Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Peripheral smear description | Peripheral smear images | Positive stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Walia R, Yeung CCS. MDS with mutated SF3B1. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/myeloproliferativeRARS.html. Accessed March 31st, 2025.
Definition / general
- Myelodysplastic syndrome with mutated SF3B1 (MDS SF3B1) is a myeloid neoplasm with cytopenia and dysplasia characterized by SF3B1 mutation and often ring sideroblasts
- Acceptable definition: MDS with low blasts and ring sideroblasts
- International Consensus Classification (ICC) 2022 nomenclature: MDS with mutated SF3B1
Essential features
- Blood counts: characterized by cytopenia of one or more lineages, without thrombocytosis
- Morphology: erythroid lineage dysplasia, ring sideroblasts are present in over 90% cases but not required for diagnosis in the presence of SF3B1 mutation, blasts < 5% in the bone marrow and < 2% in the peripheral blood
- Cytogenetics: absence of 5q deletion, monosomy 7/7q deletion or complex karyotype
- Molecular genetics: SF3B1 mutation at a variant allele frequency (VAF) of > 10% (per ICC), no biallelic TP53 or RUNX1 mutation
- In the absence of SF3B1 mutation, ≥ 15% ring sideroblasts are needed
- Reference: Blood 2020;136:157
Terminology
- WHO 4th edition equivalents: MDS RS SLD (myelodysplastic syndrome with ring sideroblasts and single lineage dysplasia) and MDS RS MLD (multilineage dysplasia)
- Formerly called refractory anemia with ring sideroblasts, refractory cytopenia with multilineage dysplasia and ring sideroblasts
ICD coding
Epidemiology
- 17% of all MDS cases (Leuk Res 2017;57:78)
- Annual incidence: 0.84/100,000 persons (Leuk Res 2011;35:1591)
- Median age: 70 - 75 years (Leuk Res 2017;57:78)
- Slight male preponderance
- Patients with low blasts and ≥ 15% ring sideroblasts without SF3B1 mutation account for 3 - 4% of all MDS cases
- Reference: Blood 2020;136:157
Pathophysiology
