Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Peripheral smear description | Positive stains | Negative stains | Flow cytometry description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Loghavi S. Chronic myelomonocytic leukemia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/myeloproliferativecmml.html. Accessed April 2nd, 2025.
Definition / general
- Clonal myeloid neoplasm defined by persistent relative (> 10%) and absolute monocytosis (≥ 0.5 x 109/L) in peripheral blood
- Features of both a myeloproliferative neoplasm and a myelodysplastic syndrome
- Inherent risk for transformation to acute myeloid leukemia
Essential features
- No evidence of BCR::ABL1, PDGFRA, PDGFRB, FGFR1 or JAK2 rearrangement
- Persistent relative (> 10%) and absolute monocytosis (≥ 0.5 x 109/L) in peripheral blood
- < 20% blasts (including myeloblasts, monoblasts and promonocytes) in peripheral blood and bone marrow
- Morphologic dysplasia involving at least 1 myeloid lineage
- Acquired clonal cytogenetic or molecular abnormality
Terminology
- Chronic myelomonocytic leukemia type I: ≤ 5% blasts in the blood and ≤ 10% blasts in the bone marrow and no Auer rods
- Chronic myelomonocytic leukemia type II: 6 - 19% blasts in the blood, 10 - 19% blasts in the bone marrow or Auer rods are present
- Myelodysplastic CMML (MD-CMML): WBC count < 13 × 109/L
- Myeloproliferative CMML (MP-CMML): WBC count ≥ 13 × 109/L
ICD coding
- ICD-10: C93.1 - chronic myelomonocytic leukemia
Epidemiology
- Annual incidence rate of ~0.4 cases per 100,000 persons with the highest incidence in patients older than 80 (Leuk Res 2015;39:177, Blood 1984;63:634)
- M:F = ~2.5:1
- Reported median age at diagnosis ranges from 65 to 75 years
Sites
- Blood and bone marrow are invariably involved
- Spleen, liver, skin and lymph nodes are the most common extramedullary sites of involvement
Pathophysiology
- Similar to other myelodysplastic syndromes, chronic myelomonocytic leukemia (CMML) often results in ineffective hematopoiesis and cytopenia
Etiology
- Acquired cytogenetic and molecular changes involving hematopoietic stem cells
Clinical features
- Heterogeneous clinical manifestations (Curr Hematol Malig Rep 2015;10:292)
