Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Intraoperative frozen / smear cytology images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Flow cytometry description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Christian H, Murga-Zamalloa CA. Primary CNS lymphoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lymphomaprimaryCNSlymphoma.html. Accessed March 30th, 2025.
Definition / general
- Diffuse large B cell lymphoma (DLBCL) that arises within the brain, spinal cord, leptomeninges or vitreous / retinal space and is not associated with immunodeficiency
Essential features
- Accounts for ~5% of all non-Hodgkin lymphomas and < 5% of brain tumors
- This is different from nodal DLBCL with secondary central nervous system (CNS) involvement
- Primary DLBCL features transcriptional and genomic profiles distinct from nodal DLBCL
- Enriched in cases that feature evasion of immune responses due to the loss of expression of HLA-DR and HLA-DQ
- Typically aggressive and occurs predominantly in adults aged > 60 years, with an overall worse prognosis than systemic DLBCL
- Other B cell lymphomas primarily involving the CNS are markedly rare and are not included in this disease category
- WHO 2022 (5th edition) classifies this type of lymphoma as primary LBCL of immune privileged sites; as of 2022, the International Consensus Classification (ICC) still categorizes this entity as primary DLBCL of the CNS
Terminology
- Outdated terminology (no longer recommended): reticulum cell sarcoma, diffuse histiocytic lymphoma, lymphomatosis cerebri and primary intraocular lymphoma (PIOL)
- The following terms for primary DLBCL of the CNS were used interchangeably: primary CNS lymphoma, B cell primary CNS lymphoma (PCL) and primary DLBCL of the CNS
- Primary DLBCL of the vitreoretina was previously called primary vitreoretinal lymphoma (PVRL)
ICD coding
Epidemiology
- Comprises 4 - 6% of non-Hodgkin lymphoma diagnoses (Lab Med 2015;46:159)
- Accounts for 1 - 4% of intracranial tumors (Lab Med 2015;46:159)
- Incidence of CNS lymphoma has statistically decreased in men from 2000 to 2019; however, the incidence has increased for women in the same period (annual percentage rate [APC] = +0.5%)
- Rising rates of primary CNS lymphoma in elderly patients may be attributed to the increased use of immunosuppressive medications to treat autoimmune disorders (Br J Cancer 2011;105:1414)
- Levasseur et al. reported the incidence of primary vitreoretinal lymphoma as 0.02 - 0.05 cases per 100,000 people (JAMA Ophthalmol 2013;131:50)
Sites
- ~72% of CNS lymphoma cases are supratentorial, with 35 - 50% involving the deeper brain structures
- ~10 - 25% of patients at diagnosis or with disease progression have ocular globe involvement
