Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Pathophysiology / etiology | Clinical features | Diagnosis | Staging / staging classifications | Prognostic factors | Case reports | Treatment | Clinical images | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology images | Peripheral smear images | Positive stains | Negative stains | Flow cytometry images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Marques-Piubelli ML, Torres-Cabala CA, Miranda RN. Sézary syndrome. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lymphomanonBsezary.html. Accessed April 1st, 2025.
Definition / general
- Leukemic variant of cutaneous T cell lymphoma (CTCL) defined by the presence of erythroderma, generalized lymphadenopathy and clonal T cells with cerebriform nuclei (Sézary cells); diagnosis is stronger if the same clone is demonstrated in the skin, lymph nodes and peripheral blood (J Am Acad Dermatol 2012;67:1189, Lancet 2008;371:945, Blood Adv 2018;2:2115, Am J Hematol 2019;94:1027, Eur J Cancer 2018;93:47, Curr Hematol Malig Rep 2016;11:468)
- Closely related to mycosis fungoides
- Sézary syndrome (SS) may be indistinguishable from mycosis fungoides when mycosis fungoides becomes erythrodermic and clinically aggressive, although mycosis fungoides usually lacks leukemic phase (J Am Acad Dermatol 2014;70:205.e1)
- Despite the overlapping features, Sézary syndrome (SS) and mycosis fungoides are considered distinct entities; SS is a disease arising de novo, while erythroderma and lymphadenopathy in a patient known to have mycosis fungoides is better referred to as SS preceded by mycosis fungoides (Eur J Cancer 2017;77:57)
Essential features
- Leukemic variant of cutaneous T cell lymphoma (CTCL) defined by the presence of erythroderma, generalized lymphadenopathy and clonal T cells with cerebriform nuclei (Sézary cells); diagnosis is stronger if the same clone is demonstrated in the skin, lymph nodes and peripheral blood (J Am Acad Dermatol 2012;67:1189, Lancet 2008;371:945, Blood Adv 2018;2:2115, Am J Hematol 2019;94:1027, Eur J Cancer 2018;93:47, Curr Hematol Malig Rep 2016;11:468)
- Histologically similar to mycosis fungoides but variable and sometimes the findings of mycosis fungoides, such as epidermotropism, are not present (Lancet 2008;371:945, Curr Hematol Malig Rep 2016;11:468, J Am Acad Dermatol 2011;64:352)
- Chronic condition and incurable: the main aim of treatment is controlling symptoms and improvement of the quality of life (Lancet 2008;371:945, Am J Hematol 2019;94:1027, Blood 2016;127:3142, J Am Acad Dermatol 2011;64:352)
Terminology
