Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Peripheral smear description | Positive stains | Flow cytometry description | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Iranzad N, Wang E. with del(5q). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/myeloproliferative5qminus.html. Accessed April 2nd, 2025.
Definition / general
- Myelodysplastic syndrome (MDS) characterized by anemia with or without other cytopenias or thrombocytosis in conjunction with chromosome 5q deletion with or without one additional cytogenetic abnormality apart from monosomy 7 or del(7q)
- Myeloblasts represent < 5% of bone marrow cellularity and < 1% of peripheral blood leukocytes
Essential features
- Subtype of MDS in low risk group with relatively good prognosis
- Requires presence of del(5q) with or without one additional cytogenetic abnormality apart from monosomy 7 or del(7q)
- TP53 mutation is associated with resistance to therapy, increased risk of transformation and decreased overall survival (J Clin Oncol 2011;29:1971, Br J Haematol 2013;160:660, Br J Haematol 2013;162:74, Haematologica 2014;99:1041)
Terminology
- MDS with 5q deletion
- 5q minus syndrome
ICD coding
- ICD-O: 9986/3 - myelodysplastic syndrome associated with isolated del(5q)
Epidemiology
- F > M
- Adults, median age approximately 60 - 70 years
Sites
- Blood and bone marrow
Pathophysiology
- Loss of tumor suppressor genes in deleted region of 5q results in haploinsufficiency of several genes (Blood 2002;99:4638, Nat Med 2007;13:78)
- RPS14
- Encodes a ribosomal structural protein (Nat Med 2010;16:59, Nature 2008;451:335, Nat Med 2016;22:288, Leukemia 2019;33:1759)
- Loss of this gene results in ineffective erythropoiesis
