Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Peripheral smear images | Positive stains | Flow cytometry description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Graham B, Syler LB, Zhang L. Atypical chronic myeloid leukemia; BCR::ABL1 negative. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/myeloproliferativeatypicalCML.html. Accessed April 3rd, 2025.
Definition / general
- Leukemic disorder with myelodysplastic / myeloproliferative features present at initial diagnosis
- Leukocytosis characterized by increased morphologically dysplastic neutrophils and their precursors
Essential features
- Clonal hematopoietic stem cell disorder that manifests with leukocytosis (always > 13 × 109/L, frequently > 25 × 109/L)
- Features are distinct from other myelodysplastic / myeloproliferative neoplasms (chronic myelomonocytic leukemia (CMML), juvenile myelomonocytic leukemia (JMML), myelodysplastic / myeloproliferative neoplasm with ring sideroblasts and thrombocytosis (MDS / MPN-RS-T))
- Does not meet WHO diagnostic criteria for BCR-ABL1 positive chronic myeloid leukemia, primary myelofibrosis, polycythemia vera or essential thrombocythemia
- No Philadelphia chromosome / BCR-ABL1 fusion gene
- No PDGFRA, PDGFRB or FGFR1 rearrangements
- No PCM1-JAK2 translocation
Terminology
- Philadelphia chromosome negative (Ph1-) atypical CML
ICD coding
- ICD-O: 9876/3 - Atypical chronic myeloid leukemia, BCR-ABL1 negative
Epidemiology
- Rare subtype of MDS / MPN