- Erythrodermic CTCL includes all primary cutaneous lymphoma that evolve to erythroderma, such as Sézary syndrome (SS) and erythrodermic mycosis fungoides
- SS was first described by Albert Sézary in 1938 (Lancet 2008;371:945, Eur J Cancer 2017;77:57)
Epidemiology
- Rare: corresponds to 2.5 - 5.0% of all CTCLs (J Am Acad Dermatol 2012;67:1189, Lancet 2008;371:945, Eur J Cancer 2017;77:57)
- Age adjusted incidence of 6.4 cases per million (Lancet 2008;371:945)
- Sixth and seventh decade (J Am Acad Dermatol 2012;67:1189, Blood Adv 2019;3:1145, Am J Clin Pathol 2011;136:944, Clin Cancer Res 2012;18:5051)
- M:F = 2:1 (J Am Acad Dermatol 2012;67:1189, Lancet 2008;371:945, Blood Adv 2019;3:1145)
- More common in Caucasians (J Am Acad Dermatol 2012;67:1189, Clin Cancer Res 2012;18:5051)
Pathophysiology / etiology
- Mycosis fungoides and SS are thought to arise from chronic antigenic stimulation; early mycosis fungoides lesions show increased numbers of dendritic cells and upregulation of their antigen presenting cell (APC) ligands B7 and CD40 and their respective T cell costimulatory ligands CD28 and CD40L (J Am Acad Dermatol 2014;70:205.e1)
- Both mycosis fungoides and SS have as cell of origin the skin resident CD45RO+ effector memory T cell (Lancet 2008;371:945)
- Antigen presenting dendritic cells could maintain the survival and proliferation of clonal T cells: higher specific human leukocyte antigen (HLA) class II alleles than the general population (Lancet 2008;371:945)
- Skin microbiomes (e.g. as Chlamydia spp) could play a role in the pathogenesis by the stimulation of clonality in T cells but it is still controversial (Lancet 2008;371:945)
- Abnormalities in pathways: NFκB / JAK STAT activation, cell cycle dysregulation / apoptosis and DNA structural dysregulation affecting gene expression (Nat Genet 2015;47:1465)
- Micro RNA expression profile detected differences between erythrodermic mycosis fungoides and SS (Acta Derm Venereol 2019;99:1148)
- Chemokines receptors (Lancet 2008;371:945, Curr Hematol Malig Rep 2016;11:468, J Am Acad Dermatol 2011;64:352)
- CCR4 and CCR10 play a role in the homing of malignant T cells to skin where they bind to ligands on endothelial cells, keratinocytes or Langerhans cells
- CCL17 is a major CCR4 ligand and it is increased in the serum of patients with mycosis fungoides and SS
- May explain the lower epidermotropism observed in SS compared with mycosis fungoides
- Loss of CD26 promotes the inactivation of CXCL12, which is the CXCR4 ligand
- CCR4 and CCR10 play a role in the homing of malignant T cells to skin where they bind to ligands on endothelial cells, keratinocytes or Langerhans cells
