Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Peripheral smear description | Peripheral smear images | Positive stains | Negative stains | Flow cytometry description | Flow cytometry images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Karamooz S, Fuda F, Jaso JM. Plasma cell leukemia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lymphomaplasmacellleukemia.html. Accessed April 3rd, 2025.
Definition / general
- Rare subtype of multiple myeloma characterized by the presence of clonal plasma cells in the peripheral blood and an aggressive clinical course
- Current definition: ≥ 5% circulating plasma cells, detected by manual differential of peripheral blood (recently updated from prior cutoff of ≥ 20% or ≥ 2.0 x 109/L) (Blood Cancer J 2021;11:192)
Essential features
- Detection of ≥ 5% circulating plasma cells by manual differential of peripheral blood, in a patient with newly diagnosed or relapsed / refractory multiple myeloma
- Aggressive clinical course marked by high tumor burden and shortened overall survival
Terminology
- Primary plasma cell leukemia (pPCL): ≥ 5% circulating plasma cells by manual differential of peripheral blood, in a patient with newly diagnosed multiple myeloma
- Secondary plasma cell leukemia (sPCL): ≥ 5% circulating plasma cells by manual differential of peripheral blood, in a patient with relapsed / refractory multiple myeloma
Epidemiology
- Incidence increases with age
- Incidence of pPCL (using updated criteria): 4 - 7% of patients with multiple myeloma (Leuk Lymphoma 2022;63:2955, J Clin Oncol 2023;41:1342)
- pPCL is more common than sPCL (60 - 70% versus 30 - 40%) (Leukemia 2013;27:780)
- Incidence of sPCL: 1 - 2% of patients with advanced or refractory multiple myeloma (Blood Cancer J 2021;11:192)
- Median time from diagnosis of multiple myeloma to development of sPCL is ~20 - 22 months (Leuk Lymphoma 2017;58:1538)
- Patients with pPCL are younger (occasionally diagnosed in second to fourth decade of life)
- Median age of presentation is ~10 years younger than for multiple myeloma (fifth to sixth decade versus sixth to seventh decade) (Leuk Lymphoma 2017;58:1538, Leukemia 2013;27:780)
- Incidence increases with age
Sites
