Table of Contents
Definition / general | Essential features | Microbiology | EBV latent nuclear and membrane antigens functions | Latency programs | Pathophysiology | Oncogenic mechanisms | Laboratory diagnosis | EBV associated reactive B cell proliferations | EBV associated B cell lymphoproliferative disorders | EBV positive T / NK lymphoproliferative disorders of childhood | Lymphomas where EBV+ is essential for diagnosis | Lymphomas where EBV+ is not essential for diagnosis | EBV positive T and NK cell aggressive lymphomas | Diagrams / tables | Clinical images | Microscopic (histologic) images | Peripheral smear images | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Segura-Rivera R, Miranda RN, Marques-Piubelli ML. EBV related lymphoid proliferations. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lymphnodesebv.html. Accessed April 2nd, 2025.
Definition / general
- EBV is the human herpesvirus 4 (HHV4) and belongs to the Herpesviridae family
- There are 4 patterns of EBV latency, defined based on the pattern of EBV proteins and small RNAs expressed in in vitro infections and in EBV induced tumors
Essential features
- Epstein-Barr virus (EBV) is the most common viral infection in humans, with 95% of the worldwide population showing an asymptomatic lifelong carrier stage state (Am J Clin Pathol 2023;159:14)
- Most EBV infections are acquired in childhood and are asymptomatic
- Patients who acquire the infection in the second decade usually develop the clinical features of infectious mononucleosis (IM), a self limited disease
- EBV plays an important role in the oncogenesis of a wide spectrum of neoplasms including solid tumors, B and T cell lymphomas, as well as classic Hodgkin lymphoma (CHL)
Microbiology
- EBV is the human herpesvirus 4 (HHV4) and belongs to the Herpesviridae family
- There are 2 subtypes: EBV1 and EBV2
- Distinguished by their sequences of the EBV nuclear antigens (EBNA) and the EBV encoded RNAs (EBER) (Viruses 2023;15:714)
- Subtype 1 is the more prevalent worldwide and has greater transforming potential (Viruses 2023;15:714)
- EBV is restricted to primate hosts and has B cell lymphotropism
- Can also infect epithelial cells where it causes growth cell arrest
- EBV can transform in vitro B lymphocytes to lymphoblastoid cell lines (LCLs) (Viruses 2023;15:714)
- EBV has a double stranded linear DNA genome containing ~100 genes surrounded by a protein capsid
- 172 kb in length
- Protein capsid contains glycoproteins important for cell tropism and receptor recognition
- Membrane antigen (MA) consists of 3 glycoproteins on the viral envelope (gp350, gp250, gp85) that mediate cell binding of virus
- Major viral envelope glycoprotein gp350 / 220 binds to complement receptor 2 (CD21) cell receptor on B lymphocytes
- Glycoprotein gp42 is essential for penetration of B cells by forming a complex with the histocompatibility complex (MHC) II
EBV latent nuclear and membrane antigens functions
- 6 EBV nuclear antigens (EBNA 1 - 6) are expressed in infected cells
- EBNA1
- Only known EBV protein to be consistently expressed in all EBV related cancers
- Main function is to ensure EBV double stranded DNA replication as cells multiply
- Avoids cytotoxic T lymphocytes surveillance
- Enhances cell survival, inhibits apoptosis
- EBNA2
- Transcriptional activation of viral EBNA and latent membrane proteins (LMP) as well as of cellular genes such as MYC
- Rewires host gene regulatory programs in multiple autoimmune diseases
- Functionally replaces the intracellular region of Notch receptor
- EBNA LP
- Assists EBNA2 mediated transcriptional activation
- Suppresses the innate cell response to viral DNA
- EBNA3A and EBNA3C
- Required for efficient transformation of B cells in vitro
- Mediate inhibition of various cyclin dependent kinase inhibitors (Bim, BLIMP1 and p16 / p15 / p18 expressions)
- Epigenetically control gene transcriptions
- EBNA3B
- Functions as a tumor suppressor
- EBNA1
- EBV has 3 LMP that are expressed in infected cells and are involved in immortalization
- LMP1
- Main transforming antigen of EBV
- Mimics CD40 signaling in B cells
- Drives growth and differentiation
- Increases cytokine production (IL6, IL8)
- Prevents p53 mediated apoptosis and upregulates BCL2
- Escape from immune surveillance via PI3K / AKT / mTOR pathway
- LMP2A
- Not essential for EBV induced transformation in vitro
- Drives proliferation and survival of B cells mimicking BCR (B cell receptor) signaling
- LMP2B
- Modulates the effects of LMP2A on BCR function
- LMP1
- EBV has 2 small noncoding RNAs (EBER1 and EBER2) and BamH1A region transcripts (BARTs, also known as miRNAs)
- EBERs can increase tumorigenicity, promote cell survival and induce interleukin 10 (IL10) expression in Burkitt lymphoma cell lines (Adv Cancer Res 2010;107:119)
- EBV is clonal in EBV associated neoplasms
- Viral clonality can be demonstrated by analysis of restriction enzyme digested EBV DNA terminal repeat fragments
- Detection of clonal EBV episomes in tumor suggests that malignant cells are also clonal
- See Table 1
- References: Viruses 2023;15:714, Pathology 2020;52:154
Latency programs
- There are 4 patterns of EBV latency, defined based on the pattern of EBV proteins and small RNAs expressed in in vitro infections and in EBV induced tumors (Viruses 2023;15:714)
- Expression of EBER is a constant feature of all EBV infected cells and therefore, is the most consistent marker to demonstrate EBV infection (see Table 1)
- EBV latency within B cells usually starts with type III in primary infection, to latency II while selecting pressure locally and type I that allows virus survival without expression of other viral genes (Pathology 2020;52:40)
- Type III: during initial infection of a resting naive B cell or in the context of immunodeficiency (Pathology 2020;52:40)
- Type II: under the selective pressure of an effective cell mediated immune response, the virus downregulates expression of several viral proteins (mostly EBNA2) entering into an intermediate stage; infected B cells differentiate into memory B cells (Pathology 2020;52:40, Semin Diagn Pathol 2020;37:32)
- Type I: restricted to the expression of EBER and EBNA 1 and is responsible for the maintenance and replication of the episomal EBV genome (Pathology 2020;52:40)
- Type 0: viral antigen expression is maximally suppressed and memory B cells are its main reservoir (Pathology 2020;52:40, Semin Diagn Pathol 2020;37:32)
- Latency III expresses all latent genes and is seen in (see
Table 2)
- EBV genome within LCLs
- Transiently seen in the normal course of EBV infection in healthy individuals
