Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Peripheral smear description | Peripheral smear images | Positive stains | Negative stains | Flow cytometry description | Flow cytometry images | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Khanlari M, Chapman J. ATLL. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lymphomanonBatlv.html. Accessed March 28th, 2025.
Definition / general
- Adult T cell leukemia / lymphoma (ATLL) is a mature T cell neoplasm associated with human T cell leukemia virus type 1 (HTLV1)
Essential features
- Lymphoma cells show monoclonal integration of HTLV1 and a mature T cell phenotype (Blood 2017;129:1071)
- Occurs in regions endemic for HTLV1 (~2.5% in HTLV1 carriers) (Int J Hematol 2002;76:240)
- Affected individuals are usually exposed to HTLV1 very early in life (Front Microbiol 2018;9:461)
- Develops after a long latency (therefore occurs in adults)
- Most often composed of highly pleomorphic small to medium sized lymphoid cells
- Widely disseminated and clinically aggressive
- Clinical spectrum of HTLV1 associated diseases is wide and includes neoplastic and nonneoplastic disorders (Clin Microbiol Rev 2010;23:577)
Terminology
- Abbreviation: adult T cell leukemia / lymphoma (ATLL)
- Synonyms: adult T cell leukemia, adult T cell lymphoma, adult T cell leukemia / lymphoma (HTLV1+)
ICD coding
Epidemiology
- HTLV1 virus is endemic in Southwestern Japan, the Caribbean basin, sub-Saharan Africa, South America and parts of the Middle East, Australia and Melanesia (Front Microbiol 2012;3:388, Viruses 2022;14:664)
- Median age of onset varies by geographic location
- Third to ninth decade
- Mean: 58 years
- M:F = 1.5:1
- Transmission: requires the presence of living HTLV1 infected cells
- Mother to infant
- Sexual fluids
- Blood and blood products
Sites
- Acute variant
- Leukemic involvement
- Any nodal and extranodal sites can be involved
- Chronic variant
- Predominant leukemic involvement
- Nodal, liver, spleen, skin and pulmonary involvement may be present
