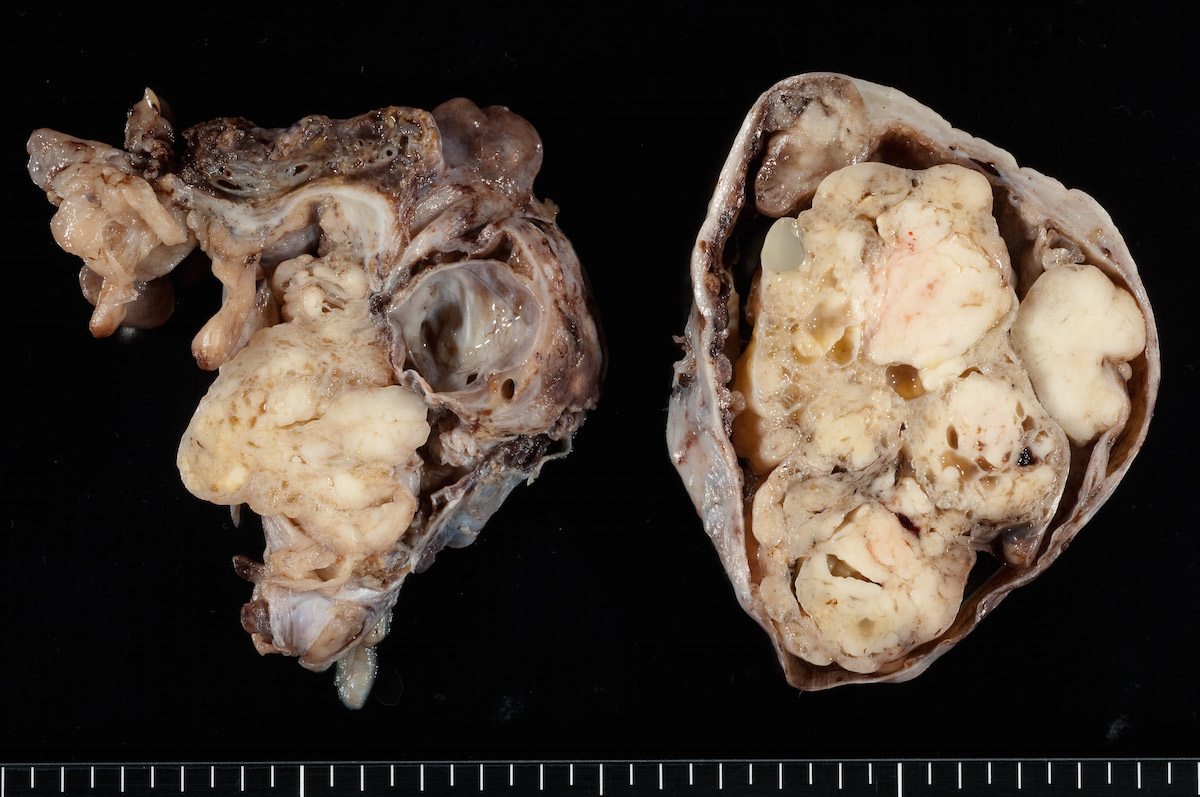
Clear cell carcinoma, case 2:
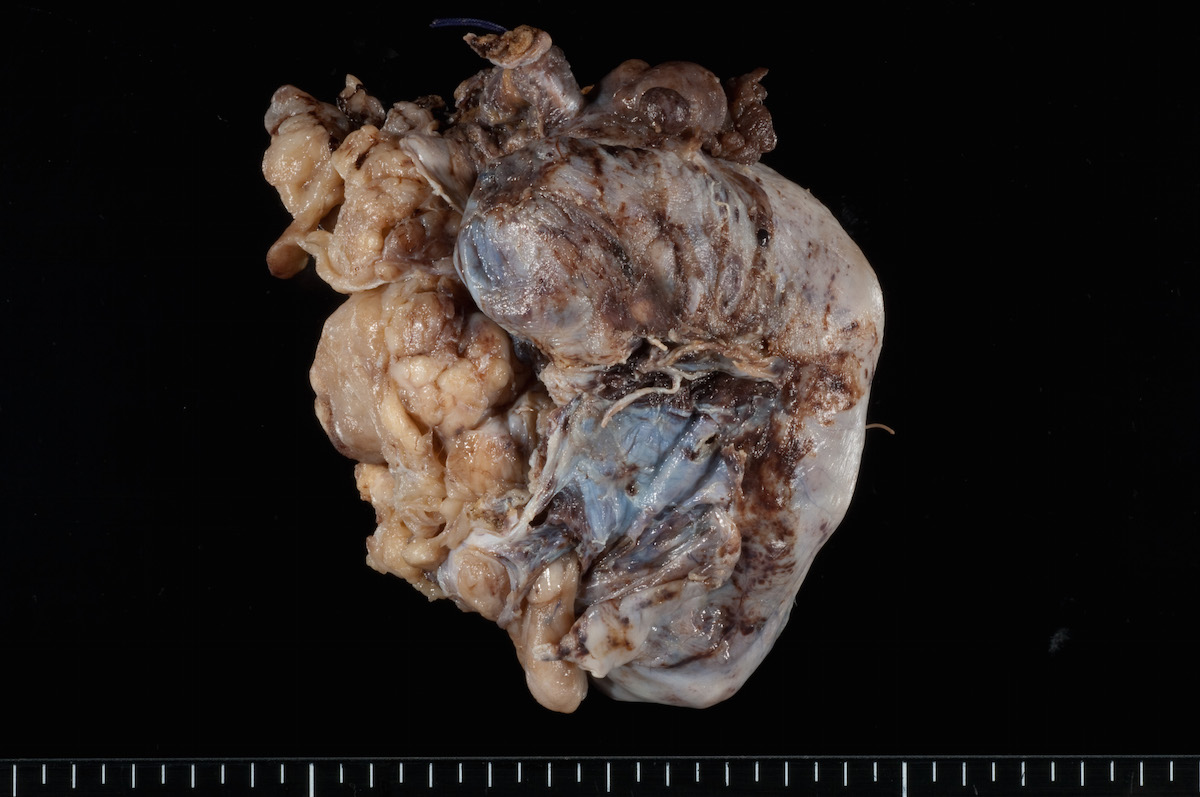
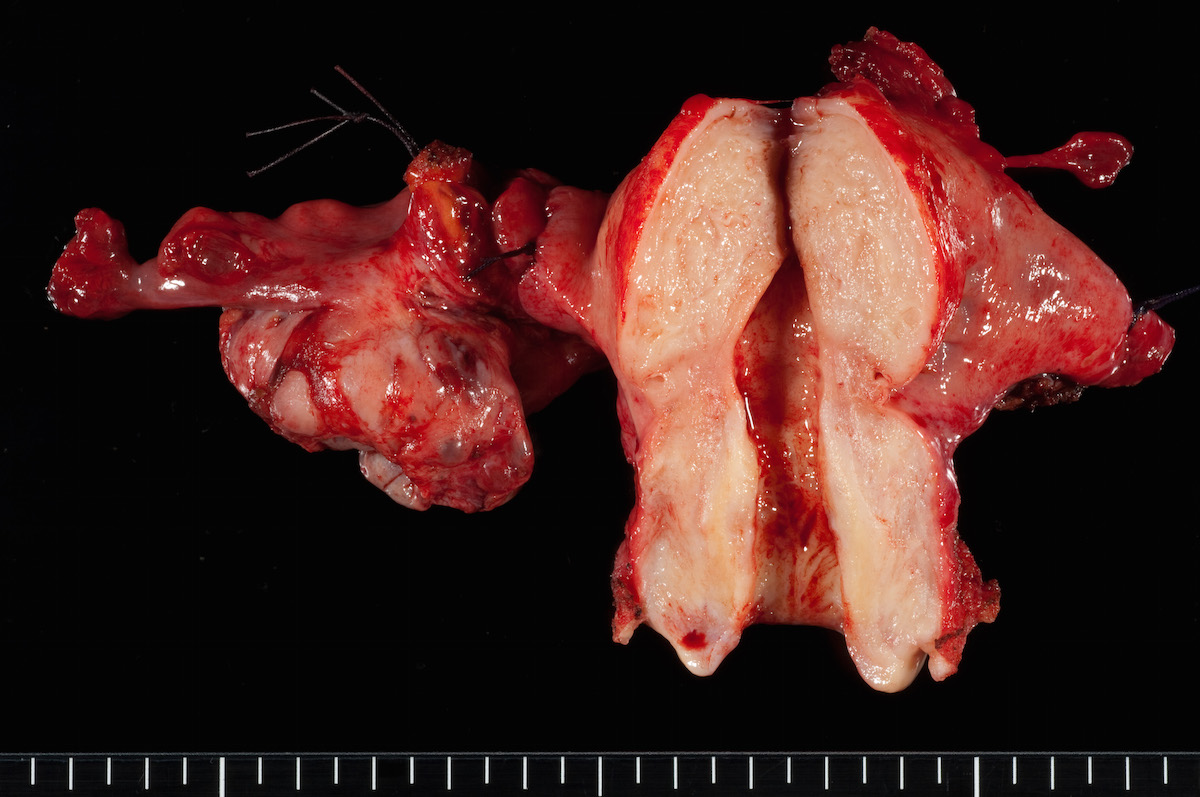
Bilateral endometrioid cancer:
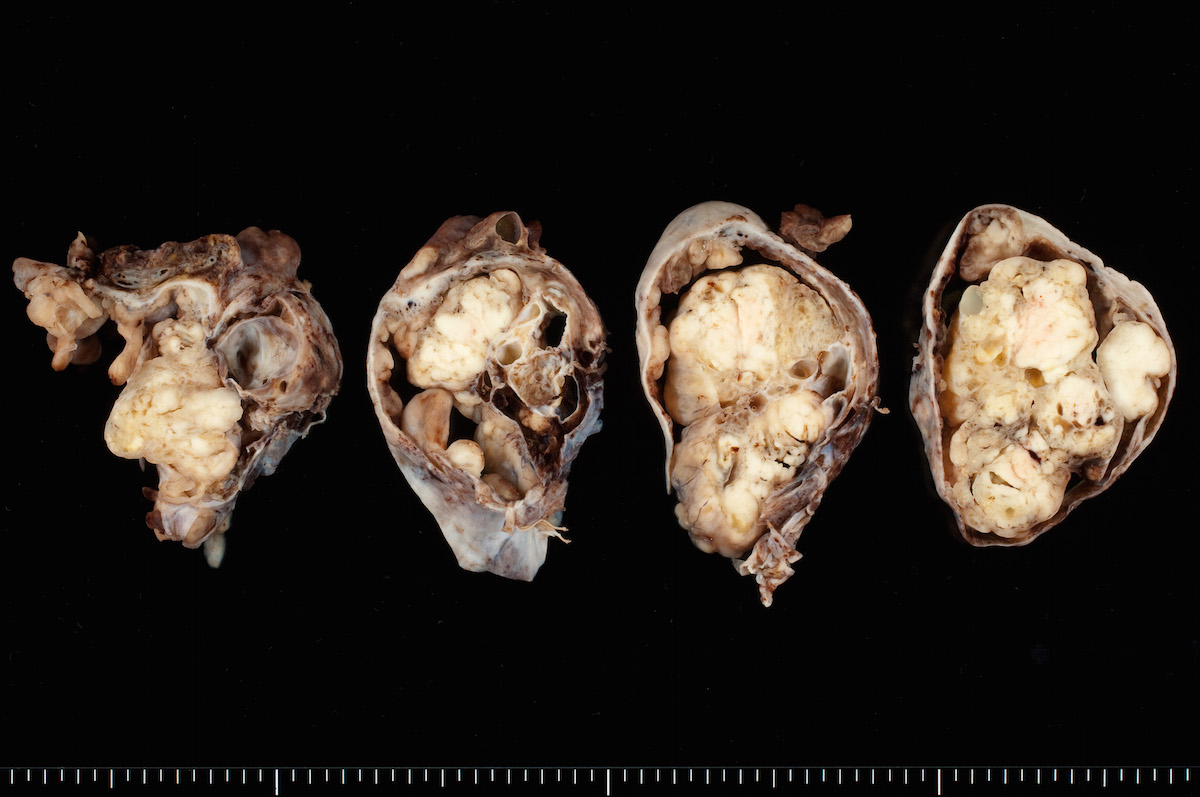
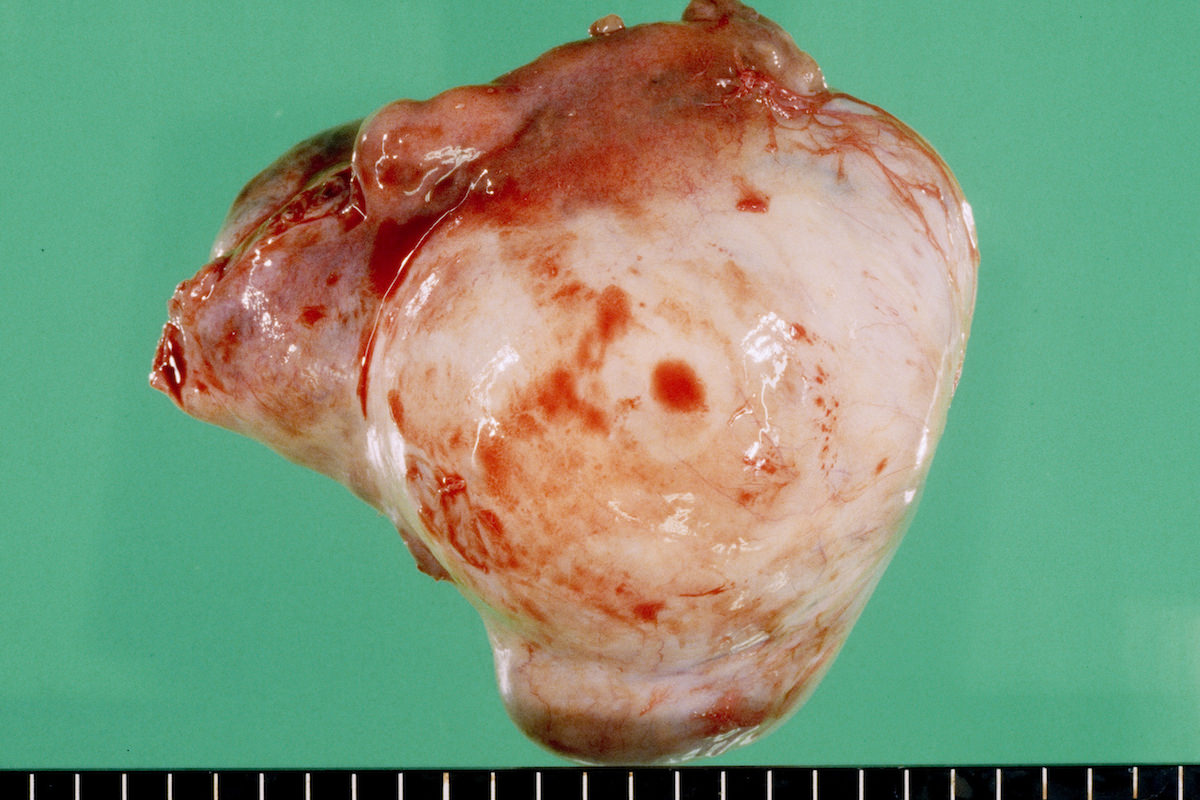
Ovarian tumor:
Microscopic (histologic) description
Putative precursor lesions
- Serous tubal intraepithelial carcinoma / low grade serous carcinoma: for high grade serous carcinoma
- Serous borderline tumor: for low grade serous carcinoma and a few high grade serous carcinomas
- Endometriosis / adenofibroma / borderline tumor: for endometrioid carcinoma, clear cell carcinoma, seromucinous carcinoma, mixed endometrioid-clear cell carcinoma