- Thought to play a role in p53 pathway activation (Blood 2011;117:2567)
- CSNK1A1
- Encodes casein kinase 1A1
- Haploinsufficiency leads to WNT / beta catenin pathway dysregulation (Cancer Cell 2014;26:509)
- Important therapeutic target
- miR-145 and miR-146a
- Plays a role in NFκB activation
- Contributes to dysmegakaryopoiesis (Nat Med 2010;16:49)
Etiology
- Unknown at this time
Clinical features
- Commonly presents with anemia, usually macrocytic, often severe
- 32 - 49% have thrombocytosis at presentation (Leukemia 2004;18:113, Blood 1993;81:1040)
- 17 - 18% have thrombocytopenia (Leukemia 2004;18:113, Blood 1993;81:1040)
- Pancytopenia is very rare and if present, should be categorized as MDS, unclassifiable (Leuk Res 2013;37:64)
Diagnosis
- Conventional cytogenetics with del(5q) involving 5q31-5q33 region with or without one additional abnormality apart from monosomy 7 or del (7q) (Leukemia 2012;26:1286, Leukemia 2011;25:110, J Clin Oncol 2012;30:820)
- FISH may be performed to detect del(5q) if conventional cytogenetics is suboptimal but is limited for providing information as to whether or not it is isolated
- TP53 mutation
- Associated with resistance to therapy, increased risk of transformation and decreased overall survival (J Clin Oncol 2011;29:1971, Br J Haematol 2013;160:660, Br J Haematol 2013;162:74, Haematologica 2014;99:1041)
- Detected in approximately 20% of cases
- Can be determined via molecular testing or immunohistochemistry
- Other mutations may be detected with molecular testing, including alterations in JAK2, MPL, SF3B1, RUNX1, WT1, ASXL1 and TET2, among others (Leukemia 2006;20:1319, Leukemia 2010;24:1283, Leuk Res 2010;34:821, Blood 2015;126:233, Blood 2011;118:6239, Am J Hematol 2011;86:393)
Laboratory
