Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Flow cytometry description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Aqil B. Plasmacytoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lymphomaplasmacytoma.html. Accessed March 30th, 2025.
Definition / general
- Solitary plasma cell neoplasm without the presence of multiple myeloma (MM) or features of end organ damage (CRAB: hypercalcemia, renal insufficiency, anemia and bone lesion)
Essential features
- 2 types: solitary plasmacytoma of bone (SPB) and extramedullary plasmacytoma (EMP)
- EMP commonly involves upper respiratory tract (nasal cavity, paranasal sinuses and nasopharynx)
- Positive stains include CD38, CD138 and MUM1
- Expression of cyclin D1 and CD56 in EMP is noted in older individuals
- Patients with SPB with minimal bone marrow involvement (< 10%) are more likely to develop multiple myeloma than those with no clonal plasma cells by flow cytometry or immunohistochemistry
- Plasmablastic morphology has been noted to have aggressive behavior (Ann Diagn Pathol 2015;19:117)
ICD coding
- ICD-11: 2A83.2 & XH4BL1 - solitary plasmacytoma & plasmacytoma, NOS
Epidemiology
- Occurs in older individuals (median age of 55 - 65 years) (ScientificWorldJournal 2012;2012:895765, BMC Cancer 2017;17:13)
- M > F (Cancer Med 2021;10:386, ScientificWorldJournal 2012;2012:895765, BMC Cancer 2017;17:13)