- SF3B1 gene encodes a component of the U2 small nuclear ribonucleoproteins (snRNP) spliceosome
- Mutations in the SF3B1 gene lead to altered splicing of mitochondrial iron transporter gene and other metabolic genes, which in turn leads to the ineffective erythropoiesis and formation of ring sideroblasts
- Ring sideroblasts are erythroid precursors with abnormal iron accumulation within the mitochondria
- These changes lead to morphologic dysplasia, ineffective erythropoiesis and iron overload
- References: Blood 2016;128:462, Blood 2012;120:3173
Etiology
- Driver mutations in SF3B1 gene in hematopoietic stem cells
Clinical features
- Anemia
- Thrombocytopenia and neutropenia in a minority
- Hepatosplenomegaly due to iron overload in some cases
- Reference: Am J Hematol 2021;96:379
Diagnosis
- Refractory anemia or bicytopenia / pancytopenia on complete blood count
- < 5% bone marrow blasts, < 2% peripheral blood blasts
- Molecular studies: next generation sequencing (NGS) panel including SF3B1 and associated myeloid neoplasm genes for diagnosis and prognosis; absence of biallelic TP53 inactivation
- Cytogenetic studies: absence of del(5q), monosomy 7 or 7q deletion, complex karyotype
- Does not fulfill criteria for other MDS / myeloproliferative neoplasm (MPN) entities
- References: Am J Hematol 2021;96:379, Blood 2016;127:2391
Laboratory
- Normochromic macrocytic or normochromic normocytic anemia (hemoglobin usually is < 10 g/dL); usually no other cytopenias
- Thrombocytosis should be absent as these are classified as MDS / MPN with SF3B1 mutation and thrombocytosis, which have concurrent JAK2 V617F, CALR or MPL mutations
- Concurrent iron deficiency can mask the presence of MDS RS (Blood 2011;117:5793)
- May demonstrate lab values of progressive iron overload (e.g., elevated serum ferritin and transferrin saturation) (Hemasphere 2020;4:e357)
Prognostic factors
- Among other WHO 5th edition and ICC 2022 described MDS subtypes, MDS with mutated SF3B1 has the best outcomes (Leukemia 2022;36:1703, Blood 2022;140:1200)