- Incidence: ~1 - 2 cases per every 100 BCR-ABL1 positive CML
- Median age at presentation: ~70 years (Am J Hematol 2017;92:542)
- M:F = ~1:1, with slight male predominance (Ther Adv Hematol 2015;6:308)
Sites
- Peripheral blood and bone marrow always involved
- Splenic and hepatic involvement common
Pathophysiology
- No specific pattern of cytogenetic or molecular abnormalities
- Karyotypic abnormalities common (80% of cases)
- Molecular abnormalities common:
- SETBP1, ETNK1, ASXL1, TET2, RAS and RUNX1 mutations commonly seen (Biomark Res 2017;5:33, Leukemia 2013;27:1852, Haematologica 2014;99:e244, Blood 2015;125:499, Blood 2015;125:422, Int J Hematol 2015;101:229, Am J Hematol 2017;92:542)
- Presence of CALR, MPL and JAK2 mutations tend to exclude the diagnosis of aCML
Etiology
- Unknown currently
Clinical features
- Moderate anemia
- Thrombocytopenia
- Splenomegaly
- Leukocytosis
- References: Haematologica 2006;91:1566, Ann Oncol 2000;11:441, J Clin Oncol 2001;19:2915, Blood 1991;78:205, Blood 2014;123:2645
Diagnosis
- WHO diagnostic criteria:
- Peripheral blood leukocytosis ~13 × 109/L, due to increased numbers of neutrophils and their precursors (i.e. promyelocytes, myelocytes and metamyelocytes), with neutrophil precursors constituting ≥ 10% of the leukocytes
- Dysgranulopoiesis, which may include abnormal chromatin clumping or projection, abnormal segmentation or hypogranularity
- No or minimal absolute basophilia; basophils constitute < 2% of the peripheral blood leukocytes