- Infectious mononucleosis
- Lymphoid proliferations and lymphomas associated with immune deficiency and dysregulation
- Primary central nervous system (CNS) lymphoma (human immunodeficiency virus [HIV] associated)
- Diffuse large B cell lymphoma (DLBCL) associated with chronic inflammation
- EBV positive breast implant associated large B cell lymphoma (Mod Pathol 2021;34:2154)
- Latency II (also referred to as the default program), in which LMPs are expressed
- Hodgkin lymphoma
- EBV positive DLBCL
- Lymphomatoid granulomatosis
- Angioimmunoblastic T cell lymphoma
- Extranodal NK / T cell lymphoma, nasal type
- Aggressive NK cell leukemia
- EBV associated T and NK cell lymphomas
- Latency I is described as a program where EBNA1 is the only viral protein expressed
- Immunocompetent / immunocompromised Burkitt lymphoma
- Primary effusion lymphoma (PEL)
- Plasmablastic lymphoma (PBL), oral type, in immunodeficient HIV patients
- Latency 0, in which none of the EBV antigens are expressed and immune recognition is avoided; typically observed in circulating memory B cells in healthy individuals
- See Table 3
Pathophysiology
- Target cells: oropharyngeal epithelium and B lymphocytes (Viruses 2023;15:714)
- B cells are the major reservoir of the virus before epithelial amplification in the oral cavity
- Oropharyngeal epithelial cells are permissive for viral replication
- EBV can shuttle between different cell types under certain circumstances: T cells, NK cells, monocytes, smooth muscle cells, follicular dendritic cells (FDC) and neurons
- First cell type to get infected is debated (Viruses 2023;15:714)
- Epithelial cells theory: the virus replicates and releases virions which then infect the colocalized B cells in the lymphoepithelial structures of the Waldeyer ring
- B cells theory: the virus enters tonsillar crypts, crosses the thin layer of epithelium and infects naive B cells residing in the follicular mantle
- Entry of EBV is through endocytosis (Viruses 2022;14:2372)
- B cells
- Viral surface glycoprotein gp350 / 220 attaches to CD21 / CD35 on the surface of B cells (Viruses 2022;14:2372)
- Second glycoprotein, gp42, binds to HLA class II molecules, triggers conformational changes and causes fusion of both membranes (Viruses 2022;14:2372)
- Epithelial cells
- BMRF2 glycoprotein facilitates EBV attachment through binding with β1, α5 and α3 integrins (Viruses 2022;14:2372)
- BMRF2 may also function in the cell to cell transmission of EBV (Viruses 2022;14:2372)
- B cells
- Initial replication occurs within oropharyngeal mucosal epithelium and spreads through infection of B cells in the lymphoid tissues in Waldeyer ring
- Infection of tonsillar naive B cells leads to a latency III program, with a full spectrum of latent proteins expressed
- Most of the proliferating cells are eliminated by NK cells and the emerging latent antigen specific primary T cell response (lytic phase)
- Some studies suggested that the infection of T and NK cells could occur through the immunological synapse, in an attempt by these cells to kill the EBV infected B cells (Front Oncol 2023;13:1240359)
- Molecule of HLA class II present on NK cells may interact with glycoproteins gp42 and gp85 from EBV, fundamental in the internalization (Front Oncol 2023;13:1240359)
- Some infected cells escape from immune surveillance by downregulating antigen expression and undergoing germinal center (GC) reaction, where a more limited set of viral genes are expressed in what constitutes the latency II program
- Stable reservoir of resting viral genome positive memory B cells is established when these EBV infected GC B cells migrate to peripheral blood, where viral antigen expression is silenced (latency 0)
- When EBNA1 is expressed intermittently during the division of these memory B cells, the viral genome is distributed to the daughter memory B cells (latency 1)
- During latent phase, EBV can persist in the host cells by replicating extrachromosomal nucleic acids (episomes)
- EBV maintains latency in memory B cell pool via expression of 2 EBV encoded small RNAs (EBER1 and EBER2), EBV nuclear antigens (EBNAs) and 3 LMPs
- EBNA1 maintains episomal state of EBV DNA in infected cells
- Protein EBNA2 blocks B lymphocyte differentiation and allows cell proliferation
- LMPs are membrane spanning proteins that function as constitutive active receptors to sustain B cell activation and differentiation independently of ligand binding
- LMP1 is a major oncogene that functions as a constitutively active member of tumor necrosis factor receptor family
- CD8 positive cytotoxic T cells and NK cells kill infected B cells
- Virus is periodically reactivated and shed in saliva
Oncogenic mechanisms
- Oncogenes lead inhibition of cell apoptosis and host cell cycle disturbances that promote G1 / S phase transition
- EBV genes activate oncogenes such as MYC and BCL2 and signaling pathways such as JNK, NFκB, PI3K / Akt and JAK / STAT
- Virus also inhibits tumor suppressor genes such as TP53, DOK1, PRDM1, PKR, PTEN, DICE1 and others
- p21cip1 and p27kip1 is downregulated by EBERs, while BCL2 is upregulated and thereby inhibits the release of CDK4 and CDK2 and promotes the cell cycle from G1 phase to S phase
- Histone modification, DNA methylation, noncoding RNA expression and chromatin remodeling
- Reference: Microb Pathog 2023;183:106292
Laboratory diagnosis
- Serology (Viruses 2023;15:656)
- Method of choice for unequivocal diagnosis
- Mainly includes the exploration of 3 or 4 markers using automated immunoassays
- Antiviral capsid antigen (VCA) or total anti-EBV immunoglobulin M (IgM)
- Anti-VCA
- Anti-early antigen (EA) or total anti-EBV immunoglobulin G (IgG)
- Anti-Epstein-Barr nuclear antigen 1 (EBNA1) IgG
- Combination of these markers can differentiate a primary from a past infection
- Antibodies to EA IgG generally occur in acute phase of illness
- VCA IgM appears with onset of symptoms and usually persists for 2 - 3 months
- With or without anti-VCA IgG but always without anti-EBNA1 IgG
- VCA IgG appears with onset of symptoms and is positive for life
- May be absent at the very start of symptoms
- VCA IgM appears with onset of symptoms and usually persists for 2 - 3 months
- Antibodies to EBNA1 IgG and EBNA2 IgG
- EBNA1 IgG appears after 2 - 3 months
- 3 - 5% of individuals and 10 - 20% of immunocompromised patients, anti-EBNA1 IgG is not detected after primary infection
- Not related to specific symptoms or risks
- EBNA2 IgG can appear early in 30% of patients in course of disease