- Repetitive with clinical features and diagnosis headings
Prognostic factors
- Median survival is approximately 5.5 - 12 years (Leukemia 2010;24:1283, Leukemia 2011;25:110, Leukemia 2004;18:113)
- Transformation to AML in < 10% of patients
- Dysgranulopoiesis associated with a poor prognosis (Leukemia 2009;23:796, Hum Pathol 2013;44:346)
- TP53 mutation associated with a poor prognosis (J Clin Oncol 2011;29:1971, Br J Haematol 2013;160:660, Br J Haematol 2013;162:74)
Case reports
- 50 year old woman with history of autoimmune scleritis treated with immunosuppressive therapy presenting with weakness, pallor and progressive anemia (Case Rep Oncol 2012;5:586)
- 60 year old man with macrocytic anemia, weakness and fatigue (Case Rep Oncol 2014;7:277)
- 66 year old woman presenting with persistent anemia (Blood 2017;129:2333)
- 71 year old woman with history of polycythemia vera presenting with anemia, thrombocytosis and leukocytosis (Mediterr J Hematol Infect Dis 2016;8:e2016050)
- 76 year old woman presenting with dizziness (Blood Res 2018;53:104)
Treatment
- Lenalidomide, a thalidomide analogue, has been shown to be beneficial (Ann Hematol 2005;84:569, N Engl J Med 2006;355:1456)
- Transfusion independence achieved in approximately 67% of patients
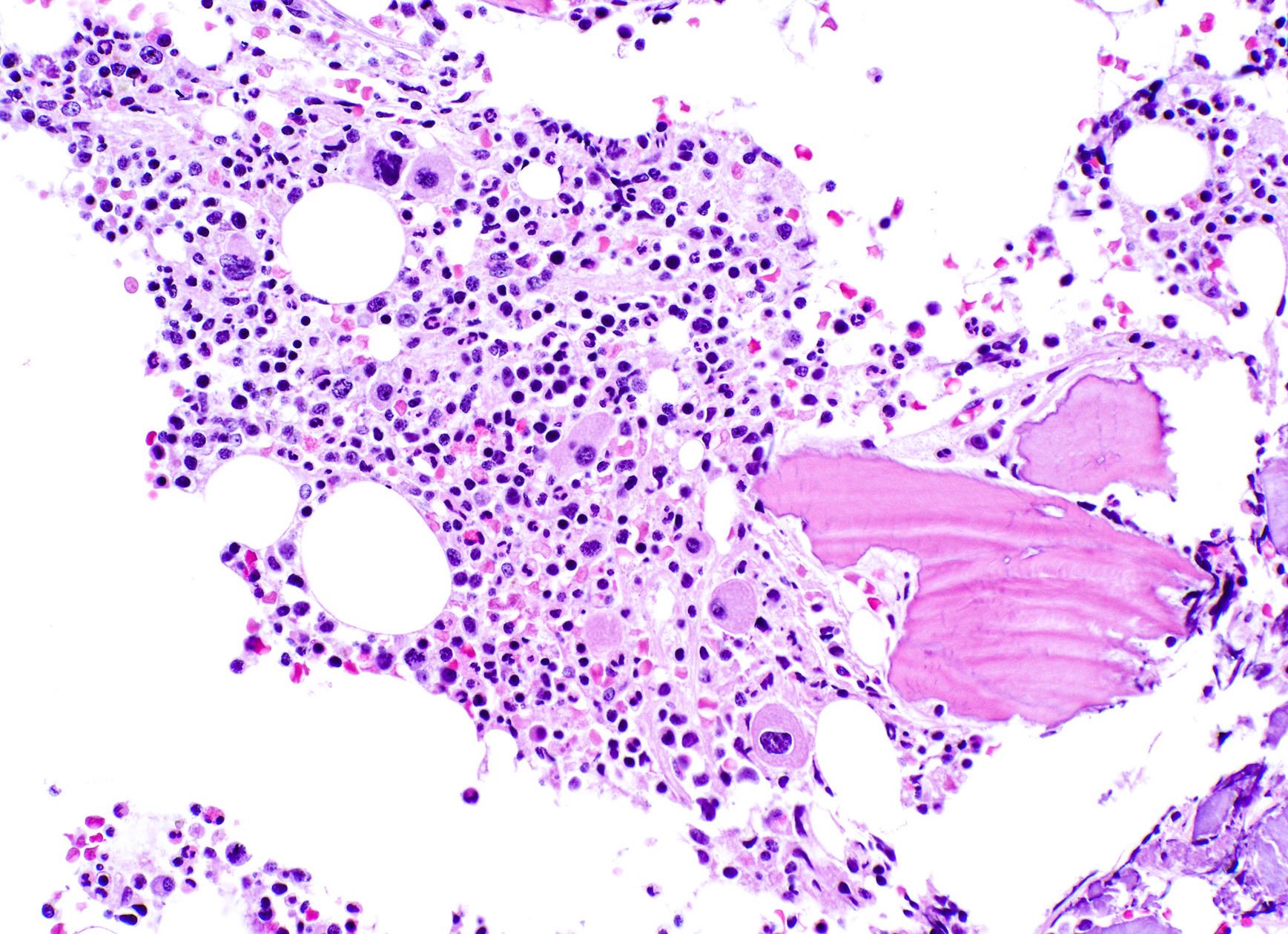
Microscopic (histologic) description
- Blast count must be < 5% in bone marrow
- Bone marrow cellularity typically hyper to normocellular (Leukemia 2004;18:113)
- Megakaryocyte hyperplasia
- Megakaryocytes with nonlobated and hypolobated nuclei
- Erythroid hypoplasia
- Dysgranulopoiesis is uncommon
Microscopic (histologic) images
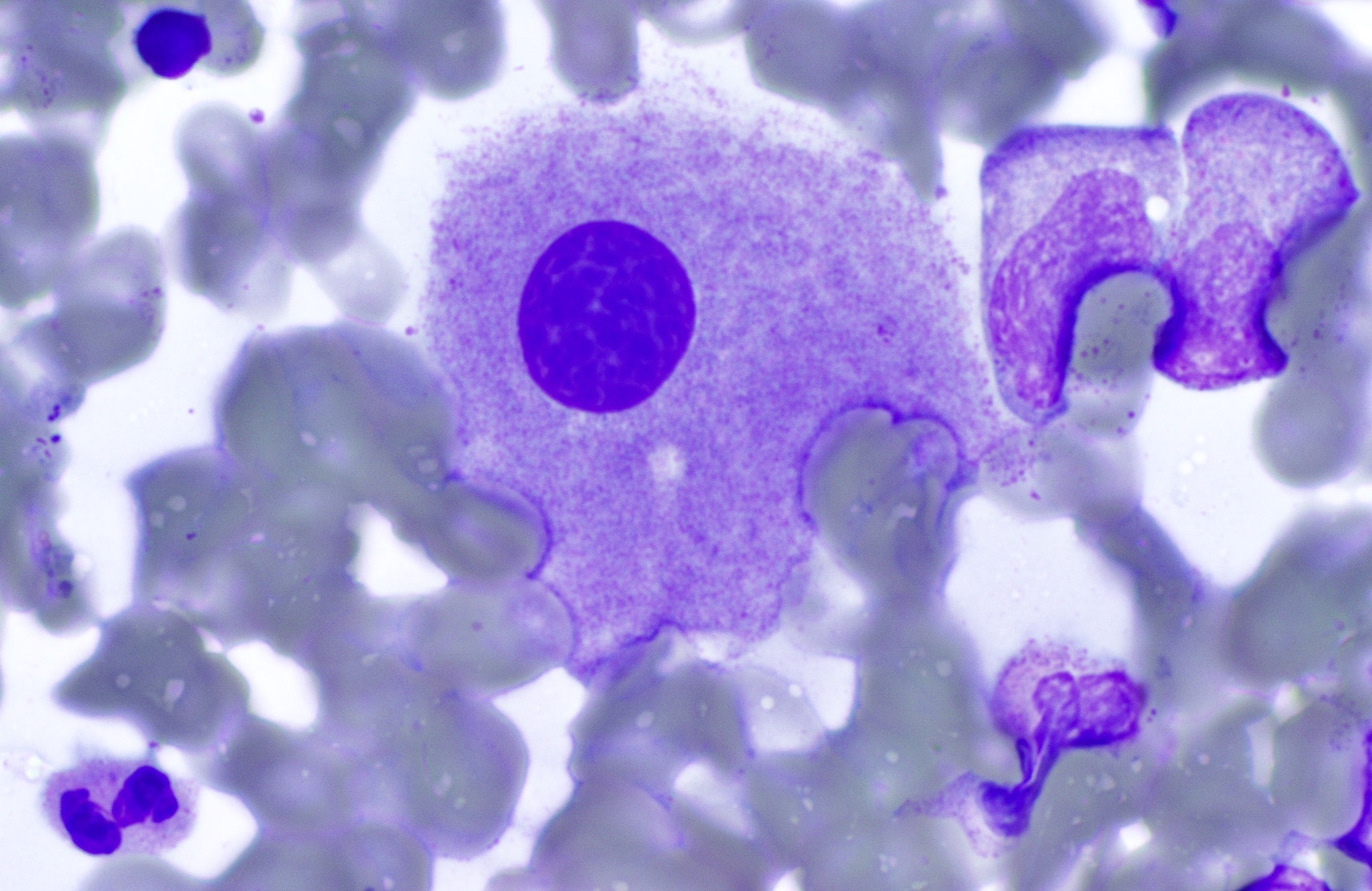
Cytology description
- Ring sideroblasts may be present
- Auer rods must be absent