- SPB accounts for ~4 - 5% and EMP comprises 1 - 3% of plasma cell neoplasms (J Hematol Oncol 2018;11:10, Eur J Haematol 2017;99:216)
- Special form of EMP with IgA expression presents in lymph nodes of the head and neck region and occurs in younger patients with various forms of immune dysregulation
Sites
- SPB involves spine, pelvis, ribs, skull and long bones (Am J Hematol 2012;87:647, Ann Hematol 2012;91:1785, Cancer Med 2021;10:462)
- EMP most commonly affects upper respiratory tract and less commonly lungs, gastrointestinal tract, lymph nodes, skin and genitourinary tract (Cancer 1999;85:2305, J Pathol 2005;205:92, Clin Med (Lond) 2020;20:e191, J Cancer Res Clin Oncol 2021;147:1773, Am J Clin Pathol 2001;115:119, BMC Cancer 2017;17:13)
Pathophysiology
- Cytogenetically, EMP and multiple myeloma are closely related; however, the distribution of IGH translocation partners is different, with the notable absence of t(11;14) in EMP (Haematologica 2008;93:623, Am J Dermatopathol 2013;35:357)
Etiology
- Hypothesis behind its occurrence is chronic antigenic exposure
- It is also known to occur in patients with immunodeficiency or immune dysregulation (Am J Clin Pathol 2017;147:129)
Clinical features
- Depending on the location, the presenting features vary; features include pain, swelling, headache, dysphagia and fracture, vary (Ann Hematol 2012;91:1785)
