Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Peripheral smear images | Flow cytometry description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Reese A, Zhang L, Nguyen L. Idiopathic hypereosinophilic syndrome. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/myeloproliferativehypereosinophilicsyndrome.html. Accessed April 2nd, 2025.
Definition / general
- Idiopathic hypereosinophilic syndrome is a disorder defined by peripheral blood eosinophilia (absolute eosinophil count ≥ 1.5 x 109/L) for at least 6 months with organ damage / dysfunction attributable to tissue hypereosinophilic infiltrate per biopsy and no discernible underlying etiology
- Diagnosis of exclusion
Essential features
- Hypereosinophilia (HE) is defined by an increase in eosinophils in the peripheral blood (≥ 1.5 x 109/L); however, if eosinophilia is sustained for ≥ 6 months and there is associated tissue damage, this should be classified as hypereosinophilic syndrome (HES)
- Clinically, if treatment is necessary to minimize tissue / organ damage, the criteria of 6 months might not be enforced and an eosinophil count ≥ 1.5 x 109/L on 2 occasions for ≥ 1 month apart may be sufficient (Pathobiology 2019;86:39)
- Idiopathic hypereosinophilia diagnostic criteria
- Eosinophil count ≥ 1.5 x 109/L for ≥ 6 months
- Tissue or organ damage associated with eosinophilic infiltration
- Cardiac and cutaneous manifestations are common but liver, CNS, muscle, pulmonary and nasal sinus involvement may occur as well
- No discernible underlying cause
- Exclude reactive causes of eosinophilia (allergy, infection including parasites, drug related, connective tissue disorder, etc.)
- Exclude acute myeloid leukemia, myeloproliferative neoplasms, myelodysplastic syndromes, myelodysplastic / myeloproliferative neoplasms, systemic mastocytosis and myeloid / lymphoid neoplasms with eosinophilia and PDGFRA, PDGFRB, FGFR1 or PCM1-JAK2 rearrangement
- Exclude cytokine producing, immunophenotypically aberrant T cell population
- No cytogenetic or molecular abnormalities, however, must exclude clonal hematopoiesis (CHIP) mutations
Terminology
- Idiopathic HES
ICD coding
- ICD-10: D72.110 - idiopathic hypereosinophilic syndrome (IHES)
Epidemiology
- Typically occurs in adults age 20 - 50 years (Blood 1994;83:2759, Pediatr Hematol Oncol 2009;26:129, Br J Haematol 2010;151:440)
- Estimated prevalence is 0.36 - 6.3 per 100,000 (J Allergy Clin Immunol 2010;126:179)
- Previously thought to have male predominance until cases with FIP1L1-PDGFRA fusion were excluded
Sites
- Skin and cardiac involvement common
- Minority of patients present with splenomegaly and lymphadenopathy
- Liver, CNS, muscle, pulmonary and nasal sinus involvement occur more commonly than in eosinophilic leukemia (Haematologica 2014;99:e148)
Etiology
- Cause is unknown
Clinical features
- Onset of symptoms is often insidious with eosinophilia being detected incidentally; however, some patients initially present with severe and life threatening problems due to the rapid progression of cardiovascular and neurologic complications
- Retrospective study of 188 patients with hypereosinophilia reported the frequency of symptoms at presentation (J Allergy Clin Immunol 2009;124:1319):
- Dermatologic symptoms were most common, followed by pulmonary, gastrointestinal, cardiac and lastly neurologic
- 6% of patients presented initially with incidentally detected asymptomatic hypereosinophilia
- Dermatologic: angioedema, dermographism, eczema, erythroderma, pruritis, urticaria
- Pulmonary: cough, dyspnea, wheezing
- Gastrointestinal: weight loss, abdominal pain, vomiting, diarrhea, hepatitis, cholangitis, Budd-Chiari syndrome
- Cardiac: cardiac damage, valvular fibrosis, thromboembolism
- Neurologic: behavioral changes, confusion, ataxia, memory loss, peripheral neuropathy
Diagnosis
- Unexplained eosinophilia of ≥ 1.5 x 109/L for at least 6 months that leads to tissue damage
- Exclude reactive causes of eosinophilia (parasites, drug related, allergy, etc.)