- Peripheral blood: by definition, plasma cells are ≥ 5% of circulating white blood cells
- Bone marrow: variable, often extensive involvement by sheets of plasma cells with varying degrees of cytologic atypia
- Extramedullary sites: liver, spleen, lymph nodes and CNS are common sites of extramedullary involvement (Curr Oncol Rep 2019;21:8, Leuk Lymphoma 2022;63:2955)
Pathophysiology
- Pathophysiology is incompletely understood
- Alterations in the bone marrow microenvironment and downregulated expression of cell adhesion molecules may decrease neoplastic cell dependence on the bone marrow and promote extramedullary involvement (Leuk Lymphoma 2017;58:1538, Leukemia 2013;27:780)
- Loss of CD56 / NCAM expression disrupts adhesion of plasma cells to bone marrow stroma (Int J Hematol Oncol 2022;11:IJH39)
- Decreased expression of chemokine receptors such as CXCR4 may disrupt plasma cell localization to bone marrow stroma
- Expression of CD27 is associated with antiapoptotic processes via the nuclear factor Κβ pathway (which can be targeted with antiproteosome agents such as bortezomib) (Blood 2014;123:3770)
Etiology
- Exact etiology of primary and secondary PCL remains under investigation
- Altered expression of adhesion molecules and extracellular matrix play an incompletely understood role in pathogenesis (Blood Cancer J 2020;10:70, Leukemia 2020;34:1866)
Clinical features
- pPCL is characterized by an aggressive clinical presentation and high tumor burden
- High tumor burden is reflected by increased serum lactate dehydrogenase (LDH) and beta 2 microglobulin (Blood Cancer J 2021;11:23)
- Compared to multiple myeloma, patients with pPCL are more likely to show the following at presentation (Blood Cancer J 2021;11:23)
- High levels of bone marrow involvement
- Elevated levels of serum LDH and beta 2 microglobulin
- Hypercalcemia
- Cytopenias (anemia and thrombocytopenia)
- Renal dysfunction
- Extramedullary disease
- Rare report of pPCL presenting with tumor lysis syndrome (Clin Case Rep 2022;10:e05933)
- Of patients with detectable monoclonal gammopathy, IgG is most common, followed by IgA, IgD and IgE
- ~35 - 40% of patients show increased light chains only or are nonsecretory (2 - 8%) (Curr Oncol Rep 2019;21:8)