- No bone, CNS or GI tract involvement is present
- No ascites or pleural effusions are present
- Lymphomatous variant
- Predominantly nodal involvement with no leukemic involvement
- Smoldering
- Atypical lymphocytes in the peripheral blood are present in low counts (~5%)
- Skin and lung lesions are often present
- There is no involvement of the liver, spleen, CNS, bone or GI tract, nor pleural / ascites effusions
- References: Br J Haematol 1991;79:428, Mod Pathol 2018;31:1046
Pathophysiology
- ATLL patients suffer from profound immunodeficiency; the concept that the cell of origin of ATLL is a T regulatory T cell provides a biologic basis for disease associated immunodeficiency (Cancer Sci 2005;96:527)
- Expression of HTLV1 proteins exposes infected cells to virus specific cytotoxic T lymphocytes (CTL)
- CTL escape is evident in ATLL with immunodominant viral epitopes and host genes involved in antigen presentation frequently altered
Etiology
- ATLL is uniformly causally associated with HTLV1 infection
- HTLV was the first human retrovirus to be identified, isolated from a patient derived T cell lymphoma cell line (Proc Natl Acad Sci USA 1980;77:7415)
- HTLV1 is a type C retrovirus, Deltaretrovirus genus
- Single strand of RNA is converted into a double strand of DNA in the host via reverse transcriptase (Viruses 2010;2:2037)
- Monoclonal
- Randomly integrates into host cell genome (Oncology 2015;89:7)
- Infection alone is not sufficient to result in neoplastic transformation of infected cells
- HTLV1 can infect immature thymocytes and mature CD4+ T cells
- Enters by cell to cell contact via 3 cellular molecules: neuropilin 1, heparan sulfate proteoglycan (HSPG) and glucose transporter type 1 (GLUT1) (J Virol 2006;80:6844)
- HTLV1 genome encodes 2 crucial proteins, Tax and HBZ (HTLV1 B zip protein)
- Tax: transcriptional transactivator that drives viral replication and activates cellular pathways involved in T cell proliferation (mainly via the NFκB and AP1 pathways)