- Constitutional symptoms
- Cytopenia
- Splenomegaly
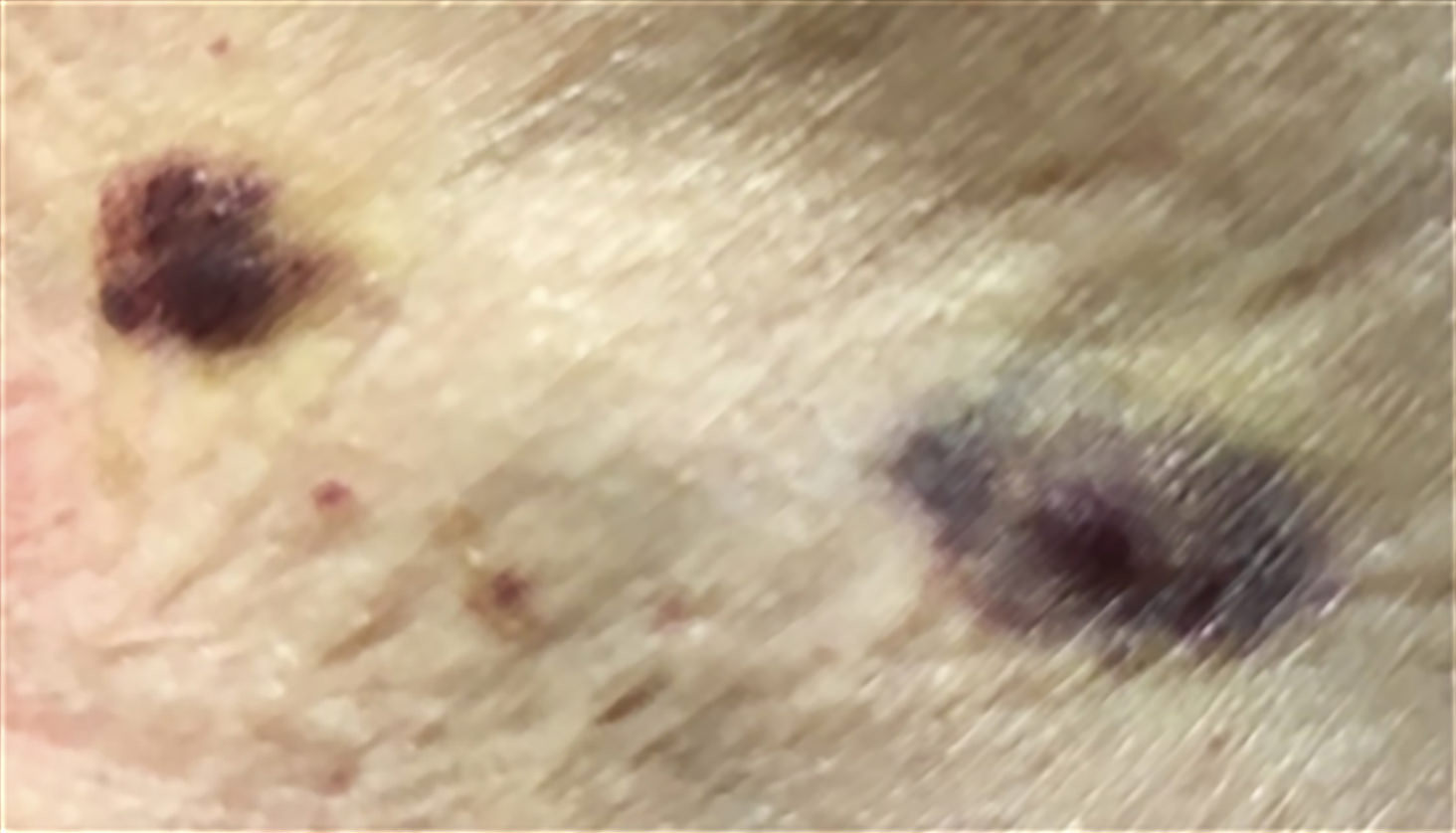
- Cutaneous lesions
- Infection
- Bleeding
- Various inflammatory or autoimmune processes (Leuk Lymphoma 2017;58:1488)
Diagnosis
- Persistent absolute (≥ 0.5 x 109/L) and relative (> 10%) peripheral blood monocytosis
- Does not meet WHO criteria for chronic myeloid leukemia, primary myelofibrosis, polycythemia vera or essential thrombocythemia
- No evidence of BCR::ABL1, PDGFRA, PDGFRB, FGFR1 or JAK2 rearrangement
- < 20% blasts (including myeloblasts, monoblasts and promonocytes) in peripheral blood and bone marrow
- Morphologic dysplasia involving at least 1 myeloid lineage
- Myelodysplasia may be absent if all of the above are present and there is evidence of an acquired clonal cytogenetic or molecular abnormality or if monocytosis has persisted ≥ 3 months and all reactive causes of monocytosis have been excluded
- Acquired clonal cytogenetic or molecular abnormality
- Reference: Leukemia 2022;36:1703
Laboratory
- Leukocytosis or leukopenia with relative and absolute monocytosis and neutrophilia or neutropenia
- Anemia
- Thrombocytopenia or rarely thrombocytosis
- Increased serum lactate dehydrogenase (Am J Hematol 2016;91:631)
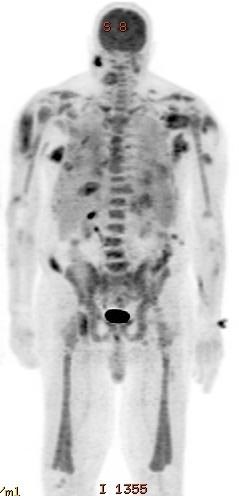
Radiology description
- Hepatosplenomegaly
- Fluorodeoxyglucose (FDG) avid extramedullary lesions may be seen (Clin Nucl Med 2014;39:811)
Prognostic factors
- Prognosis is poor, with a median overall survival of 2 - 3 years (Blood 2002;99:840)
- 15 - 30% risk of transformation to acute myeloid leukemia
- Associated with worse outcome
- Older age
- Higher white blood cell (WBC) count
- Low platelet count
- ASXL1 (frameshift or nonsense mutations)
- Certain cytogenetic alterations including trisomy 8, alterations of chromosome 7 and a complex karyotype (Haematologica 2011;96:375, J Clin Oncol 2013;31:2428)
- Associated with worse outcome
- Several risk stratification systems have been proposed; these risk stratification models incorporate
a variety of clinical, laboratory, cytogenetic or molecular parameters (see table below) (Curr Hematol Malig Rep 2018;13:446)