- No or minimal absolute monocytosis; monocytes constitute < 10% of the peripheral blood leukocytes
- Hypercellular bone marrow with granulocytic proliferation and granulocytic dysplasia, with or without dysplasia in the erythroid and megakaryocytic lineages
- < 20% blasts in the blood and bone marrow
- No evidence of PDGFRA, PDGFRB or FGFR1 rearrangement or of PCM1-JAK2 translocation
- WHO criteria for BCR-ABL positive chronic myeloid leukemia, primary myelofibrosis, polycythemia vera or essential thrombocythemia are not met
- History of MPN, the presence of MPN features in the bone marrow or MPN associated mutations (e.g. JAK2, CALR or MPL) should be excluded
- References: PLoS One 2014;9:e98258, Haematologica 2006;91:1566, Ann Oncol 2000;11:441, Blood 1991;78:205, Blood 2014;123:2645
Laboratory
- Increased LDH
Radiology description
- Splenomegaly
Prognostic factors
- Poor prognosis
- Median survival = 15 months, 5 year overall survival = 25% (Ther Adv Hematol 2015;6:308)
- Poorer outcomes associated with (Haematologica 2006;91:1566, Ann Oncol 2000;11:441):
- Age > 65 years
- White blood cells > 50 × 109/L
- Hemoglobin < 10 g/dL
- Thrombocytopenia
- ~30 - 40% of aCML transforms to acute myeloid leukemia (Blood 2014;123:2645)
Case reports
- 19 year old man with hepatosplenomegaly and leukocytosis (Ann Hematol 2020;99:1145)
- 30 year old son and 59 year old father with SETBP1 positive aCML (Braz J Med Biol Res 2015;48:583)