- Past infection is defined by the presence of anti-VCA IgG and anti-EBNA1 IgG without anti-VCA IgM
- Antibodies to VCA IgG, VCA IgM and EBNA1 IgG can be used to distinguish acute and past infections in immunocompetent patients
- VCA IgG and VCA IgM in absence of EBNA1 IgG indicates acute infection
- VCA IgM antibodies decrease and VCA IgG antibodies increase during convalescence
- VCA IgG and EBNA1 IgG in absence of VCA IgM indicate past infection
- Antibodies to EA IgG generally occur in acute phase of illness
- Rapid antibody responses to EBV are correlated with a reduced severity of primary infection
- Serology is less useful in immunocompromised patients who may have atypical antibody profiles
- Peripheral blood smear
- Patients with IM can present with atypical lymphocytes (Downey cells)
- 3 types (Lancet 2020;395:225)
- Type I: slightly larger than normal lymphocytes, with indented or so called bean shaped nuclei, reduced cytoplasm with few azurophilic granules and vacuoles
- Type II: larger with abundant pale cytoplasm and often abutting or partly surrounding adjacent red blood cells
- Type III: also large but they have one or more prominent nucleoli and abundant, deeply basophilic cytoplasm
- They represent activated CD8 T lymphocytes responding to EBV infected cells (Curr Top Microbiol Immunol 2015;390:211)
- 3 types (Lancet 2020;395:225)
- Patients with IM can present with atypical lymphocytes (Downey cells)
- Heterophile antibodies (Curr Top Microbiol Immunol 2015;390:211)
- Not specific
- Heterophile test detects IgM class antibodies that agglutinate mammalian erythrocytes
- Peak 2 - 5 weeks after onset of clinical symptoms and are useful in diagnosis of primary EBV infection
- Heterophile antibodies have sensitivity of 63 - 84% and specificity of 84 - 100%
- 25% false negative rate in first week of illness and in young children
- False positive tests may occur with malignancies, autoimmune disease, other infections
- Polymerase chain reaction (Methods Mol Biol 2017;1532:33)
- Mainly used to monitor EBV viral loads in specific clinical situations
- Diagnosis and monitoring antiviral therapy in patients with posttransplant lymphoproliferative disorder (PTLD)
- Immunocompromised patients who may have defective antibody production
- Because serological reactivation does not correlate with viral load in this patient group, EBV serological testing is not useful at all
- Young children with heterophile negative IM
- Most used EBV gene targets in these assays are BALF5 gene (coding for thymidine kinase), BamHIW region, EBNA1 gene, EBER1 gene
- Whole blood is the specimen of choice
- EBV DNA is located within memory B cells
- Cerebrospinal fluid can be tested by PCR to diagnose EBV infection of central nervous system
- Mainly used to monitor EBV viral loads in specific clinical situations
- See Table 2
EBV associated reactive B cell proliferations
Infectious mononucleosis
- Acute infection that usually follows primary infection
- Fatigue, fever, exudative pharyngitis, anterior and posterior cervical lymphadenopathy
- Infection is usually self limited
- Microscopic features
- Histologic changes are variable and depend on disease duration
- Early stage: reactive follicles, with mild paracortical expansion
- Progression: follicles sparse due to interfollicular expansion
- Advanced stage: follicles may become effaced, with interfollicular expansion
- Interfollicular areas with numerous immunoblasts or mixed infiltrate
- Immunoblasts can be binucleated, mimicking Hodgkin / Reed-Sternberg (HRS) cells
- Focal necrosis may be present
- Immunohistochemistry
- HRS-like cells are CD45 positive, CD15 negative, CD30 (weak positive) admixed with B and T immunoblasts
- EBV latency type III
- EBV encoded RNA (EBER) present in numerous infected cells, ranging from small and intermediate lymphocytes to HRS-like immunoblasts
- EBNA1 positive, EBNA2 variable and EBNA3 positive
- LMP1 positive and LMP2 positive
- Most T lymphocytes in the background are CD8+
- Histologic changes are variable and depend on disease duration
- Reference: Semin Diagn Pathol 2018;35:54
EBV associated B cell lymphoproliferative disorders
EBV positive mucocutaneous ulcer
Lymphoproliferative disorders associated with immune deficiency and dysregulation
- Occurs in the context of attenuated immunosurveillance, such as the use of iatrogenic immunosuppression, acquired immunodeficiency, primary immunodeficiency, posttransplant setting or age associated immunodeficiency
- Isolated, self limited, shallow, well circumscribed lesion in the oropharyngeal mucosa, skin or gastrointestinal tract
- Presence of ≥ 2 lesions is best classified as EBV+ polymorphic B cell lymphoproliferative disorder (LPD) (nodal and extranodal, recently proposed entity) or EBV+ DLBCL
- No lymphadenopathy, organomegaly or bone marrow infiltration, no lactate dehydrogenase (LDH) elevation
- EBV DNA in blood is not increased
- Useful criterion in the differential diagnosis with EBV positive DLBCL, NOS
- Most cases regress spontaneously or with reduction of immunosuppression
- Microscopic features
- Sharply circumscribed ulcer; skin or mucosal sites
- Atypical intermediate size or large lymphoid cells
- HRS-like cells can be present
- Large cells can be numerous (large cell lymphoma-like)
- Background shows variable number and proportion of small lymphocytes, histiocytes, plasma cells and eosinophils
- Base of lesion is demarcated by rim of small T lymphocytes
- Immunophenotype
- Large lymphoid cells are B cells
- Non-germinal center phenotype: CD20 positive, PAX5 positive, OCT2 positive, MUM1 / IRF4 positive, CD10 negative
- CD30 positive, CD15 (~50%)
- Rim of CD8+ lymphocytes at base of lesion
- Large lymphoid cells are B cells
- EBV evaluation
- EBER might be positive mainly in the large HRS-like cells but it is frequently observed in a wide range of background cells, including small lymphocytes
- LMP1 positive and EBNA2 positive (50% of cases)
- Small lymphocytes within the lesion are T cells; increased CD8 / CD4
- References: Pathology 2020;52:40, Hum Pathol 2018;79:18
Lymphoproliferative disorders associated with immune deficiency and dysregulation
- Hyperplasias arising in immune deficiency / dysregulation (WHO terminology): mass lesion with preserved tissue architecture (nondestructive)
- Typically lack monoclonal Ig gene rearrangements
- Polymorphic lymphoproliferative disorders arising in immune deficiency / dysregulation (WHO terminology): mass lesion with effaced / destroyed tissue architecture arising after transplant
- Monoclonal or oligoclonal Ig gene rearrangements in ~60% of cases