- MDAPS: MD Anderson Prognostic System (Blood 2002;99:840)
- Global MDAPS (Cancer 2008;113:1351)
- CPSS: CMML Specific Prognostic Scoring System (Blood 2013;121:3005)
- CPSS-Mol: Molecular CPSS (Blood 2016;128:1408)
- Groupe Francophone des Myelodysplasies (J Clin Oncol 2013;31:2428)
- Mayo Prognostic Model (Leukemia 2013;27:1504)
- Mayo Molecular Model (Blood Cancer J 2015;5:e333)
Chronic myelomonocytic leukemia prognostic models
Case reports
- 30 year old woman with therapy related disease (Int J Hematol 2009;89:699)
- 64 year old man with progression to acute myeloid leukemia FIP1L1::PDGFRA responsive to imatinib (J Hematol Oncol 2014;7:26)
- 64 year old man with blastic plasmacytoid dendritic cell neoplasm associated with chronic myelomonocytic leukemia (Blood 2016;128:1664)
- 67 year old man with a 3 year history of chronic myelomonocytic leukemia (J Cutan Pathol 2017;44:1075)
- 87 year old woman with enlarging neck mass due to involvement of thyroid gland (Br J Haematol 2003;120:548)
Treatment
- Allogeneic hematopoietic stem cell transplantation remains the only curative treatment option
- Other therapeutic means are also used to alleviate the symptoms related to the associated cytopenia and cytoses; these include the use of erythropoiesis stimulating agents, cytoreductive chemotherapy and hypomethylating agents (Blood 2017;130:126)
- Wait and watch approach may be used in patients with low risk and clinically stable disease
- Ruxolitinib may be considered in order to abrogate the effects of aberrant RAS signaling pathway activation (Clin Cancer Res 2016;22:3707)
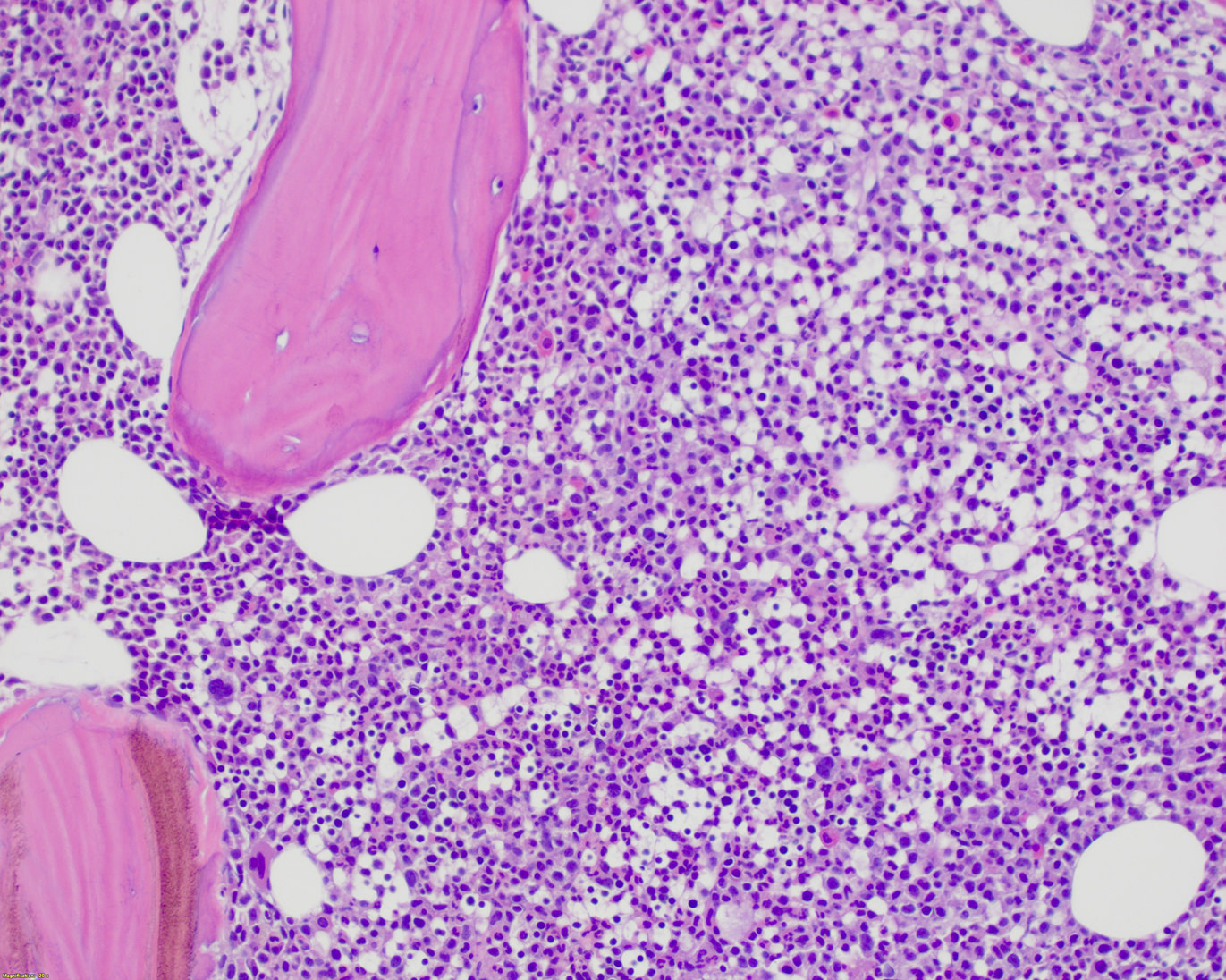
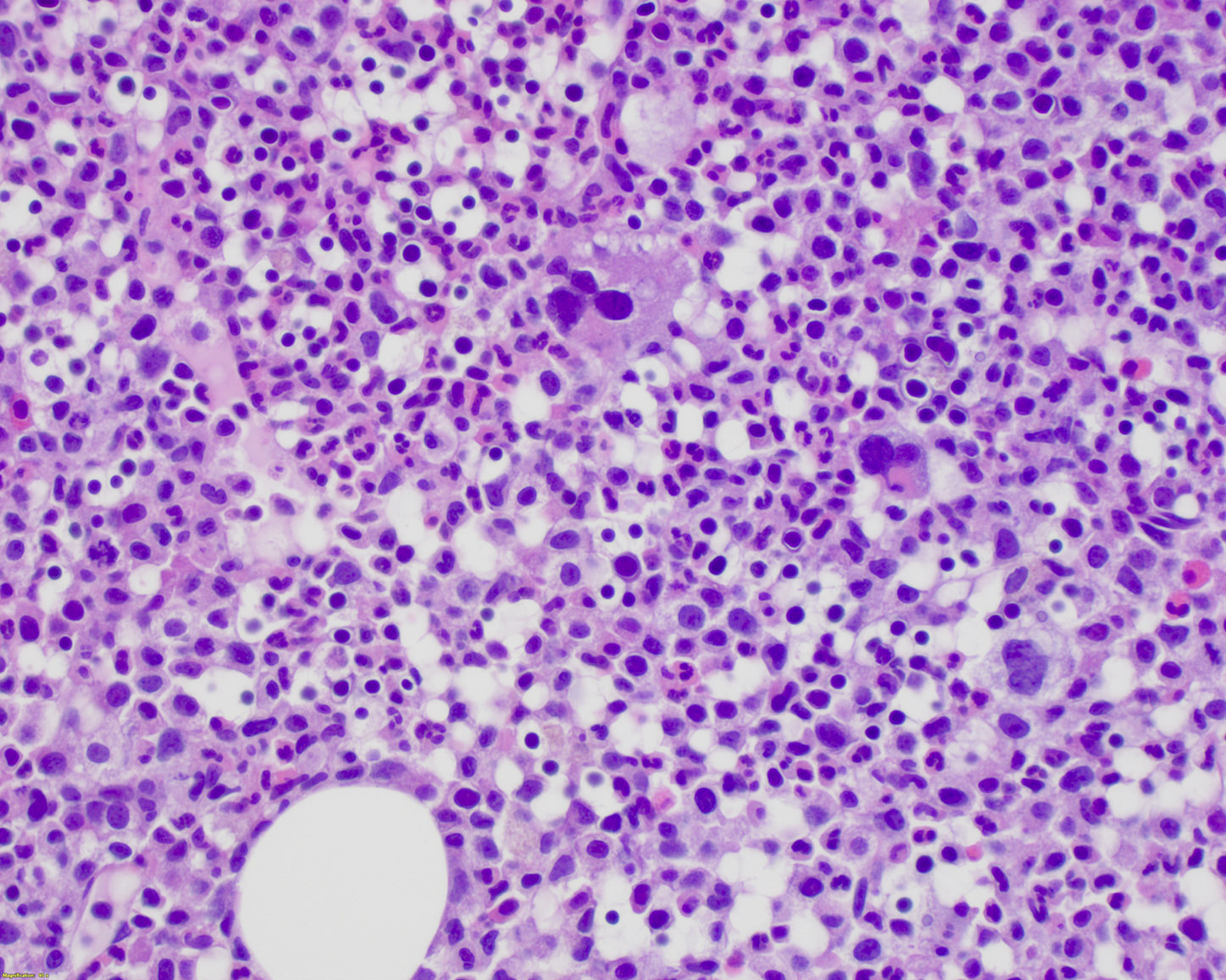
Microscopic (histologic) description
- Bone marrow is often hypercellular and shows a myelomonocytic proliferation resulting in increased myeloid:erythroid (M:E) ratio but an increase in erythroid precursors may also be seen in some cases
- Monocytic proliferation is always present but may be difficult to appreciate on routine H&E stained sections
- Dysgranulopoiesis is almost always present
- Dyserythropoiesis is also commonly observed but is less striking
- Dysmegakaryopoiesis is seen in up to 80% of cases