- T helper 2 (Th2) response is characteristic of SS as well as that of tumor stage mycosis fungoides; overexpression of CD47 in Sézary cells is under the influence of Th2 cytokines, such as IL-4, IL-7 and IL-13 (Blood Adv 2019;3:1145, Curr Hematol Malig Rep 2016;11:468, J Am Acad Dermatol 2011;64:352)
Clinical features
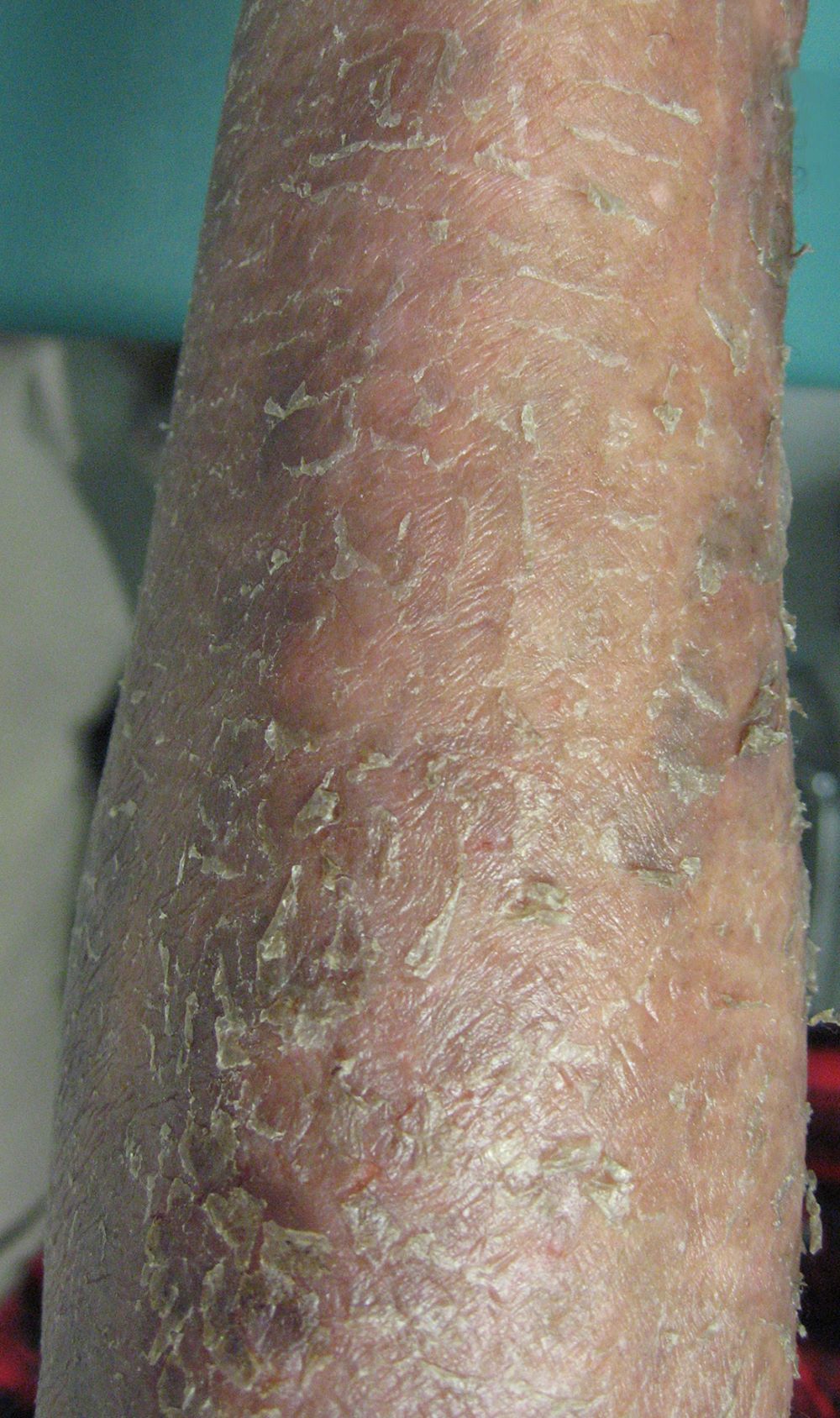
- Erythroderma is the key feature for diagnosis and is defined by involvement of > 80% of body surface and may range from mild to intense (J Am Acad Dermatol 2012;67:1189, Lancet 2008;371:945, Eur J Cancer 2018;93:47, Curr Hematol Malig Rep 2016;11:468)
- Alopecia and plaques at initial clinical presentation are uncommon (systemic symptoms usually absent)
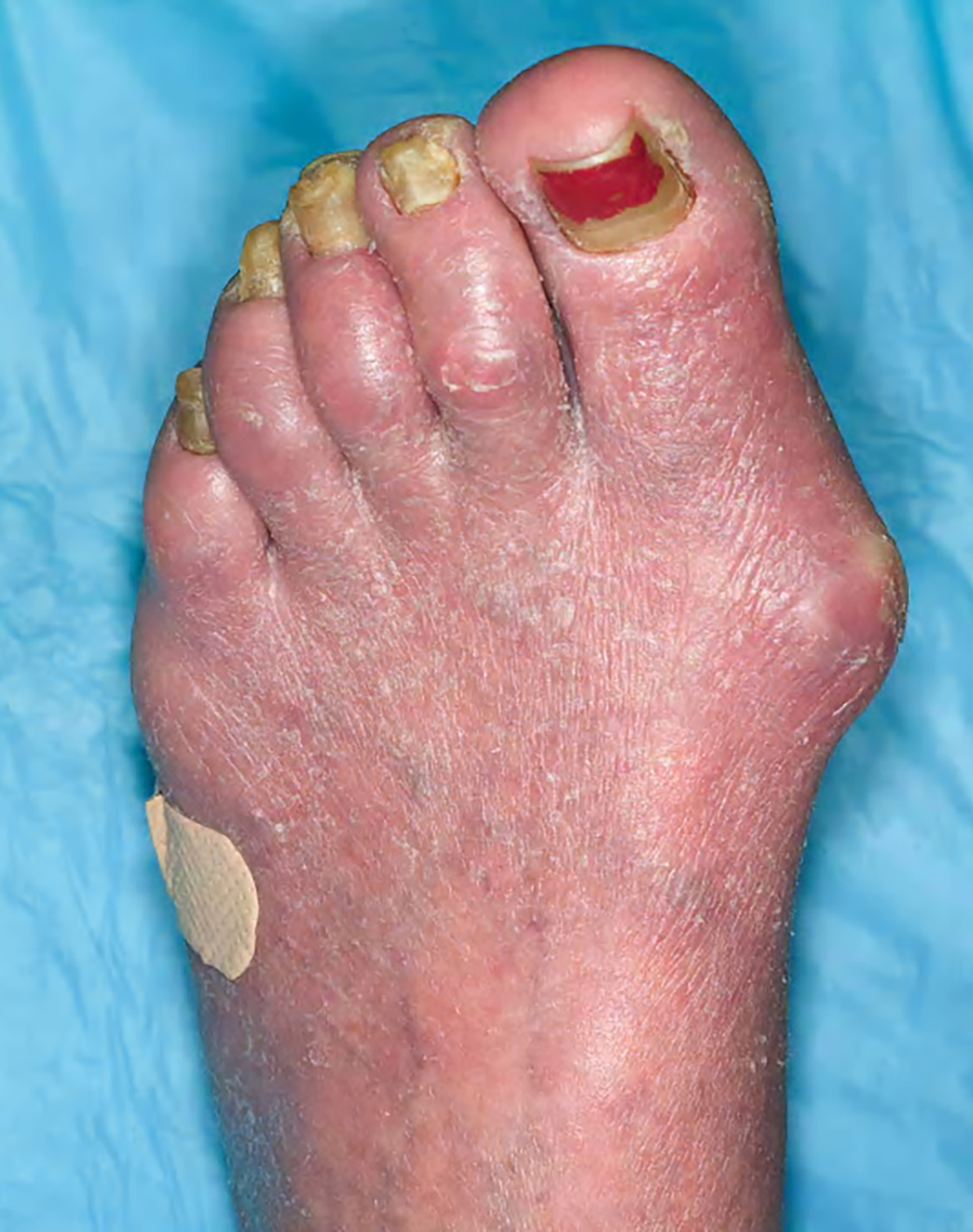
- Nail dystrophy, blepharoconjunctivitis and ectropium can be present in advanced disease
- Palms and sole usually show thickening, scales and fissures
- Median duration of all dermatologic symptoms before diagnosis: 3.5 years
- Erythroderma is present usually 1.7 years before diagnosis
- Clinical presentation with erythroderma with < 1 x 109/L is defined as pre-Sézary and some patients progress to SS, while others follow a more indolent clinical course
- Pruritus is often present (Lancet 2008;371:945)
- Systemic symptoms usually absent (J Am Acad Dermatol 2012;67:1189)
- Adenopathy is usually present (J Am Acad Dermatol 2012;67:1189)
- Bone marrow involvement in approximately 20% of the cases (J Am Acad Dermatol 2012;67:1189)
- Cases may be preceded by mycosis fungoides and should be designated as SS preceded by mycosis fungoides; these cases are different from de novo SS (Am J Hematol 2019;94:1027)
Diagnosis
- Characterized by the presence of erythroderma, generalized lymphadenopathy and clonal T cells (same clone in skin, lymph node and peripheral blood) in addition to 1 or more of the following criteria (Am J Hematol 2019;94:1027, Eur J Cancer 2018;93:47, Am J Clin Pathol 2011;136:944, Curr Hematol Malig Rep 2016;11:468, J Am Acad Dermatol 2011;64:352):
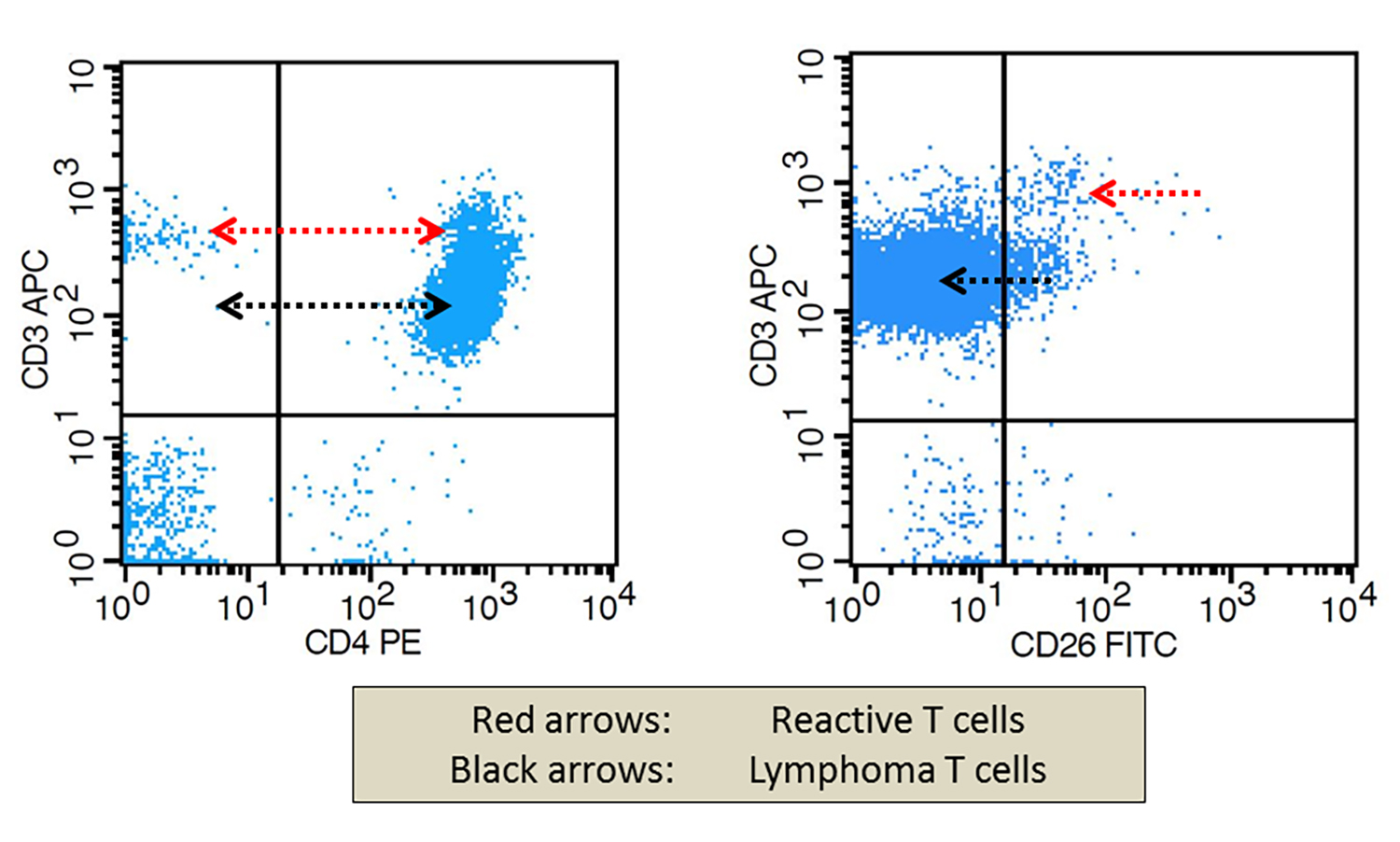
- Absolute Sézary cell count ≥ 1 x 109/L (manual count or by flow cytometry)
- Alternative diagnostic criteria: ≥ 40% of CD4+ / CD7- and ≥ 30% of CD4+ / CD26- T cells (flow cytometry)
- TRBC1 is more sensitive and specific for detection of clonal T cells by flow cytometry
- Expanded CD4+ T cell population with CD4:CD8 ≥ 10
- It is reliant on the absolute CD8 count and the ratio may decrease after treatment
- Loss of one or more T cell antigens
- Demonstration of T cell clonality by southern blot or PCR based methods
