Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Electron microscopy description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Rastegar S, Kalir T. Leydig cell hyperplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/ovaryleydigcellhyperplasia.html. Accessed December 25th, 2024.
Definition / general
- Initially described by Rutgers and Scully (Int J Gynecol Pathol 1986;5:319)
- Benign proliferation of Leydig (hilus) cells in ovarian hilum; polygonal cells with abundant eosinophilic cytoplasm and Reinke crystals, closely associated with neurovasculature
Essential features
- Postmenopausal occurrence
- Predominantly located in the ovarian hilus, closely associated with neurovasculature
- Microscopic aggregates of Leydig (hilus) cells
- Reinke crystals and lipofuscin deposits present
- May present with virilization due to androgen production
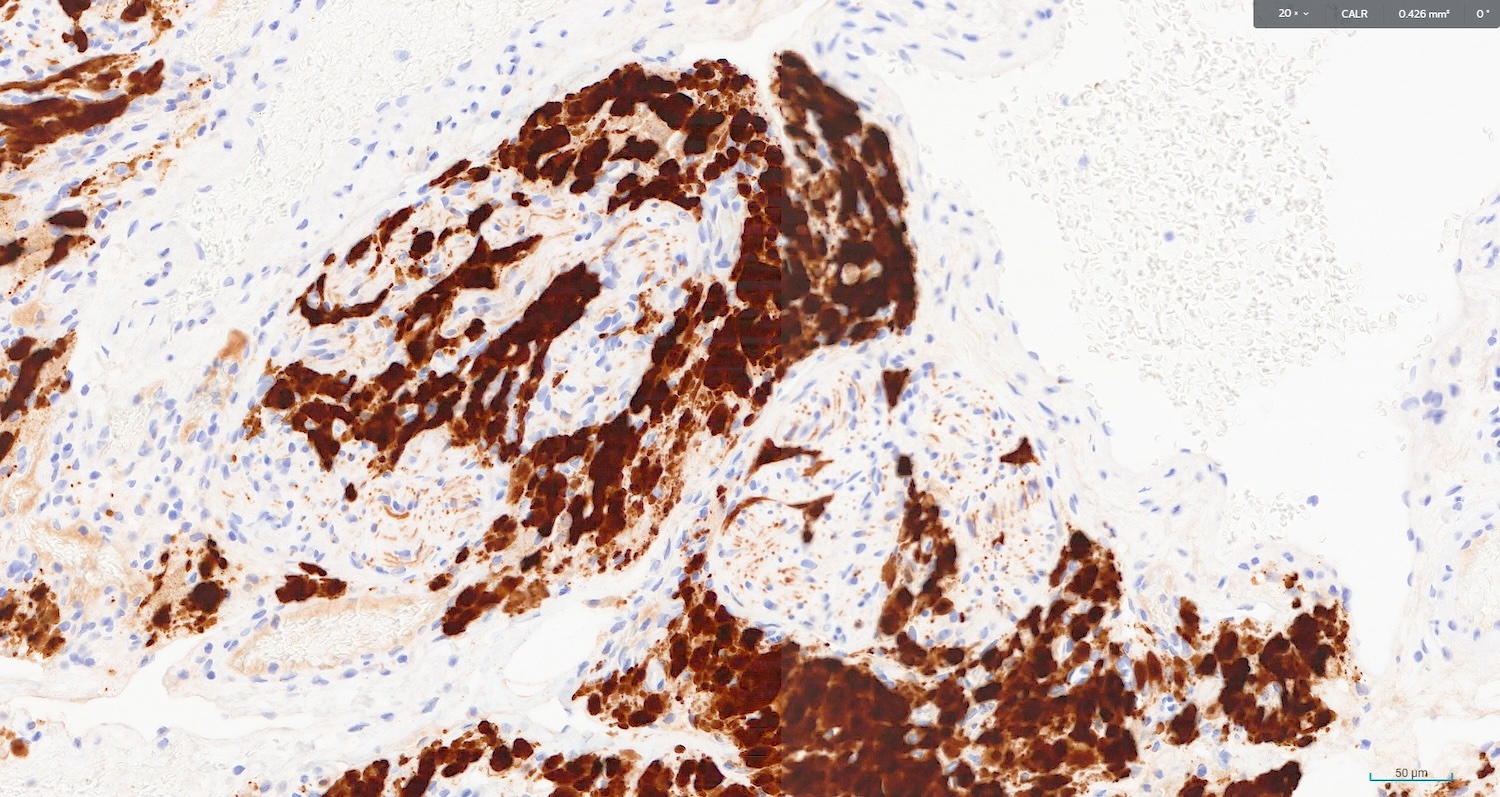
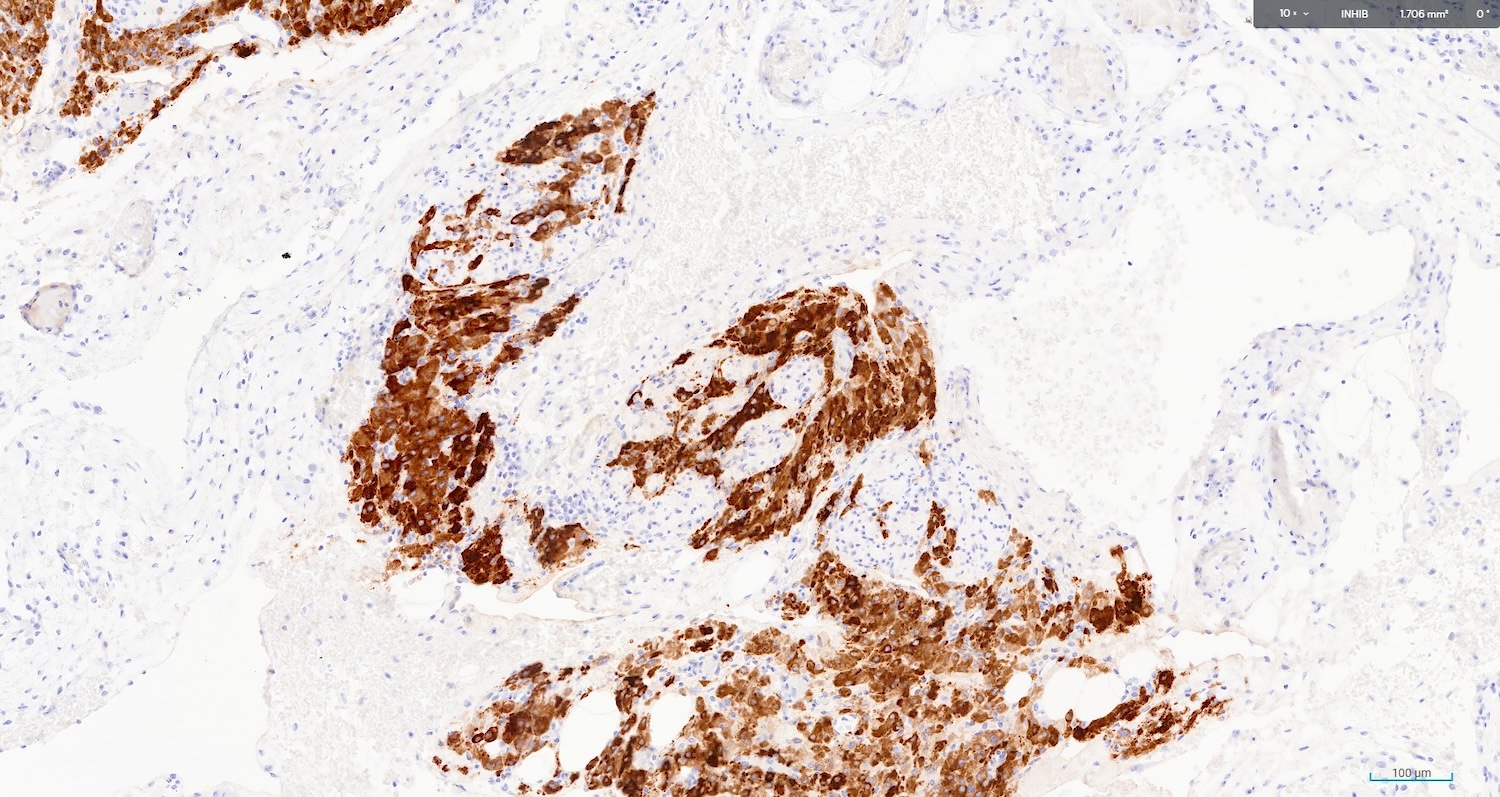
- Stain positive for calretinin and inhibin
Terminology
- Acceptable: hilus cell hyperplasia (Int J Gynecol Pathol 1986;5:319)
