Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Diagrams / tables | Classification | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Features to report | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Roden AC. Thymoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/mediastinumthymoma.html. Accessed November 27th, 2024.
Definition / general
- Malignant epithelial tumor of the thymic gland
Essential features
- Malignant
- Lobulated architecture comprised of cellular lobules intersected by fibrous bands
- Neoplastic cells: epithelial cells
- Thymocytes are reactive
- Prognostic parameters:
- Stage
- Completeness of resection
- Morphology (as defined by WHO)
Terminology
- WHO type B3 (by some authors referred to as atypical thymoma)
- Anterior (prevascular) mediastinum (J Thorac Oncol 2014;9:S97)
- Middle (visceral) mediastinum
- Posterior (paravertebral) mediastinum
ICD coding
Epidemiology
- Most common solitary lesion in mediastinum and prevascular (anterior) mediastinum in adults (J Thorac Oncol 2020;15:568)
- 1 - 5 per million population per year in adults
- Any age, peak 40 - 60 years old (Mayo Clin Proc 1993;68:1110, J Thorac Oncol 2015;10:691)
- M = F
- 34 - 40% associated with paraneoplastic / autoimmune syndromes
- Myasthenia gravis (MG)
- 15 - 21% of patients with MG have thymoma
- May present with thymic follicular hyperplasia
- 33 - 40% of patients with thymoma have MG (J Thorac Oncol 2015;10:691, J Thorac Oncol 2018;13:436)
- Other neuromuscular syndromes / diseases (Lambert-Eaton syndrome, myotonic dystrophy)
- Autoimmune diseases (systemic lupus erythematosus, polymyositis, myocarditis, Sjögren syndrome, ulcerative colitis)
- Endocrine disorders (Addison disease, hyperthyroidism)
- Hematologic diseases (hypogamaglobulinemia, red cell aplasia, pancytopenia), aplastic anemia
- Others (hypertrophic pulmonary osteoarthropathy) (Mayo Clin Proc 1993;68:1110)
- Patients with thymoma and paraneoplastic / autoimmune syndrome: more commonly younger, female, smaller tumors, B2 or B3 subtype, lower stage, R0 resection (J Thorac Oncol 2018;13:436)
- Myasthenia gravis (MG)
Sites
- Anterior (prevascular) mediastinum (J Thorac Oncol 2020;15:568)
- Rarely at ectopic sites: middle (visceral) and posterior (paravertebral) mediastinum, neck, pleura, lung, pericardium, thyroid gland, others (Virchows Arch 2016;469:245)
Etiology
- Tumor cells originate from thymic epithelial cells
Diagrams / tables