- Vitreoretinal DLBCL rarely extends into the optic nerve at the time of diagnosis and ~65 - 90% of patients will have their disease progress to the brain (Oncologist 2011;16:1589, Virchows Arch 2020;476:647)
- Most patients who relapse (~85%) will likely have disease confined to the CNS (J Neurooncol 2006;80:159)
- Relapse from primary brain DLBCL can occur in the testis or the vitreoretinal space (Leukemia 2016;30:361)
Pathophysiology
- Most cases are considered as mature germinal center exit B cells with evidence of ongoing somatic hypermutation in the IGHV genes
- As this lymphoma is considered to arise in an immune privileged site, lymphoma cells adapt to escape immune regulation and downregulate certain immune reactions
- Combined transcriptional profiles and immunohistochemistry identified 3 subgroups with distinct immune subtypes correlating with immune cell infiltrates (Theranostics 2021;11:3565)
- Nearly half of cases (44%) are grouped in the immune intermediate group with variable infiltrates of activated T cells and tumor associated macrophages
- 37% of cases are grouped in an immune rich subset characterized by higher frequencies of activated CD8 T cells, macrophages and Tregs, with a predominance of NFκB signaling, likely corresponding to patients with mutations in MYD88
- Third group consists of immune poor cases and is characterized by hyperactivation of WNT / β catenin, HIPPO and NOTCH signaling
- Frequent mechanism of immune evasion is the loss of expression of HLA-DR and HLA-DQ due to deletions and mitotic recombination (Blood 2000;96:3569)
Etiology
- Oncogenic toll-like receptor (TLR) and B cell receptor signaling are enriched due to mutations in the MYD88 and CD79B genes (Blood 2016;127:869, Leuk Lymphoma 2015;56:2141, Blood 2014;124:3896)
Clinical features
- Symptoms vary according to the site involved; the most common symptoms at presentation include cognitive impairment (74%) and balance disorders (59%) (J Neurooncol 2022;158:99)
- Headache and nausea / vomiting may also occur due to increased intracranial pressure (J Radiol Case Rep 2022;16:1)
- Patients with vitreoretinal involvement usually complain of floaters and painless loss of vision
- Most cases with vitreoretinal involvement have a bilateral presentation with multifocal, yellowish white, cream colored retinal lesions and substantial vitreous infiltration (Cancers (Basel) 2021;13:3921)
- Patients typically do not present with B symptoms (fever, night sweats, weight loss)
- Seizures are uncommon as the deeper brain structures are typically involved (Cancer 2017;123:4314)
Diagnosis
- Ophthalmic examination findings may include
- Vitreous infiltration by white cells, typically larger than those seen in inflammatory responses, on slit lamp examination, arranged in sheets or keratotic precipitates