- Poor overall survival (OS) (5 - 30 months) (Curr Oncol Rep 2019;21:8, Leuk Lymphoma 2022;63:2955)
- References: Leuk Lymphoma 2017;58:1538, Leuk Lymphoma 2022;63:2955, J Clin Oncol 2023;41:1342, Leukemia 2013;27:780
Diagnosis
- pPCL is defined by detection of ≥ 5% circulating plasma cells, identified by manual differential of the peripheral blood, in a patient with a new diagnosis of multiple myeloma (Blood Cancer J 2021;11:192)
- sPCL is defined by detection of ≥ 5% circulating plasma cells, identified by manual differential of the peripheral blood, in a patient with relapsed or refractory multiple myeloma
- Detection of ≥ 2% circulating plasma cells by flow cytometry in patients with multiple myeloma portends a pPCL-like clinicopathologic course but is not yet recognized as pPCL (J Clin Oncol 2023;41:1383)
- When present, the percentage of circulating plasma cells and the method used for detection (e.g., manual differential, flow) should be included in the pathology report
Laboratory
- Monoclonal gammopathy by serum or urine protein electrophoresis
- IgG is most common, followed by IgA, IgD, light chain only and nonsecretory
- Serum and urine immunofixation to characterize the monoclonal gammopathy
- Elevated levels of serum or urine free light chains
- Abnormal kappa:lambda free light chain ratio
- Anemia and thrombocytopenia
- Renal dysfunction and elevated creatinine
- Elevated serum LDH and beta 2 microglobulin
- Bone marrow with variable, often extensive involvement by plasma cells
- ≥ 5% circulating plasma cells by morphologic examination of concurrent peripheral blood
- Flow cytometry of peripheral blood and bone marrow shows aberrant plasma cells with monotypic, cytoplasmic light chain expression
- Background B lymphocytes are polytypic and lack abnormalities
- Hypodiploidy and high risk cytogenetic abnormalities, by fluorescence in situ hybridization
- Mutation of TP53 is frequently observed in both pPCL and sPCL
- Frequent detection of t(11;14) and cyclin D1 expression observed in both pPCL and sPCL
- References: Leuk Lymphoma 2017;58:1538, Leuk Lymphoma 2022;63:2955, Leukemia 2013;27:780, J Clin Oncol 2023;41:1342
Prognostic factors
- pPCL has an unfavorable prognosis with decreased overall survival as compared to multiple myeloma