- When SF3B1 mutations are seen, the presence of multilineage dysplasia has not demonstrated significant prognostic impact
- Transfusion dependence is associated with worse outcomes and higher risk of transformation to acute myeloid leukemia (AML)
- In comparison to patients with MDS with low blasts and SF3B1 mutations, MDS with low blasts, ring sideroblasts and wild type SF3B1 has a less favorable overall survival and leukemia free survival (Crit Rev Oncol Hematol 2019;133:74)
- Presence of excess blasts has a worse prognosis even in the presence of SF3B1 mutation
- Additional mutations in TP53, RUNX1, EZH2 genes have been associated with a worse outcome as compared to patients with SF3B1 mutation alone (Blood 2015;126:233)
Case reports
- 16 year old girl with hypochromic microcytic anemia (Arch Pathol Lab Med 2005;129:e199)
- 59 year old man with beta thalassemia trait, MDS with ring sideroblasts and SF3B1 and TET2 mutations (NAJMS 2017;10:32)
- 73 year old woman with MDS with ring sideroblasts (ASH: MDS with Ring Sideroblasts [Accessed 9 April 2024])
Treatment
- Recombinant erythropoietin (EPO) for anemia
- Iron chelation therapy for iron overload
- Lusatercept and sotatercept: soluble fusion proteins that increase hemoglobin levels by a different mechanism than EPO (Am J Hematol 2019;94:475)
Microscopic (histologic) description
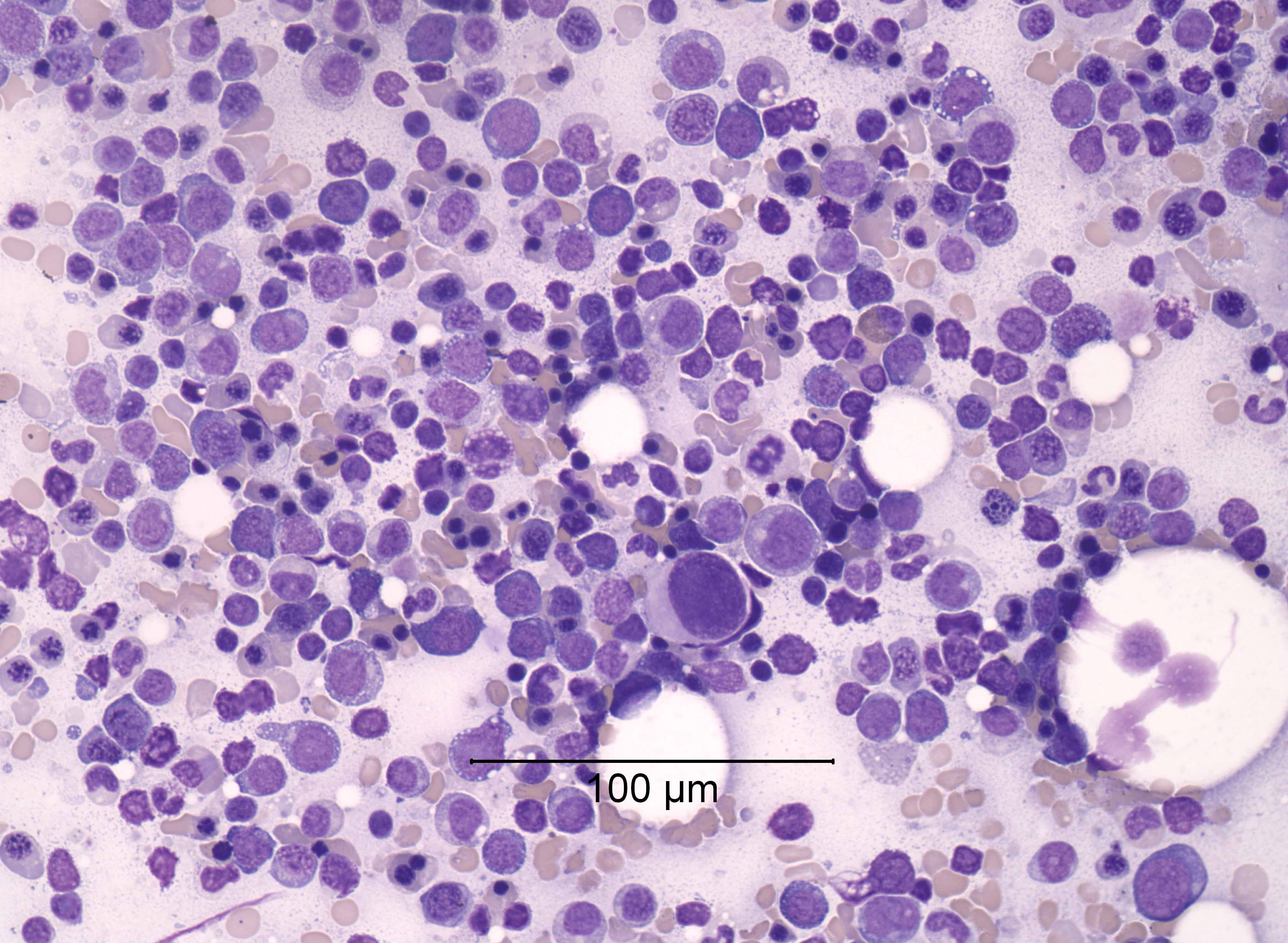
- Microscopic criteria
- Characterized by cytopenias (1 - 2 MDS RS SLD, 1 - 3 MDS RS MLD)
- Ring sideroblasts and morphological dysplasia (1 lineage [erythroid] or 2 - 3 lineages), often with concomitant SF3B1 mutations
- In the absence of SF3B1 mutations, ≥ 15% ring sideroblasts are needed, whereas > 5% ring sideroblasts are required for diagnosis in the presence of SF3B1 mutations
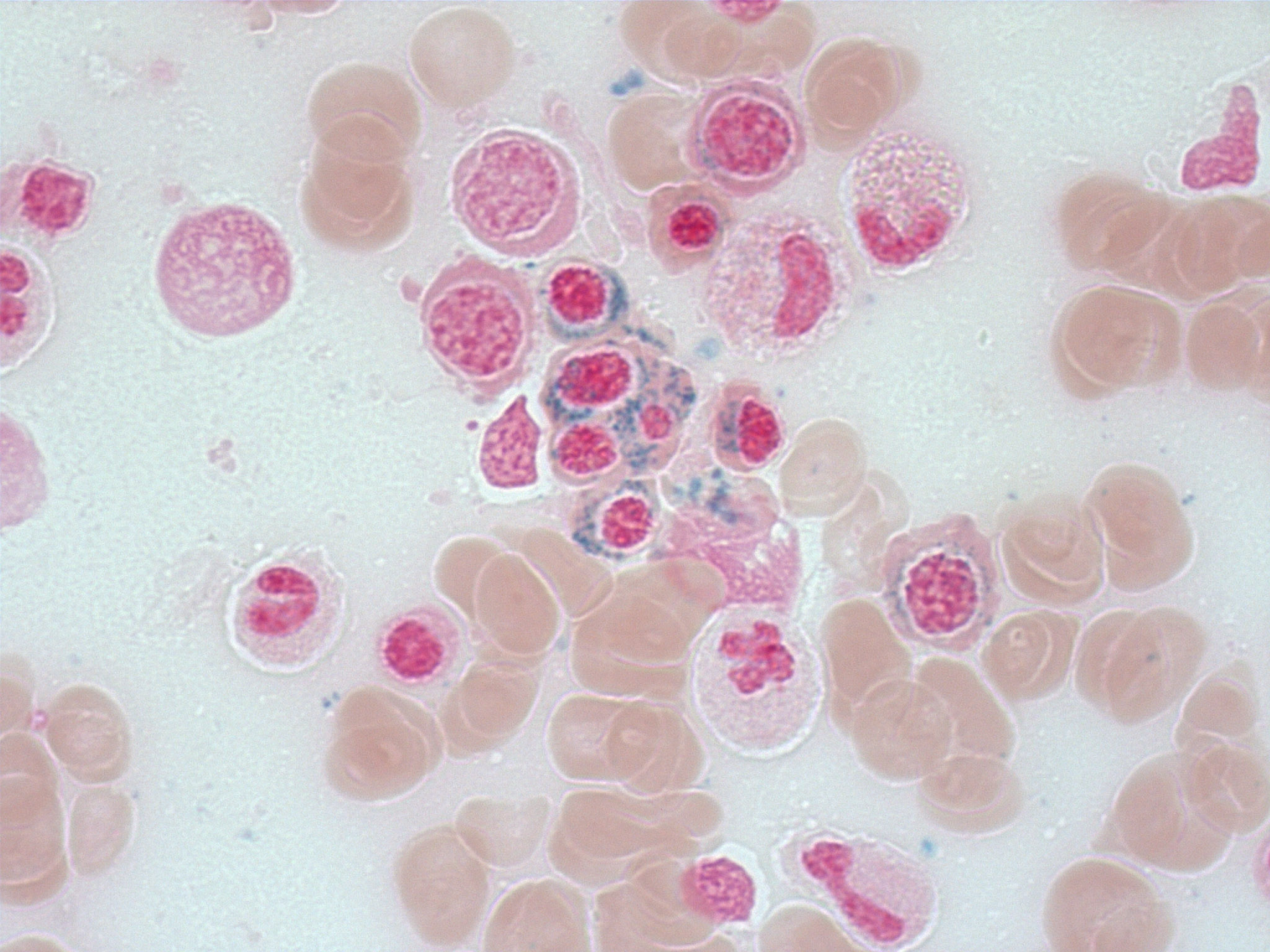
- Ring sideroblasts are erythroid precursors with ≥ 5 iron granules encircling one - third or more of the nucleus (Haematologica 2008;93:1712)