- Exclude acute myeloid leukemia, myeloproliferative neoplasms, myelodysplastic syndromes, myelodysplastic / myeloproliferative neoplasms and systemic mastocytosis
- Exclude cytokine producing, immunophenotypically aberrant T cell population
- Tissue damage present due to hypereosinophilia
- Bone marrow with increased eosinophilic precursors; proposed criteria of ≥ 20% marrow cellularity with or without peripheral blood eosinophilia (Pathobiology 2019;86:39)
- Affected organs may have increased eosinophils with eosinophil degranulation (Pathobiology 2019;86:39)
Laboratory
- Serum IgE level is elevated in about half of patients
- Serum tryptase is occasionally elevated (Blood 2003;101:4660)
- Serum B12 and platelet counts are typically normal (Haematologica 2014;99:e148)
Prognostic factors
- Slowly progressive clinical course with death occurring due to cardiac damage in some cases
Case reports
- 26 year old man with ascites (JRSM Open 2020;11:2054270419894826)
- 36 year old man with erythroderma (An Bras Dermatol 2018;93:451)
- 43 year old man with pulmonary hypertension (Pulm Circ 2019;9:2045894018793999)
- 83 year old woman with dyspnea (Am J Case Rep 2019;20:381)
Treatment
- Patients are typically observed during the initial stages of the disease
- As HES progresses, initial treatment is often systemic glucocorticoid therapy with second line treatments including imatinib, interferon alfa and hydroxyurea
- Recent study showed that mepolizumab could be effective at treating flares in HES (J Allergy Clin Immunol 2020;146:1397)
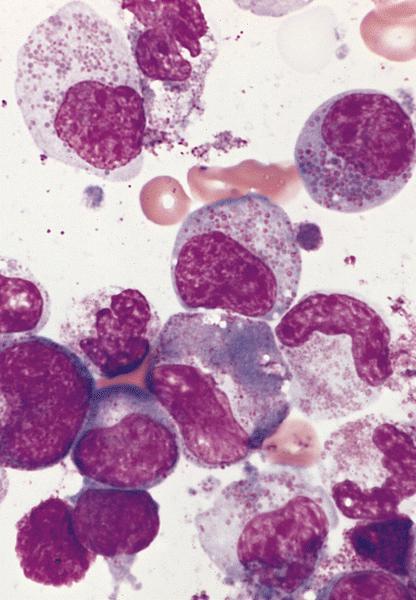
Microscopic (histologic) description
- Bone marrow
- Normocellular or hypercellular marrow with an increase in eosinophils and eosinophilic precursors with orderly maturation
- Bone marrow eosinophilia proposed criteria: ≥ 20% marrow cellularity with or without peripheral blood eosinophilia (Pathobiology 2019;86:39)
- Normal differential counts
- No increase in blasts (< 5%)
- No neoplastic process
- Tissue / organ
- Eosinophils are typically scattered or absent in normal tissue; affected organs may have increased eosinophils with eosinophil degranulation (Pathobiology 2019;86:39)
- Possible eosinophilic microabscesses
- Organs affected include gastrointestinal tract, lung, lymph nodes, spleen and thymus
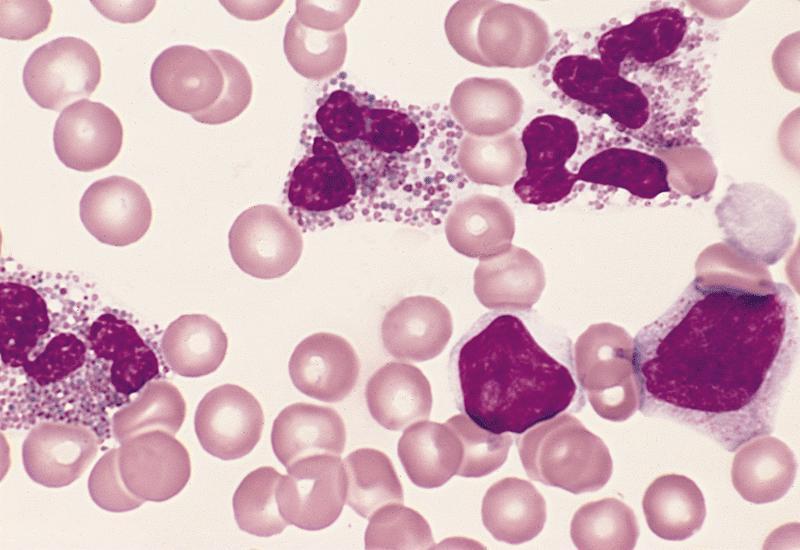
Peripheral smear description
- Marked eosinophilia (≥ 1.5 x 109/L) with normal eosinophil morphology (Foucar) (J Allergy Clin Immunol 2012;130:607)
- No increase in blasts (< 2%)
Flow cytometry description
- Flow cytometry on blood, bone marrow or other tissue specimen may be important for excluding a potential associated lymphoid, myeloid or mast cell neoplasm
- No clonal myeloid or lymphoid cells
- If an aberrant T cell population is identified, lymphocytic variant of hypereosinophilic syndrome should be considered (Arch Pathol Lab Med 2016;140:1060, J Allergy Clin Immunol 2012;130:607, Immunol Allergy Clin North Am 2007;27:389, Medicine (Baltimore) 2014;93:255)
- Generally, not useful when examining eosinophils