- Aurora borealis sign may be seen in fundus photography; it is caused by the infiltration of vitreous fibrils by neoplastic lymphocytes and is characterized by a wavy appearance that resembles the northern lights in the sky
- Multiple creamy white to yellow-orange subretinal lesions are visualized on fundus photography or by other imaging modalities (see Clinical images)
- Stereotactic / open biopsy for brain parenchymal involvement or cerebrospinal fluid (CSF) in the case of leptomeningeal / spinal involvement (Neuro Oncol 2019;21:296)
- Molecular testing is important to determine the presence of clonal IGH gene rearrangements and testing for IL10 levels or MYD88 L265P mutations (Br J Haematol 2021;193:497)
- Positron emission tomography (PET) / computed tomography (CT) scan and bone marrow biopsy are currently recommended to exclude systemic involvement (Neuro Oncol 2019;21:296, Ann Hematol 2023;102:1897)
- Diagnostic work up for patients with primary vitreoretinal lymphoma includes the following (Ocul Immunol Inflamm 2021;29:507)
- Diagnostic vitrectomy is preferred to be obtained using a low cut rate (600 cuts per minute); the specimen should be immediately analyzed if possible (Int J Retina Vitreous 2018;4:18)
- Determine immunophenotype (flow cytometry or immunohistochemistry) with cell morphology and molecular testing for IGH gene clonal rearrangements
- Testing for IL10/IL6 ratio or MYD88 L265P is highly recommended; the presence of MYD88 L265P supports the presence of primary DLBCL of the vitreoretina in cases with paucicellular / poor quality specimens
- Imaging: B scan ultrasound, fundus fluorescein angiography, indocyanine green angiography, fundus autofluorescence or spectral domain optical coherence tomography
- Whole body PET / CT scan must be performed to exclude systemic involvement
Laboratory
- Serum lactate dehydrogenase (LDH) levels may be elevated (JAMA Neurol 2013;70:311, Blood 2011;118:510)
- At least 1 of the routine CSF indices (increased cell counts, low CSF glucose level or increased CSF protein) is abnormal in nearly 80% of cases; of interest, CSF is not obtained in many cases to avoid complications of elevated intracranial pressure (Neurology 2007;68:1674, Ann Neurol 1995;38:202, Leuk Res 2008;32:1196, Acta Neurol Scand 1992;86:129)
- Immunoglobulin index in CSF can be elevated in 20 - 29% of cases (oligoclonal bands in the CSF can be detected in 17 - 67% of patients) (Acta Neurol Scand 1992;86:129, Acta Neurol 1987;44:915)
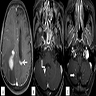
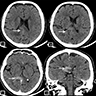
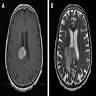
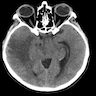