- HBZ: drives cell proliferation, inhibits apoptosis and induces TIGIT and CCR4 expression on the cell surface; it has been implicated in the proliferation and trafficking of malignant cells (Philos Trans R Soc Lond B Biol Sci 2017;372:20160272)
- Together with HTLV1 derived molecular alterations, driver somatic genetic and epigenetic lesions accumulate over time and modify the neoplastic behavior; among those, the following are included (Blood 2018;131:215, Blood 2022;139:967, Nat Genet 2015;47:1304, Am J Pathol 2010;176:402)
- Recurrent loss of function mutations in the CICL / ATXN1 complex
- PRKCB mutations
- PDL1 gene amplification
- IRF4 mutations
- CDKN2a deletions
- REL splice variant mutations
Diagrams / tables
Clinical features
- 4 clinically defined variants in Shimoyama classification: acute, lymphomatous, chronic and smoldering (Br J Haematol 1991;79:428)
- Acute variant: ~55 - 60% of cases; survival usually < 1 year
- Leukocytosis (often > 100 x 109/L)
- Peripheral blood involvement with numerous flower cells
- Skin rash and lymphadenopathy
- Hepatosplenomegaly
- Hypercalcemia
- Elevated serum lactate dehydrogenase (LDH)
- Rapidly progressive course and frequent opportunistic infections
- Lymphomatous variant: ~20% of cases; survival usually < 1 year
- Lymphadenopathy
- Skin lesions
- No / minimal peripheral blood involvement
- Hypercalcemia is less frequent (compared with acute variant)
- Chronic variant: ~15 - 20% of cases; survival > 2 years but < 5 years due to progression to acute or lymphomatous ATLL
- Lymphocytosis
- Exfoliative skin lesions
- Lymphocyte count is much lower than in the acute variant
- Few atypical lymphocytes in peripheral smear review
- Mild hepatosplenomegaly
- Mild lymphadenopathy
- No hypercalcemia
- Smoldering variant: ~5% of cases; survival > 2 years but < 10 years due to progression to
acute ATLL or infectious complications
- Skin or lung lesions
- > 5% atypical lymphocytes in the absence of leukocytosis
- No hepatosplenomegaly, hypercalcemia or lymphadenopathy