- Endometriosis / mucinous borderline tumor: for mucinous carcinoma
- Teratoma / Brenner tumor / mucinous borderline tumor: for mucinous carcinoma
- Benign Brenner tumor: for borderline Brenner tumor
Positive stains
- p53 (high grade serous carcinoma, high grade endometrioid carcinoma, mucinous carcinoma)
- nuclear β catenin (endometrioid carcinoma)
- HER2 (mucinous carcinoma)
- Refer to histopathology for lineage specific markers
Negative stains
- p53 (30% of high grade serous carcinoma)
- MLH1, MSH2, MSH6, PMS2 (endometrioid carcinoma)
- PTEN (endometrioid carcinoma)
- ARID1A (endometrioid carcinoma, clear cell carcinoma, seromucinous carcinoma) (Int J Gynecol Cancer 2012;22:1310, Int J Gynecol Pathol 2012;31:297)
- Refer to histopathology for lineage specific markers
Molecular / cytogenetics description
Common mutations
NOTE: High grade serous carcinoma and low grade serous carcinomas have a mutually exclusive mutational signature
| Tumor type | Acronym | Prevalence |
| High grade serous carcinoma | BRCA1 | 12% |
| BRCA2 | 11% | |
| CSMD3 | 6% | |
| FAT3 | 6% | |
| TP53 | 95% | |
| Low grade serous carcinoma | BRAF | 38% |
| KRAS | 19% | |
| Endometrioid carcinoma | ARID1A | 55% |