- Cytogenetic abnormalities in ~33% of cases
- Both types are due to impaired host immunosurveillance
- Posttransplant setting
- Most common scenario for polymorphic LPDs
- Cancer therapy associated
- Autoimmune diseases
- HIV setting
- Posttransplant setting
- Almost always associated with EBV infection
- Latency type III pattern: EBERs positive, LMPs positive, all EBV nuclear antigens positive
- Follicular hyperplasia type: EBER positive in 40 - 50%
- EBER is often localized to reactive GCs
- Microscopic features
- 3 types of nondestructive lesions are recognized
- Plasmacytic hyperplasia: medullary and interfollicular plasma cells, lymphocytes
- IM-like hyperplasia: paracortical expansion by CD30 positive immunoblasts
- Follicular hyperplasia: widely spaced, reactive follicles, usually reflect seroconversion from EBV negative to EBV positive
- Germinal centers of hyperplastic lymphoid follicles may contain numerous cells positive for EBER
- Polymorphic LPDs
- Effacement by variable size lymphocytes, immunoblasts, histiocytes and plasma cells
- Reed-Sternberg and Hodgkin-like cells are common
- These lesions do not meet criteria for lymphoma
- There are no sheets of large cells
- Effacement by variable size lymphocytes, immunoblasts, histiocytes and plasma cells
- 3 types of nondestructive lesions are recognized
- See Table 6
- References: Pathology 2020;52:40, Hum Pathol 2018;79:18, Am J Surg Pathol 2018;42:116, Laryngoscope 2011;121:1718
EBV positive T / NK lymphoproliferative disorders of childhood
Hydroa vacciniforme lymphoproliferative disorder
Severe mosquito bite allergy
Chronic active EBV disease (T and NK cell phenotype)
- Chronic EBV+ LPD of childhood with a broad spectrum of clinical aggressiveness and usually a long clinical course
- Pathogenesis is unknown; however, genetic predisposition might play a role (Blood 2019;133:2753)
- Classic
- Indolent, self limited, more common in White people
- Localized papulovesicular eruptions on sun exposed skin
- No systemic symptoms; rarely progresses to a more aggressive form (systemic)
- Systemic
- More common in Asia and Latin America
- Skin lesions in sun exposed and nonexposed areas
- Mild to severe disease
- Systemic symptoms: fever, lymphadenopathy, liver involvement
- Patients might respond initially to immunomodulating therapies but eventually will require treatment similar to chronic active EBV (CAEBV) disease
- Classic
- EBV DNA is elevated in the blood and the levels are not discriminatory between the 2 forms
- Most cases have clonal TCR gene rearrangements
- Microscopic features
- Intraepidermal spongiotic vesiculation
- Lymphoid infiltrate predominantly in the dermis but may extend into the subcutaneous tissue
- Mainly perivascular and periadnexal, often with angiodestructive features
- Epidermotropism (exocytosis) and ulcers are common
- Small neoplastic cells without atypia
- Immunophenotype
- Mostly CD8 positive, some cases are CD4 positive; rarely CD4+ / CD8+
- Occasional cases have an NK cell phenotype; tend to be more panniculitis-like
- Might mimic subcutaneous panniculitis-like T cell lymphoma (SPTCL)
- TIA1 positive, granzyme B positive, perforin positive; CD30 is often expressed
- EBER is positive in a variable number of cells; LMP1 is negative
- Reference: Virchows Arch 2023;482:227
Severe mosquito bite allergy
- Rare disease; exaggerated reaction to mosquito bites in children
- Localized, clear or hemorrhagic bullae in mosquito bitten areas
- Cases with systemic manifestations with organ infiltration should be classified as CAEBV disease
- Patients have an increased risk of developing hemophagocytic syndrome and progression to an overt NK / T cell lymphoma
- Symptomatology comes and goes every time a mosquito bites
- Microscopic features
- Tends to be panniculitis-like
- Features of angiodestruction are usually seen
- Most of the infiltrating cells have an NK cell phenotype
- EBV evaluation
- Association with decreased CD4 positive cells, high serum IgE titers and high EBV DNA copy number
- EBV positive NK cell lymphocytosis in peripheral blood
- LMP1 is rarely positive by IHC in tissue but can be detected by PCR in peripheral blood (latency II)
- EBER is positive in a fraction of the NK cells
- Much higher density of EBV positive cells should raise suspicion of NK cell lymphoma / leukemia
- Reference: Hum Pathol 2018;79:18
Chronic active EBV disease (T and NK cell phenotype)
- CAEBV disease refers only to T and NK cell lineage disease
- Excludes cases of B cell lineage that usually arise in patients with primary immunodeficiency
- Absence of underlying immunosuppression
- More often in children but also in young adults
- IM-like illness persistent for > 3 months
- Fever, lymphadenopathy, hepatosplenomegaly, anemia, skin rash, diarrhea and uveitis
- Rare manifestations: myocarditis, coronary aneurism, interstitial pneumonia, digestive tract ulcer / perforation, CNS involvement
- Symptoms range from mild to severe
- Protracted clinical course
- Patients with EBV infected T cells have a shorter survival time than patients with NK cell disease, prominent systemic symptoms and high titers of EBV DNA in blood
- Increased risk of progression to EBV+ T or NK cell lymphoma
- Microscopic features
- Small lymphocytic inflammatory changes without evidence of malignant lymphoproliferation
- Diagnosis is usually made in liver or a lymph node biopsy
- Liver: sinusoidal and portal infiltration suggestive of viral hepatitis
- Lymph nodes: either follicular or paracortical hyperplasia, focal necrosis or small granulomas
- Ancillary testing
- Lineage: T cells (60%) or NK cells (40%)
- CD4 positive > CD8 positive > γ δ T cells
- EBER is positive
- CD56 is expressed in 40% of cases
- Monoclonal TCR (40 - 63%); monoclonal EBV (84%)
- Somatic mutations in DDX3D and KMT2D have been demonstrated
- References: Am J Clin Pathol 2023;159:14, Virchows Arch 2023;482:227
Lymphomas where EBV+ is essential for diagnosis
EBV positive diffuse large B cell lymphoma, NOS
Diffuse large B cell lymphoma with chronic inflammation
Fibrin associated diffuse large B cell lymphoma
Lymphomatoid granulomatosis
- No history of immunodeficiency or previous lymphoma
- Preferential distribution among elderly patients might be related to physiological immunosenescence
- Extranodal mass with or without lymphadenopathy in ~70% of cases
- Only lymphadenopathy in ~30% of cases
- Microscopic features
- 2 types, no clinical or prognostic relevance
- Polymorphous
- Most frequent
- HRS-like and LP-like cells often present, scattered in a reactive background with small lymphocytes, histiocytes and plasma cells