- Lacks Auer rods, < 5% bone marrow blasts, < 2% peripheral blood blasts
- Bone marrow histology
- Cellularity
- Hypercellular or normocellular marrow
- Erythropoiesis
- Erythroid hyperplasia (with mild to moderate dysplasia, including irregular nuclear contours, nuclear budding, megaloblastic change)
- Myelopoiesis
- Myeloblasts < 5% (or call MDS with excess blasts)
- Megakaryopoiesis
- Normal numbers and morphology (in SLD)
- In addition to the presence of ring sideroblasts, erythroid hyperplasia and dysplasia, > 10% dysplastic cells in other lineages (myeloid or megakaryocyte) noted in multilineage dysplasia subtype
- Increased storage iron
- Cellularity
- References: Mediterr J Hematol Infect Dis 2015;7:e2015035, Blood 2016;127:2391
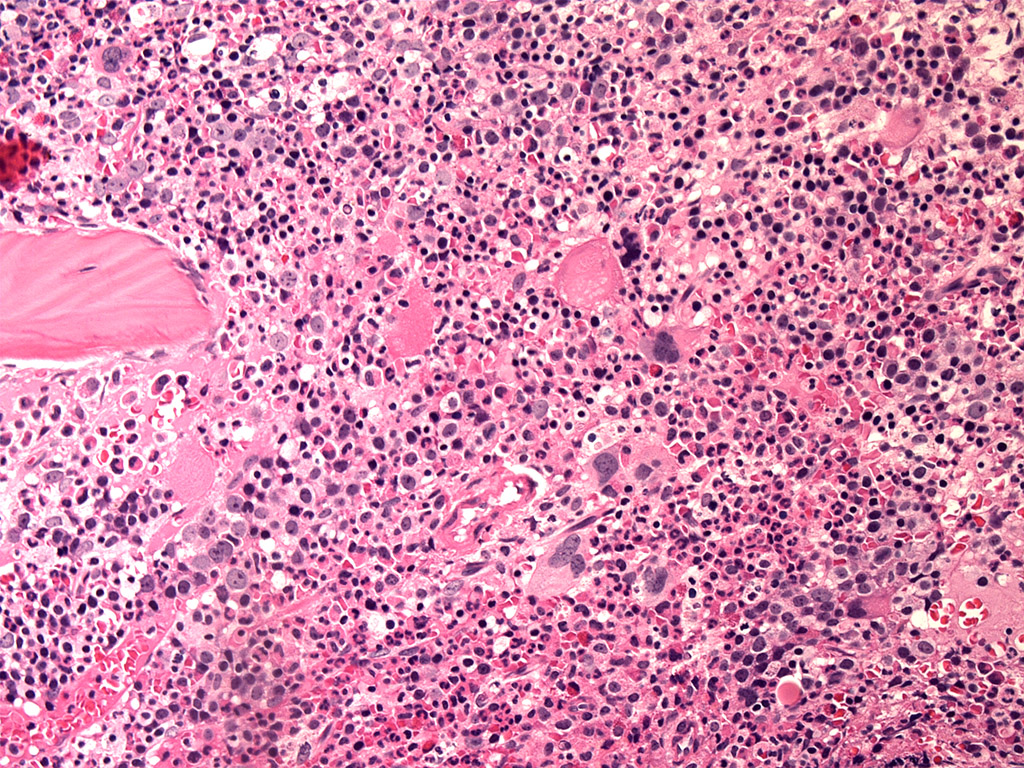
Microscopic (histologic) images
Cytology description
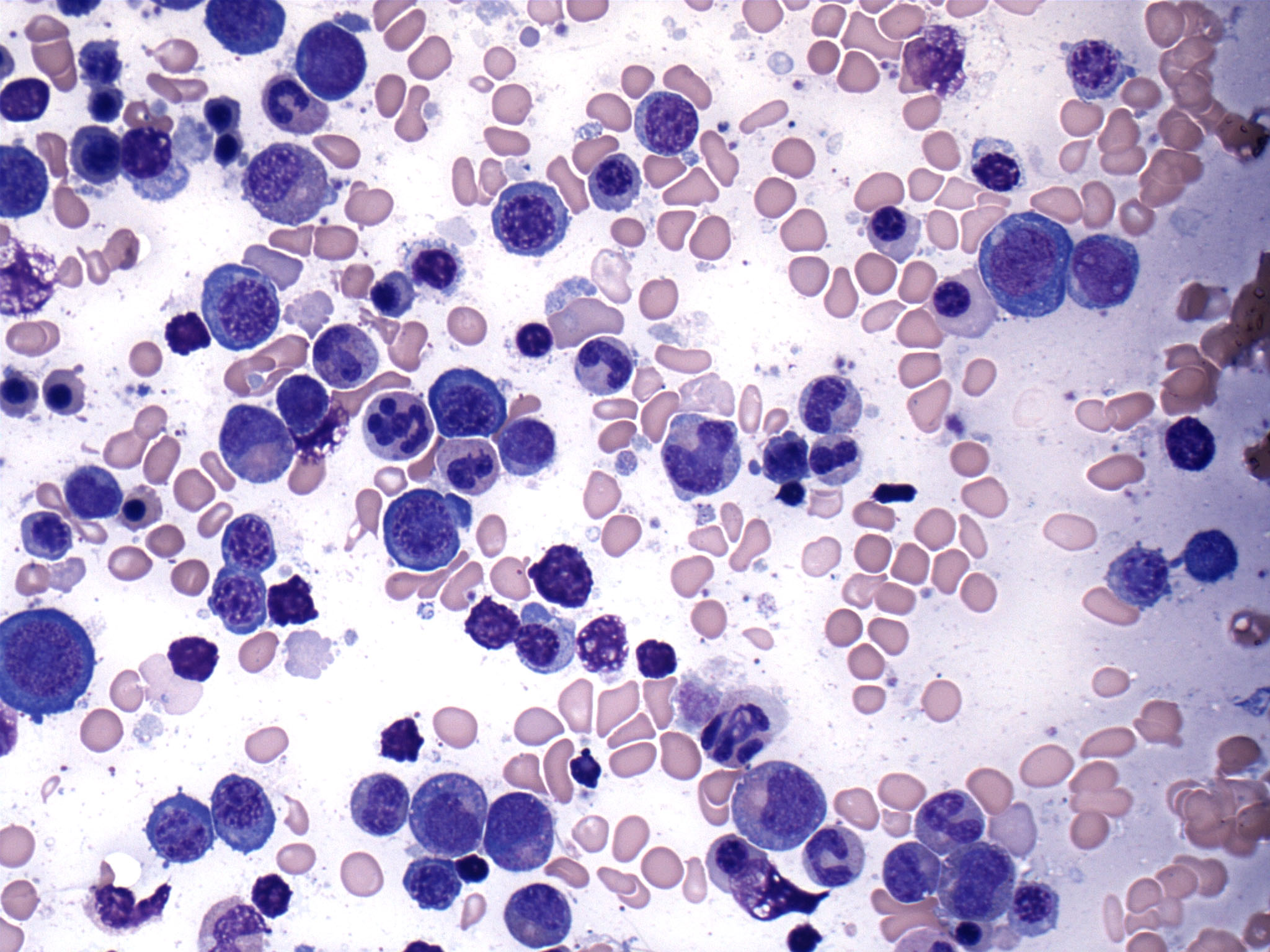
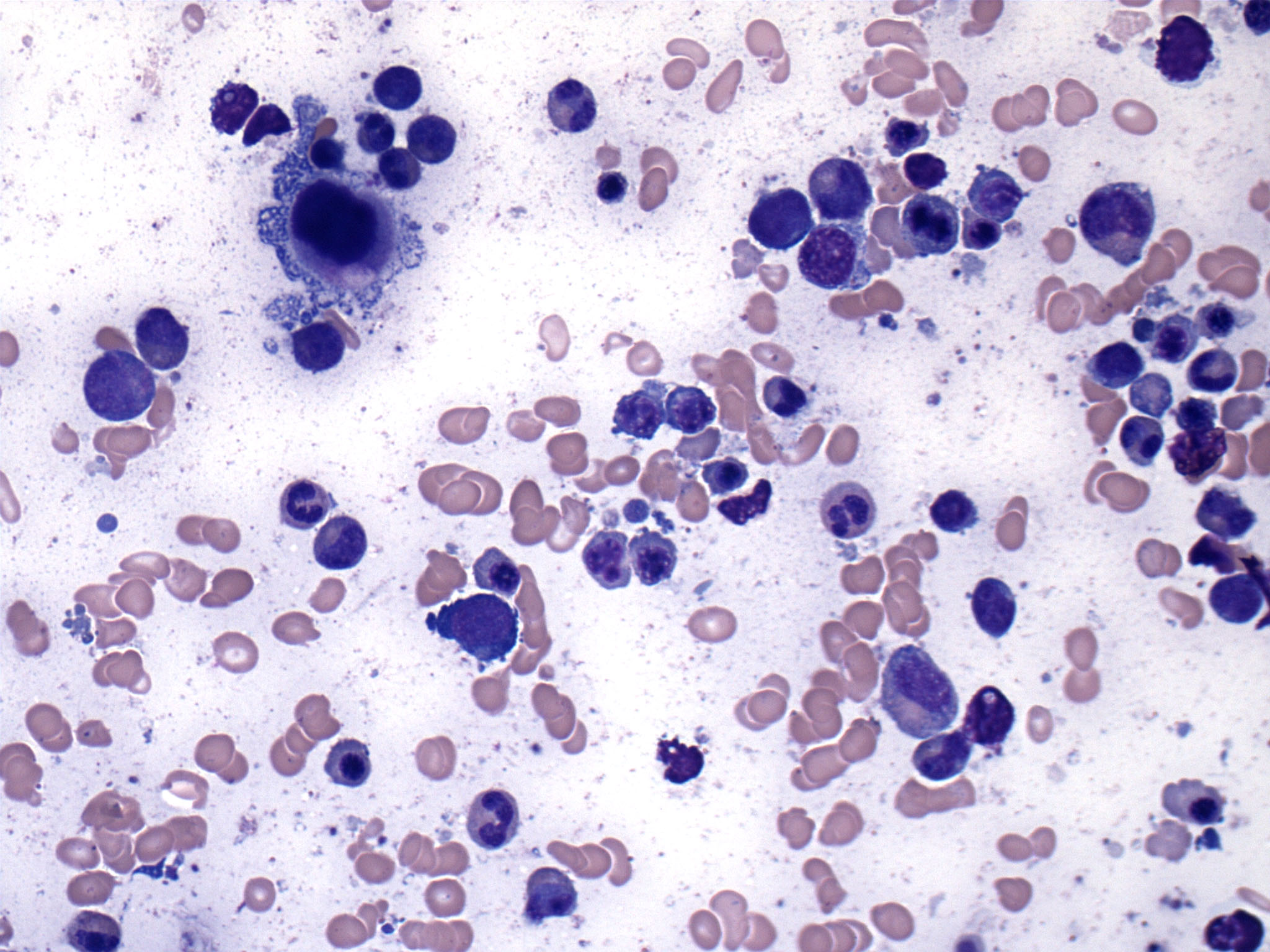
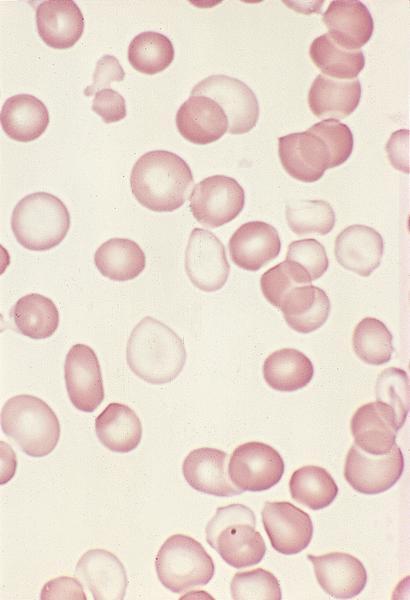
Peripheral smear description
- Peripheral blood
- Dimorphic red blood cell populations, normal and microcytic hypochromic
- Occasional coarse basophilic stippling and Pappenheimer bodies
- < 1% myeloblasts
- Evaluate for cytopenias and granulocytic dysplasia to subclassify
- Reference: Blood 2020;136:157
Positive stains
- Iron stain highlights the ring sideroblasts
- Reticulin stain is used to assess the degree of fibrosis in the bone marrow biopsy
- CD34 is used to highlight the blasts in the bone marrow biopsy
- Reference: Hematol Transfus Cell Ther 2019;41:279
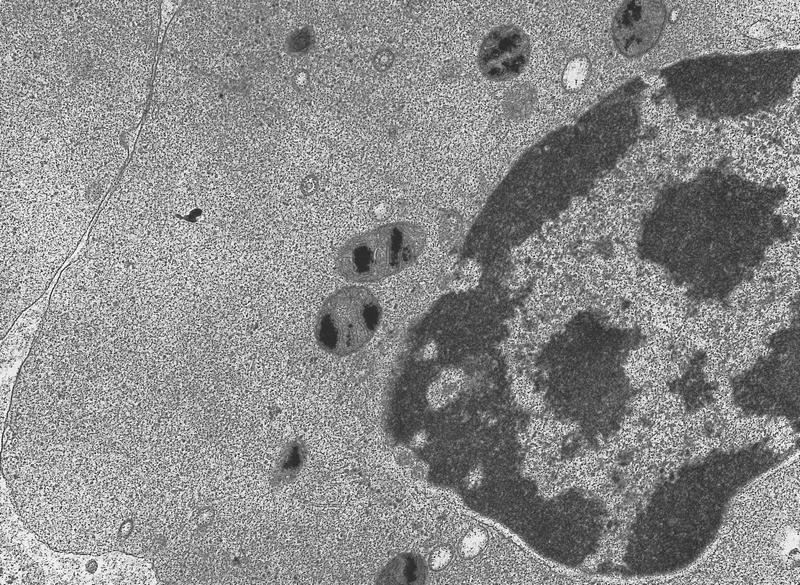
Electron microscopy description
- Transmission electron microscopy analysis of ring sideroblasts reveals features of apoptosis with condensed nuclear chromatin and fragmentation of the erythroid cells due to iron deposition in mitochondria (Oncogene 2006;25:4757)
Molecular / cytogenetics description
- Mutation in the spliceosome gene SF3B1 is found in 90% of cases, usually at a high VAF (35 - 43%)