- Some cases may have an associated paraneoplastic syndrome (Lancet 2020;396:e21, Am J Hematol 2021;96:872)
- TEMPI syndrome (telangiectasia, elevated erythropoietin and erythrocytosis, monoclonal gammopathy, perinephric fluid collection and intrapulmonary shunting)
- POEMS (polyneuropathy, organomegaly, endocrinopathy, myeloma protein and skin changes)
- AESOP syndrome (adenopathy and extensive skin patch overlying plasmacytoma)
Diagnosis
- Clonal plasma cells without associated monotypic B cells in a biopsy demonstrating involvement of bone or extramedullary site
- No other lesions detected on imaging studies
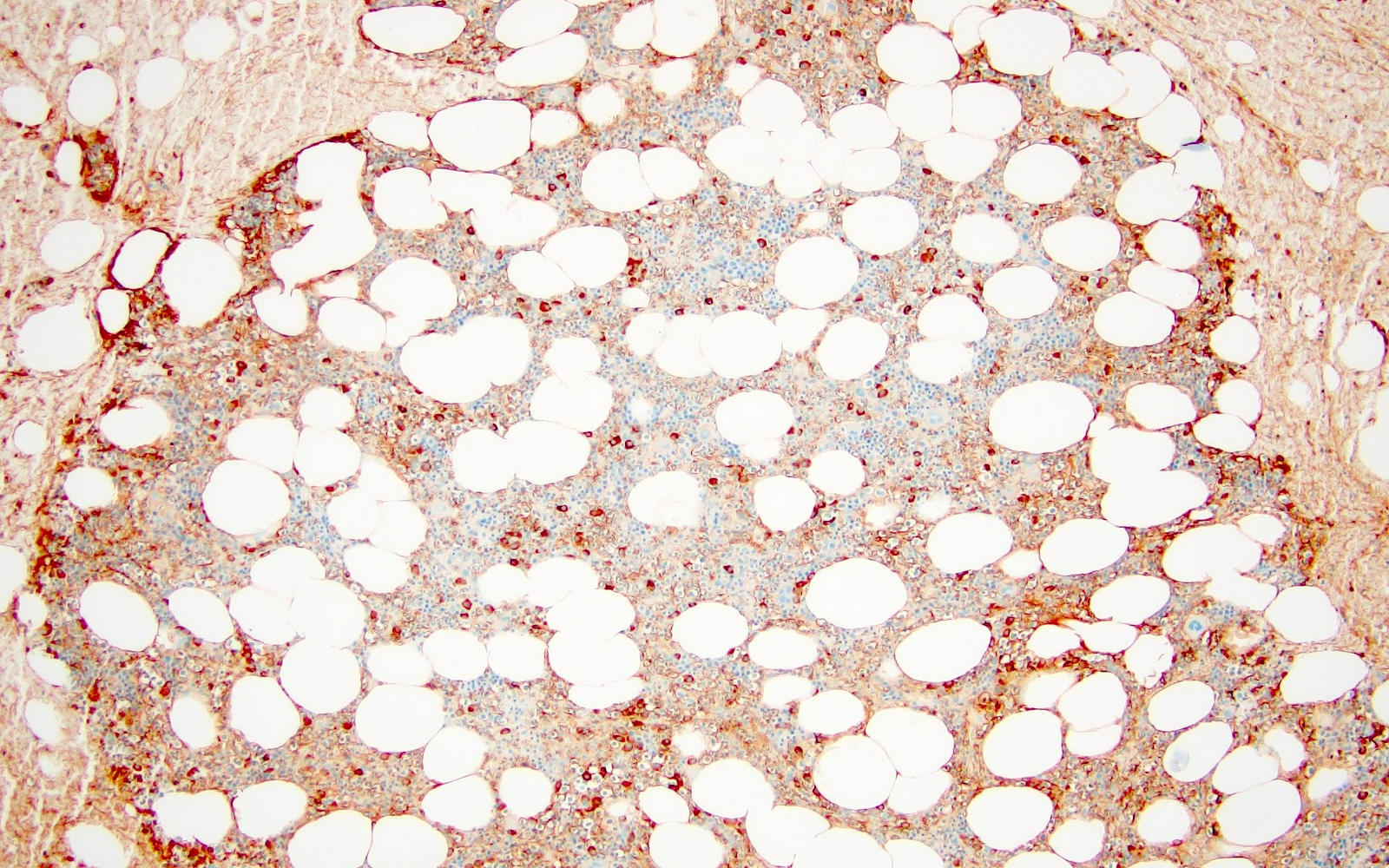
- < 10% monotypic plasma cells on bone marrow biopsy
- No evidence of end organ damage (CRAB)
- References: Mediterr J Hematol Infect Dis 2017;9:e2017052, Lancet Oncol 2014;15:e538
Laboratory
- Detection of serum or urine paraprotein on electrophoresis / immunofixation
- Increased free kappa or lambda light chains
- Most common paraproteins include IgG, IgA and light chains (Bence-Jones protein) (Ann Hematol 2012;91:1785, Int J Radiat Oncol Biol Phys 2020;106:589, Clin Med (Lond) 2020;20:e191)
- Elevated β2 microglobulin
- Evaluation to rule out multiple myeloma: serum calcium, creatinine and bone marrow biopsy
Radiology description