pTNM staging for thymic epithelial tumors (8th edition of the AJCC / UICC staging)
Recommended reporting of thymic epithelial tumors with multiple components (2021 WHO)
Reference: Amin: AJCC Cancer Staging Manual, 8th Edition, 2017
| Stage | T | N | M | Tumor Extension |
| I | 1a 1b | 0 | 0 | Encapsulated or extending into mediastinal fat, no involvement of mediastinal pleura Invasion of mediastinal pleura |
| II | 2 | 0 | 0 | Invasion of pericardium |
| IIIA | 3 | 0 | 0 | Invasion into lung, brachiocephalic vein, superior vena cava, chest wall, phrenic nerve or extrapericardial pulmonary artery or veins |
| IIIB | 4 | 0 | 0 | Invasion into aorta (ascending, arch, or descending), arch vessels, intrapericardial pulmonary artery, myocardium, trachea, esophagus |
| IVA | Any | 1 0, 1 | 0 1a | Anterior (perithymic) lymph nodes Separate pleural / pericardial nodule(s) (implants) |
| IVB | Any | 2 Any | 0, 1a 1b | Deep intrathoracic / cervical lymph nodes Pulmonary intraparenchymal nodule, distant organ metastasis |
Recommended reporting of thymic epithelial tumors with multiple components (2021 WHO)
| Thymoma with > 1 histologic pattern (except AB thymoma) | List all thymoma subtypes, including percentages in 10% increments; start with predominant component |
| Thymoma and thymic carcinoma | List all components, including % in 10% increments; start with thymic carcinoma component independent of extent |
| Thymoma and thymic carcinoid tumor | List all components, including % in 10% increments; start with thymic carcinoid tumor component independent of extent |
Classification
Classification of thymomas according to 2021 WHO
| Type A thymoma (including atypical subtype) |
| Type AB thymoma |
| Type B1 thymoma |
| Type B2 thymoma |
| Type B3 thymoma |
| Micronodular thymoma with lymphoid stroma |
| Metaplastic thymoma |
| Lipofibroadenoma |
Clinical features
- Malignant; thymomas can invade, recur, metastasize and rarely lead to death (J Thorac Oncol 2015;10:691)
- May be incidental and asymptomatic (Mayo Clin Proc 1993;68:1110)
- Symptoms:
- Due to compression of adjacent structures: shortness of breath, cough, wheezing, superior vena cava syndrome (swollen face, neck, upper extremities)
- Systemic: fever, weight loss
- Due to paraneoplastic syndrome
- Overall 5 year disease free survival: 91.7% (J Thorac Oncol 2015;10:691)
- 10 year survival based on histology (Ann Thorac Surg 2006;81:2328):
- Type A: 97% (76 - 100)
- Type AB: 95% (74 - 100)