Molecular / cytogenetics description
- No cytogenetic or molecular genetic abnormalities identified by definition (Jaffe: Hematopathology, 2nd Edition, 2016)
- Must exclude neoplasms with rearrangements of BCR-ABL1, PDGFRA, PDGRB or FGFR1 or PCM1-JAK2, ETV6-JAK2, BCR-JAK2 or FLT3 fusions as well as activating PDGFRA gene mutation
Sample pathology report
- Bone marrow, posterior iliac crest, core biopsy, clot section, aspirate smears and touch imprint:
- Hypereosinophilic syndrome (see comment)
- Hypercellular (80 - 90%) bone marrow with myeloid hyperplasia with markedly increased eosinophils and no overt increase in blasts (< 5%)
- No evidence of PDGFRA, PDGFRB, FGFR1 or PCM1-JAK2 gene rearrangements
- No evidence of BCR-ABL1, CBFB-MYH11 / inv(16) gene rearrangements
- No gene mutations identified per next generation sequencing (NGS) panel
- Comment: There is no morphologic evidence of a myeloid neoplasm associated with eosinophilia. Clinically there are no identifiable etiologies of reactive eosinophilia such as allergic reactions, parasitic infections, autoimmune diseases and medications. The patient presents with a persistent eosinophilia for over 6 months (ranging from 1600 - 5500/µL). Electrocardiogram and echocardiograms were reportedly abnormal. Imaging studies showed subendothelial fibrosis within the ventricles. The corresponding tissue biopsy showed eosinophilic infiltration (20 - 40 eosinophils/high power field) with degranulation within the myocardium.
- Flow cytometric analysis demonstrates occasional CD34+ myeloblasts which comprise about 1.0% of total events. Hematogones are absent. No clonal B cell population or immunophenotypically abnormal T cells (e.g. CD3- / CD4+) were noted to suggest lymphoid variant of hypereosinophilic syndrome or T cell lymphoma. No clonal plasma cell population is identified.
- Additional immunohistochemical stains performed on the core biopsy show no increase in CD34 or CD117 positive immature myeloid precursors. CD117 also highlights occasional normal appearing mast cells which do not form aggregates. CD3 and CD20 highlight scattered T and B cells respectively. CD138 stains plasma cells, which comprise < 1% of the marrow cellularity.
- Peripheral smear: Manual review of the peripheral blood shows normochromic, normocytic anemia, mild leukocytosis, absolute eosinophilia and essentially normal platelets. There is no absolute monocytosis or circulating blasts. Morphologically, RBCs show normochromic, normocytic anemia with mild anisopoikilocytosis. WBCs: slightly increased in number (13000/µL) without neutrophilia, blastosis or immature monocytosis. Occasional circulating immature granulocytic precursors, e.g. metamyelocytes and myelocytes, are noted. A manual 500 cell differential count reveals 42.0% of eosinophils, resulting in an absolute eosinophilic count of 5500/µL and 3.0% monocytes, resulting in an absolute monocyte count of 390/µL. No dysplastic granulocytes such as hypogranular or hyposegmented forms are identified. Platelets: normal by count with occasional large platelets.