Radiology description
- Lesions typically involve the periventricular / deep white matter
- Magnetic resonance imaging (MRI): T1 weighted hypointense to isointense lesion, gadolinium contrast homogenous enhancement
- Immunocompetent patients usually do not have lesion associated hemorrhage, necrosis, calcifications or ring enhancement (Hematol Oncol Clin North Am 2019;33:597)
- CT: hyperdense, with contrast homogenous or heterogenous enhancement (J Radiol Case Rep 2022;16:1)
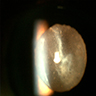
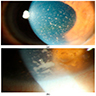
Radiology images
Images hosted on other servers:
Prognostic factors
- Overall 5 year survival rate is 30 - 40%, with a median overall survival of 16 months (Brain Behav 2018;8:e00928*)
- International Extranodal Lymphoma Study Group (IELSG) prognostic model includes 5 variables associated with adverse prognosis (J Clin Oncol 2003;21:266)
- Age > 60 years
- Involvement of the deep regions of the brain
- Elevated serum LDH
- Elevated CSF protein; this criterion is of limited use since CSF is not usually obtained because of increased intracranial pressure
- Performance status
- Memorial Sloan Kettering Cancer Center (MSKCC) prognostic model identifies 3 prognostic classes (J Clin Oncol 2006;24:5711, Brain Behav 2018;8:e00928*)
- Class 1 (age < 50 years)
- Class 2 (age ≥ 50 years, Karnofsky performance score ≥ 70)
- Class 3 (age ≥ 50 years, Karnofsky performance score < 70)
*This study did not identify immune status as an independent prognostic factor
Case reports
- 25 year old man with 1 week history of severe headaches and changes in vision (Can J Ophthalmol 2019;54:e134)
- 54 year old woman with symptoms of numbness and weakness in the right leg (BMC Neurol 2019;19:90)
- Man in his late 60s presented with 6 week history of decreased mood, decreased cognitive function and fatigue (J Neuropsychiatry Clin Neurosci 2022;34:84)
- 71 year old man presented with cerebellar symptoms (dizziness, nausea, vomiting and gait imbalance) (J Med Case Rep 2018;12:341)
- 75 year old woman presented with subacute progressive confusion and unusual behavior (Neurooncol Pract 2017;4:46)
Treatment
- There is no standard treatment in cases with primary brain involvement; it varies by institution
- Initial treatment includes corticosteroids followed by high dose methotrexate (MTX), rituximab and additional chemotherapeutic agents
- Ibrutinib resistance may be seen in tumors with CARD11 coiled coil domain mutations (Cancer Discov 2017;7:1018)
- There is no consensus in management for cases with primary vitreoretinal involvement; current treatment options include high dose MTX, systemic chemotherapy or localized therapy (intravitreal MTX, rituximab or ocular external beam radiotherapy) (Neuro Oncol 2023;25:37)
