Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy description | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Hiser W, Kresak JL. Centronuclear myopathy. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/musclecentronuclearmyopathy.html. Accessed April 1st, 2025.
Definition / general
- Genetically heterogeneous group of myopathies defined by the presence of multiple centrally placed nuclei on histologic sections
Essential features
- Diagnosis requires the clinical features of a congenital myopathy in combination with histologic finding of multiple centrally placed nuclei on muscle biopsy
- Most frequent mutations include MTM1, DNM2 and BIN1
Terminology
- X linked form is often referred to as myotubular myopathy
ICD coding
- ICD-10: G71.2 - congenital myopathies
Epidemiology
- Uncertain incidence and prevalence but less frequent than central core and nemaline myopathies
- X linked myotubular myopathy estimated at 1 per 100,000 male births per year (Brain Behav 2013;3:476)
Sites
- Predominantly involves proximal musculature but may extend distally
- Ocular muscles are typically involved
- Autosomal recessive form more frequently involves facial muscles of mastication (Orphanet J Rare Dis 2008;3:26)
Pathophysiology
- Disease occurs as a result of varying mutations affecting different proteins involved with multiple cellular pathways
- Most proteins affected are involved with various pathways of membrane trafficking and remodeling, including endocytosis and autophagy
Etiology
- Multiple mutations with differing inheritance patterns have been implicated in CNM:
- X linked: MTM1 (90% of affected men)
- Autosomal dominant: DNM2 and CCDC78
- Autosomal recessive: BIN1 and TTN
- Less frequently mutations of RYR1, MTMR14, SPEG (Neuropathol Appl Neurobiol 2017;43:5)
Clinical features
- Clinical presentation is variable and partly based on mutation
- Marked proximal muscle weakness but may also involve distal musculature, particularly on lower extremities
- X linked form is severe and presents at birth with significant weakness, hypotonia, external ophthalmoplegia and respiratory distress
- Fetal signs include polyhydramnios, reduced fetal movement and thinning of the ribs