- 50% of SF3B1 mutations affect codon 700, with other hotspots at codons 666, 662, 622, 625
- Presence of comutations involving BCOR, BCORL1, NRAS, RUNX1, SRSF2 or STAG2 are associated with significantly different outcomes in comparison to mutation in SF3B1 alone (NEJM Evidence 2022;1:EVIDoa2200008)
- Most cases of MDS SF3B1 have a normal karyotype or abnormalities involving a single chromosome
- Cases with 5q deletion, monosomy 7/7q deletion or complex karyotype are excluded
Sample pathology report
- Bone marrow, right posterior iliac crest, aspirate smears, core biopsy, particle prep and peripheral blood smear:
- Hypercellular marrow with megakaryocytic and erythroid dysplasia, 1+ reticulin deposition and no increased blasts (see comment)
- Increased ring sideroblasts are present
- Anemia with no circulating blasts
- Comment: The sum of the morphological findings (notably, the presence of ring sideroblasts and a dominant SF3B1 mutation [VAF 44%]) is diagnostic of a myeloid neoplasm and compatible with myelodysplastic neoplasm with low blasts and SF3B1 mutation (MDS SF3B1) under the WHO classification system. Under the International Consensus Classification system, this would be called myelodysplastic syndrome with mutated SF3B1 (also called MDS SF3B1). Myelodysplastic / myeloproliferative neoplasm with SF3B1 mutation and thrombocytosis (MDS / MPN SF3B1 T) was a consideration but the lack of thrombocytosis argues against this entity.
- Cytogenetics
- Normal male, 46, XY
- Molecular studies
- Positive for clinically significant variants: SF3B1 (p.N626S, NM_012433.3:c.1877A>G, VAF 44%)
- Flow cytometry
- 3% myeloid blasts (see comment)
- Comment: Bone marrow flow cytometry reveals the presence of 3% myeloid blasts expressing CD33, CD13, CD117 and MPO. There is no abnormal B cell clone identified and B cells constitute ~4% of all nucleated cells. Plasma cells comprise 2% of all nucleated cells and normally express CD138 and CD38.