- Monomorphous (large cell lymphoma)
- Large neoplastic cells with immunoblastic or centroblastic morphology, without a polymorphous inflammatory background
- Areas of necrosis; often geographic pattern
- Angiocentric / angiodestructive lesions can be present
- Polymorphous
- Immunophenotype
- CD20 positive, CD22 positive, CD79a positive, PAX5 positive
- 90% of cases are CD10 negative and BCL6 negative
- CD30 expression associated with a poorer prognosis in elderly patients but not in young patients; only a few are CD15 positive
- IRF4 / MUM1 positive
- EBV evaluation
- EBV DNA load may have prognostic significance in DLBCL patients regardless of tumor EBV status (Viruses 2023;15:656)
- Supports the hypothesis that EBV could disappear from the tumor after contributing to lymphomagenesis in an earlier phase of cancer development
- It has been suggested that high levels of pretreatment EBV DNA in whole blood (> 30,800 copies/mL) are associated with a worse prognosis (Viruses 2023;15:656)
- EBER positive: no defined cutoff; WHO classification suggests a cutoff of 80%
- EBER highlights more positive cells than LMP1
- Lymphoma cells usually have EBV type II latency pattern
- EBNA2 is expressed only in some cases (14%)
- EBV DNA load may have prognostic significance in DLBCL patients regardless of tumor EBV status (Viruses 2023;15:656)
- 2 types, no clinical or prognostic relevance
- References: Pathology 2020;52:40, Viruses 2023;15:656
Diffuse large B cell lymphoma with chronic inflammation
- Rare; associated with hypoxic microenvironment
- Mass producing DLBCL that occurs in a setting of longstanding chronic inflammation
- Mostly involves body cavities or narrow spaces
- Presents with large tumor mass
- Reference: Pathology 2020;52:40
Fibrin associated diffuse large B cell lymphoma
- Arises from fibrinous material in wall of pseudocysts
- It has been suggested that EBV positive DLBCL associated with breast implants is related with this subset of neoplasms
- This is a controversial concept since fibrin is not a known pathogenic mechanism
- Minute tumor burden; associated with hypoxic microenvironment
- Consistently associated with EBV
- Does not produce mass
- Does not directly produce symptoms
- Discovered incidentally on histologic examination of surgical pathology specimen
- Highly favorable clinical outcome with complete surgical excision
- Chemotherapy for DLBCL is not required
- Reference: Pathology 2020;52:40
Lymphomatoid granulomatosis
- Extranodal, predominantly pulmonary angiocentric or angiodestructive lymphoproliferative disorder
- Lung is the most frequent site of involvement
- Multiple bilateral pulmonary nodules (most frequent)
- Other sites of involvement: skin and central nervous system
- Microscopic features
- Neoplastic B cells are EBV positive
- Latency III: LMP1 positive, EBNA2 positive
- Many admixed T cells, histiocytes, plasma cells and variable number of immunoblasts
- Histologic grade based on number of EBV positive large B cells and extent of necrosis
- < 5 EBV positive B cells per high power field (HPF) in grade 1
- 5 - 50 EBV positive cells per HPF in grade 2
- > 50 EBV positive cells per HPF in grade 3; this latter criterion may not be fulfilled when tumor is extensively necrotic and reactivity for EBER is decreased
- Cases with sheets of EBV positive large B cells should be classified as EBV+ DLBCL
- Ancillary tests
- Large cells are B cells
- CD45RB / LCA positive, EBER positive, CD30 variable, CD15 negative
- Smaller reactive cells are T cells: CD4 > CD8
- Monoclonal IGH rearrangements in grade 2 (50%) and grade 3 (70%) of lymphomatoid granulomatosis cases
- Neoplastic B cells are EBV positive
- References: Hum Pathol 2018;79:18, Pathology 2020;52:40
Lymphomas where EBV+ is not essential for diagnosis
Plasmablastic lymphoma
Burkitt lymphoma
Acquired immunodeficiency syndrome (AIDS) related diffuse large B cell lymphoma
Classic Hodgkin lymphoma
Primary effusion lymphoma
- Diffuse proliferation of large neoplastic cells with plasmablastic cytologic and immunophenotypic features
- Generally associated with immunodeficiency, a subset occurs in immunocompetent patients
- Predominantly extranodal disease
- Aggressive clinical course, poor prognosis
- Microscopic features
- Large neoplastic cells with a variable degree of immunoblastic or plasmablastic features
- Starry sky pattern can be seen
- High mitotic and apoptotic rates
- Ancillary tests
- Negative for B cell markers (CD20, CD79a, PAX5) and CD45
- CD138 positive, MUM1 / IRF4 positive, CD38 positive
- EBER positive in ~70% of cases
- High proliferation rate
- MYC rearrangements in 40 - 50% of cases
- References: Pathology 2020;52:40, Hum Pathol 2018;79:18
Burkitt lymphoma
- Highly aggressive B cell neoplasm characterized by monomorphic, intermediate size cells with basophilic cytoplasm
- Germinal center B cell immunophenotype with MYC overexpression
- CD20 positive, CD10 positive, BCL6 positive, BCL2 negative, LMO2 negative and Ki67 proliferation rate ~100%
- MYC translocation partnered with immunoglobulin (Ig) gene loci
- IGH: 80 - 85%, IGL: 10%, IGK: 5%
- Germinal center B cell immunophenotype with MYC overexpression
- Infection by Epstein-Barr virus (EBV) in a subset of cases
- EBER positive, EBV LMP1 negative, EBNA1 positive, consistent with latency type 1
- Association with epidemiologic variants
- > 95% of cases of endemic Burkitt lymphoma
- ~20 - 30% of cases of sporadic Burkitt lymphoma
- ~30 - 40% of cases of immunodeficiency associated Burkitt lymphoma
- Recent studies recommend dividing Burkitt lymphoma into 2 groups: EBV positive and EBV negative, regardless of epidemiologic context and geographic location
- EBV positive Burkitt lymphoma shows significantly higher levels of somatic hypermutation and harbors fewer driver mutations
- References: Pathology 2020;52:40, Leukemia 2022;36:1720
Acquired immunodeficiency syndrome (AIDS) related diffuse large B cell lymphoma
- Most common lymphoma arising in the context of HIV infection
- No correlation between viral load and occurrence of lymphoma
- Mostly nodal disease, gastrointestinal tract is the most common extranodal site of involvement (19%)
- EBER is positive in ~40% of the cases
- Lymphomagenesis is induced by multiple mechanisms
- Genetic alterations
- Chronic B cell activation by immune dysfunction
- Loss of immunoregulatory control of oncogenic herpesviruses (EBV, HHV8)
- Similar morphological, immunophenotypic and molecular features to HIV negative cases
- Usually has a non-germinal center immunophenotype, may express CD30