- Imaging shows solitary lytic bone lesion (in SPB) or an extramedullary mass (in EMP)
Prognostic factors
- Cytogenetic abnormalities similar to multiple myeloma are seen in plasmacytoma but they do not have much prognostic significance
- Progression risk to multiple myeloma is higher for SPB (60 - 85% after 10 years) than for EMP (12 - 35% after 10 years) but the presence of a minimal infiltrate of clonal plasma cells (< 10%) in the bone marrow (BM) as detected by flow cytometry or immunohistochemistry has a strong association (Blood 2014;124:1300, Blood 2014;124:1296, Lancet Oncol 2014;15:e538)
- 3 year progression rate to multiple myeloma is ~60% for SPB with the presence of clonal BM plasma cells versus 6 - 12% without, compared to ~20% for EMP with and 6% without clonal BM plasma cells
- Risk factors associated with progression to multiple myeloma (Hematology Am Soc Hematol Educ Program 2005;2005:373, BMC Cancer 2006;6:118, ScientificWorldJournal 2012;2012:895765, Clin Med (Lond) 2020;20:e191, Am J Clin Oncol 2020;43:709)
- Size of lesion > 5 cm
- Advanced age
- Persistent paraprotein > 1 year after therapy
- References: Cancer 1999;85:2305, Eur J Haematol 2017;99:216, Br J Haematol 2010;151:525, J Hematol Oncol 2018;11:10, J Pathol 2005;205:92
Case reports
- 33 year old woman presented with vertigo and peripheral facial nerve palsy (World Neurosurg 2020;134:10)