- Peripheral blood
- Differential
- Complete blood count: 3,500/mm3
- Neutrophils: 60%
- Lymphocytes: 30%
- Eosinophils: 4%
- Monocytes: 4%
- Basophils: 2%
- Blasts: 0%
- Morphology
- Red blood cells: macrocytic anemia with anisopoikilocytosis
- White blood cells: leukopenia, no circulating blasts, normal morphology
- Platelets: mild thrombocytopenia, few giant platelets seen
- Differential
- Bone marrow aspirate
- Differential
- Erythroid: 65%
- Myeloid: 32%
- Lymphocytes: 3%
- Plasma cells: 2%
- Blasts: 3%
- M:E ratio = 1:2
- Morphology
- Erythroid: erythroid hyperplasia with features of dyserythropoiesis - binucleation, nuclear budding, nucleocytoplasmic asynchrony
- Myeloid: normal maturation with mild dysgranulopoiesis
- Megakaryocytes: normal in numbers with hypolobated and monolobated forms
- Plasma cells: normal in number and morphology
- Lymphocytes: normal in number and morphology
- Iron stain: 40% ring sideroblasts are identified with presence of perinuclear iron granules
- Differential
- Bone marrow biopsy
- Morphology
- Hypercellular (70% cellularity) bone marrow with erythroid hyperplasia, dyserythropoiesis, dysgranulopoiesis and dysmegakaryopoiesis
- Reticulin stain shows grade I fibrosis
- Morphology
Differential diagnosis
- MDS / MPN with SF3B1 mutation and thrombocytosis:
- Anemia associated with dysplastic erythropoiesis and ≥ 15% ring sideroblasts
- Persistent thrombocytosis with platelet count ≥ 450 x 109/L
- Detection of SF3B1 mutation concurrently with JAK2 p.V617F
- Various nonclonal conditions can present with ring sideroblasts in the bone marrow; thorough clinical history and absence of diagnostic criteria for clonal MDS RS (> 15% RS or SF3B1 mutation) are essential for differential diagnosis
- Genetically inherited congenital sideroblastic anemia (Br J Haematol 2016;174:847):
- Characterized by mutations in 1 of 3 mitochondrial pathways: heme synthesis, iron sulfur cluster biogenesis and protein synthesis