- 68 year old woman with fever and generalized bone pain (Clin Case Rep 2018;6:915)
Treatment
- No standard treatment
- Allogeneic stem cell transplant (SCT) is only treatment that achieves long term remission (Blood 2017;129:838, Blood 2013;122:1707)
- Hypomethylating agents for non-SCT candidates (Blood 2017;129:838)
- Other therapies, such as mutation targeted agents, are becoming more readily available (Blood 2017;129:838, Blood 2013;122:1707)
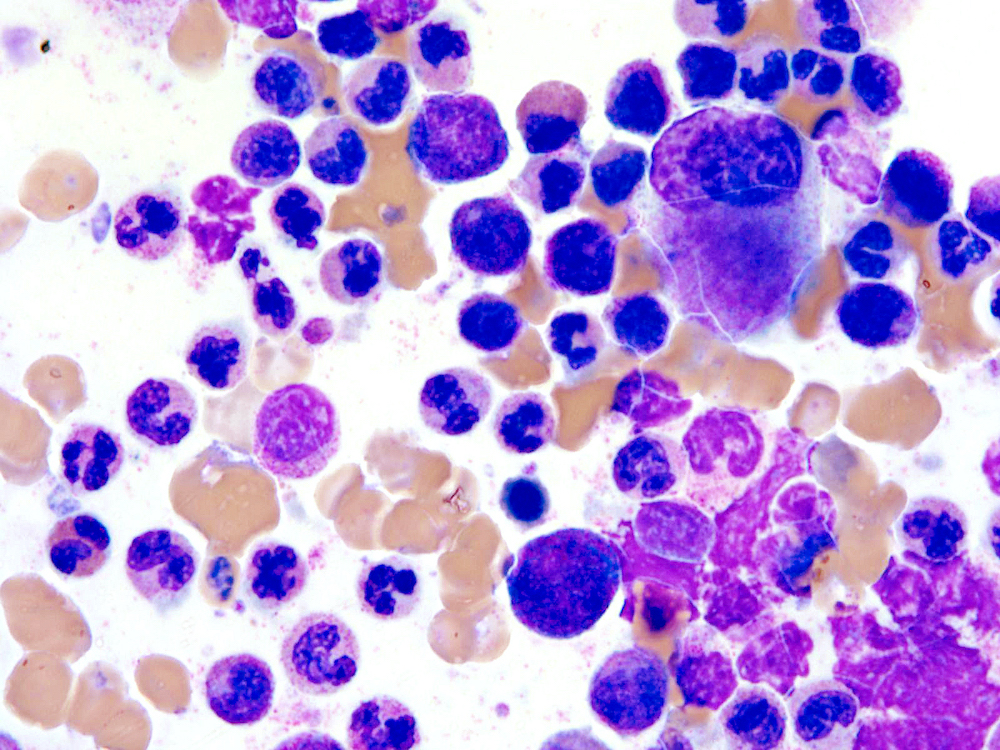
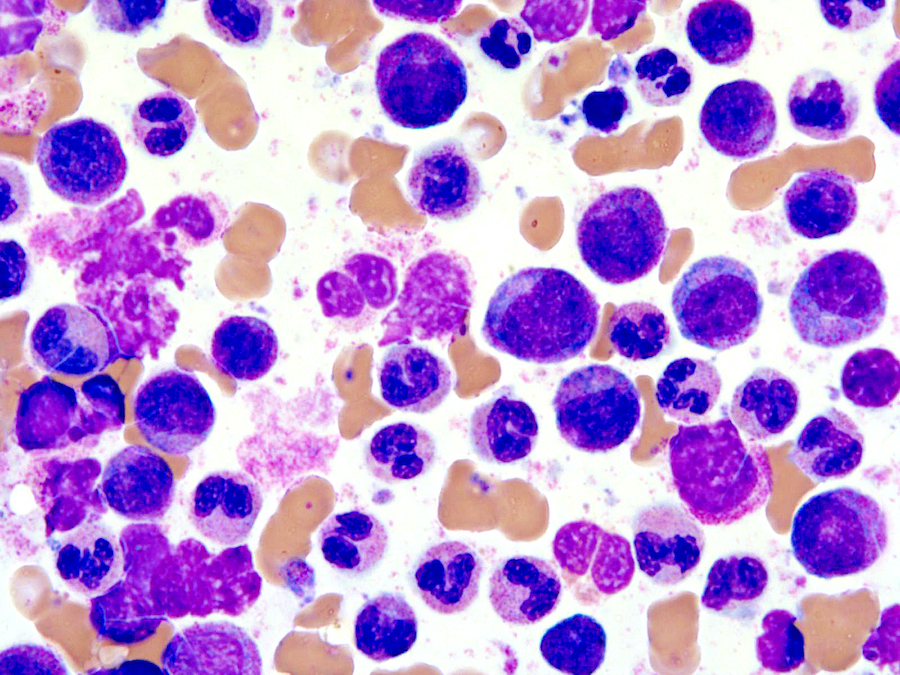
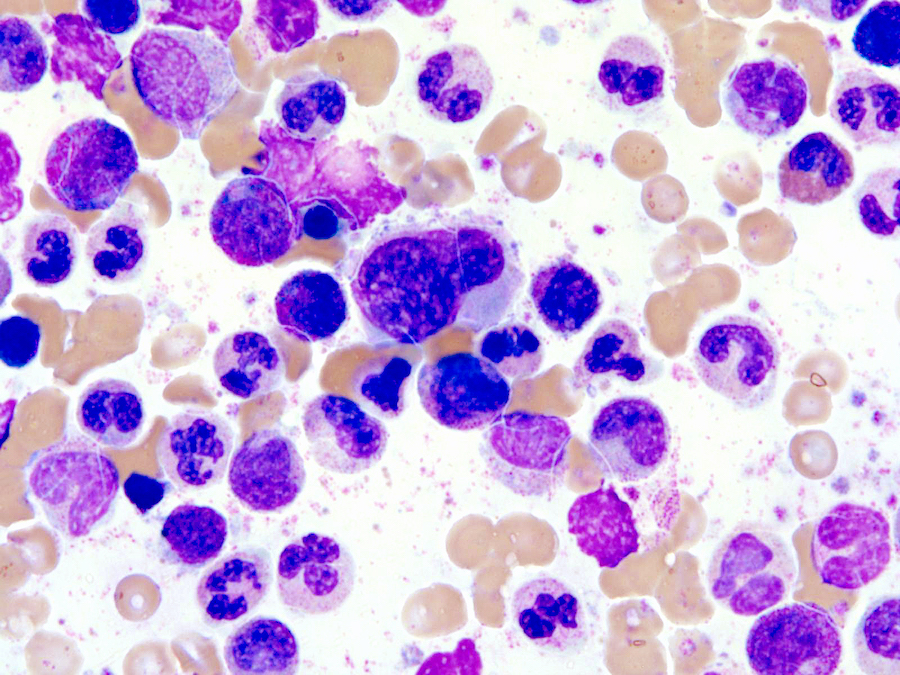
Microscopic (histologic) description
- Bone marrow (Jaffe: Hematopathology, 2nd Edition, 2016, Blood 2014;123:2645):
- Hypercellular marrow with increased granulocytic proliferation
- Increased myeloid:erythroid ratio > 10:1
- May have increased blasts but < 20%
- Granulocytic dysplasia often marked
- Dyserythropoiesis can be present in up to half of cases
- Dysmegakaryopoiesis common, including small or micromegakaryocytes and hypolobated forms
- Reticulin fibrosis increased in some patients
- Syndrome of abnormal chromatin clumping (Haematologica 1990;75:532, Nouv Rev Fr Hematol 1995;37:245):
- Considered a variant of aCML
- Characterized by high percentage of granulocytes and precursors with exaggerated chromatin clumping identified in peripheral blood or bone marrow
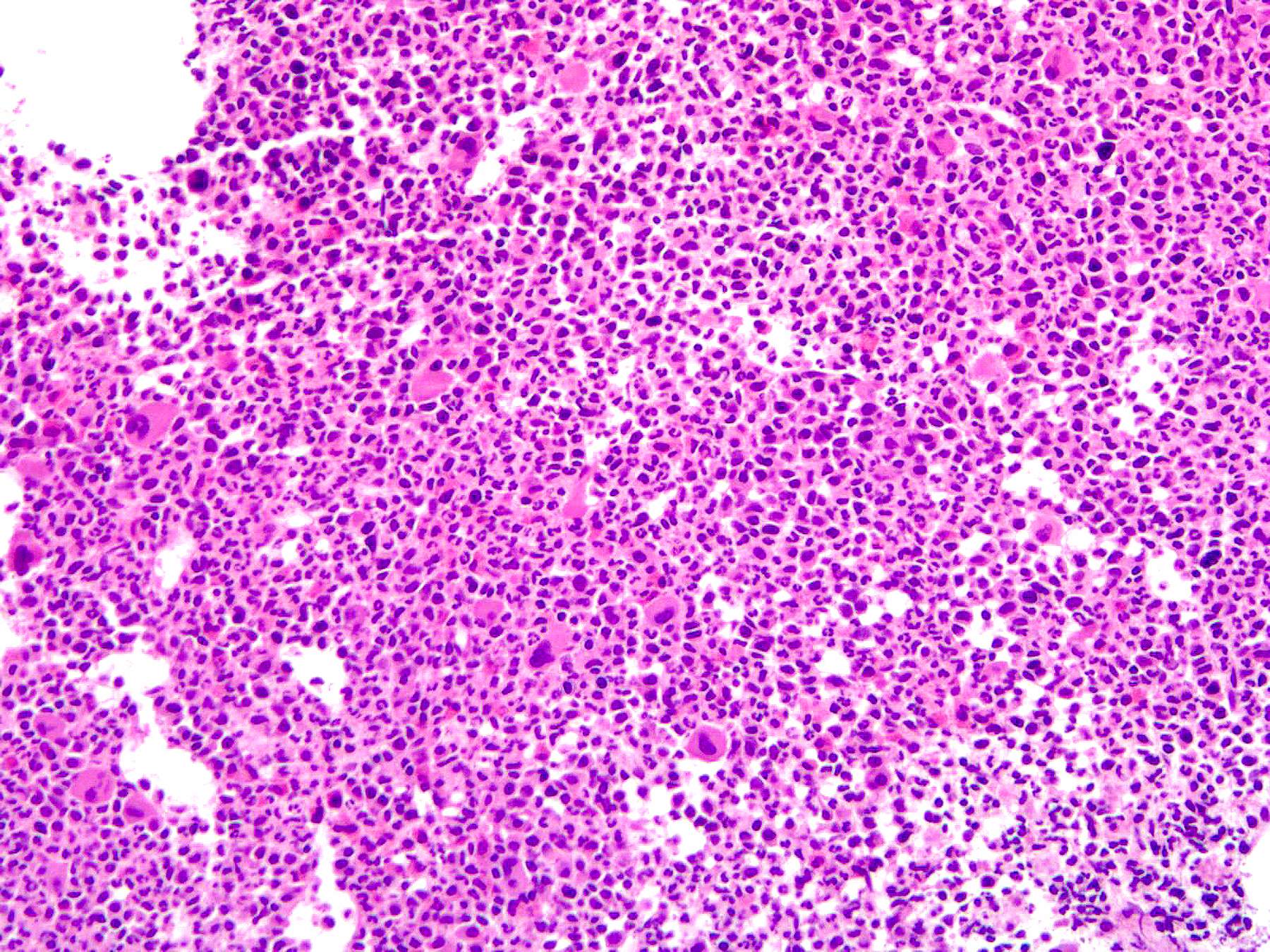
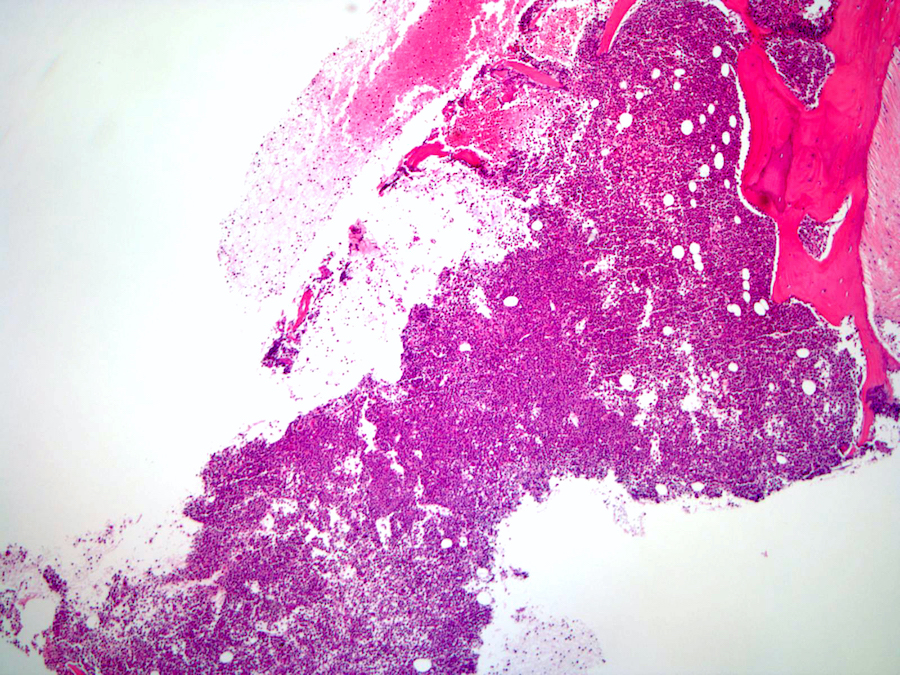
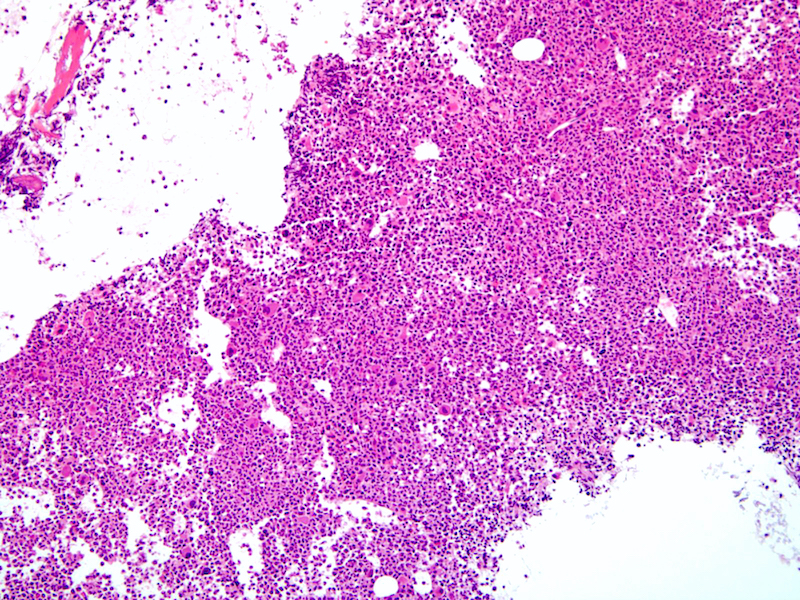