- Mild to moderate reticulin fibrosis may be seen
- Neoplastic proliferation of mature plasmacytoid dendritic cells (CD123+) is seen in a subset of cases (Am J Hematol 2010;85:893)
- Splenic red pulp is usually infiltrated by chronic myelomonocytic leukemia
Microscopic (histologic) images
Cytology description
- Monocytes may be comprised of mature or immature forms (monoblasts, promonocytes)
- Immature forms in this context are regarded as blast equivalents
- There is typically background dysplasia in one or more of the myeloid lineages including myeloid, erythroid or megakaryocytic lineages
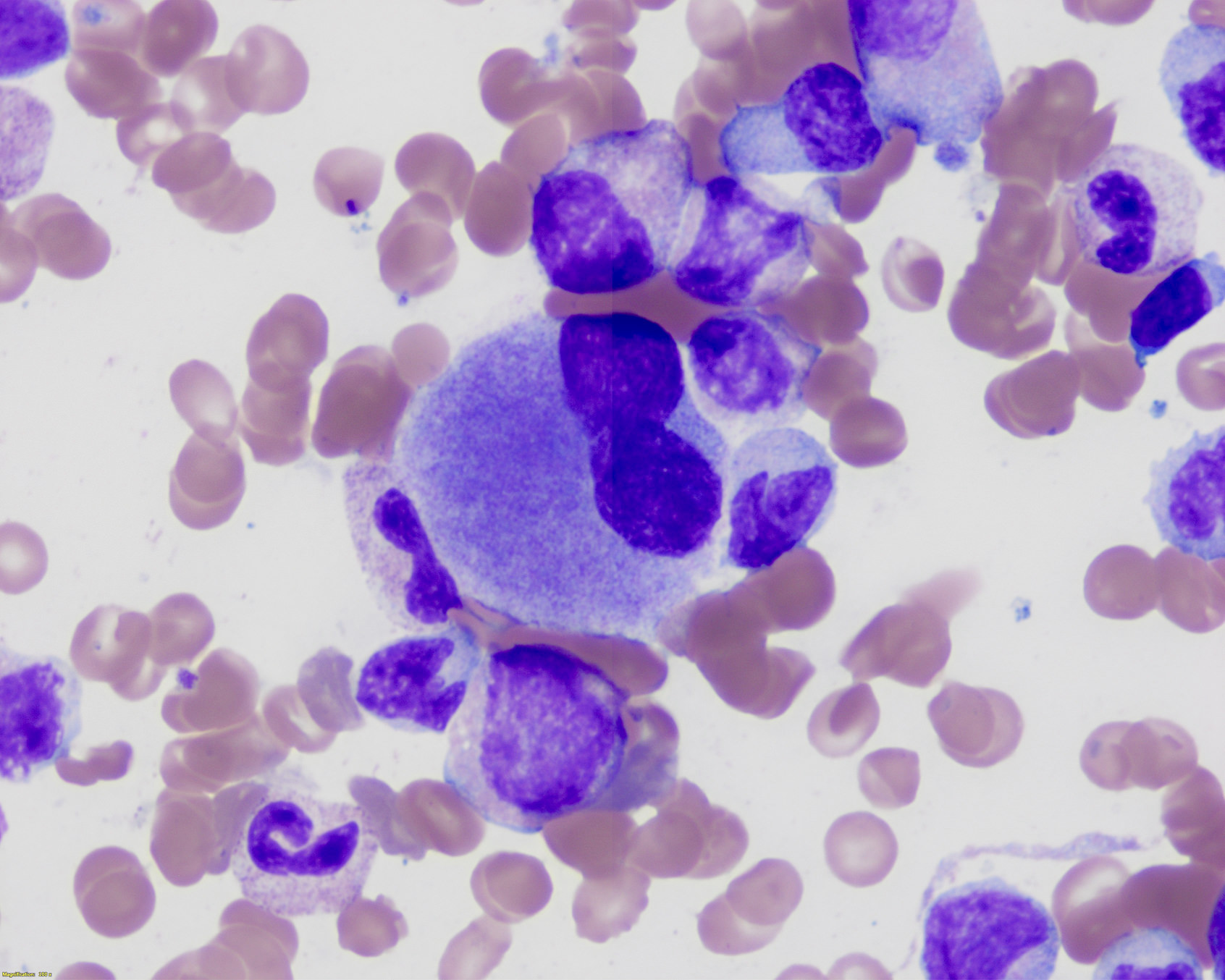
Peripheral smear description
- Relative and absolute monocytosis, variable increased immature monocytes (promonocytes, monoblasts)
- May show neutrophilia or neutropenia with variable dysplastic neutrophils (hypogranular, hypolobated)
- Left shifted granulocytes may be seen but usually account for < 10% of leukocytes and blasts are < 20% by definition
- Variable anemia, with normocytic or macrocytic red blood cells
- Variable thrombocytopenia
- Reference: Leukemia 2022;36:1703
Positive stains
- Butyrate esterase (cytochemistry) highlights increased monocytes on bone marrow and peripheral blood smears
- CD11c, CD14, CD68 and CD163 and lysozyme (immunohistochemistry): highlights increased myelomonocytic cells in bone marrow biopsy and clot
- Reference: Leukemia 2022;36:1703
Negative stains
- Myeloperoxidase may be weakly positive or negative
Flow cytometry description
- Flow cytometry can differentiate reactive monocytosis from chronic myelomonocytic leukemia but findings are not specific and may be seen in other myeloid neoplasms (Eur J Haematol 2015;95:168)
- CD34+ myeloid blasts show antigenic aberrancies (median of 6 aberrancies per case) including increased intensity of CD117, CD123, CD13 and CD34 expression and decreased intensity of CD38 expression as well as altered pattern of CD45 / side scatter and aberrant expression of lineage infidelity markers such as CD2, CD5, CD7, CD19 and CD56, among others
- Monocytic and granulocytic cells show frequent immunophenotypic aberrancies (96% and 83%, respectively)