- Use of V beta antibodies allows quantification of the clone (obsolete)
- Clonality by flow cytometry: constant chain of T cell receptor: TRBC1 (Int J Mol Sci 2021;22:1817)
- Use of high throughput sequencing is more sensitive but not widely used
- Cytogenetic demonstration of an abnormal clone
- Bone marrow involvement is not a defined criteria for SS
- Criteria for blood response (see staging table below):
- Complete response (CR): B2 should change to B0
- Partial response (PR): at least 50% reduction of tumor burden
- Progressive disease (PD): B0 or B1 to B2 with an increase in absolute counts of ≥ 50% or B2 with an increase in absolute counts of ≥ 50% or loss of response with an increase in absolute counts ≥ 1x109/L and ≥ 50% from nadir
- Relapse: increase in absolute counts of ≥ 1x109/L in the context of CR
Staging / staging classifications
- Staging of mycosis fungoides and SS is performed according to the International Society for Cutaneous Lymphomas (ISCL) and the European Organization for Research and Treatment of Cancer (EORTC) (Eur J Cancer 2017;77:57, Blood 2016;127:3142)
| Skin | |
| T1 | Limited patches, papules or plaques covering < 10% of the skin surface: T1a: patch only T1b: plaque with or without patch |
| T2 | Patches, papules or plaques covering ≥ 10% of the skin surface: T2a: patch only T2b: plaque with or without patch |
| T3 | One or more tumors (≥ 1cm diameter) |
| T4 | Confluence of erythema covering ≥ 80% body surface area |
| Node | |
| N0 | No clinically abnormal peripheral lymph node |
| N1 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grade 1 or NCI LN0-2 N1a: clone negative N1b: clone positive |
| N2 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grade 2 or NCI LN3 N2a: clone negative N2b: clone positive |
| N3 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grade 3 - 4 or NCI LN4; clone positive or negative |
| Nx | Clinically abnormal peripheral lymph nodes with no histological confirmation |
| Visceral | |
| M0 | No visceral organ involvement |
| M1 | Visceral involvement (must have pathological confirmation) |
| Peripheral Blood (PB) | |
| B0 | Absence of significant blood involvement: ≤ 5% Sézary cells in PB B0a: clone negative B0b: clone positive |
| B1 | Low blood tumor burden: > 5% Sézary cells in PB and does not meet criteria of B2 B1a: clone negative B1b: clone positive |
| B2 | High blood tumor burden: ≥ 1000/µL Sézary cells in PB with clone positive |
- SS is defined as stage IVA1 (T1-T4, N0-N2, M0, B2) and above
Prognostic factors