- X linked sideroblastic anemia is the most common form attributed to mutation in aminolevulinic acid (ALA) synthase
- Microcytic hypochromic anemia and iron overload
- Acquired sideroblastic anemia due to chronic alcohol use (Postgrad Med 1992;92:147):
- History of chronic alcoholism
- Elevated serum aspartate aminotransferase (AST):alanine aminotransferase (ALT) ratio
- Macrocytic anemia
- < 15% ring sideroblasts
- Drug induced sideroblastic anemia - chloramphenicol, penicillamine, linezolid, isoniazid (Haematologica 2013;98:e138):
- History of medication use
- May have pure red cell aplasia
- Reversible after discontinuation of drug
- Lead poisoning:
- High serum lead levels, lead lines (Burton lines) on teeth and gingiva
- < 15% ring sideroblasts
- Basophilic stippling in red cells on peripheral smear, microcytic and hemolytic anemia
- Copper deficiency / zinc toxicity (Clin Case Rep 2015;3:325):
- Low serum copper levels or high serum zinc levels
- < 15% ring sideroblasts
- Cytoplasmic vacuoles in erythroid and myeloid cells
- Genetically inherited congenital sideroblastic anemia (Br J Haematol 2016;174:847):
- Reference: Am J Hematol 2021;96:379
Additional references
Board review style question #1
Board review style answer #1
D. SF3B1 mutation. SF3B1 mutations are associated with ringed sideroblasts in almost 90% of cases. Answer A is incorrect because isolated del 5q may or may not be associated with ring sideroblasts and these MDS cases are classified as MDS with 5q deletion irrespective of ring sideroblasts. Answer B is incorrect because JAK 617F mutation is frequently associated with myeloproliferative neoplasms like polycythemia vera and essential thrombocytosis; however, some cases of myelodysplastic / myeloproliferative neoplasm with SF3B1 mutation and thrombocytosis can have JAK2 V617F comutation and these cases show the presence of ring sideroblasts. Answer C is incorrect because MDS with monosomy 7 is not usually associated with ring sideroblasts.
Comment Here