- Bone marrow biopsy: Quality: adequate. Cellularity: 80 - 90%. Hematopoiesis: trilineage maturation with mild myeloid hyperplasia and relatively normal erythropoiesis. Megakaryocytes are identified and normally distributed. There is no apparent increase in blasts or abnormal localization of immature myeloid precursors. Mature eosinophils are increased, making up approximately 30 - 40% of marrow cellularity. Special stains: reticulin: loose network of reticulin without significant intersections (minimal reticulin fibrosis). Small loose lymphoid aggregates composed of small mature lymphocytes are focally noted, overall < 5% of marrow cellularity.
- Bone marrow clot section: Quality: adequate. Cellularity: 80 - 90%, morphologic features are similar to those observed in the core biopsy.
- Bone marrow aspirate: Quality: adequate. Granulocytes: mildly increased; mild left shifted maturation without morphologic dysplasia. Eosinophilic precursors, predominantly mature forms, are markedly increased comprising approximately 40 - 50% of cellularity. Erythrocytes exhibit normal normoblastic maturation. Megakaryocytes appear normal by morphology and adequate in number. Myeloblasts: overall ~3% of nucleated cells. There is no monocytosis or increase promonocytes. Iron: storage iron is adequate (2+ on a scale of 0 - 4). No ring sideroblasts are present. Scattered mature mast cells are present (2%).
Differential diagnosis
- Acute myeloid leukemia with inv(16)(p13.1;q22) or t(16;16)(p13.1;q22) / CBFB / MYH11:
- Immature eosinophils containing rough basoeosinophilic granules
- Oligoblastic myelogenous leukemia or increased blasts, not necessarily ≥ 20%
- FISH or cytogenetic evidence of inv(16) or t(16;16) / CBFB-MYH11
- Atypical chronic myeloid leukemia (aCML), BCR-ABL1 negative:
- Besides features of myeloproliferative neoplasms and eosinophilia
- Leukocytosis, often > 25 x 109/µL
- Dysplasia is present in > 10% of neutrophilic precursors
- No absolute monocytosis
- < 20% blasts
- NGS study with frequent mutations involving SETBP1
- Besides features of myeloproliferative neoplasms and eosinophilia
- Chronic myelomonocytic leukemia (CMML) with eosinophilia:
- Normal or leukocytosis
- Sustained monocytosis ≥ 1 x 109/L, > 10% of WBCs for > 6 months
- < 20% blasts
- Bone marrow morphologic findings: hyper or normocellular, myeloproliferative or myelodysplastic predominance with or without marrow monocytosis
- Negative for t(5;12) or PDGFRB gene rearrangement
- Negative for BCR-ABL1 gene rearrangement
- NGS study with frequent mutations involving TET2, SRSF2, ASXL1 and SETBP1
- Chronic eosinophilic leukemia, NOS:
- Persistent eosinophilia (≥ 1.5 x 109/L) in which reactive causes have been excluded
- Does not meet diagnostic criteria for chronic myeloid leukemia with t(9;22)(q34.1;q11.2) / BCR-ABL1 gene fusion, polycythemia vera, essential thrombocythemia, primary myelofibrosis, chronic neutrophilic leukemia, chronic myelomonocytic leukemia, atypical chronic myeloid leukemia (BCR-ABL1 gene fusion negative) or systemic mastocytosis
- Blasts < 20% of peripheral blood and bone marrow cells and does not have the following cytogenetic aberrations:
- PDGFRA, PDGFRB or FGFR1 rearrangements
- t(8;9)(p22;p24.1) / PCM1-JAK2, t(9;12)(p24.1;p13.2) / ETV6-JAK2 or t(9;22)(p24.1;q11.2) / BCR-JAK2 fusions
- inv(16)(p13.1;q22) or t(16;16)(p13.1;q22) / CBFB / MYH11, t(15;17)(q22;q11-12) / PML-RARA or t(8;21)(q22;q22.1) / RUNX1 / RUNX1T1
- t(9;22)(q24;q31) / BCR-ABL1 fusions
