Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Cheung V, Gupta R. Dysplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/oralcavitydysplasia.html. Accessed April 3rd, 2025.
Definition / general
- Abnormal proliferation of oral epithelial squamous cells showing cytologic and architectural atypia; associated with risk of progression to squamous cell carcinoma
Essential features
- Oral epithelial dysplasia describes a spectrum of changes in the oral squamous mucosa, as it transforms from normal epithelium into a premalignant lesion with potential to develop invasive carcinoma (if not adequately treated)
- Various grading systems have been proposed to capture this spectrum of changes, to reduce inter / intraobserver variability and to guide patient management; the current widely used system is a 3 tier system, with some authors advocating for a binary system to increase reproducibility
- Clinical correlation and good communication with the surgeons / dentists is important in distinguishing dysplasia from mimics
Terminology
- Oral epithelial dysplasia (OED): histologically defined entity indicating premalignant changes in the epithelium
- Oral potentially malignant disorders (OPMD): clinically defined entities that may or may not show histologic features of dysplasia
- These include leukoplakia, erythroplakia, erythroleukoplakia, oral submucous fibrosis, oral dysplasia
ICD coding
Epidemiology
- Epidemiology for high grade dysplasia / carcinoma in situ is similar to oral squamous cell carcinoma (SCC) and includes:
- Gender: worldwide incidence higher among males than females
- Age: older adult population
- Geographic (IARC: Global Cancer Observatory [Accessed 28 February 2022])
- Higher incidence of oral cavity cancer in southern Asia, Melanesia; associated with areca / betel nut use
- High incidence also in Australia, eastern and western Europe; associated with smoking / tobacco use
- Certain oral potentially malignant disorders (OPMD) have specific predilection:
- Leukoplakia: middle aged men (> 40 years old)
- Proliferative verrucous leukoplakia: older (> 60 years old), women
- Oral submucous fibrosis: Asian, broad age range, affects both male and female with betel nut chewing habits
Sites
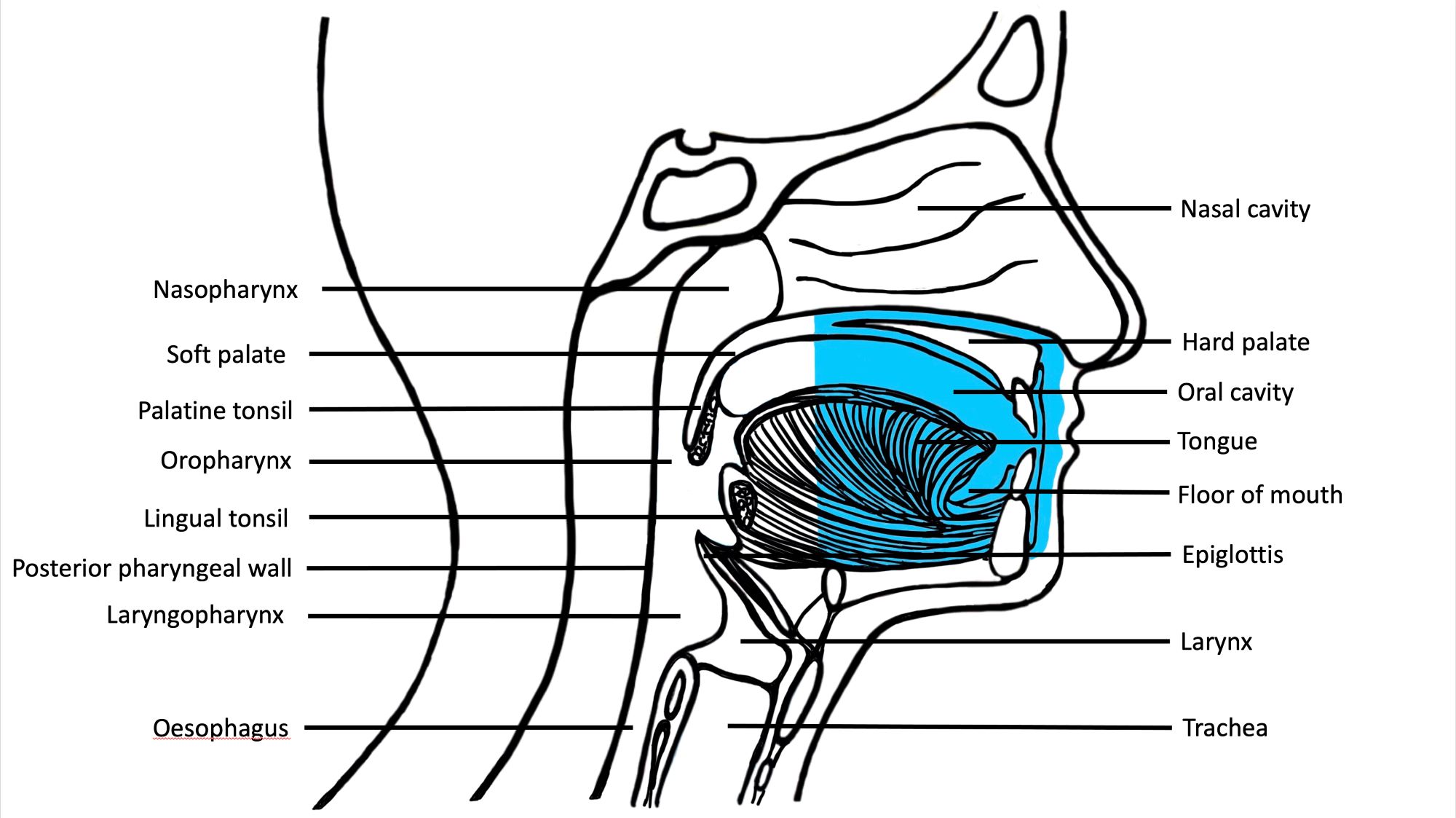
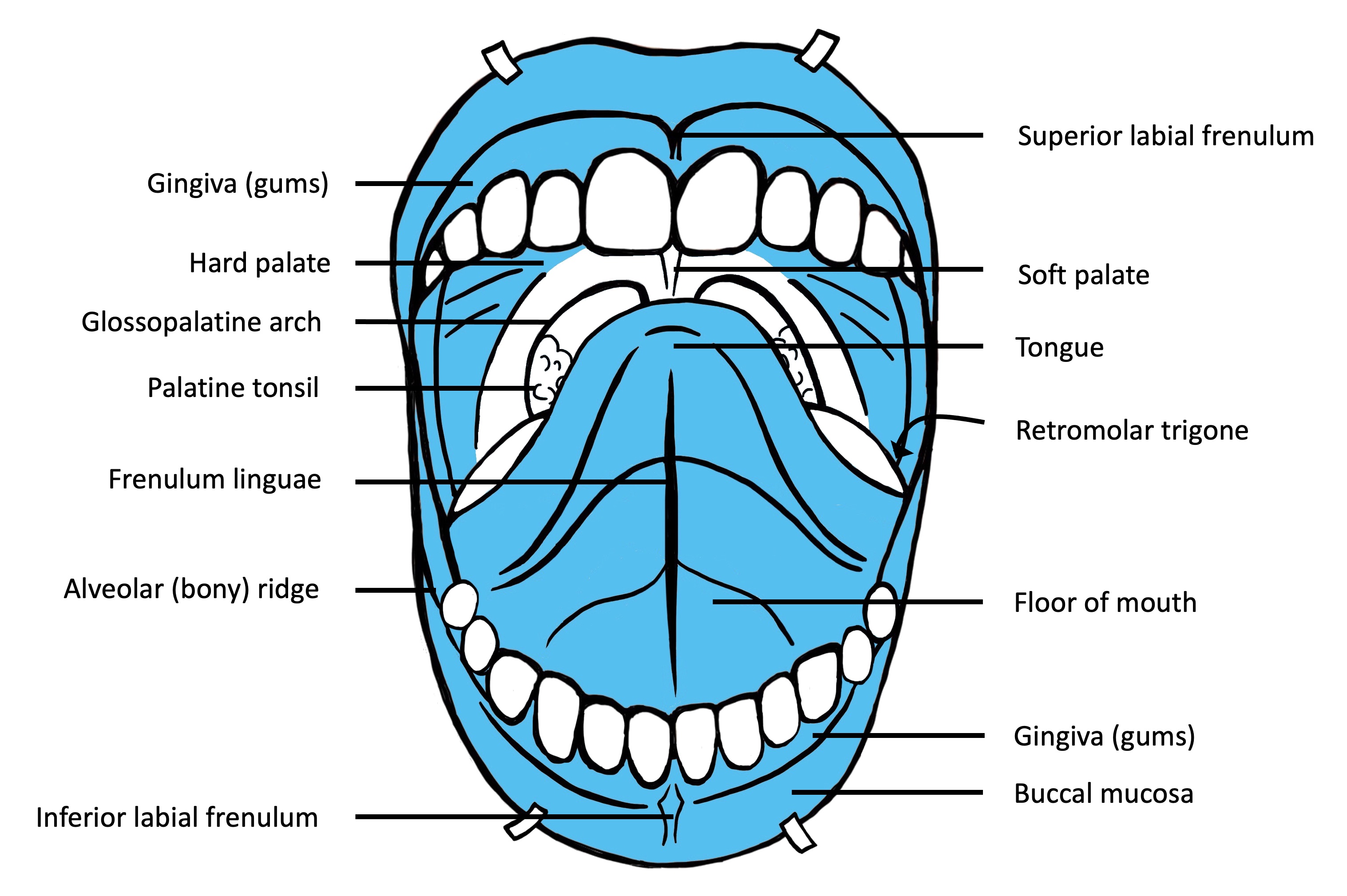
- Oral cavity
- Tongue: anterior two - thirds from circumvallate papillae to the junction at floor of mouth
- Floor of mouth: inner surface of lower alveolar ridge to ventral tongue