- 47 year old man, previously diagnosed with chronic osteomyelitis, presented with repeated discharge and ulceration in right tibia (Medicine (Baltimore) 2023;102:e33307)
- 54 year old woman with EMP of the right mainstem bronchus (Ann Thorac Surg 2019;108:e119)
- 71 year old man with progressive fatigue in the setting of diffuse hypermetabolic lymphadenopathy throughout his chest, abdomen and pelvis (J Med Case Rep 2019;13:153)
- 74 year old woman with EMP involving larynx (Radiologia (Engl Ed) 2022;64:69)
Treatment
- Treatment of plasmacytoma depends on the type (SPB or EMP) and the location
- SPB: radiation therapy (most common) or rarely surgery (kyphoplasty, vertebroplasty) (World Neurosurg 2020;134:e790)
- EMP: radiation, surgery, chemotherapy or immunotherapy
- Chemotherapy is considered for patients with persistent disease or tumors > 5 cm in size (Int J Radiat Oncol Biol Phys 2020;106:589, Ann Oncol 2021;32:309)
Gross description
- Soft gelatinous mass on cut section
Microscopic (histologic) description
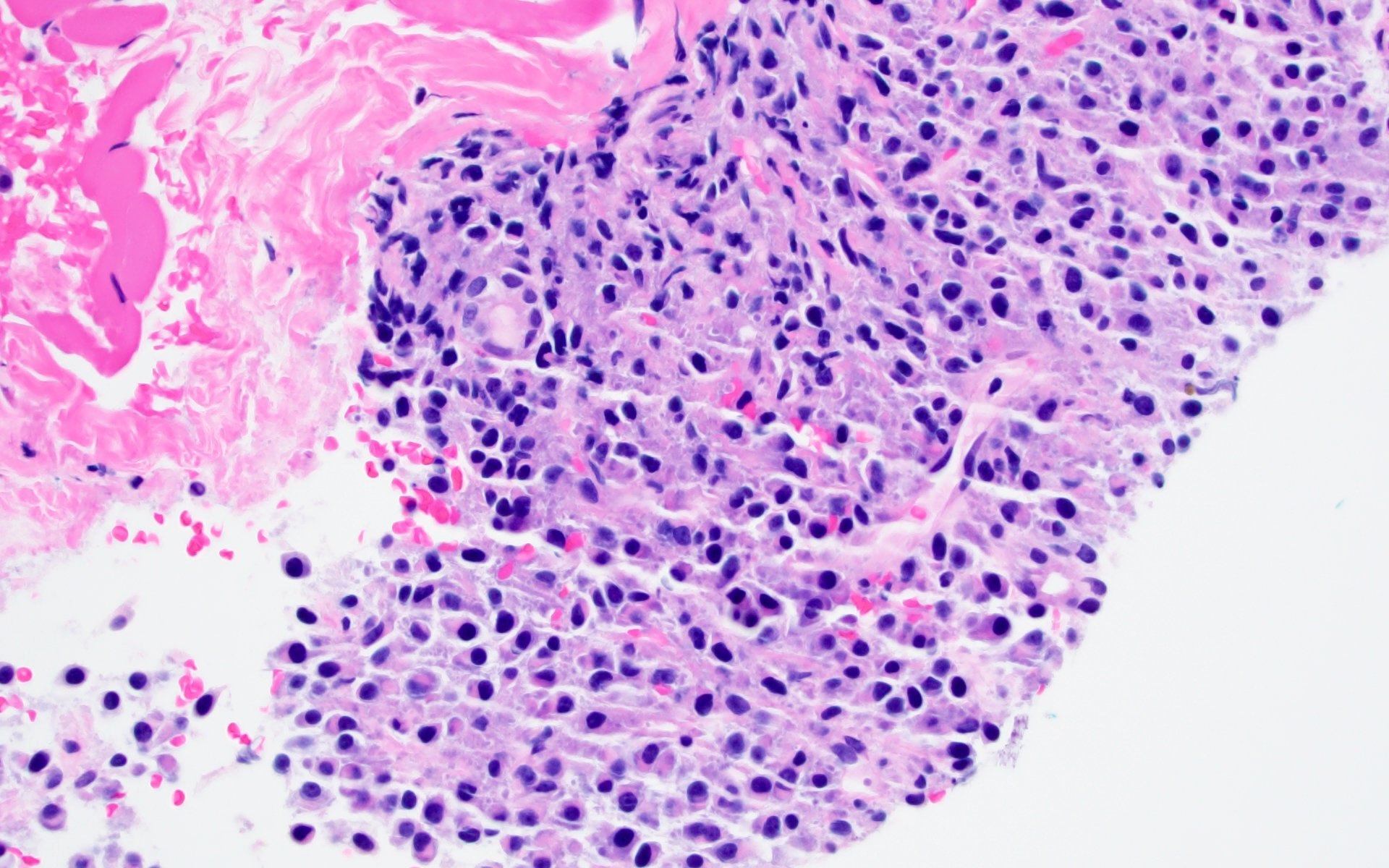
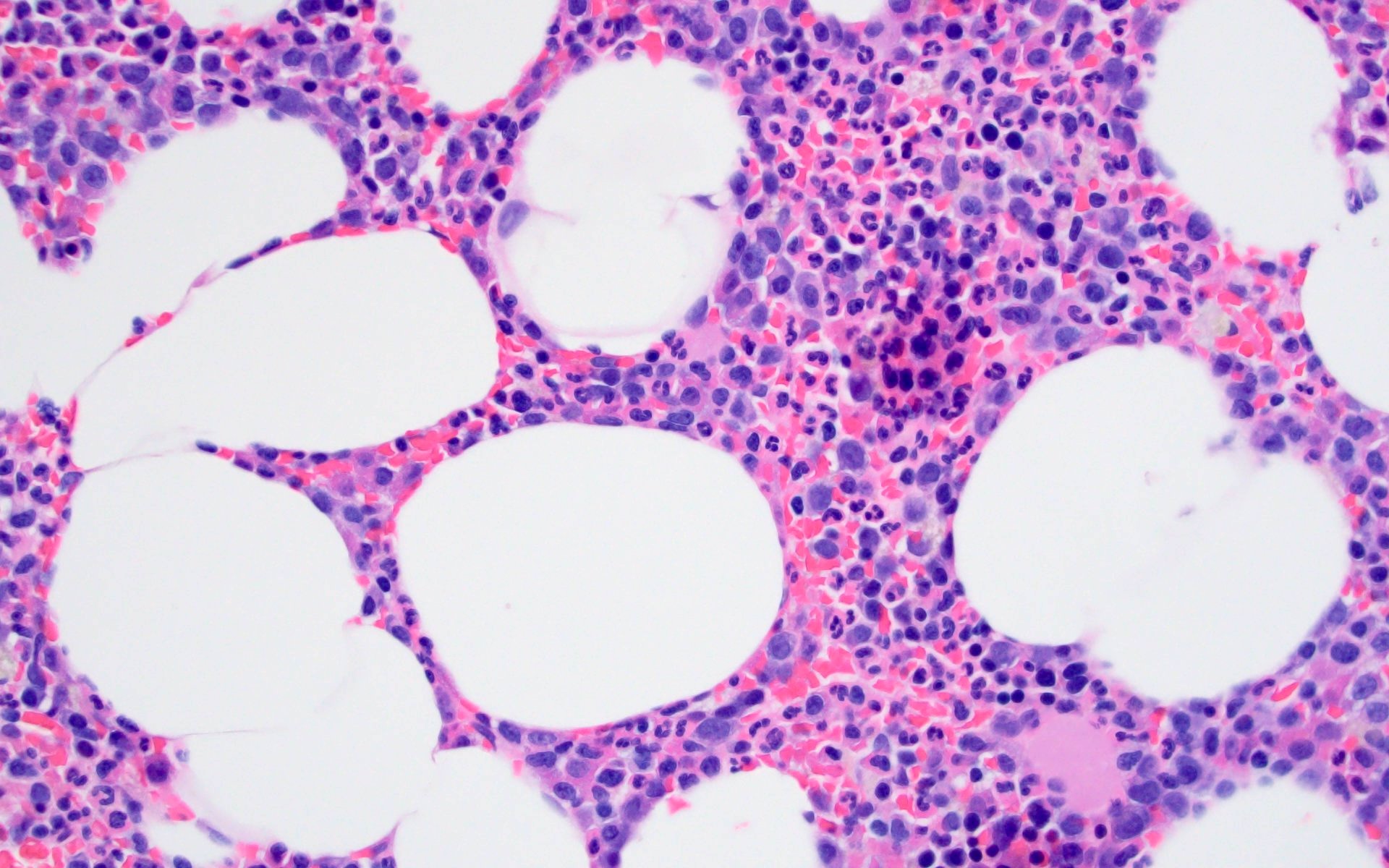
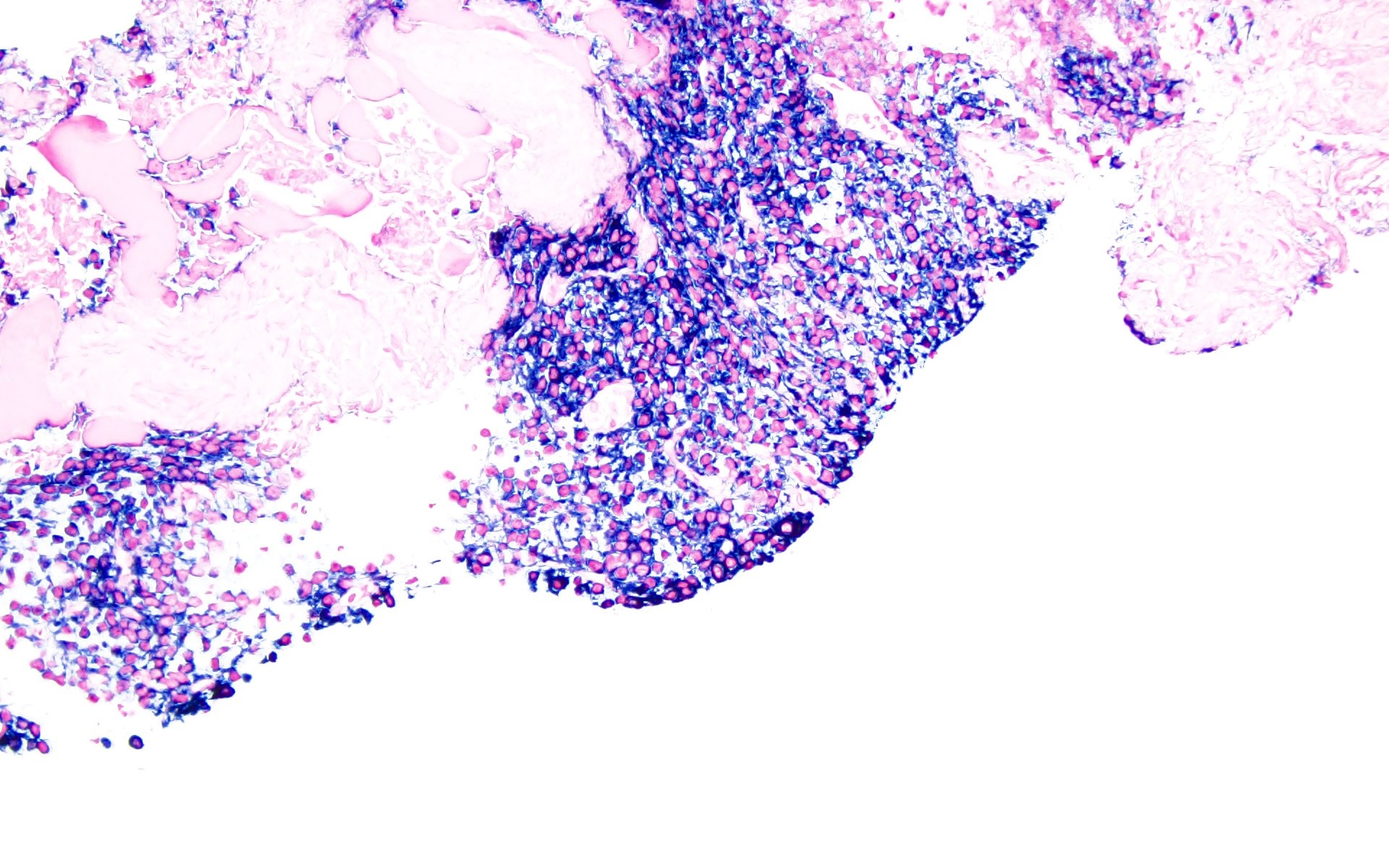
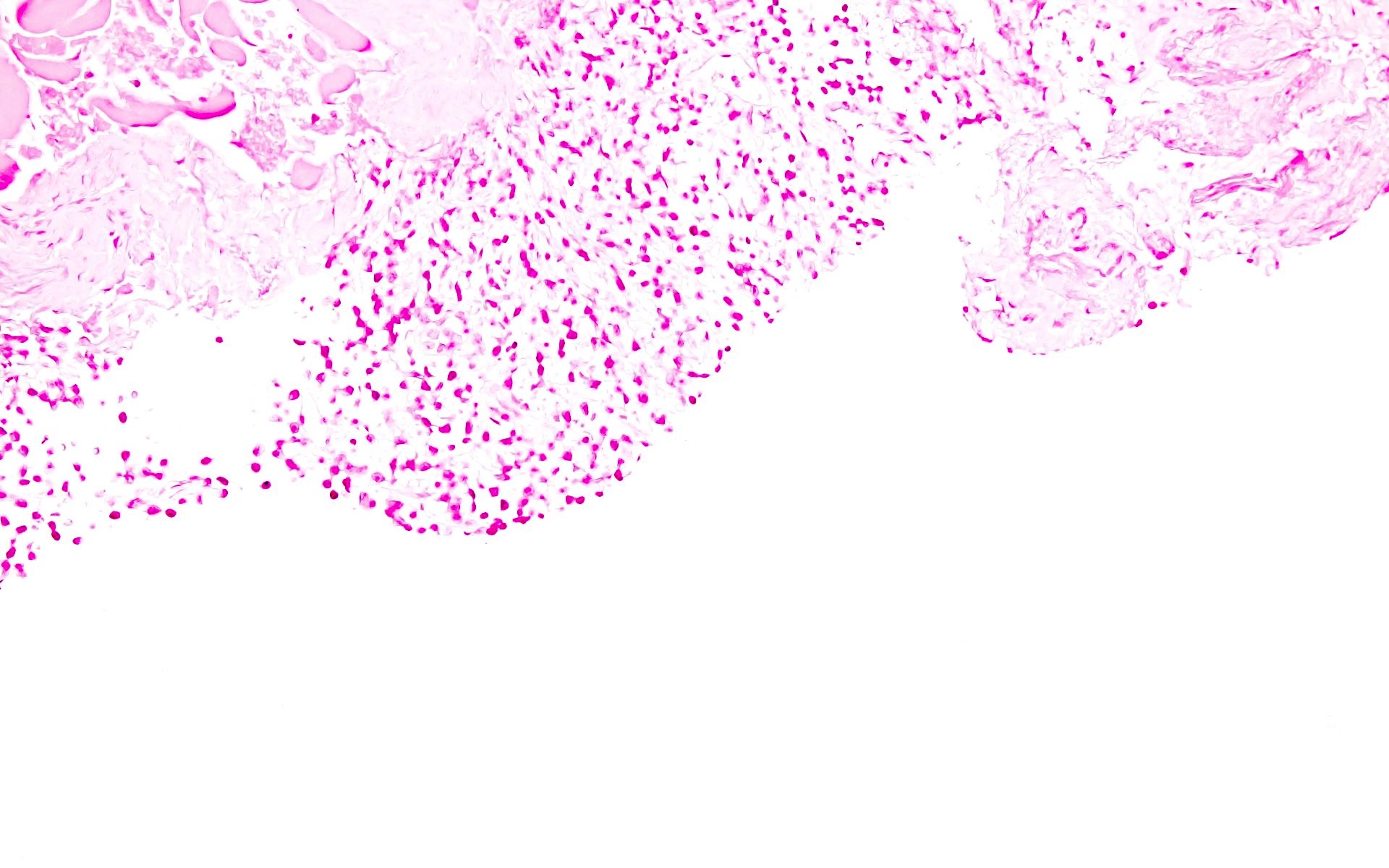
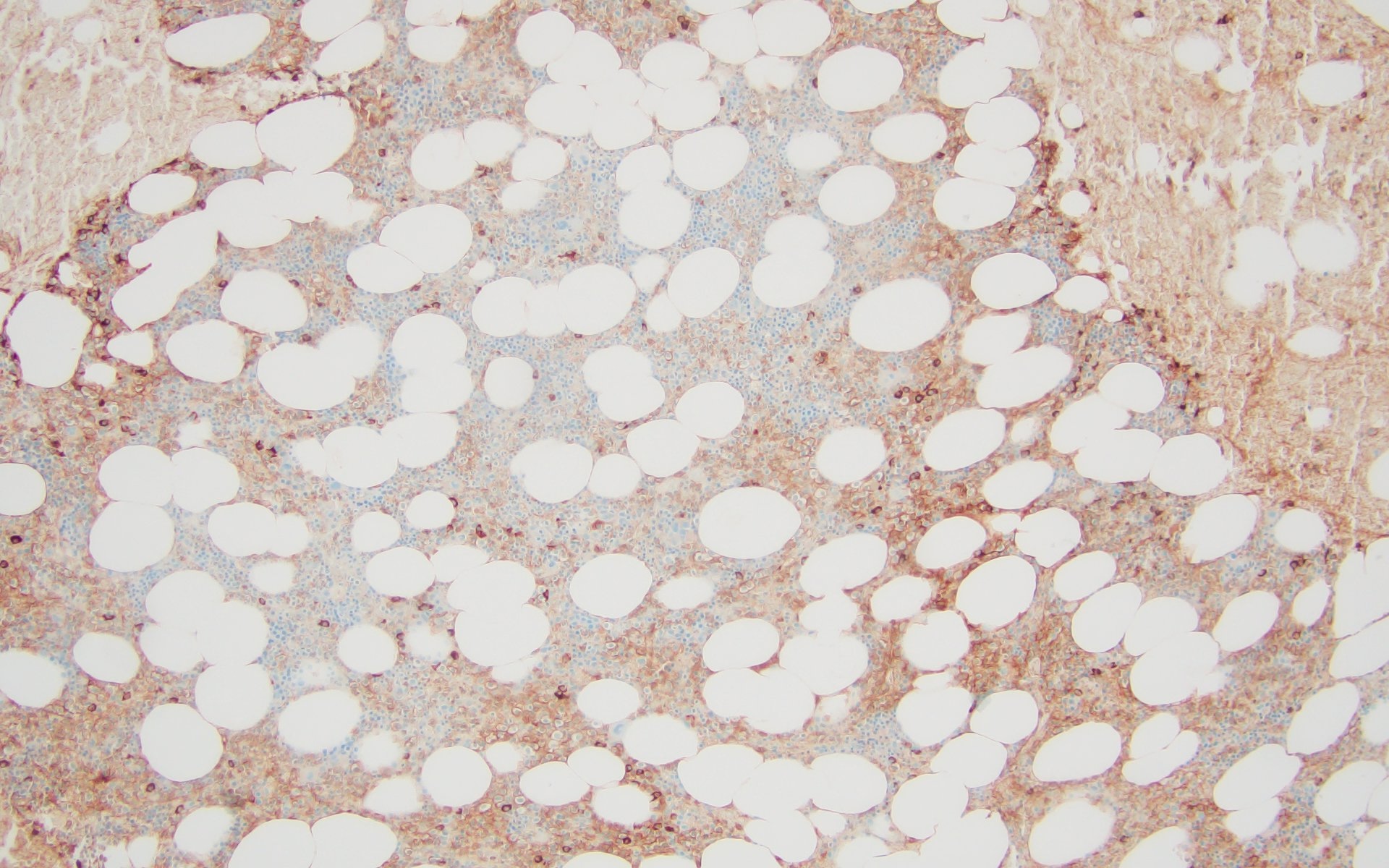