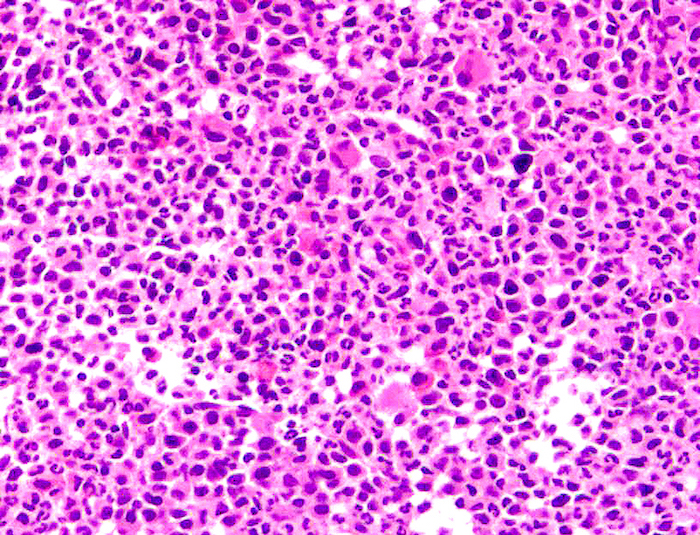
Microscopic (histologic) images
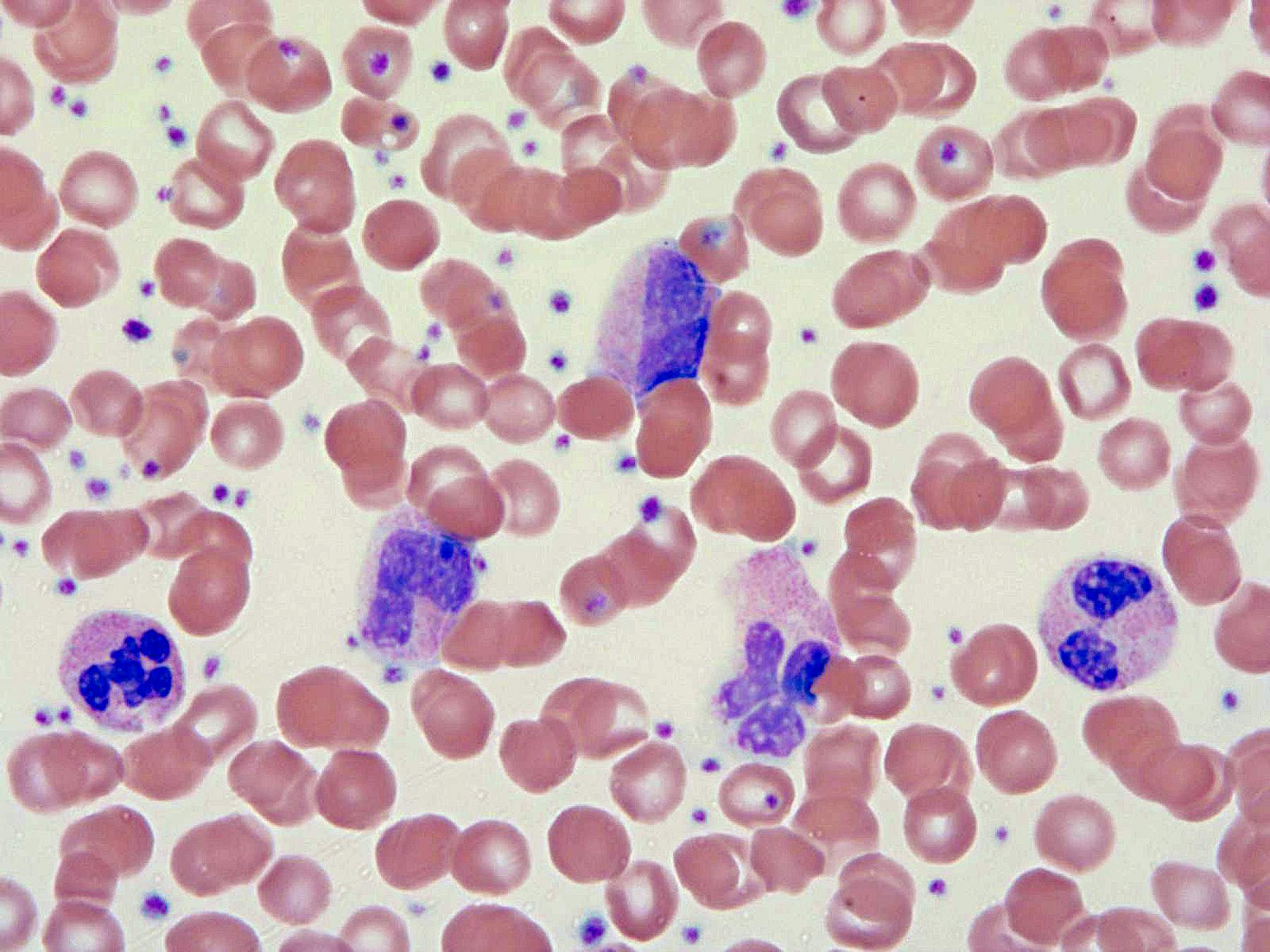
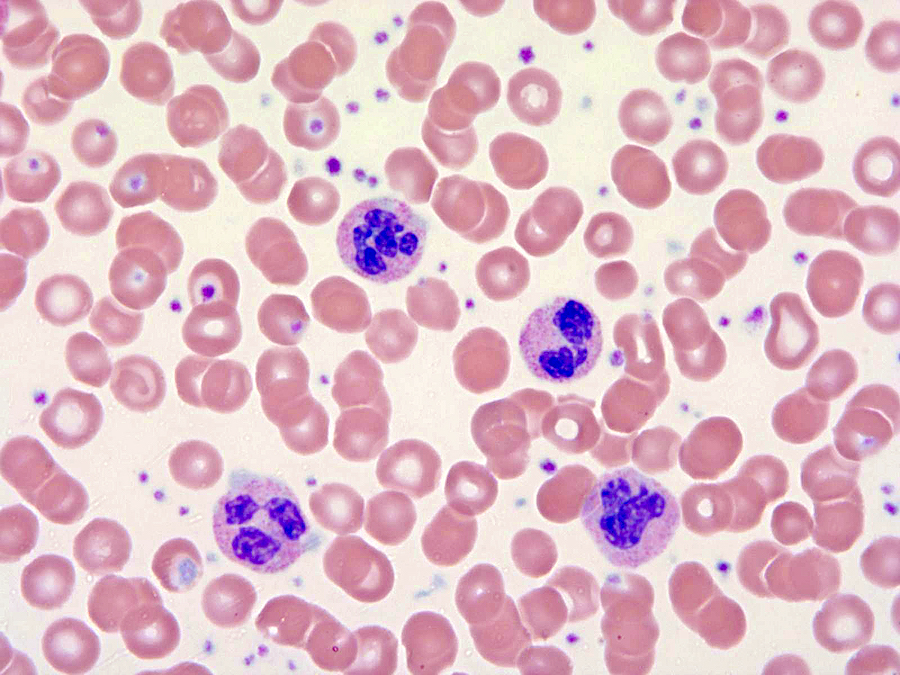
Peripheral smear description
- Leukocytosis with white blood cells (WBC) ≥ 13 × 109/L with median values of 24 - 96 × 109/L
- < 20% blasts in peripheral blood and bone marrow; usually < 5%
- Neutrophil precursors (promyelocytes, myelocytes and metamyelocytes) comprising > 10% of WBC
- Prominent dysgranulopoiesis
- Acquired Pelger-Huët abnormality
- Clumped chromatin
- Hypersegmentation of nuclei
- Abnormal nuclear projections
- Cytoplasmic hypogranularity
- Absent or minimal monocytosis; < 10% of leukocytes
- Absent or minimal basophilia; < 2% of leukocytes
- Anemia and thrombocytopenia are common
- References: PLoS One 2014;9:e98258, Haematologica 2006;91:1566, Ann Oncol 2000;11:441, Blood 1991;78:205, Blood 2014;123:2645
Positive stains
- No specific immunophenotype
- Granulocytes positive for MPO, CD33, CD13, CD15
- Monocytes positive for CD14 and CD68; monocytosis on biopsy should call into question a diagnosis of aCML
- References: J Clin Oncol 2001;19:2915, Blood 1991;78:205
Flow cytometry description
- No specific flow cytometry findings
Molecular / cytogenetics description
- Cytogenetics
- Karyotypic abnormalities present in up to 80% of cases
- Most common abnormalities:
- Gain of chromosome 8
- del(q20)
- Rarely i17q
- Abnormalities of 12, 13, 14, 17 and 19 also reported
- Molecular study
- NGS mutation findings:
- SETBP1, ETNK1, ASXL1, TET2, RAS, RUNX1 and PTPN1 detected
- Most cases have more than 1 mutation
- Mutations of SETBP1 on chromosome 18q21.1 seen in ~10 - 25% cases
- ETNK1 mutations slightly less common than SETBP1 (seen in ~8% of cases)
- CSF3R mutation present in < 10% of aCML
- Presence of MPN associated mutations (JAK2, CALR or MPL) tend to exclude diagnosis of aCML
- References: Biomark Res 2017;5:33, Leukemia 2013;27:1852, Haematologica 2014;99:e244, Blood 2015;125:499, Blood 2015;125:422, Int J Hematol 2015;101:229, Am J Hematol 2017;92:542, N Engl J Med 2013;368:1781
- NGS mutation findings:
Sample pathology report
- Peripheral blood:
- Mild normocytic anemia
- Marked leukocytosis with neutrophilia (70%) and circulating immature granulocytic precursors (22%)
- Dysplastic neutrophils
- No circulating blasts or mature monocytosis identified
- Mild thrombocytopenia
- Bone marrow, right posterior iliac crest, core biopsy, clot section and aspirate smear:
- Hypercellular bone marrow with marked granulocytic hyperplasia and dysgranulopoiesis consistent with atypical chronic myeloid leukemia (see comment)
- Comment: Of note, there is a marked leukocytosis with overt dysmyelopoiesis. FISH study is reportedly negative for t(9;22) / BCR-ABL1. Gene mutation profile showed a mutation involving the SETBP1 gene (VAF of 66%). Karyotyping was reportedly normal female karyotype, 46,XX[20]. The overall findings are consistent with atypical chronic myeloid leukemia.