- Buccal: inner surface of cheek
- Labial: inner surface of lips
- Alveolar ridge: mucosa over the alveolar process of maxilla and mandible
- Retromolar trigone: mucosa over the ramus of the mandible, posterior to the last molar tooth
- Hard palate: area between the upper alveolar ridge extending to posterior edge of palatine bone (OED is rare on the hard palate)
- Higher risk of malignant transformation in tongue (lateral / ventral) and floor of mouth dysplastic lesions
Pathophysiology
- Progression of dysplasia to carcinoma is a stepwise event with accumulation of genetic changes with loss of heterozygosity, copy number variations, somatic mutations and hypermethylation leading to increasing severity of dysplasia and eventually to carcinoma
Etiology
- Causes are similar to those of oral squamous cell carcinoma
- Lifestyle factors:
- Smoking, tobacco use
- Areca nut chewing
- Excessive alcohol consumption
- Alcohol use has synergistic effect with smoking / areca nut chewing (Br J Cancer 2015;112:580, Eur J Cancer Prev 2010;19:431)
- Medical background:
- Autoimmune disorders
- Immunosuppressed state
- Genetic syndromes (Cancers (Basel) 2021;13:3696):
- Dyskeratosis congenita
- Bloom syndrome (congenital telangiectatic erythema)
- Xeroderma pigmentosum
- Fanconi anemia
- Nutritional deficiencies (Cancers (Basel) 2021;13:3696):
- Iron deficiency
- Vitamin A, B and C deficiencies
- HPV infection (HPV oncogenic type 16 and 18) (Anticancer Res 2010;30:3435, Mod Pathol 2017;30:1646, Mod Pathol 2013;26:1288):
- Prevalence of HPV in oral cavity SCC 4 - 6%
- Prevalence of HPV in OPMD and oral dysplasia reported 7 - 34%
Diagrams / tables
Clinical features
- Presentation can be variable, ranging from asymptomatic lesions to nonhealing ulcers
- Examination and detection require visual inspection and palpation for induration
- OED can present clinically as leukoplakia or erythroplakia (Head Neck Pathol 2019;13:423)
- Leukoplakia:
- Defined as white plaque of uncertain risk, provided that other known diseases / disorders with known cancer risk have been excluded
- Localized leukoplakia more common in men and can present as
- Homogeneous leukoplakia: uniform plaque, partially well delineated
- Nonhomogeneous leukoplakia: warty / verrucous appearance with nodular or red areas, higher risk of dysplasia and carcinoma
- Majority are keratosis of unknown significance but a proportion (40 - 46%) can show dysplasia, carcinoma in situ or invasive carcinoma at presentation (Oral Surg Oral Med Oral Pathol Oral Radiol 2014;118:713)