- Large head circumference and length > 90th percentile
- Cryptorchidism, pyloric stenosis and hepatic cavernous hemangiomas may also be seen
- Most carriers are asymptomatic but some may have mild muscle weakness
- Autosomal dominant form tends to be the mildest and occur later than the X linked
- Severity varies based on what part of the protein is affected
- Presentation frequently in adolescence / early adulthood but some mutations present in neonatal period
- Progressive and typically begins in adolescence but rarely results in loss of ambulation before the sixth decade
- May present with exercise induced myalgias
- Neonatal presentation is often more severe but symptoms typically improve over time
- Ocular involvement with ptosis is almost always seen
- Autosomal recessive form is characterized by facial muscle weakness, particularly those involved with mastication, in addition to ocular involvement with ptosis and external ophthalmoplegia
- Intermediate severity between X linked and autosomal dominant
- Skeletal abnormalities (scoliosis, high arched palate) often seen
- Variable degrees of respiratory distress but may be severe
- Associated cardiomyopathy has been reported (Orphanet J Rare Dis 2008;3:26)
Diagnosis
- Must have characteristic histologic findings of centrally placed nuclei in addition to compatible clinical presentation
- DNA sequencing is used for molecular confirmation of the diagnosis
- Screening for MTM1 mutations should be performed in females with appropriate clinical or histologic findings
Laboratory
- Creatinine kinase (CK) normal to slightly elevated
Radiology description
- Muscle MRI of patients with DNM2 mutations shows a characteristic progressive pattern of early ankle plantarflexor involvement with later changes in hamstring muscles and ending with anterior thigh
- Also shows adductor longus and rectus femoris involvement (Orphanet J Rare Dis 2008;3:26)
Prognostic factors
- X linked: typically fatal within first few months of life, though a small portion may live into teenage years or beyond with significant medical intervention
- Autosomal recessive: More favorable prognosis with the absence of cardiorespiratory involvement
Case reports