- Poor prognosis (J Am Acad Dermatol 2012;67:1189, Blood Adv 2019;3:1145, Blood 2016;127:3142, J Am Acad Dermatol 2014;70:223.e1, Clin Cancer Res 2012;18:5051)
- Median overall survival after diagnosis is 3.1 - 4 years
- Cases with visceral involvement have a median overall survival of 2.5 years
- Median time to death after diagnosis is 3.5 years
- 5 year overall survival range is 18 - 37% and 10 year overall survival range is 0 - 18%
- Median overall survival after diagnosis is 3.1 - 4 years
- Overexpression of CD47 by Sézary cells correlates with worse overall survival (Blood Adv 2019;3:1145)
- Poor prognostic factors (J Am Acad Dermatol 2012;67:1189, Blood 2016;127:3142, Leuk Lymphoma 2012;53:868, Clin Cancer Res 2012;18:5051)
- Advanced age at diagnosis (> 65 years)
- Diagnosis of mycosis fungoides before diagnosis of SS
- Presence of TCR gene rearrangement in skin or blood
- Usually present
- Higher lactate dehydrogenase levels at presentation
- Number of Sézary cells in the peripheral blood does not appear to be a prognostic factor (J Am Acad Dermatol 2014;70:223.e1)
- Increased risk of a secondary lymphoma in patients with SS / mycosis fungoides (Arch Dermatol 2007;143:45)
Case reports
- 49 year old man with clinical presentation of erythematous papules (J Dermatol 2019;46:61)
- 64 year old man with pulmonary involvement by Sézary syndrome (Int J Dermatol 2016;55:81)
- 66 year old man with renal involvement by Sézary syndrome (Am J Kidney Dis 2018;72:890)
- 70 year old woman with bullous Sézary syndrome (Dermatol Online J 2020;26:13030)
Treatment
- Chronic condition and incurable: the main aim of treatment is control of symptoms and improvement of quality of life (Lancet 2008;371:945, Am J Hematol 2019;94:1027, Blood 2016;127:3142,J Am Acad Dermatol 2011;64:352)
- Systemic therapies
- Retinoids (derivates of vitamin A) and bexarotene (Lancet 2008;371:945, Am J Hematol 2019;94:1027, Curr Hematol Malig Rep 2016;11:468, Blood 2016;127:3142, J Am Acad Dermatol 2014;70:223.e1)
- Well tolerated
- Efficacy in cases of extracutaneous involvement
- Moderate rates of response can be achieved if used as monotherapy
- Chlorambucil associated with prednisone
- Methotrexate (MTX) low dose
- Recombinant interferon α (Lancet 2008;371:945, Blood 2016;127:3142, J Am Acad Dermatol 2014;70:223.e1)
- Used as monotherapy or associated with retinoids
- Conventional chemotherapy (Lancet 2008;371:945, Am J Hematol 2019;94:1027, Blood 2016;127:3142)
- Cyclophosphamide, hydroxydaunorubicin, oncovin and prednisone (CHOP) or CHOP-like regimen
- Rarely achieves complete durable remission
- Target therapy (Lancet 2008;371:945, Am J Hematol 2019;94:1027, Blood Adv 2019;3:1145, Blood 2016;127:3142, J Am Acad Dermatol 2014;70:223.e1)