- Sheets of plasma cells with varying morphology ranging from normal appearing to large atypical plasma cells with prominent nucleoli to plasmablastic or anaplastic morphology (Am J Clin Pathol 2001;115:119, J Clin Oncol 2012;30:e91, Skeletal Radiol 2018;47:995)
- Russell or Dutcher bodies may be seen
- Associated amyloid deposition (Ann Diagn Pathol 2015;19:117)
Microscopic (histologic) images
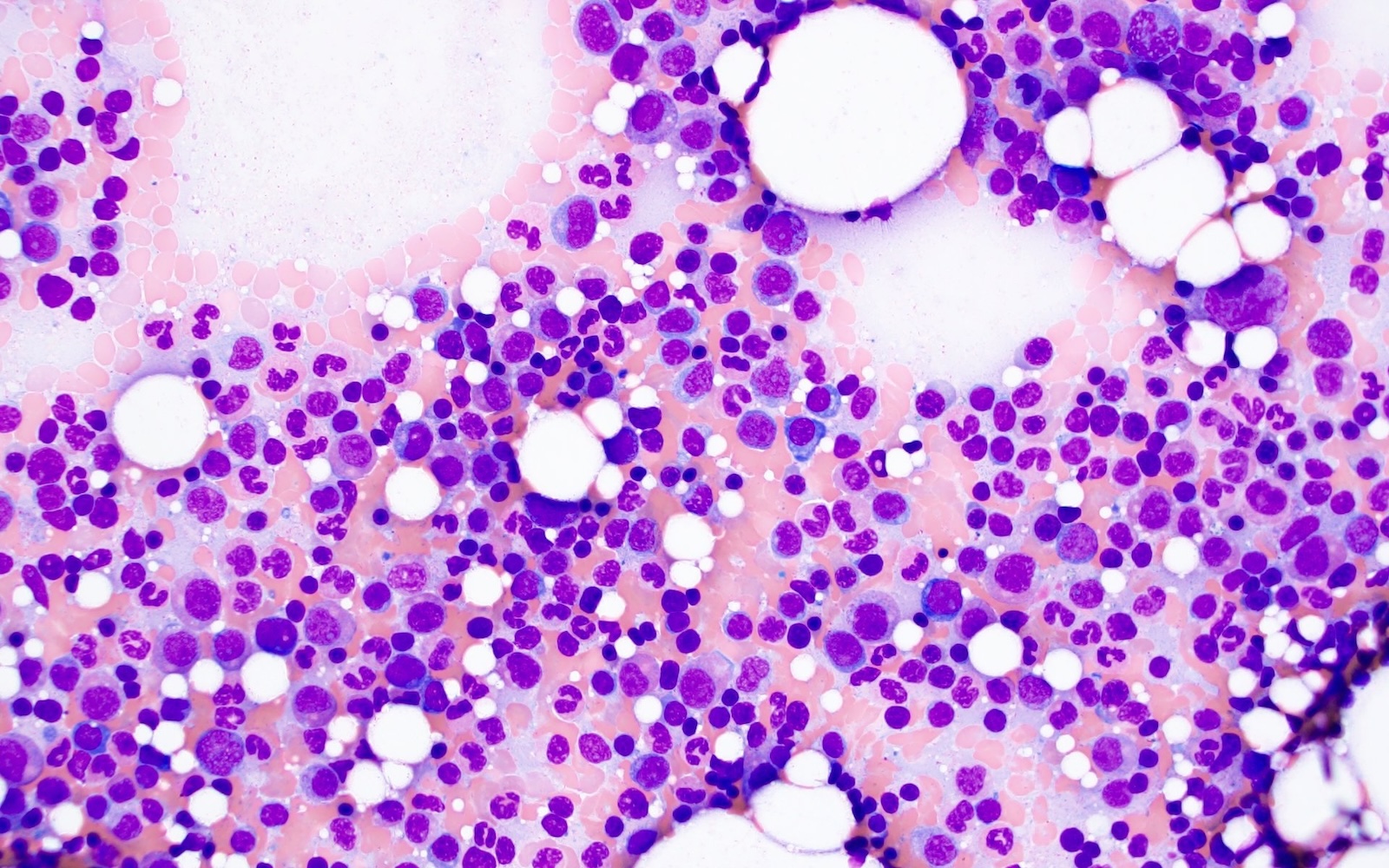
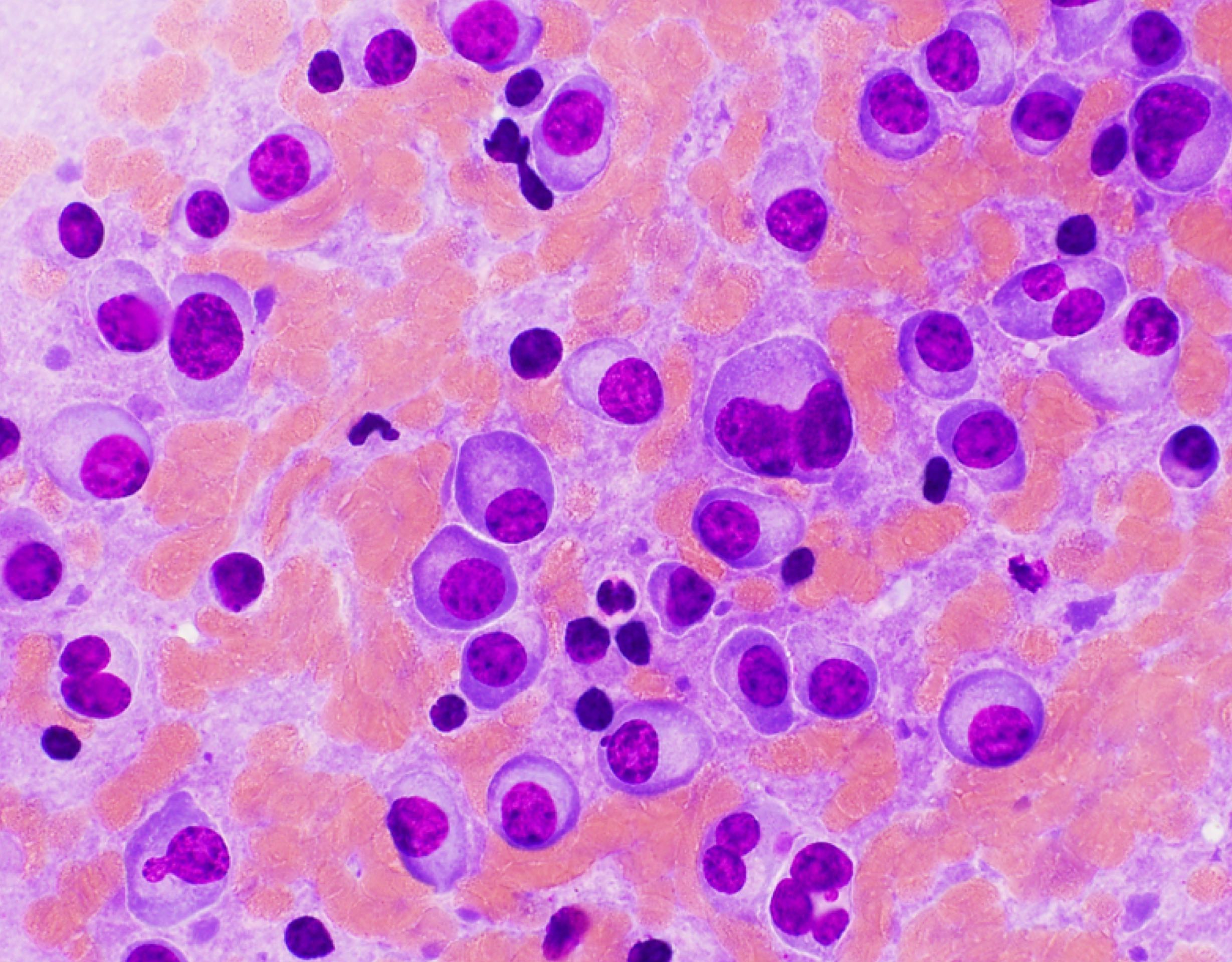
Cytology description
- Increased plasma cells with variable morphology, including small unremarkable plasma cells to atypical plasma cells (prominent nucleoli, plasmablastic / anaplastic morphology)
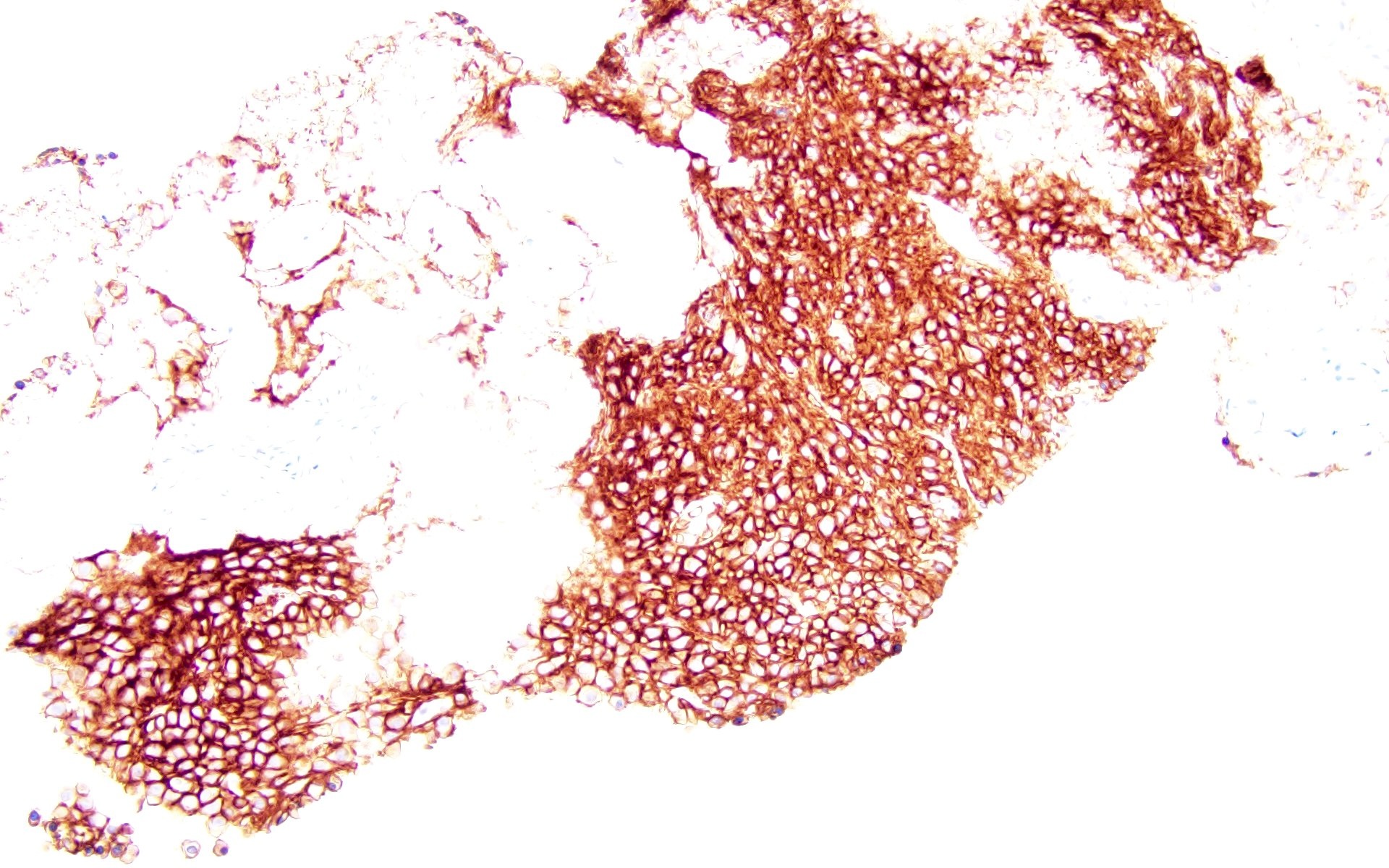
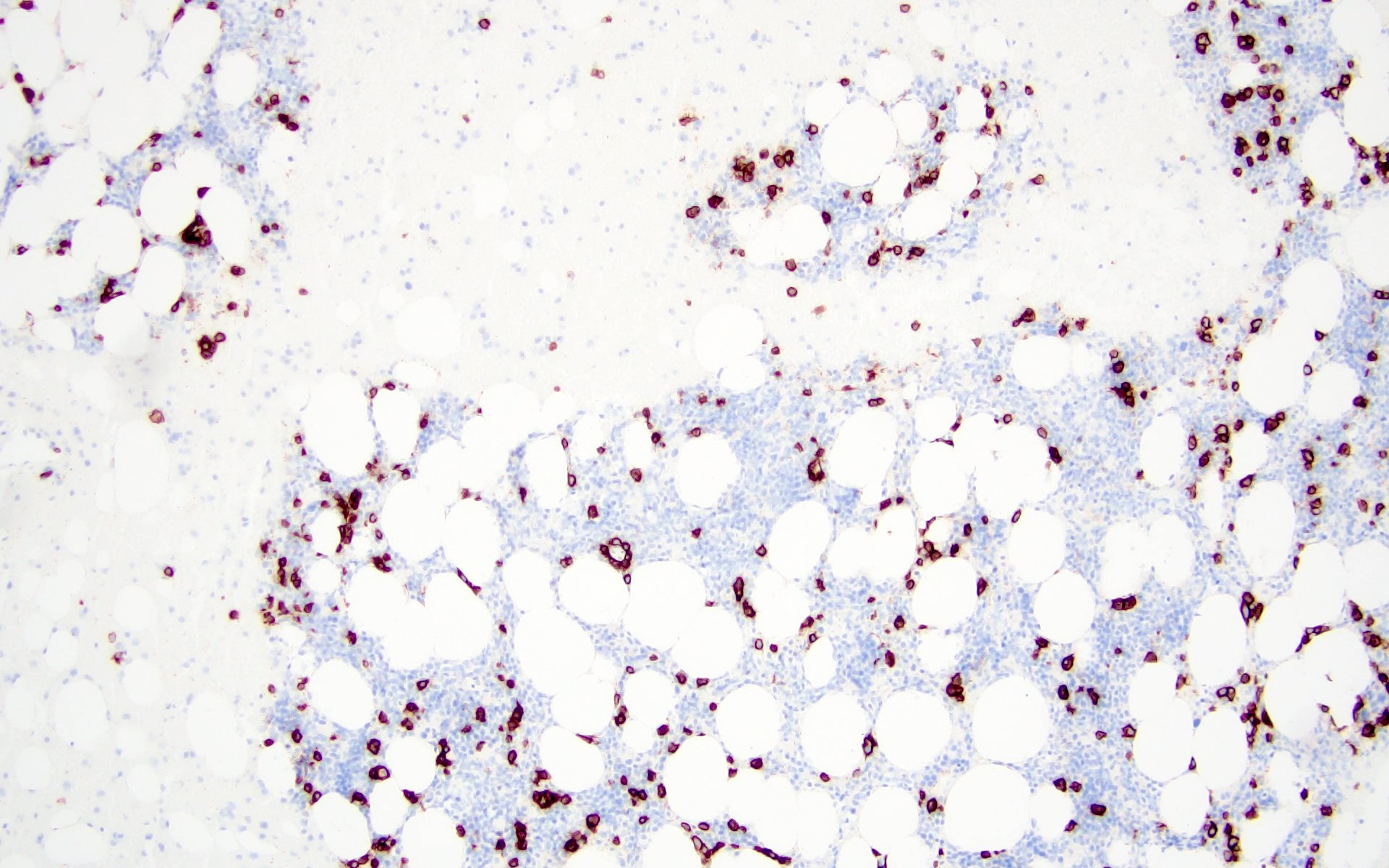
Positive stains
- CD38, CD138, MUM1, CD229, CD48, CD272, CD319 (Cytometry B Clin Cytom 2016;90:91, Cytometry B Clin Cytom 2019;96:338, Cytometry B Clin Cytom 2016;90:81)
- Some cases with expression CD117, CD20, CD33, CD10 (Haematologica 2006;91:1234, Cytometry B Clin Cytom 2010;78:239, Blood Cancer J 2018;8:e621)
- IgG, IgA (Cancer 1999;85:2305)
- Either kappa or lambda cytoplasmic immunoglobulin light chain expression
- Low Ki67 proliferative index
Negative stains
- CD19, CD45
- Cyclin D1 (J Pathol 2005;205:92)
- CD56
- EBV (mostly negative)
- Rare cases of EBV+ EMP in immunocompetent patients (EPIC) have been reported, which have brisk CD8+ and TIA1+ cytotoxic T cell infiltrate (Histopathology 2015;67:225)