ICD coding
Epidemiology
- Most common in postmenopausal and pregnant women (Int J Gynecol Pathol 1993;12:108)
- Postmenopausal age range is 72 - 82 years old (average: 75) (Int J Gynecol Pathol 1986;5:319)
Sites
- Usually bilateral in ovarian hilus (Eur J Gynaecol Oncol 2009;30:338)
- May be located in mesovarium, mesosalpinx and in medullar and cortical areas of the ovaries (Histol Histopathol 2017;32:1089)
Pathophysiology
- Fetal Leydig cells differentiate from mesenchymal cells during the eighth week of life; after the eighteenth week, the Leydig cells start to involute (Z Anat Entwicklungsgesch 1971;135:43)
- Fetal Leydig cells disappear completely during the first years of life; new generation of Leydig cells differentiate at puberty (Histopathology 1988;12:307)
- In adult women, the ovarian Leydig cells are possibly derived from the neural crest or the ganglia (Histol Histopathol 2017;32:1089)
Etiology
- Leydig cell proliferation is stimulated by human chorionic gonadotropin (hCG) in pregnancy or luteinizing hormone (LH) in menopause (Case Rep Womens Health 2023;39:e00537)
Clinical features
- Ovarian Leydig cells produce androgens and are associated with increased serum levels of testosterone and androstenedione and eventually virilization (Hum Pathol 2019;85:119)
Diagnosis
- Diagnosis of Leydig cell hyperplasia needs a systematic approach; it is aided by hormone levels rather than imaging and is confirmed by histopathological examination of the ovaries (Case Rep Womens Health 2023;39:e00537)
Laboratory
- Increased serum levels of testosterone and androstenedione (Hum Pathol 2019;85:119)
Radiology description
- Imaging is not helpful; normal findings on imaging can be seen in Leydig cell hyperplasia (Gynecol Endocrinol 2013;29:213)
- Transvaginal ultrasound scan may show prominent ovaries (Case Rep Endocrinol 2022;2022:8804856)
- Nodules can be found on ultrasound or computed tomography (CT) scan (Gynecol Endocrinol 2013;29:213)
Prognostic factors
- Usually, benign; may increase cardiovascular risk due to increased levels of testosterone and androstenedione (Gynecol Endocrinol 2013;29:213)