- Proliferative verrucous leukoplakia:
- Affects older women
- Presents as white plaque, verrucoid / warty keratotic surface and multifocal disease
- May demonstrate a ring around the collar appearance, extending along the marginal gingiva (J Periodontol 2021;92:273)
- Demonstrates a higher rate of malignant transformation
- Erythroplakia:
- Granular red plaque, painless (much less common than leukoplakia)
- More frequently associated with dysplasia, carcinoma in situ and invasive malignancy
- Oral submucous fibrosis:
- Mucosal pallor
- Palpable fibrous area
- Burning sensation
- Associated with areca nut chewing
- Leukoplakia:
Diagnosis
- Diagnosis is based on histological examination of biopsy specimen of the lesion
- Biopsies should aim to sample the most representative area with dysplasia / malignancy; e.g., thick white plaques, erythematous, indurated areas and adjacent to ulcer (J Can Dent Assoc 2008;74:283, J Can Dent Assoc 2012;78:c75)
- Biopsies of the lesion periphery can be useful in certain cases (e.g., verruciform lesions) as it allows for comparison with adjacent normal mucosa
- Large lesions may require multiple biopsy samples of heterogeneous area
- Types of biopsies (J Can Dent Assoc 2012;78:c75):
- Incisional biopsy
- Method of choice in examining representative area of tissue, especially where there is concern for malignancy
- Excisional biopsy
- Complete removal of a lesion is usually more appropriate for benign lesions
- Punch biopsy
- This technique can be used for incisional or excisional biopsy of a small lesion that is easy to access
- Laser technique
- Greater value in wound management over scalpel biopsy where wound closure is difficult or inappropriate
- Used for complete excision of lesions
- Avoid use during incisional biopsy to avoid cytologic alterations, which may interfere with diagnosis
- Incisional biopsy
Prognostic factors
- Presence of risk factors:
- Persisting lifestyle factors (smoking / tobacco, excess alcohol, areca nut) increases risk of recurrence / progression
- Precursor lesions:
- Nonhomogeneous leukoplakia, erythroleukoplakia and erythroplakia are associated with higher risk of dysplasia and transformation
- Multifocal disease increases risk of recurrence
- Incomplete resection increases risk of recurrence
- Size of the lesion (> 200 mm2) increases risk of malignant transformation (J Oral Pathol Med 2016;45:155)