- Acute variant: ~55 - 60% of cases; survival usually < 1 year
- Primary cutaneous tumoral type
- No leukemic phase, lymphadenopathy, hepatosplenomegaly or hypercalcemia
- Proposed as a fifth clinical type in the Shimoyama classification (Int J Dermatol 2010;49:1099)
- Nonneoplastic HTLV1 associated diseases
- Tropical spastic paraparesis / HTLV1 associated myelopathy (TSP / HAM) (Rev Neurol (Paris) 2012;168:257)
- HTLV1 associated infective dermatitis
- Uveitis
- Thyroiditis
- Pneumonitis
- Myositis
| Comparison of clinical forms of adult T cell leukemia / lymphoma | ||||
|---|---|---|---|---|
| Clinical manifestation | Acute | Lymphomatous | Smoldering | Chronic |
| Lymphocytosis | Increased | No | No | Mildly increased |
| Blood abnormal lymphocyte | Increased | No* | > 5% | Mildly increased |
| Increased LDH | Yes | No | No | Minimal |
| Hypercalcemia | Yes | Variable | No | No |
| Skin rash | Variable: > 50% | Variable: > 50% | Erythematous rash | Rash, papules |
| Lymphadenopathy | Usually present | Yes | No | Mild |
| Hepatosplenomegaly | Usually present | Often present | No | Mild |
| Bone marrow infiltration | May be present | No | No | No |
| Median survival | < 1 year | < 1 year | > 2 years | > 2 years |
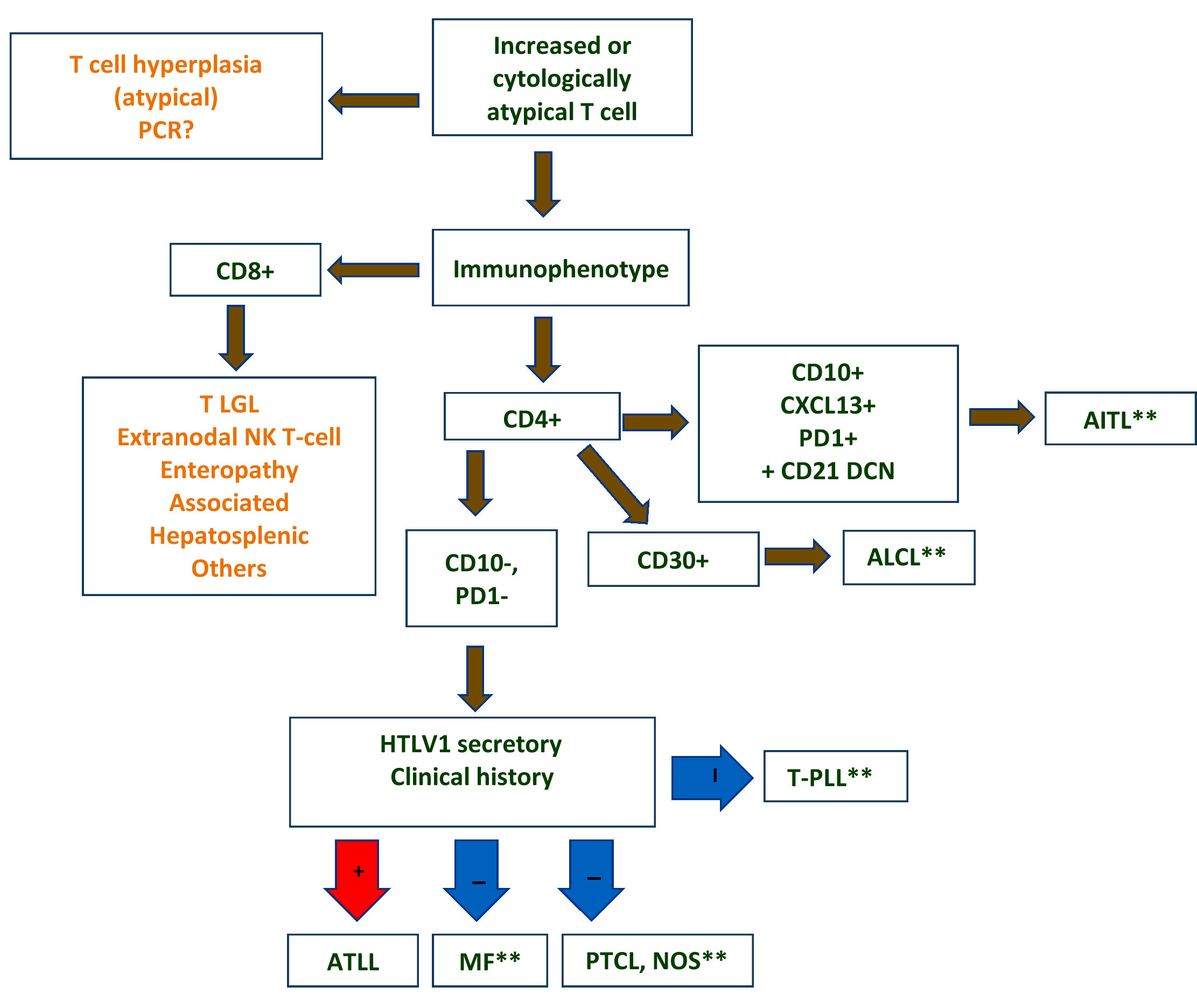
Diagnosis
- Strongest support for a diagnosis of ATLL: demonstration of viral integration and presence of viral transcripts of HBZ and TAX genes in the tumor cells
- New diagnostic algorithm has been proposed in biopsy specimens (see Diagrams / tables), assigning the diagnosis of ATLL if HBZ is positive by RNAscope or if the tumor population is ≥ 30% and TAX quantitative PCR is positive in ≥ 10% of cells (Mod Pathol 2021;34:51)
- Clinical features favoring the diagnosis: appropriate patient demographic (patient derived from HTLV1 endemic area), hypercalcemia, skin lesions and a leukemic phase (J Clin Pathol 2007;60:1373)
- Histologic features favoring the diagnosis: pleomorphic T cell lymphoma, leukemic phase, CD4+ αβ type with T regulatory immunophenotype, expression of CD25