- Overall survival (using > 20% circulating plasma cells as cutoff): ~6.8 - 12.6 months (Leukemia 2013;27:780)
- Overall and progression free survival is approximately doubled by use of lower cutoffs, novel therapeutic agents and autologous stem cell transplant
- Overall survival rates of 16 - 30 months have been reported (Leuk Lymphoma 2022;63:2955, Leuk Lymphoma 2017;58:1538)
- Presence of any amount of circulating plasma cells in patients with multiple myeloma negatively affects prognosis and survival rates progressively decline with increasing amounts of circulating cells (J Clin Oncol 2023;41:1342)
- A recent study using flow cytometry based identification and quantitation of circulating plasma cells reported that achievement of minimal residual disease (MRD) negativity improved progression free survival in patients with multiple myeloma and circulating tumor cells (J Clin Oncol 2022;40:3120)
- Majority of patients with pPCL have high (II or III) Revised International Staging System (R-ISS) scores due to elevated beta 2 microglobulin and LDH, indicating a high tumor burden and poor prognosis (Leuk Lymphoma 2022;63:2955, Curr Oncol Rep 2019;21:8, J Clin Oncol 2015;33:2863)
- Prognostic influence of various cytogenetic abnormalities, molecular genetic abnormalities and prognosis remains under investigation
Case reports
- 60 year old woman with secondary plasma cell leukemia, successfully treated with chimeric antigen receptor T cell therapy targeting B cell maturation antigen (BCMA; CD269) (Front Oncol 2022;12:901266)
- 62 year old man presenting with hypercalcemia, tumor lysis syndrome and primary plasma cell leukemia (Clin Case Rep 2022;10:e05933)
Treatment
- Combination of immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs) may be the optimal induction therapy for pPCL (Leuk Lymphoma 2017;58:1538, Curr Oncol Rep 2019;21:8, Leukemia 2013;27:780)
- Autologous stem cell transplant (ASCT) after induction therapy and maintenance therapy reduced early relapse and improved survival in eligible patients (Expert Rev Hematol 2019;12:245)
- Venetoclax (BCL2 inhibitor) as a single agent or in combination with daratumumab, dexamethasone and bortezomib can be considered in patients with complex genomic characteristics and t(11;14) (Haematologica 2023;108:941, Eur J Haematol 2018;100:215, Blood 2022;139:2666)