Peripheral smear description
- Blast percentage must be < 1%
- Anemia common, often severe and typically macrocytic
- Thrombocytosis in 33% of cases
- Thrombocytopenia less commonly seen
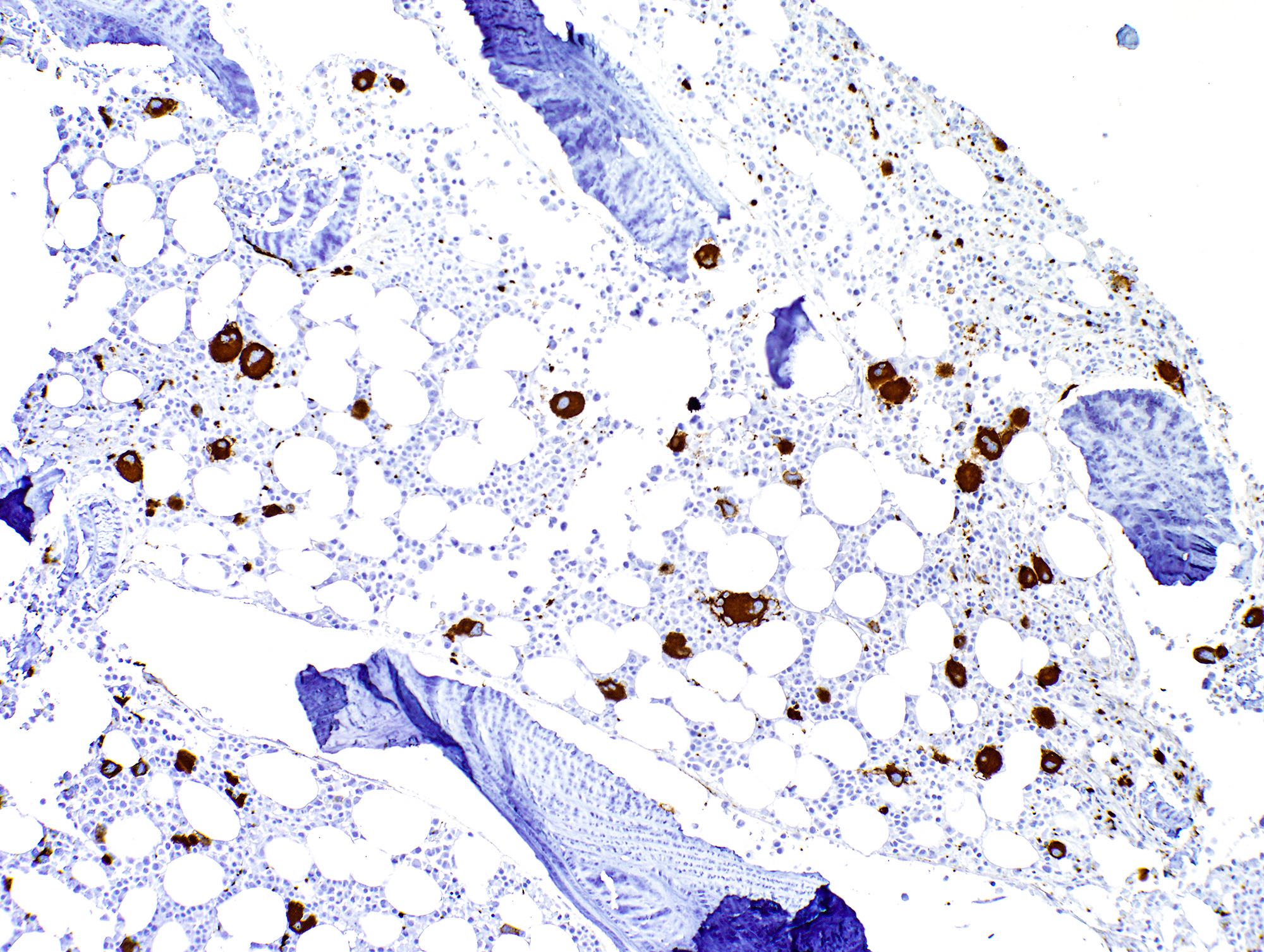
Positive stains
- CD61 highlights increased / dysplastic megakaryocytes
Flow cytometry description
- No findings specific to this entity
- Myeloblasts with aberrant phenotype may be detected
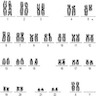
Molecular / cytogenetics description
- Repetitive with diagnosis heading
Sample pathology report
- Bone marrow, aspirate smear, touch imprint, clot and core biopsy:
- Normocellular bone marrow (30%) with megakaryocyte dysplasia
- 3% bone marrow blasts (see comment)
- Comment: The presence of dysplastic micromegakaryocytes in this patient with longstanding macrocytic anemia is concerning for myelodysplastic syndrome with single lineage dysplasia (MDS SLD). However, a normocellular marrow would be highly unusual for this diagnosis as MDS typically presents with hypercellularity and rarely with hypocellularity. Thus, a definitive diagnosis of MDS cannot be made at this time without evidence of myeloid clonality, increased blasts or hyper / hypocellularity. Correlation with the pending chromosome analysis and FISH studies are required to establish clonality. In addition, myeloid NGS studies may be diagnostically useful to provide evidence of clonality with additional prognostic or potentially therapeutic information; this test may be performed on the peripheral blood if clinically indicated. The corresponding flow cytometric analysis did not detect any phenotypic abnormalities in myeloid or monocytic lineages or increase in blasts, in keeping with the morphologic findings.