Reference: MDS with mutated SF3B1
Comment Here
Reference: MDS with mutated SF3B1
Board review style question #2
Which of the following statements is true regarding myelodysplastic syndrome (MDS) with ring sideroblasts?
- MDS with mutated SF3B1 has poor disease outcomes as compared to other subtypes of MDS
- Presence of excess blasts has a worse prognosis, even in the presence of SF3B1 mutation
- Presence of ring sideroblasts is an essential diagnostic requirement for MDS with mutated SF3B1
- Ring sideroblasts as well as SF3B1 mutation are not detected in other MDS types or other myeloid neoplasms
Board review style answer #2
B. Presence of excess blasts has a worse prognosis, even in the presence of SF3B1 mutation. The favorable outcome associated with SF3B1 mutation is lost as soon as an excess of blasts is observed. Answer C is incorrect because the presence of ring sideroblasts is not a diagnostic requirement if SF3B1 mutation is present at allele fraction > 35%. Ring sideroblasts are seen in more than 90% of these cases; however, if SF3B1 is not available, > 15% ring sideroblasts are required for diagnosis of MDS with low blasts and ring sideroblasts. Answer A is incorrect because MDS with low blasts and SF3B1 mutation has a better prognosis than other MDS types with low blasts. Answer D is incorrect because ring sideroblasts as well as SF3B1 mutation can be detected in other MDS types or other myeloid neoplasms, including acute myeloid leukemia and MDS with increased blasts. Cases that fulfill the criteria for MDS with low blasts and 5q deletion should be classified as such, even if ring sideroblasts or SF3B1 mutation are identified. In patients who present with thrombocytosis, detection of SF3B1 mutation concurrently with JAK2 p.V617F, CALR or MPL mutations qualifies for a diagnosis of myelodysplastic / myeloproliferative neoplasm with SF3B1 mutation and thrombocytosis.
Comment Here
Reference: MDS with mutated SF3B1
Comment Here
Reference: MDS with mutated SF3B1