- Location of lesion:
- Higher risk of malignant transformation in tongue and floor of mouth lesions (J Oral Pathol Med 2016;45:155, Cancer 2001;91:2148)
- Grade of dysplasia:
- Not all dysplastic lesions progress to invasive carcinoma
- Transformation rate (annual) reported at 12% with higher rates associated with higher grades of dysplasia (Head Neck 2009;31:1600, Head Neck 2020;42:539)
- Transformation rates over 15 year period:
- Low grade (mild) dysplasia ~6%,
- Moderate dysplasia ~18%,
- High grade (severe) dysplasia ~39% (Cancer Prev Res (Phila) 2013;6:822)
- Not all dysplastic lesions progress to invasive carcinoma
Case reports
- 18 year old man with right lateral tongue leukoplakia (Oral Oncol 2021;122:105565)
- 39 year old woman (no history of smoking or alcohol use), on treatment for rheumatoid arthritis, develops a right lateral tongue lesion (J Oral Pathol Med 2005;34:447)
- 46 year old man with a left buccal mucosa nodule of several years duration (Int J Surg Pathol 2014;22:248)
- 54 year old man, with myeloma treated with hematopoietic stem cell transplantation, develops diffuse white lesions of the buccal, tongue and palate mucosa (Spec Care Dentist 2019;39:51)
Treatment
- Surgical management is the treatment of choice
- Excision or laser ablation
- Does not completely eliminate the risk of recurrence or transformation; therefore, regular follow up is required
- Nonsurgical management
- Topical and systemic treatments (e.g., bleomycin, cyclooxygenase [COX] inhibitors, vitamin A and beta carotene) have been examined but show no benefit in reducing risk of recurrence or malignant transformation (Cochrane Database Syst Rev 2016;7:CD001829, Periodontol 2000 2019;80:126)
- Elimination of risk factors is essential to reduce the risk of recurrence
Clinical images
Gross description
- Identification of OED may be easier on excisions rather than small biopsies
- Mucosal resections can show ill defined, pale areas or plaque-like lesions with / without ulceration
- Thickened, verruciform appearance in lesions with verrucous hyperplasia
- OED is confined to surface mucosa
Frozen section description
- Dysplastic changes may be seen in mucosal margins of radical resections for oral squamous cell carcinoma (Cancer 2006;107:2792, Head Neck Oncol 2011;3:56)
- Frozen sections should not be performed for diagnosis of OED as it can compromise evaluation of grade and invasion
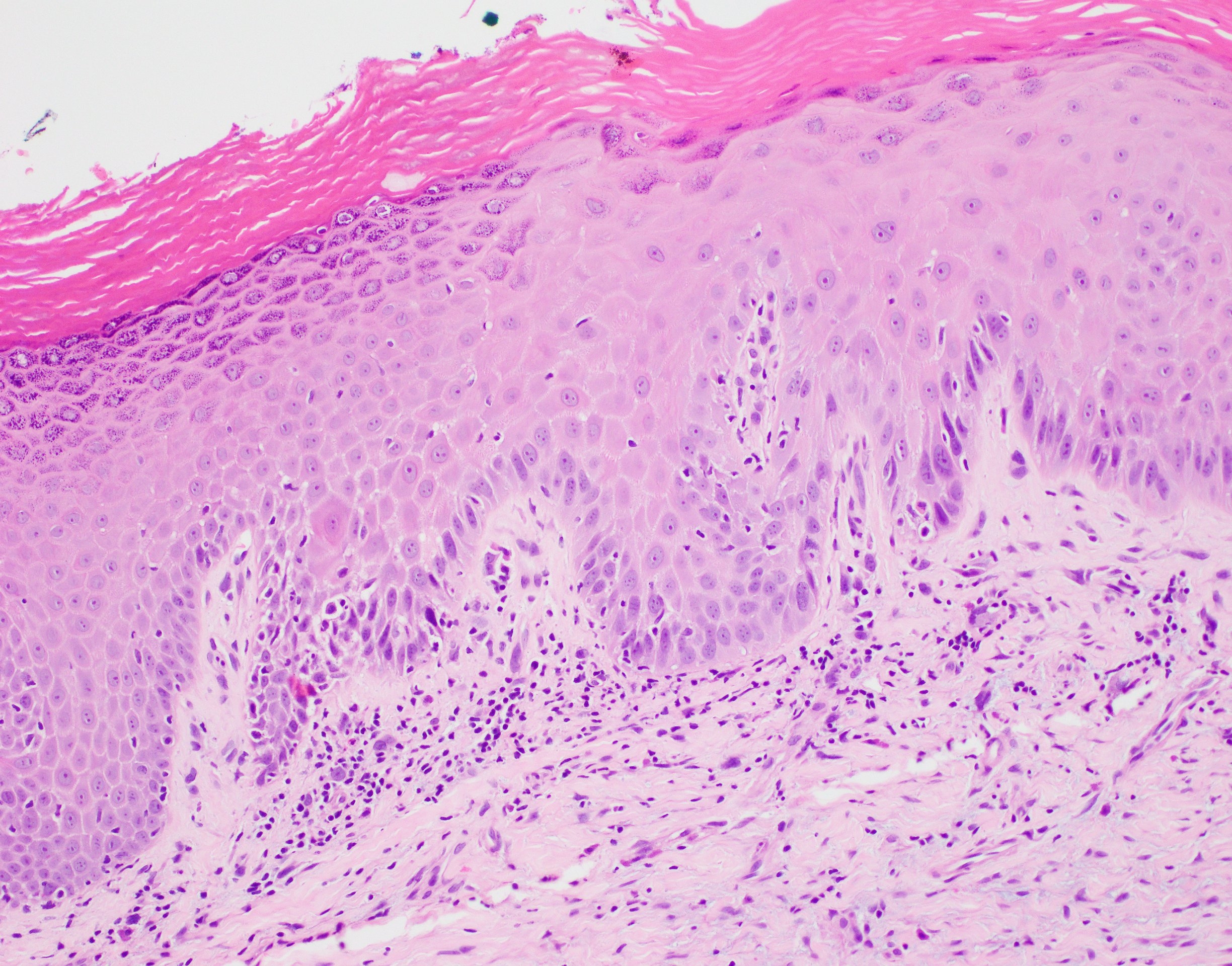
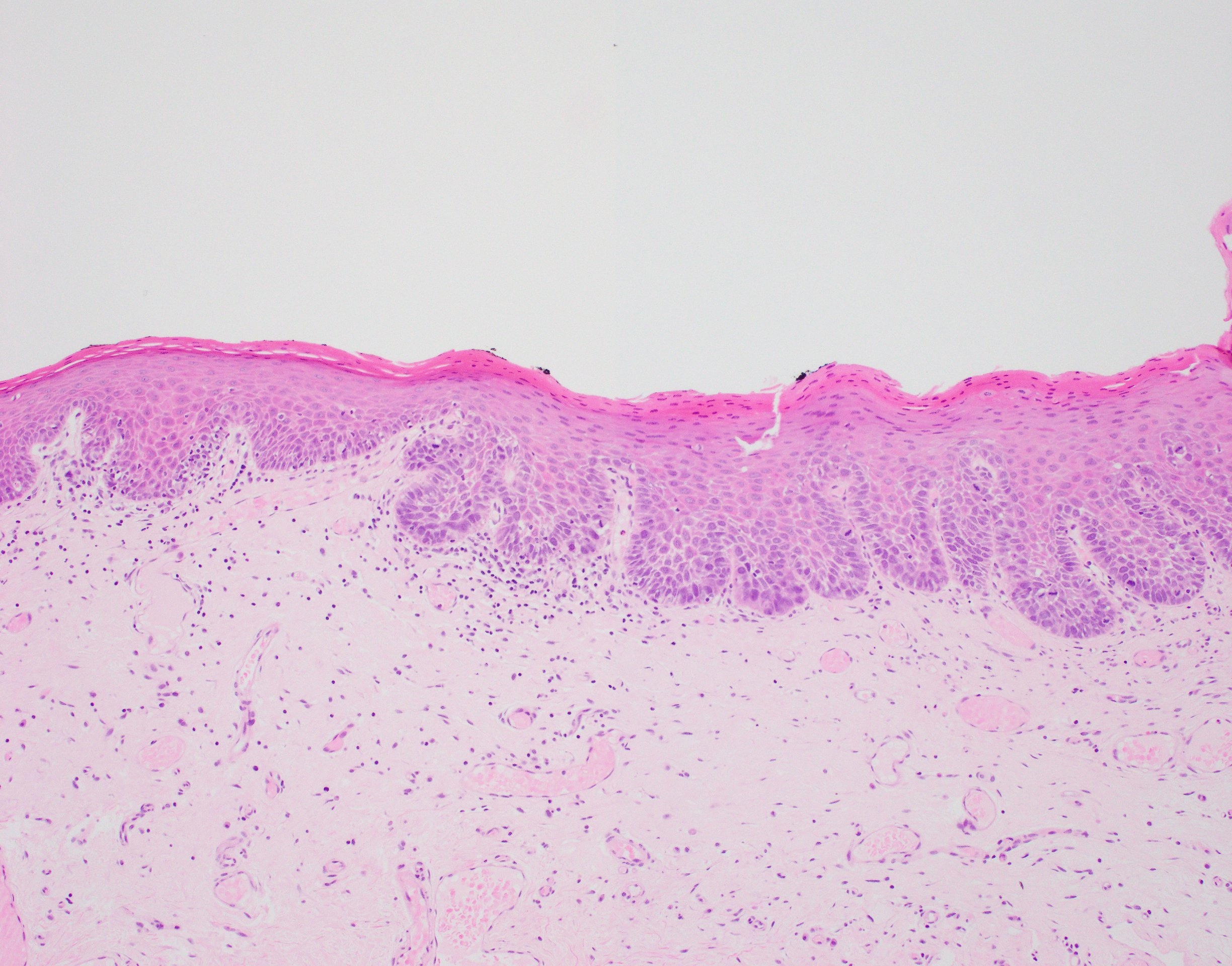