- Granulocytes tend to show altered patterns of maturation markers CD11b / CD13 / CD16
- Monocytes show decreased expression of CD45 and HLA-DR, altered (increased or decreased) expression of CD64 and CD14 and aberrantly increased expression of CD56 (positive in ≥ 25% of monocytes) and CD2
- Hematogones are completely absent in ~93% of cases
- Classical monocyte (CD14+ / CD16-) population is expanded (Blood 2015;125:3618)
- Expansion of classical monocyte subsets (≥ 94%) has also been shown to be able to discriminate from other myelodysplastic syndrome subtypes with relatively good sensitivity (72%) and specificity (86%)
- Flow cytometry immunophenotyping can distinguish from other myeloproliferative with monocytosis using a classical monocyte fraction cutoff of 92% with a sensitivity of 93% and specificity of 100% (Blood Cancer J 2017;7:e584)
Molecular / cytogenetics description
- Clonal cytogenetic abnormalities are seen in 20 - 30%
- Most frequent chromosomal alterations include +8 (23%), -Y (20%), -7/del 7q (14%), +21 (8%) and del20q (8%) and der(3q) (8%) (Haematologica 2011;96:375)
- Cytogenetic changes have important prognostic implications and are included in all major risk stratification models (Am J Hematol 2014;89:813)
- Clones have up to 15 mutations per kilobase of coding DNA
- Most commonly mutated genes including epigenetic and transcriptional regulation
- TET2 (60%)
- ASXL1 (40%)
- EZH2 (< 5%; more common in myeloproliferative chronic myelomonocytic leukemia), DNMT3A (~5%), IDH1 (< 2%) and IDH2 (~5%), mutations in the spliceosome machinery including SRSF2 (50%), U2AF1 (7%), SF3B1 (5 - 10%), ZRSR2 (3%), PRPF8 (Nat Commun 2016;7:10767)
- Epigenetic and splicing gene mutations frequently co-occur with mutations involving genes in the cell signaling pathways (~30%) including JAK2 (5%), KRAS (5 - 10%), NRAS (10 - 20%), CBL (13%), BRAF (7%), PTPN11 (< 5%), FLT3 (< 5%), NPM1 (< 3%) (J Clin Oncol 2013;31:2428, Am J Hematol 2014;89:499)
- Signaling pathway mutations are more frequent in myeloproliferative chronic myelomonocytic leukemia
- Mutations in the BRAF kinase domain in ~7%