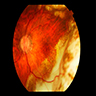
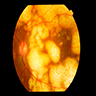
Clinical images
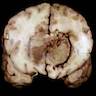
Gross description
- Variable appearance
- Brown to gray-tan to yellow
- Firm and homogenous with central necrosis and areas of hemorrhage
- Variable demarcation from the surrounding brain parenchyma
- Reference: Arch Pathol Lab Med 2008;132:1830
Frozen section description
- Histologic features in frozen section are like those observed in permanent sections
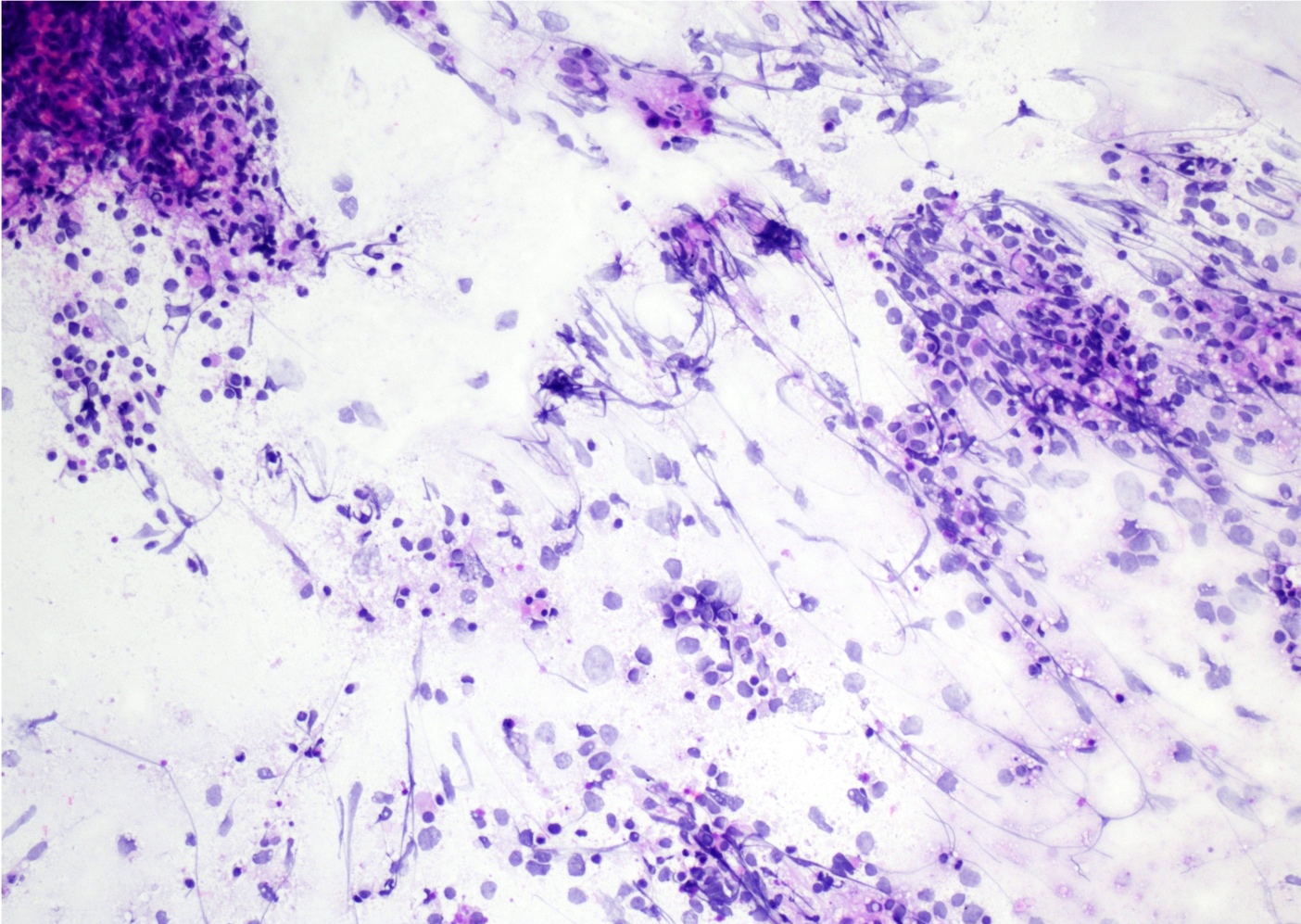
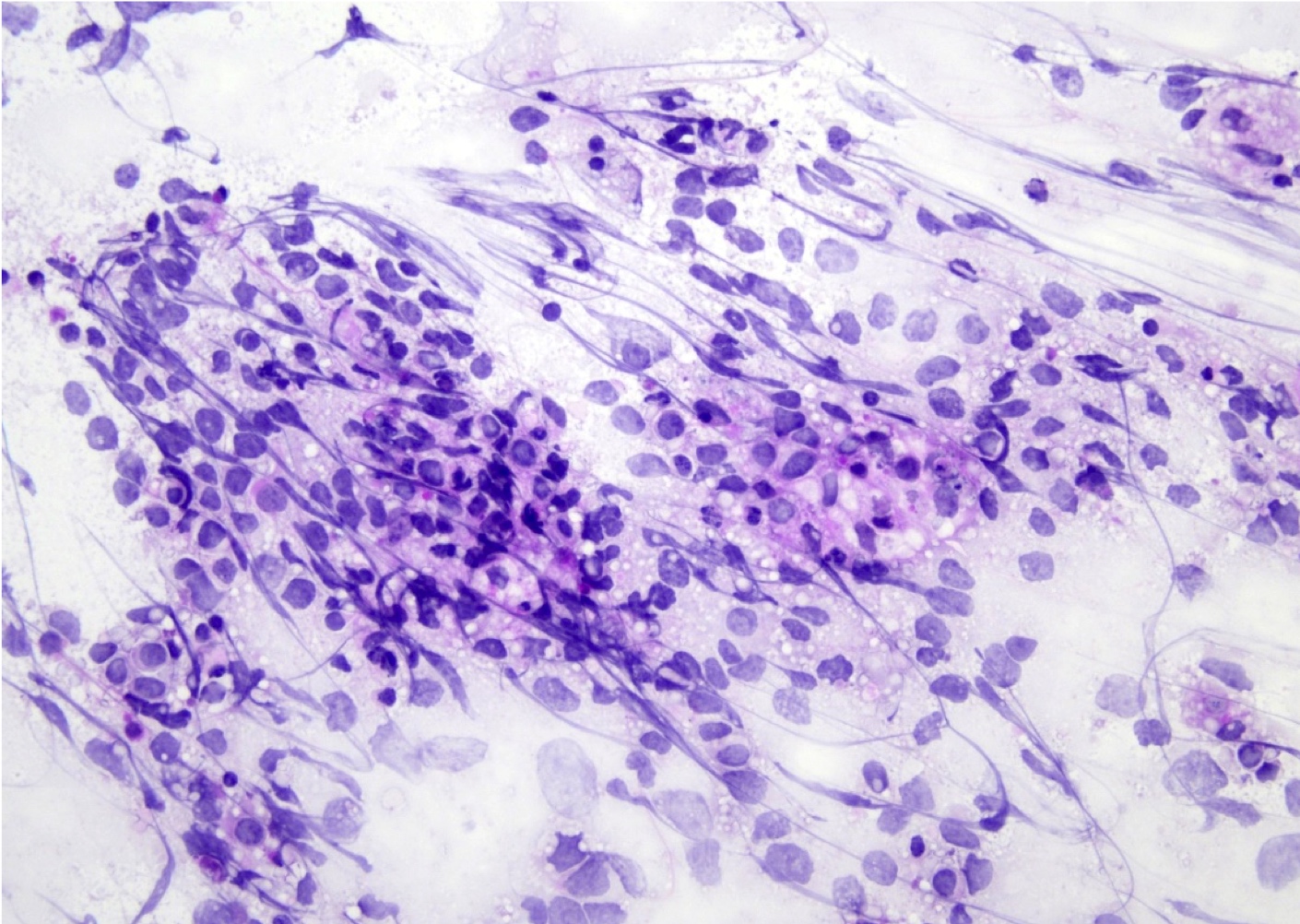
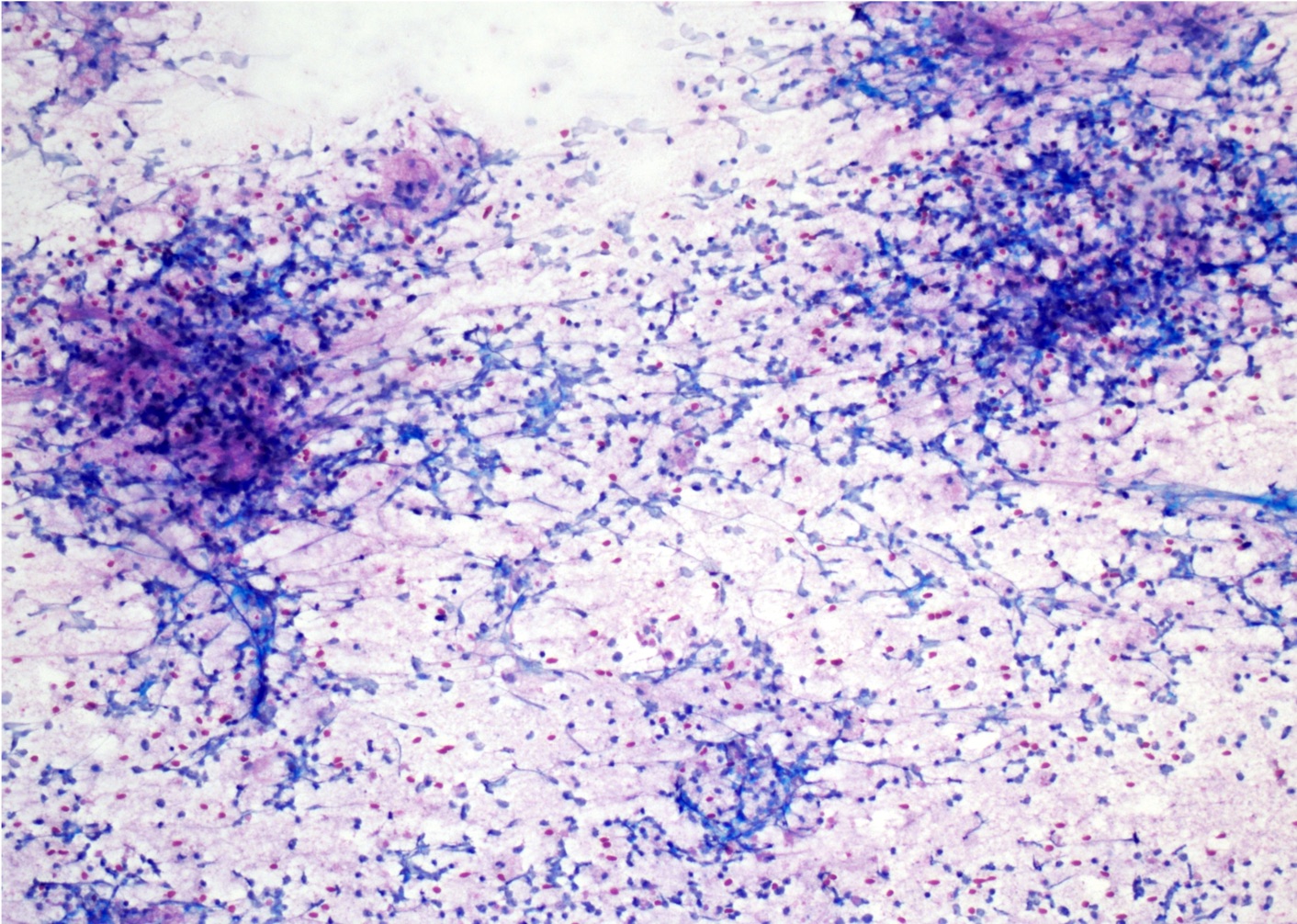
- Cytology evaluation via touch preps / squash preps is also essential to intraoperative evaluation (see Microscopic (histologic) images)
Intraoperative frozen / smear cytology images
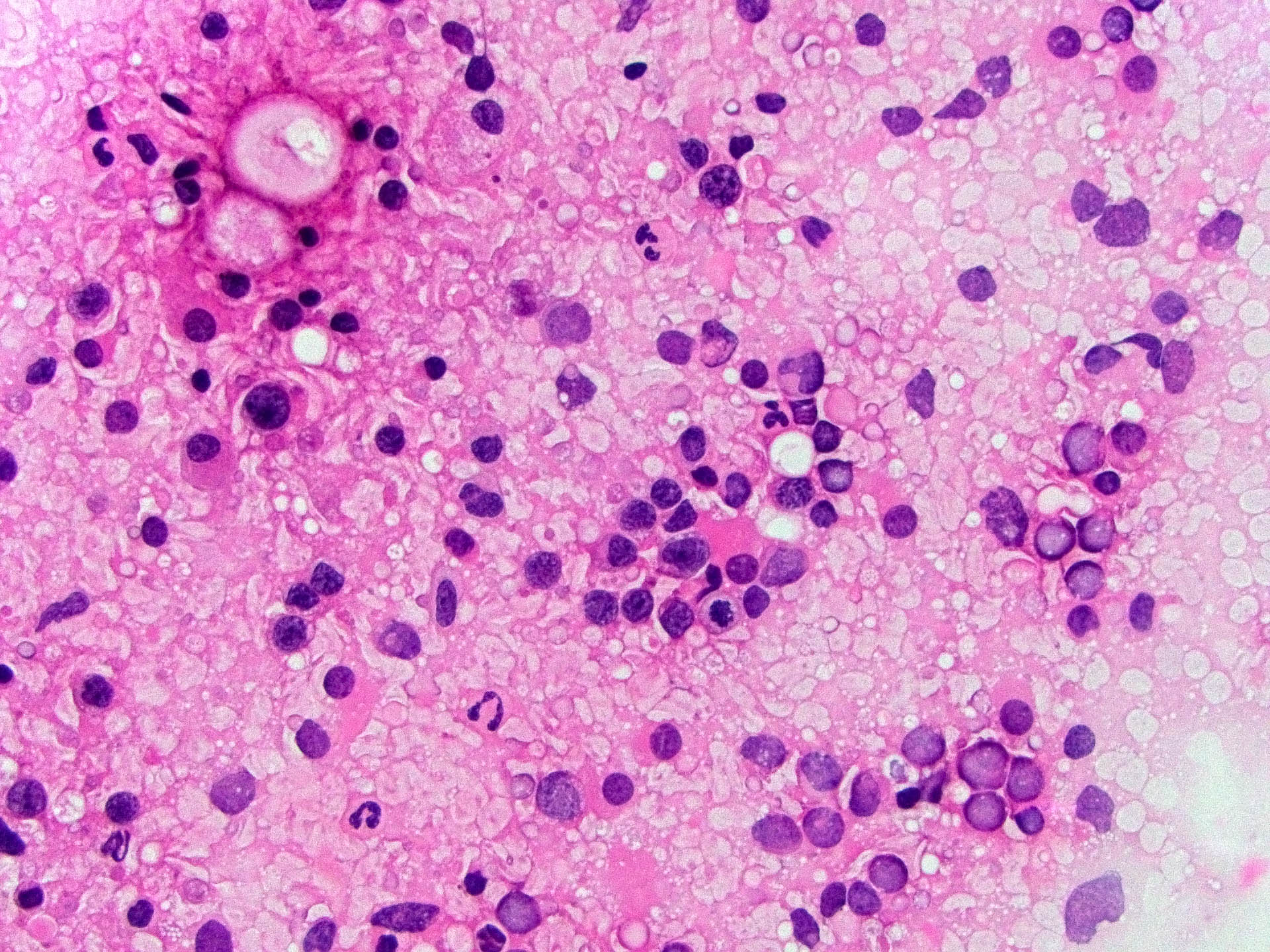
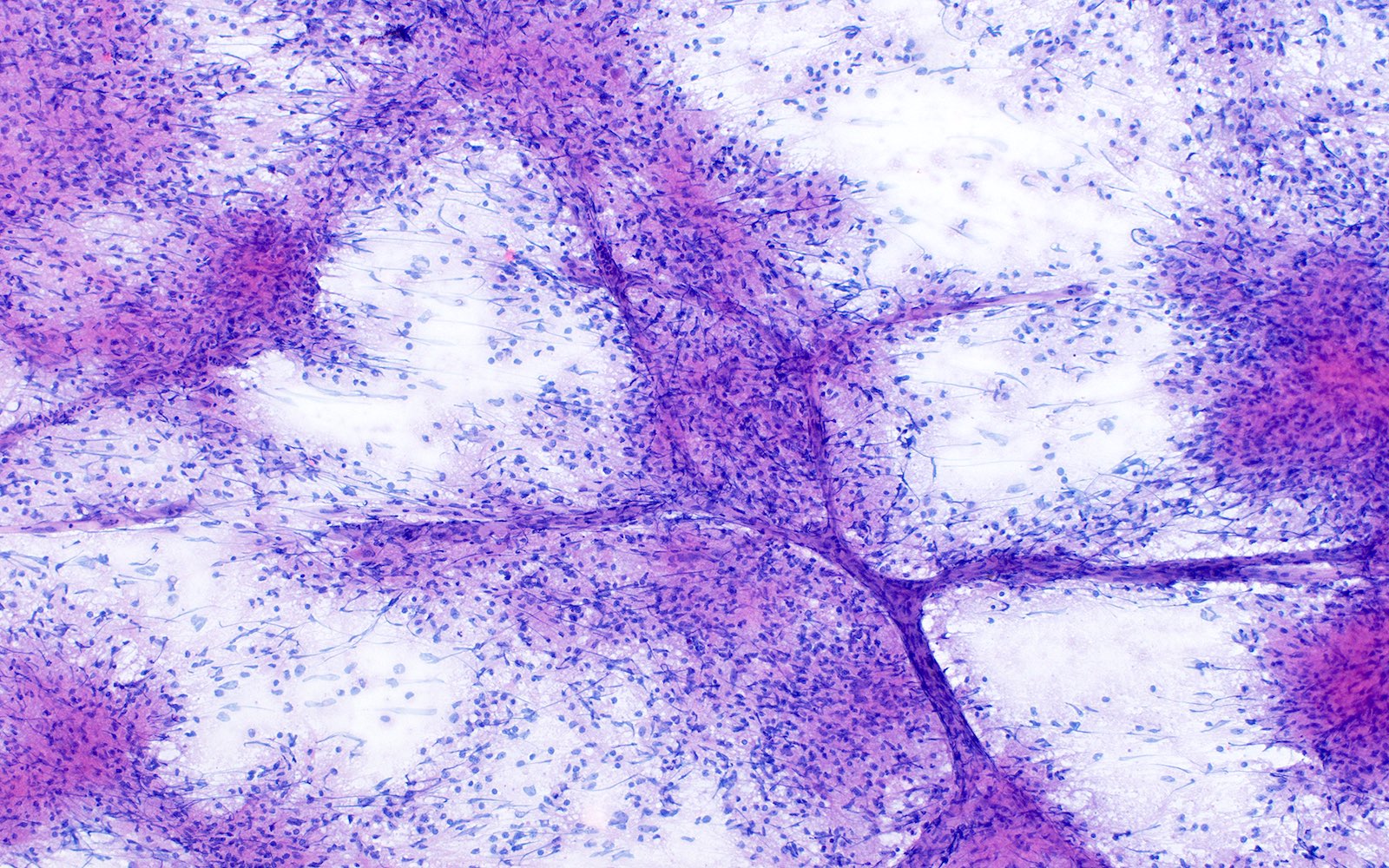
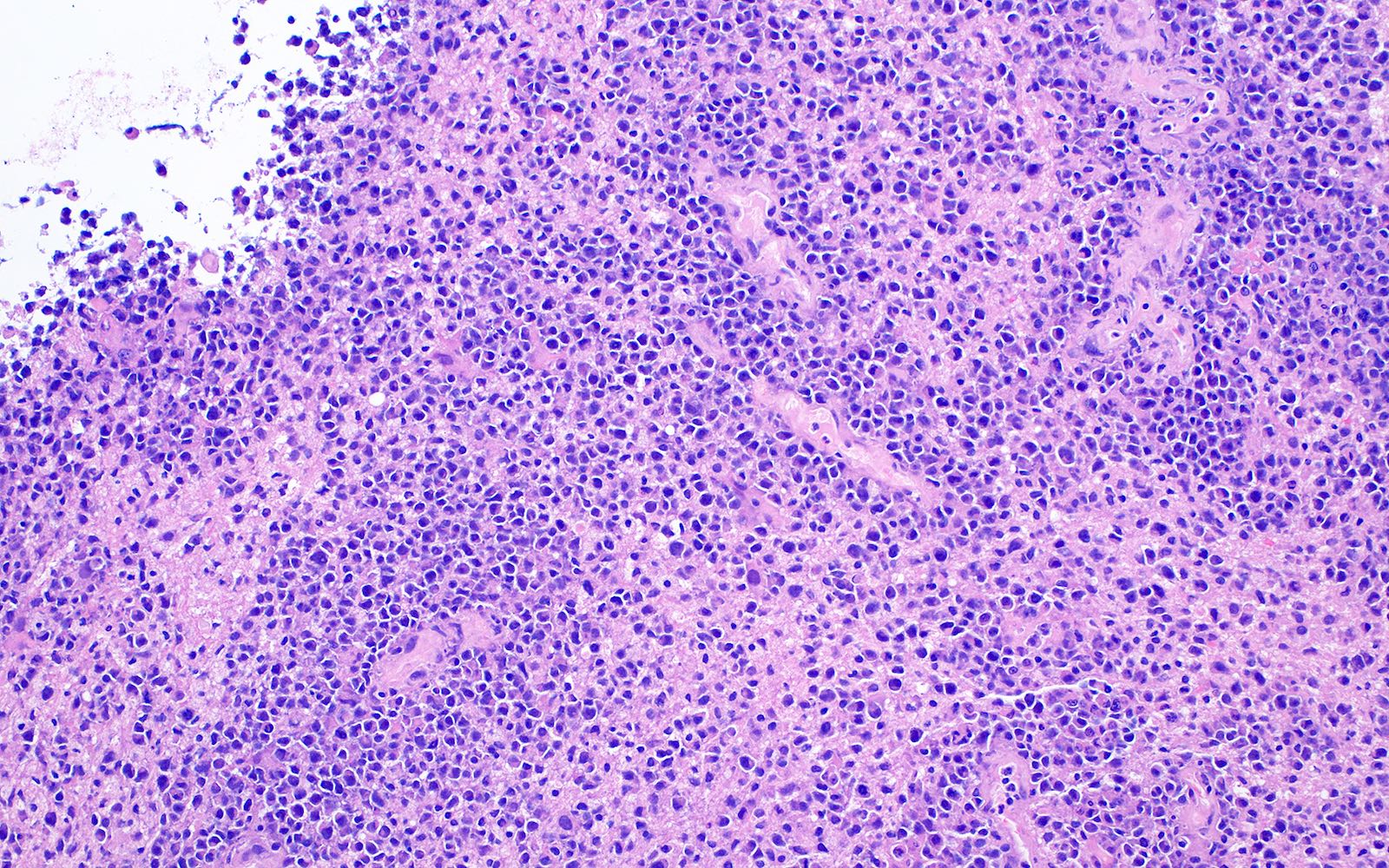
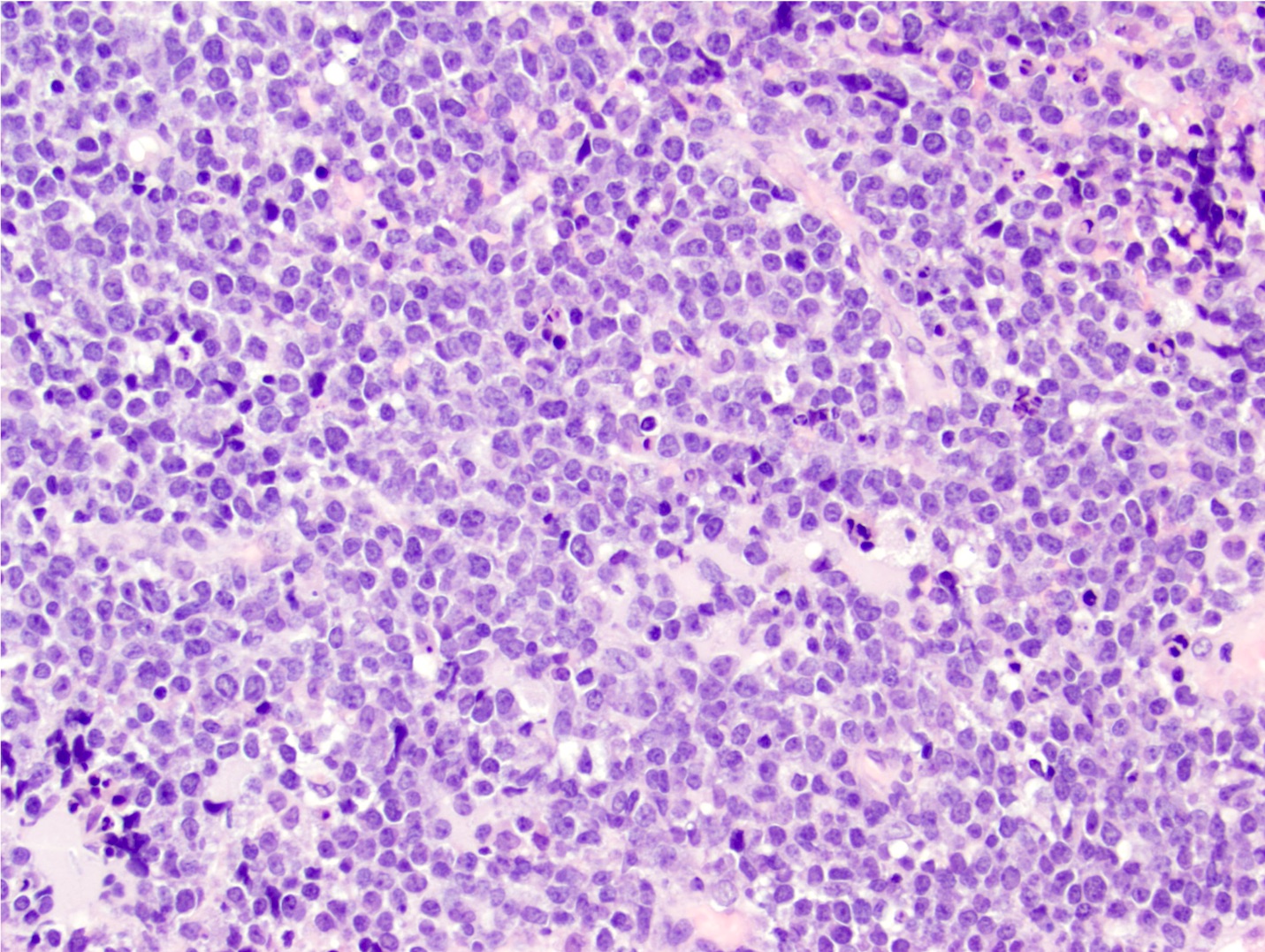
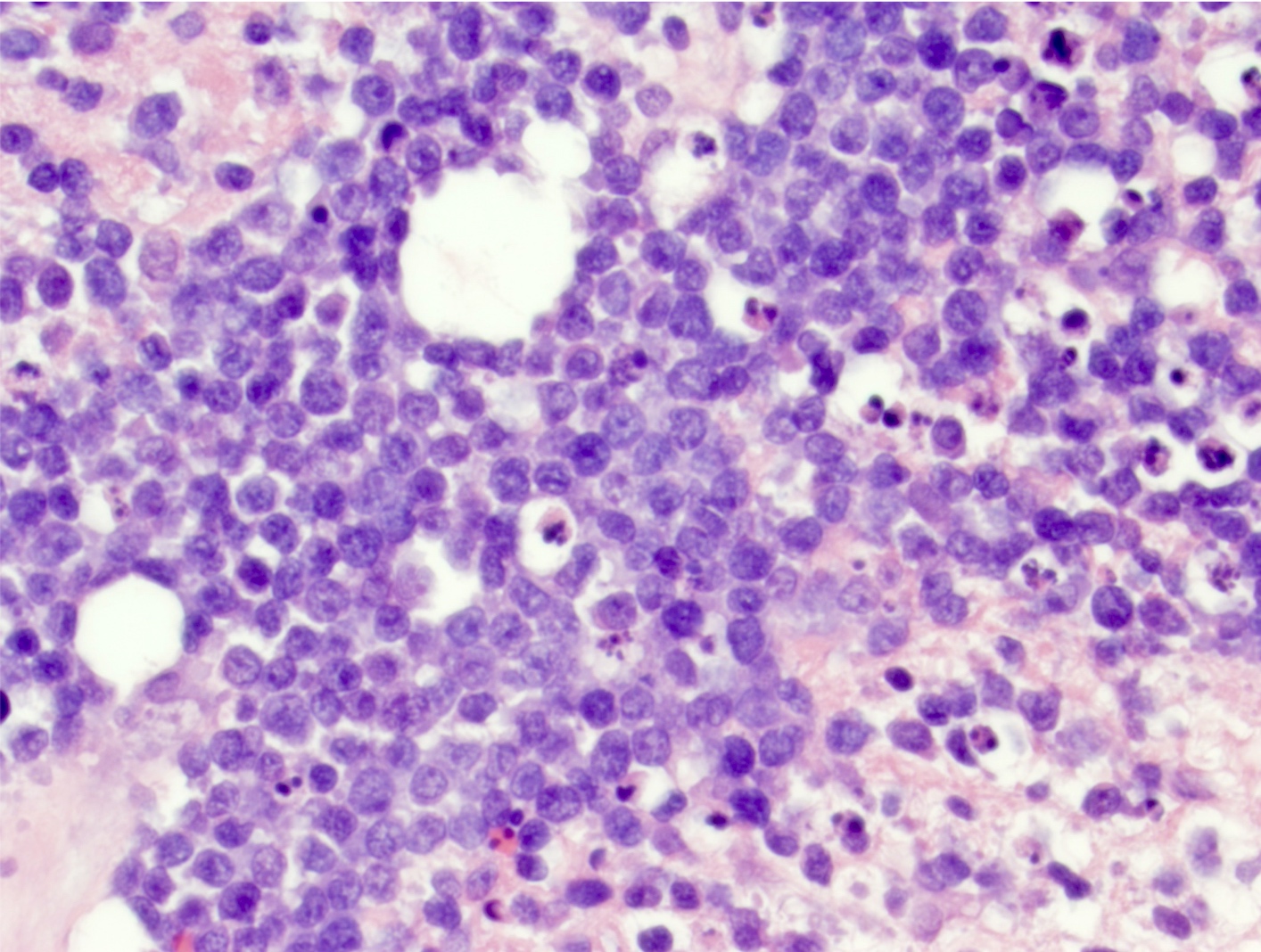
Microscopic (histologic) description
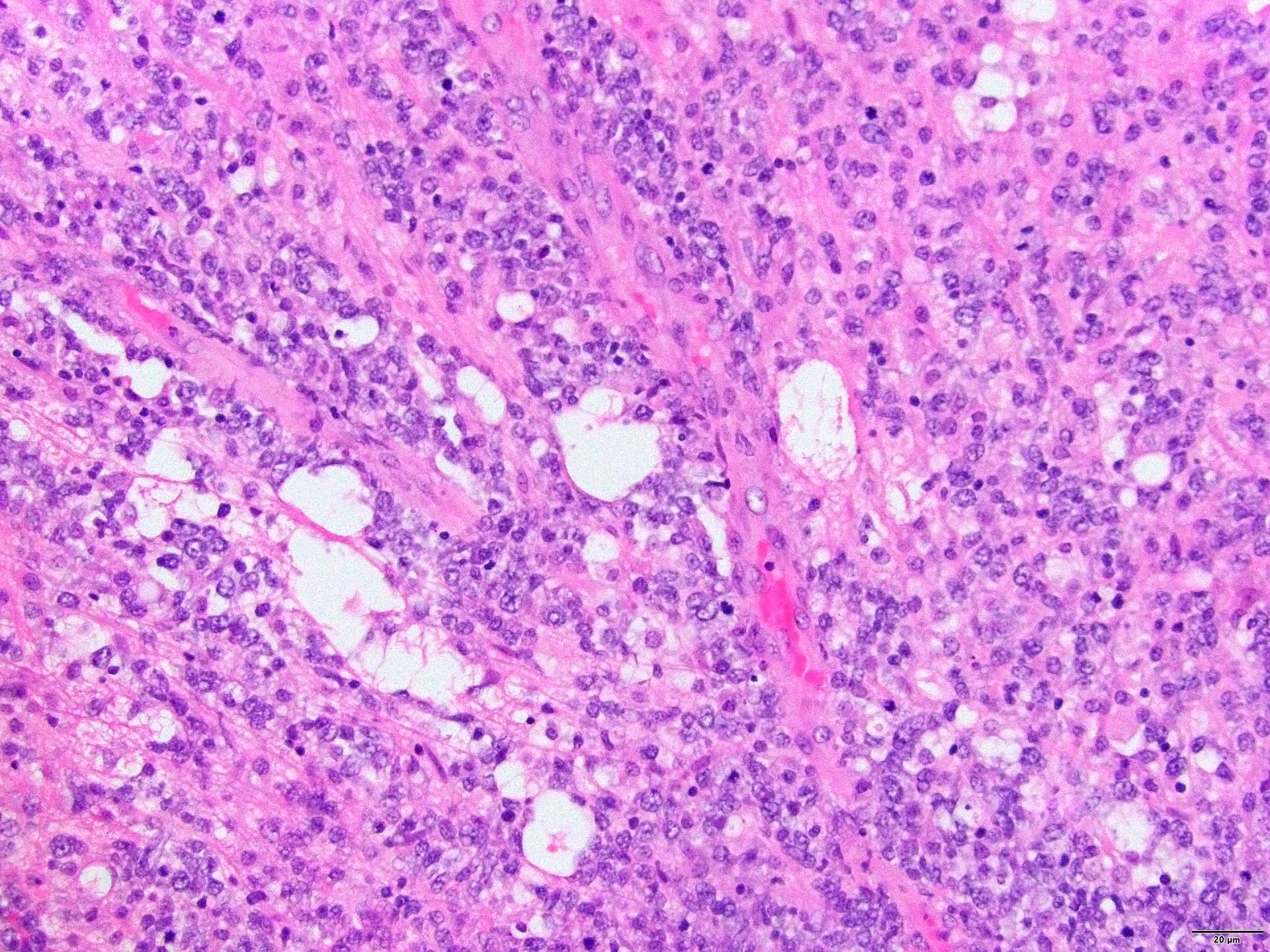
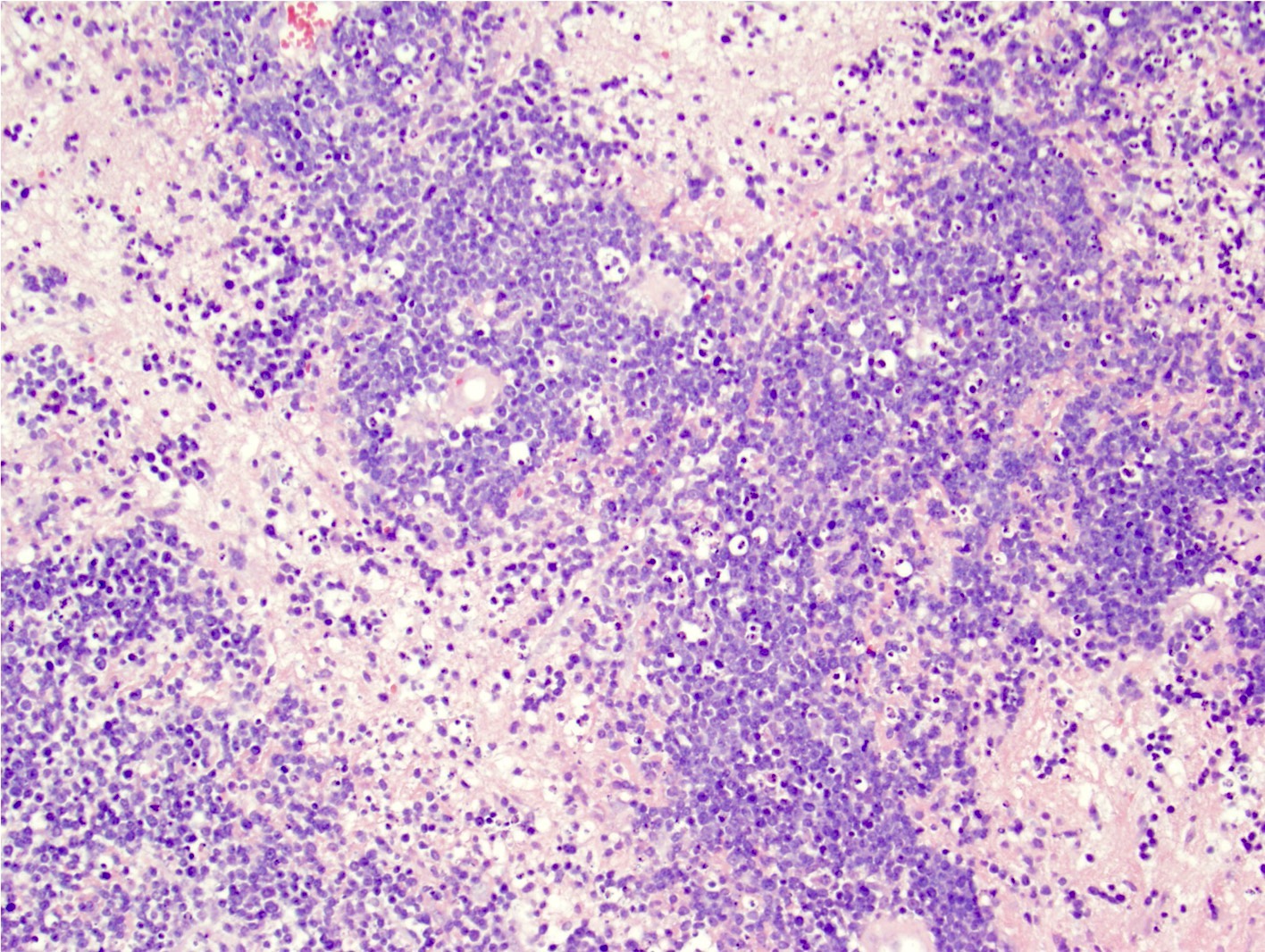
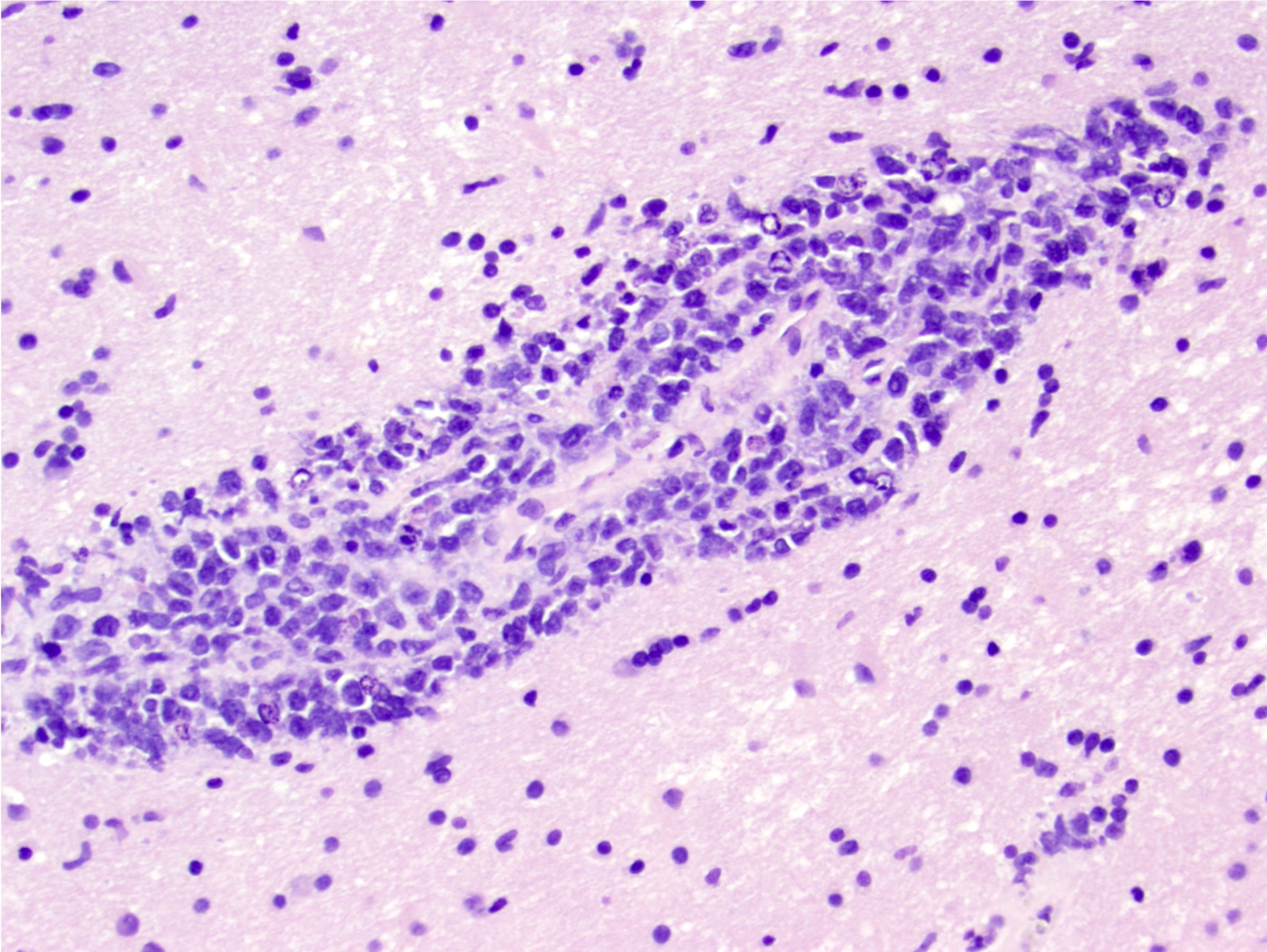
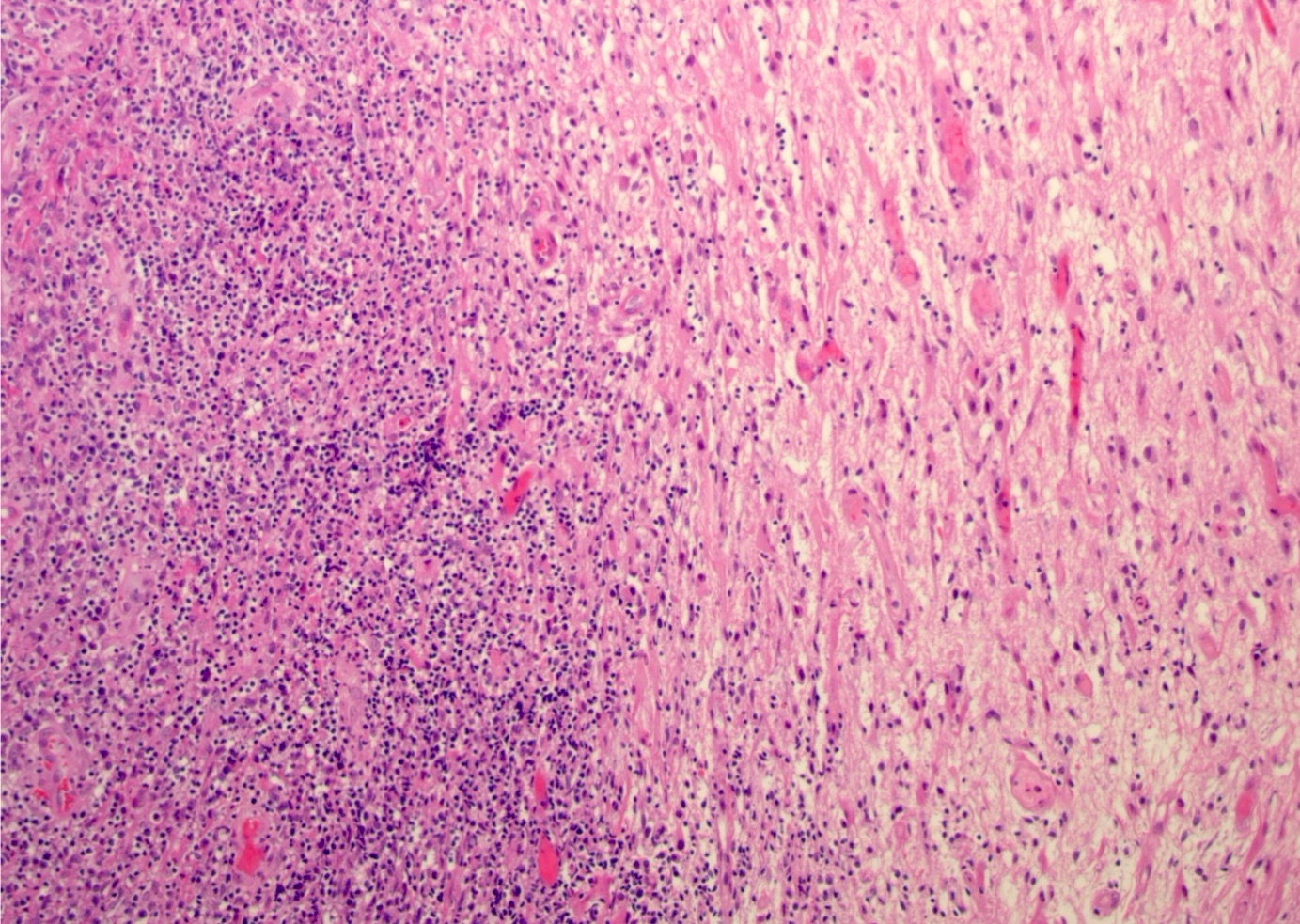
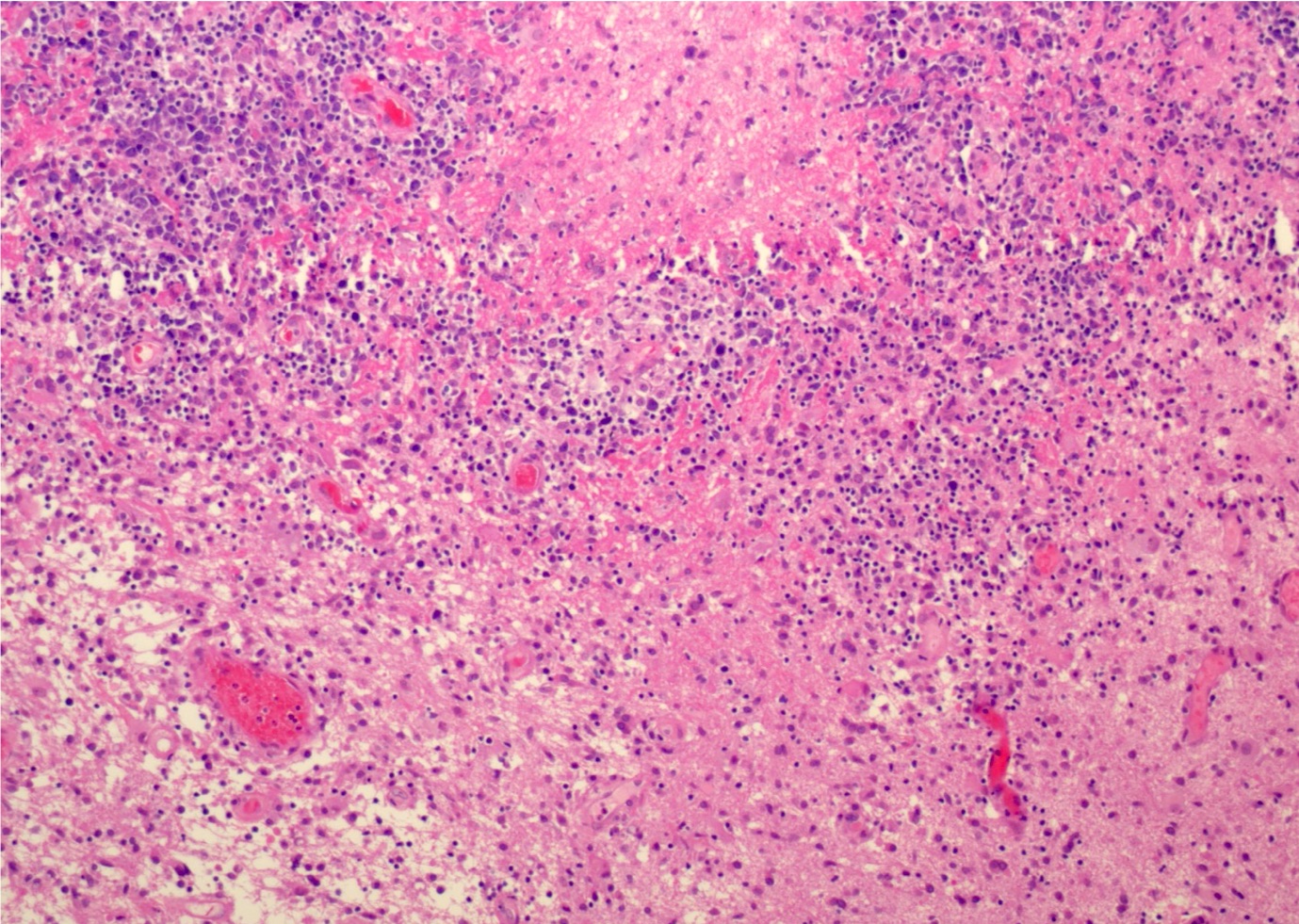
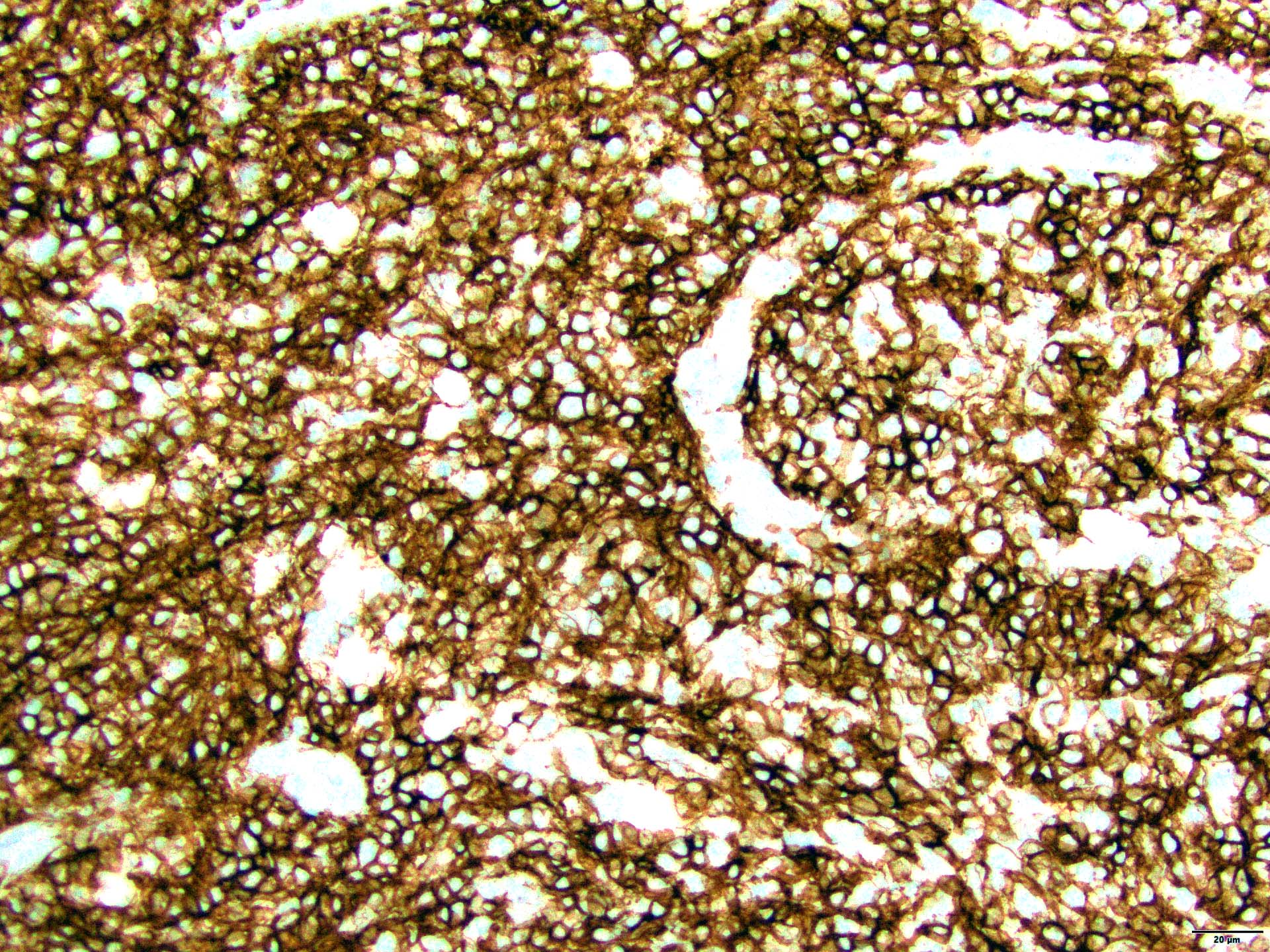
- Characteristic angiocentric growth pattern with cuffs of neoplastic lymphocytes (Arch Pathol Lab Med 2008;132:1830)
- This pattern may not be as readily apparent in cases or areas with extensive and hypercellular involvement
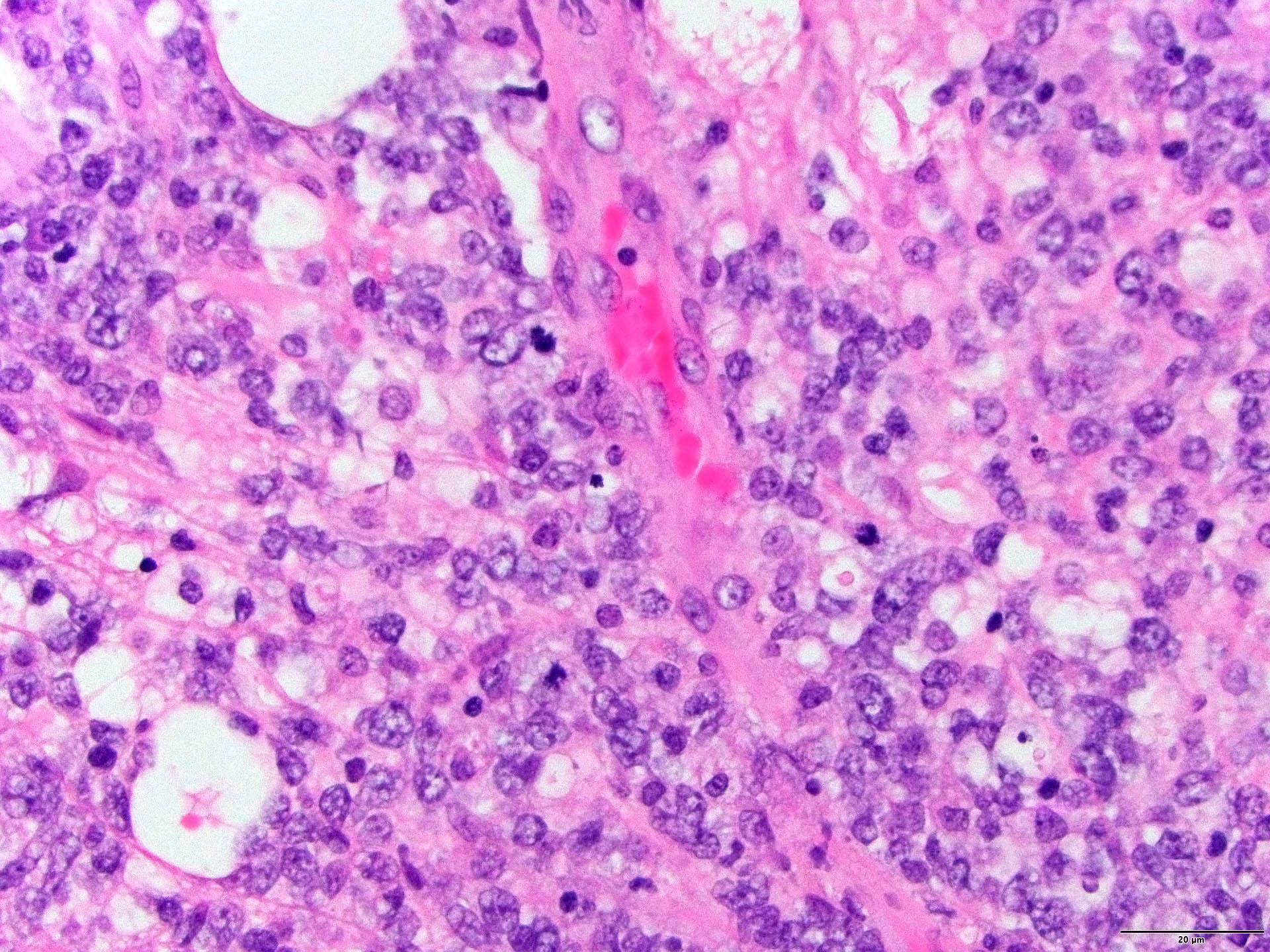
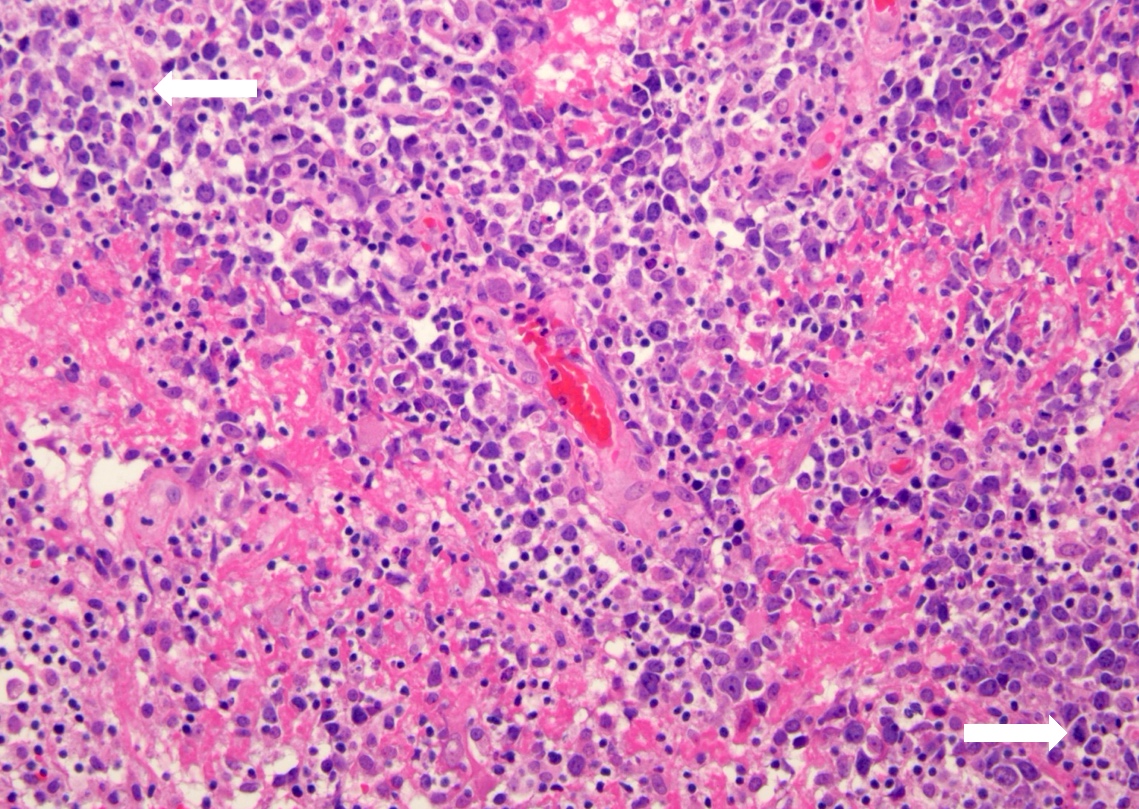
- Diffuse infiltration of brain parenchyma singly or in small clusters; necrosis is common
- Accompanied by activated astrocytes and microglia; reactive inflammatory infiltrate may also be present
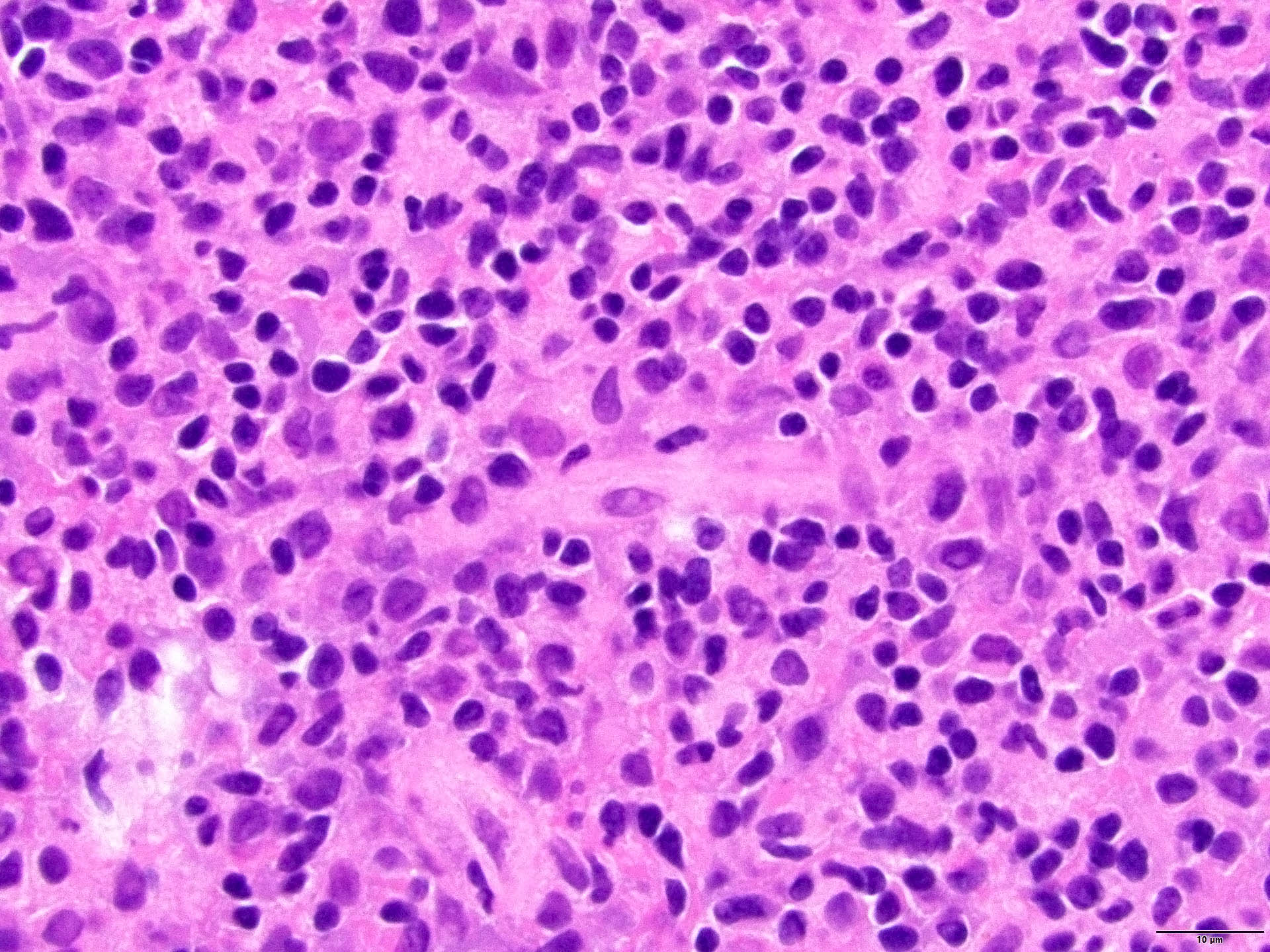
- Neoplastic cells are medium to large lymphocytes, frequently displaying vesicular chromatin and prominent nucleoli
- Corticosteroid administration before biopsy is common for this disease and may induce dramatic tumor shrinkage and cause tumor areas to appear hypocellular with increased cellular debris and macrophages
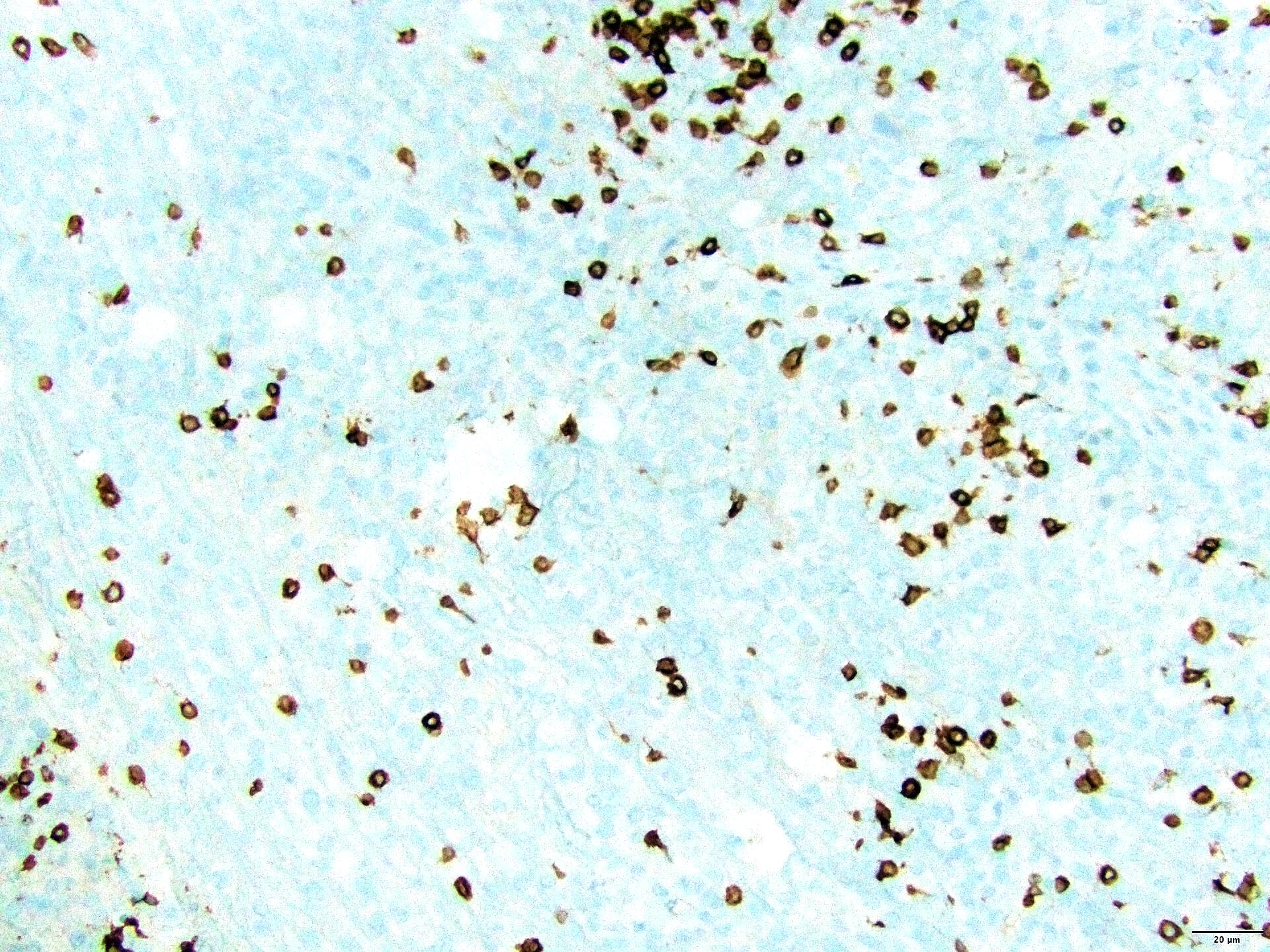
Microscopic (histologic) images
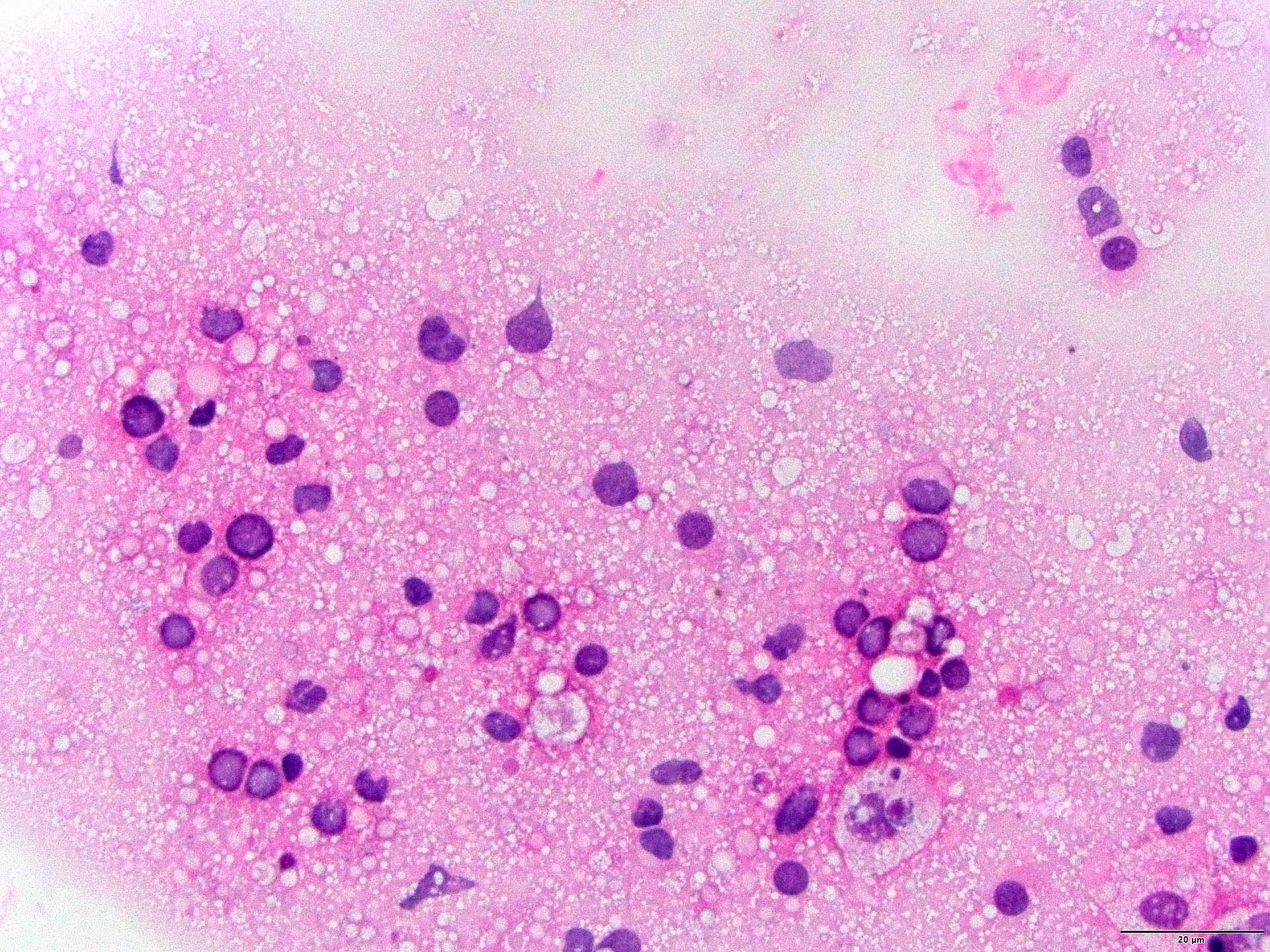
Cytology description
- Cerebrospinal fluid (CSF) cytology: medium to large neoplastic lymphoid cells that frequently show irregular nuclear contours, abnormal chromatin and prominent nucleoli with background reactive lymphocytes
- Squash preparation: discohesive groups of monomorphic, medium to large basophilic lymphoid cells with prominent smearing artifacts and background reactive glial cells