- ATLL in patients with HTLV1 infection histologically mimics more common T cell lymphomas, such as peripheral T cell lymphoma or anaplastic large cell lymphoma and therefore can be difficult to distinguish by histology alone (Mod Pathol 2018;31:1046)
Laboratory
- Evidence of HTLV1 or 2 sequences at the molecular level
- Seropositivity for anti-HTLV1 antibody as a surrogate for demonstration of monoclonal integration of the virus
- Only useful in areas with low prevalence of HTLV1 infection
- In areas of high HTLV1 prevalence, viral integration must be demonstrated
- Expression of CD25 by lymphoma cells (Mod Pathol 2023;36:100169)
- Other laboratory tests / results
- Complete blood count (CBC): elevated leukocyte count and circulating neoplastic lymphocytes (leukemic phase)
- Elevated serum LDH level reflects disease burden / activity
- Hypercalcemia: more common in patients with acute variant; variably associated with lytic bone lesions
- Eosinophilia and neutrophilia are common
- Elevated levels of soluble IL2 receptor α chain in patients with aggressive ATLL
Radiology description
- May have lytic bone lesions (Insights Imaging 2018;9:845)
- Skull, pelvis, spine and long bones can be affected
- Punched out lesions similar to those seen in plasma cell myeloma can be found
- F18 deoxyglucose positron emission tomography (PET) / computed tomography (CT) is usually positive in sites of disease activity
- CT detects sites of nodal and extranodal disease
Prognostic factors
- Clinical variant, age, performance status, serum calcium, soluble interleukin 2 receptor (sIL2R) and LDH levels are major prognostic factors
- Death often stems from opportunistic infections
- Acute and lymphomatous variants
- Aggressive clinical course
- Recurrent infection: parasitic (Strongyloides stercoralis) and viral infections (Parasite Immunol 2004;26:487)
- p16 gene deletion and p53 mutation: more aggressive clinical course
- Chronic and smoldering variants