- Peripheral smear: Manual review of the peripheral blood shows normochromic, normocytic anemia with mild thrombocytopenia. RBCs: mild normochromic, normocytic with minimal anisopoikilocytosis. WBCs: marked leukocytosis with markedly increased dysplastic neutrophils and left shifted myeloid precursors. Platelets: thrombocytopenia with rare large forms.
- Bone marrow biopsy: Quality: adequate. Cellularity: 90%. The bone marrow shows hypercellularity (90%) with marked granulocytic hyperplasia and diminished erythroid precursors. Megakaryocytes are increased and many of them are smaller than normal in size. The M:E ratio is increased. Reticulin stain highlights mild reituculin fibrosis (MF1). Iron stores are decreased to absent.
- Bone marrow clot section: Quality: adequate. Cellularity: 90% morphologic features are similar to those observed in the core biopsy.
- Bone marrow aspirate: Quality: adequate. Granulocytes: markedly increased; left shifted maturation without overt increase in blasts; marked dysplasia (abnormal chromatin pattern, hyposegmented nuclei or hypogranular cytoplasm). Erythrocytes: markedly decreased with progressive maturation, mild dysplasia (slight nuclear irregularities, basophilic stippling of cytoplasm or N:C maturation asynchrony). Megakaryocytes: adequate in number; dysplastic (variable in size, increased hypolobated forms, frequently with nuclear hyperchromasia). Blasts: overall ~2% of nucleated cells. No ring sideroblasts are present.