- Alemtuzumab
- Monoclonal antibody against CD52 cell surface glycoprotein
- May induce long term remission
- Second line therapy
- Anti-PD1
- CD47 / SIRPα
- Alemtuzumab
- Extracorporeal photopheresis (ECP) (Lancet 2008;371:945, Am J Hematol 2019;94:1027, Blood 2016;127:3142, J Am Acad Dermatol 2014;70:223.e1)
- Considered first line of treatment
- May induce long term remission
- Excellent safety profile and response rates up to 60%
- Alone or in combination with systemic therapy
- Histone deacetylase inhibitors (HDACi) (Lancet 2008;371:945, Am J Hematol 2019;94:1027, Curr Hematol Malig Rep 2016;11:468, Blood 2016;127:3142, J Am Acad Dermatol 2014;70:223.e1)
- Vorinostat and romidepsin
- Overall response rate 24 - 38%
- Vorinostat and romidepsin
- Allogenic stem cell transplantation (Blood 2016;127:3142, J Clin Oncol 2010;28:2365)
- There is no consensus regarding the indication
- May be preceeded by skin electron beam radiation
- Acute and chronic graft versus host disease occur in more than 50% of patients
- Retinoids (derivates of vitamin A) and bexarotene (Lancet 2008;371:945, Am J Hematol 2019;94:1027, Curr Hematol Malig Rep 2016;11:468, Blood 2016;127:3142, J Am Acad Dermatol 2014;70:223.e1)
Clinical images
Microscopic (histologic) description
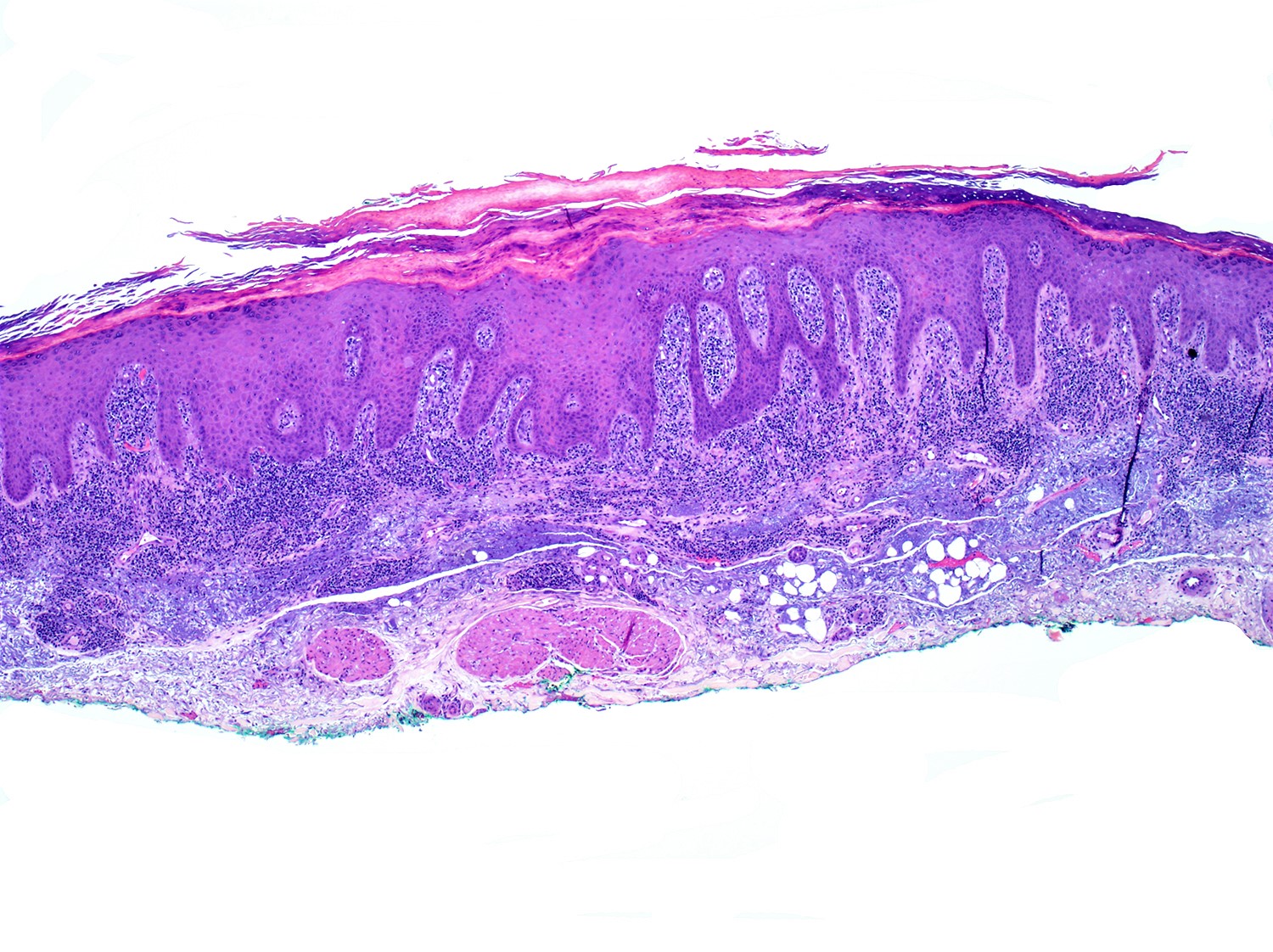
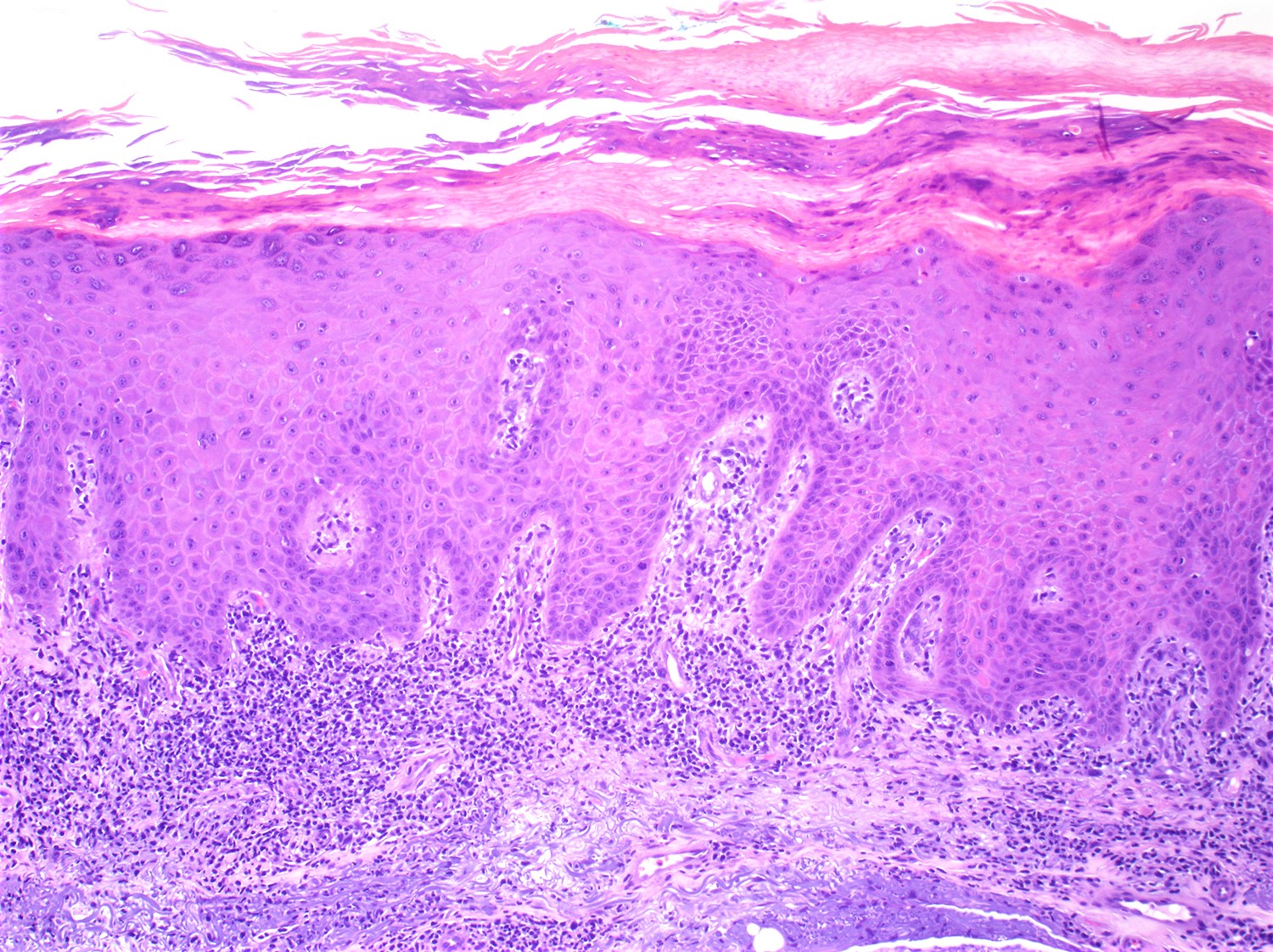
- SS is histologically similar to mycosis fungoides but sometimes epidermotropism is not present (Lancet 2008;371:945, Curr Hematol Malig Rep 2016;11:468, J Am Acad Dermatol 2011;64:352)
- May range from limited to superficial perivascular lymphocytic and eosinophilic dermatitis with or without spongiosis (atopic-like lesions) to lichenoid lymphocytic infiltrate
- Pautrier microabscesses are not common
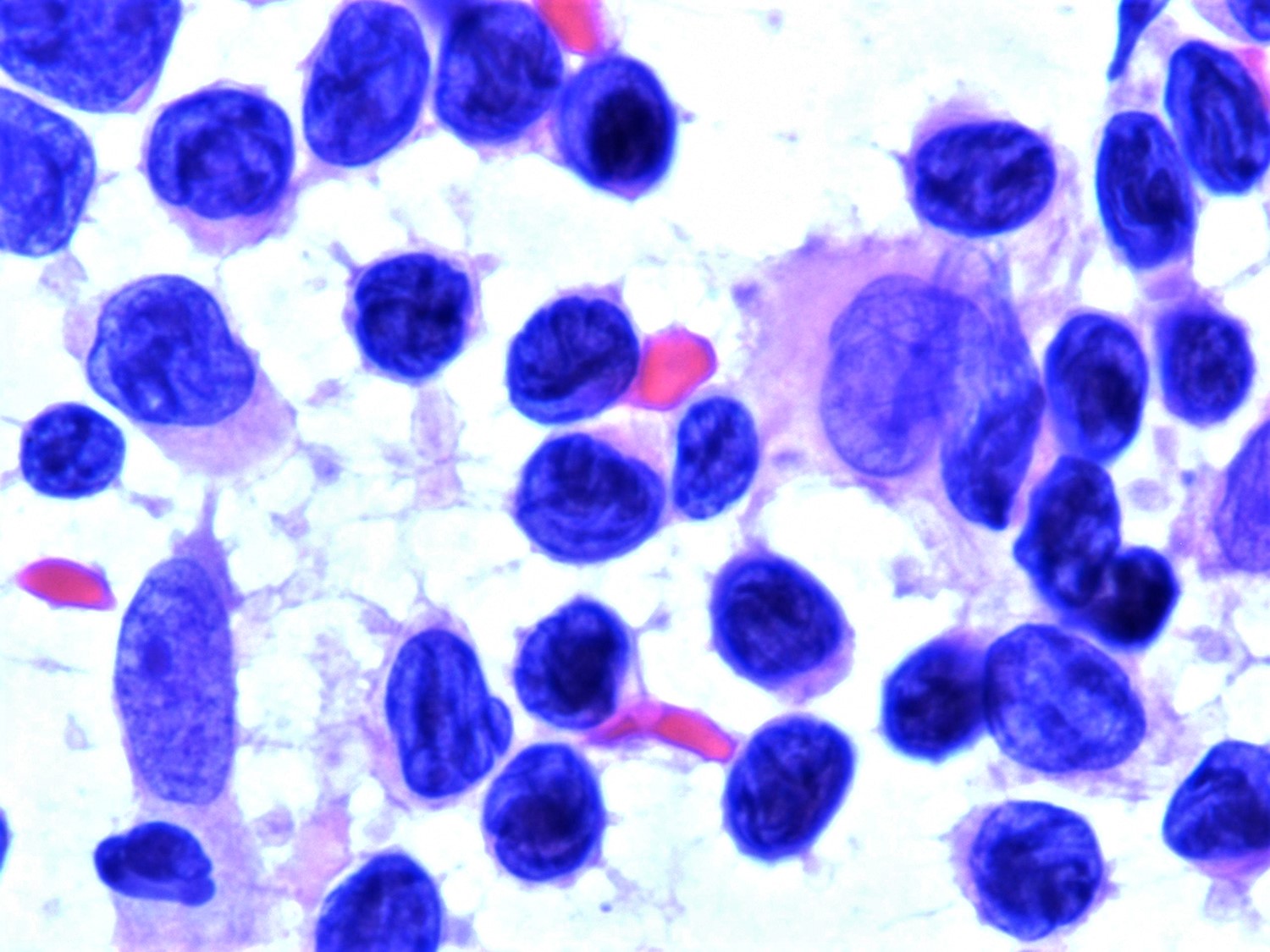
- Peripheral blood (Lancet 2008;371:945)
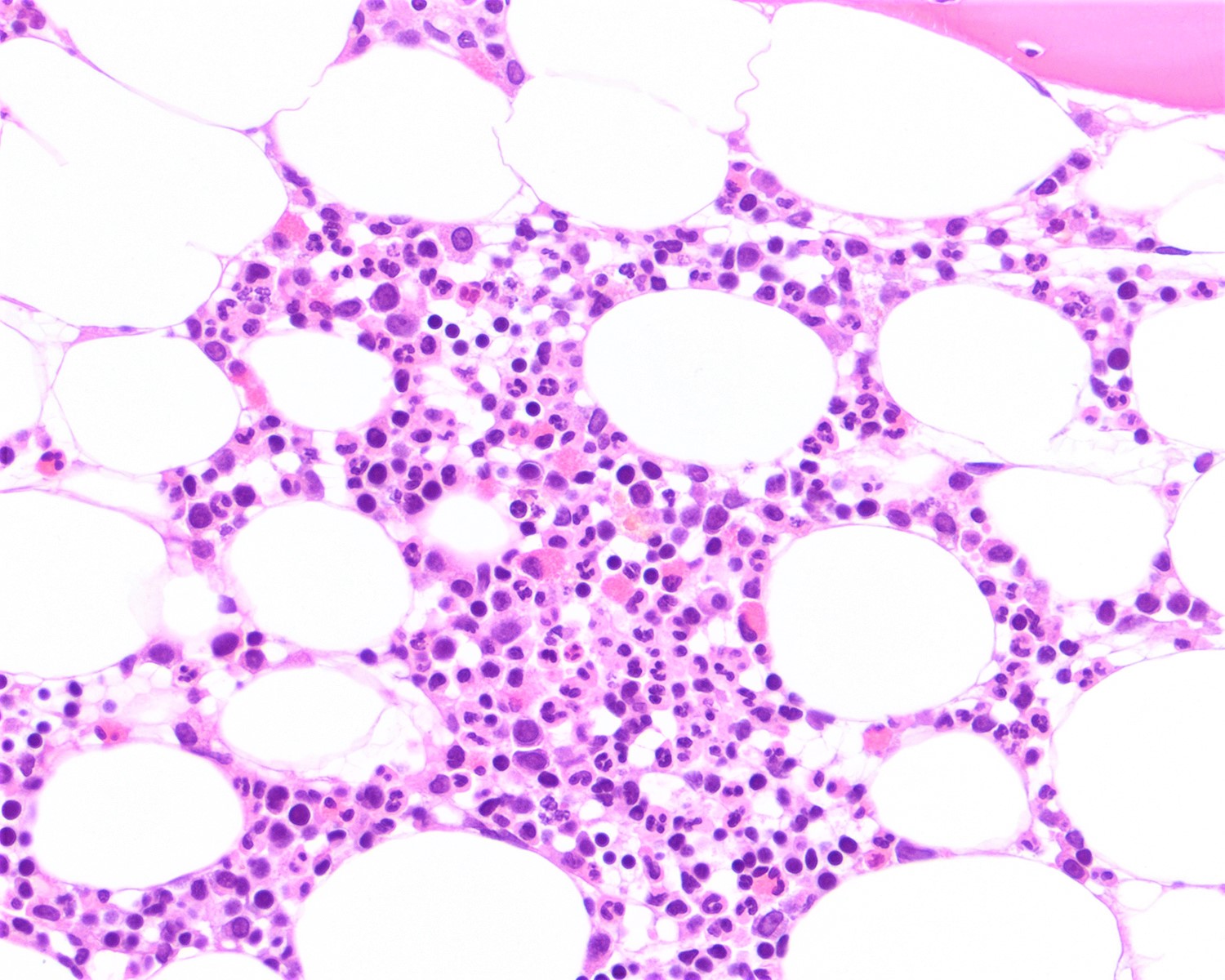
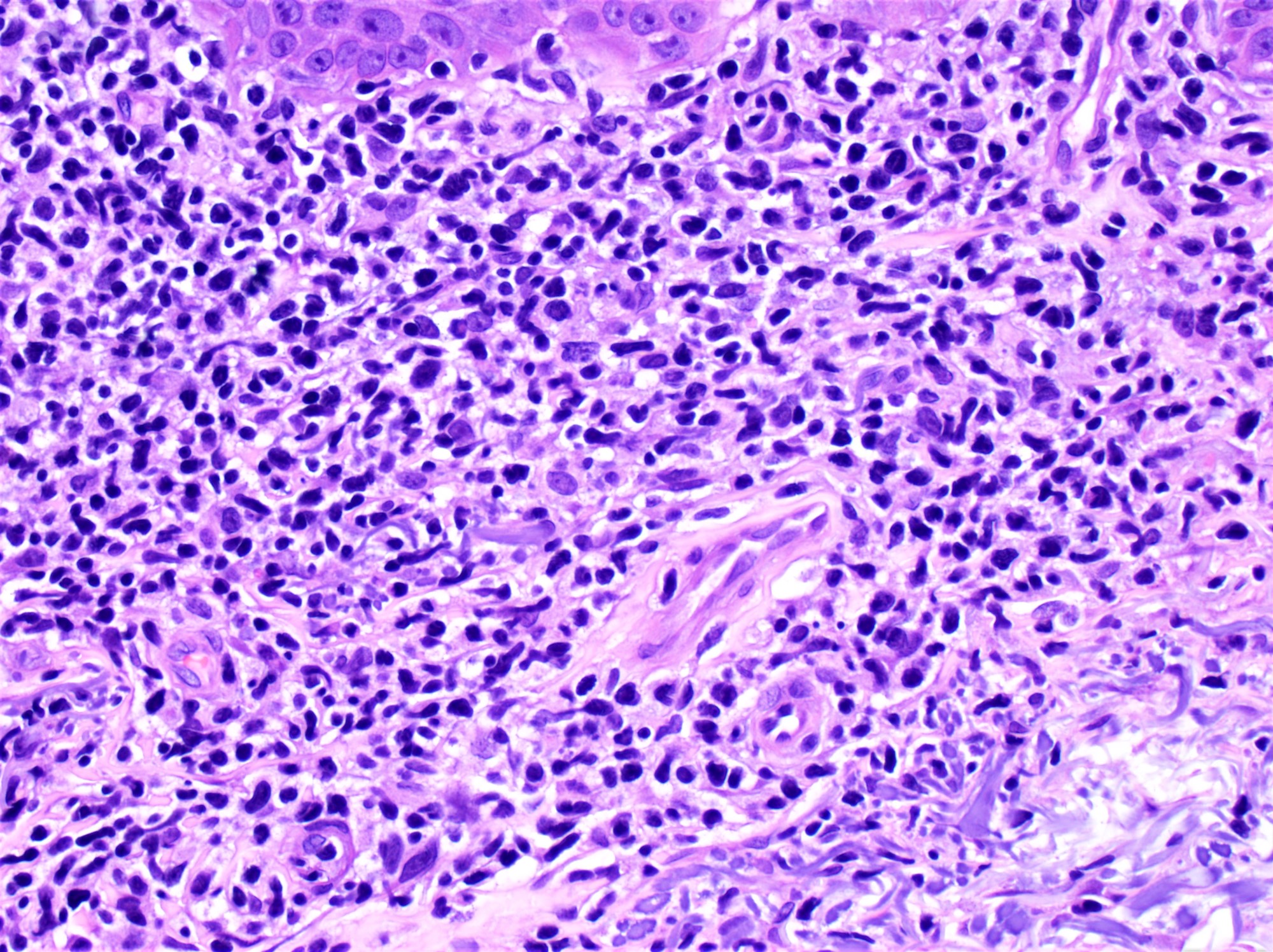
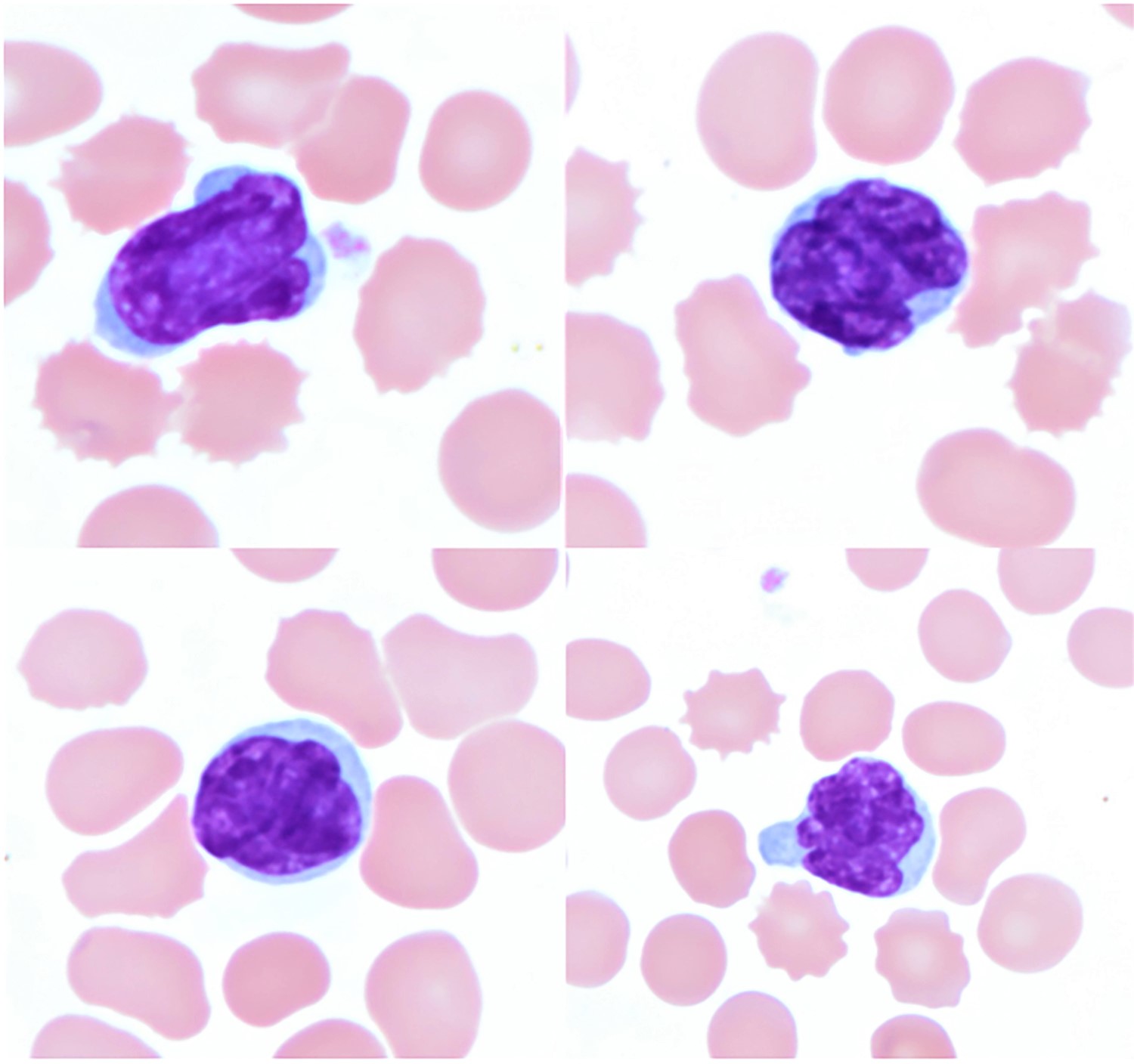
- Sézary cells: atypical lymphocytes of intermediate to large size and cerebriform nuclei
- Similar cells, albeit few or rare may be found in healthy individuals or in patients with other inflammatory skin diseases
- Sézary cells: atypical lymphocytes of intermediate to large size and cerebriform nuclei
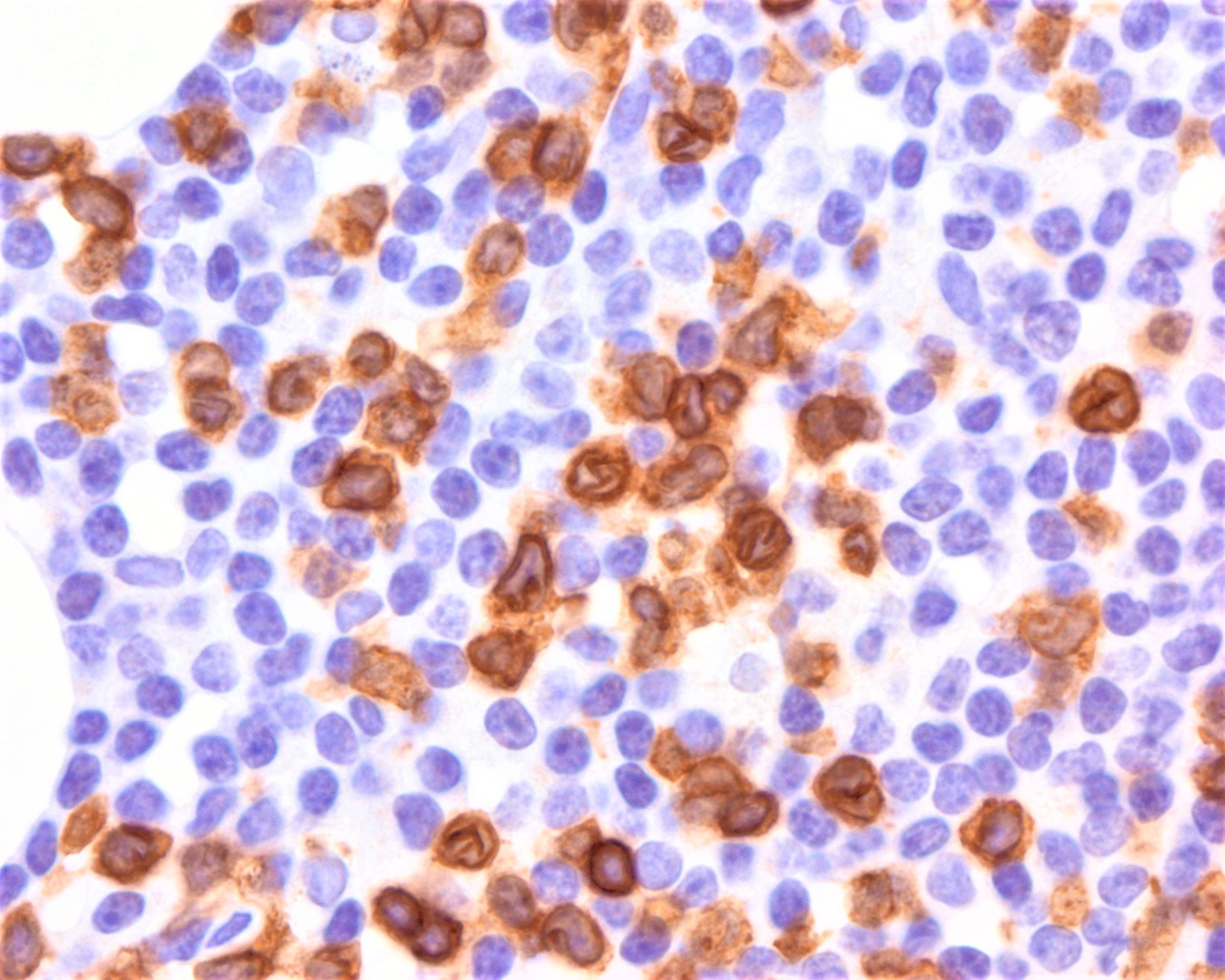
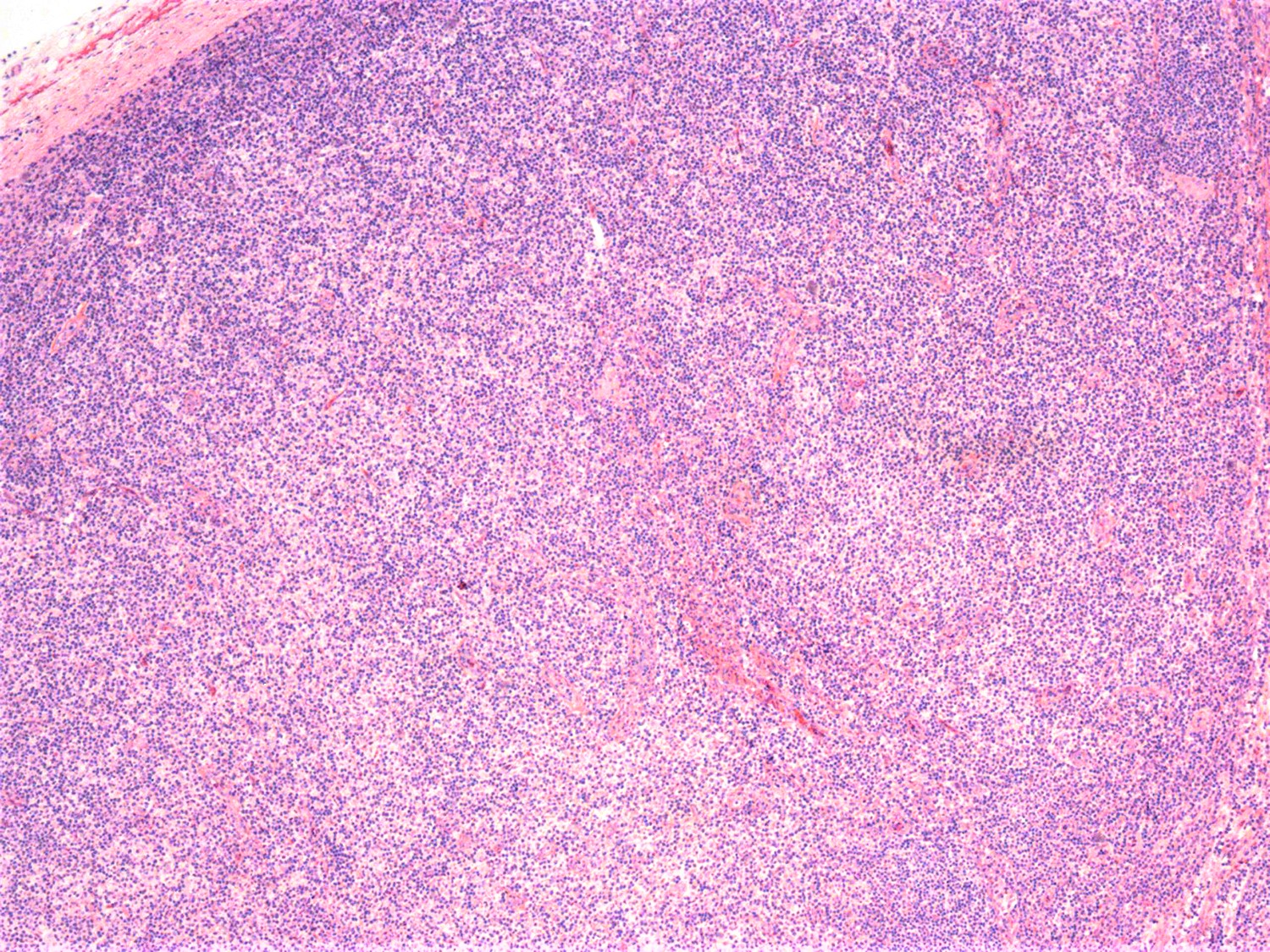
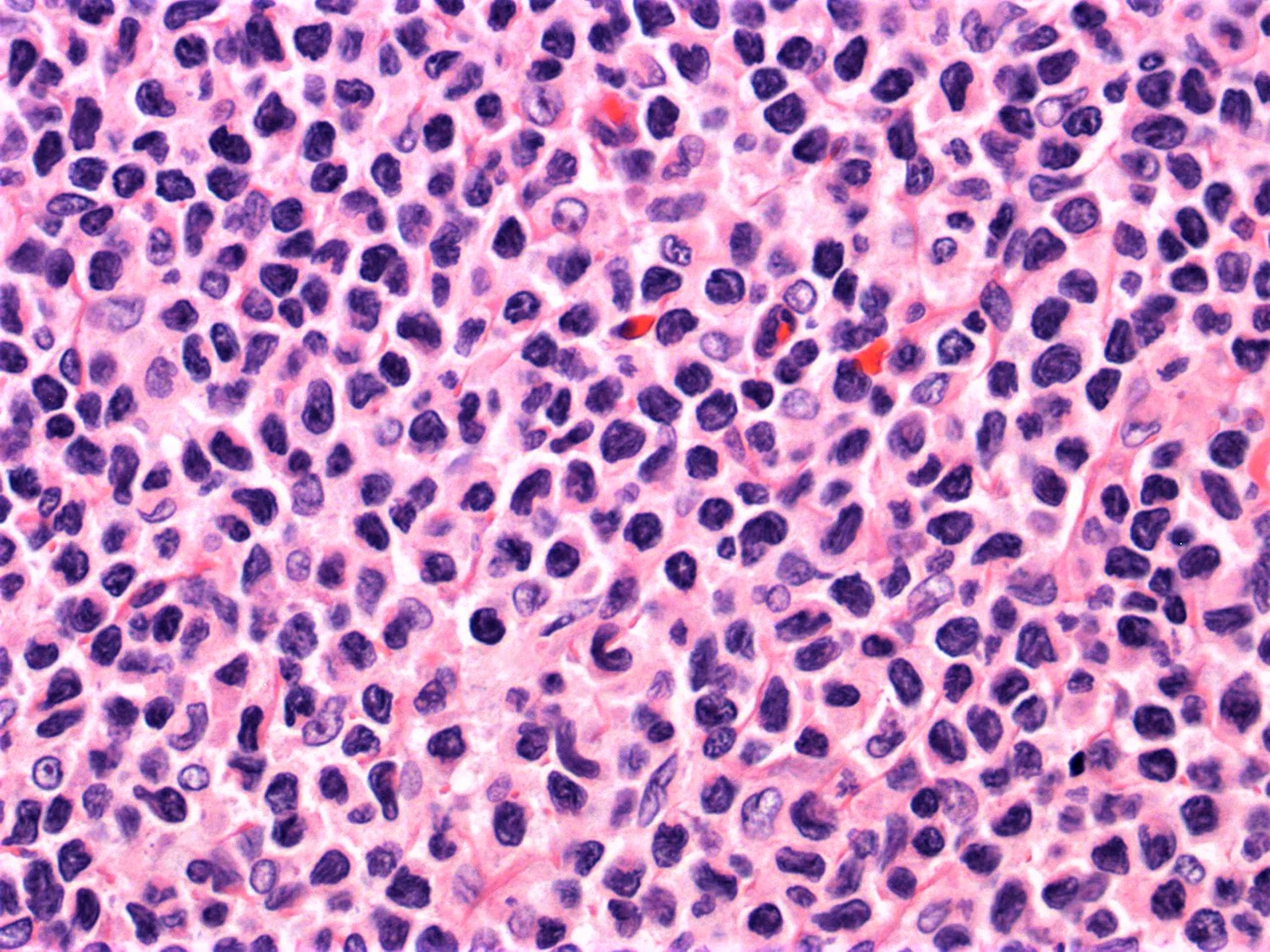
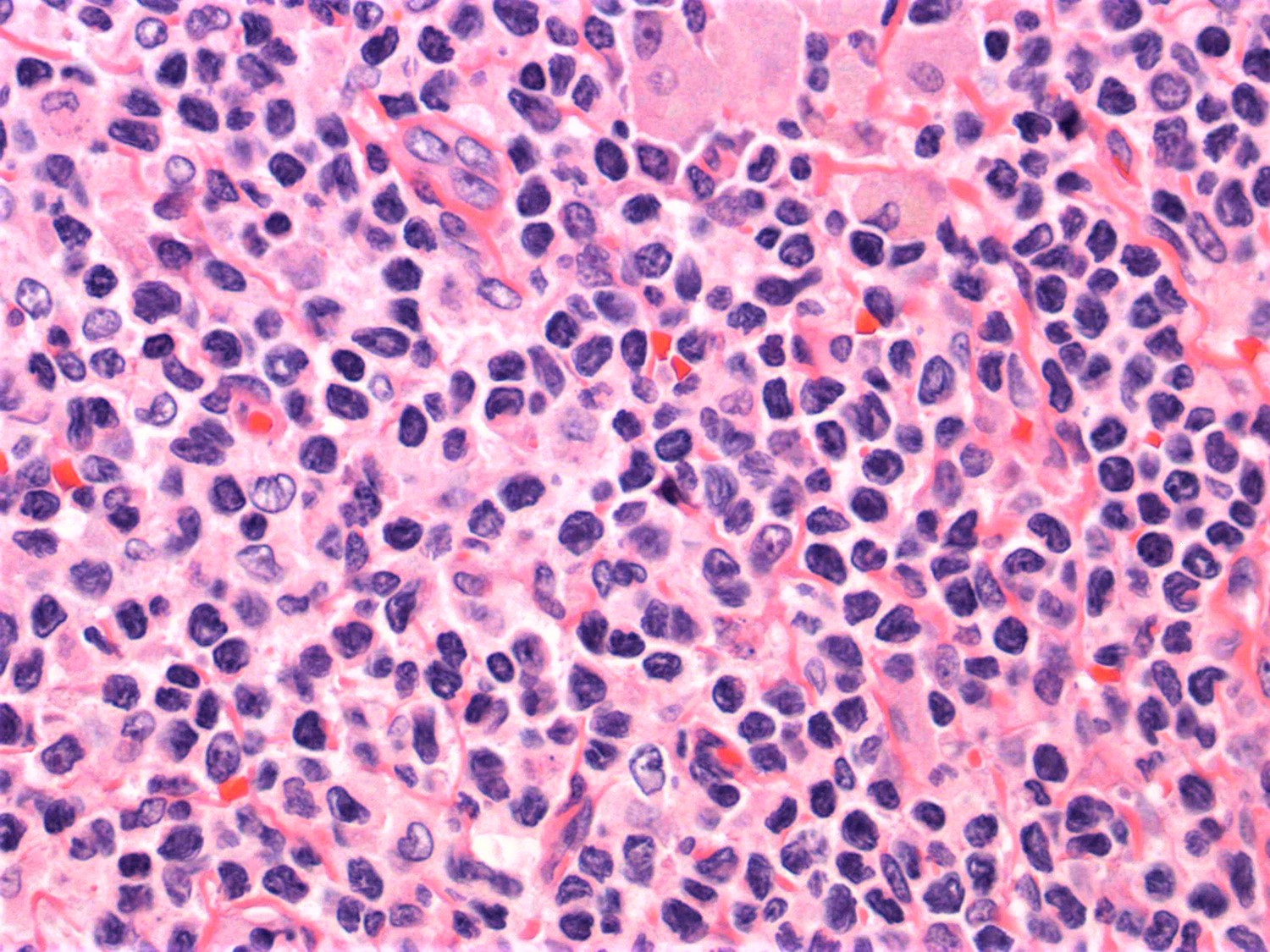
- Lymph node (Am J Hematol 2019;94:1027, Cancer 1986;57:237)
- Complete effacement of the nodal architecture by monotonous infiltrating population of Sézary cells