- Co-occurrence of SRSF2 and TET2 mutations is highly specific (~98%) to myeloid neoplasms with a CMML-like phenotype (Blood 2014;124:1513)
- Worse survival
- Truncating ASXL1 mutations (frameshift and nonsense) (Leukemia 2014;28:2206)
- ASXL1 / EZH2 comutations
- DNMT3A mutations (Blood Cancer J 2016;6:e385)
- Favorable outcome
- Clonal TET2 mutations with wild type ASXL1 (Blood Cancer J 2016;6:e385)
- RUNX1 mutations
- Associated with more severe cytopenia and progression to acute myeloid leukemia
- No impact on survival (Leukemia 2009;23:1426)
- TP53 mutations
- Uncommon (~4%, more common in myeloproliferative chronic myelomonocytic leukemia)
- May be clonal or subclonal
- Mostly detected at initial diagnosis
- In contrast to other cases with TP53 mutations, usually have a noncomplex karyotype
- Prognostic significance is unclear (Leuk Res 2018;70:97)
Sample pathology report
- Bone marrow, right posterior iliac crest, core biopsy, clot section, aspirate smears and touch imprint:
- Chronic myelomonocytic leukemia (CMML 1) (see comment)
- Hypercellular (90%) bone marrow showing myelomonocytic and megakaryocytic hyperplasia, trilineage dysplasia, monocytosis and 5% blasts
- Comment: Flow cytometric analysis demonstrates changes consistent with myelodysplastic syndrome or related stem cell disorder. CD34+ myeloblasts are ~1.2% (note that hemodilution and other technical factors may limit the precision of blast counts by flow cytometry). They have an altered immunophenotype (CD34+, CD117+, CD13+, CD33+, CD123+, CD38 decreased). Hematogones are absent. Granulocytes show decreased side scatter, in keeping with aberrant hypogranularity. Monocytes are increased (~12%) show increased expression of CD2. No monotypic B cells or immunophenotypically abnormal T cells are detected. Fluorescence in situ hybridization is negative for BCR::ABL1. The monocyte count as indicated by the accompanying CBC / differential is noted; however, a manual 500 cell differential count of the peripheral blood smear (10/2/2020) reveals 11% monocytes resulting in an absolute monocyte count of (1.02 K/uL). A CBC obtained on 04/29/2022 also revealed relative (19%) and absolute (1.4 K/uL) monocytosis. Additional cytogenetic and molecular studies are in progress and will be reported separately.