| CTNNB1 | 60% | |
| KRAS | 7% | |
| MLH1/MSH2/MSH6/PMS2 | 20% | |
| PIK3CA | 35% | |
| PTEN | 16% | |
| PPP2R1A | 10-15% | |
| TP53 | 7% | |
| Clear cell carcinoma | ARID1A | 75% |
| KRAS | 10% | |
| PIK3CA | 35% | |
| PPP2R1A | 10% | |
| TERT | 16% | |
| Seromucinous tumor | ARID1A | 33% |
| KRAS | 69% | |
| Mucinous carcinoma | BRAF | 12% |
| HER2 | 50% | |
| KRAS | 54% | |
| RNF43 | 20% | |
| TP53 | 50% | |
| Borderline Brenner tumors | KRAS | 14% |
| PIK3CA | 28% |
NOTE: High grade serous carcinoma and low grade serous carcinomas have a mutually exclusive mutational signature
- Germline/somatic mutations in BRCA1, BRCA2 and other genes predict:
- Platinum sensitivity and longer survival in women with high grade serous carcinoma
- Benefit from PARP inhibitors (Journal of Cancer 2016;7:1441)
Additional references
- Kuhn E, Ayhan A., The non-ovarian origin and pathogenesis of ovarian carcinomas: update on the pathological and the molecular clues (PDF)
- Kurman RJ, Shih IeM., The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded (Am J Pathol 2016 Apr;186:733)
- Prat, J., Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features (Virchows Arch 2012 Mar;460:237)
- Lim D, Oliva E., Precursors and pathogenesis of ovarian carcinoma (Pathology 2013 Apr;45:229)