- More protracted clinical course
- Progression to an acute phase with an aggressive course in ~25%
- p16 gene deletion and chromosomal deletion detected via comparative genomic hybridization (CGH) in the chronic phase is a negative prognostic factor (J Clin Oncol 1997;15:1778)
- Aggressive (acute / lymphoma) variants (Blood 2018;131:215)
- Higher frequencies of TP53 and IRF4 mutations
- Many copy number alterations (CNAs), including PDL1 amplifications and CDKN2A deletions, compared with indolent (chronic / smoldering) subtypes
Case reports
- 49 year old man with multiple cranial nerve palsies and meningeal lymphoma (Am J Hematol 2017;92:397)
- 50 year old woman with rheumatoid arthritis and panbronchitis-like pulmonary lesions (J UOEH 2017;39:55)
- 58 year old woman with erythematous and itchy plaques (Br J Haematol 2017;177:507)
- 70 year old man with TCL1 positive lymphoma (Mod Pathol 2018;31:1046)
- 73 year old woman with unilateral conjunctival infiltration (J Clin Exp Hematop 2017;57:143)
Treatment
- Chronic or smoldering ATLL: observation may be appropriate for patients who are asymptomatic
- ATLL is resistant to most chemotherapy
- No standard chemotherapy regimen
- Intensive high dose combination chemotherapy and bone marrow transplantation have been used in limited numbers of patients (Blood 2010;116:1369)
- Monoclonal antibody based therapies have been attempted directed against
- IL2R (anti-Tac)
- CCR4 (mogamulizumab)
- CD52 (alemtuzumab)
- Recent clinical trials use arsenic trioxide, interferon α and zidovudine (Adv Ther 2018;35:135)
Gross description
- Skin lesions have been classified as erythema, papules or nodules (Semin Diagn Pathol 2020;37:81)
- Rare cases show tumor-like lesions or erythroderma as seen in mycosis fungoides / Sézary syndrome
- Enlarged and effaced lymph nodes
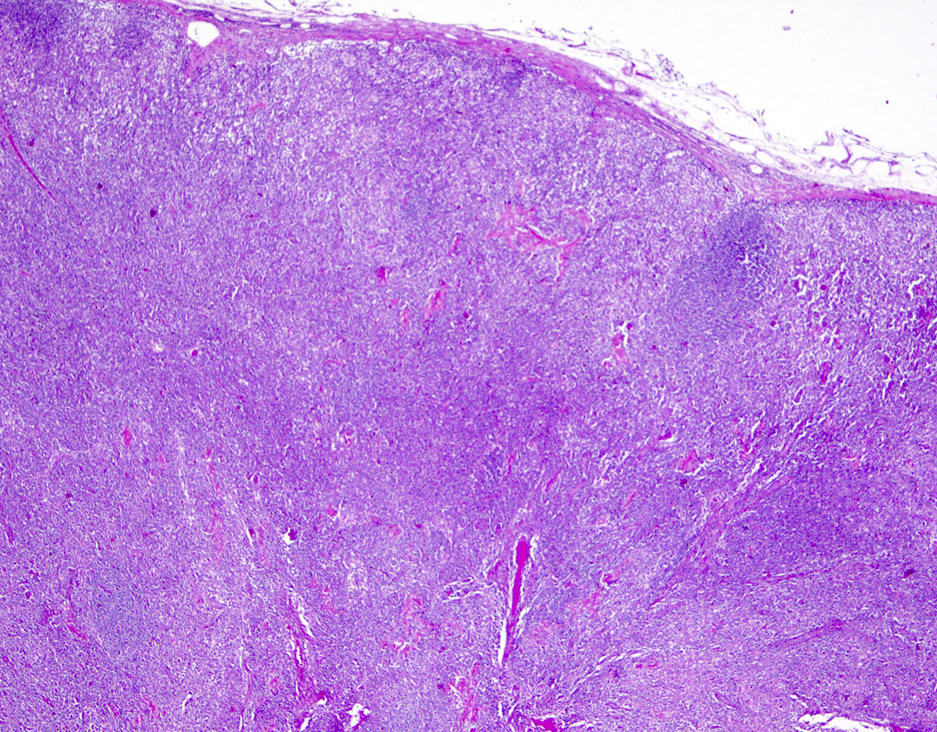
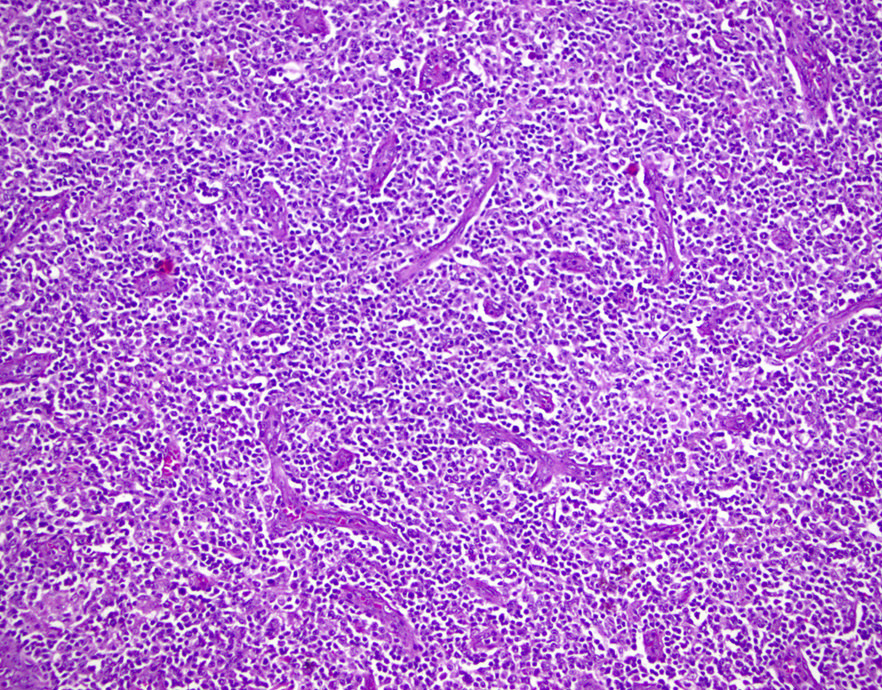
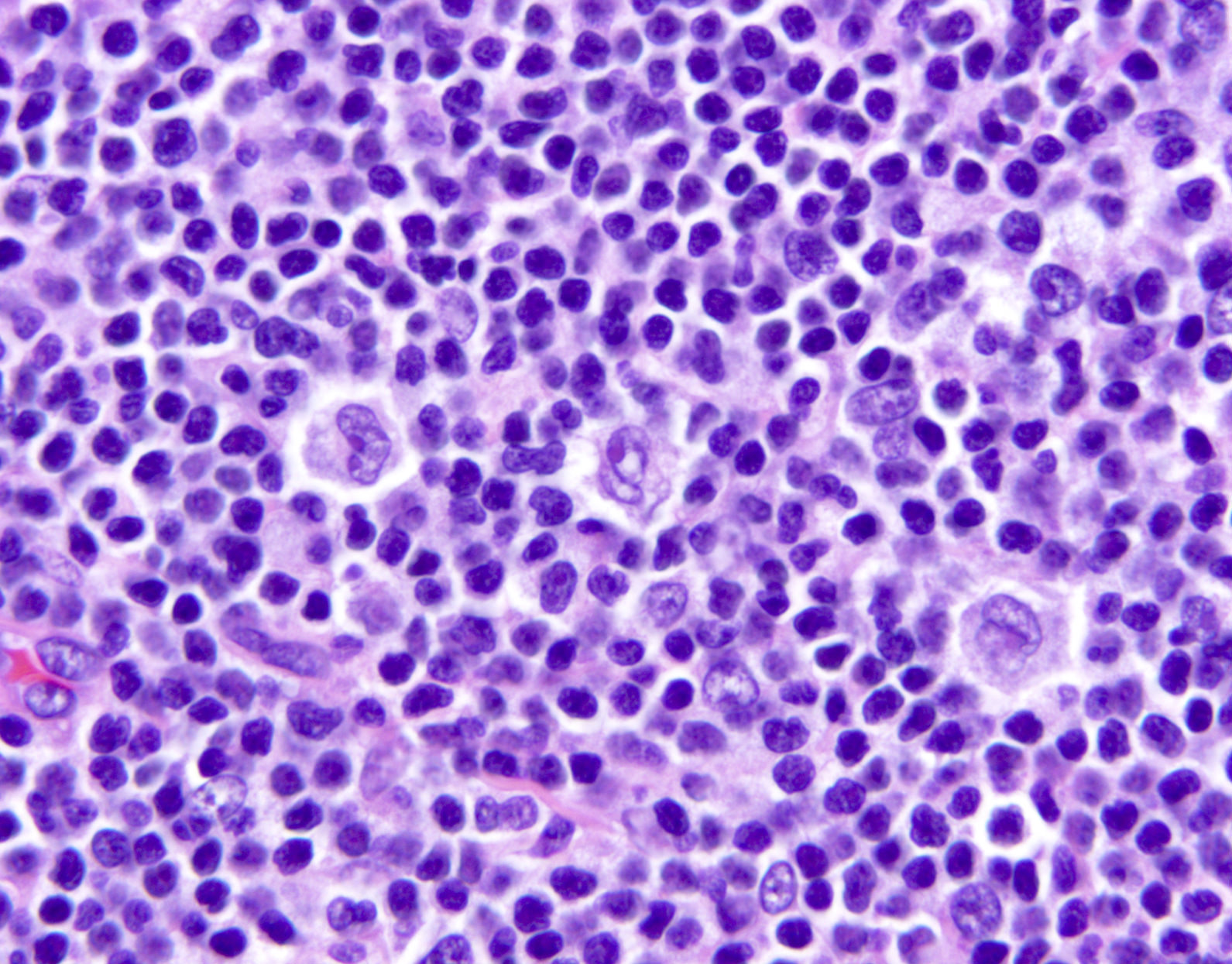
Microscopic (histologic) description
- Bone marrow