- Type B1: 92% (83 - 100)
- Type B2: 81% (33 - 92)
- Type B3: 62% (35 - 82)
- Patients with thymoma: increased risk of developing secondary cancer (Future Oncol 2018;14:1943, Histopathology 2012;60:437)
Diagnosis
- Suggested on chest Xray, confirmed by CT; possibly MRI
- Preresection biopsy usually not performed or necessary
Laboratory
- None for thymoma
- Acetylcholine receptor binding antibodies if myasthenia gravis
- Possibly workup for other paraneoplastic / autoimmune syndromes if clinically suspicious
Radiology description
- Chest Xray: usually ovoid or lobulated, smooth, well marginated mass, projecting over the mediastinum, more commonly protruding unilaterally (J Thorac Oncol 2010;5:S296)
- Chest CT: typically well defined, round or lobulated, homogeneous lesion that enhances after contrast injection
- Can be heterogeneous or cystic
- Used to evaluate for invasion
- MRI increasingly utilized, specifically in differentiating thymoma from thymic cyst or thymic hyperplasia, to identify phrenic nerve involvement or to evaluate for invasion in patients with contraindication to iodinated contrast (Magn Reson Imaging Clin N Am 2015;23:165, J Cancer 2019;10:3208)
Prognostic factors
- Stage (TNM stage; previously Masaoka-Koga stage), in some studies independent of resection status and WHO subtype (J Thorac Oncol 2015;10:691)
- Complete resection associated with overall survival
- WHO subtype associated with overall and disease free survival
- Size of thymoma, age, weight loss are associated with outcome in some studies
Case reports
- 35 year old man with 7 cm anterior mediastinal mass on chest Xray (J Pathol Transl Med 2017;51:165)
- 49 year old woman with productive cough ( Surg Case Rep 2021;2021:rjaa545)
- 58 year old woman with crushing chest pain (J Radiol Case Rep 2020;14:16)
- 61 year old woman with 1 month history of nonproductive cough and 5 kg weight loss (Respir Med Case Rep 2021;33:101423)
- 66 year old woman with incidental 13 cm mediastinal tumor (J Thorac Dis 2017;9:E808)
Treatment
- Thymectomy with or without resection of adjacent structures depending on extent of tumor, whenever possible
- Possibly neoadjuvant chemotherapy or radiation or postoperative chemotherapy or radiation, depending on stage and involvement of margins
- Immune checkpoint inhibitors: prospective trials have shown durable responses and appears to improve survival in patients with relapsed thymoma