- Touch preparation: discohesive, medium to large, atypical basophilic cells with a high N:C ratio and open chromatin with immature (lymphoblast) features; in posttreatment tumors, only necrotic cells or reactive T cells may remain (Asian J Neurosurg 2013;8:195)
- Examination of the vitreous fluid to detect primary vitreoretinal lymphoma presents a challenge as specimens are often paucicellular and have poor preservation of the cells present
Positive stains
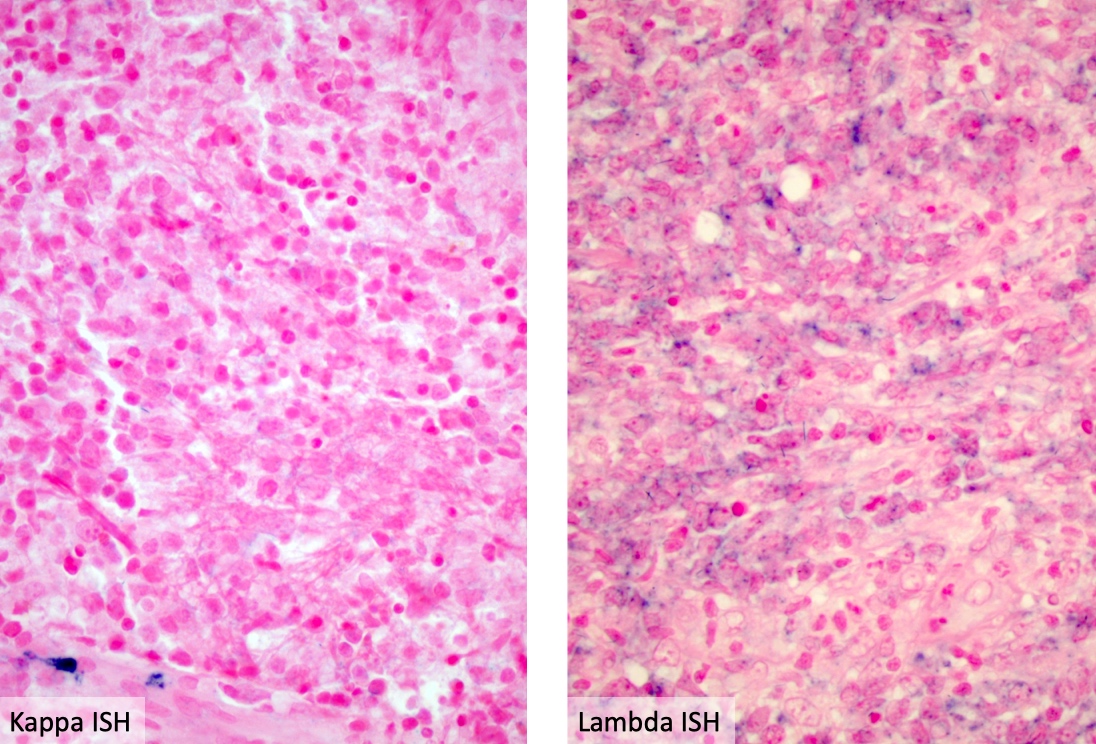
- PAX5, CD19, CD20, CD22, CD79a (B cell markers)
- BCL2 (90%)
- BCL6 (60 - 80%)
- MUM1 / IRF4 (60 - 80%)
- MYC (92%)
- Ki67 (MIB1) index (40 - 90%) (Arch Pathol Lab Med 2008;132:1830, Hematol Oncol 2014;32:57, Acta Neuropathol 2013;126:603)
Negative stains
- EBV positivity suggests underlying immunodeficiency
- CD10: < 10% of primary DLBCL of the CNS are positive; CD10 positivity is seen more frequently in systemic DLBCL and should prompt a careful search for systemic disease (Hematol Oncol 2014;32:57)
- CD38, CD138 (plasma cell markers)
- Pertinent negative stains also include ALK1, CD30 and HHV8
Flow cytometry description
- Brain biopsies
- Tissue biopsy for flow cytometric analysis can be challenging in obtaining interpretable results due to debris following processing or poor cell yield
- As typically seen with primary DLBCL of the CNS, these specimens are CD19+ / CD20+, light chain restricted with coexpression of CD27 and CD38, a variable expression of CD5 and CD10 negative
- Vitreoretinal specimens
- Analysis of vitreoretinal specimens can be challenging due to poor cellular preservation and typically paucicellular yield
- Vitreous fluid should be processed promptly as the neoplastic cells begin to degrade within 60 minutes
- If this cannot be done, placement of the specimen into an appropriate preservative, such as PreservCyt, is necessary (Ocul Immunol Inflamm 2021;29:430)
- If flow cytometric analysis is successful, a CD19+ / CD20+ light chain restricted B cell population can be identified (Front Mol Biosci 2021;7:611017)
- CSF
- Analysis of CSF can be challenging due to poor cellular preservation and typically paucicellular yield; ideally, ~10.5 mL or more should be available for analysis
- Cells may begin to degrade within 30 minutes of collection and analysis should be performed within 60 minutes; adding media, such as Roswell Park Memorial Institute (RPMI) medium, may help extend the flow cytometric analysis window (Int J Lab Hematol 2022;44:45)
Molecular / cytogenetics description
- MYD88 L265P mutation is the most common variant seen in primary DLBCL of the CNS (Lancet Haematol 2024;11:e540)
- Mutations in genes in the NFκB pathway (CARD11, CD79B, MYD88 and TNFAIP3) have also been reported (Clin Cancer Res 2015;21:3986, Oncologist 2023;28:e26, Medicine (Baltimore) 2023;102:e34931, Blood 2016;127:869, Nat Med 2018;24:679)