- Degree of bone marrow infiltration is less than expected, given the marked lymphocytosis that is often present
- Pattern of marrow involvement can be diffuse, interstitial or sinusoidal
- Often evidence of bone resorption and osteoclastic activity
- Bone trabeculae may show remodeling
- Lytic bone lesions can be present even in the absence of tumoral bone infiltration
- Lymph nodes
- Involves paracortical T cell zones
- Typically show diffuse architectural effacement
- May be subdivided according to cell type and pattern into
- Pleomorphic small cell
- Pleomorphic medium and large cell type / pattern (most common)
- Anaplastic large cell-like (resembling anaplastic large cell lymphoma [ALCL])
- Angioimmunoblastic T cell lymphoma-like (AITL)
- Hodgkin lymphoma-like: seen in early phase of some adult T cell lymphomas
- Leukemic pattern of infiltration with preservation or dilation of lymph node sinuses that contain malignant cells
- Size or shape of the neoplastic cells or identification / classification as one of the patterns above does not impact the clinical course
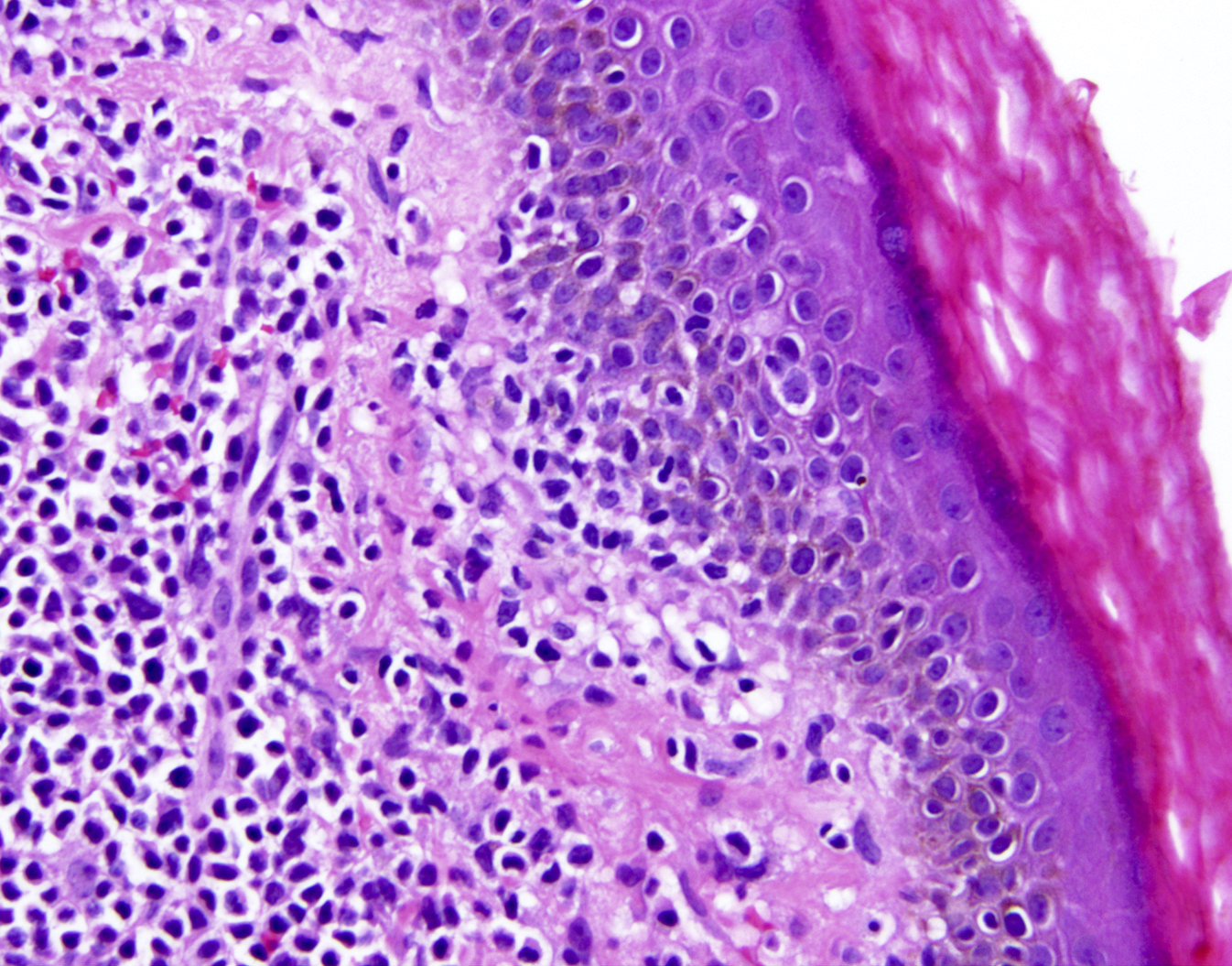
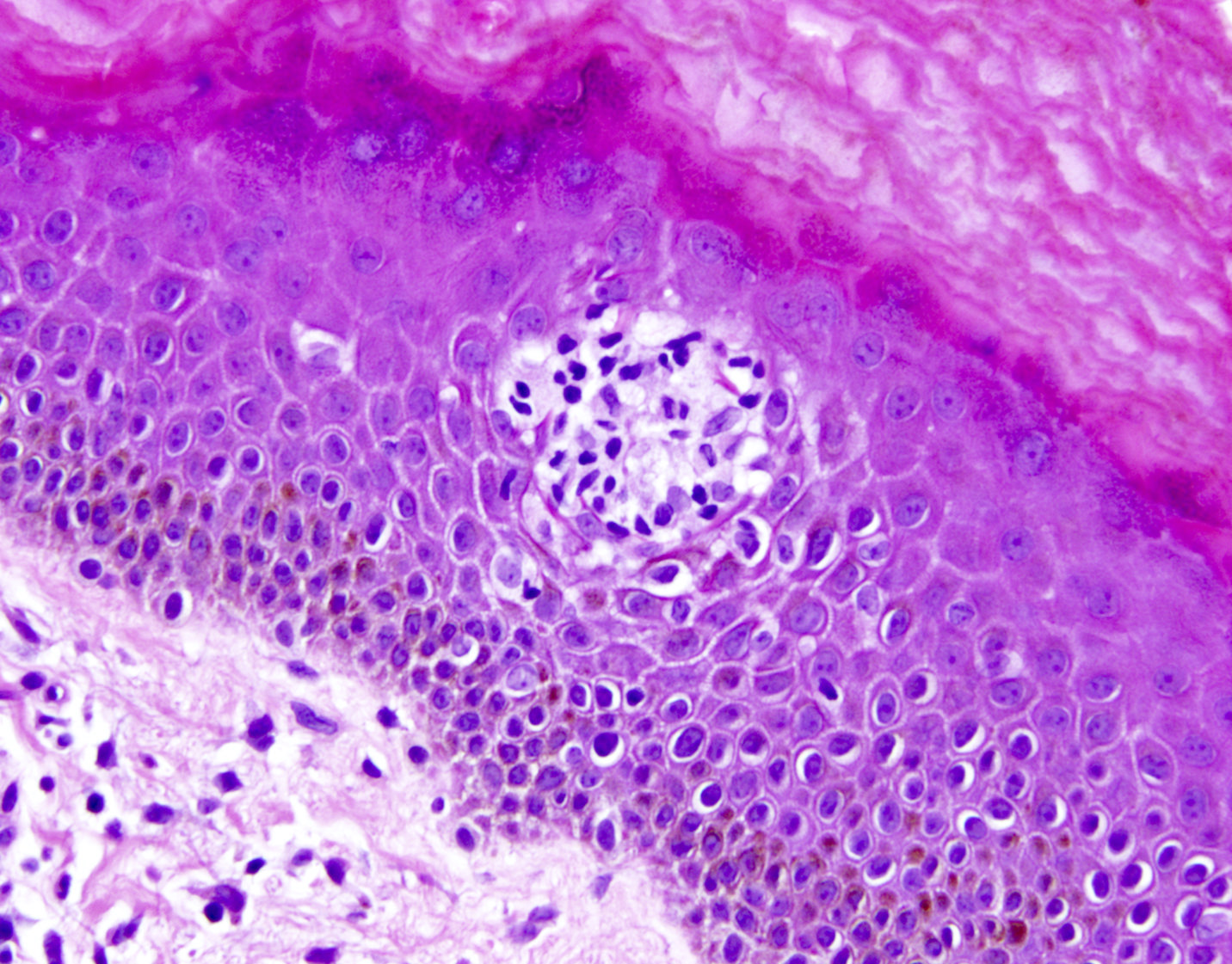
- Skin lesions
- Epidermal infiltration with Pautrier-like microabscesses is common (Mod Pathol 2018;31:1046)
- Hyperparakeratosis is variably present in the overlying epidermis
- Dermal infiltration: mainly perivascular but larger tumor nodules with extension to subcutaneous fat may be observed
- Erythematous lesions: composed of smaller cells in a perivascular pattern in the dermis
- Papules and nodules: composed of larger cells that replace the dermis
- Other sites commonly involved by ATLL include liver, spleen, lungs and central nervous system
- Grading: there is no formal grading system for ATLL
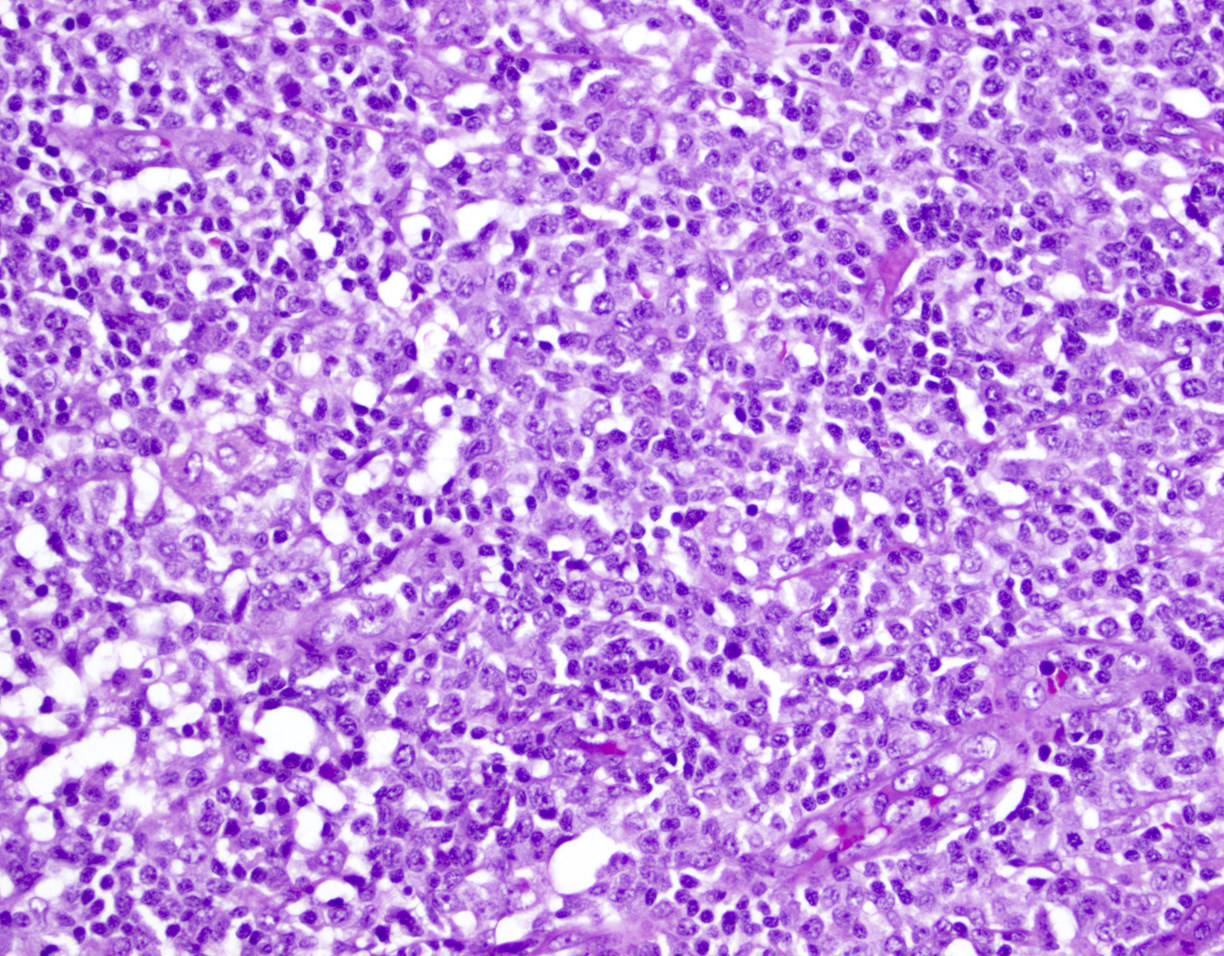
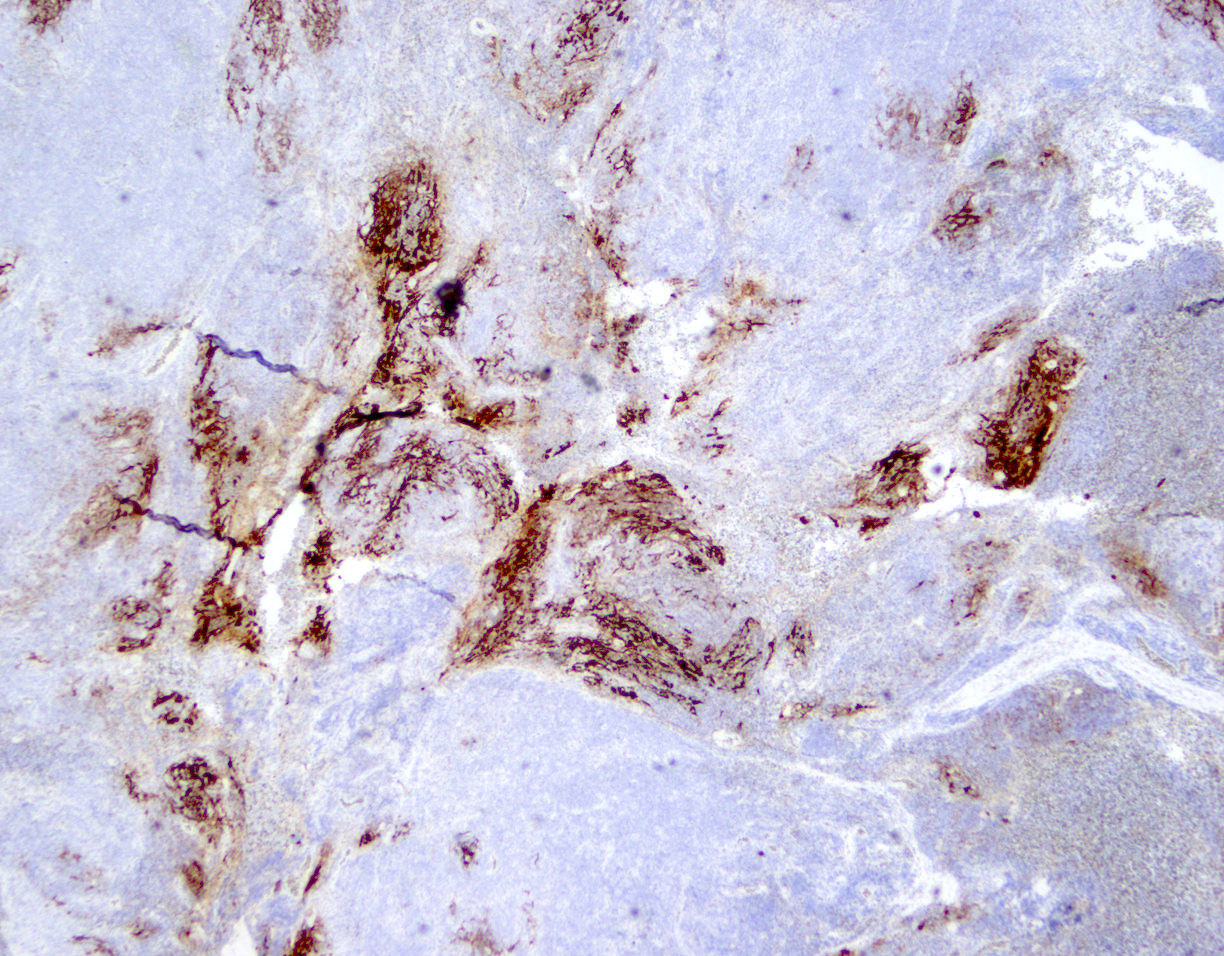
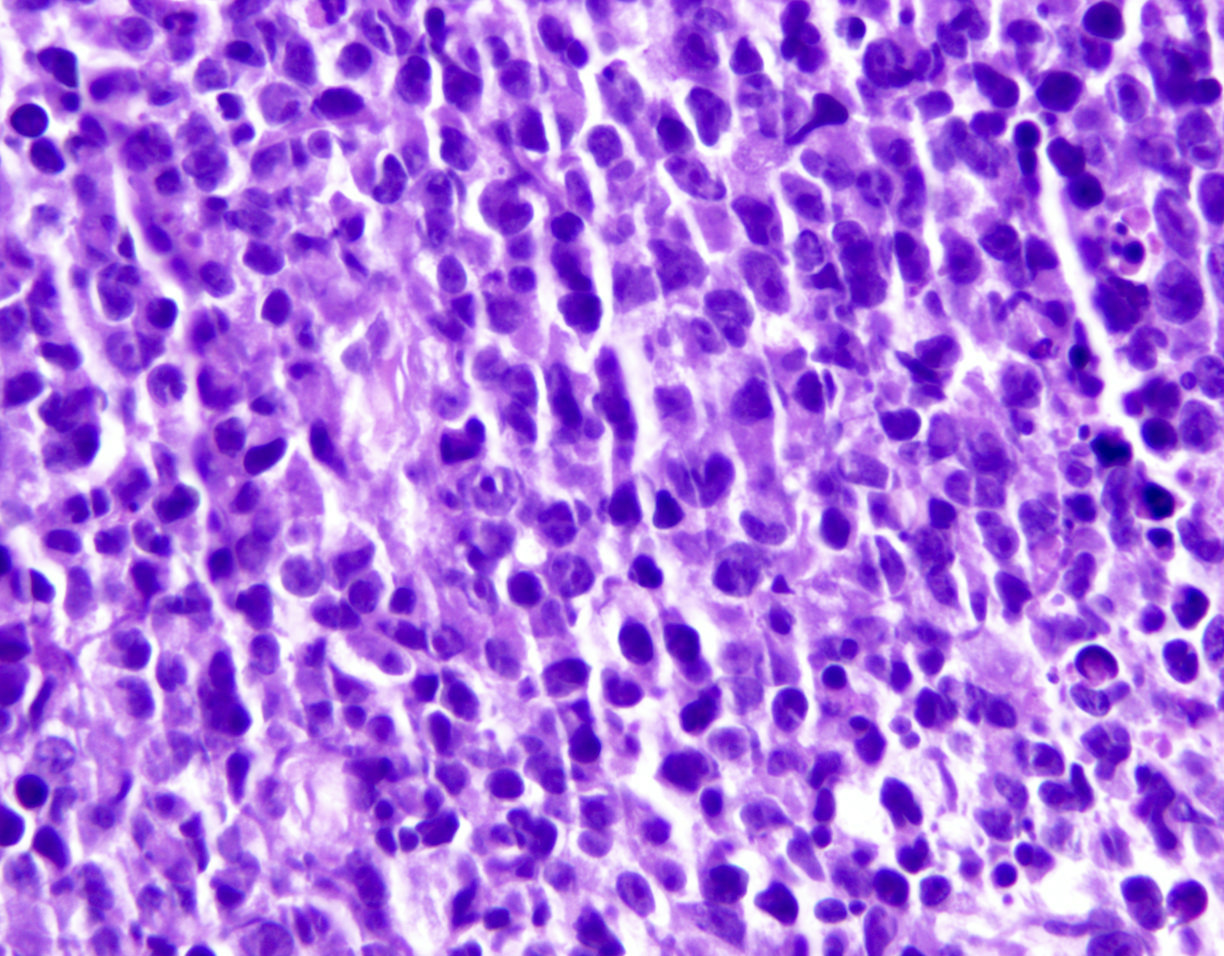
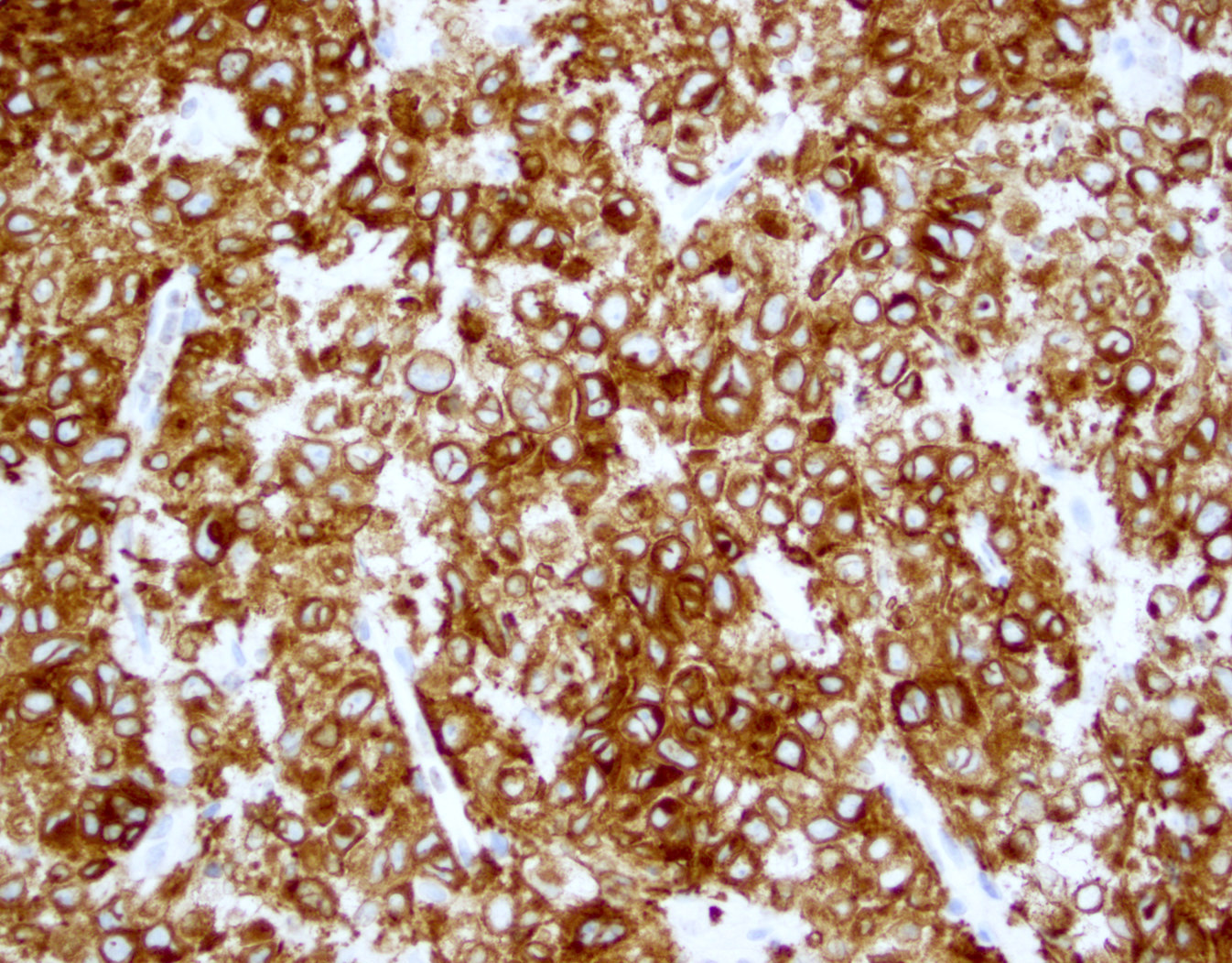
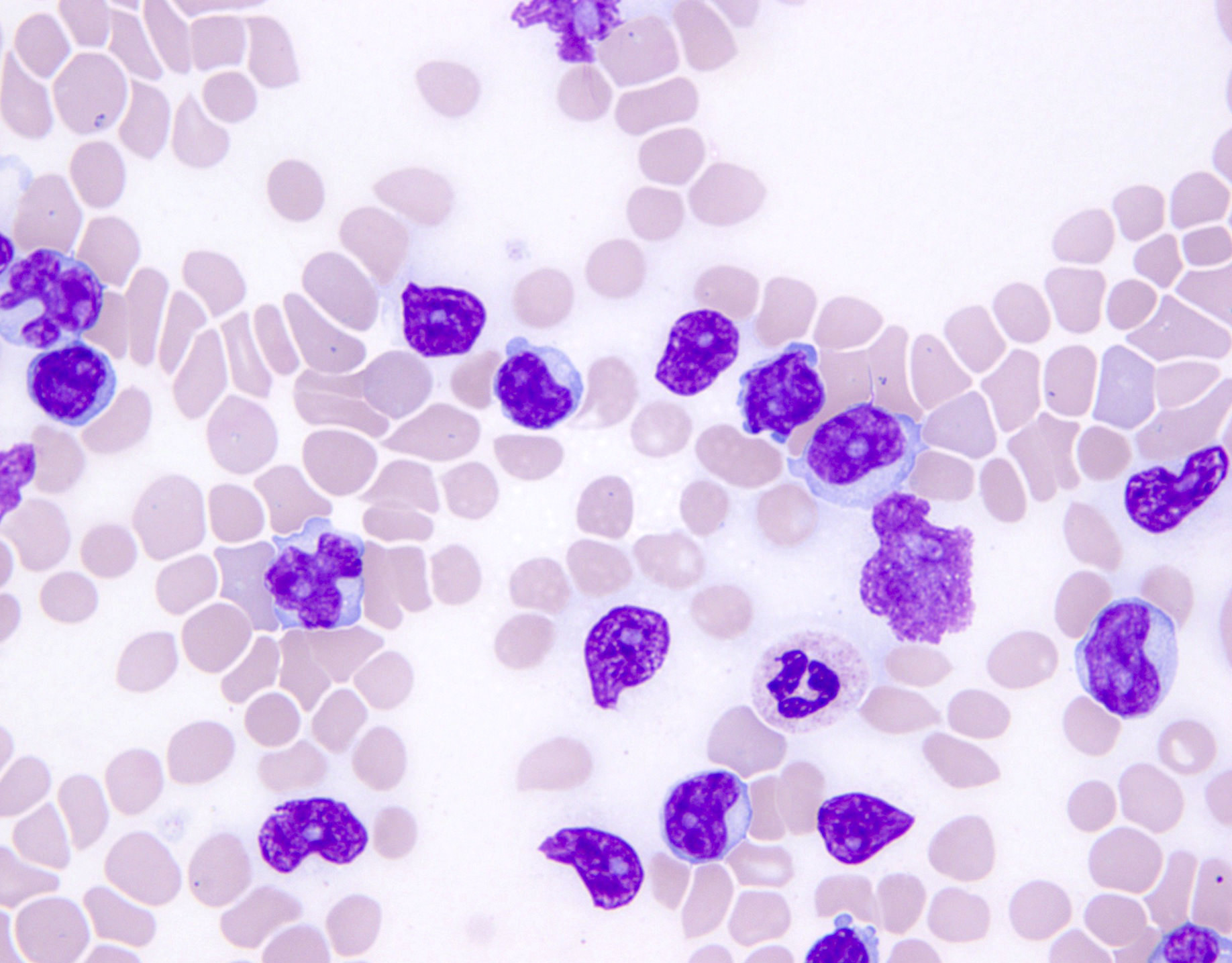
Microscopic (histologic) images
Contributed by Jennifer Chapman, M.D.