- Controversial, possibly contraindicated; severe and life threatening immune toxicity can occur and therefore extreme caution is necessary while using immunotherapy in patients with thymoma
- Currently, this treatment is only recommended in the setting of clinical trials with close monitoring (Mediastinum 2021;5:23)
- Targeted therapies: only limited activity (Lung Cancer 2018;126:25)
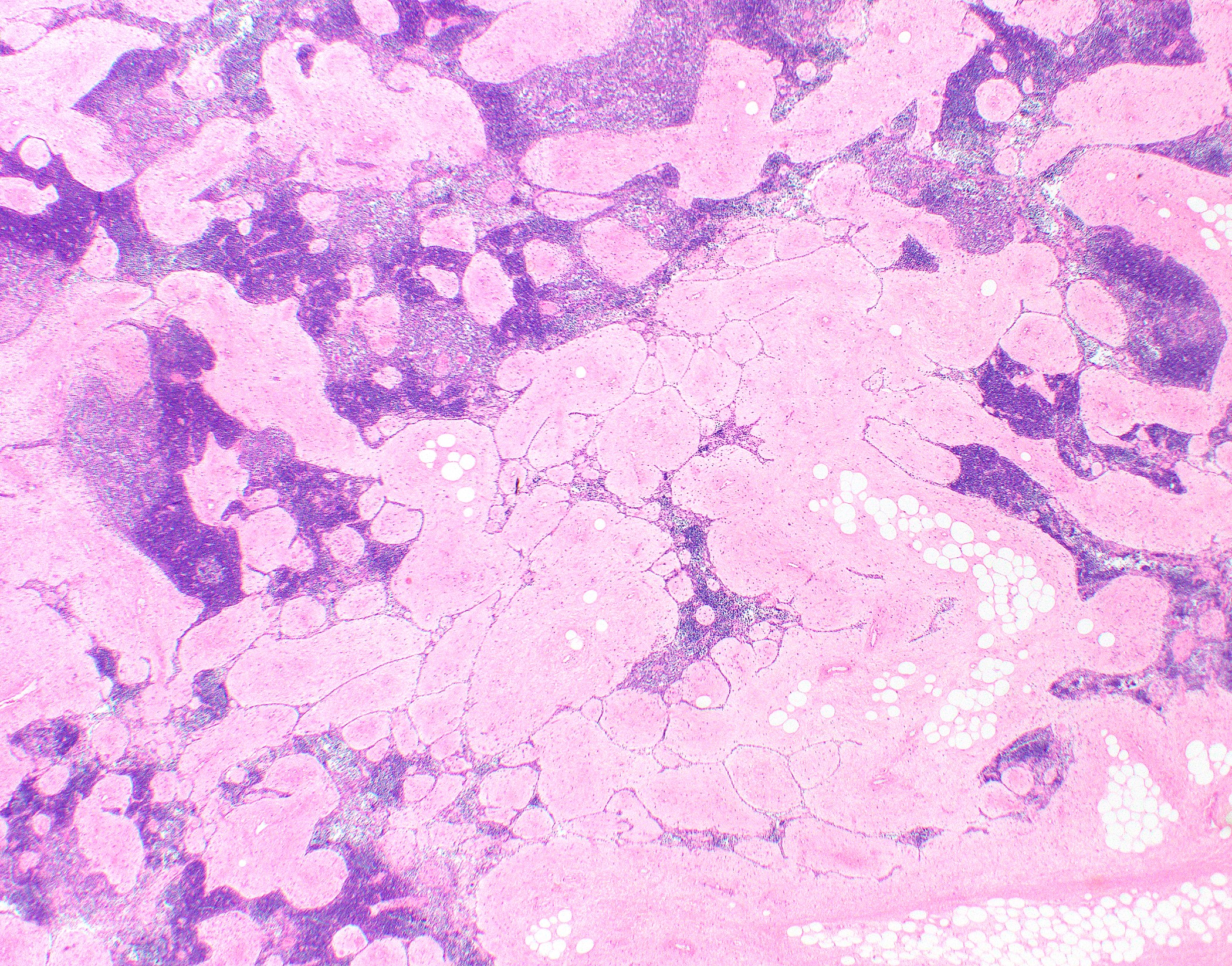
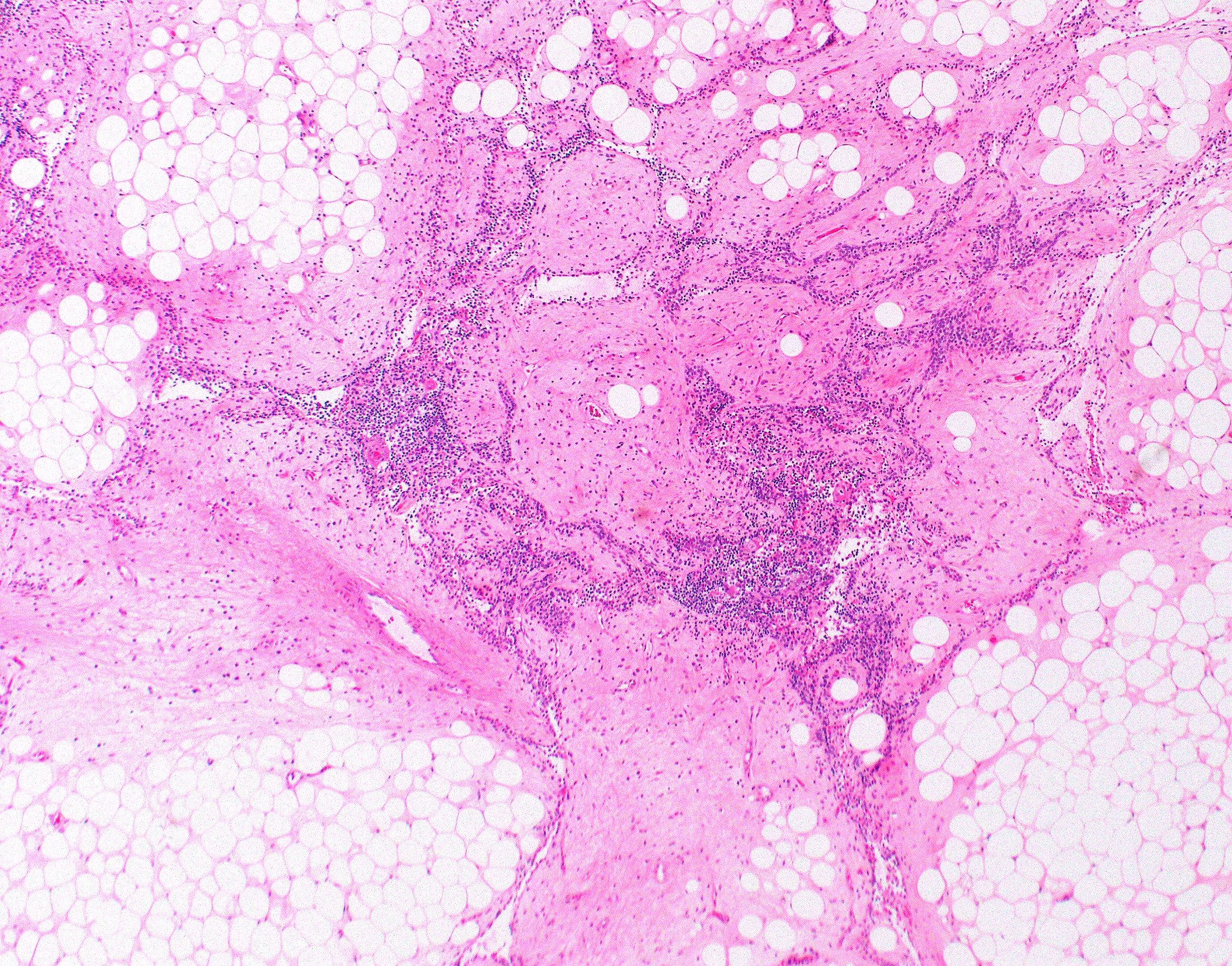
Gross description
- Lobulated
- Gray-tan
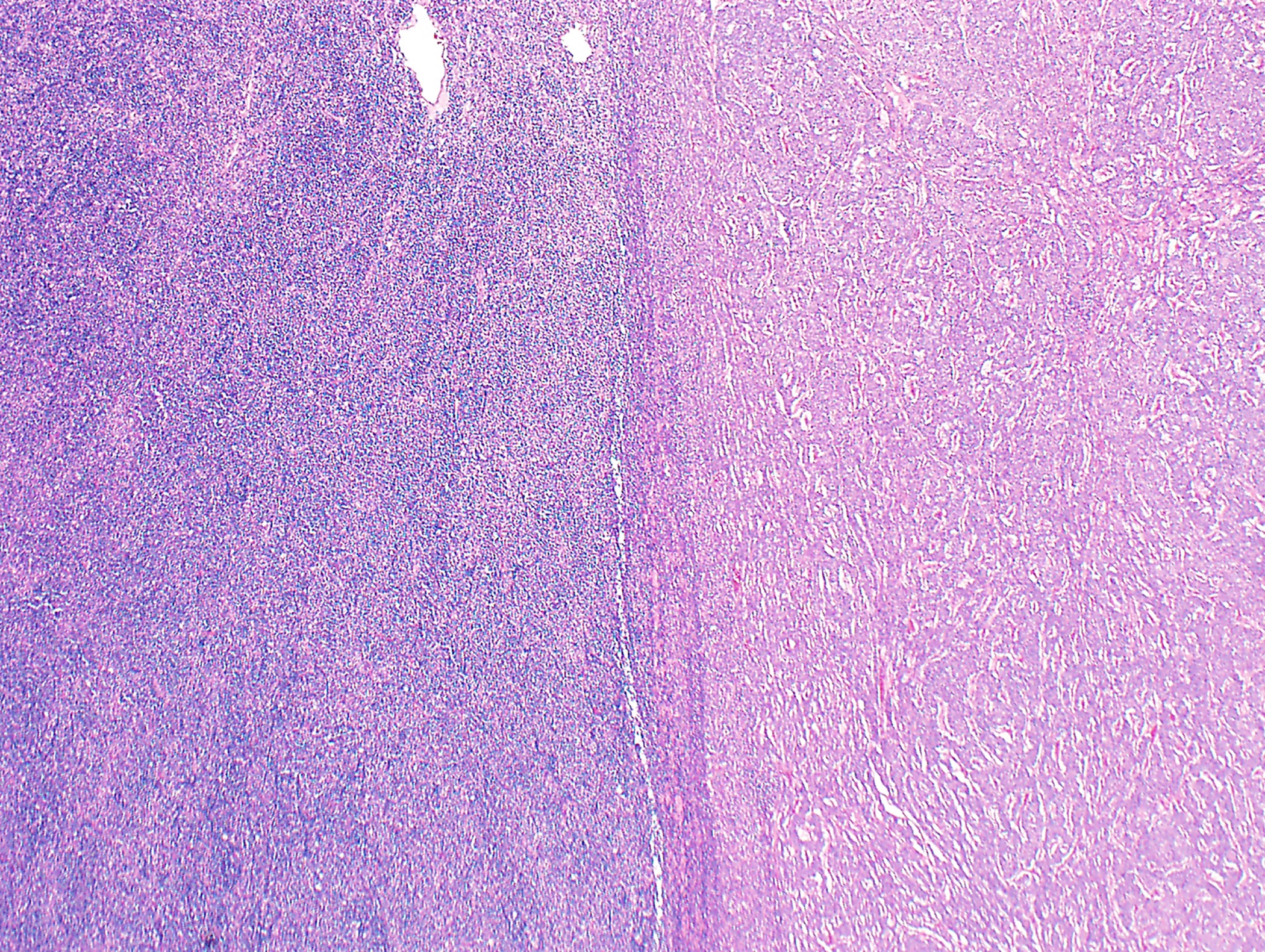
- Often encapsulated
- Possibly with cystic changes
- Sampling of perithymic lymph nodes important for staging
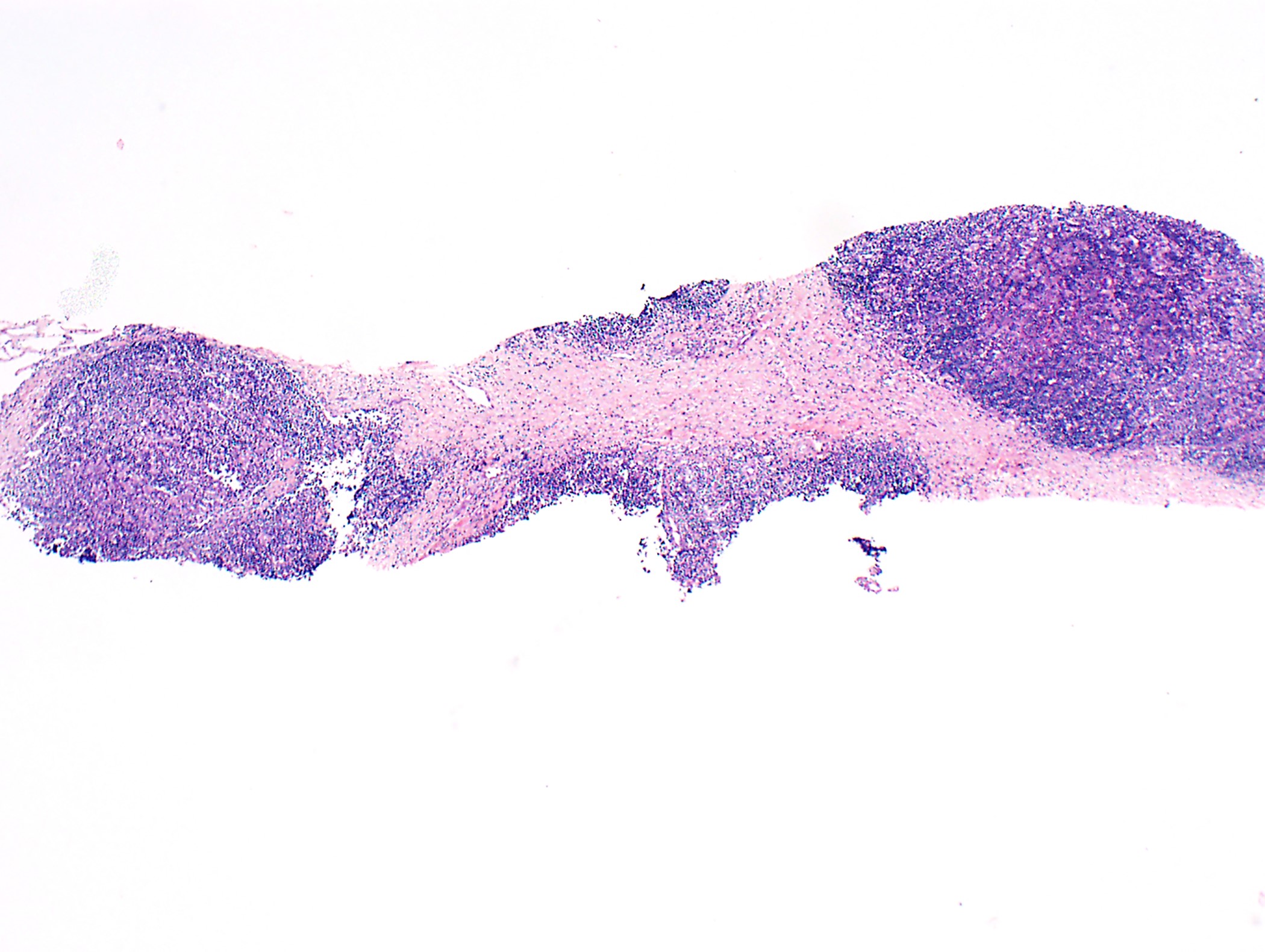
Frozen section description
- Lobulated neoplasm with fibrous bands and cellular lobules
- Sometimes only a few fibrous bands present
- Cellular lobules comprised of (i) a mixture of lymphocytes and large polygonal (epithelial neoplastic) cells, (ii) polygonal cells only without or only with a few lymphocytes or (iii) bland short spindle cells
- Associated cyst(s) possible
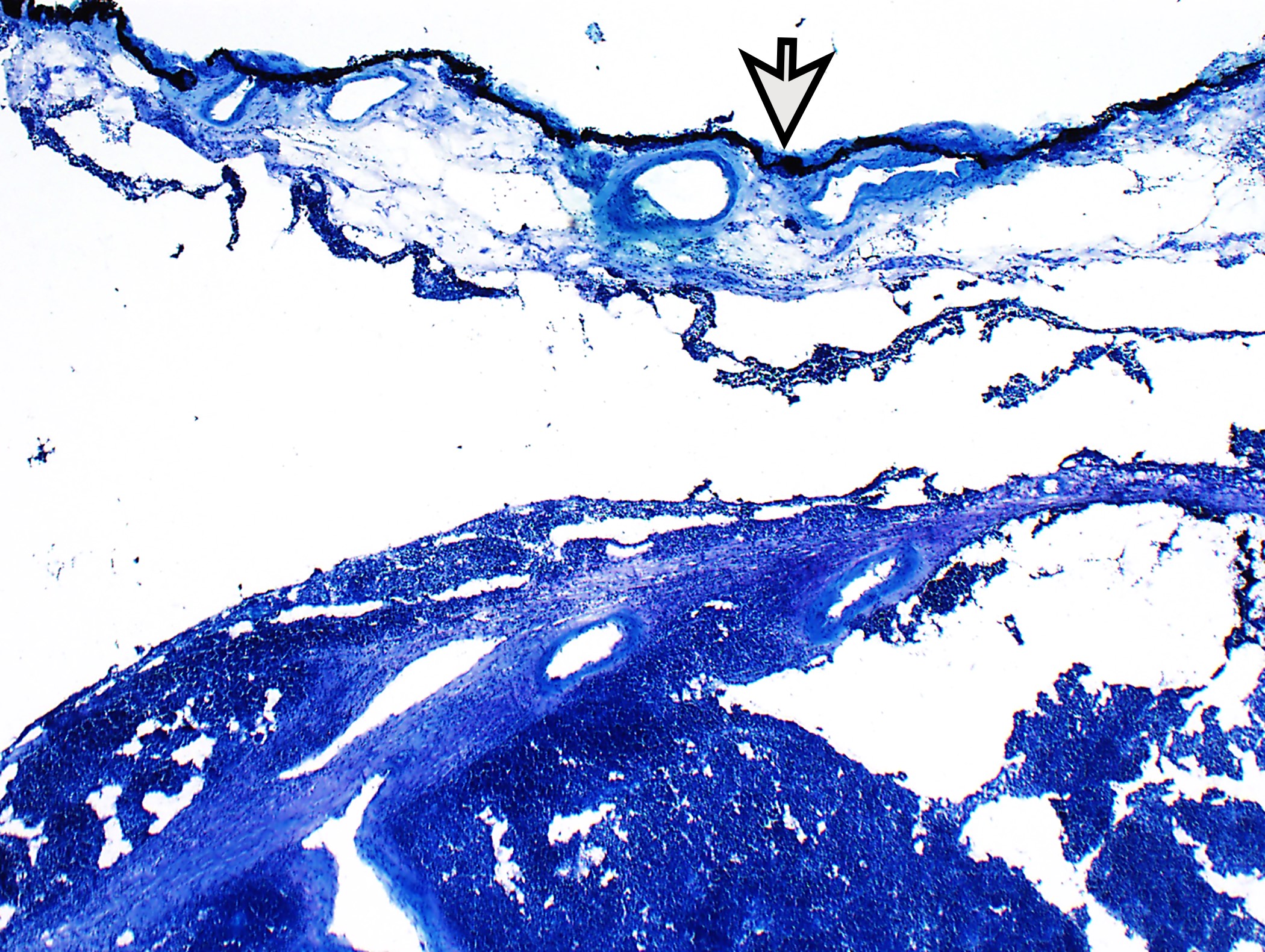
- Evaluation of margins: discussion with surgeon important to identify true surgical margin as not the entire specimen surface is considered surgical margin
- Invasion into adjacent structures (mediastinal pleura, pericardium, phrenic nerve, lung, great vessels, among others)
Frozen section images
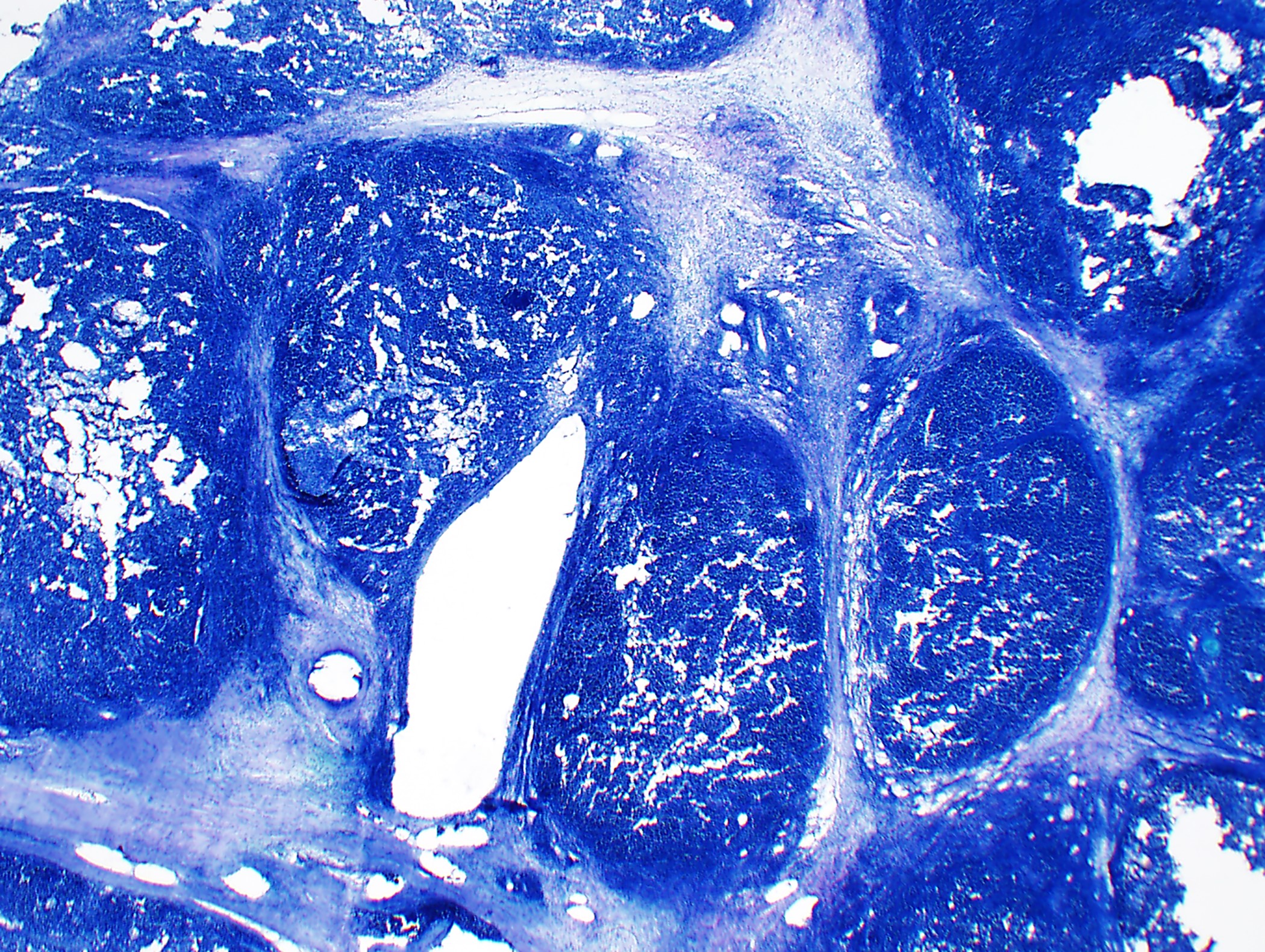
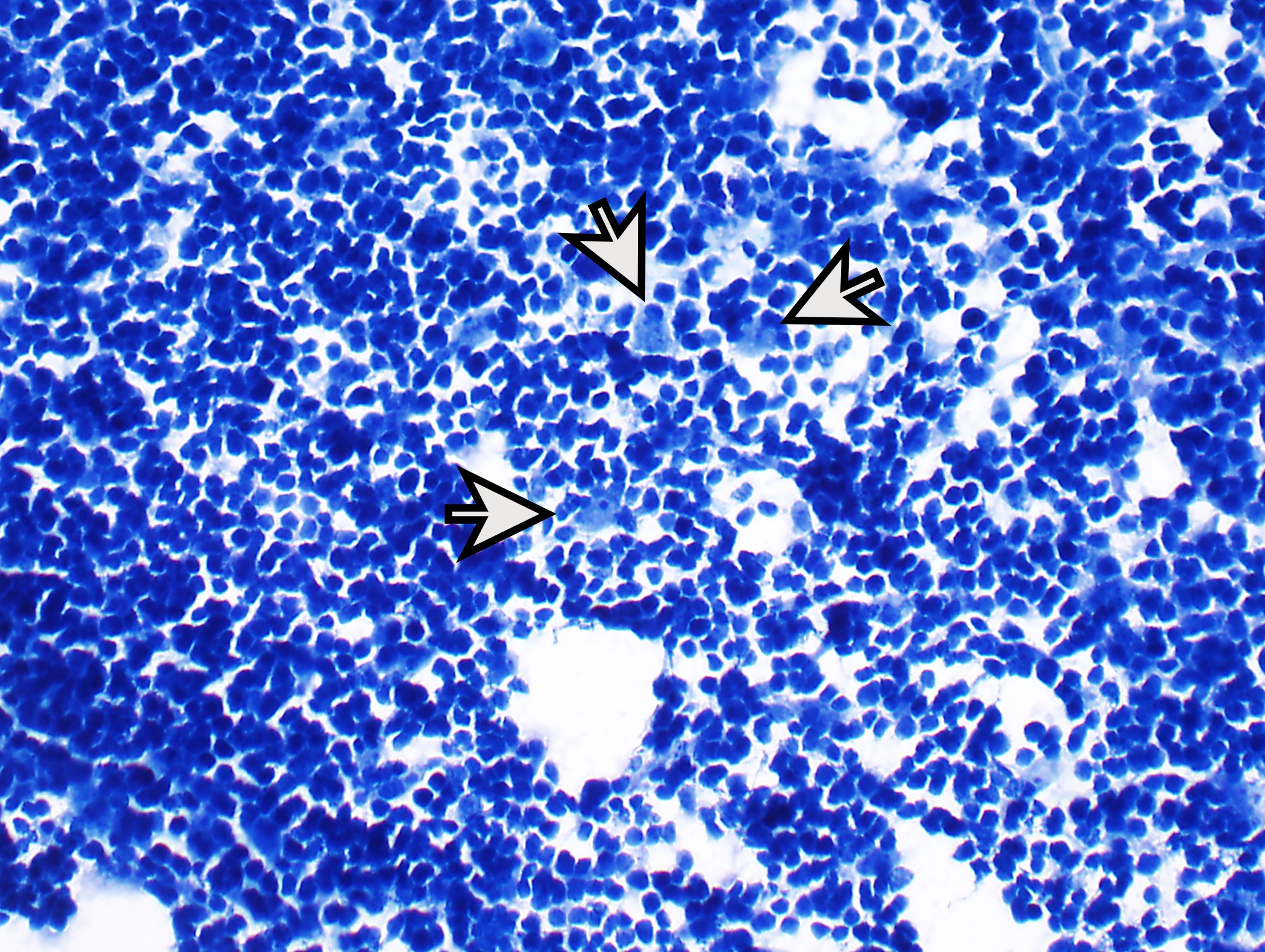
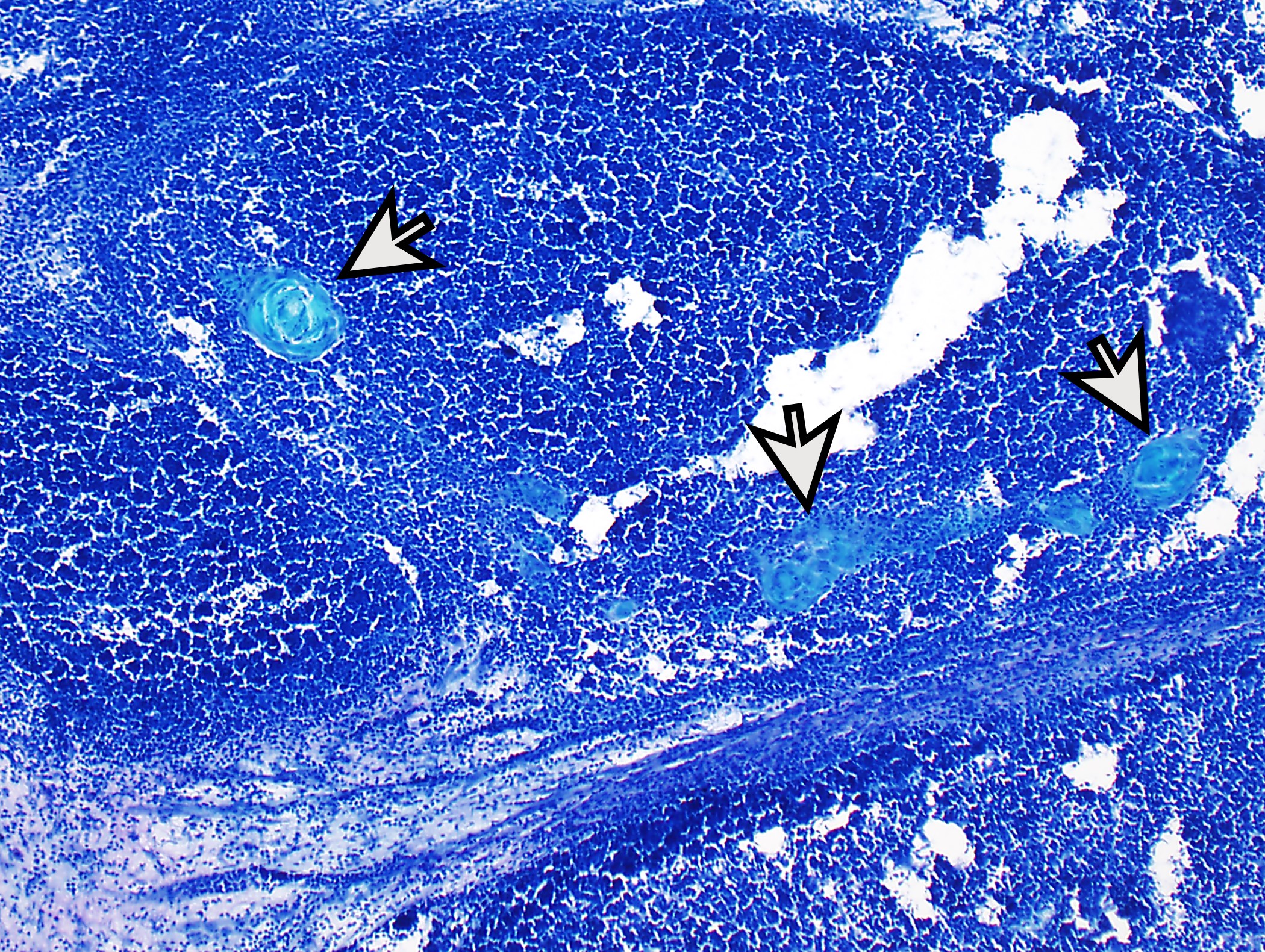
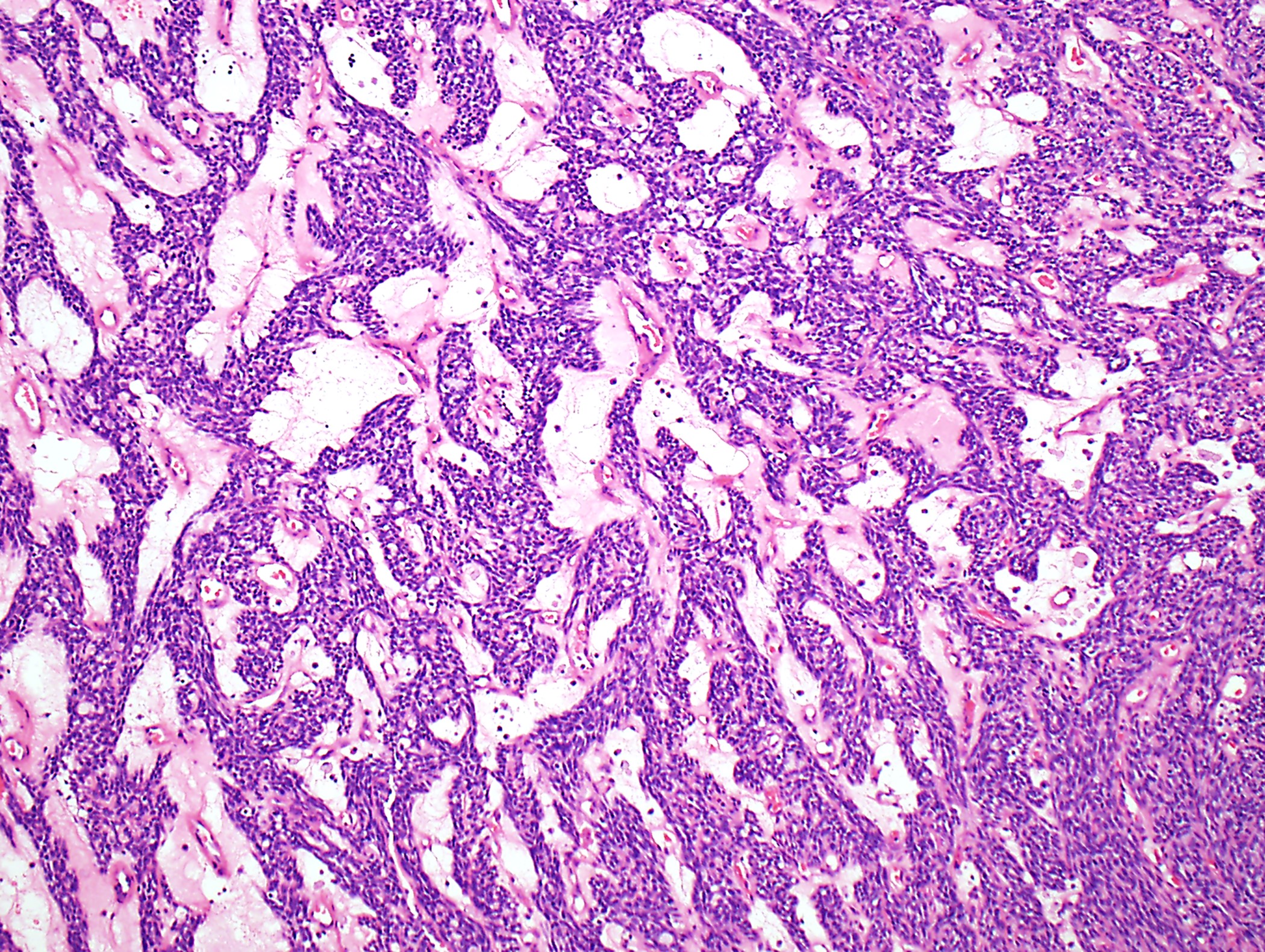
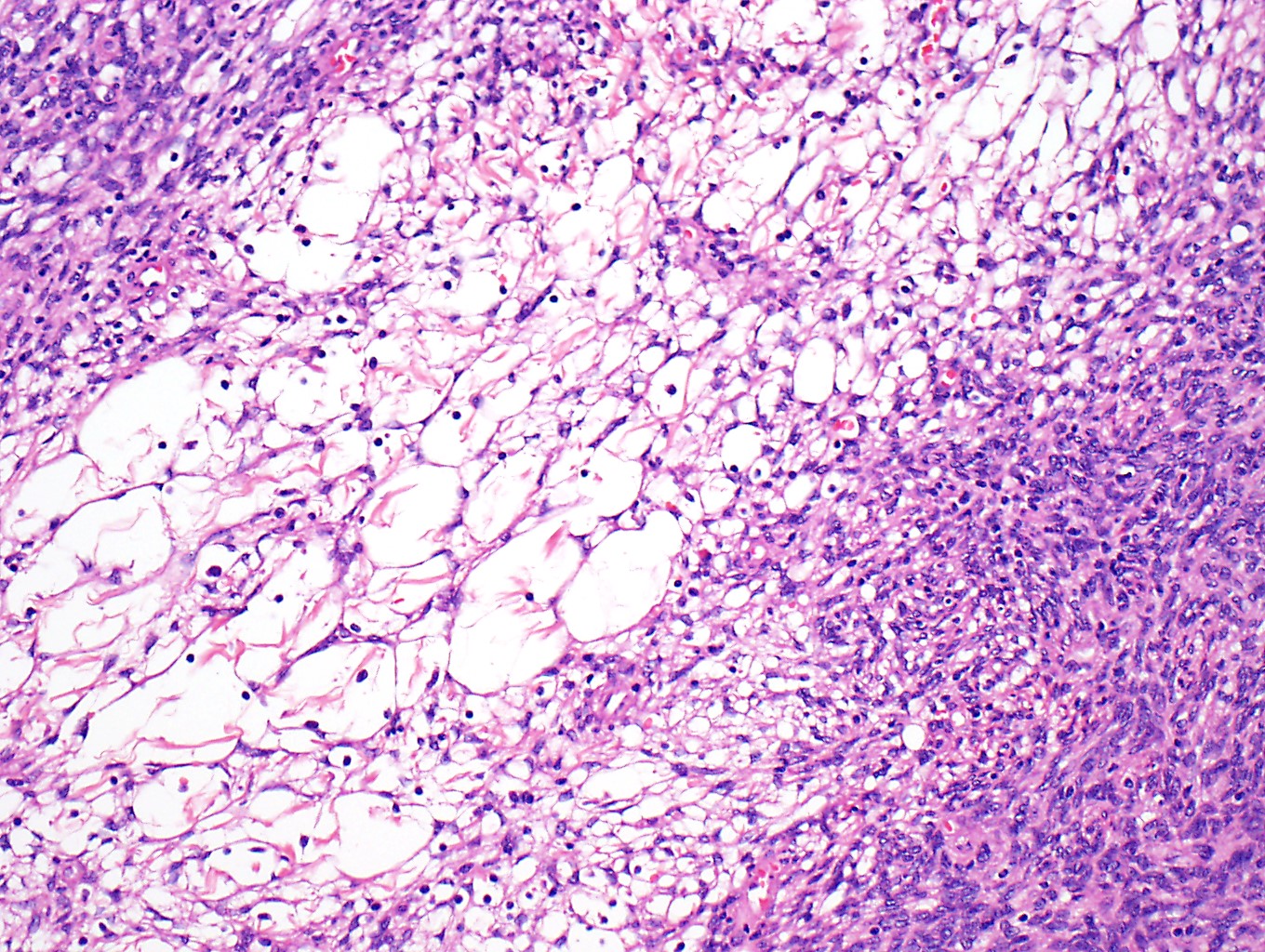
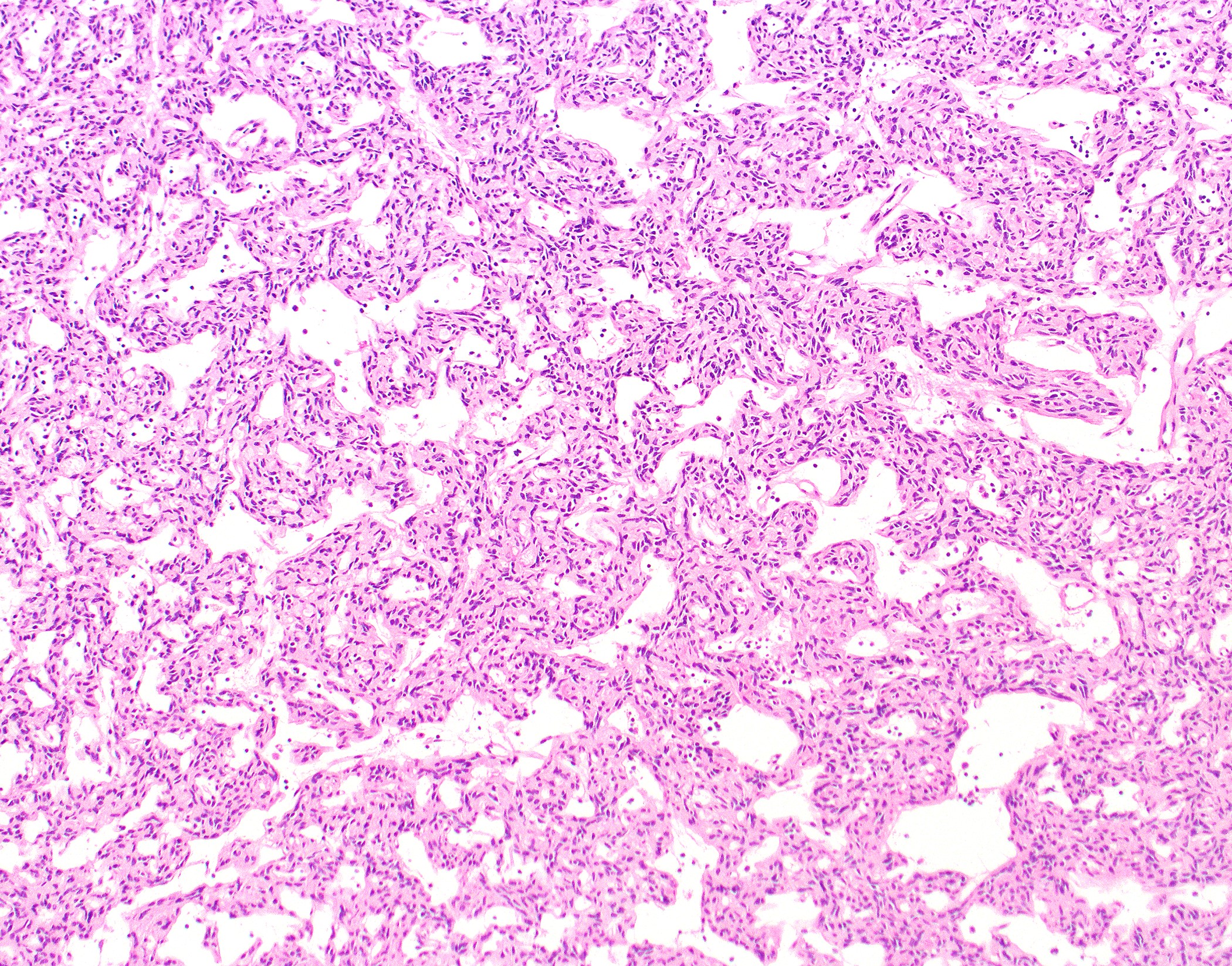
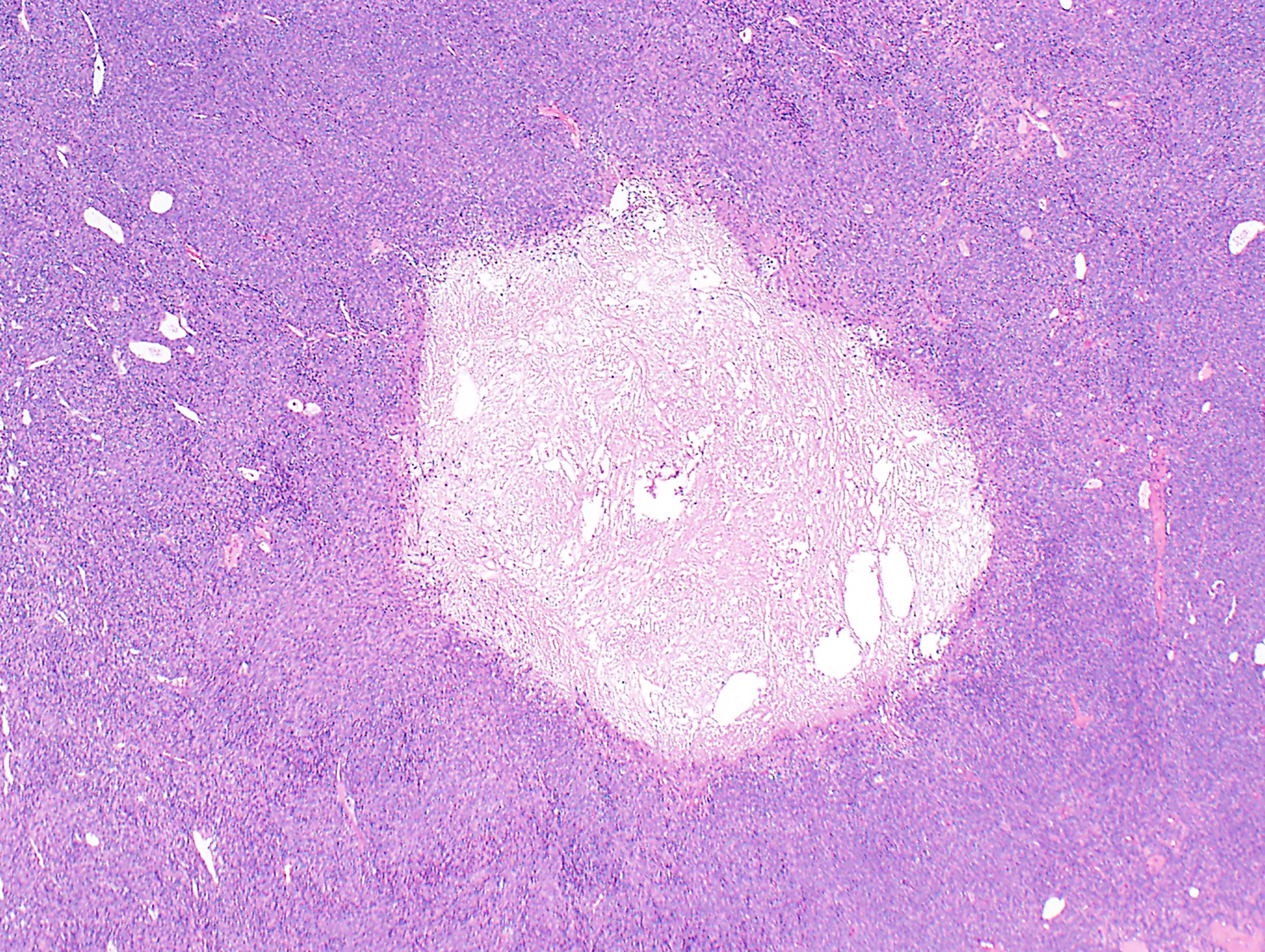
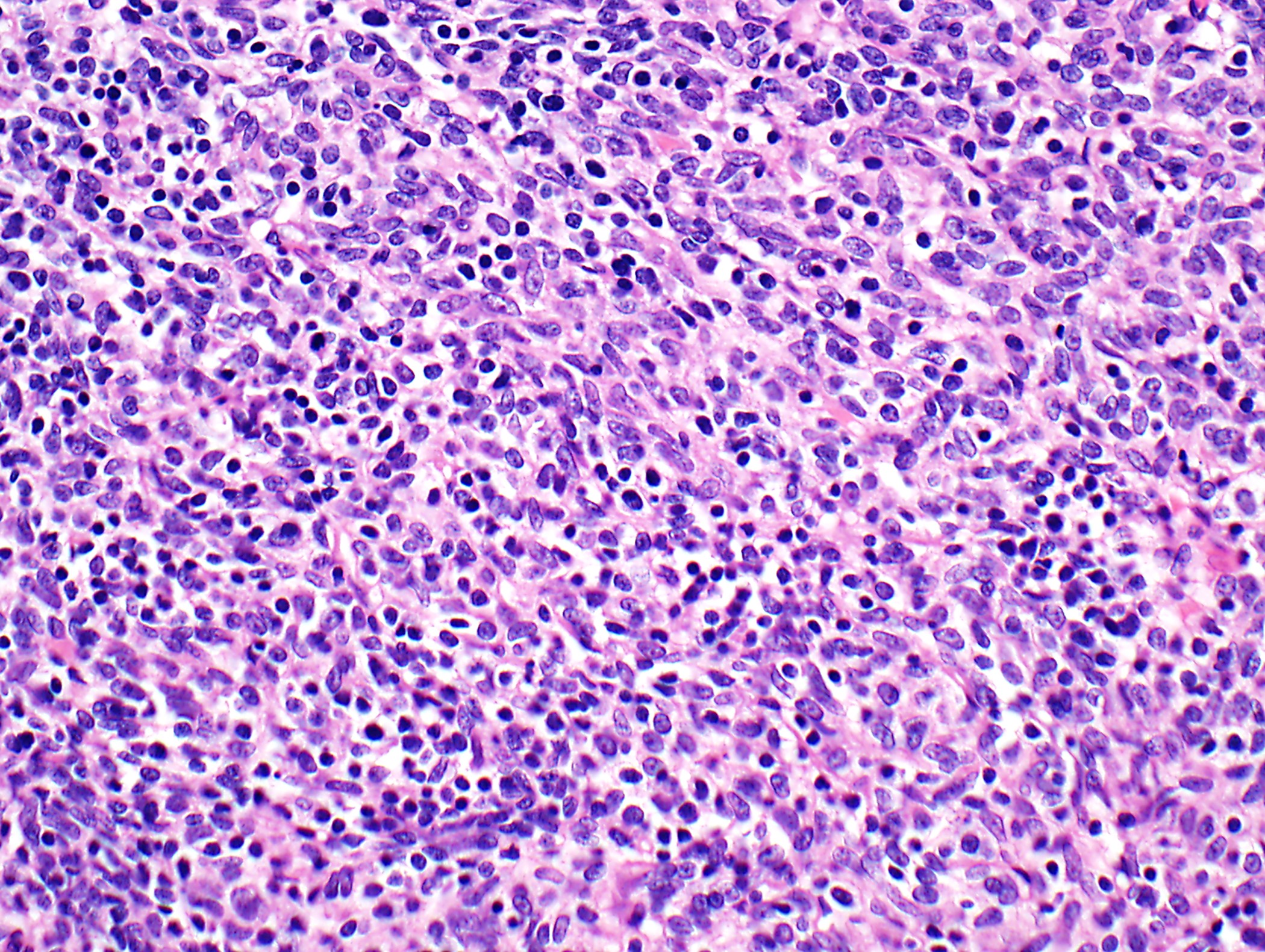
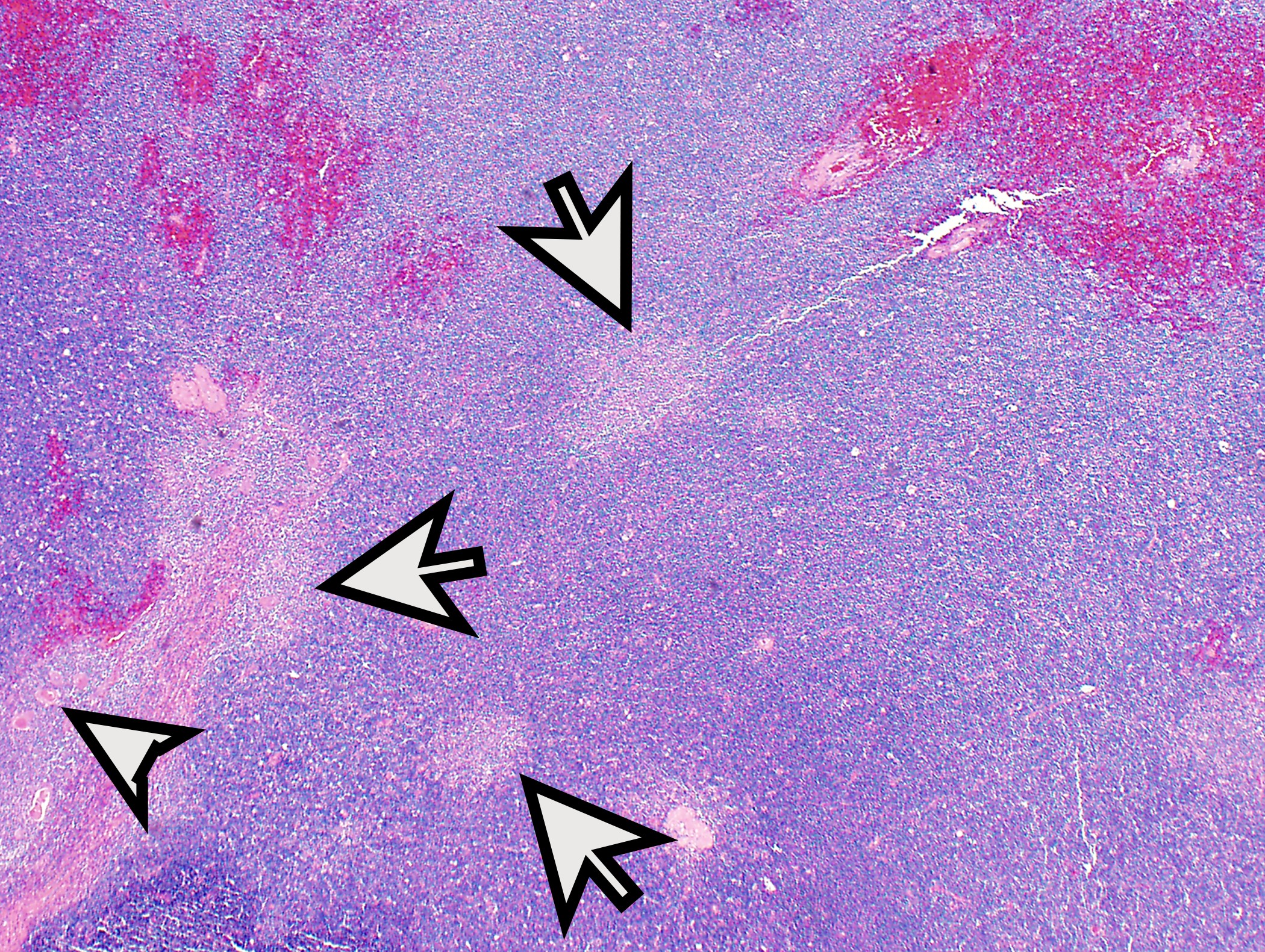
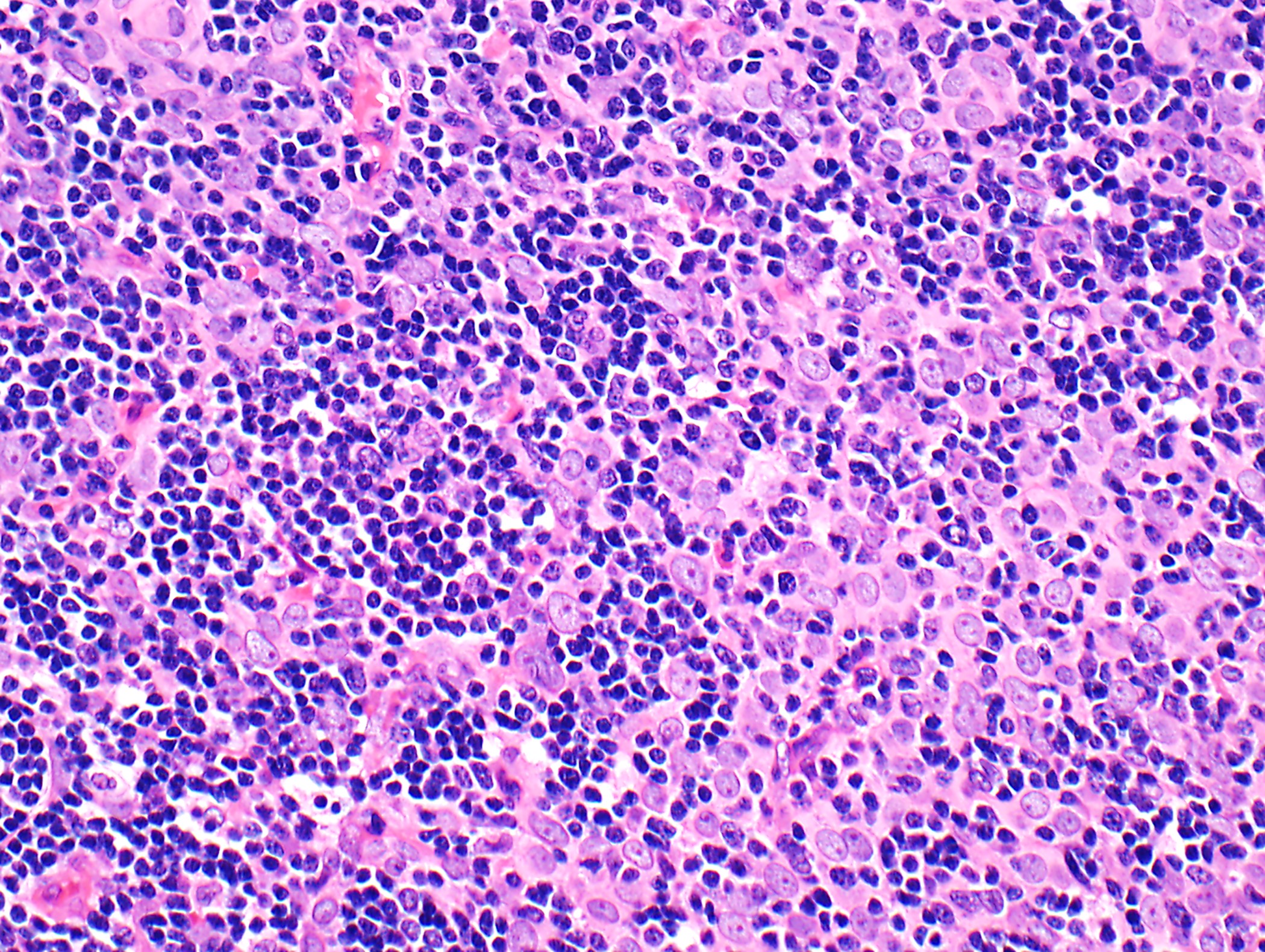
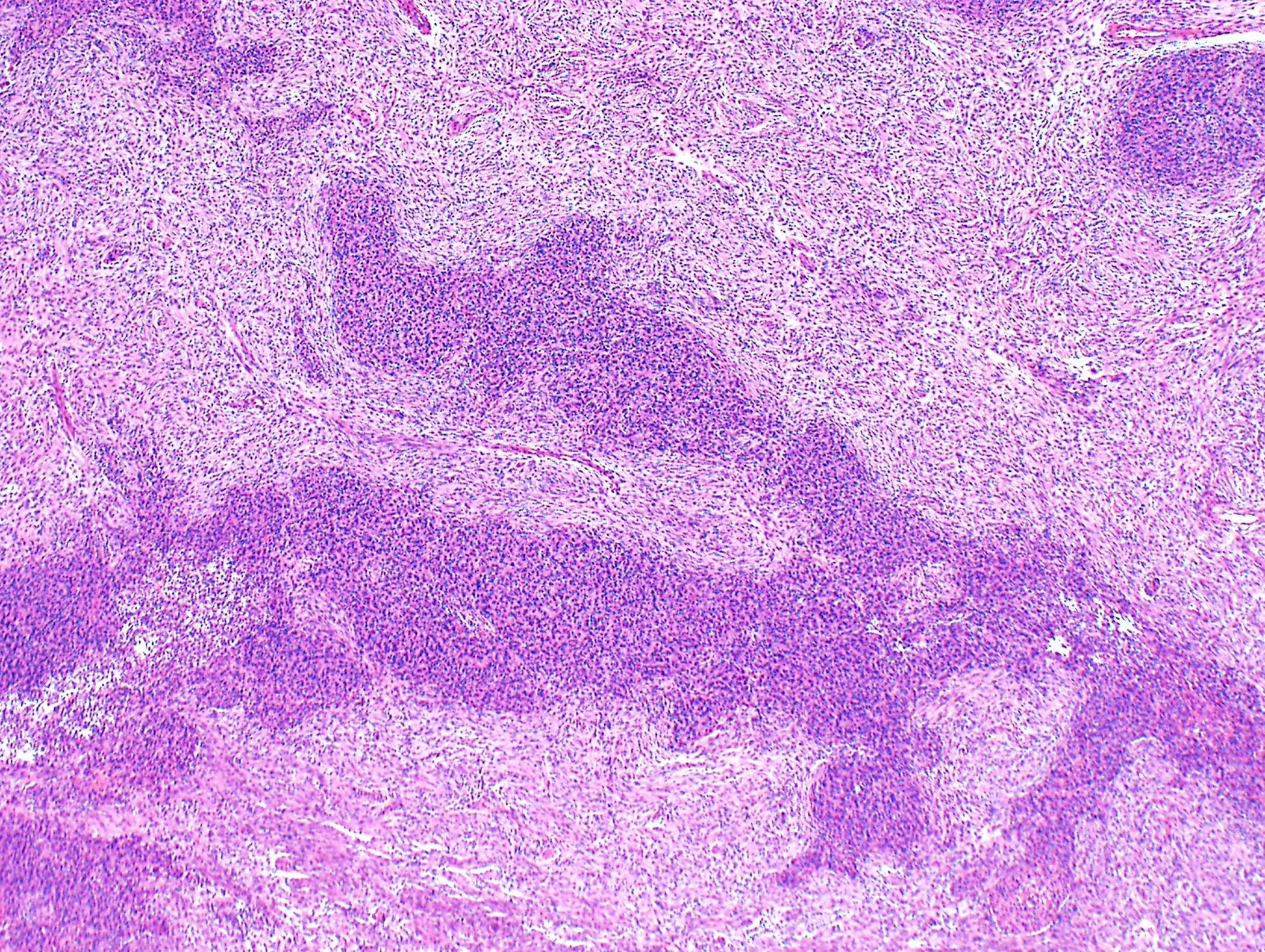
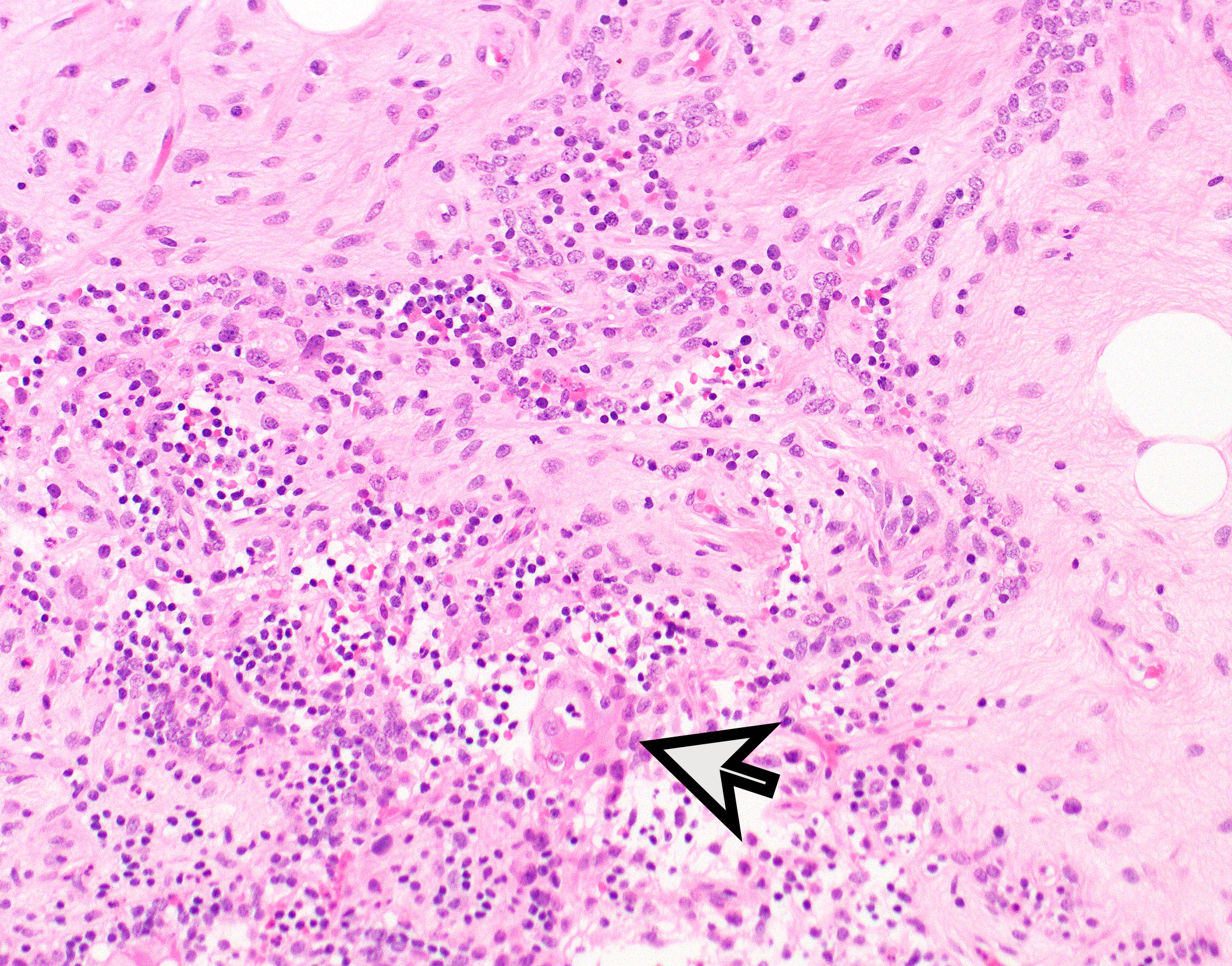
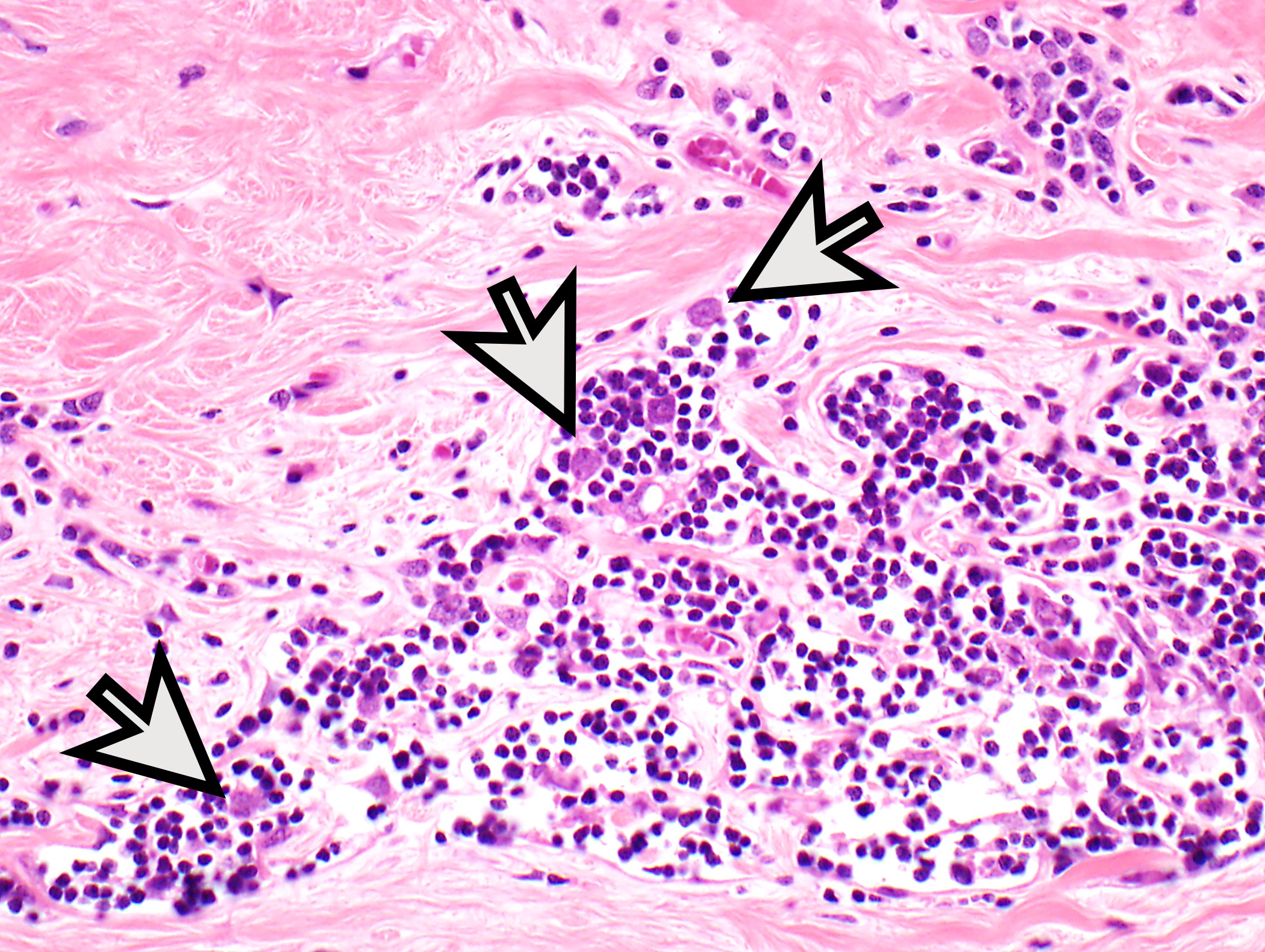
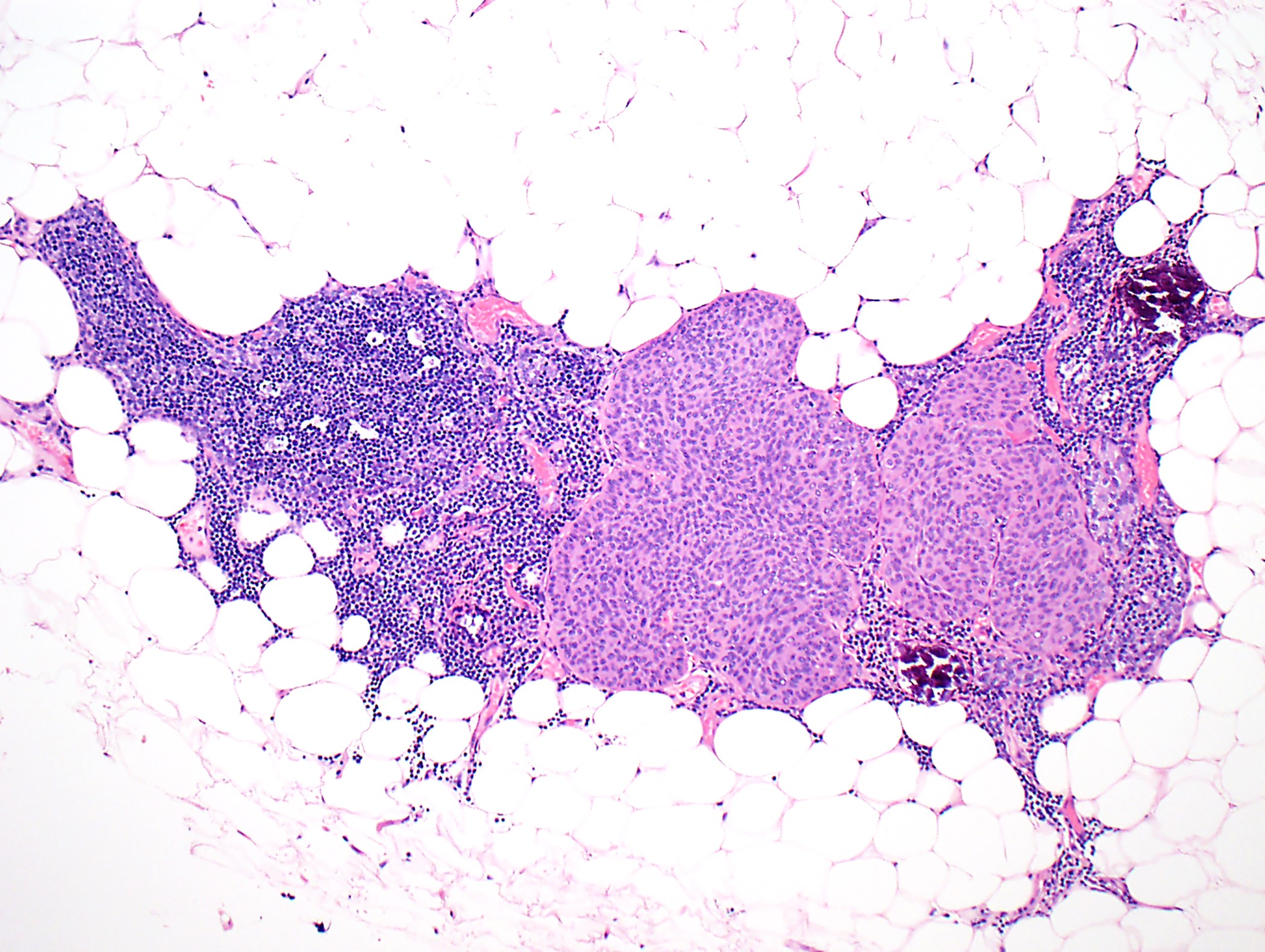
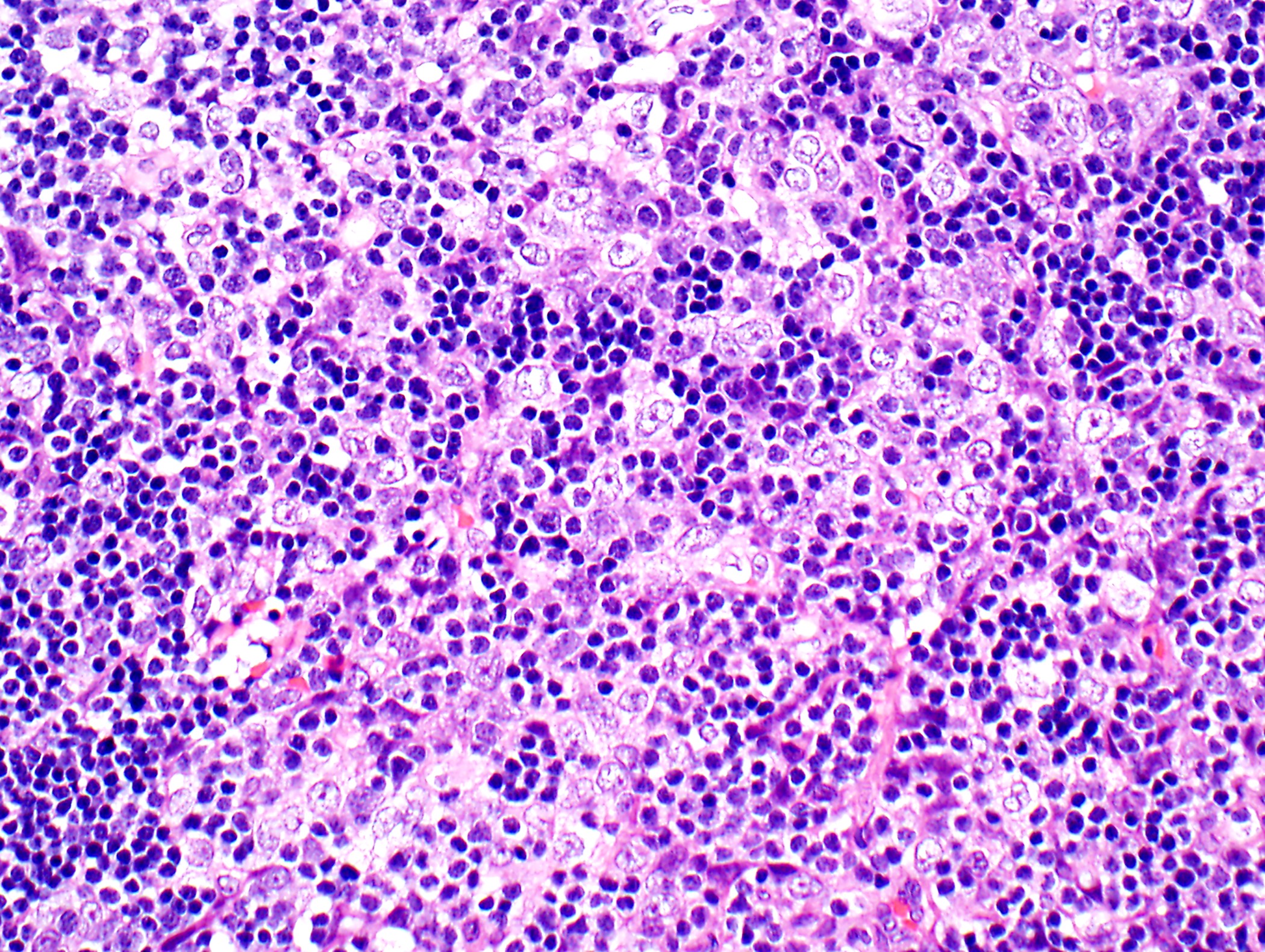
Microscopic (histologic) description
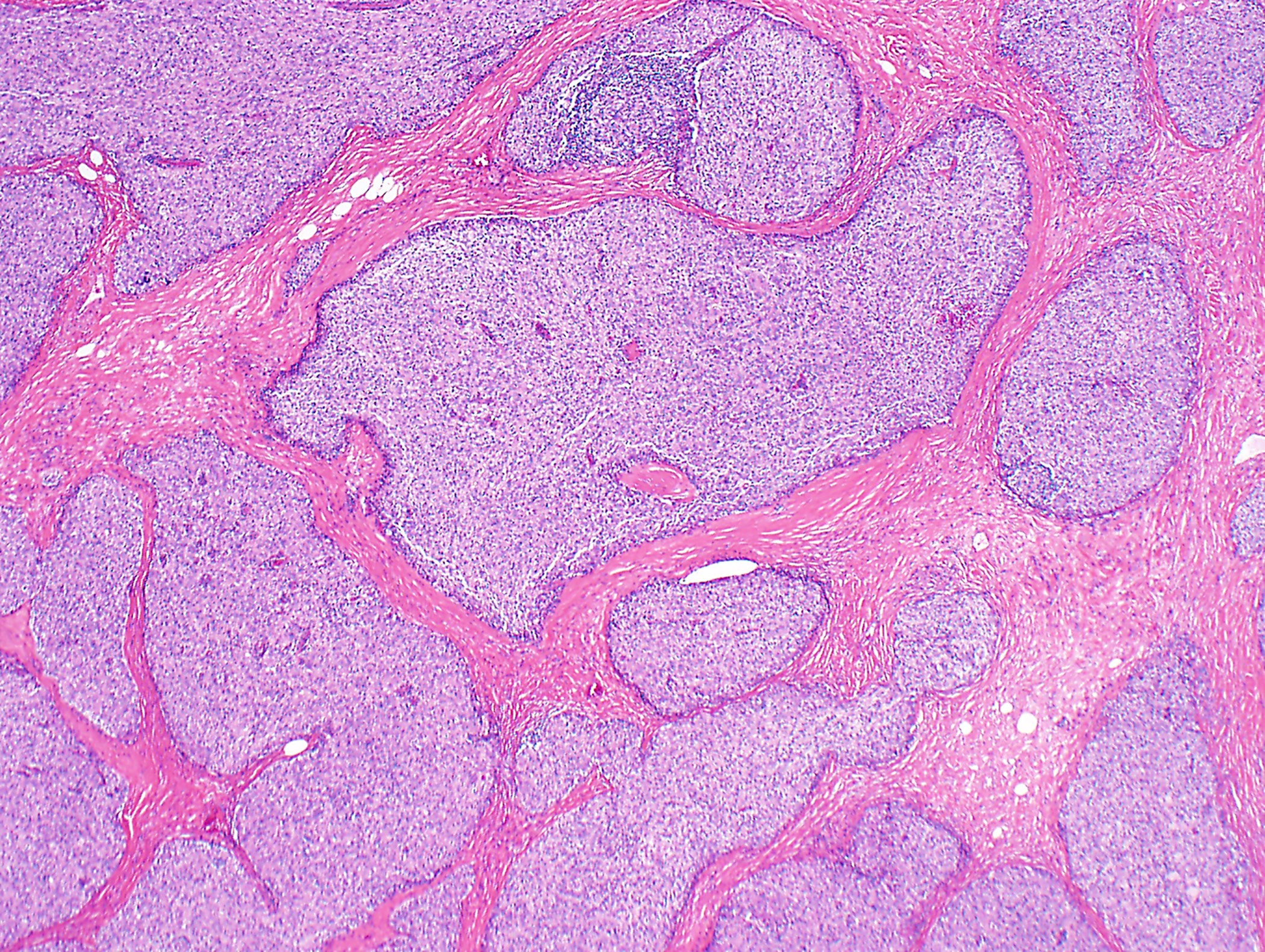
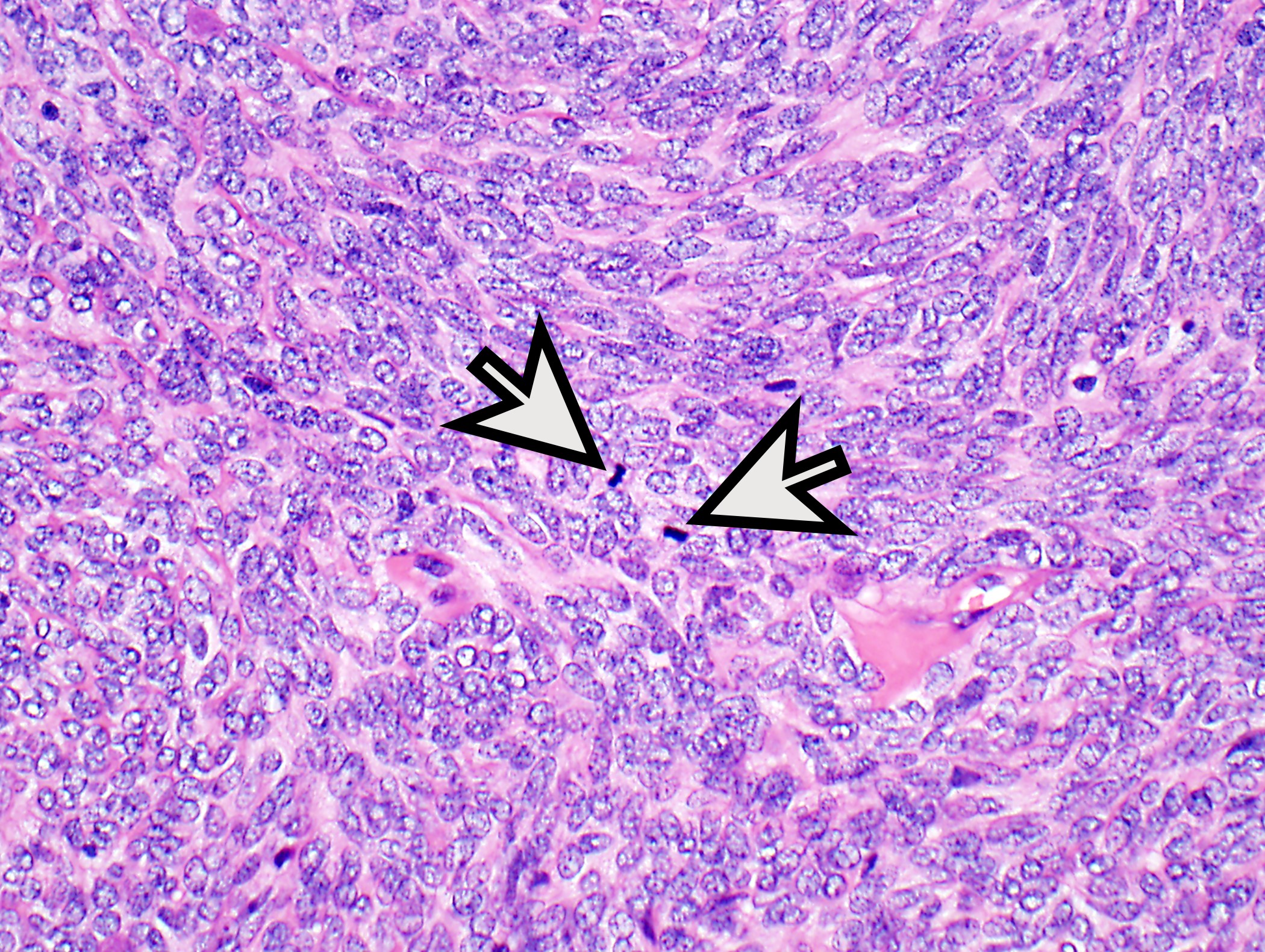
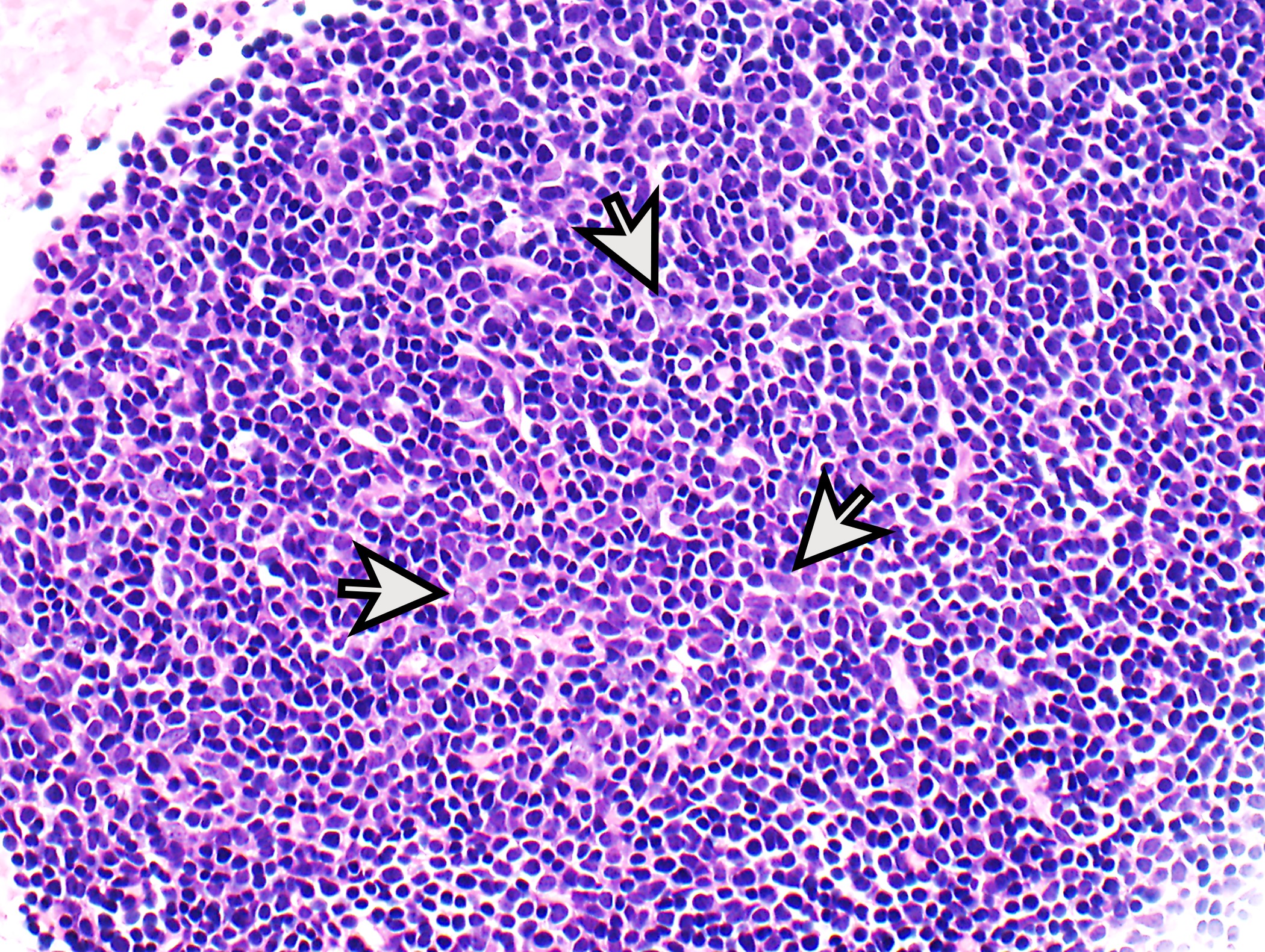
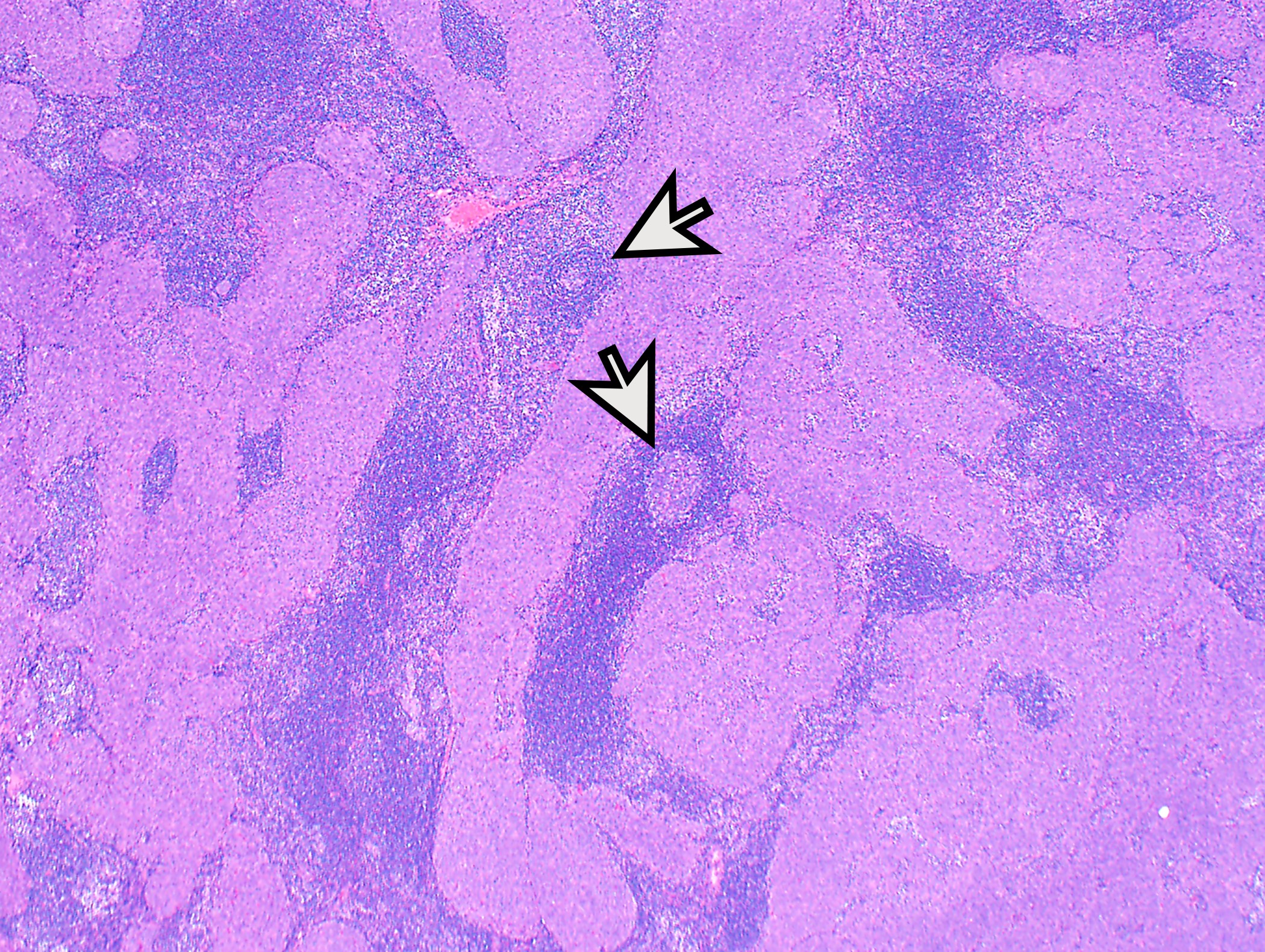
- Low magnification: lobulated architecture with cellular lobules and intersecting fibrous bands; usually at least partially encapsulated
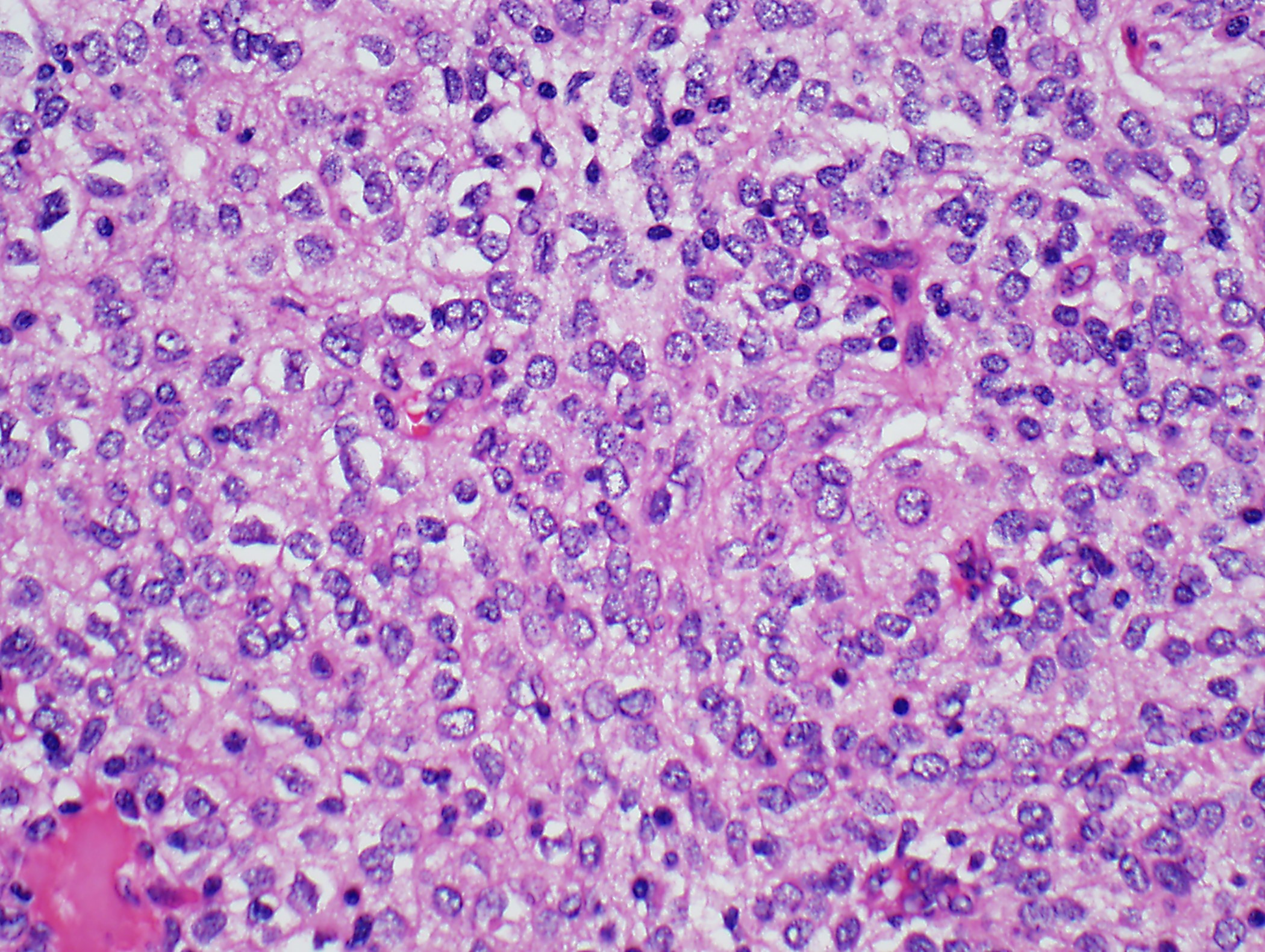
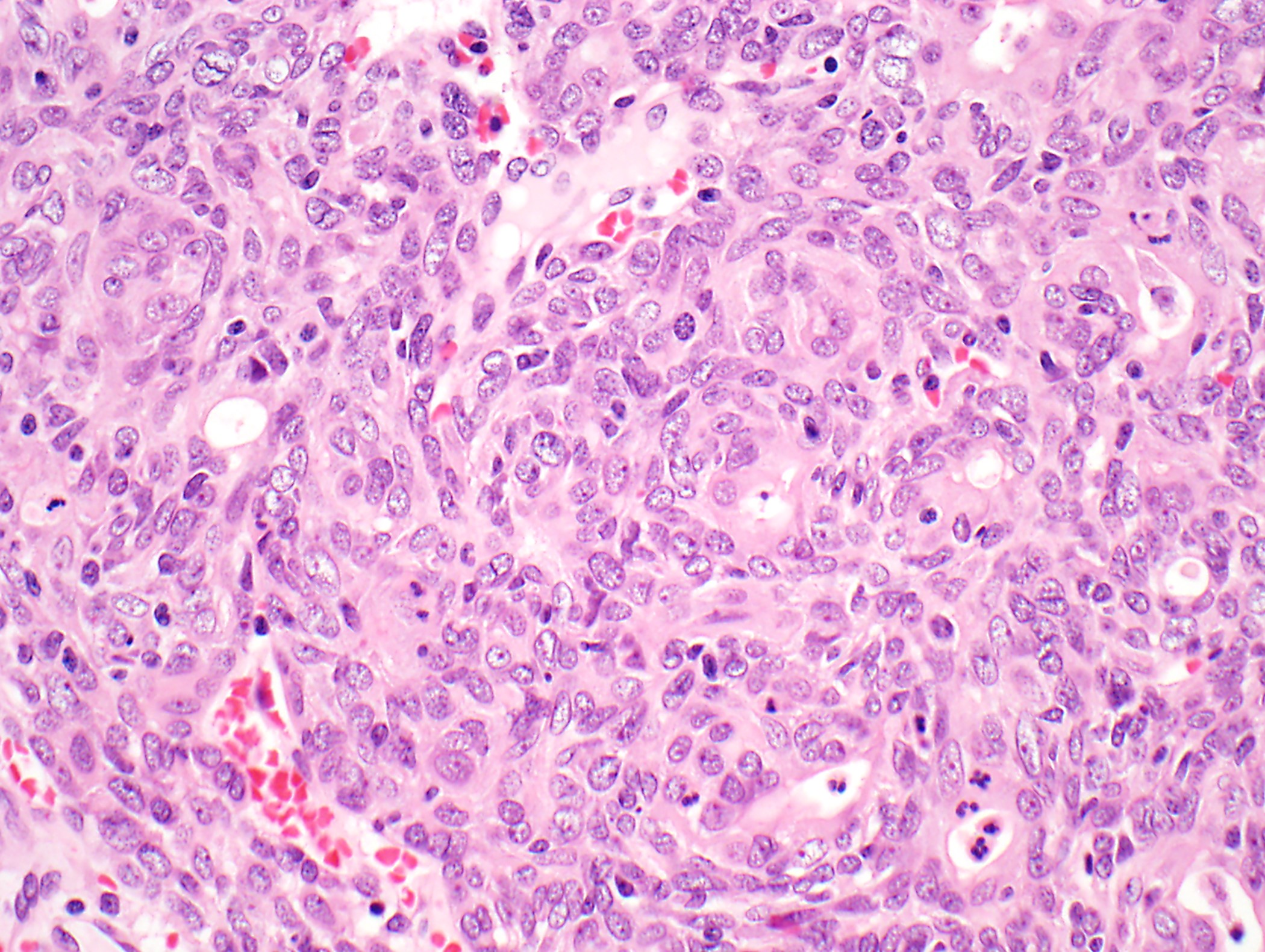
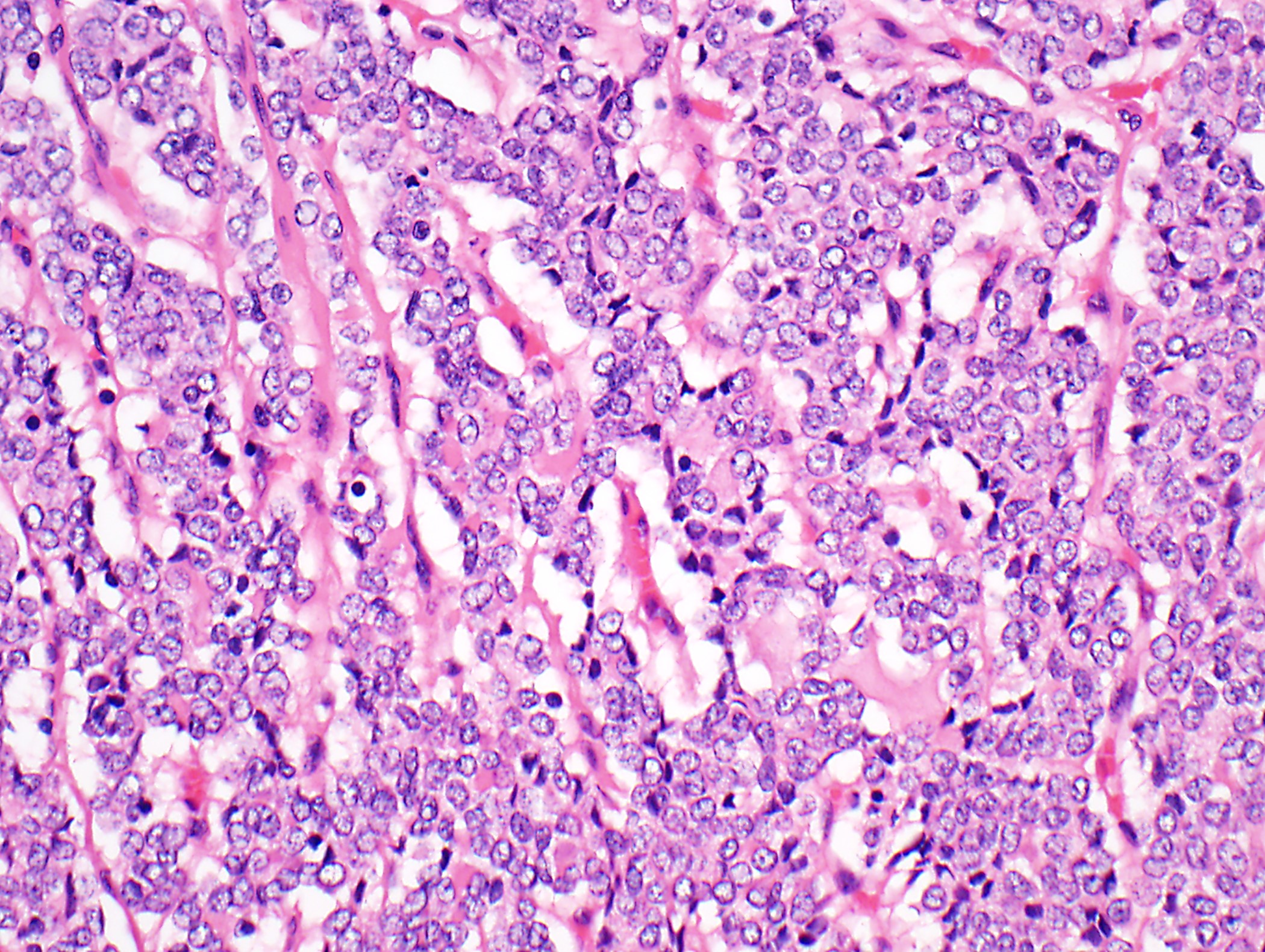
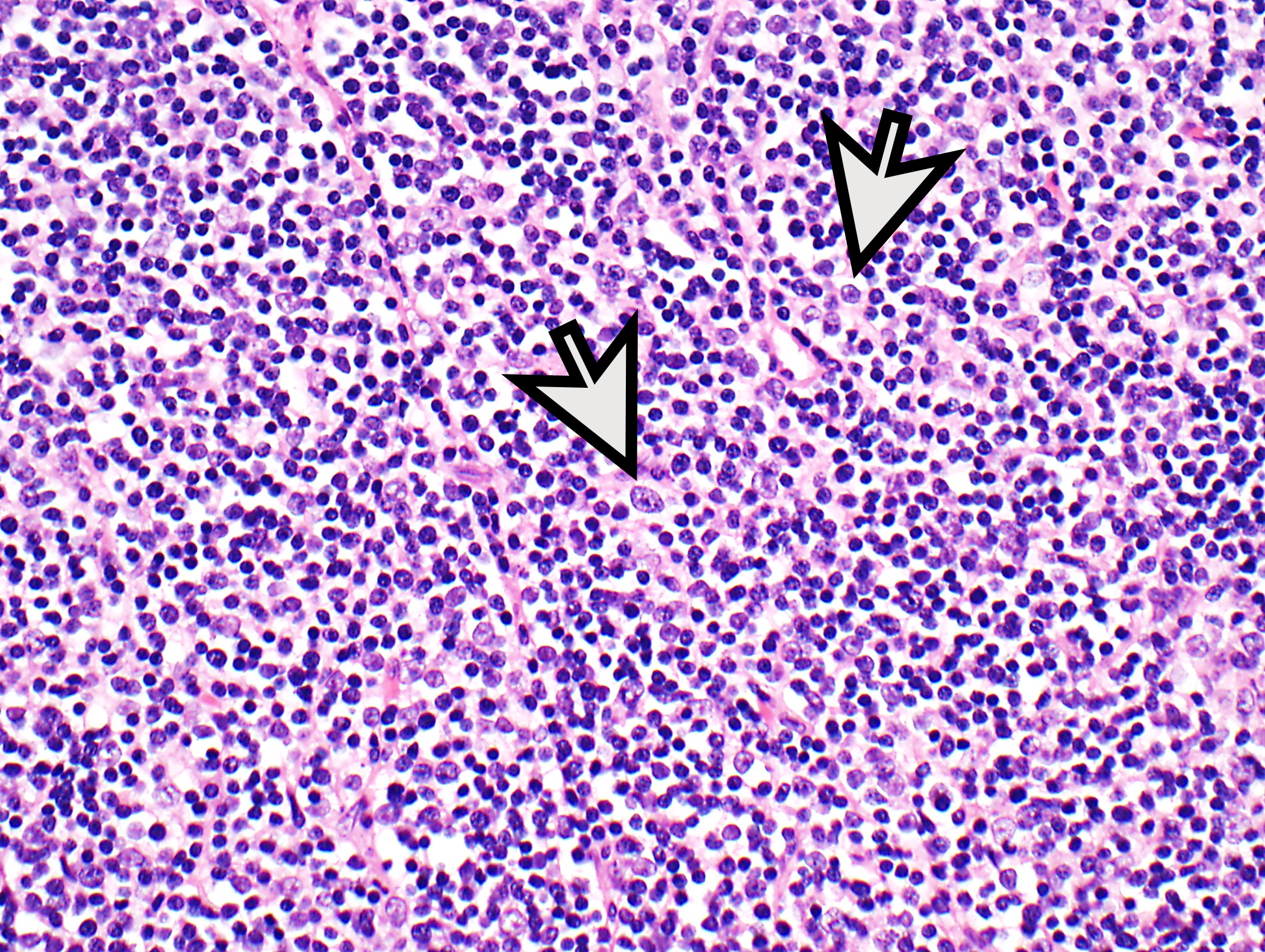