- Monoclonal IGH rearrangements, frequent somatic hypermutation of IGH variable regions
- May present as primary central nervous system lymphoma, usually with immunoblastic morphology
- Long history and advanced stage of AIDS
- Very low CD4 positive count
- Immunoblastic morphology common
- EBV positive in 80 - 100% of cases
- Reference: Pathology 2020;52:40
Classic Hodgkin lymphoma
- Overall EBV positivity rate is 30 - 50% in the U.S. and Europe but ~100% in Vietnam, Kenya and Honduras
- More frequent in childhood (< 10 years) and in older adults (> 60 years)
- Possibly represents 2 distinct phenomena, one related to earlier age of EBV infection and the other to the decline in immune function
- Also correlates with lower socioeconomic status
- More frequent in childhood (< 10 years) and in older adults (> 60 years)
- EBV is detectable only in HRS cells and absent in background cells
- EBV infected HRS cells show latency pattern II
- Positive for EBERs, LMP1, LMP2A and EBNA1
- EBV infected HRS cells show latency pattern II
- Epidemiologically, there are 3 different incidence patterns
- Pattern 1: seen in developing nations and in patients of low socioeconomic status, there is an early childhood peak in incidence with tumors that are predominantly mixed cellularity (MC) subtype and EBV positive
- Pattern 2: seen in countries with transitional economies, has peaks of incidence in childhood and a second decade peak and shows a relatively more equal frequency of the MC and nodular sclerosis (NS) subtypes
- Pattern 3: seen in developed countries and in patients of high socioeconomic status, shows a peak incidence in the third decade with tumors that are mainly NS subtypes and EBV negative
- Association with EBV is also variable among pathological subtypes (Pathology 2020;52:154)
- Nodular sclerosis: 10 - 25%
- Mixed cellularity: 72 - 85%
- Lymphocyte rich: 57%
- Lymphocyte depleted: 75%
- It has been proposed that EBV rescues crippled germinal center B cells from apoptosis
- EBV provides the necessary signals required for cellular growth, survival and cellular genetic alterations, thus generating a pool of cells that may become progenitors of HRS cells
- Full contribution of EBV to CHL molecular pathogenesis remains to be fully elucidated
- Association of EBV and CHL in the setting of iatrogenic immunodeficiency has been identified in up to 80% of cases
- References: Pathology 2020;52:154, Viruses 2023;15:656
Primary effusion lymphoma
- Arises from HHV8 infected B cells with plasmablastic differentiation
- HHV8 / KSHV (Kaposi sarcoma herpes virus) positive is essential for diagnosis
- Pan B cell markers are negative
- EBER positive in ~70% of cases
- Diagnosis is usually established on cytologic preparations of effusion fluid
- Biopsy specimens may show neoplastic cells adherent to mesothelial surfaces
- EBV is constant in setting of HIV infection
- Latency pattern I
- Potential role in promoting establishment of latency in HHV8 by inhibiting its lytic replication
- See Table 4
- References: Pathology 2020;52:40, Hum Pathol 2018;79:18
EBV positive T and NK cell aggressive lymphomas
Extranodal NK / T cell lymphoma
Aggressive NK cell leukemia
Primary nodal EBV positive T / NK cell lymphoma (EBV TNKL)
Systemic EBV positive T cell lymphoma of childhood
- Predominantly extranodal lymphoma of either NK or T cell lineage
- Nasal cavity, nasopharynx, paranasal sinuses, palate (upper aerodigestive tract)
- Extranasal presentation: skin and intestines are the most common sites
- Geographical predilection for Asia and Latin America
- HLA::DPB1 is postulated as the susceptibility gene
- Microscopic features
- Characterized by necrosis, angiocentricity and angiodestruction, cytotoxic immunophenotype and EBV infection
- Ancillary testing
- CD2 positive, cytoplasmic CD3ε positive, CD94 positive, CD4 negative, CD5 negative, CD8 negative, TCRβ (BF1) negative
- Both T and NK cells express CD3ε
- TIA1 positive, granzyme B positive, perforin positive, CD56 variable
- CD30 positive in 30 - 50% of cases, PDL1 positive in 40 - 100% of cases
- EBER positive, presence of EBV in the majority of viable lymphoma cells
- Strong association, regardless of ethnicity
- Mechanism of infection of T cells by EBV is not entirely clear
- Latency pattern II has been reported
- CD2 positive, cytoplasmic CD3ε positive, CD94 positive, CD4 negative, CD5 negative, CD8 negative, TCRβ (BF1) negative
- Reference: Hum Pathol 2018;79:18
Aggressive NK cell leukemia
- Overlaps with extranodal NK / T cell lymphoma when there is multiorgan involvement
- Considered leukemic counterpart of extranodal NK / T cell lymphoma
- Characteristically in adults, may present in young adults
- High fever, general malaise, hepatosplenomegaly, liver failure and pancytopenia
- Neoplastic NK cells in peripheral blood (PB) and bone marrow (BM)
- Fulminant clinical course
- Common association with hemophagocytic lymphohistiocytosis (HLH)
- Microscopic features
- Varying degrees of leukemic infiltrate in BM, liver, lymph nodes and spleen
- Lymph nodes with diffuse infiltration by neoplastic cells and striking hemophagocytosis
- Broad cytologic spectrum from subtle to large pleomorphic cells
- Immunophenotype
- CD3ε positive, CD56 positive, CD2 positive, FASL positive, CD16 positive (75%)
- Surface CD3 negative, CD5 negative, CD57 negative
- EBER positive (90%)
- Reference: Am J Clin Pathol 2023;159:14
Primary nodal EBV positive T / NK cell lymphoma (EBV TNKL)
- Rare, aggressive lymphoma
- Mainly elderly patients in East Asia
- Associated with underlying immunodeficiency (autoimmune [AI], HIV, PTLD)
- Microscopic features
- Primarily in lymph nodes but can involve extranodal sites
- If nasal involvement, consider a diagnosis of extranodal NK / T cell lymphoma
- Monomorphic infiltrate, lack angiodestruction or coagulative necrosis, mainly T cell lineage
- CD3 positive, CD2 positive, CD8 positive, CD56 negative or positive, CD5 negative, CD4 negative
- TIA1 positive, granzyme B positive, perforin positive
- EBV latency II
- Poor outcome: median overall survival of 4 - 6 months
- Low genomic complexity compared with extranodal NK / T cell lymphoma (ENKTL) or panniculitis-like T cell lymphoma (PTCL), NOS
- Mutation in epigenetic modifier genes: TET2, DNMT3A
- Favor nodal EBV TNKL over extranasal ENKTL
- Mutation in epigenetic modifier genes: TET2, DNMT3A
- Primarily in lymph nodes but can involve extranodal sites
- Reference: Virchows Arch 2023;482:227
Systemic EBV positive T cell lymphoma of childhood
- More often in children and young adults