- Large cell or blastoid morphology may occur in advanced stages.
Microscopic (histologic) images
Peripheral smear images
Positive stains
- Heterogeneous expression of T cell markers (Blood Adv 2018;2:2115, Am J Hematol 2019;94:1027, Eur J Cancer 2018;93:47, Am J Clin Pathol 2011;136:944, Exp Dermatol 2017;26:668, Curr Hematol Malig Rep 2016;11:468, Blood 2013;122:3511, Leuk Lymphoma 2012;53:868)
- T central memory phenotype: CD45RO, CD62L and CD197 (Blood Adv 2018;2:2115, Exp Dermatol 2017;26:668, Curr Hematol Malig Rep 2016;11:468)
- Some cases may have a T naïve or stem cell memory T and express CD45RA
- CCR4 and CCR7 (Lancet 2008;371:945, Curr Hematol Malig Rep 2016;11:468)
- CD47 (Blood Adv 2019;3:1145)
- Possible target therapy
- CTLA4 (Exp Dermatol 2017;26:668)
- PD-1 (Arch Dermatol 2012;148:1379)
- When positive, it is usually expressed in > 50% of cells
- NOTCH1 (N terminal sequence of icNOTCH1 antibody) (J Invest Dermatol 2012;132:2810)
- Syndecan-4 (Exp Dermatol 2017;26:668)
- Aberrant expression of MHC class I killer immunoglobulin-like receptor KIR / CD158κ (Am J Hematol 2019;94:1027, Exp Dermatol 2017;26:668, Curr Hematol Malig Rep 2016;11:468)
- TCRVβ (Blood Adv 2018;2:2115, Exp Dermatol 2017;26:668, Leuk Lymphoma 2012;53:868, Mod Pathol 2010;23:284)
Negative stains
- Loss of one or more conventional T cell markers: CD2, CD5, CD7, CD8, CD25, CD26, CD30, FOXP3 (Blood Adv 2018;2:2115, Am J Hematol 2019;94:1027, Eur J Cancer 2018;93:47, Am J Clin Pathol 2011;136:944, Exp Dermatol 2017;26:668, Curr Hematol Malig Rep 2016;11:468,Leuk Lymphoma 2012;53:868, J Am Acad Dermatol 2011;64:352)
- B cell markers (Exp Dermatol 2017;26:668)
Molecular / cytogenetics description
- No driver mutations identified (Blood Adv 2018;2:2115)
- Somatic mutations in TCR/NFκB signaling, Th2 differentiation (ZEB1), cell survival (e.g. JAK / STAT), epigenetic regulation (e.g. DNMT3A, TET2, SMARCA4), homologous recombination (e.g. BRCA2) and cell cycle control (e.g. TP53) (Exp Dermatol 2017;26:668, Curr Hematol Malig Rep 2016;11:468, Nat Commun 2015;6:8470, Nat Genet 2015;47:1465)
- Activation of NOTCH1 signaling (J Invest Dermatol 2012;132:2810)
- Heterogeneous expression of transcripts (Blood Adv 2018;2:2115, Exp Dermatol 2017;26:668, Curr Hematol Malig Rep 2016;11:468)
- S100A4, S100A10, GATA3, Twist1, TOX, IL7R, CCR7 and CXCR4 may be upregulated
- ARID1A, SATB1, STAT4 and Fas may be downregulated
- Genomic complexity (Exp Dermatol 2017;26:668, Curr Hematol Malig Rep 2016;11:468, Nat Commun 2015;6:8470, Blood 2013;122:3511)