Differential diagnosis
- Myeloproliferative neoplasm (MPN):
- No dysplasia (except for abnormal megakaryocytes)
- Cytosis (see CML and CNL below for differential findings)
- BCR-ABL fusion
- JAK2, CALR, MPL, CSF3R mutations
- Chronic myeloid leukemia (CML):
- Presence of Philadelphia chromosome / BCR-ABL fusion gene
- Dysplasia is minimal in CML
- Basophilia > 2% in CML
- Chronic neutrophilic leukemia (CNL):
- Leukocytosis comprised mostly of neutrophils
- No significant left shift in proliferating neutrophils
- CSF3R mutation more common in CNL and favor this entity
- Myelodysplastic syndrome (MDS):
- Dysplasia in ≥ 1 lineage
- No leukocytosis
- Cytopenias predominate
- Myeloid / lymphoid neoplasms with eosinophilia and gene rearrangement:
- Chronic myelomonocytic leukemia (CMML):
- Monocytosis, > 10% of WBCs
- More severe dysplasia in aCML
- Higher survival
- Myelodysplastic / myeloproliferative neoplasm, unclassifiable (MDS / MPN, U):
- Leukocytosis and dysplastic changes in MDS / MPN, U raises differential diagnosis of aCML
- Presence of SETBP1 mutations favor the diagnosis of aCML
- Often lower WBC count when compared to aCML
- Distinction cannot be made in a subset of cases
- Clinically they are indistinguishable
- Significant blood and bone marrow morphological overlapping features
- aCML carries a worse prognosis (overall survival of 12.4 months versus 21.8 months for MDS / MPN, U)
- Reference: Jaffe: Hematopathology, 2nd Edition, 2016
Additional references
Board review style question #1
A 71 year old man presented to his primary care physician with complaints of fatigue and nausea. Upon further workup, he was found to have normocytic anemia, thrombocytopenia and marked leukocytosis (70 × 109/L) with no monocytosis. Imaging studies demonstrated a mildly enlarged spleen with no discernible lesions. A bone marrow biopsy was performed showing no increase in blasts with representative images shown above. FISH studies for BCR-ABL were negative. Gene mutation profile demonstrated a mutation involving the ETNK1 gene. Which of the following is the most likely diagnosis?
- Chronic myeloid leukemia (CML)
- Chronic neutrophilic leukemia (CNL)
- Acute myeloid leukemia (AML)
- Chronic myelomonocytic leukemia (CMML)
- Atypical chronic myeloid leukemia (aCML)
Board review style answer #1
E. aCML is a myeloproliferative / myelodysplastic (MPN / MDS) neoplasm associated with leukocytosis with no associated monocytosis. Clinically, patients often present with anemia, thrombocytopenia and splenomegaly. Bone marrow biopsy shows marked granulocytic hyperplasia and dysplastic neutrophils and left shifted myeloid precursors. FISH studies are characteristically negative for BCR-ABL with molecular studies often showing presence of SETBP1 and ETNK1 mutations. Answer choices A, B and C all represent additional MPN / MDS overlap neoplasms and can be all be ruled out with the findings above: CML is characterized by the presence of a BCR-ABL translocation. CNL is characterized by markedly increased neutrophils with no significant dysplasia or left shifted myeloid precursors. CMML shows a persistent monocytosis, not seen in the above case. AML is characterized by increased blasts > 20% of the total cellularity, wherein the blast count is reportedly normal above.
Comment Here
Reference: Atypical chronic myeloid leukemia
Comment Here
Reference: Atypical chronic myeloid leukemia
Board review style question #2
Which of the following cytogenetic or molecular alterations is most frequently identified in aCML?
- CSF3R
- JAK2
- PCM-JAK2
- PDGFR
- SETBP1
Board review style answer #2
E. SETBP1. PCM-JAK2 and PDGFRA / B gene rearrangements are independent entities listed in 2017 WHO that should be excluded before a diagnosis of aCML is established. JAK2 gene mutation is extremely rarely identified in aCML and when positive, aCML should be excluded. Although CSF3R mutation was reported in a subset of aCML, the overall incidence rate (< 10%) is much lower than SETBP1 (24.3%). The presence of CSF3R mutations, in conjunction with neutrophilia (> 80% of differential count) without dysplasia, favors a diagnosis of chronic neutrophilic leukemia (CNL) over aCML (Nat Genet 2013;45:18).
Comment Here
Reference: Atypical chronic myeloid leukemia
Comment Here
Reference: Atypical chronic myeloid leukemia