- No underlying immunodeficiency
- Occurs shortly after acute primary EBV infection and rare in the setting of CAEBV disease
- Abnormal serology against EBV with lack of anti-VCA IgM
- High fever, hepatosplenomegaly, pancytopenia, coagulopathy, abnormal liver function
- Lymphadenopathy might occur and be the dominant feature
- Fulminant clinical course that results in death within days to weeks
- Virtually always associated with HLH
- Overlapping clinical and morphologic features with HLH
- Microscopic features
- Cytologic atypia ranges from minimal to severe
- Histiocytic hyperplasia and striking hemophagocytosis in BM, spleen and liver
- Lymph nodes look depleted with paracortical EBV+ CD8+ T cells
- Ancillary tests
- CD8+ cytotoxic αβ T cells, CD3, CD2, TIA1 and granzyme B but lack CD56
- Rarely, cases associated with CAEBV show a CD4+ immunophenotype
- EBER+ cells are less numerous than the infiltrating CD8+ cells
- See Table 5
- Reference: Am J Clin Pathol 2023;159:14
Diagrams / tables
Table 1: EBV viral gene expression patterns during different types of latency
| Genes | Latency III | Latency II | Latency I | Latency 0 |
| Epstein-Barr nuclear antigen 1 (EBNA1) | + | + | + | - |
| Epstein-Barr nuclear antigen 2 (EBNA2) | + | - | - | - |
| Epstein-Barr nuclear antigen 3 (EBNA3) | + | - | - | - |
| Epstein-Barr nuclear antigen (EBNA) LP | + | - | - | - |
| Latent membrane protein 1 (LMP1) | + | + | - | - |
| Latent membrane protein 2 (LMP2) | + | + | - | - |
| Epstein-Barr encoded RNAs (EBERs) | + | + | + | + |
| BHRF1 micro RNAs (miRNAs) | + | - | - | - |
| BamHI A rightward transcript (BART) micro RNAs (miRNAs) | + | + | + | + |
Table 2: Main Epstein-Barr virus serological profiles
| Anti-viral capsid antigen (VCA) IgG | Anti-viral capsid antigen (VCA) IgM | Anti-Epstein-Barr nuclear antigen (EBNA) IgG | Interpretation |
| - | - | - | Seronegative individual |
| Variable | + | - | Primary infection |
| + | - | + | Past infection |
| + | - | - | Past infection (adults) or primary infection (children) |
| + | + | + | Past infection or end of primary infection |
| - | - | + | Indeterminate |
Table 3: Viral latency type in EBV associated lymphoproliferative disorders and lymphomas
| Disease | Percentage of EBV related cases | Latency pattern | Viral proteins expressed | EBER expression pattern | ||||
| EBNA1 | EBNA2 | LMP1 | LMP2 | EBER | ||||
| B cell lymphoproliferative disorders | ||||||||
| Infectious mononucleosis | 100 | III | + | + | + | + | + | Many small and large cells are EBV+ (mainly B cells and rare positive T and NK cells); most of the EBV+ cells are present in the paracortical area |
| EBV positive mucocutaneous ulcer | 100 | I or II | + | - | + | + | + | EBV expression is variable with most cases showing scattered EBV+ cells |
| Lymphoproliferative disorders associated with immune deficiency and dysregulation | > 90 | III or II | + | + | + | + | + |
|
| T / NK lymphoproliferative disorders of childhood | ||||||||
| Hydroa vacciniforme lymphoproliferative disorder | 100 | II | + | - | + | + | + | EBV expression in around 50% of lesional cells |
| Severe mosquito bite allergy | 100 | II | + | - | - | - | + | EBV is positive in few of the lesional NK cells; a much higher density of EBV+ cells should raise suspicion of NK cell lymphoma |
| Chronic active EBV disease (CAEBVD) | 100 | II | + | - | + | + | + | EBV is uniformly expressed in many cytotoxic T cells in most cases |
| B cell lymphomas | ||||||||
| EBV positive diffuse large B cell lymphoma, NOS | 100 | II or III | + | + | + | + | + | Most of the large atypical lymphoma cells are EBV+, a cutoff of 80% has been proposed |
| Diffuse large B cell lymphoma with chronic inflammation | 100 | II or III | + | + | + | + | + | Most of the lymphoma cells are diffusely positive for EBER |
| Fibrin associated large B cell lymphoma | 100 | II or III | + | + | + | + | + | Most of the lymphoma cells are diffusely positive for EBER |
| Primary effusion lymphoma | Most of the lymphoma cells are positive for EBER in the EBV positive cases | |||||||
| HIV associated | 100 | I | + | - | - | - | + | |
| HIV unrelated | 70 - 90 | I | + | - | - | - | + | |
| Lymphomatoid granulomatosis | 100 | III | + | + | + | + | + | The large neoplastic B cells are EBV positive; the number of EBV+ cells determines the grade |
| Plasmablastic lymphoma | 60 - 75 | I | + | - | - | - | + | Most of the lymphoma cells are diffusely EBV positive |
| Burkitt lymphoma | Most lymphoma cells are positive for EBER in EBV positive cases | |||||||
| Endemic | > 95 | I | + | - | - | - | + | |
| Sporadic | 20 - 80 | I | + | - | - | - | + | |
| AIDS related DLBCL | The pattern of EBV expression coincides with the histological subtype of lymphoma occurring in the immunocompetent state | |||||||
| Immunoblastic | 70 - 100 | III | + | + | + | + | + | |
| Nonimmunoblastic | 10 - 30 | III | + | + | + | + | + | |
| CNS lymphomas | 80 - 100 | III | + | + | + | + | + | |
| Hodgkin lymphoma |
| |||||||
| EBV unrelated | 20 - 90 | II | + | - | + | + | + | |
| EBV associated | 100 | II | + | - | + | + | + | |
| EBV positive T and NK cell lymphomas | ||||||||
| Extranodal, NK / T cell lymphoma | 100 | I or II | + | - | Variable | Variable | + | Virtually all lymphoma cells are positive for EBER |
| Aggressive NK cell leukemia | 90 | II | + | - | + | + | + | Most lymphoma cells are EBV positive |
| Primary nodal EBV positive T / NK cell lymphoma | 100 | II | + | - | + | + | + | Most lymphoma cells are EBV positive |
| Systemic EBV positive T cell lymphoma of childhood | 100 | II | + | - | + | + | + | Most lymphoma cells are EBV positive |
Table 4: Differential diagnosis of EBV positive B cell lymphoproliferative disorders and lymphomas
| Features | Infectious mononucleosis | EBV+ mucocutaneous ulcer | EBV+ classic Hodgkin lymphoma | EBV+ diffuse large B cell lymphoma |
| Clinical | ||||
| Age | Young, elderly | Elderly | Young and elderly | Elderly |
| Lymphadenopathy | Present | Absent | Present, nodal or mediastinal | Present, high stage |
| LDH elevation | Present, mild to moderate | Absent | Present | Present |
| Extranodal disease | Absent | Present | Extremely rare as primary disease | Can be present, late stages |
| Clinical course | Self limited in majority of cases | Waxing and wanning | Progressive | Aggressive, poor outcome |
| Morphology | ||||
| Architecture | Paracortical | Ulcer | Effacement | Effacement |
| Circumscription | Absent | Present, lymphocytic rim at base | Absent | Absent, diffuse involvement |
| Large cells | Reed-Sternberg-like cells | Reed-Sternberg-like cells | Reed-Sternberg cells | Sheets of large neoplastic cells, some RS-like cells |