- Chimeric antigen receptor (CAR) T cell therapy, other immunotherapies (mAbs, bispecific T cell engagers, antibody drug conjugates) and small molecule inhibitors are under more investigation (Haematologica 2023;108:941)
- A therapeutic focus on achieving minimal residual disease (MRD) negativity, rather than complete remission, may improve a patient’s outcome (Clin Lab Med 2017;37:821, J Clin Oncol 2023;41:1342, J Clin Oncol 2022;40:3120)
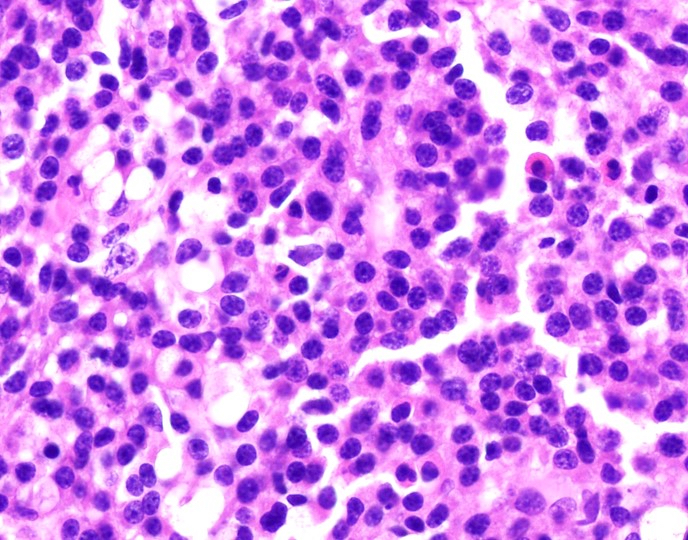
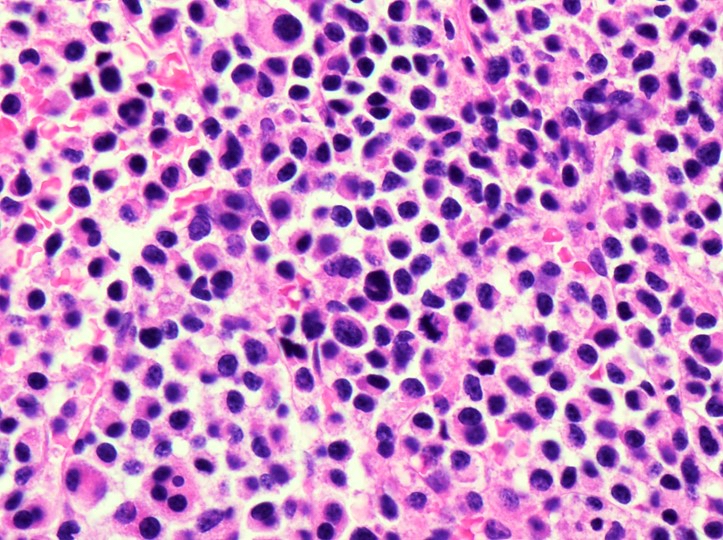
Microscopic (histologic) description
- Peripheral blood
- White blood cells may be decreased or markedly increased with ≥ 5.0% plasma cells detected by manual differential
- Plasma cells are usually larger than background lymphocytes with eccentric nuclei, condensed chromatin with peripherally located chromatin clumps imparting a clock face-like appearance, abundant, basophilic cytoplasm and a perinuclear hof of clear cytoplasm
- Anemia and thrombocytopenia are often present
- Rouleaux formation is often present
- Bone marrow aspirate
- Aspirate contains increased plasma cells
- Background hematopoietic elements may be markedly reduced
- Bone marrow core biopsy and clot section
- Marrow usually shows infiltration by sheets and clusters of plasma cells
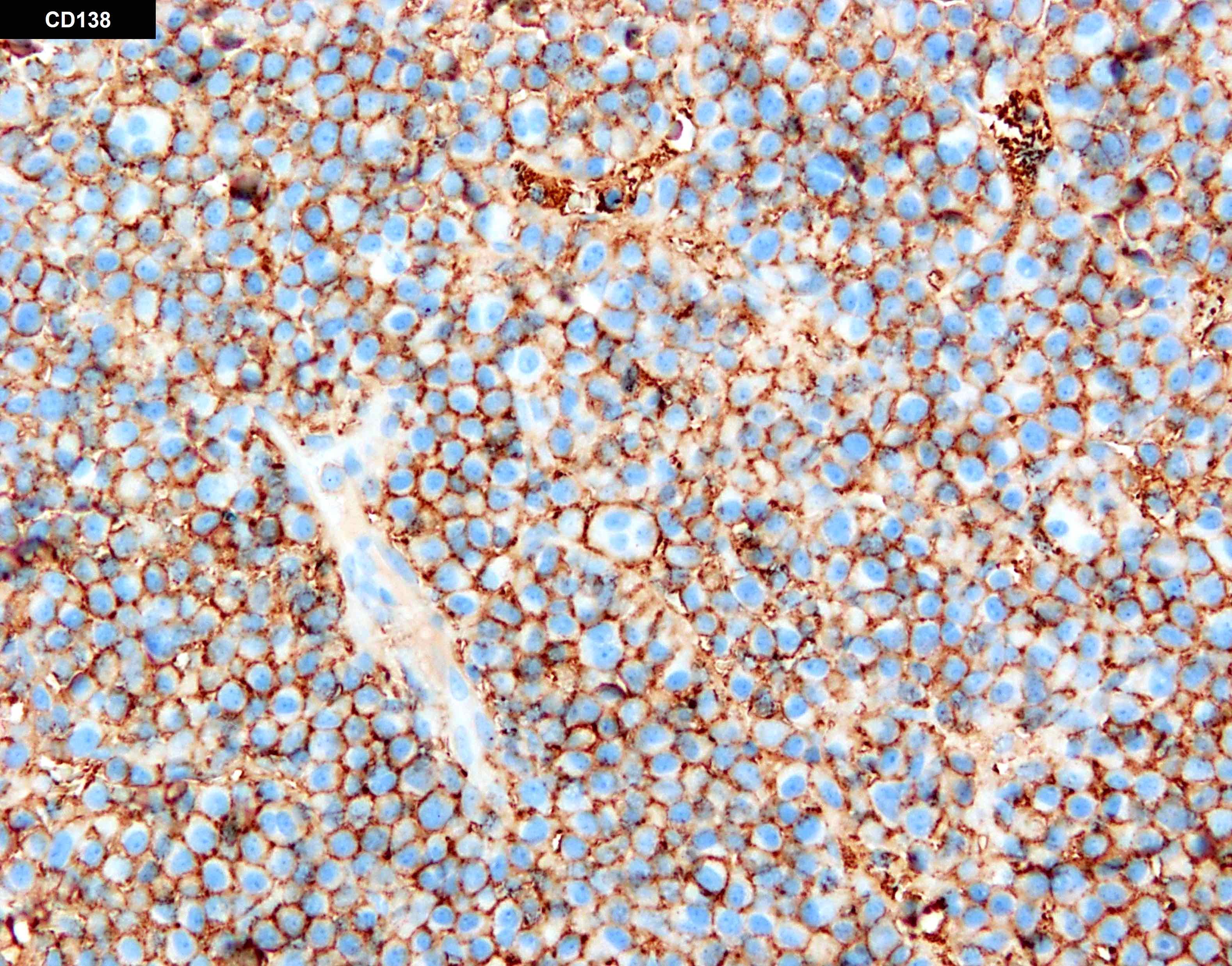
- CD138 immunohistochemistry is used to estimate the percentage of bone marrow involvement and to confirm diagnosis in cases with atypical morphology
- Bone lesions and extramedullary lesions
- Sheets and clusters of plasma cells (morphologic features of osseous and nonosseous plasmacytoma, respectively)
- Reference: Semin Diagn Pathol 2003;20:211
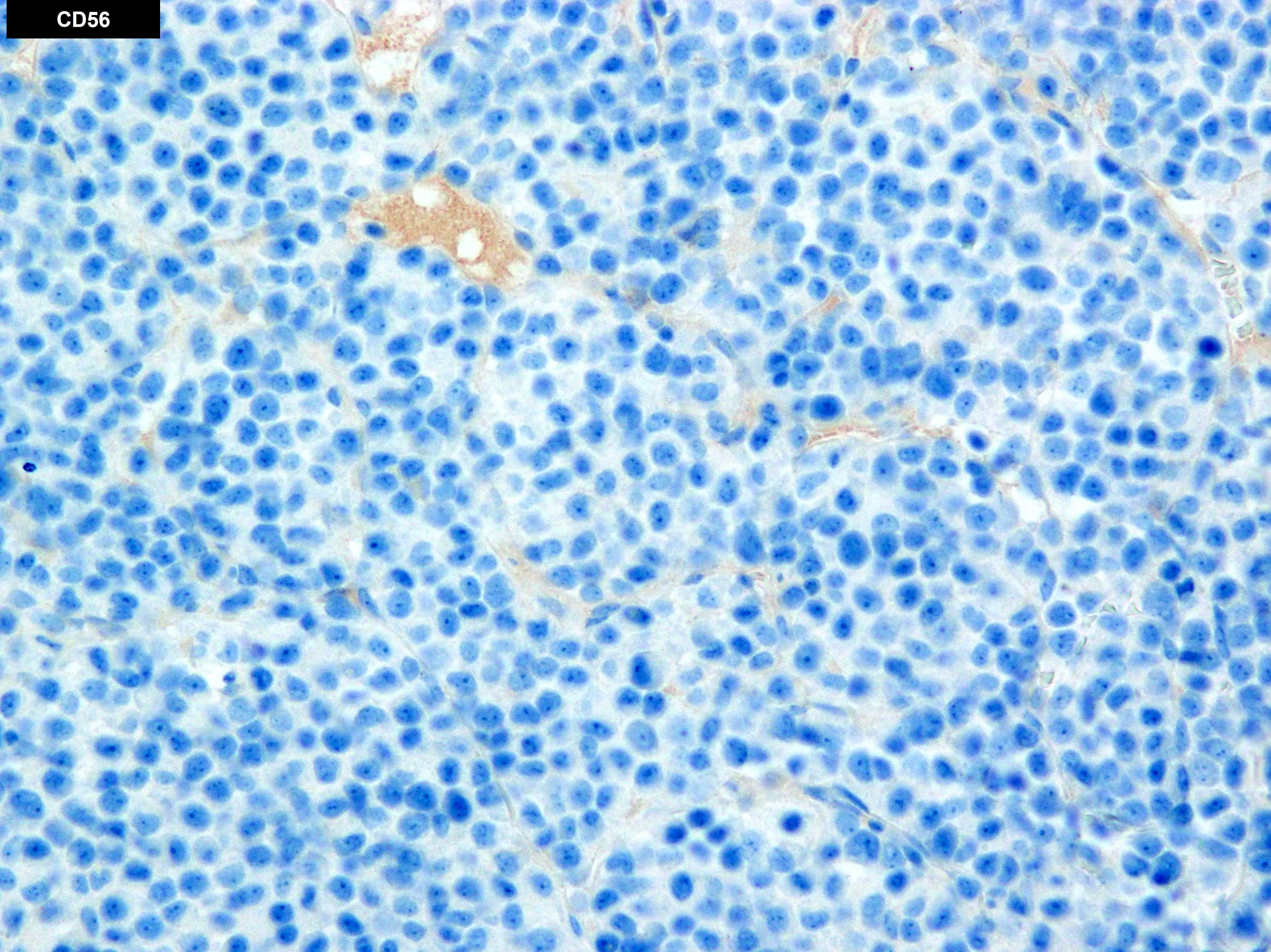
Microscopic (histologic) images
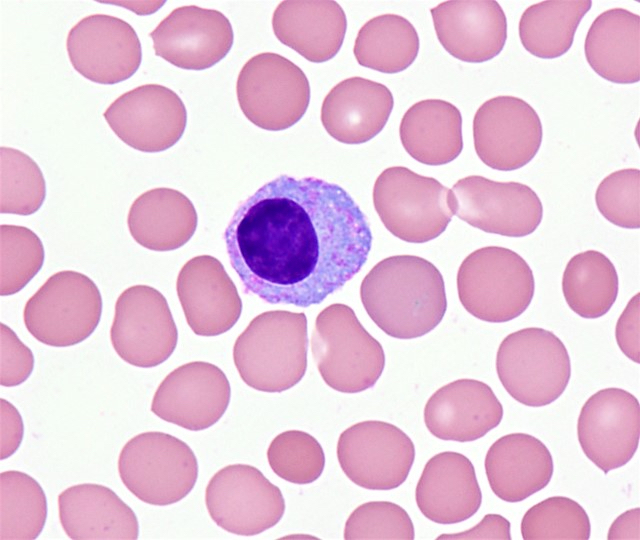
Cytology description
- Medium sized cells with eccentric nuclei, condensed, clock face or spoke wheel-like chromatin, abundant basophilic cytoplasm and a perinuclear clearing
- Cells may show variable amounts of morphologic atypia
- Intranuclear (Dutcher bodies), cytoplasmic (Russell bodies) or extracellular immunoglobulin may be present