- Peripheral smear: Manual review of the peripheral blood shows normochromic, normocytic anemia, thrombocytopenia, relative (11%) and absolute (1.02 K/uL) monocytosis and rare circulating blasts. RBCs: mild normochromic, normocytic anemia with minimal anisopoikilocytosis. WBCs: overall adequate in number with relative neutrophilia and relative and absolute monocytosis. A manual 500 cell differential count reveals 11% monocytes, resulting in an absolute monocyte count of 1.02 K/uL). Granulocytes show left shifted maturation and are dysplastic (increased hypogranular or hyposegmented forms). Monocytes include atypical forms. Rare (~1%) circulating blasts are noted. Platelets: thrombocytopenia with occasional large, hypogranular platelets.
- Bone marrow biopsy: Quality: adequate. Cellularity: 90%. Hematopoiesis: trilineage maturation with myelomonocytic hyperplasia and relative erythroid hypoplasia. Megakaryocytes are increased (15/high power field on average) and are dysplastic (pleomorphic increased small or hypolobated forms, many with hyperchromatic nuclei, rarely with separation of nuclear lobes. Also identified are large forms with abnormal nuclear lobulation). Infiltrate: no apparent increase in blasts. Special stains: loose network of reticulin without significant intersections (minimal reticulin fibrosis); trichrome is negative for collagen deposition.
- Bone marrow clot section: Quality: adequate. Cellularity: 90% morphologic features are similar to those observed in the core biopsy.
- Bone marrow aspirate: Quality: adequate. Granulocytes: increased; left shifted maturation; dysplastic (hyposegmented nuclei or hypogranular cytoplasm). Erythrocytes: progressive maturation, mild dysplasia (slight nuclear irregularities, basophilic stippling of cytoplasm or N:C maturation asynchrony). Megakaryocytes: increased; dysplastic (variable in size, increased hypolobated forms, frequently with nuclear hyperchromasia). Blasts: overall ~5% of nucleated cells, including monocytic precursors. Iron: storage iron is adequate (2+ on a scale of 0 - 4). Occasional (9%) ring sideroblasts are present.