| EBV latency type | III | II / III | II | III / II |
| Immunohistochemistry | ||||
| CD45 | Positive in most cells | Positive in most cells | Negative in HRS cells | Positive in neoplastic cells |
| CD20 | Positive in large cells | Positive in large cells | Mostly negative in HRS cells, faint reactivity in HRS cells in ~20% of cases | Positive in large cells |
| PAX5 | Positive, strong | Positive, strong | Positive, weak | Positive, strong |
| BOB.1 | Positive, strong | Positive, strong | Negative, can be weak | Positive, strong |
| MUM1 | Positive | Positive | Positive | Positive |
| BCL6 | Negative | Negative | Negative | Can be positive |
| CD10 | Negative | Negative | Negative | Negative |
| CD30 | Positive in HRS-like cells, usually dim | Positive in HRS-like cells, usually dim | Positive in HRS cells, strong | Positive |
| CD15 | Positive in up to 50% of cases | Positive in up to 50% of cases | Positive, variable | Positive in up to 50% of cases |
| PDL1 | Negative | Negative | Positive in > 80% | Can be positive (40 - 60%, extranodal) |
| Diagnostic molecular testing | ||||
| B cell | Polyclonal | Clonal in 50% of the cases | Clonal | Monoclonal IGH gene rearrangements |
| T cell | Polyclonal | Oligoclonal and restricted TCR rearrangement patterns | Polyclonal, restricted pattern in elderly patients | Oligoclonal and restricted TCR rearrangement patterns |
| Genetic features | No immune evasion features | No immune evasion features | Immune evasion (host evasion) | Immune evasion (host evasion) |
Table 5: Differential diagnosis of EBV positive T and NK lymphomas
| Feature | Extranodal NK / T cell lymphoma | Aggressive NK cell leukemia | EBV positive nodal T and NK cell lymphoma | Systemic EBV+ T cell lymphoma of childhood |
| Clinical presentation | ||||
| Age | Adults | Young to middle aged adults | Older adults | Children, young adults |
| Site at presentation | Nasopharynx (70 - 80%), others (20 - 30%): skin, gastrointestinal (GI) | Bone marrow, spleen, peripheral blood, rarely lymph nodes (20%) | Lymph nodes, no nasal involvement by definition | Systemic proliferation: bone marrow, liver or spleen, CNS |
| Behavior | Localized disease, frequent dissemination | Fatal | Aggressive | Fulminant |
| Median survival | 26 - 76 months | Weeks | 4 months | Days to weeks |
| Hemophagocytic syndrome | Generally absent | Present | Uncommon | Always present |
| Morphology | ||||
| Cytology of neoplastic cells | Variable atypia, spectrum from small to large cells | Large granular atypical lymphocytes, distinct nucleoli and clear cytoplasm (smears) | Pleomorphic medium sized cells, with centroblastic, anaplastic or plasmacytoid features | Small to intermediate sized with subtle to absent atypia (most common) or large atypical cells |
| Necrosis | Common | Frequently present | Variable | Absent |
| Angiocentricity and angiodestruction | Present | Frequently present | Uncommon | Absent |
| Apoptosis | Present | Frequently present | Variable | Absent |
| Ancillary testing | ||||
| CD2 | Positive | Positive | Positive | Positive |
| CD3 | Often negative; subset is positive | Negative | Positive | Positive |
| CD3ε | Positive | Positive | Positive | Positive |
| CD4 | Negative | Negative | Negative | Usually negative |
| CD8 | Positive | Usually negative | Positive (> 80%) | Usually positive |
| CD56 | Positive | Positive | Mostly negative (positive < 20%) | Negative |
| Cytotoxic granules | Positive | Positive | Positive | Positive |
| EBER | All neoplastic cells | All neoplastic cells, a subset is negative (< 15%) | All neoplastic cells | Majority of neoplastic cells |
Table 6: Differential diagnosis of EBV+ B cell lymphoproliferations with Hodgkin-like features
| Disease | Clinical features | Morphology | Immunophenotype | Lineage, clonality and molecular features |
| EBV+ mucocutaneous ulcer |
|
|
|
|
| EBV+ diffuse large B cell lymphoma, NOS |
|
|
|
|
| EBV+ classic Hodgkin lymphoma |
|
|
|
|
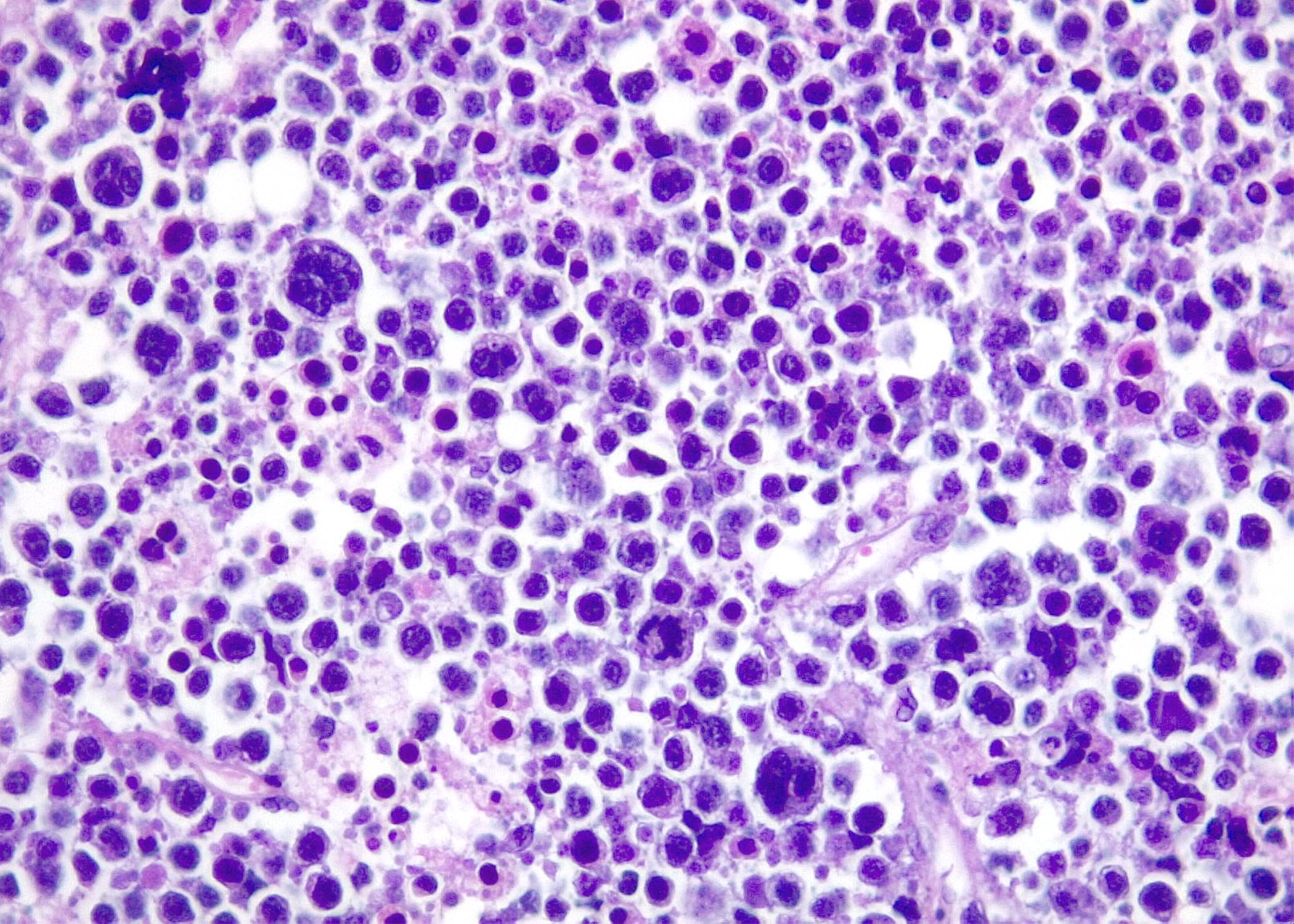
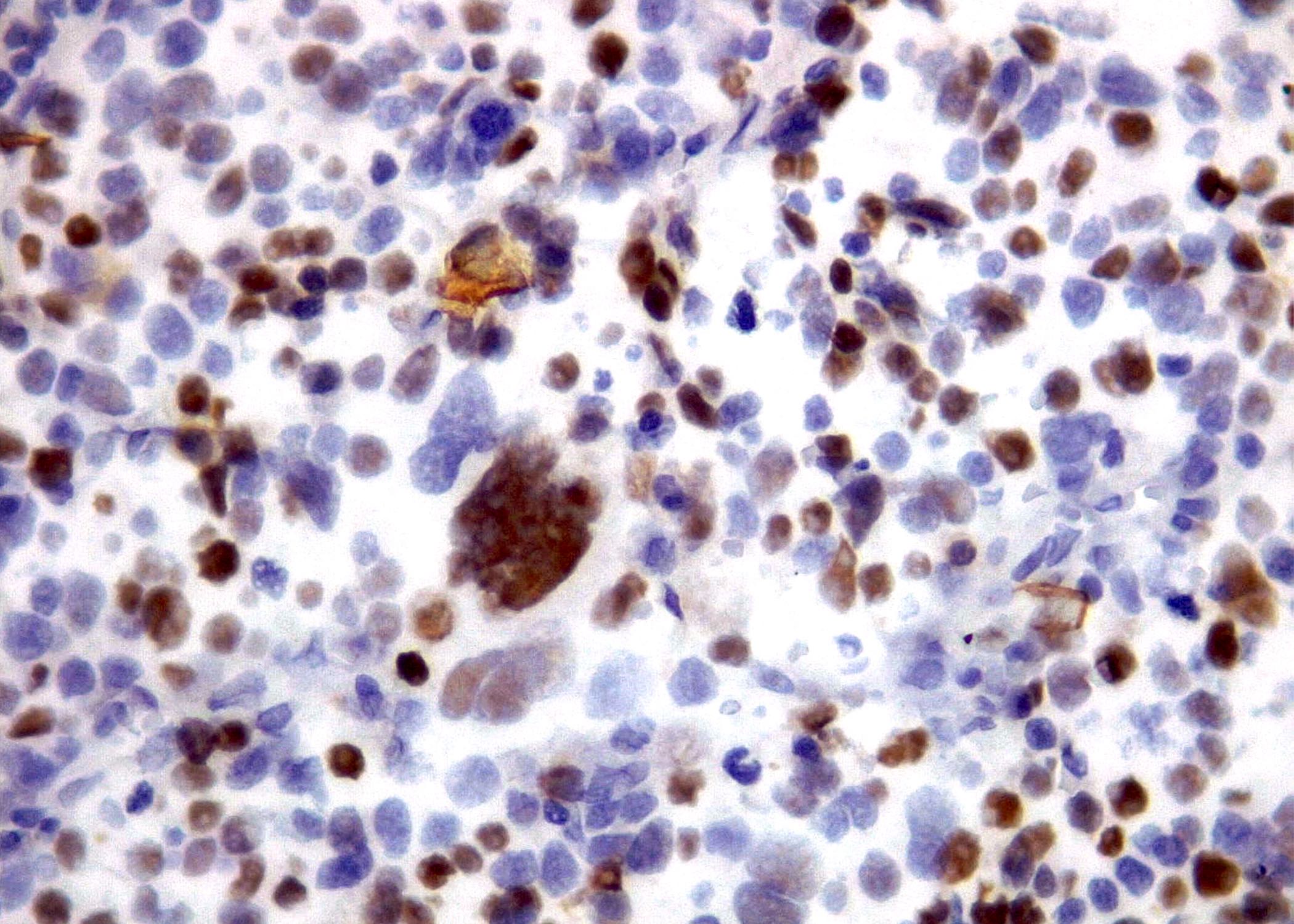
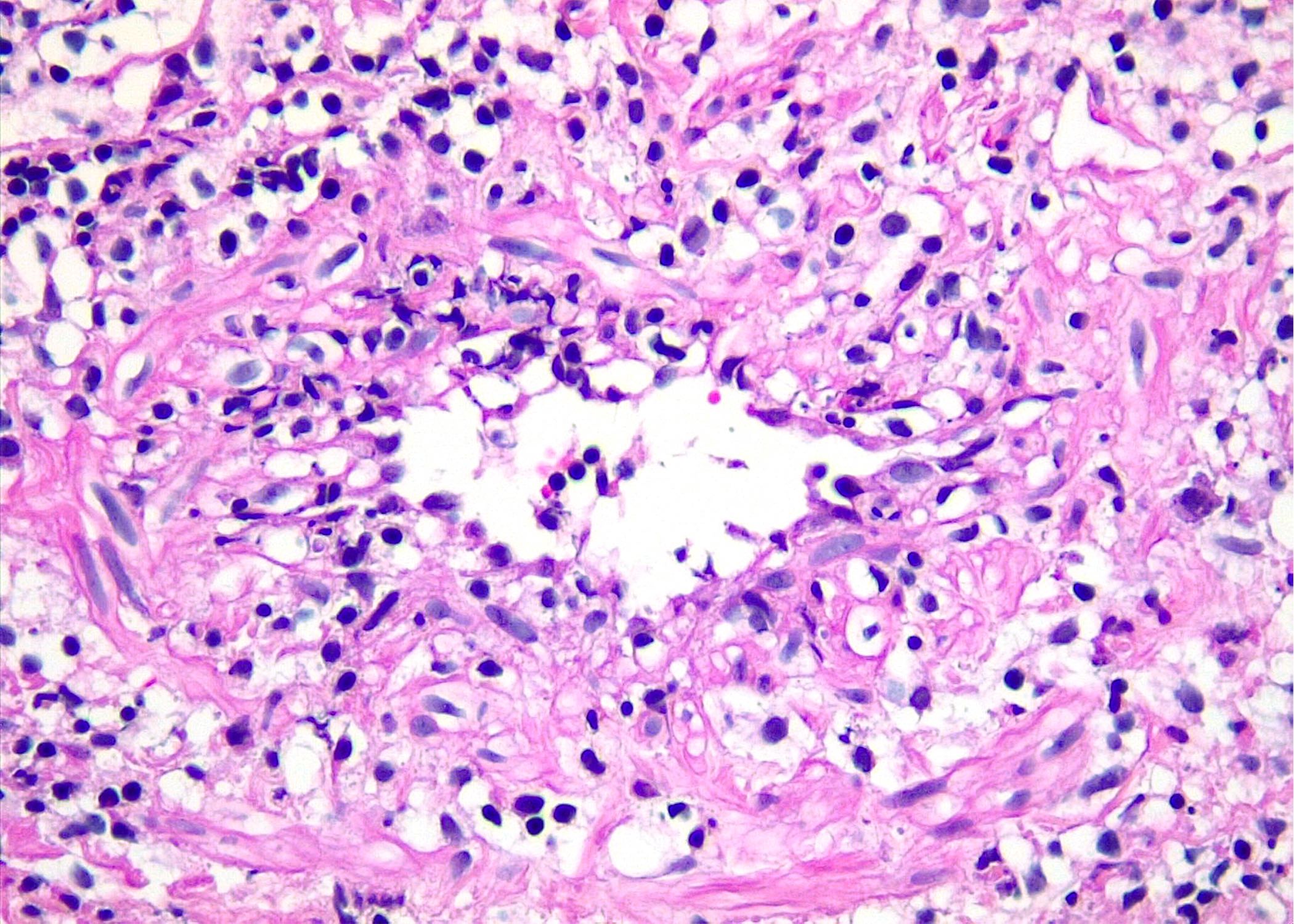
Microscopic (histologic) images
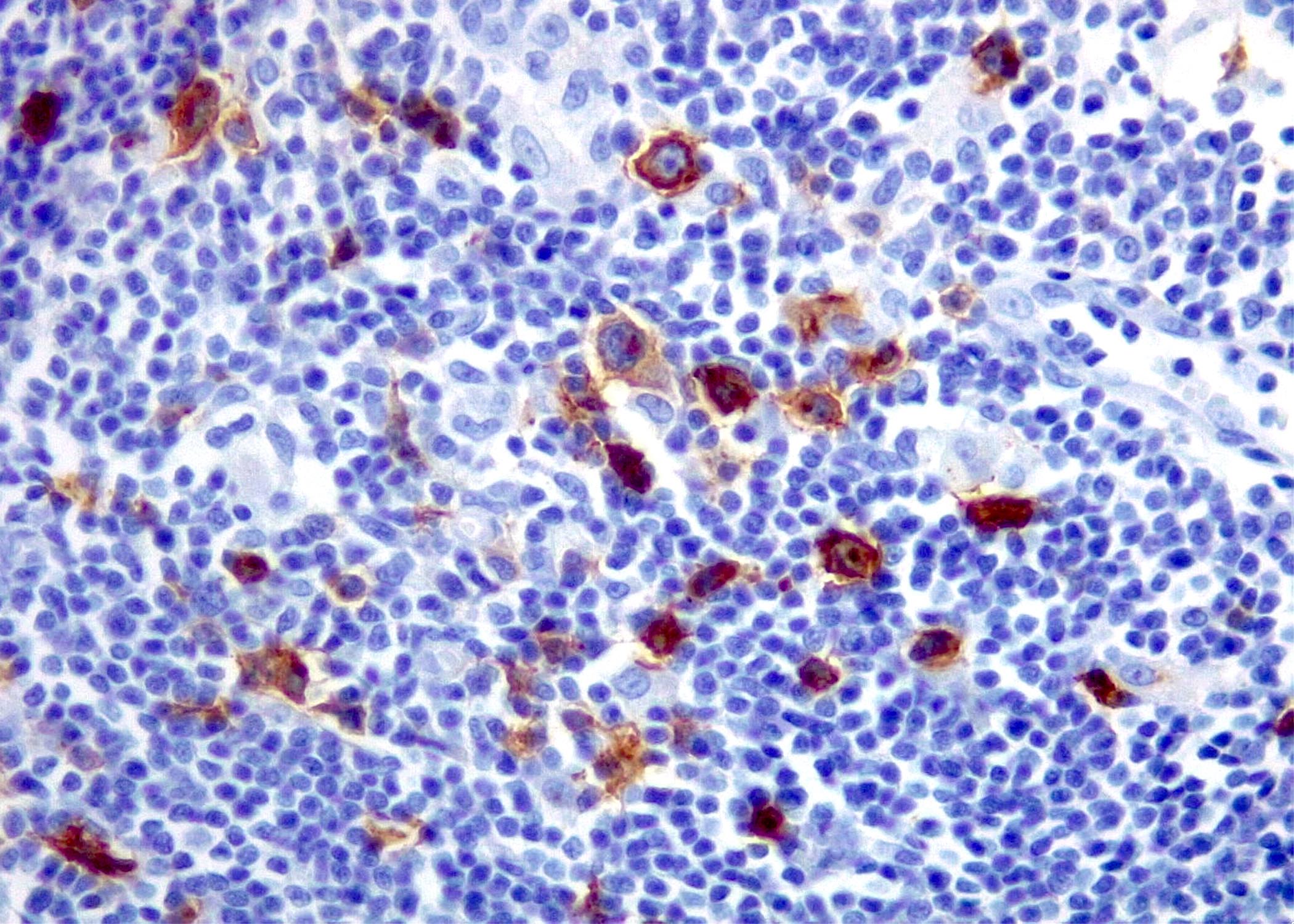
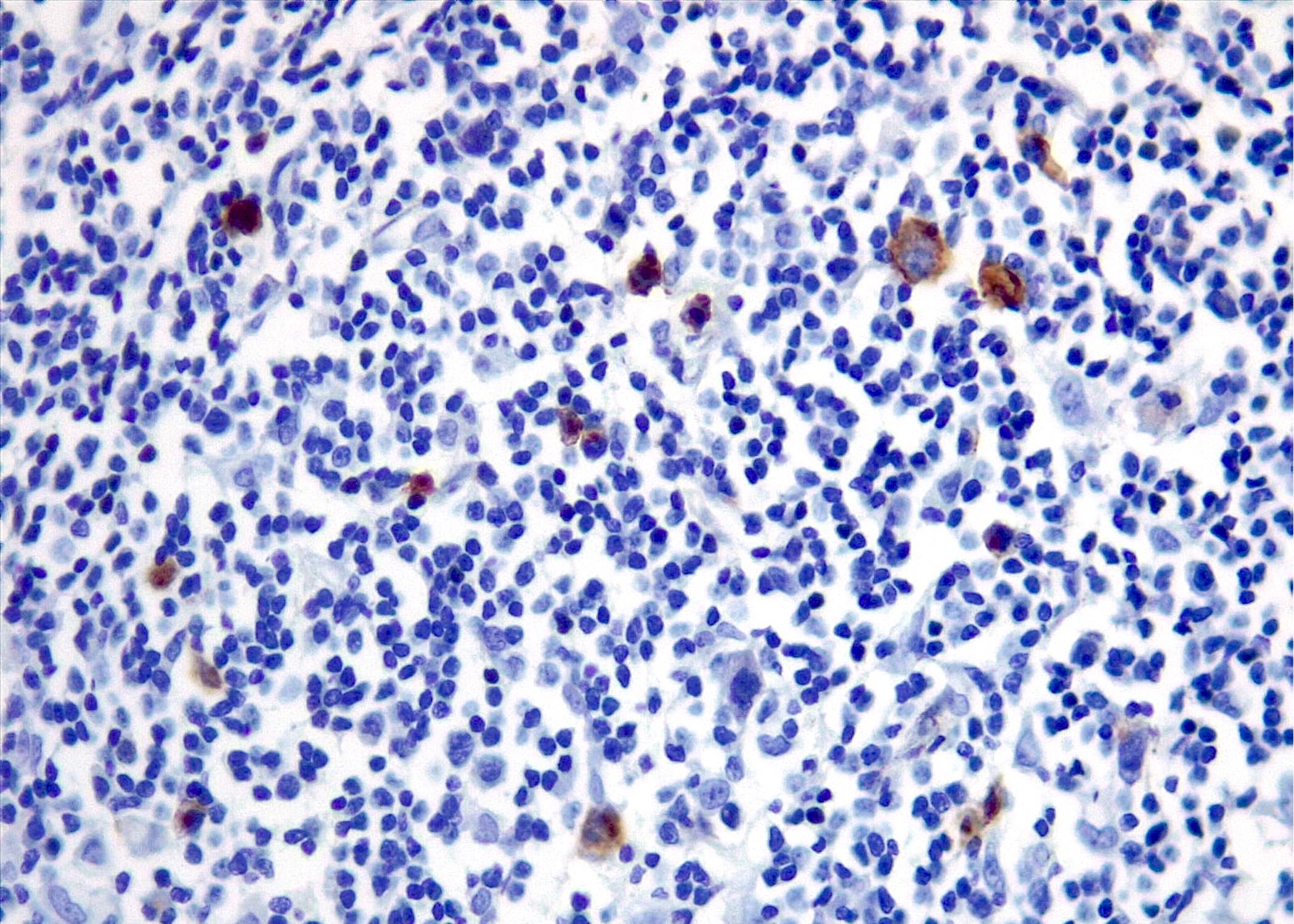
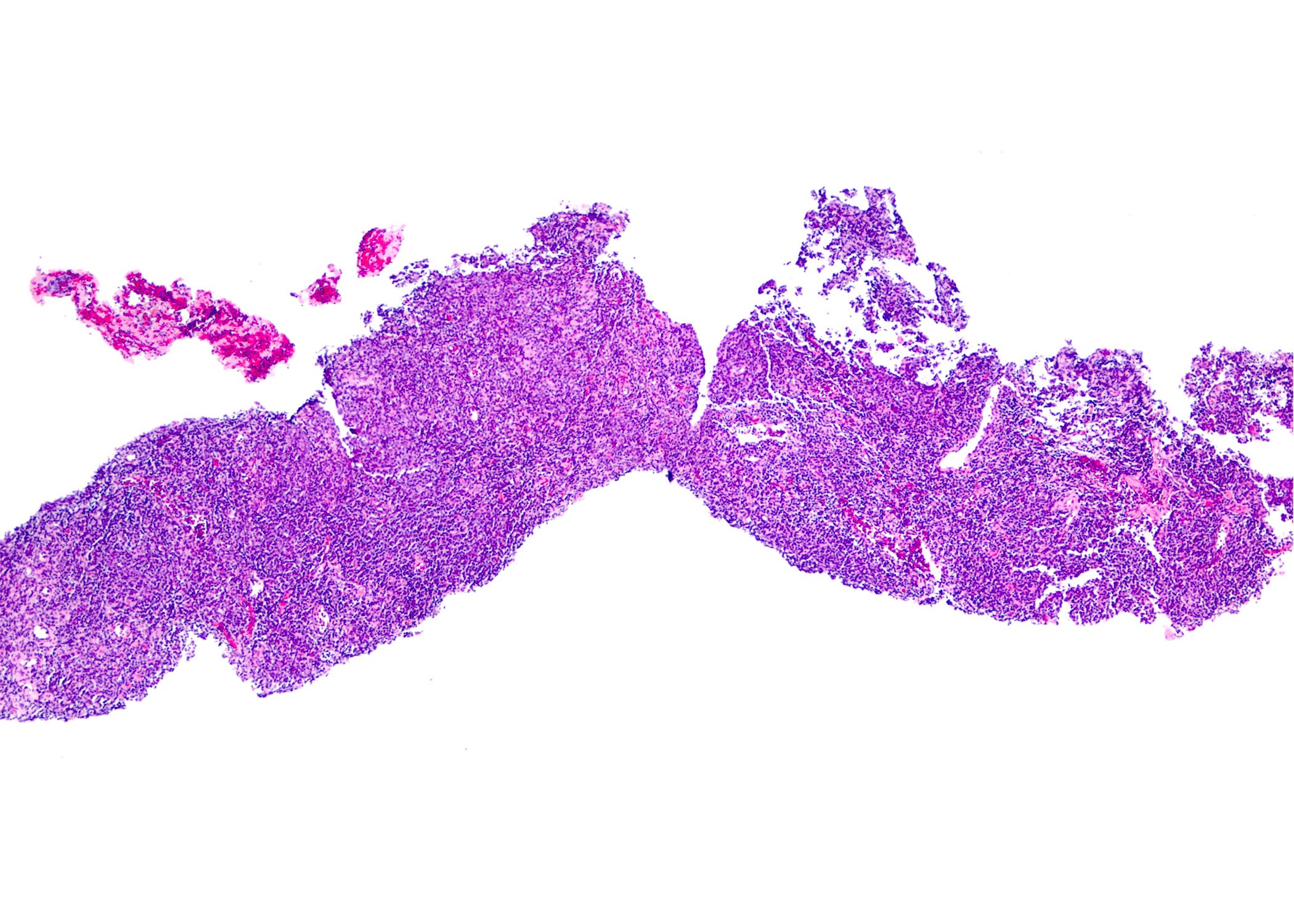
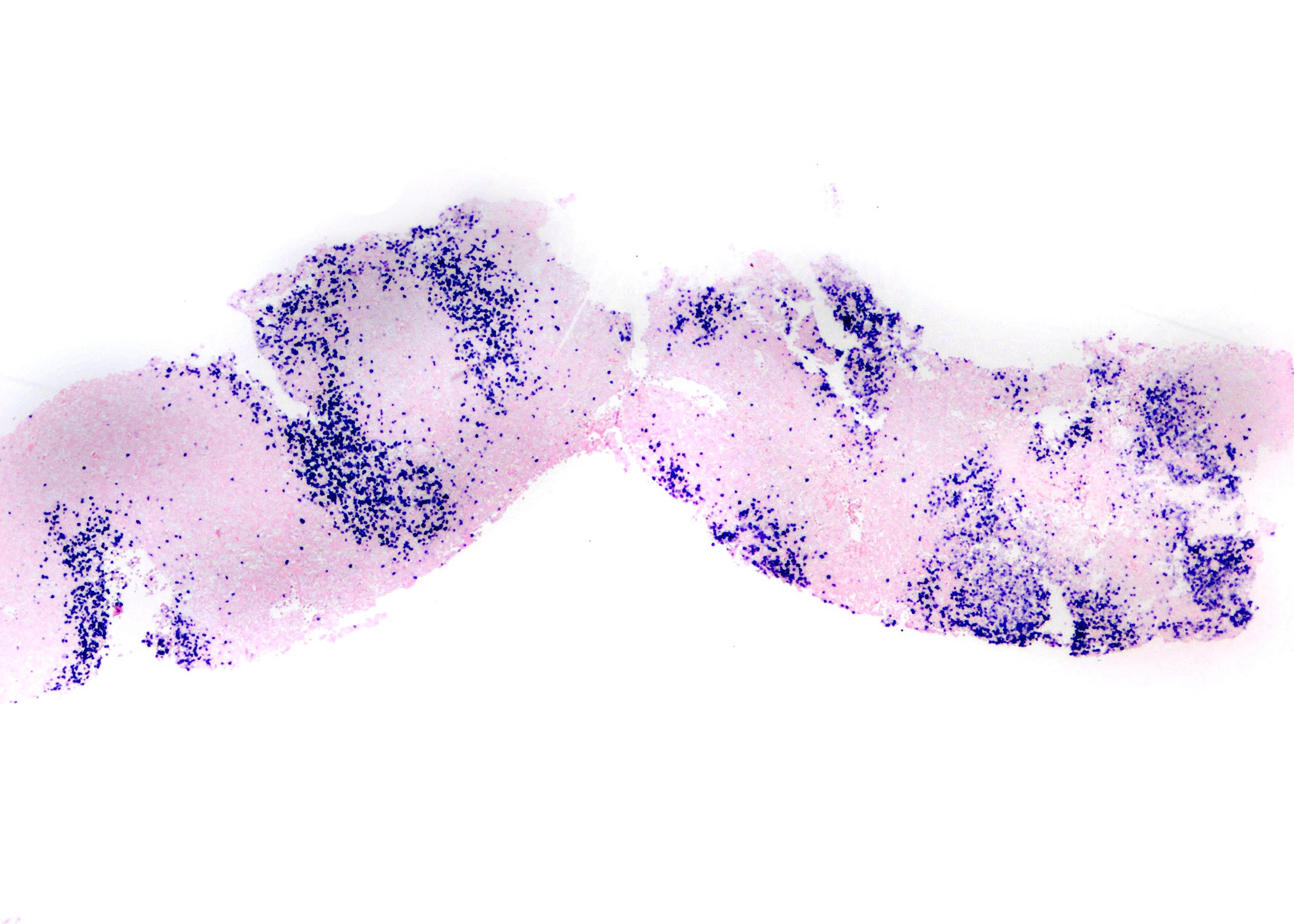
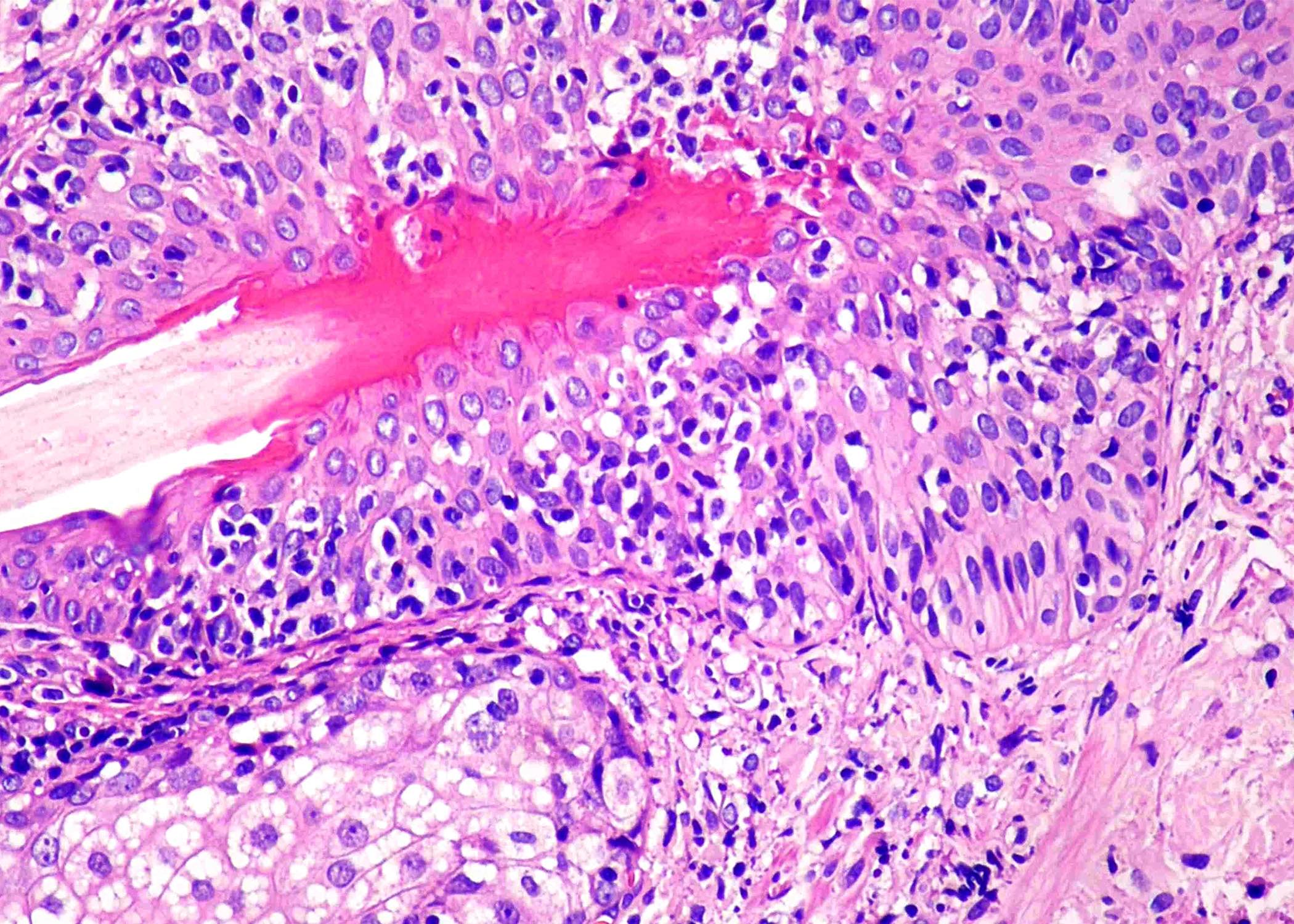
Contributed by Roberto N. Miranda, M.D. and Roman Segura-Rivera, M.D.
Peripheral smear images
Additional references
Board review style question #1
Which of the following is true about Epstein-Barr virus (EBV) infection?
- EBV is the human herpesvirus 5 (HHV5) and belongs to the Herpesviridae family
- EBV subtype 2 is the more prevalent worldwide and has greater transforming potential
- Expression of EBER is a constant feature of all EBV infected cells, independent of the latency pattern acquired
- Most EBV infections are acquired in adulthood and are asymptomatic
Board review style answer #1
C. The expression of EBER is a constant feature of all EBV infected cells, independent of the latency pattern acquired. Answer A is incorrect because EBV is the human herpesvirus 4 (HHV4) and part of the Herpesviridae family. Answer D is incorrect because most EBV infections are acquired in childhood and are asymptomatic. Answer B is incorrect because subtype 1 is the most prevalent worldwide and has a greater transforming potential.
Comment Here
Reference: Epstein-Barr virus
Comment Here
Reference: Epstein-Barr virus
Board review style question #2
What lymphomas and lymphoproliferative disorders (LPDs) commonly present with Epstein-Barr virus (EBV) type I latency pattern?
- Primary effusion lymphoma, hydroa vacciniforme LPD and Hodgkin lymphoma
- Primary effusion lymphoma, lymphomatoid granulomatosis and acquired immunodeficiency syndrome (AIDS) related diffuse large B cell lymphoma
- Primary effusion lymphoma, plasmablastic lymphoma and Burkitt lymphoma
- Primary effusion lymphoma, severe mosquito bite allergy and Hodgkin lymphoma
Board review style answer #2
C. Primary effusion lymphoma, plasmablastic lymphoma and Burkitt lymphoma. Primary effusion lymphoma, plasmablastic lymphoma and Burkitt lymphoma are usually latency type I. Answers A and D are incorrect because hydroa vacciniforme, LPD, Hodgkin lymphoma and severe mosquito bite allergy are usually latency type II. Answer B is incorrect because lymphomatoid granulomatosis and AIDS related diffuse large B cell lymphoma are usually latency pattern III.
Comment Here
Reference: Epstein-Barr virus
Comment Here
Reference: Epstein-Barr virus