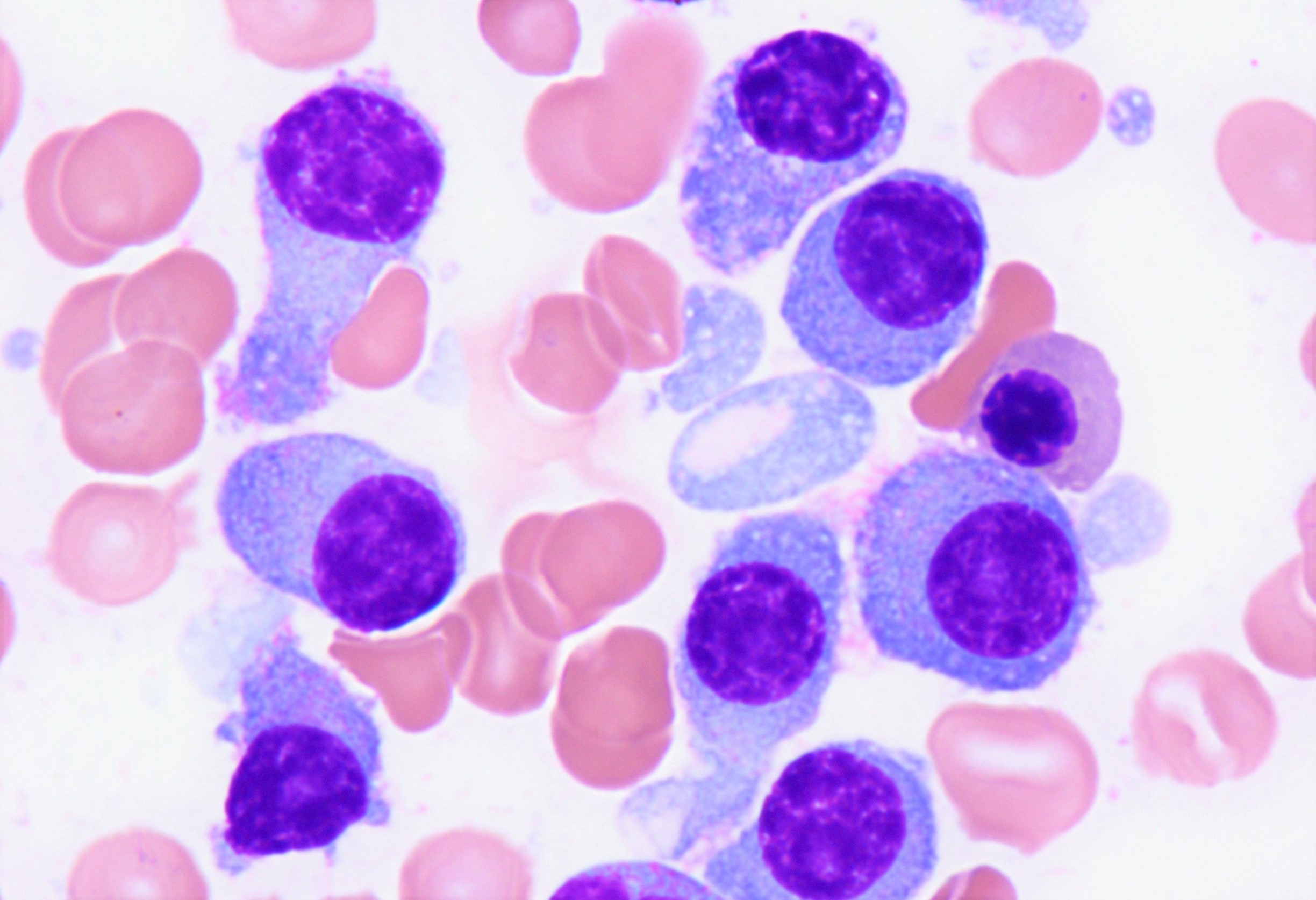
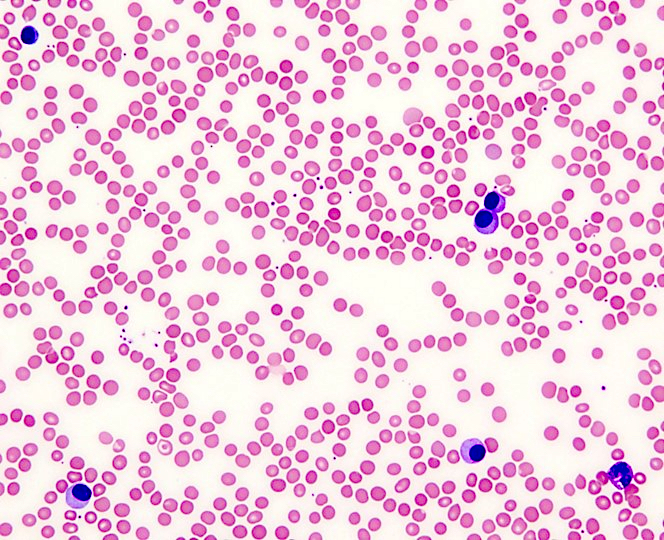
Peripheral smear description
- Peripheral blood
- White blood cells may be decreased or markedly increased with ≥ 5.0% plasma cells detected by manual differential
- Plasma cells are usually larger than background lymphocytes with eccentric nuclei, condensed chromatin with peripherally located chromatin clumps imparting a clock face-like appearance, abundant, basophilic cytoplasm and a perinuclear hof of clear cytoplasm
- Reference: Semin Diagn Pathol 2003;20:211
Positive stains
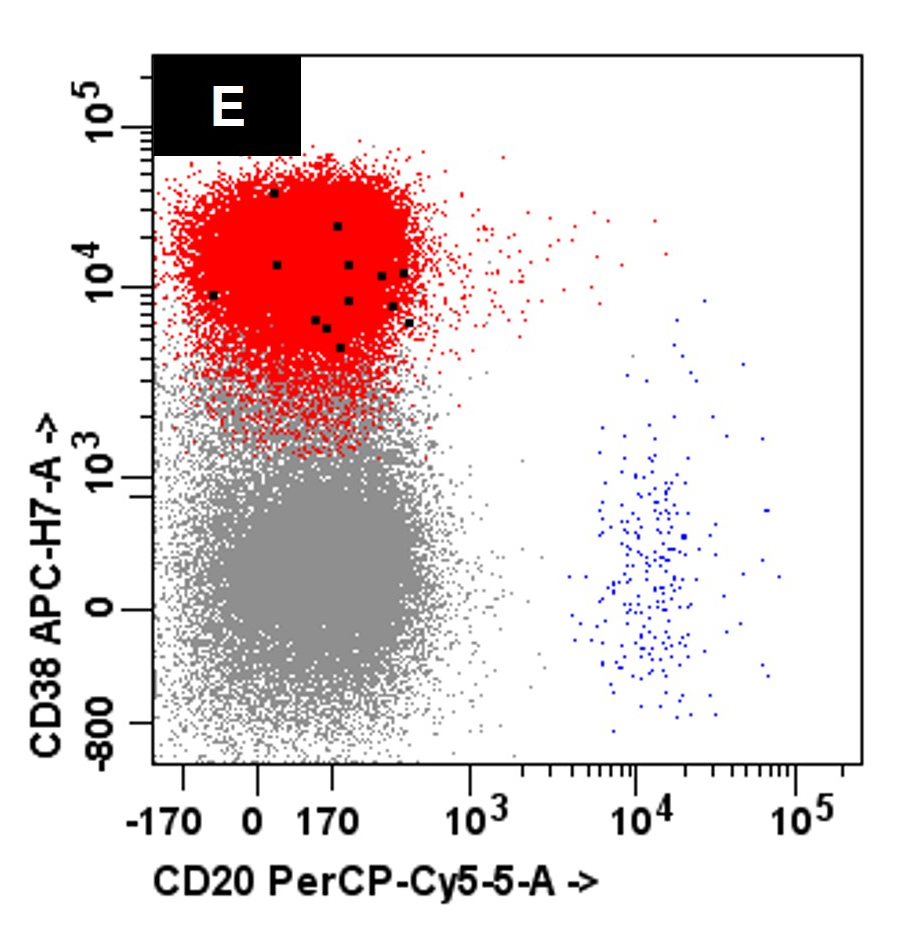
- Flow cytometry: neoplastic plasma cells in primary and secondary PCL are variably positive for CD38 and CD138
- Monotypic expression of cytoplasmic kappa or lambda light chains can be assessed via flow cytometry, immunohistochemistry or in situ hybridization
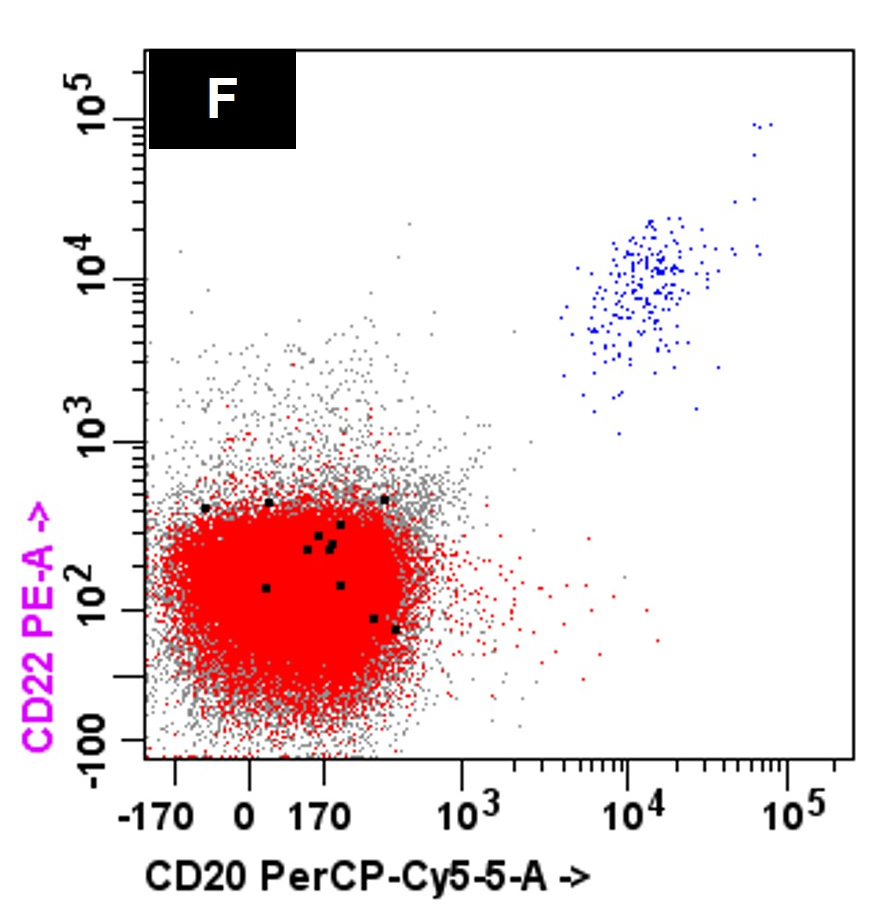
- Neoplastic plasma cells express variable amounts of CD20, CD23, CD44 and CD45
- CD20 expression may be associated with the presence of t(11;14) CCND1::IGH (Leuk Res 2013;37:1251, Front Oncol 2022;12:1061438)
- CD23 expression is highly associated with the presence of t(11;14) CCND1::IGH (Br J Haematol 2010;150:724, Br J Haematol 2010;149:292, Leukemia 2013;27:780)
- CD28 is more frequently expressed in sPCL versus pPCL; its expression has been linked with proliferation / progression and chemotherapeutic resistance in multiple myeloma (Leuk Lymphoma 2017;58:1538, Blood 2014;123:3770)
- CD27 is more frequently expressed in pPCL than sPCL and is associated with antiapoptotic pathway activation (Leukemia 2013;27:780)
- Immunohistochemistry: positive for CD138 with more frequent expression of CD20, CD45 and cyclin D1 in primary and secondary PCL
Negative stains
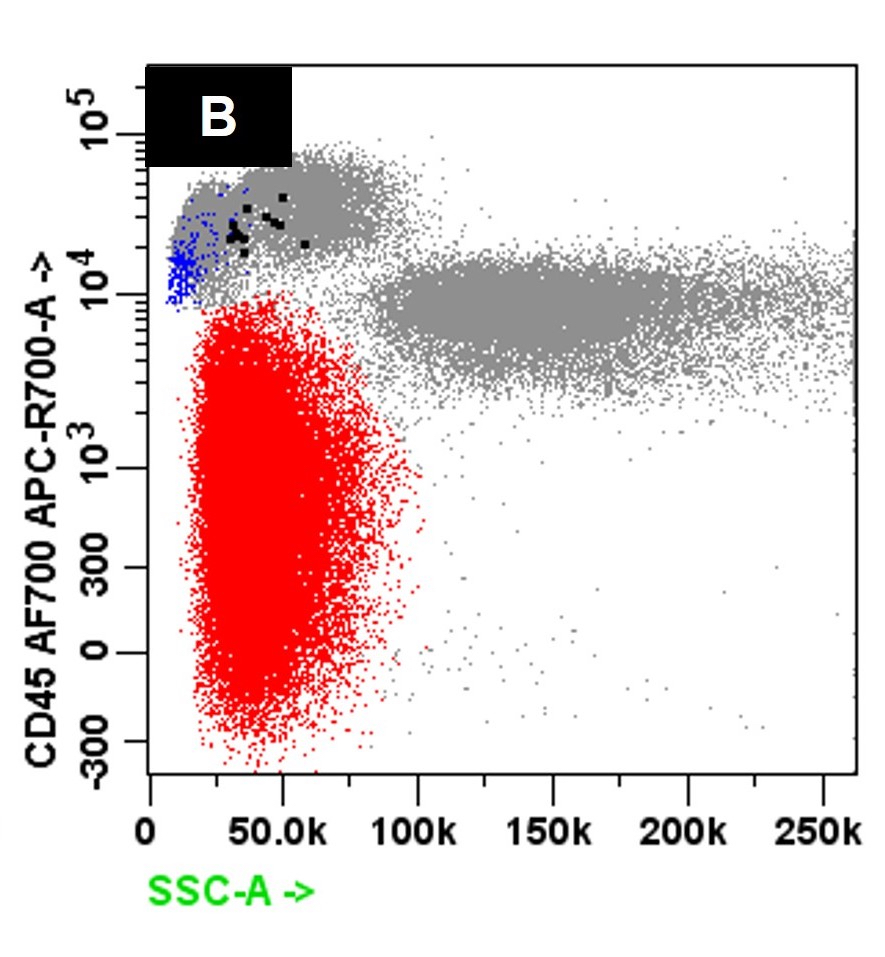
Flow cytometry description
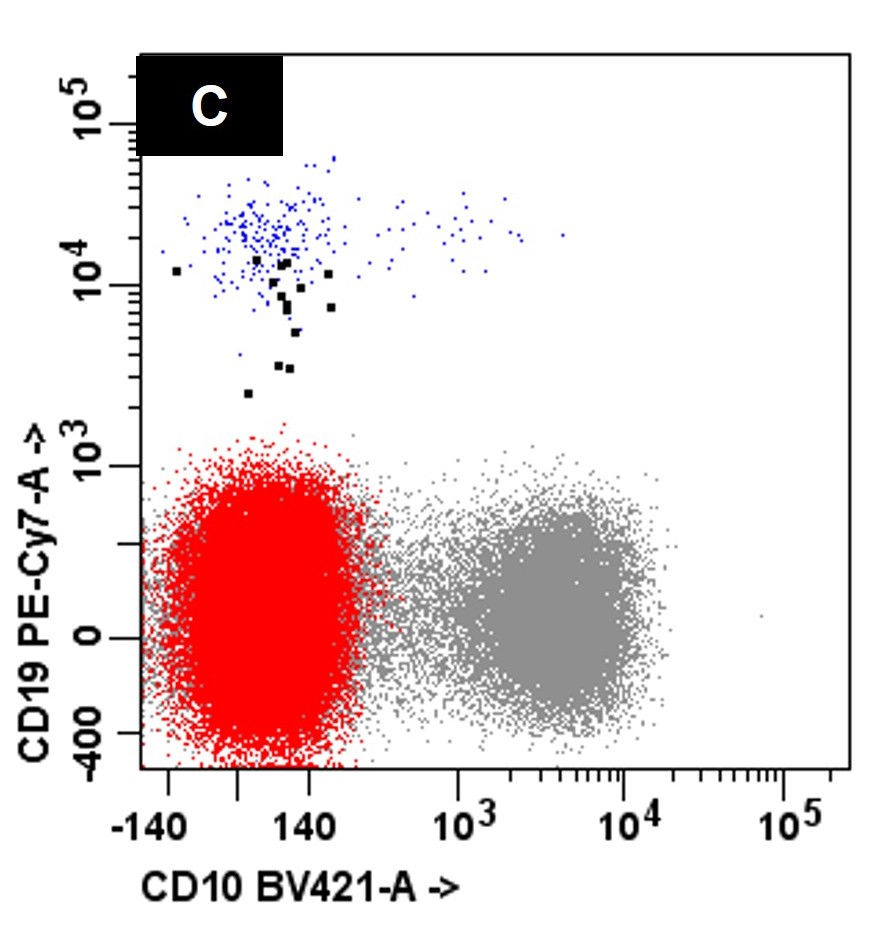
- Neoplastic cells express CD38 and CD138 and typically lack expression of CD19 and CD45 with monotypic cytoplasmic light chain expression (Leuk Res 2011;35:169)
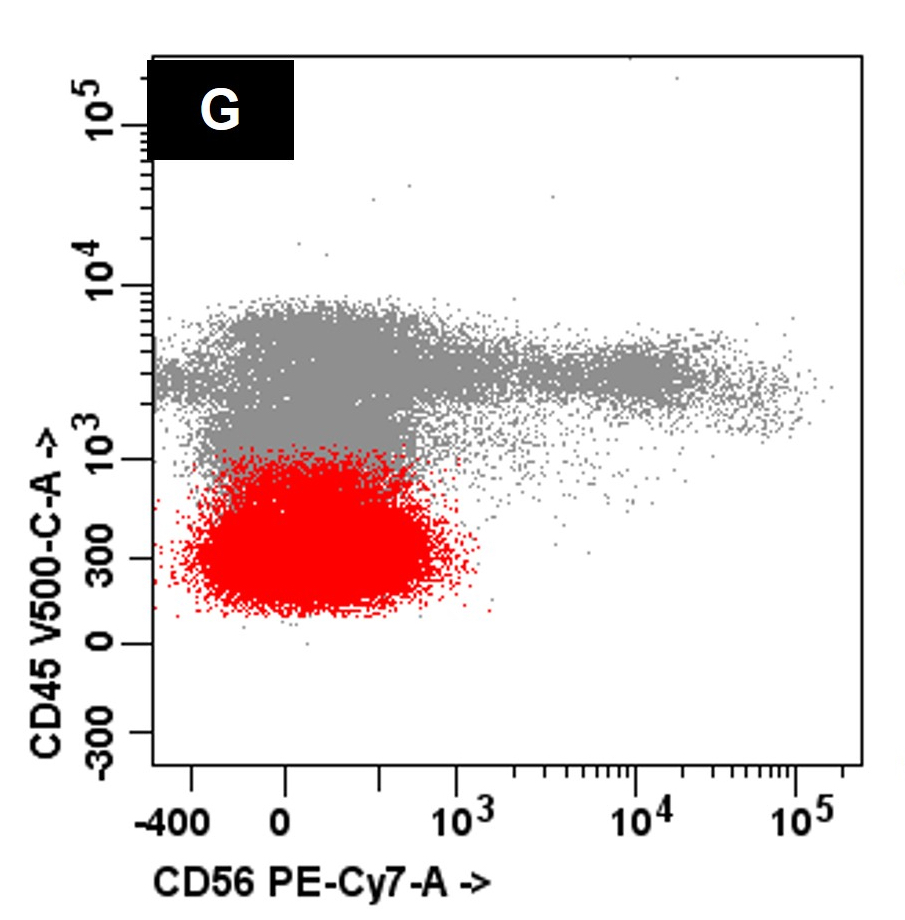
- Compared to MM, the neoplastic cells in PCL less frequently express CD56 (Br J Haematol 2021;195:95)