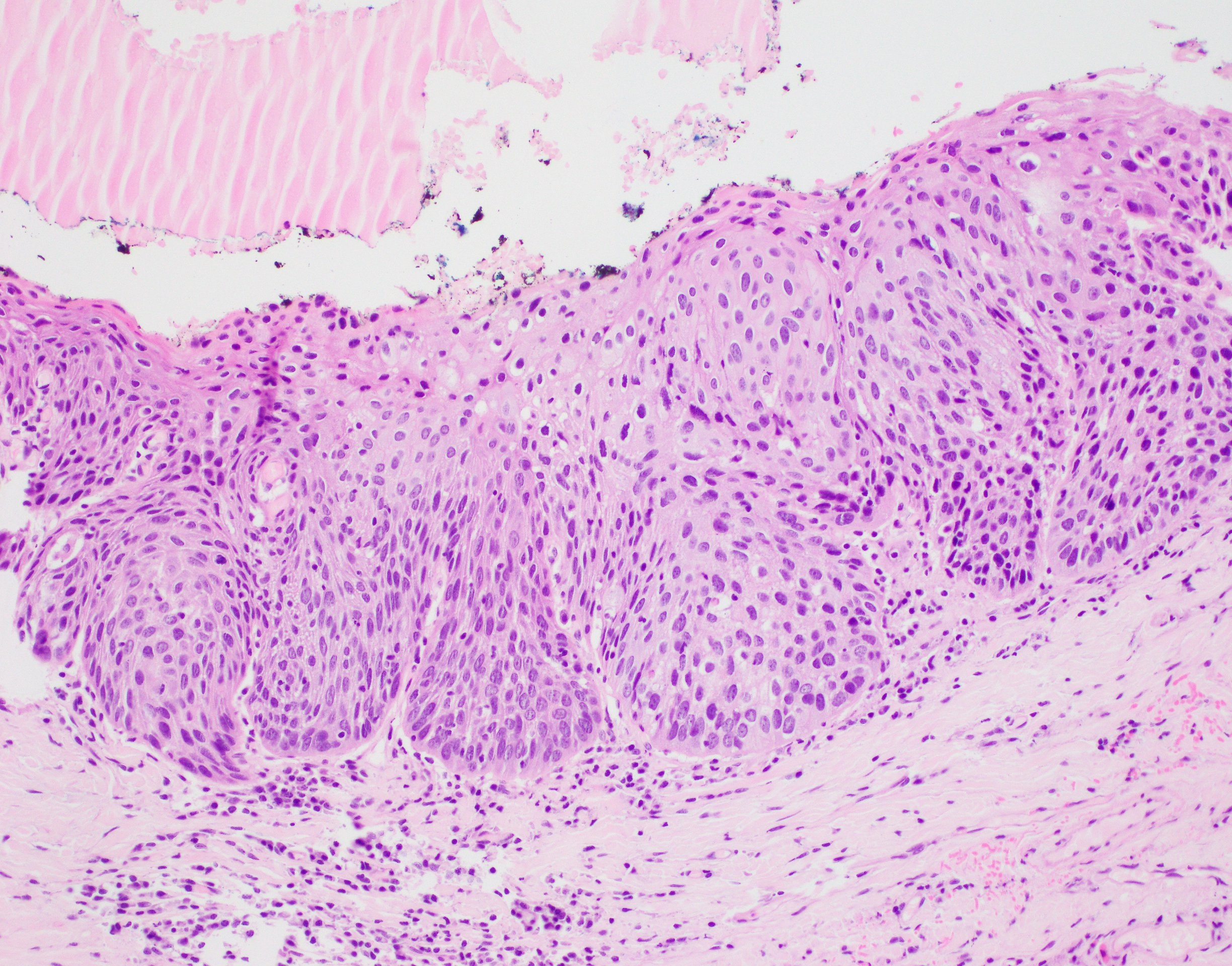
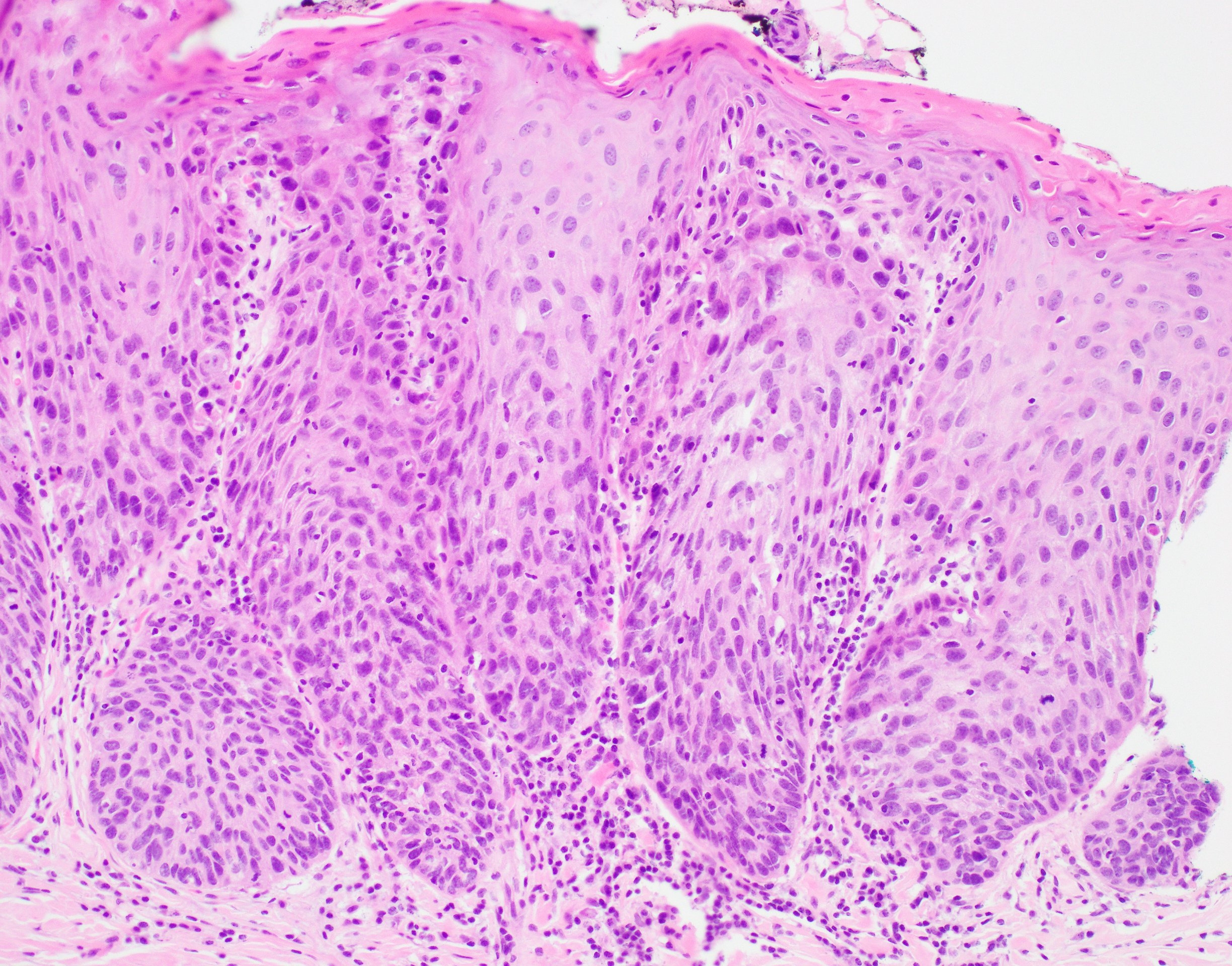
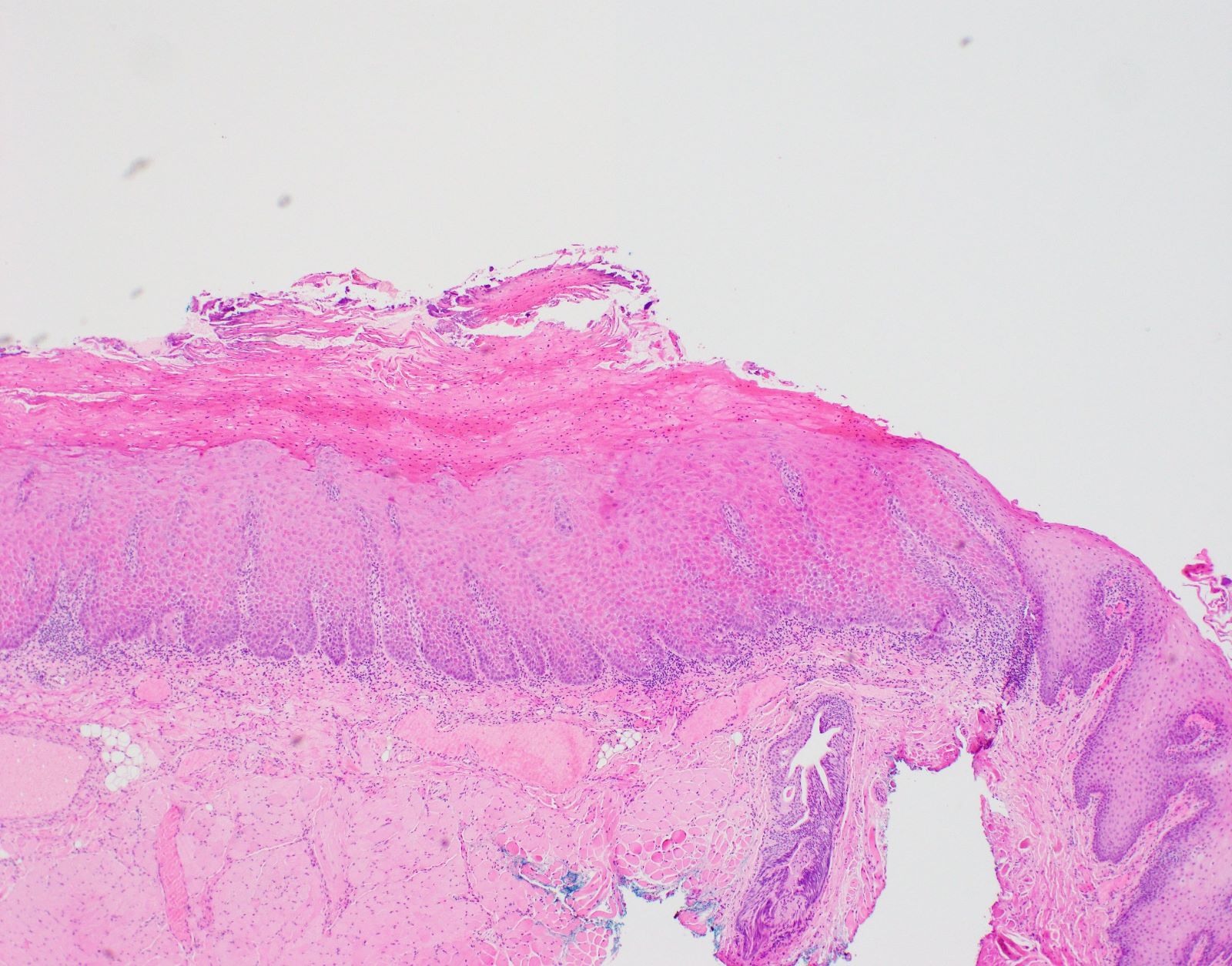
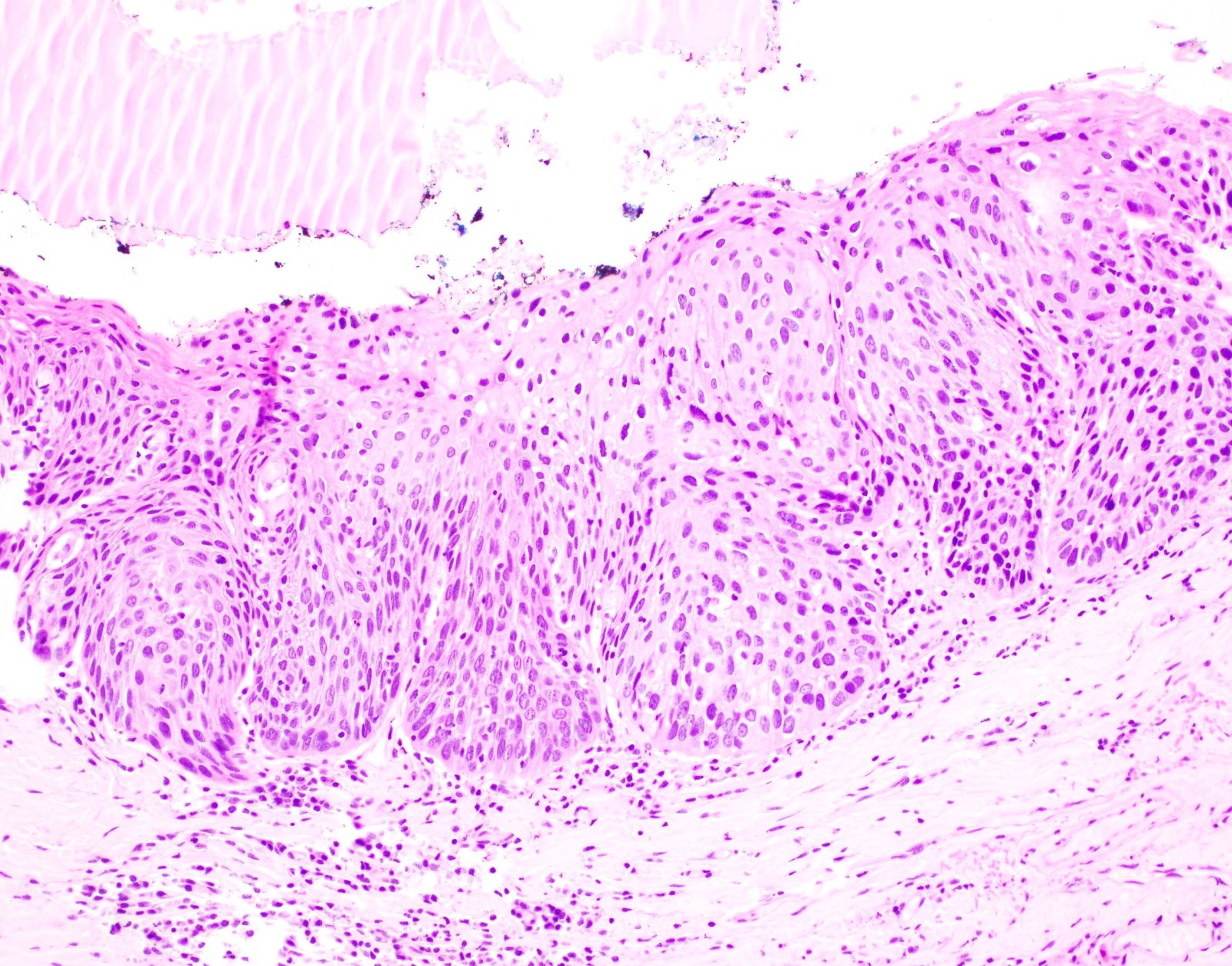
Microscopic (histologic) description
- Diagnostic criteria for epithelial dysplasia are divided into 2 types of features, architectural and cytological; various combinations of these features lead to a specific grade of dysplasia
- Dysplasia is divided into 3 grades of severity ranging from:
- Mild / low: atypia involves < one - third
- Moderate / intermediate: atypia involves one to two - thirds of the mucosal thickness
- Severe / high: atypia involves > two - thirds of the epithelial thickness
- However, the degree of cytologic atypia can increase the grade even if involving lower part of mucosa
- Carcinoma in situ is synonymous with severe dysplasia and involves full thickness of the oral mucosa
- Diagnostic criteria for epithelial dysplasia adapted from WHO Classification of Head and Neck Tumors, 2017:
- Architectural features:
- Basal hyperplasia and increased stratification
- Loss of epithelial cell cohesion
- Bulbous drop shaped rete ridges
- Confluence of the rete
- Keratin pearls within rete ridges
- Abnormal superficial mitotic figures
- Lack of maturation towards the surface
- Verrucous / papillary architecture
- Bulky epithelial hyperplasia
- Sharp demarcation from the adjacent inflamed or normal mucosa
- Extension of squamous atypia along minor salivary gland ducts
- Cytological features:
- Abnormal variation in cell size
- Abnormal variation in cell shape
- Dyskeratosis and glassy cytoplasm
- Abnormal variation in nuclear size
- Abnormal variation in nuclear shape
- Increased nuclear:cytoplasmic ratio
- Increased number of mitotic figures
- Atypical mitotic figures
- Premature keratinization in single cells
- Increased number and size of nucleoli
- Hyperchromasia
- Architectural features:
- Further additions to the architectural and cytologic criteria outlined in WHO have been proposed, including hyperkeratosis with epithelial atrophy (Head Neck Pathol 2019;13:423)
- Purpose of grading dysplasia is for risk stratification and to direct appropriate patient management (when considered together with other risk / prognostic factors)
- History of grading oral dysplasia (J Oral Biol Craniofac Res 2020;10:788):
- Smith and Pindborg 1969
- Use of standardized photographs for each criterion of dysplasia (total of 13 criteria) (J Oral Maxillofac Pathol 2015;19:198)