- Most common chromosomal abnormalities identified are
- Loss: 6p21.32 (most common), 6q14.1-q16.3, 6q16.3, 6q21.1-q25
- Gain: 12q12-q22, 7q21.11-q21.12, 7q31.1-q31.2 (Clin Cancer Res 2012;18:5203, Blood 2016;127:869)
Sample pathology report
- Brain mass, biopsy:
- Diffuse large B cell lymphoma (see comment)
- Comment: In the absence of systemic disease, the morphologic and immunophenotypic findings are consistent with a diagnosis of primary diffuse large B cell lymphoma of the central nervous system. The WHO 5th edition (2023) classifies this lymphoma under the umbrella group denominated as primary LBCL of immune privileged sites. Clinical correlation is required, such as excluding an immunodeficiency associated lymphoproliferative disorder.
Differential diagnosis
- Systemic malignant lymphoma (i.e., diffuse large B cell lymphoma) involving the CNS:
- CD10 expression should raise suspicion for secondary CNS lymphoma
- Imaging studies are required to exclude the presence of systemic lymphoma with secondary CNS involvement
- Lymphoma associated with HIV infection:
- EBV positive
- Immunodeficiency associated CNS lymphoma
- Posttransplant lymphoproliferative disorder (PTLD):
- EBV positive
- History of immunosuppression after allograft
- High grade glioma:
- Lacks the diffuse monomorphic lymphocyte infiltrate
- Negative for CD45 and B cell markers
- Intravascular large B cell lymphoma:
- Typically involves the skin, CNS and hematopoietic system
- Proliferation of neoplastic cells, especially in capillaries and postcapillary venules with no parenchymal involvement
- Germinoma:
- Involves midline structures (pineal gland, suprasellar, sellar)
- More common in younger patients
- Large discohesive cells distributed singly, in lobules or in sheets
- Accompanied by chronic inflammation, sometimes with noncaseating granulomas
- Neoplastic cells are positive for PLAP, CD117 and OCT4 and negative for CD45
- Lymphomatoid granulomatosis:
- Extranodal; usually presents with pulmonary involvement
- EBV positive B cells with reactive T cells
- Angiocentric and destructive disease
Additional references
Board review style question #1
A 60 year old woman presents with progressive confusion and lethargy. Clinical history and laboratory testing show no evidence of immunosuppression or prior surgeries. Imaging shows a solitary lesion in the parietal lobe. A brain biopsy is performed and intraoperative touch preparations show discohesive, monomorphic, basophilic cells with extensive crushed artifacts. Permanent sections show perivascular cuffing by intermediate to large mononuclear cells with scant cytoplasm, vesicular chromatin and prominent nucleoli. By immunohistochemical stains, the mononuclear cells are positive for CD20, PAX5, CD79a, BCL6 and MUM1 and negative for CD10 and EBV. Extensive additional imaging studies revealed no lymphadenopathy or suspicious lesions in other body organs. What is the most likely diagnosis?