- Presence of clonal cytogenetic or molecular genetic abnormality(ies) or a blast count ≥ 2% in the peripheral blood or ≥ 5% in the bone marrow
- Systemic mastocytosis with eosinophilia:
- Clonal proliferation of atypical mast cells
- Major diagnostic criteria: aggregates of ≥ 15 mast cells in the bone marrow or extracutaneous organ + 1 minor criterion or
- Minor diagnostic criteria (≥ 3 of the following 4 present)
- Morphology in BM or extracutaneous organ
- Infiltrate: > 25% mast cells are spindled or have abnormal morphology or
- BM aspirate: > 25% of mast cells are immature or atypical
- Molecular: KIT D816V point mutation, negative for FIP1L1-PDGFRA gene rearrangement
- Flow cytometry / immunohistochemistry: mast cells express aberrant CD25 +/- CD2 in addition to normal mast cell markers
- Laboratory findings: serum tryptase > 20 ng/ml persistently
- Morphology in BM or extracutaneous organ
- Bone marrow: eosinophils are present or increased, which could be a secondary reaction or derived from a neoplastic clone
- Idiopathic hypereosinophilia:
- When criteria 1 - 4 are met but there is no end organ damage
- Eosinophil count ≥ 1.5 x 109/L for ≥ 6 months
- Exclude reactive causes of eosinophilia
- Exclude acute myeloid leukemia, myeloproliferative neoplasms, myelodysplastic syndromes, myelodysplastic / myeloproliferative neoplasms and systemic mastocytosis
- Exclude cytokine producing, immunophenotypically aberrant T cell population
- Subset of patients harbor certain gene mutations, such as ASXL1 (43%), TET2 (36%), EZH2 (29%), SETBP1 (22%), CBL (14%) and NOTCH1 (14%) (Mod Pathol 2016;29:854)
- Presence of clonal genetic aberration favors chronic eosinophilic leukemia, not otherwise specified
- Lymphocytic variant of hypereosinophilic syndrome (L-HES):
- Abnormal cytokine release by immunophenotypically abnormal or clonal T cells
- Most commonly aberrant CD3- / CD4+ T cell population which can be detected by flow cytometry; absolute CD3- / CD4+ T cell count 0.01 - 28.3 k/L with median 0.35 k/L
- TCRγδ rearrangements were frequently identified (76%) (Medicine (Baltimore) 2014;93:255)
- High frequency of cutaneous manifestations, soft tissue involvement and lymphadenopathy (Medicine (Baltimore) 2014;93:255)
- Other phenotypic changes, e.g. CD3+ / CD4+ / CD7- and CD3+ / CD4- / CD8- TCRαβ+ have been reported (Immunol Allergy Clin North Am 2007;27:389, N Engl J Med 1999;341:1112)
- Elevated serum IgE and polyclonal hypergammaglobulinemia produced by aberrant T cells via T helper 2 cytokines (i.e. IL5, IL4, IL13, GM-CSF) (Br J Haematol 2000;109:540)
- 33% of cases have elevated absolute lymphocyte counts (Medicine (Baltimore) 2014;93:255)
- T cell clone infected by EBV can also produce cytokines that stimulate eosinophil production (Blood 2013;121:2364)
- Unknown molecular mechanism; however, STAT3 gain of function mutation, upregulation of STAT3 pathways and STAT5B gain of function mutations have been described independently (Blood 2016;127:948, Blood 2017;129:650)
- Medication related eosinophilia:
- Administration of cytokines IL2, IL3, IL5 or GMCSF
- Myeloid neoplasms with rearrangement of PDGFRA, PDGFRB, FGFR1 or PCM1-JAK2, ETV6-JAK2 or BCR-JAK2 fusion (Blood 2017;129:704)
- Paraneoplastic eosinophilia:
- T cell lymphoma, Hodgkin lymphoma, systemic mastocytosis, acute lymphoblastic leukemia, myeloproliferative neoplasms or certain solid tumors (e.g. lung cancer, metastatic renal cell carcinoma, etc.) associated with abnormal release of IL2, IL3, IL5 or GMCSF (Arch Pathol Lab Med 2013;137:259, Rinsho Ketsueki 1991;32:874, Acta Clin Belg 2011;66:293, Am J Hematol 2007;82:234, N Z Med J 1990;103:537)