Flow cytometry description
- Positive for CD138, CD38, restricted cytoplasmic light chain and negative for CD19
Molecular / cytogenetics description
- Positive for clonal immunoglobulin heavy chain gene rearrangement
- Negative for TP53 mutation (J Pathol 2005;205:92)
- Negative for MYC gene rearrangement
- Cytogenetic abnormalities (Blood 2016;127:2955, Am J Hematol 2016;91:719, Nat Rev Clin Oncol 2018;15:409, Br J Haematol 2000;111:1116, Leukemia 2001;15:981, Haematologica 2017;102:e364, Leukemia 2019;33:159, Blood 2006;108:1724, Leukemia 2006;20:2034, Blood Cancer J 2019;9:94)
- Hyperdiploidy: trisomies of odd numbered chromosomes (3, 5, 7, 9, 11, 15, 19 and 21)
- CCND translocations
- t(12;14) / CCND2::IGH
- t(6;14) / CCND3::IGH
- t(11;14) / CCND1::IGH (least commonly seen)
- MAF translocations
- t(14;16) / IGH::MAF
- t(8;14) / MAFA::IGH
- t(14;20) / IGH::MAFB
- NSD2 translocation
- t(4;14) / IGH::NSD2
- Alterations of chromosome 1 (+1q, -1p) and -17p
- Deletion of chromosome 13q (or monosomy 13)
Sample pathology report
- Nasal cavity, biopsy:
- Plasma cell neoplasm (see comment)
- Flow cytometric analysis revealed a cytoplasmic lambda light chain restricted monotypic plasma cell population (~23% of total CD45+ leukocytes) that is CD138+, CD38+, CD19-, CD20-, CD81-, partial CD27+, CD56- and CD117-. A polytypic B cell population is identified.