- No specific recurrent translocations or gene fusions
- Cytogenetic abnormalities
- Gain of 17p11.2-q25.3 and 8q24.1-8q24.3
- Loss of 17p13.2-p11.2 and 10p12.1-q26.3
- Deletion of PTEN on locus 10q23 and it is usually monoallelic
Sample pathology report
- Skin, shave biopsy of the arm:
- Primary cutaneous T cell lymphoma, most consistent with Sézary syndrome (see comment)
- Comment: Section shows an adequate skin shave biopsy with mild spongiosis and perivascular and lichenoid infiltrate in superficial dermis with mild epidermotropism. The infiltrate is composed of small lymphocytes, with irregular nuclear contours (cerebriform-like), hyperchromatic nuclei and scant cytoplasm. Immunohistochemical studies show that the abnormal lymphocytes are diffusely positive for CD3, CD4, CD5 and CD45RO and negative for CD2, CD7 and CD30. The concurrent complete blood count (CBC) reveals lymphocytosis (7 x 109/L) with abnormal Sézary cell count and the flow cytometry immunophenotype of the peripheral blood revealed CD4+ lymphocytosis (5 x 109/L) with low CD8+ count (0.8 x 109/L) and expanded CD4+ CD26- (63%) and CD4+ CD7- (58%). According to clinical notes, the patient is a 67 year old man who presented to the department of dermatology with a 1 month history of generalized erythroderma (90% of skin involvement) and inguinal and axillary bilateral lymphadenopathy. In summary, patient presented with erythroderma and skin is involved by cerebriform T lymphocytes with minimal epidermotropism. Peripheral blood is involved morphologically by cerebriform lymphocytes that by flow cytometry express CD3, CD4 and lack CD7 and CD26. These features support the diagnosis of Sézary syndrome.
Differential diagnosis
- Nonneoplastic erythroderma (pseudo E CTCL) (J Cutan Pathol 2006;33:27)
- Adult T cell leukemia / lymphoma (Semin Diagn Pathol 2020;37:81)
- Leukemia cutis (Int J Dermatol 2012;51:383)
- Secondary cutaneous involvement of peripheral T cell lymphoma (Am J Clin Pathol 2013;139:491)
- Exclusion diagnosis: always rule out other defined entities that involve skin secondarily
- Small size morphology and CD4+
- Usually do not present cerebriform cells
- Secondary skin involvement; unusual confined to cutaneous sites
Additional references
Board review style question #1
Board review style answer #1
Board review style question #2
What is a consistent feature for the diagnostic criteria of Sézary syndrome (SS)?
- Absolute Sézary cell count ≥ 1 x 109/L
- Different T cell clones in skin, blood or lymph node
- Expanded CD8+ T cells with CD8:CD4 ≥ 10
- Loss of CD3 (T cell marker) expression
Board review style answer #2