- Glioblastoma multiforme
- Intravascular large B cell lymphoma
- Lymphomatoid granulomatosis
- Posttransplant lymphoproliferative disorder
- Primary central nervous system lymphoma
Board review style answer #1
E. Primary central nervous system lymphoma. This entity is positive for B cell markers and is primarily localized in the brain parenchyma in immunocompetent patients. Answer A is incorrect because glioblastoma multiforme is negative for B cell markers (CD20, PAX5 and CD79a) and positive for GFAP and S100. Answer B is incorrect because the neoplastic B cells are limited to the vascular space in intravascular large B cell lymphoma. Answer C is incorrect because lymphomatoid granulomatosis is usually primarily localized in the lung with secondary brain involvement (in a subset of cases) and the neoplastic cells are EBV positive. Answer D is incorrect because the patient doesn't have a solid organ transplant clinical history.
Comment Here
Reference: Primary CNS lymphoma
Comment Here
Reference: Primary CNS lymphoma
Board review style question #2
A 63 year old woman recently presented to the ophthalmology clinic due to concern for painless, progressive vision loss and floaters in her right eye, then her left eye, over 2 weeks. Ophthalmologic examination reveals multiple yellowish white, cream colored lesions of the retinas bilaterally. MRI of head and orbit support localized vitreoretinal lesions. Vitreous fluid is analyzed by cytology; unfortunately, the specimen is paucicellular with few degenerated lymphocytes. Which test should be performed next to help support the most likely diagnosis?
- Bone marrow biopsy
- Chromosomal analysis
- Obtain another specimen
- Testing for MYD88 mutation
Board review style answer #2
D. Testing for MYD88 mutation. MYD88 L265P, seen in ~69% of cases of primary diffuse large B cell lymphoma (DLBCL) of the vitreoretinal region, would support the diagnosis, specifically in cases of suboptimal cytologic specimens. Answer B is incorrect because cytogenetic aberrancies (although present) are not as frequent as the MYD88 L265P mutation. Answer C is incorrect because obtaining another specimen would be difficult and will likely demonstrate the exact yield as the original specimen. Answer A is incorrect because a bone marrow biopsy will not demonstrate primary CNS DLBCL (vitreoretinal) involvement.
Comment Here
Reference: Primary CNS lymphoma
Comment Here
Reference: Primary CNS lymphoma