- Associated with endometrioid endometrial carcinoma due to increased androgen production (Hum Pathol 2019;85:119)
Case reports
- 33 year old virilized woman with pure gonadal dysgenesis and Leydig cell hyperplasia of both gonads (J Clin Endocrinol Metab 1969;29:1429)
- 55 year old postmenopausal woman post neoadjuvant chemotherapy for carcinoma of the right fallopian tube (J Midlife Health 2022;13:247)
- 61 year old woman with hirsutism and virilization (AACE Clin Case Rep 2020;7:26)
- 64 year old postmenopausal woman with gradual onset progressive excessive hair growth (Case Rep Endocrinol 2022;2022:8804856)
- 68 year old woman with hirsutism and temporal balding (Gynecol Endocrinol 2013;29:213)
Treatment
- Medical or surgical treatment
- Surgical treatment consists of uni or bilateral oophorectomy, usually accompanied by salpingectomy
- Cyproterone acetate as an androgen receptor competitive inhibitor
- GnRH analogue followed by surgical treatment (Gynecol Endocrinol 2013;29:213)
Gross description
- Not grossly visible (Case Rep Womens Health 2023;39:e00537)
- Cell clusters do not form large, aggregated nodules leading to either significant ovarian enlargement or shape distortion (Case Rep Endocrinol 2022;2022:8804856)
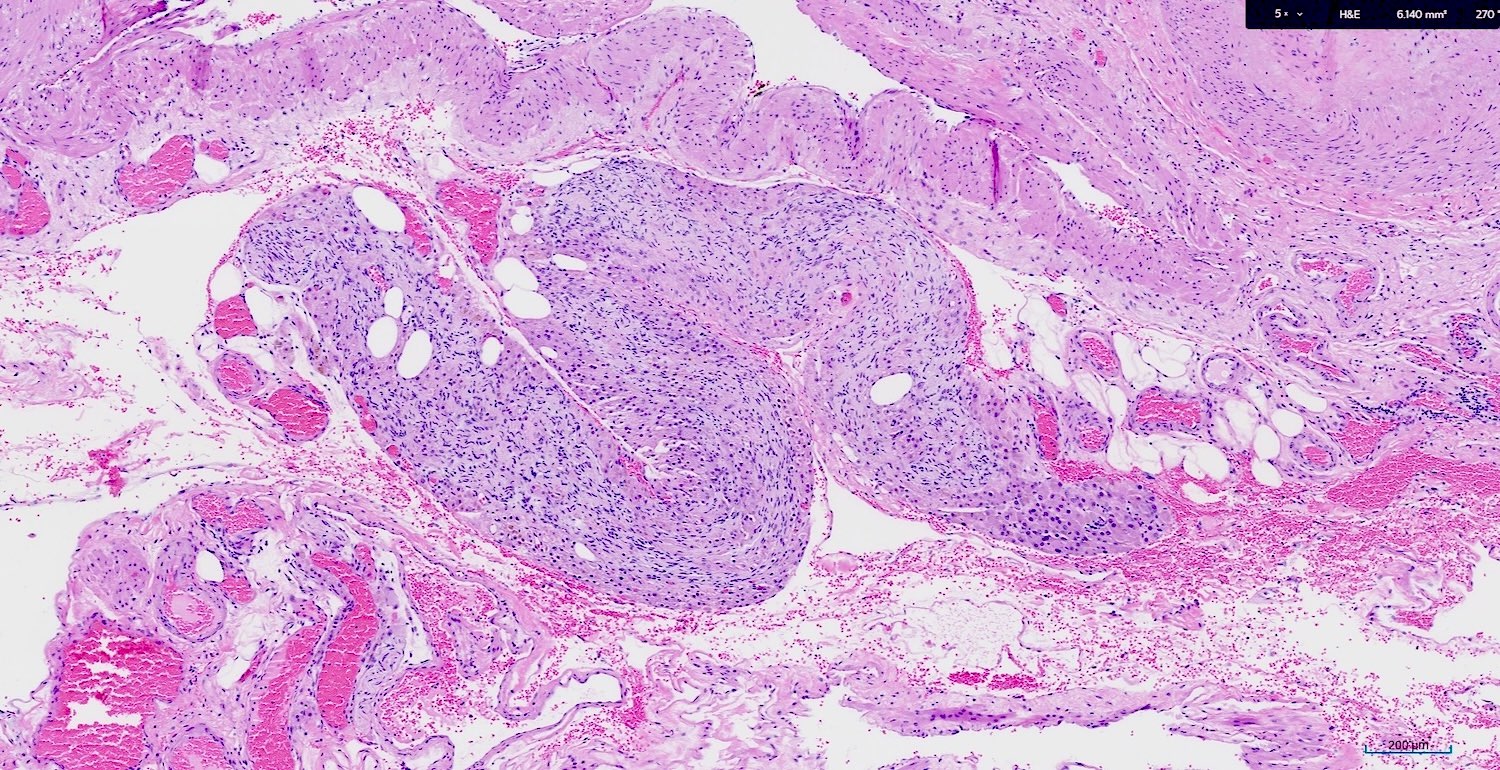
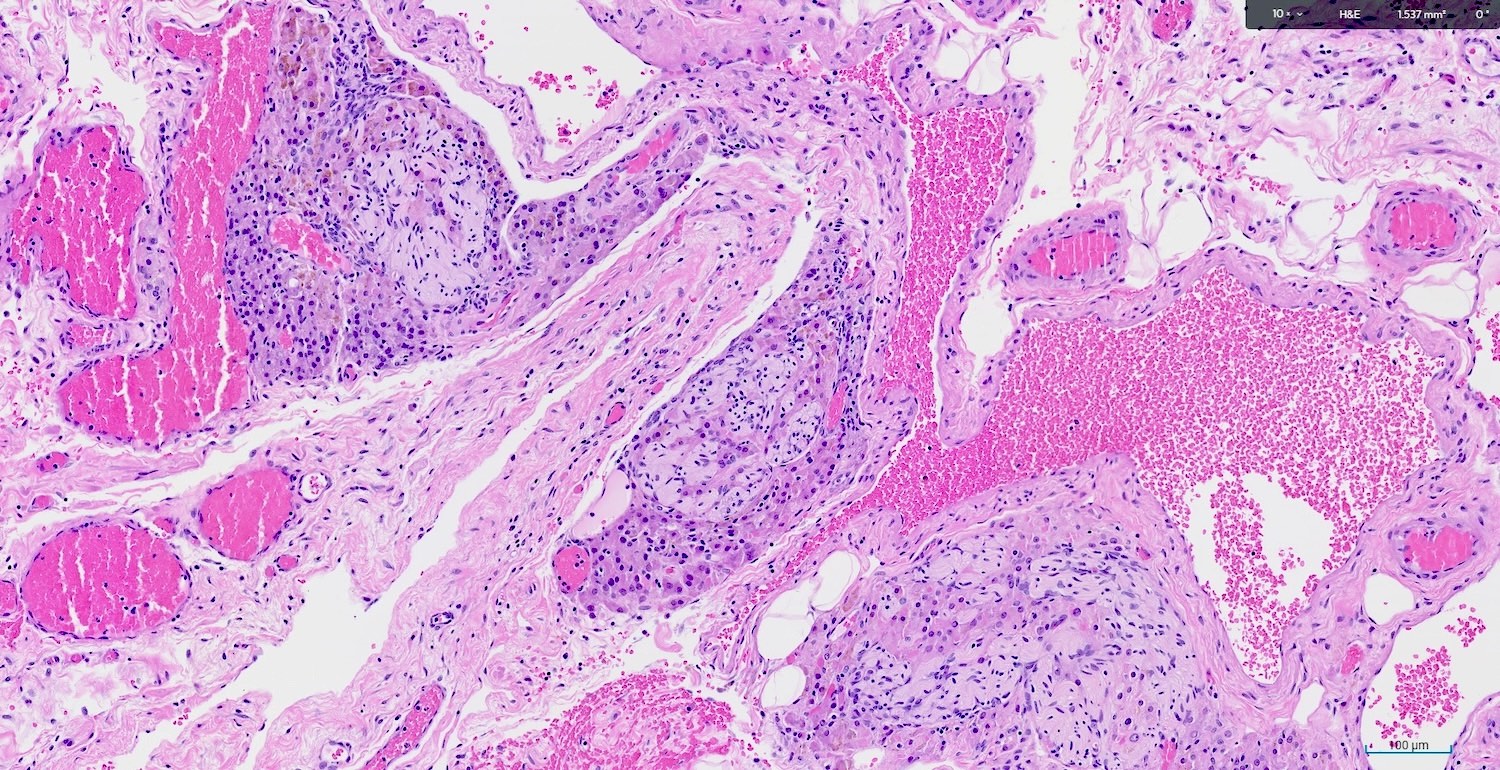
Microscopic (histologic) description
- Microscopic aggregates measuring < 1 cm in size (Gynecol Endocrinol 2013;29:213)
- Some have suggested a cut off value of > 300 cells in aggregates (Hum Pathol 2019;85:119)
- No evidence of nuclear atypia or increased mitotic activity (Case Rep Endocrinol 2022;2022:8804856)
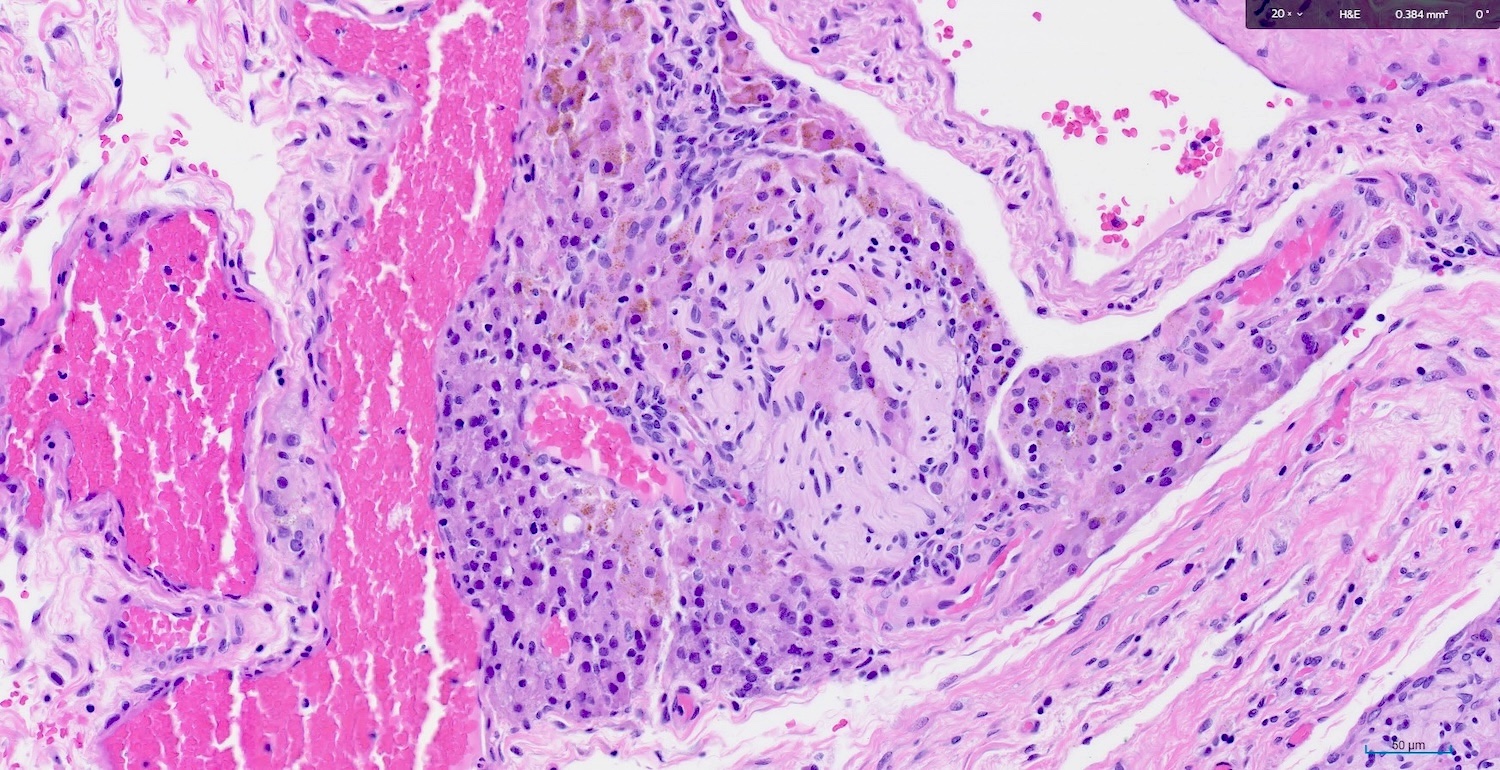
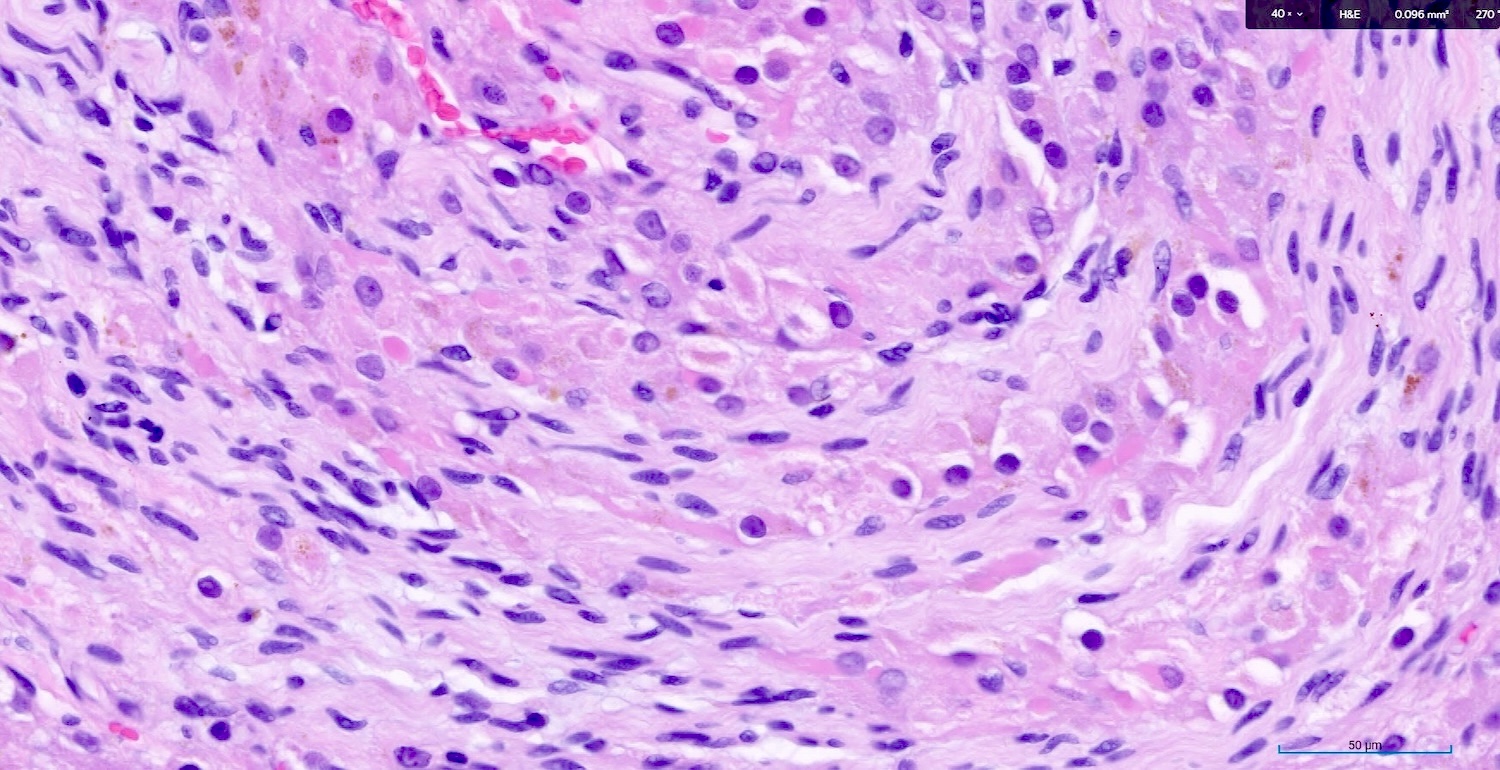
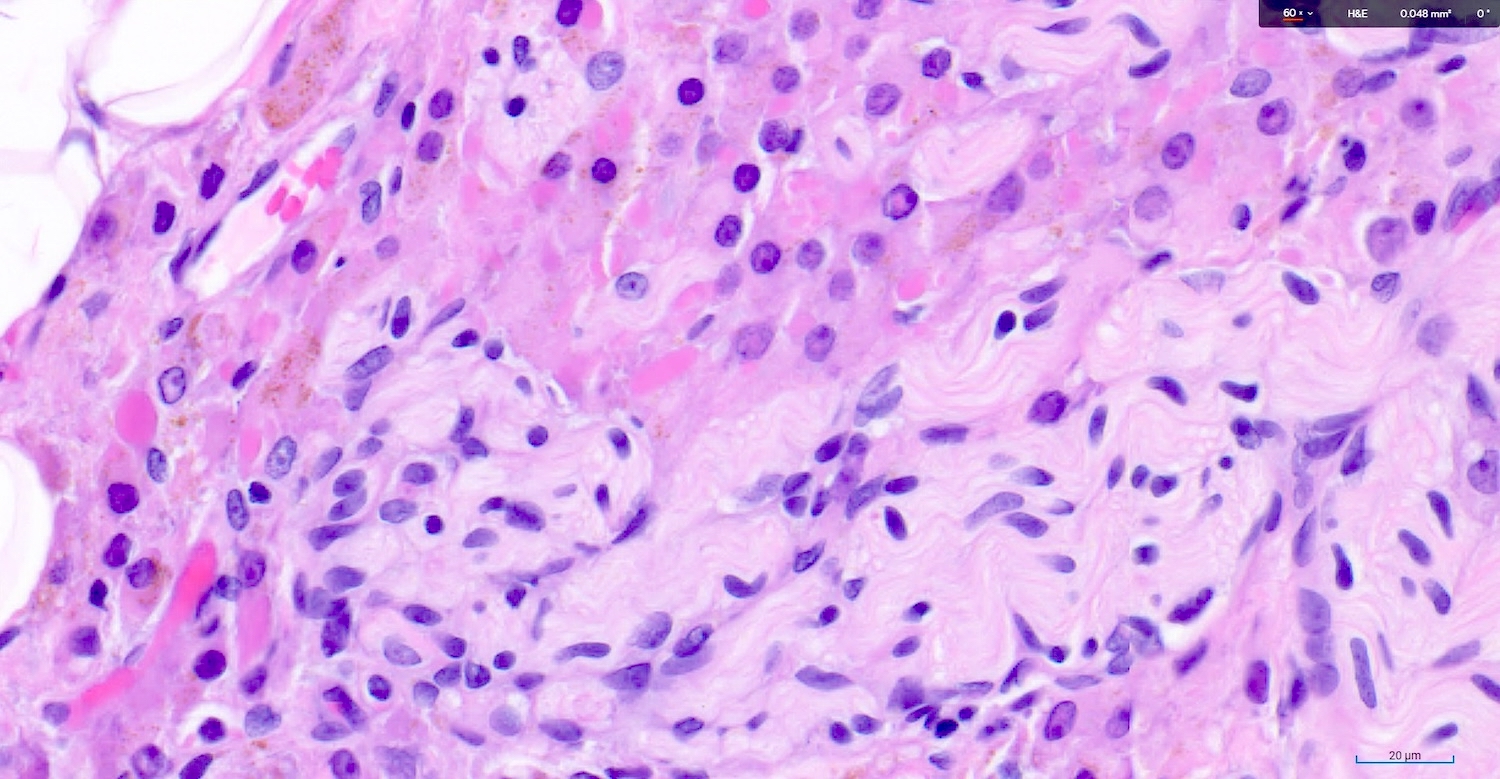
- Large epithelioid cells are closely associated with nerve fibers and blood vessels or sometimes intermixed with rete varies
- Cells are oval, polygonal or fusiform
- Nucleus is spherical and vesicular but may be ovoid
- Nucleoli are conspicuous, basophilic and variable in number (1 - 3)