- Each criterion was graded as absent, slight and marked; then were assigned specific scores that were added to give a final epithelial atypia index
- This system was found to be tedious and time consuming
- Ljubljana classification 2003
- System proposed for classifying laryngeal dysplasia was also proposed for lesions of the oral cavity (J Craniomaxillofac Surg 2003;31:75)
- Initial 4 grade system: squamous hyperplasia, basal parabasal hyperplasia, atypical hyperplasia and carcinoma in situ (Histopathology 1999;34:226)
- Differences in interpretation of terminology for each grade led to variability in grading and difficulty in categorizing dysplasia in atrophic mucosa
- Brothwell 2003 (Community Dent Oral Epidemiol 2003;31:300)
- 5 tier system: no dysplasia, mild, moderate, severe and carcinoma in situ
- Diagnostic criteria for dysplasia based on 4 histologic features only: basal and parabasal hyperplasia, nuclear hyperchromasia, nuclear pleomorphism and bulbous retes
- WHO 2005
- Recognized 5 stages of precursor lesion: squamous hyperplasia, mild dysplasia, moderate dysplasia, severe dysplasia and carcinoma in situ
- Dysplasia was based on changes in architecture and cytology and the epithelium was divided into thirds in order to classify the lesion as mild, moderate or severe
- Binary system 2006
- Due to the variability in interpretation of each cytological / architectural dysplasia criterion (presence of and degree of change), it was proposed that a 2 tier classification would increase concordance (J Oral Pathol Med 2008;37:127, Oral Oncol 2006;42:987)
- WHO 2017
- Squamous hyperplasia and carcinoma in situ were removed (carcinoma in situ synonymous with high grade dysplasia in oral cavity), leaving a 3 tiered system
- WHO recommends consensus grading by more than 1 pathologist to enhance reproducibility
- Smith and Pindborg 1969
- HPV associated oral dysplasia (Mod Pathol 2017;30:1646, Mod Pathol 2013;26:1288)
- Predilection for tongue and floor of mouth location
- Associated with high risk HPV subtype 16
- Distinct histologic features include parakeratosis, epithelial hyperplasia, prominent karyorrhexis and apoptosis in all levels of the mucosa and conventional carcinoma in situ histology; koilocytes may be focal / few in number
Microscopic (histologic) images
Positive stains