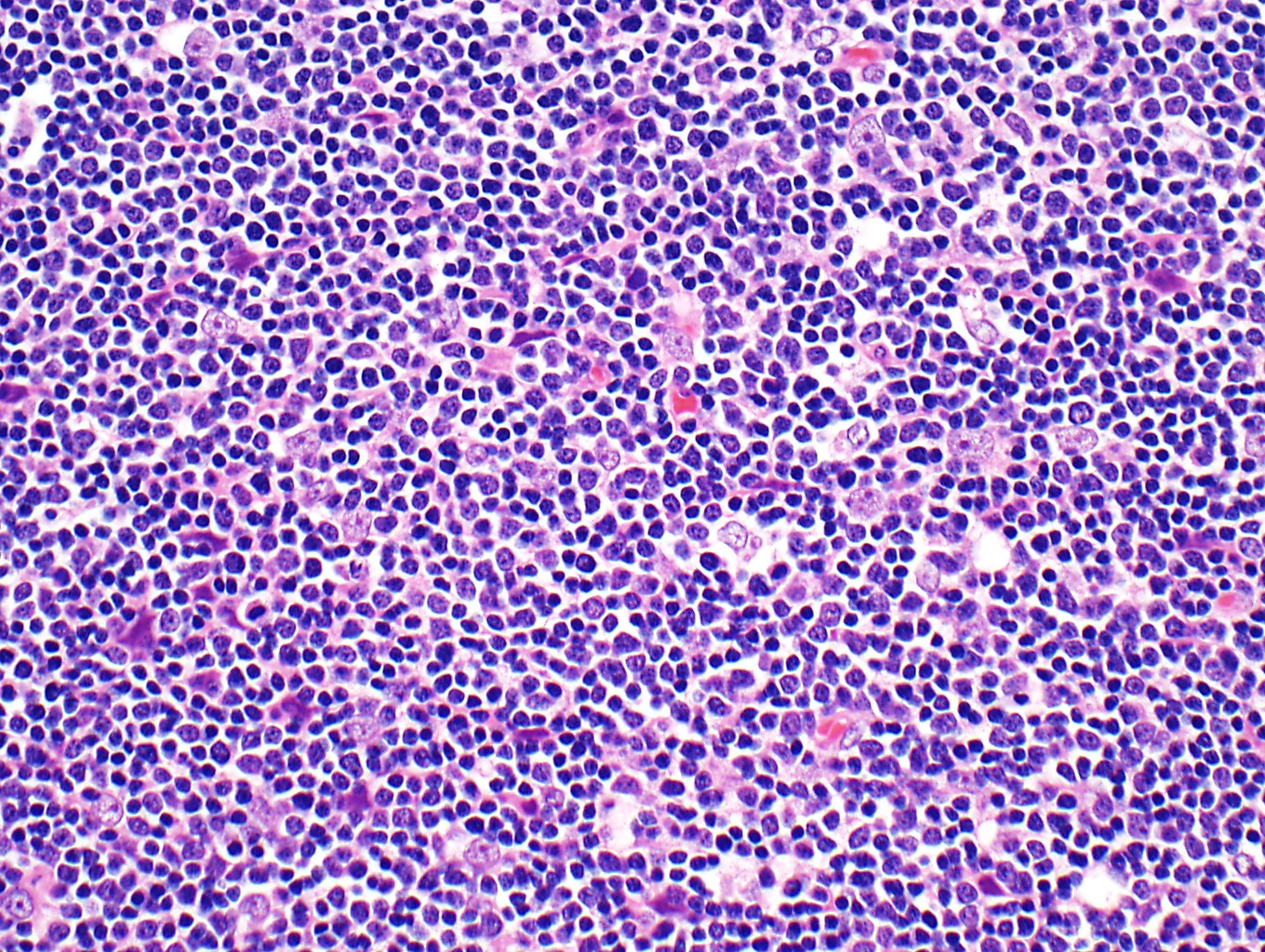
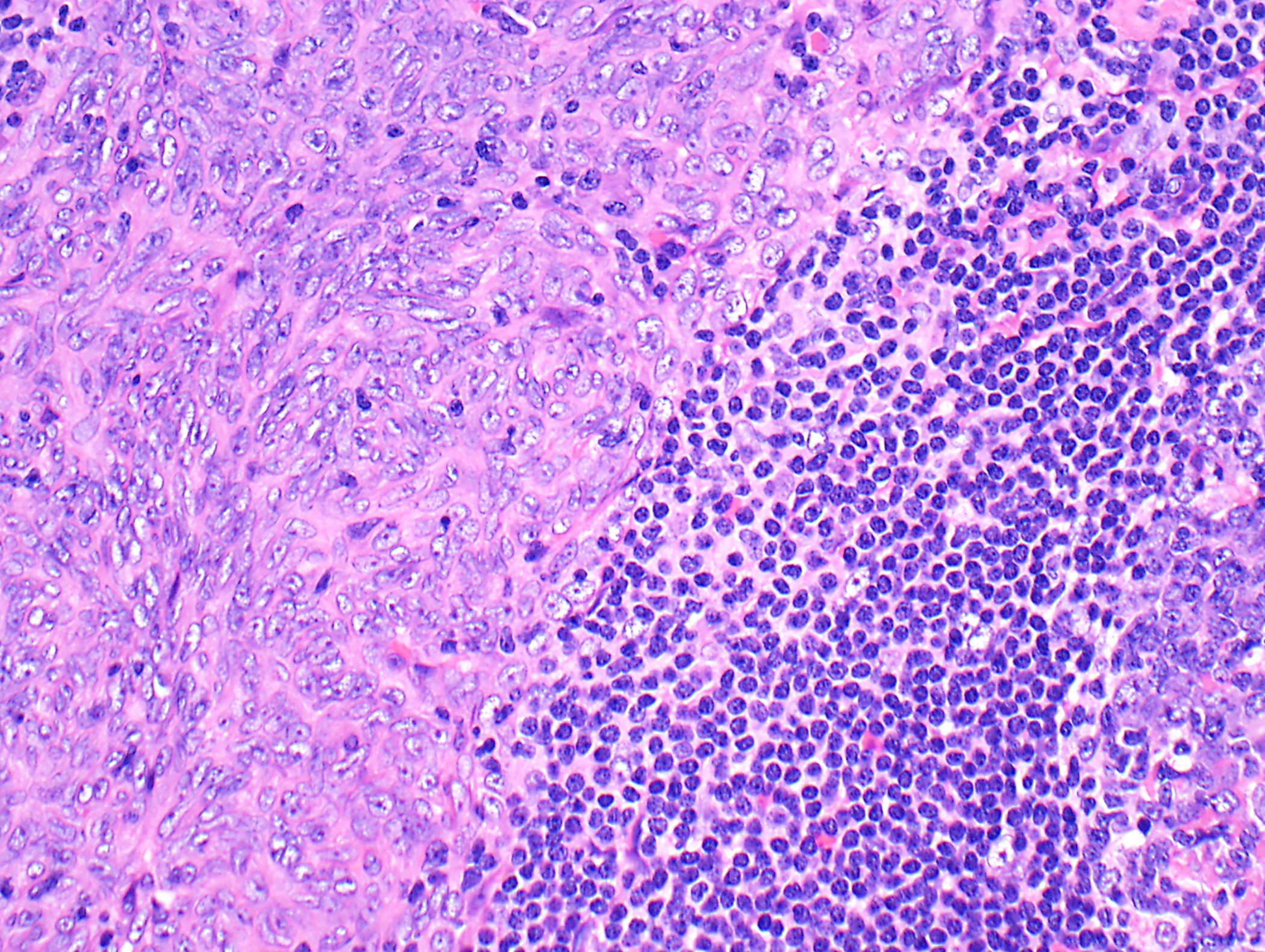
- High magnification: neoplastic epithelial cells (polygonal or spindled), various numbers of lymphocytes (thymocytes)
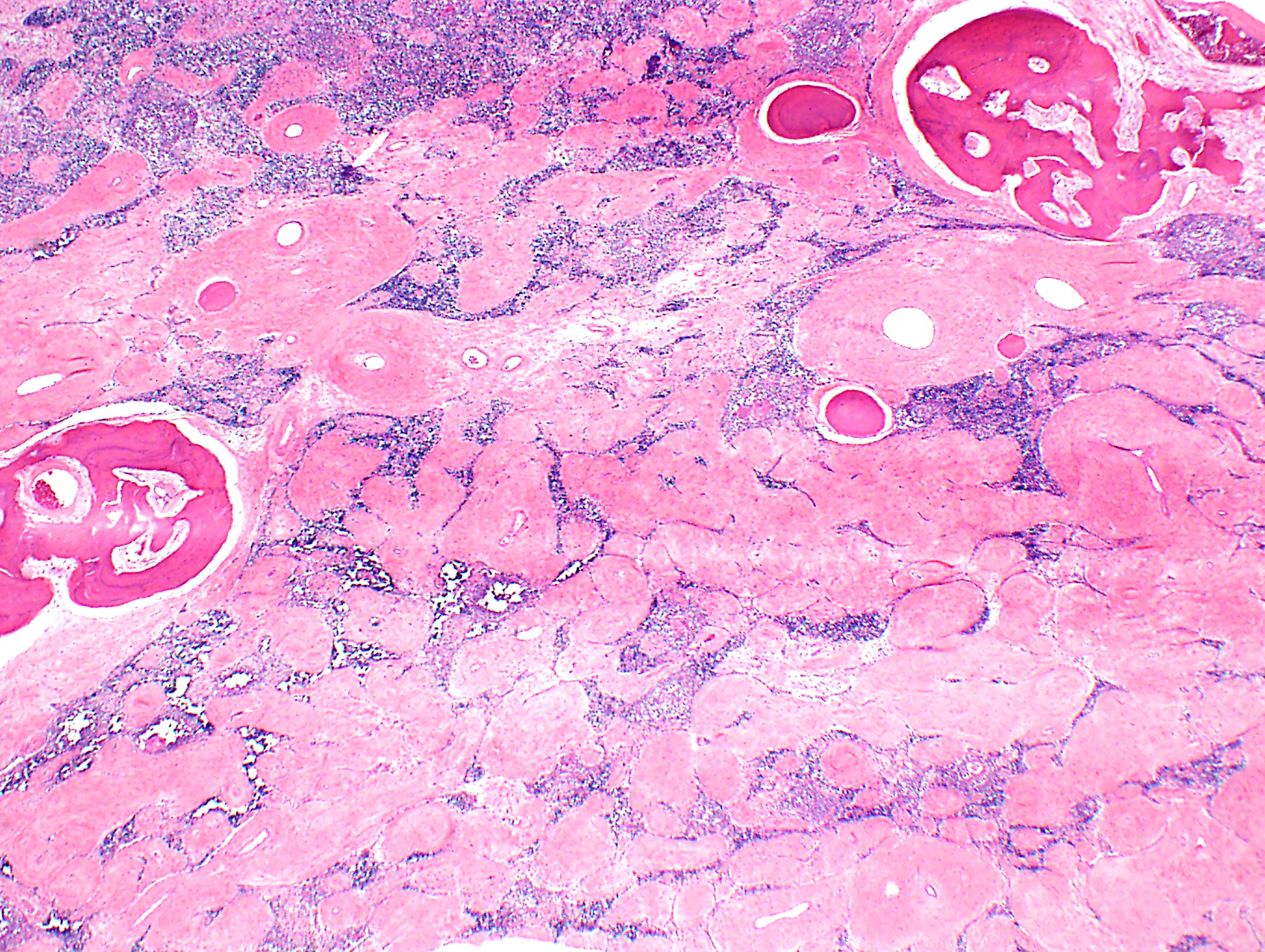
- Sometimes associated with cyst(s) or focal cystic changes
- Thymomas may be almost entirely necrotic, sclerosed or ossified
- Morphologic classification according to 2021 WHO
- Letters based on shape of neoplastic cells (A spindled, B polygonal)
- Numbers in type B thymoma (B1 → B3) based on:
- Increase in ratio of neoplastic cells to thymocytes
- Increase in cytologic atypia of neoplastic cells
- Medullary islands present in B1; occasional in B2; absent in B3
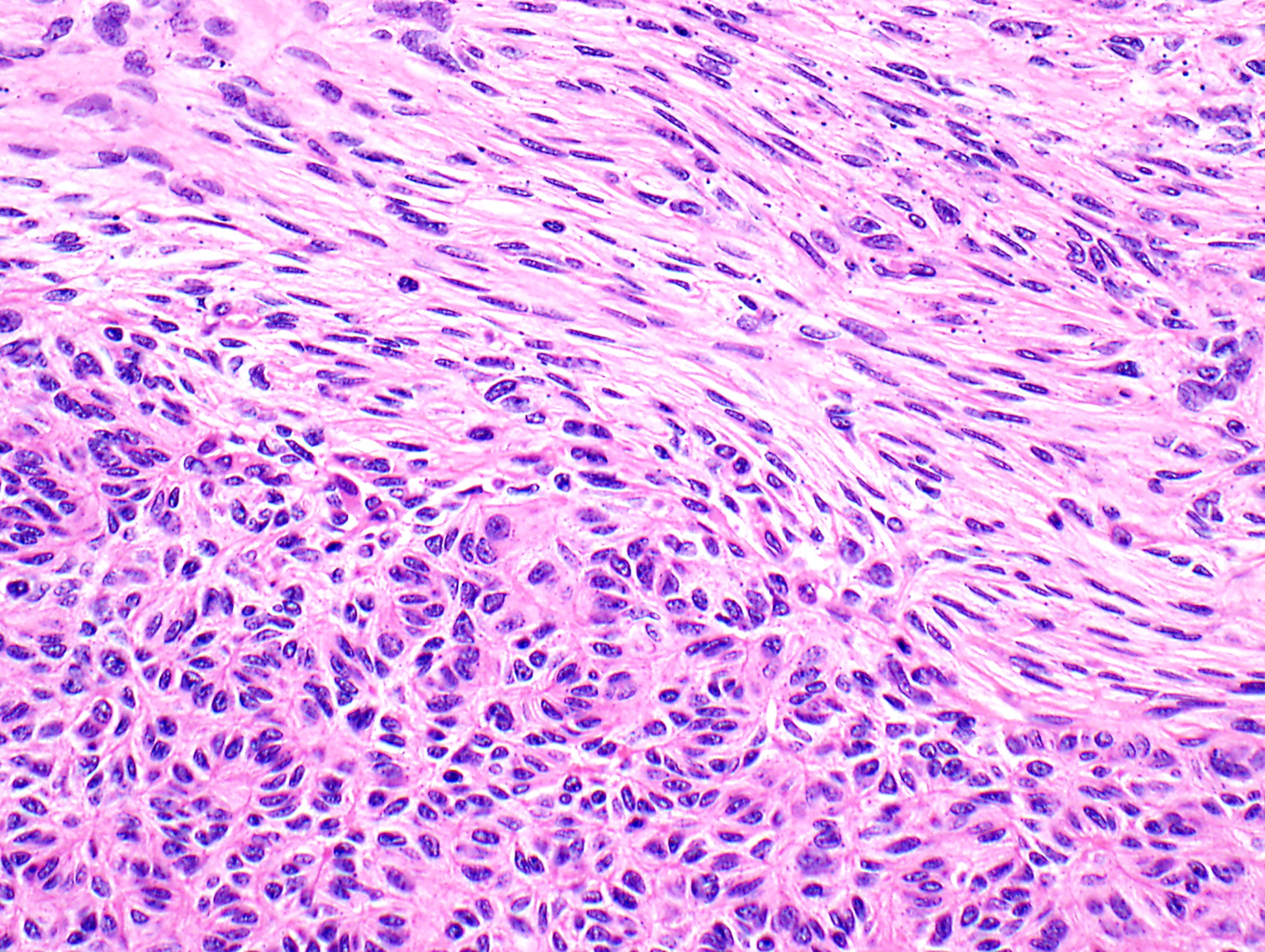
- Type A: bland oval / spindled cells
- Rare or no thymocytes
- Various patterns: hemangiopericytoma-like vascular pattern, rosettes / pseudorosettes, microcystic changes
- Atypical A
- Same as A and focal
- Hypercellularity or
- Increased mitotic count or
- Coagulative tumor necrosis
- Same as A and focal
- Type AB: morphology of A + B1 or A + B2, components can be intermingled or separated
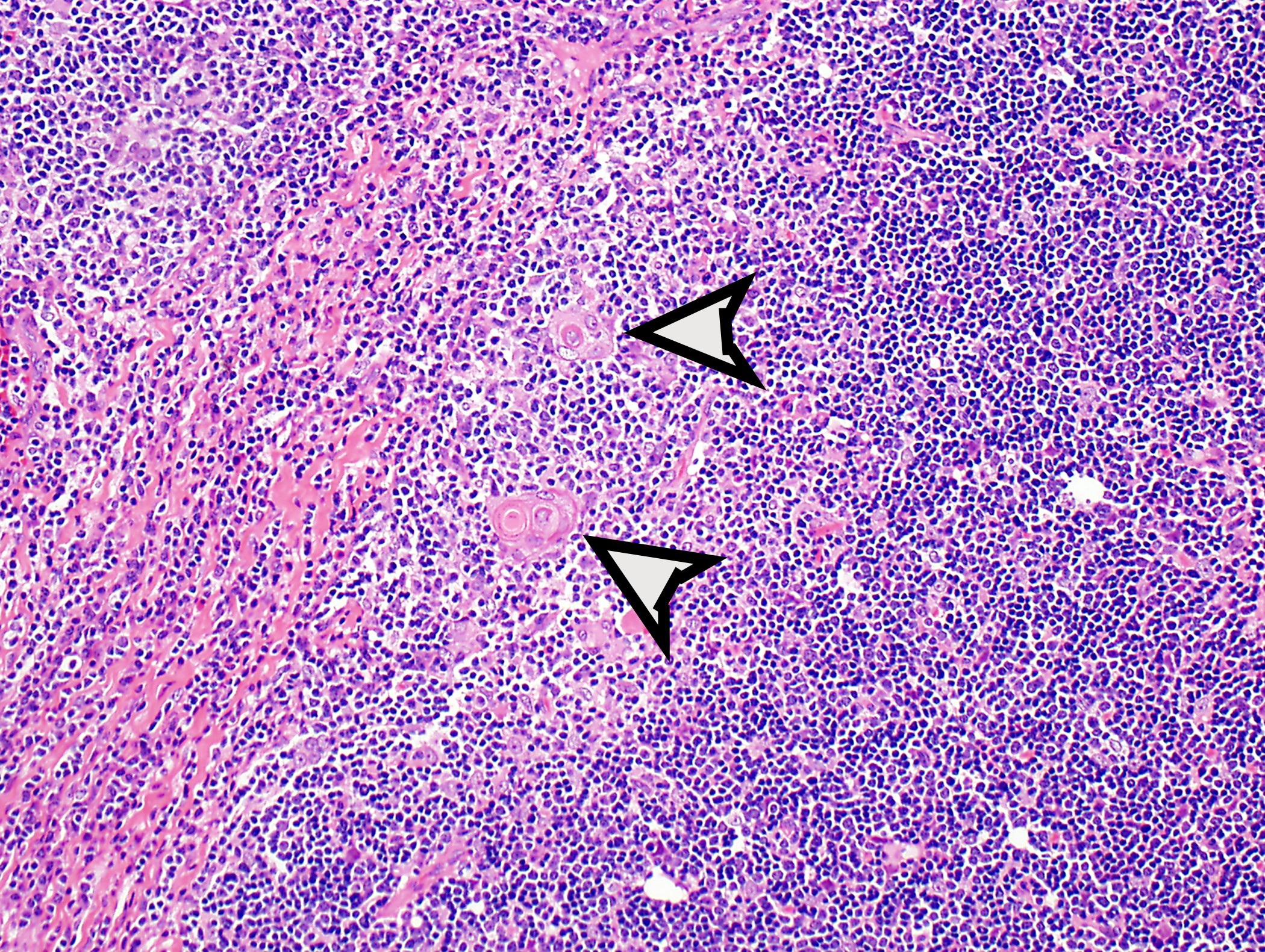
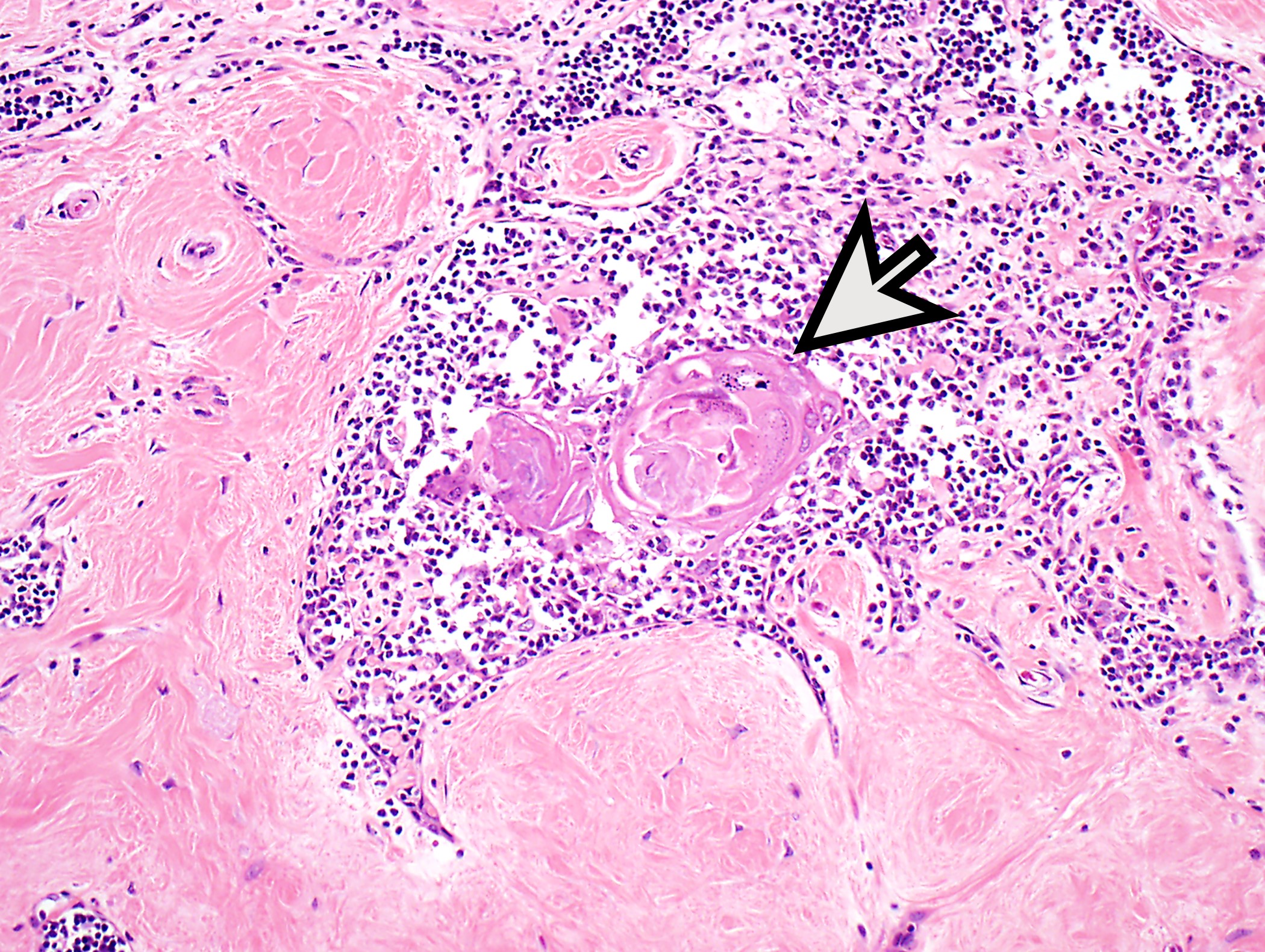
- Type B1: medullary islands (Hassall corpuscle-like elements, paler areas), scattered neoplastic cells (< 3 contiguous epithelial cells)
- Type B2: mixed neoplastic cells and thymocytes, medullary islands occasionally present
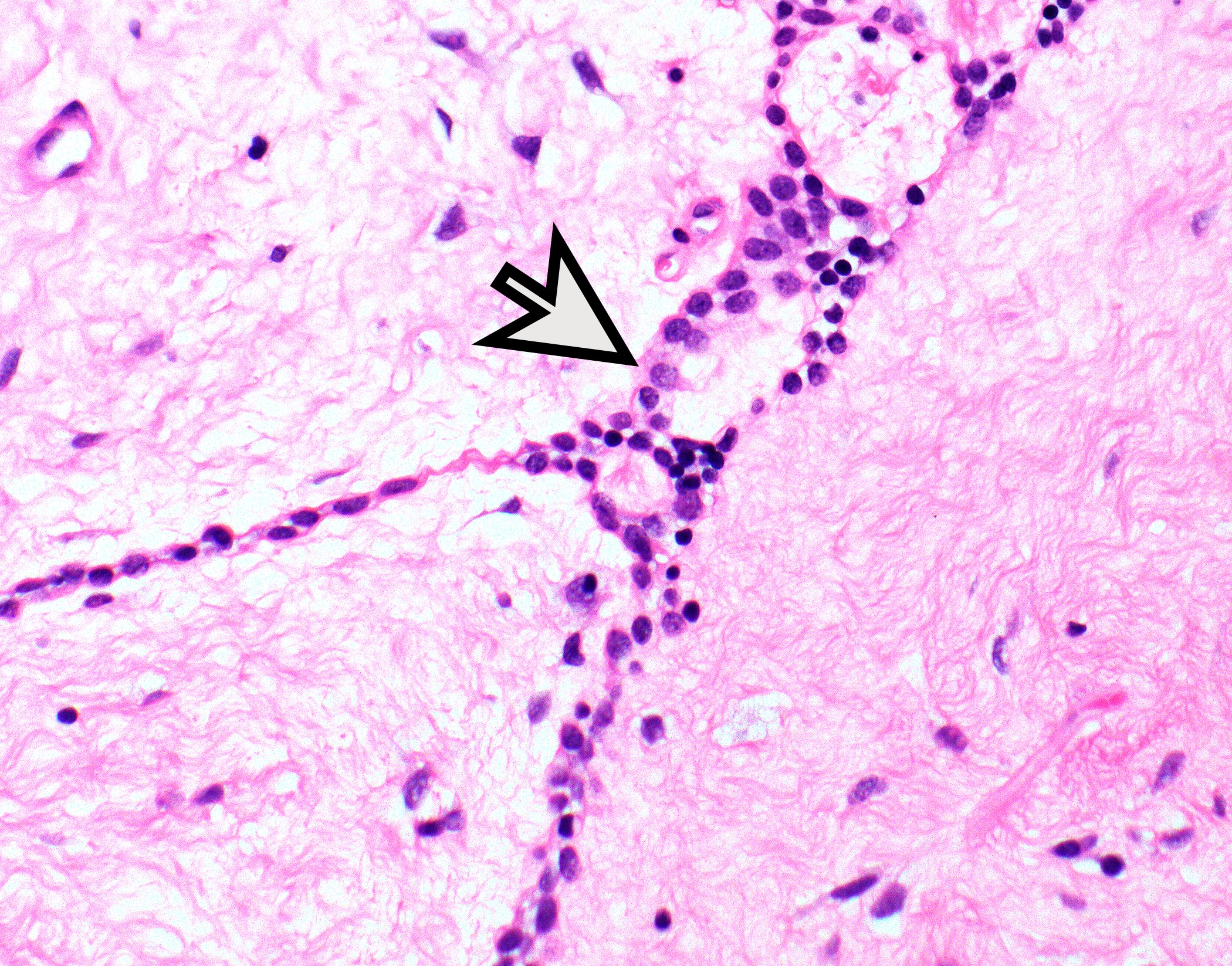
- Type B3: neoplastic cells
- Rare or no thymocytes
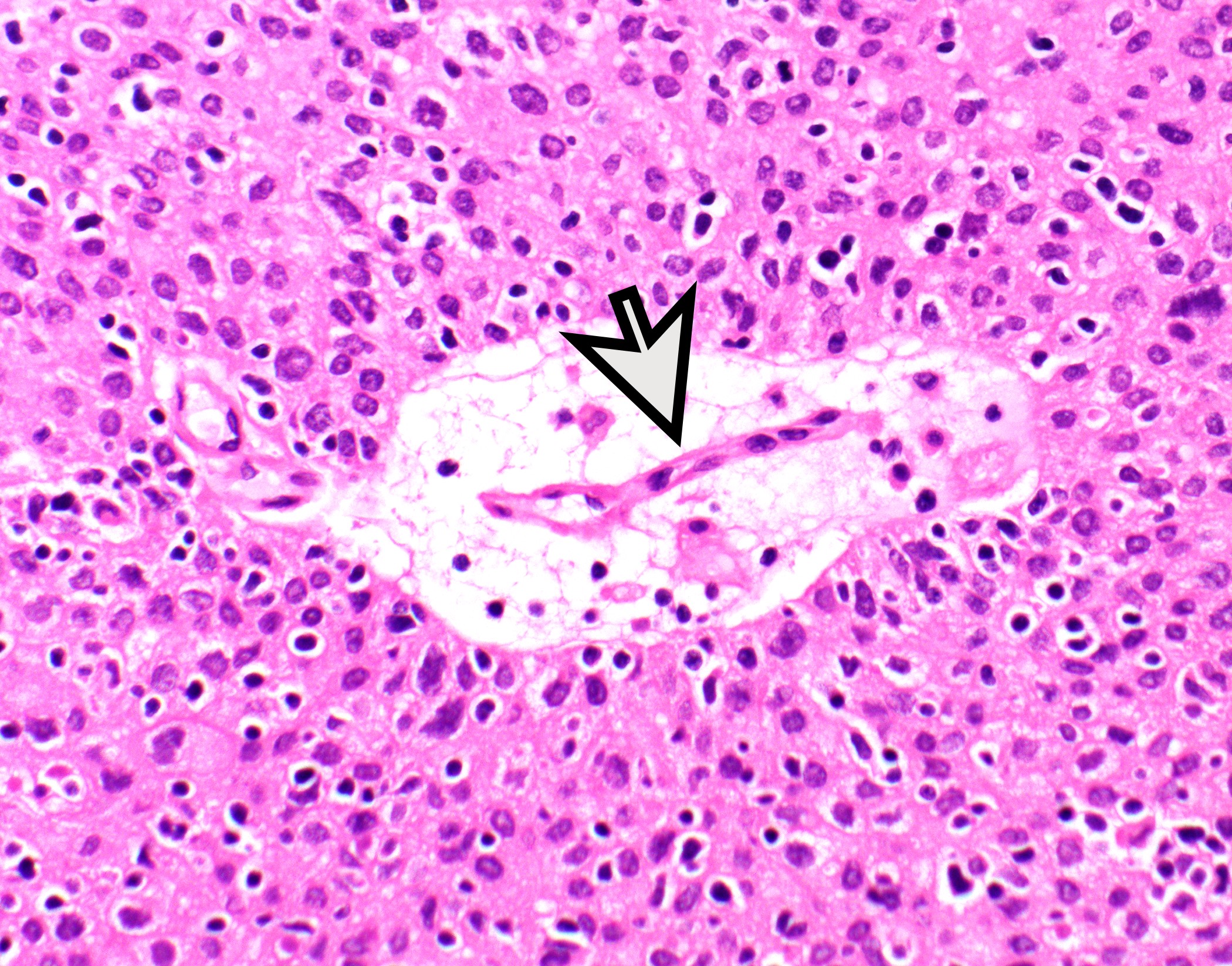
- Perivascular spaces with palisading of neoplastic epithelial cells are common (although not specific to type B3 thymomas)
- Subtyping not recommended on biopsies because of potential heterogeneity
- Uncommon thymomas:
- Micronodular thymoma with lymphoid stroma:
- Nodular appearance due to demarcated nodules or interlacing strands of neoplastic cells that are reminiscent of those of type A thymoma
- Background predominantly B cells with scattered secondary follicles
- Only scattered thymocytes
- Metaplastic thymoma:
- Biphasic
- Neoplastic cells reminiscent of type A thymoma
- Stromal spindled cells
- YAP::MAML2 rearrangement (Mod Pathol 2020;33:560)
- Entities that have been obsoleted in the 2021 WHO: microscopic thymoma (small thymic epithelial cell nests unlikely to be neoplastic; do not appear to be precursors of thymoma), sclerosing thymoma (appears to be sclerotic change of existing thymoma rather than distinct form of thymoma)