- Reactive eosinophilia:
- Parasitic or fungal infections, allergies, pulmonary disease (Löffler syndrome), cyclical eosinophilia, skin diseases (angiolymphoid hyperplasia), collagen vascular diseases such as Churg-Strauss syndrome and Kimura disease
- Myeloproliferative variants of HES (M-HES):
- HES with features of myeloproliferative disorder:
- Anemia, thrombocytopenia, circulating leukocyte precursors
- Hepatosplenomegaly
- Increased serum vitamin B12
- Chromosomal abnormalities
- Myeloid / lymphoid with following abnormalities
- FIP1L1-PDGFRA fusion
- PDGFRB rearrangement
- FGFR1 rearrangement
- PCM1-JAK2 fusion
- PDGFRA fusion with other partner genes have been described (Leukemia 2006;20:827, Br J Haematol 2007;138:77, Blood 2011;117:2935)
- KIT mutated systemic mastocytosis
- Myeloid / lymphoid with following abnormalities
- Often refractory to glucocorticoid therapy but may respond to tyrosine kinase inhibitors depending on mutation profile
- HES with features of myeloproliferative disorder:
- Familial HES:
- Autosomal dominant inheritance
- Family history of eosinophilia with or without endomyocardial fibrosis
- Aberrant gene was mapped to chromosome 5q21-33 in one affected family (Am J Hum Genet 1998;63:1086)
- May present as single organ damage, e.g. eosinophilic esophagitis and eosinophilic fasciitis (Ann Rheum Dis 1994;53:281, Clin Gastroenterol Hepatol 2008;6:621, Arthritis Rheum 1989;32:96)
- Organ restricted hypereosinophilic conditions:
- Disorder with single organ involvement together with eosinophilia ≥ 1.5 x 109/L or lower in the setting of clear single organ involvement
- When eosinophilia is ≥ 1.5 x 109/L, can be difficult to distinguish from IHES
- Can overlap with single organ restricted eosinophilic disorders, such as eosinophilic gastrointestinal disorders, chronic eosinophilic pneumonia and Wells syndrome
- Specific / defined syndromes associated with hypereosinophilia:
- Associated with immune dysregulation
- Autoimmune lymphoproliferative syndrome (ALPS) (Am J Hematol 2007;82:615, Blood Cells Mol Dis 1999;25:227)
- CARD9 deficiency collagen vascular diseases (N Engl J Med 2013;369:1704)
- HIV infection (Arch Dermatol 1994;130:119, Allergy 1997;52:110)
- Hyperimmunoglobulin E syndromes
- IgG4 related disease (Eur J Haematol 2017;98:378)
- Inflammatory bowel disease (i.e Crohn's, ulcerative colitis) (Cardiology 2013;125:258, Postgrad Med J 1986;62:1101)
- Sarcoidosis (Mayo Clin Proc 2000;75:586)
- Etiology of eosinophilia (≥ 1.5 x 109/L) is unknown but correlates with the severity of the disease
- Associated with immune dysregulation
Additional references
Board review style question #1
In addition to tissue damage, what minimum level and duration of eosinophilia is required to make a diagnosis of hypereosinophilic syndrome?
- 1.0 x 109/L for at least 3 months
- 1.0 x 109/L for at least 6 months
- 1.0 x 109/L for at least 12 months
- 1.5 x 109/L for at least 6 months
- 1.5 x 109/L for at least 12 months
Board review style answer #1
Board review style question #2
Which of the following is true of idiopathic hypereosinophilic syndrome?
- Aberrant T cell population must be present
- Can accompany systemic mastocytosis
- Carries an autosomal dominant inheritance
- End organ damage is absent
- No cytogenetic or molecular abnormalities are present
Board review style answer #2
E. No cytogenetic or molecular abnormalities are present
Comment Here
Reference: Hypereosinophilic syndrome
Comment Here
Reference: Hypereosinophilic syndrome