- While immunohistochemistry stains (p53, cyclin D1, Ki67, BCL2, EGFR) have been utilized in studying the progression of epithelial dysplasia and in an attempt to assess degree of dysplasia, the gold standard for diagnosis and grading of OED is based on routine histological examination (Anticancer Res 2008;28:2535, Int J Biol Markers 2007;22:132)
Molecular / cytogenetics description
- Loss of heterozygosity (LOH) (Cancer Prev Res (Phila) 2012;5:1081, Clin Cancer Res
2000;6:357)
- LOH at 3p and 9p associated with dysplasia with slight increase in relative risk of developing invasive cancer
- Increased risk of progression in the presence of LOH at other chromosomes including 4q, 8p, 11q, 13q and 17p
- LOH at 3p and 9p associated with dysplasia with slight increase in relative risk of developing invasive cancer
- Other molecular alterations detected in premalignant lesions include increased expression of EGFR, cyclin D1 amplification, loss of Rb and disruption / overexpression of p53 (J Oral Pathol Med 2009;38:737, J Oral Pathol Med 2021;50:632, Mod Pathol 2022;35:177)
Sample pathology report
- Lateral tongue, mucosal excision - stitch anterior:
- Low to high grade mucosal dysplasia (see comment)
- Comment: The entire specimen has been embedded and the sections studied through levels to show oral tongue mucosa with superficial skeletal muscle. There is a broad area of mucosal dysplastic changes ranging from low to high grade dysplasia. The latter is characterized by moderately thickened mucosa with atypical squamous cells involving almost full thickness of the epithelium. There is loss of polarity, increased nuclear size, hyperchromasia and pleomorphism with readily seen nucleoli. Suprabasal mitoses are noted including a few atypical forms. There is focal superficial excoriation and acute inflammation, with isolated fungal hyphae seen on dPAS stain. No invasive carcinoma is identified. The high grade dysplasia is 2 mm away from the closest peripheral margin, midline, while low grade dysplasia is seen focally at the anterior margin.
Differential diagnosis
- Clinical history is often useful to distinguish OED from mimics
- Traumatic / reactive keratoses:
- Clues to reactive keratosis: superficial changes, shredding keratin, shaggy parakeratosis, uniform cytologic atypia, balloon cells and intracellular edema
- Can show basal cell hyperchromasia and pleomorphism but usually less severe than true dysplasia
- Candida:
- Causes hyperplasia, parakeratosis, superficial neutrophilic infiltrate
- Fungal hyphae: PAS and PAS diastase
- Oral lichen planus (OLP):