- Variable expression of CD20, CD23, CD45 and CD200 may be seen (Blood Cancer J 2021;11:23, Leukemia 2013;27:780)
- CD23 expression is often associated with presence of t(11;14) and cyclin D1 expression (Leukemia 2013;27:780)
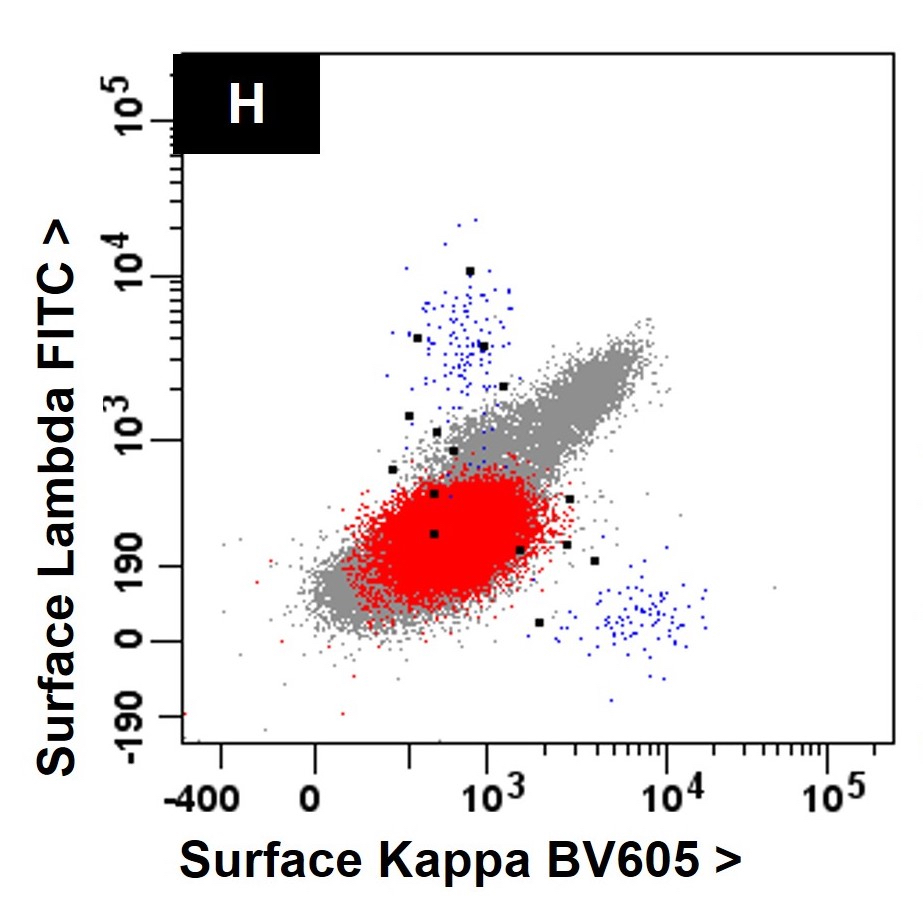
Flow cytometry images
Molecular / cytogenetics description
- Complex cytogenetic and molecular genetic abnormalities are more frequent in PCL than in multiple myeloma; the most commonly encountered findings are
- Abnormal karyotype
- High risk cytogenetics: t(4;14) or t(14;16) or del(17p13) or t(8q24) (Biomedicines 2022;10:209)
- Hypodiploidy (Leukemia 2013;27:780)
- Loss of chromosome 1p
- Gain of chromosome 1q
- Del 13q (Cancers (Basel) 2022;14:1594)
- Loss of chromosome 16 and 7 (80%, 11%) (Cancers (Basel) 2022;14:1594)
- Trisomy 8: 43% (Cancers (Basel) 2022;14:1594)
- Translocations involving the IGH locus (14q32)
- t(11;14) CCND1::IGH (cyclin D1) with atypical breakpoint in almost half of pPCL (Biomedicines 2022;10:209)
- t(4;14) IGH::FGFR3, t(14;16) IGH::MAF: 13 - 25% may be seen (Cancers (Basel) 2022;14:1594, Biomedicines 2022;10:209)
- Commonly encountered molecular genetic abnormalities
- High prevalence of TP53 mutation or deletion 17p by FISH (Blood 2022;139:2666)
- In patients with t(11;14), commonly associated with a deletion 17p
- In non-t(11;14) commonly associated to biallelic inactivation (Oncotarget 2015;6:17543, Curr Oncol Rep 2019;21:8, Blood Cancer J 2020;10:70)
- High prevalence of MYC abnormalities (loss, rearrangement, amplification) (Biomedicines 2022;10:209, Genes Chromosomes Cancer 2009;48:624)
- Overexpression of MYC by RT-PCR (Leuk Lymphoma 2017;58:1538)
- High prevalence of TP53 mutation or deletion 17p by FISH (Blood 2022;139:2666)
Sample pathology report
- Bone marrow, aspirate, clot, core biopsy and peripheral blood:
- Primary plasma cell leukemia, 25% circulating plasma cells (see comment)
- Plasma cell neoplasm, involving > 95% of bone marrow
- Comment:
- The peripheral blood shows leukocytosis with numerous circulating plasma cells (25%). The bone marrow is hypercellular (95 - 100%) and shows near total replacement by sheets of atypical, pleomorphic plasma cells (67% by manual differential; > 95% by immunohistochemistry for CD138).