- Cytoplasm is granular, with multiple vacuoles or with a clear perinuclear halo
- They contain cytoplasmic lipid and lipofuscin deposits
- In ~30% of the cells, crystalloids of Reinke are present: eosinophilic long, round or rectangular inclusions usually surrounded by a clear halo
- Inclusions can be acidophilic spherical or as hyaline globules that are precursors of the crystal bodies (Histol Histopathol 2017;32:1089)
Microscopic (histologic) images
Positive stains
- Intense cytoplasmic and nuclear calretinin (J Midlife Health 2022;13:247)
- Moderate cytoplasmic for inhibin (Histol Histopathol 2017;32:1089)
- Nuclear staining for CDK4; androgen receptor (Case Rep Womens Health 2023;39:e00537)
Negative stains
Electron microscopy description
- Stacks of rough endoplasmic reticulum, microfilaments, lipid droplets and lipofuscin granules
Videos
Ovarian Leydig cell hyperplasia in woman
Sample pathology report
- Ovary, oophorectomy:
- Leydig (hilus) cell hyperplasia (see comment)
- Comment: Nodular, microscopic aggregates of hilus cells are present in close association with nerve and vascular in the ovarian hilum. The cells are variable in size and polygonal. The nucleus is spherical, conspicuous and variable in number. Acidophilic spherical or hyaline globules and rare crystalloids of Reinke are present. There is no evidence of nuclear atypia or increased mitotic activity.
Differential diagnosis
- Leydig cell tumor:
- Fibrinoid degeneration of vessel walls (characteristic)
- Visible on ultrasound
- Grossly visible, well circumscribed mass, with a mean size of 2 cm
- Adrenal rests:
- Frequently detected in retroperitoneal, pelvic or groin areas
- Vesicular eosinophilic to clear cytoplasm
- Composed of 3 layers of adrenal cortex
- Pregnancy luteoma:
- Occurs during pregnancy and puerperium
- Follicle-like spaces
- Not restricted to ovarian hilum
- Luteinized thecoma:
- Ovarian stromal neoplasm usually not in hilum
- Grossly visible mass composed of theca cells
- Hyaline plaques
Additional references
Board review style question #1
Microscopic perineural clusters of benign looking epithelioid cells containing crystalloids of Reinke have been found incidentally in the hilar area of bilateral ovaries of a 75 year old woman. Positivity for which of the following immunostains would support the diagnosis?
- Calretinin
- Cytokeratin AE1 / AE3
- EMA
- WT1
Board review style answer #1
A. Calretinin. Leydig cell hyperplasia is a benign proliferation of steroid producing Leydig (hilus) cells that show positivity for calretinin, inhibin and androgen receptors and are negative for cytokeratin, EMA and WT1 immunostains. Answer C is incorrect because Leydig cells are considered sex cord stromal cells and they typically do not stain positive for epithelial markers. Answer B is incorrect because Leydig cells are considered sex cord stromal cells and they typically do not stain positive for keratin markers. Answer D is incorrect because WT1 stains Sertoli cells and not Leydig cells.
Comment Here
Reference: Leydig cell hyperplasia
Comment Here
Reference: Leydig cell hyperplasia