- OLP tends to present as bilateral, multifocal, migrating / evolving lesions in contrast to an isolated, nonmigrating lesion of epithelial dysplasia
- Reactive atypia from lichenoid inflammation can be difficult to distinguish from mild dysplasia
- Look for classic features of OED: loss of polarity, drop shaped rete, mitoses, premature keratinization, sharp distinction from normal adjacent mucosa
- Lymphocyte predominant infiltrate versus mixed infiltrate in epithelial dysplasia with lichenoid features may be helpful
- In some situations, it may be impossible to differentiate between reactive atypia and true dysplasia
- Discussion with surgeon / dentist and recommend treatment or healing / resolution of inflammation before re-evaluation and rebiopsy, if clinically indicated
- Good communication with the clinician is important in ensuring appropriate patient management
Additional references
Board review style question #1
You receive a wedge biopsy from a 48 year old woman with a longstanding tongue lesion. Which of the following is true in relation to this histological finding?
- Ensure the entire specimen is embedded and examined with levels
- Following complete excision, the patient will no longer require any follow up
- Immunohistochemistry for p16 can be used to confirm diagnosis
- Presence of fungal hyphae will confirm the mucosal changes are reactive and are not dysplasia
Board review style answer #1
A. Ensure the entire specimen is embedded and examined with levels. Small mucosal biopsy / excisions should be entirely embedded and examined through levels to identify highest grade of dysplasia and the presence / absence of (micro) invasive disease.
Comment Here
Reference: Dysplasia
Comment Here
Reference: Dysplasia
Board review style question #2
Which of the following is the most favorable prognostic factor for oral epithelial dysplasia?
- 45 year old woman with recurrent lateral tongue erythroplakia
- Complete resection of low to intermediate grade dysplasia of lip buccal mucosa
- Floor of mouth dysplasia recurrence
- Multifocal intermediate to high grade dysplasia
- Tobacco chewing in patient with previous tongue cancer
Board review style answer #2
B. Complete resection of low to intermediate grade dysplasia of lip buccal mucosa. The most favorable prognostic factor is the complete resection of a low risk lesion in a lower risk site (compared with tongue and floor of mouth sites that have higher risk of malignant transformation).
Comment Here
Reference: Dysplasia
Comment Here
Reference: Dysplasia