- Infant boy at birth presenting with severe hypotonia (J Child Neurol 2007;22:447
- Two infant boys with X linked MTM (J Clin Neurol 2013;9:57)
- 2 month old boy with hypotonia (Brain Pathol 2015;25:651)
- 8 year old boy with genetically confirmed X linked myotubular myopathy (Pediatr Neurol 2009;40:483)
- 17 year old girl with proximal muscle weakness (Autops Case Rep 2017;7:43)
- Two first degree cousins with a novel BIN1 stop mutation (Orphanet J Rare Dis 2010;5:35)
- Patient with dynamin centronuclear myopathy (J Clin Neuromuscul Dis 2016;18:84)
Treatment
- No curative treatment
- Supportive therapy
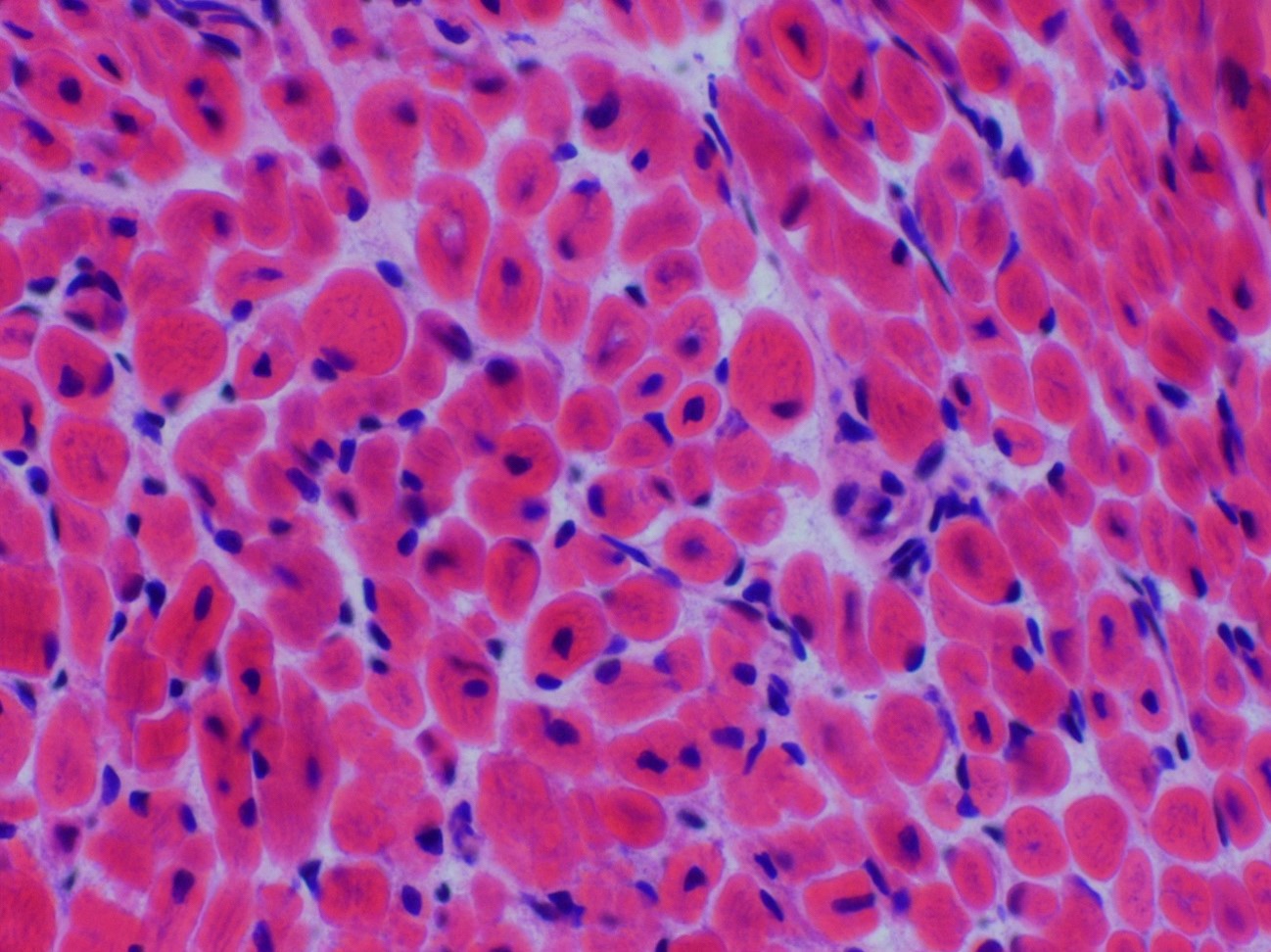
Microscopic (histologic) description
- Small muscle fibers with centrally located nuclei, often with a peripheral halo
- Halos lack mitochondria and are highlighted by oxidative stains (Neuropathol Appl Neurobiol 2017;43:5)
- Type 1 myofiber predominance, myofiber size variation and fatty infiltration
- Necklace fibers: basophilic ring along the periphery of the cell membrane visible with H&E, PAS, Gömöri trichrome and oxidative stains in patients with MTM1 mutation (Acta Neuropathol 2009;117:283)
- Radial arrangement of sarcoplasmic strands, visible with NADH stain, seen with DNM2 mutation (Orphanet J Rare Dis 2008;3:26)
Positive stains
- Desmin
- Variable vimentin
- RYR1 and DHPR (Brain Behav 2013;3:476)
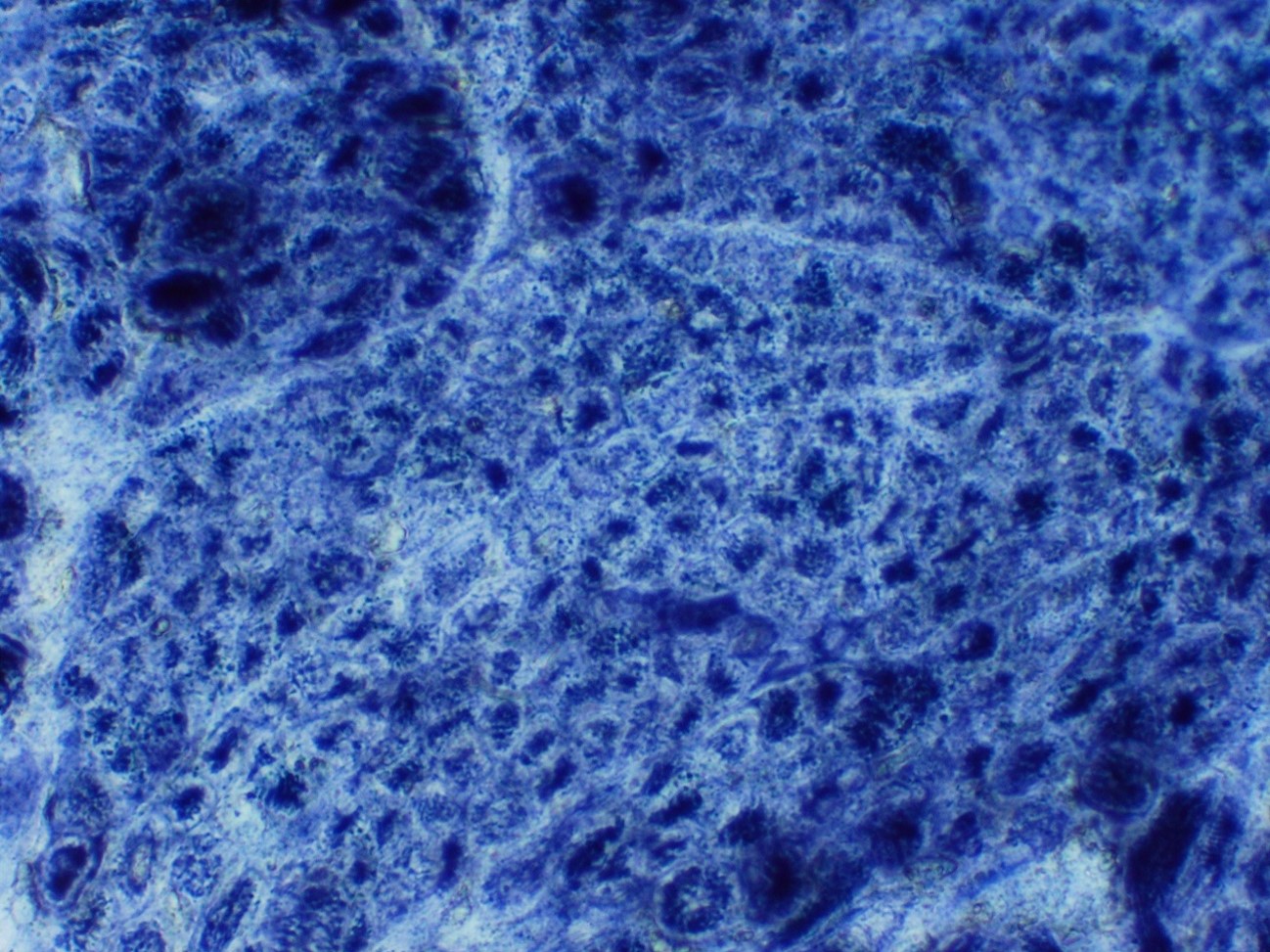
Electron microscopy description
- X linked: prominent nucleoli and central region with mitochondrial aggregates, glycogen granules and reduced myofilaments; increased mitochondria, glycogen granules and sarcoplasmic reticulum within necklace fibers
- Autosomal dominant: central region with radial sarcoplasmic strands, tapering toward the center
- Autosomal recessive: central region with filamentous, amorphous material comprised of mitochondria, tubules and glycogen (Brain Pathol 2015;25:651)
Differential diagnosis
- Congenital myotonic dystrophy
- Motor neuropathies
- Myasthenia
- Other congenital myopathies
- Spinal muscular atrophy
Additional references
Board review style question #1
Necklace fibers are seen in association with which mutation involved with centronuclear myopathy?
- BIN1
- DNM2
- MTM1
- MTMR14
- RYR1
Board review style answer #1