Differential diagnosis
- Myeloid neoplasms with PDGFRA, PDGFRB, FGFR1 or JAK2 rearrangement:
- Typically show eosinophilia
- Specifically exclude these entities using FISH in cases of monocytosis with prominent eosinophilia
- Chronic myeloid leukemia:
- May present with monocytosis
- Presence of BCR::ABL1
- Reactive monocytosis:
- Lacks clonal cytogenetic and molecular alterations
- Bone marrow morphology is unremarkable, dysplasia is absent
- Monocyte partitioning studies may be helpful
- Primary myelofibrosis with monocytosis:
- Megakaryocytes are large, atypical and clustered in primary myelofibrosis
- Primary myelofibrosis with monocytosis is reported to have a higher JAK2 V617F allelic burden (median: 43%; range: 20 - 62%) compared with CMML cases in which the reported JAK2 V617F allelic burden is lower (median: 17%; range: 5 - 36%) (Hum Pathol 2019;85:290)
- SRSF2 and RAS mutations
- Rare gray zone cases exist with hybrid features (Am J Surg Pathol 2018;42:799, Hum Pathol 2019;85:290)
Additional references
Board review style question #1
Which of the following is a required criterion for the diagnosis of chronic myelomonocytic leukemia according to WHO 5th criteria?
- < 10% blasts (including monoblasts and promonocytes) in the peripheral blood and bone marrow
- Presence of ASXL1 mutation of BCR::ABL1, PDGFRA, PDGFRB or FGFR1 rearrangement or PCM1::JAK2
- Persistent absolute (≥ 0.5 x 109/L) and relative (> 10%) peripheral blood monocytosis
- Persistent absolute (≥ 1 x 109/L) and relative (> 10%) peripheral blood monocytosis
- Presence of SRSF2 mutation
Board review style answer #1
C. The prerequisite criteria for a diagnosis of CMML per WHO 5th are:
1. Persistent absolute (≥ 0.5 × 109/L) and relative (≥ 10%) peripheral blood monocytosis.
2. Blasts constitute < 20% of the cells in the peripheral blood and bone marrow.
3. Not meeting diagnostic criteria of chronic myeloid leukemia or other myeloproliferative neoplasms.
4. Not meeting diagnostic criteria of myeloid/lymphoid neoplasms with eosinophilia and defining gene rearrangements (e.g. PDGFRA, PDGFRB, FGFR1, or JAK2).
Answer A is incorrect because up to 19% blasts are permitted for the diagnosis. Answer B is incorrect because ASXL1 mutation is not a required criterion and BCR::ABL1, PDGFRA, PDGFRB or FGFR1 rearrangement or PCM1::JAK2. Exclude the diagnosis of CMML. Answer D is incorrect because the minimum absolute monocyte count has been lowered to (≥ 0.5 × 109/L) in the WHO 5th edition. Answer E is incorrect because SRSF2 mutation is not a required criterion.
Comment here
Reference: Chronic myelomonocytic leukemia
1. Persistent absolute (≥ 0.5 × 109/L) and relative (≥ 10%) peripheral blood monocytosis.
2. Blasts constitute < 20% of the cells in the peripheral blood and bone marrow.
3. Not meeting diagnostic criteria of chronic myeloid leukemia or other myeloproliferative neoplasms.
4. Not meeting diagnostic criteria of myeloid/lymphoid neoplasms with eosinophilia and defining gene rearrangements (e.g. PDGFRA, PDGFRB, FGFR1, or JAK2).
Answer A is incorrect because up to 19% blasts are permitted for the diagnosis. Answer B is incorrect because ASXL1 mutation is not a required criterion and BCR::ABL1, PDGFRA, PDGFRB or FGFR1 rearrangement or PCM1::JAK2. Exclude the diagnosis of CMML. Answer D is incorrect because the minimum absolute monocyte count has been lowered to (≥ 0.5 × 109/L) in the WHO 5th edition. Answer E is incorrect because SRSF2 mutation is not a required criterion.
Comment here
Reference: Chronic myelomonocytic leukemia
Board review style question #2
Board review style answer #2
D. TET2 is the most commonly mutated gene in CMML. Answers A - C are incorrect because while these mutations may be seen in CMML, they occur substantially less frequently compared to TET2 mutations.
Comment here
Reference: Chronic myelomonocytic leukemia
Comment here
Reference: Chronic myelomonocytic leukemia