- Micronodular thymoma with lymphoid stroma:
- Lipofibroadenoma:
- Resembles fibroadenoma of the breast
- Fibrotic and hyaline stroma, focal adipose tissue, strands of bland appearing epithelial cells and a few lymphocytes
- May contain Hassall corpuscles or calcifications
- Considered benign
- Reference: Semin Diagn Pathol 2022;39:99, Adv Anat Pathol 2021;28:291
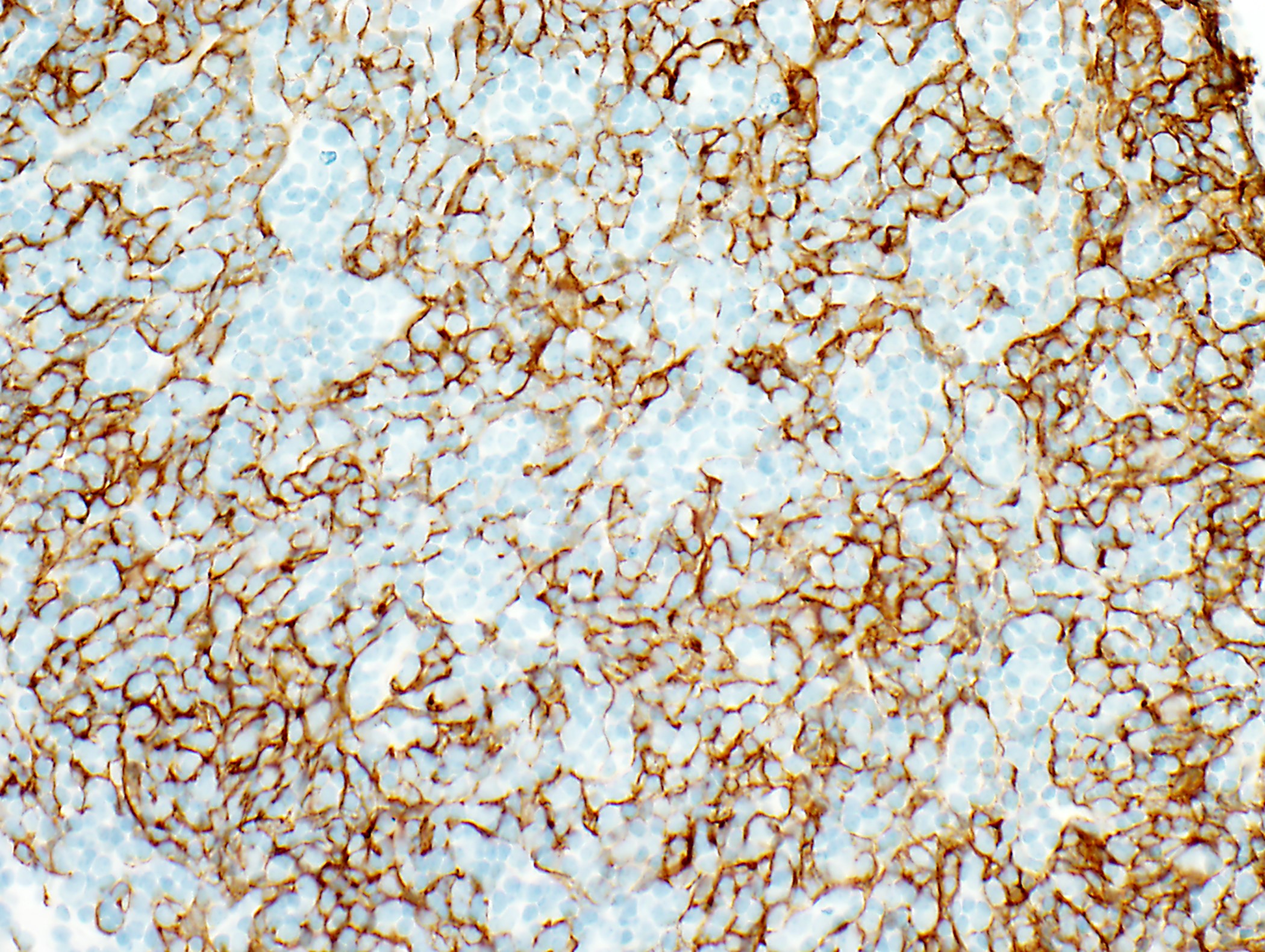
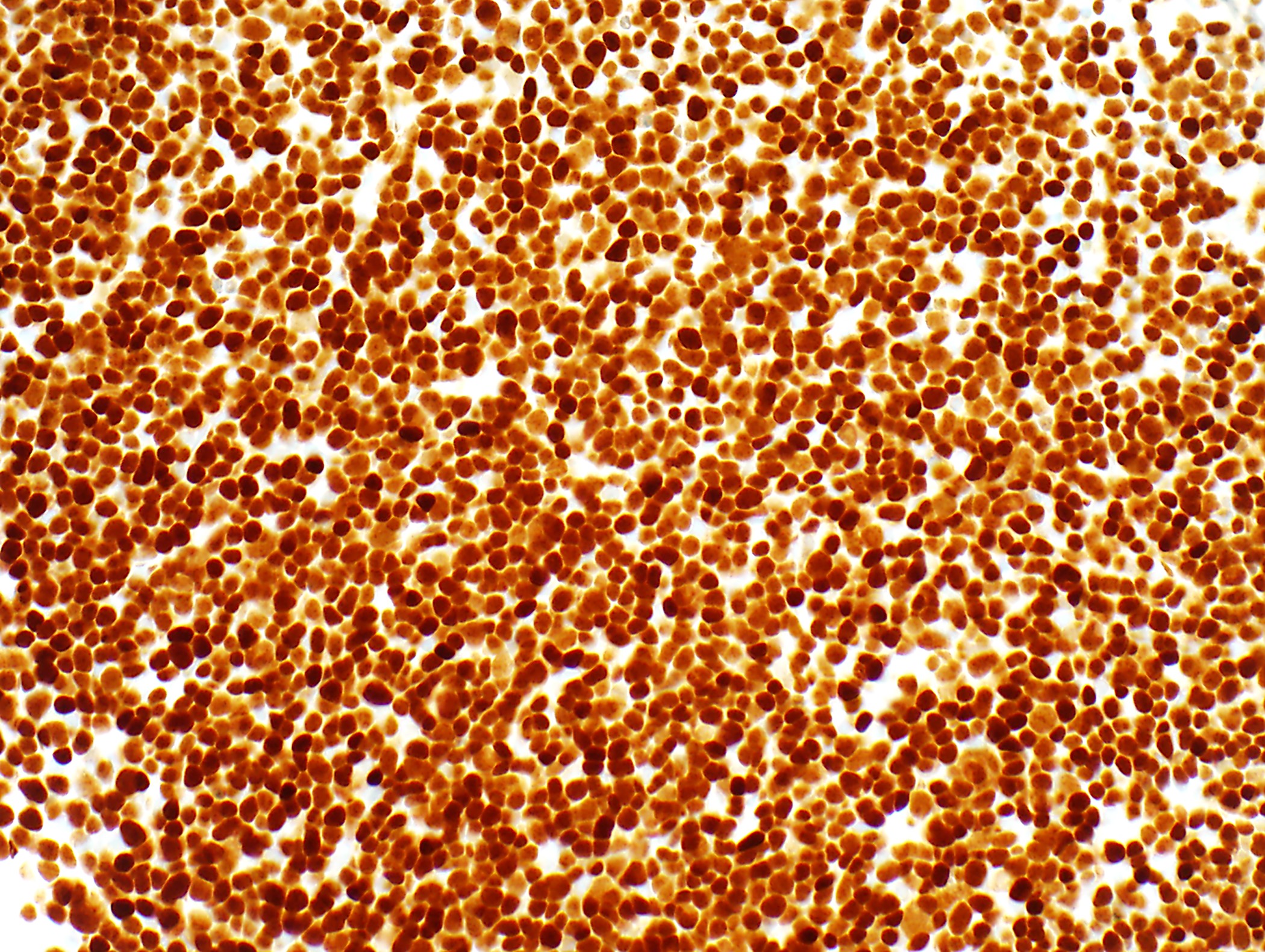
Microscopic (histologic) images
Contributed by Anja C. Roden, M.D.
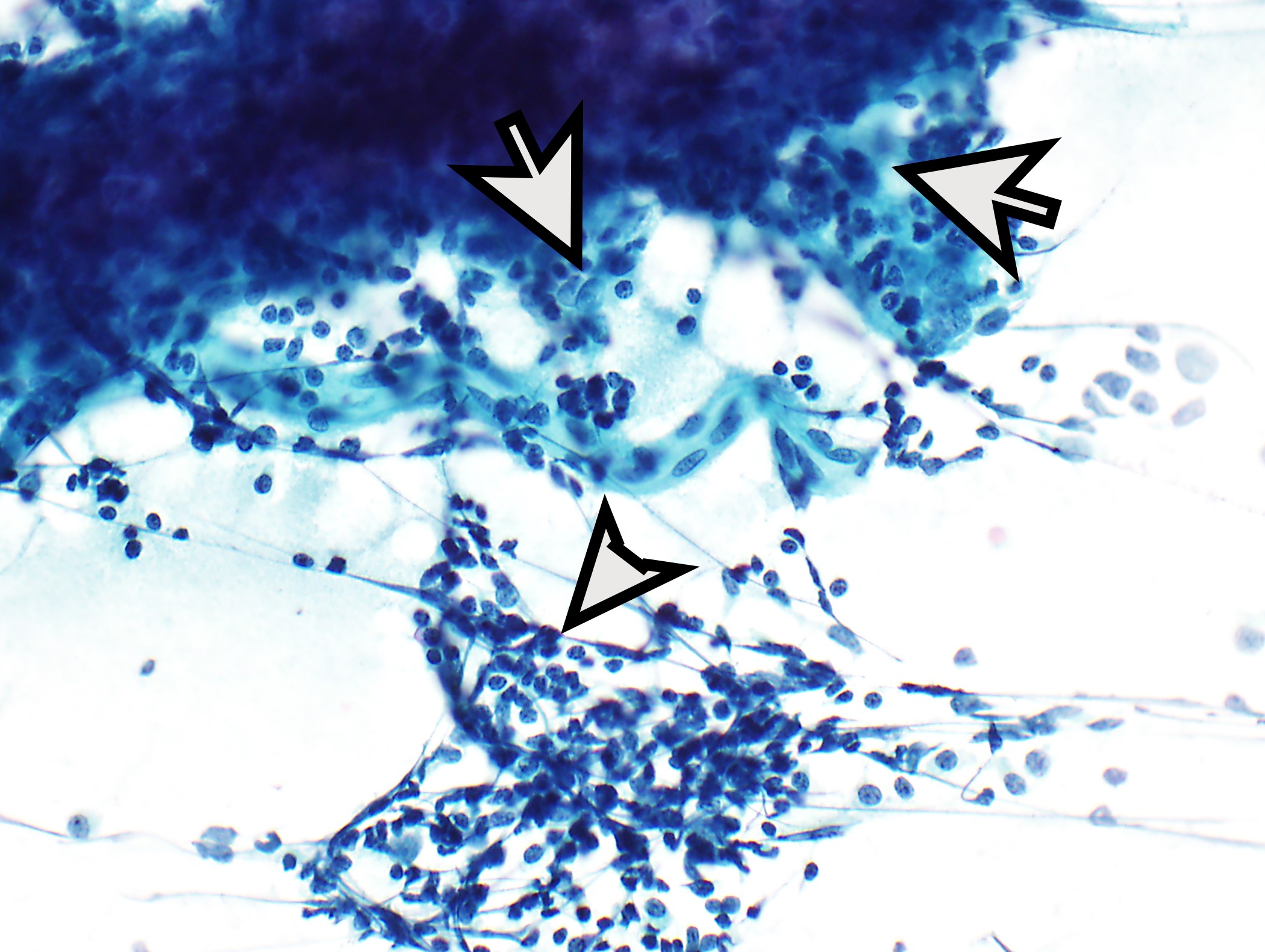
Cytology description
- Dual cell population: small lymphocytes and polygonal epithelial cells (isolated or clustered); possibly sheets of bland, elongated or spindled cells or polygonal epithelial cells
- Subtyping is not recommended
- Reference: J Thorac Oncol 2010;5:S281
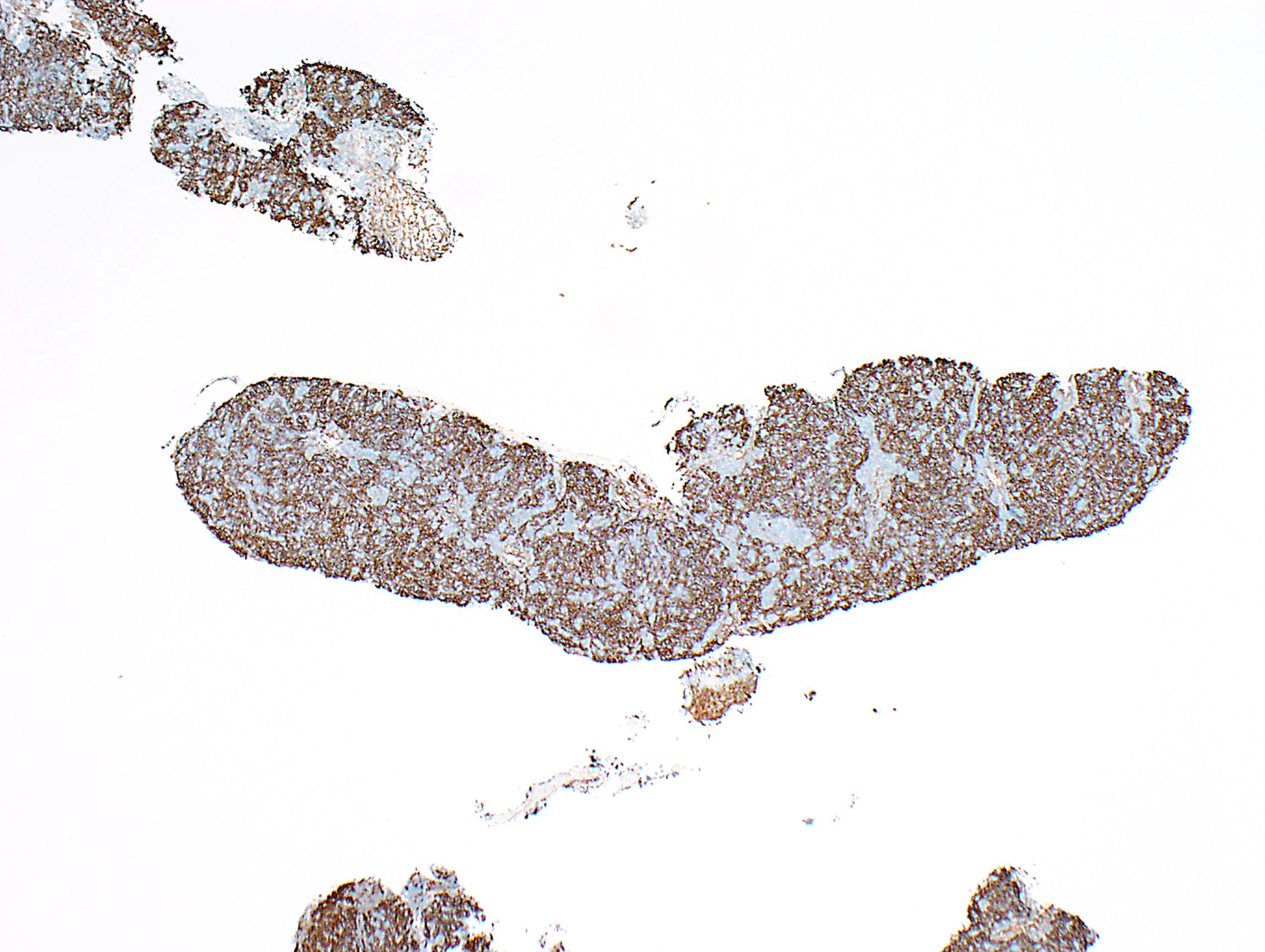
Positive stains
- Neoplastic epithelial cells:
- Thymocytes: TdT, CD1a, CD99 (might be absent in types A, B3)
- Reference: Diagnostics (Basel) 2020;10:460
Negative stains
Molecular / cytogenetics description
- Low tumor mutation burden (TMB), mean 0.48 mutations / megabase (Cancer Cell 2018;33:244)
- Loss of heterozygosity in 6q25.2-25.3: most common chromosomal deletion, FOXC1 is potential target (tumor suppressor gene) (Clin Cancer Res 2013;19:1960)
- GTF2I missense mutations (codon L424H) in 74 - 82% of type A and AB thymomas and 21 - 32% of B1 - B3 thymomas, appears thymoma specific oncogene (Cancer Cell 2018;33:244, Nat Genet 2014;46:844)
- HRAS, NRAS and TP53 mutations in some thymomas (Cancer Cell 2018;33:244)
- YAP1::MAML2 gene fusion in metaplastic thymomas (Mod Pathol 2020;33:560)
Features to report
- Report of resection specimen should include:
- Procedure
- Morphology (2021 WHO classification); all components with percentages (in 10% increments)
- Margin status
- Extent of invasion
- Treatment effect if applicable
- Pleural or pericardial implants if applicable
- Metastases if applicable
- Lymph node status
- Pathologic tumor stage (pTNM stage; optional in addition: Modified Masaoka Stage)
- Background thymic gland if present
Sample pathology report
- Thymus, thymectomy:
- WHO type B1 invading into lung (see synoptic report)
- The surgical margins are negative by < 0.1 cm. Multiple (6) regional lymph nodes are negative.
- Synoptic report:
- Procedure: thymectomy
- Tumor size:
- Greatest dimension: 6.5 cm
- Additional dimensions: 5.1 x 3.2 cm
- Histologic type: type B1 thymoma
- Transcapsular invasion: present
- Tumor extension: tumor involves pulmonary parenchyma, left lower lobe lung
- Margins: uninvolved by tumor
- Distance of tumor from closest margin: < 1 mm
- Treatment effect: no known presurgical therapy
- Lymphovascular Invasion: not identified
- Regional lymph nodes:
- Number of lymph nodes involved: 0
- Number of lymph nodes examined: 6
- Pathologic staging (AJCC, 8th edition)
- TNM descriptors: not applicable
- Primary tumor: pT3
- Regional lymph nodes: pN0
- Distant metastasis: pMX
- Modified Masaoka Stage: stage III
- Additional pathologic findings: thymic follicular hyperplasia
Differential diagnosis
- Lymphoma / leukemia (e.g., T lymphoblastic lymphoma / leukemia, small B cell lymphomas, T cell lymphomas, classic Hodgkin lymphoma):
- No diffuse meshwork of keratin positive neoplastic cells
- Effaced architecture in lymphoma versus lobulated architecture in thymoma
- LMO2 expressed in neoplastic lymphoblasts of T acute lymphoblastic leukemia (T ALL) and absent in thymoma (Am J Clin Pathol 2016;145:180)
- CD20 positive B cells in small B cell lymphomas; classic Hodgkin lymphoma can show lobulated architecture but is comprised of mixed chronic inflammation often with eosinophils and scattered large Reed-Sternberg cells and Hodgkin cells
- Thymic carcinoma:
- Distorted architecture with desmoplasia and often increased cytologic atypia
- Increased Ki67 labeling index (> 14% of tumor cell nuclei staining) in a subset of thymic carcinomas but not thymoma (Hum Pathol 2015;46:17)
- Diffuse coexpression of CD5 and CD117 in tumor cells in a subset of thymic squamous cell carcinomas but not thymomas (Diagn Pathol 2015;10:210)
- Metastatic carcinoma:
- Distorted architecture, increased cytologic atypia
- Benign thymic gland (e.g., thymic follicular hyperplasia, true thymic hyperplasia, sampling bias):
- Lacks lobulated architecture and lacks the diffuse meshwork of keratin positive neoplastic cells (although there are keratin positive epithelial cells scattered throughout the benign thymic gland)
- Usually intimately associated with adipose tissue without capsule
- Lymphoid follicles in thymic follicular hyperplasia (caveat: can also be seen in micronodular thymoma with lymphoid stroma or on rare occasions in thymoma)
- Thymic cyst:
- Cyst with adjacent benign thymic parenchyma with or without chronic inflammation