- Concurrent flow cytometry shows an aberrant plasma cell population with monotypic, cytoplasmic light chain expression.
- Review of the electronic medical record indicates that the patient does not have a prior diagnosis of plasma cell neoplasm or other malignancy.
- The combined findings are diagnostic of primary plasma cell leukemia. Clinical correlation and correlation with pending ancillary and staging studies is recommended.
- Bone marrow, aspirate, clot, core biopsy and peripheral blood:
- Secondary plasma cell leukemia, 35% circulating plasma cells (see comment)
- Relapsed plasma cell myeloma, involving 80 - 90% of bone marrow
- Comment:
- The peripheral blood shows leukocytosis with numerous circulating plasma cells (35%). The bone marrow is hypercellular (95 - 100%) and shows near total replacement by sheets of atypical, pleomorphic plasma cells (45% by manual differential; 80 - 90% by immunohistochemistry for CD138).
- Concurrent flow cytometry shows an aberrant plasma cell population with monotypic, cytoplasmic light chain expression.
- The combined findings are diagnostic of relapse of the patient’s previously diagnosed plasma cell myeloma with development of secondary leukemic transformation (secondary plasma cell leukemia). Clinical correlation and correlation with pending ancillary and staging studies is recommended.
Differential diagnosis
- Florid polytypic plasmacytosis:
- In rare cases, patients with autoimmune disease, HIV and other infectious / inflammatory disorders may have a florid increase in reactive bone marrow plasma cells
- Plasma cells will lack immunophenotypic aberrancies by flow cytometry or immunohistochemistry
- Plasma cells will show polytypic, cytoplasmic kappa and lambda light chain expression by flow cytometry or immunohistochemistry
- B cell lymphoma with plasmacytic differentiation:
- B cell non-Hodgkin lymphoma shows a variable propensity for plasmacytic differentiation (e.g., marginal zone lymphoma and lymphoplasmacytic lymphoma)
- Flow cytometry will demonstrate the presence of 2 monoclonal cell populations: an aberrant B cell and plasma cell population with monotypic expression of the same light chain (assessed on the cell surface for B cells and within cytoplasm / intracellularly for plasma cells)
- Neoplastic B cells will express CD19, CD20, CD22 and monotypic, surface light chain expression
- Neoplastic plasma cells will show monotypic, cytoplasmic expression of the same light chain as the B cells
- Neoplastic plasma cells often express CD19, CD20 (partial) and CD45 and lack expression of CD56
- Neoplastic plasmacytic cells often show monotypic, surface light chain expression similar to the neoplastic B lymphocytes
- B cell non-Hodgkin lymphoma shows a variable propensity for plasmacytic differentiation (e.g., marginal zone lymphoma and lymphoplasmacytic lymphoma)
Board review style question #1
A 45 year old patient presents with weight loss, leukocytosis, anemia and thrombocytopenia. Serum protein electrophoresis shows a monoclonal IgG kappa protein. Bone marrow examination shows ~90% involvement by sheets of atypical plasma cells with monotypic, intracytoplasmic kappa light chain expression. Review of a concurrent peripheral blood smear shows ~6% atypical cells (see images above). Which of the following statements regarding these cells is most correct?
- Neoplastic cells have monotypic, surface light chain expression by flow cytometry
- Neoplastic cells typically lack expression of CD38 by flow cytometry
- Neoplastic cells typically lack expression of CD56 by flow cytometry
- Neoplastic cells with bright expression of CD19 by flow cytometry
- Neoplastic cells with bright expression of CD45 by flow cytometry
Board review style answer #1
C. Neoplastic cells typically lack expression of CD56 by flow cytometry. The clinical and pathologic features are consistent with a diagnosis of primary plasma cell leukemia. Answer D is incorrect because by flow cytometry, the neoplastic plasma cells are characteristically negative for expression of CD19, CD56 and surface immunoglobulin expression. Answers A, B and E are incorrect because the neoplastic plasma cells express monotypic, cytoplasmic light chain and they are positive for CD38 and CD138 with variable expression of CD20, CD23 and CD45.
Comment Here
Reference: Plasma cell leukemia
Comment Here
Reference: Plasma cell leukemia
Board review style question #2
A 56 year old patient is diagnosed with secondary plasma cell leukemia. Which of the following laboratory values would provide the best assessment of tumor burden and disease prognosis?
- Serum beta 2 microglobulin
- Serum calcium
- Serum creatinine
- Urine immunofixation electrophoresis
- White blood cell count
Board review style answer #2
A. Serum beta 2 microglobulin. Serum beta 2 microglobulin, LDH and albumin level have been shown to correlate with tumor burden and disease prognosis in multiple myeloma and plasma cell leukemia and are major components of the Revised International Staging System (R-ISS) for multiple myeloma. Answers B - E are incorrect because serum creatinine, calcium, white blood cell count and immunoglobulin heavy or light chain isotype assist with diagnosis but do not correlate with tumor burden.
Comment Here
Reference: Plasma cell leukemia
Comment Here
Reference: Plasma cell leukemia