FISH - MDS panel:
- Interpretation
- 18.5% of cells showed deletion of 5q (EGR1) by interphase FISH. No evidence of numeric abnormalities or deletions involving chromosomes 7, 8 or 20 observed by interphase FISH. Correlation with other laboratory and clinical information is recommended.
- nuc ish (D5S721/D5S23x2,EGR1x1)[37/200]
- nuc ish (D7Z1,D7S486)x2[200]
- nuc ish (D8Z2,D20S108)x2[199]
Summary table:
| Locus | Result |
| Monosomy 5/5q- | 18.5% deletion 5q |
| Monosomy 7/7q- | within normal limits |
| Trisomy 8 | within normal limits |
| Deletion of 20q | within normal limits |
Chromosome analysis:
- Interpretation
- 46,XX,del(5)(q12q33)[4]/46,XX[14]
- Abnormal clone with deletion 5q in 4/18 cells, consistent with concurrent interphase FISH analysis showing 18.5% of cells with deletion 5q (EGR1). Please see the Objective Findings section for details. Evaluation for an acquired TP53 mutation is recommended in patients with MDS with isolated del(5q) to help identify an adverse prognostic subgroup in this generally favorable prognosis MDS entity. Correlation with other laboratory and clinical information is recommended. (Blood 2016;127:2391)
Differential diagnosis
- Myelodysplastic syndrome (MDS), unclassifiable (Blood 2016;127:2391):
- Diagnosis of exclusion
- Cases otherwise meeting criteria for MDS with del(5q) but with 1% blasts on peripheral blood smear on ≥ 2 occasions or pancytopenia, defined as:
- Hemoglobin < 10 g/dL
- Absolute neutrophil count < 1.8 x 109/L
- Platelet count < 100 x 10109/L
- MDS with excess blasts (EB):
- As the name suggests, with 2 distinct subcategories depending on the blast count:
- MDS EB1
- 5 - 9% blasts in bone marrow
- 2 - 4% blasts in peripheral blood
- MDS EB2
- 10 - 19% blasts in bone marrow
- 5 - 19% blasts in peripheral blood
- MDS EB1
- As the name suggests, with 2 distinct subcategories depending on the blast count:
- MDS with single / multilineage dysplasia:
- Lacks characteristic 5q deletion
- MDS with ring sideroblasts:
- Lacks characteristic 5q deletion
- Ring sideroblasts can be seen in both
- SF3B1 mutation can be seen in both
Additional references
Board review style question #1
A 73 year old woman presents with macrocytic anemia and fatigue. Nutritional deficiency as a cause of her anemia has been ruled out clinically. Bone marrow biopsy shows a normocellular marrow. Aspirate smear shows the findings seen above. On chromosomal analysis, the patient is found to have isolated del(5q).
Which of the following is true about this disease?
- Approximately 40% of patients progress to AML
- Monosomy 7 is commonly seen in this entity
- SF3B1 mutation excludes the diagnosis
- TP53 mutation is associated with shorter overall survival
Board review style answer #1
D. TP53 mutation is associated with shorter overall survival
Comment Here
Reference: Isolated del(5q)
Comment Here
Reference: Isolated del(5q)