- Germ cell tumor (e.g., seminoma):
- Usually morphology is different; however, seminoma can have lymphoid hyperplasia, fibrous bands and remnant thymic tissue
- Immunostains are important
- Thymic carcinoid tumor:
- Might have morphologic similarities with type A thymoma, expresses neuroendocrine markers
- Solitary fibrous tumor:
Additional references
Board review style question #1
Board review style answer #1
A. Epithelial cell. In thymomas, epithelial cells are the neoplastic cells.
(B) Incorrect. A few scattered benign histiocytes might be present in these tumors. (C) Incorrect. Lymphocytes, which are predominantly thymocytes, are considered reactive. (D) Incorrect. Although classic Hodgkin lymphomas can exhibit a lobulated architecture, usually they do not have so sharply demarcated fibrous bands. Reed-Sternberg cells or Hodgkin cells are not identified. (E) Incorrect. Thymocytes are considered reactive in these tumors.
Comment Here
Reference: Thymoma
(B) Incorrect. A few scattered benign histiocytes might be present in these tumors. (C) Incorrect. Lymphocytes, which are predominantly thymocytes, are considered reactive. (D) Incorrect. Although classic Hodgkin lymphomas can exhibit a lobulated architecture, usually they do not have so sharply demarcated fibrous bands. Reed-Sternberg cells or Hodgkin cells are not identified. (E) Incorrect. Thymocytes are considered reactive in these tumors.
Comment Here
Reference: Thymoma
Board review style question #2
What is an important prognostic parameter in thymomas?
- Association with a cyst
- Male sex
- Presence of fibrous bands
- Surgical resection margin
- Thymic follicular hyperplasia
Board review style answer #2
D. Surgical resection margin. Complete resection is an important prognostic parameter in thymomas.
(A) Incorrect. Cystic changes have no impact on prognosis. (B) Incorrect. Sex is not associated with prognosis. (C) Incorrect. The presence of fibrous bands is not associated with prognosis. (E) Incorrect. Thymic follicular hyperplasia in the background thymic gland has no prognostic implication.
Comment Here
Reference: Thymoma
(A) Incorrect. Cystic changes have no impact on prognosis. (B) Incorrect. Sex is not associated with prognosis. (C) Incorrect. The presence of fibrous bands is not associated with prognosis. (E) Incorrect. Thymic follicular hyperplasia in the background thymic gland has no prognostic implication.
Comment Here
Reference: Thymoma