- Comment: The biopsy shows sheets of small plasma cells which are lambda restricted based on kappa / lambda in situ hybridization studies. EBER ISH is negative. Ki67 shows low proliferative index (< 5%). Clinical correlation with serology, imaging and bone marrow biopsy is suggested.
Differential diagnosis
| Extramedullary plasmacytoma (EMP) | Multiple myeloma (MM) with extramedullary disease | Lymphoplasmacytic lymphoma (LPL) | Extranodal marginal zone lymphoma (EMZL) | Plasmablastic lymphoma | |
| Presentation | Localized | Advanced stage of MM or at relapse | IgM paraprotein with macroglobulinemia | Localized | Aggressive disease, usually in immunocompromised patients (HIV) |
| Location | Upper respiratory tract | Localized / systemic disease | Systemic disease | Stomach, ocular adnexa, salivary gland, skin, lung, breast, thyroid | Nasal / oral cavity, gastrointestinal tract, lymph nodes |
| Precursor lesion | None | History of MM | IgM MGUS | Sites of chronic infection / inflammation (Sjögren syndrome and Hashimoto thyroiditis) | None |
| Morphology | Mostly mature appearing plasma cells | Variable normal to anaplastic / plasmablastic morphology | Small lymphocytes, plasmacytoid cells and plasma cells | Small lymphocytes, monocytoid cells and plasma cells | Large cells with round eccentric nucleus and prominent nucleoli |
| Phenotype | Cytoplasmic light chain restriction; CD38+, CD138+, MUM1+, CD19-, CD56 variable, CD20-, PAX5-, cyclin D1-, MYC-, no p53 overexpression | Cytoplasmic light chain restriction; CD38+, CD138+, MUM1+, CD19-, CD56 variable, CD20-, PAX5-, cyclin D1-/+, MYC and p53 overexpression | Surface light chain restriction; CD20+, PAX5+, CD43-/+, CD10-, CD5-/+, CD56-, CD117-, cyclin D1- | Surface light chain restriction; CD20+, PAX5+, CD43-/+, CD10-, CD5-/+, CD56-, CD117-, cyclin D1- | CD38+, CD138+, MUM1+, CD79a-/+, CD20-, PAX5-, CD45-, LANA-, ALK-, MYC and PDL1 overexpression |
| EBV | EBV variable (15% positive) | Negative | Negative | Negative | Positive (60 - 80%) |
| Bone marrow involvement | No / minimal (< 10%) | No / present | Present | Rare | No / present |
| Cytogenetics | IGH translocations with exception of t(11;14), hyperdiploidy | High risk cytogenetics and secondary alterations including del(17p), 1q gains, MYC rearrangement | Lacks MM hyperdiploidy and translocations | Lacks MM hyperdiploidy, trisomy of chromosomes 3 and 18, t(11;18)(q21;q21) / BIRC3::MALT1, t(1;14)(p22;q32) / IGH::BCL10, t(14;18)(q32;q21) / IGH::MALT1, t(3;14)(p14;q32) / IGH::FOXP1 | Lacks MM hyperdiploidy and translocations, MYC rearrangement |
| Molecular | Negative for MYC rearrangement and TP53 mutation | TP53 mutation | MYD88 L265P (> 90%), CXCR4 mutation (30 - 40%) | TNFAIP3 mutation, GPR34 mutation, TET2, CD274 and TNFRSF14 mutations | Mutations of JAK / STAT, MAPK / ERK and NOTCH pathways, TP53 mutation |
| Prognosis | Excellent | Poor | Good | Excellent | Poor |
Additional references
Board review style question #1
A 43 year old man presents with nasal obstruction and epistaxis. Imaging showed a 2.2 cm nasopharyngeal mass. Nasopharyngeal and bone marrow biopsies were performed (see images above). Which of the following risk factors in this patient increases the chances of progression to multiple myeloma?
- Age
- Bone marrow involvement
- Morphology
- Tumor size
Board review style answer #1
B. Bone marrow involvement is present in this case, which increases the risk of progression to multiple myeloma. Answers A, C and D are incorrect because the patient is young and the tumor size is < 5 cm with bland morphology (not plasmablastic / anaplastic), so none of the factors accounted for by these options increase risk of progression to multiple myeloma.
Comment Here
Reference: Plasmacytoma
Comment Here
Reference: Plasmacytoma
Board review style question #2
Which of the following genetic abnormalities is commonly associated with extramedullary plasmacytoma?
- MYC rearrangement
- t(11;14)
- t(12;14)
- TP53 mutation
Board review style answer #2
C. t(12;14). CCND translocations, which are seen in extramedullary plasmacytoma, include t(12;14) / CCND2::IGH and t(6;14) / CCND3::IGH. Answer B is incorrect because t(11;14) / CCND1::IGH is least commonly detected in extramedullary plasmacytoma. Answers A and D are incorrect because TP53 mutation is noted in multiple myeloma and both MYC rearrangement and TP53 mutation are identified in plasmablastic lymphoma.
Comment Here
Reference: Plasmacytoma
Comment Here
Reference: Plasmacytoma