Cytology description
- ATLL cells show variable appearances
- Irregular / polylobulated nuclei, homogeneous, condensed chromatin, small nucleoli (Proc (Bayl Univ Med Cent) 2014;27:235, Diagn Cytopathol 2016;44:416)
- Agranular basophilic cytoplasm
- Tumor cells in lymph nodes are frequently pleomorphic and most cases have a mixture of small to large pleomorphic cells
Peripheral smear description
- Medium to large sized lymphocytes with multilobulated nuclei (flower cells) in the peripheral blood of acute (leukemic) ATLL (Hematol Oncol Clin North Am 2008;22:781)
- Slightly larger than normal peripheral blood lymphocytes with moderately condensed chromatin, absent or small nucleoli, scant slightly basophilic cytoplasm and some with multilobulated nuclei in chronic ATLL
Peripheral smear images
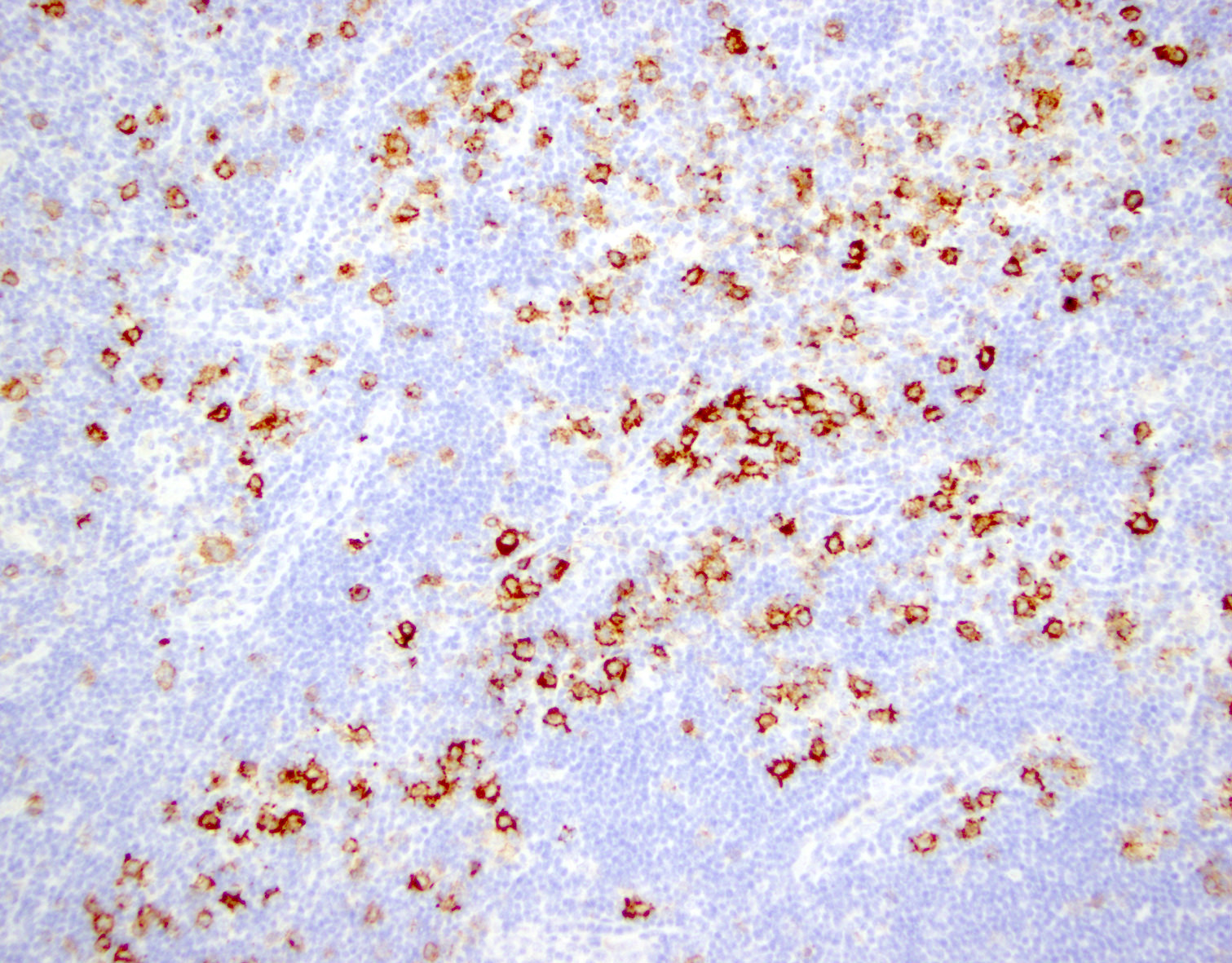
Positive stains
- CD2, CD3, CD4 (most), CD5, CD25 (IL2R), T cell receptor (TCR) αβ, CD45RO, HLA-DR variable, FOXP3 (68%), CD52 variable, CD62 / selectin L variable and CCR4 (88%) (Leukemia 2005;19:2247, Clin Cancer Res 2003;9:3625)
- Frequent expression of IRF4 / MUM1
- Can be negative for CD30 or can express weak or diffuse and strong CD30 (in large transformed cells)
- EBV+ reactive B cells (immunoblasts) may be present in the background
Negative stains
Flow cytometry description
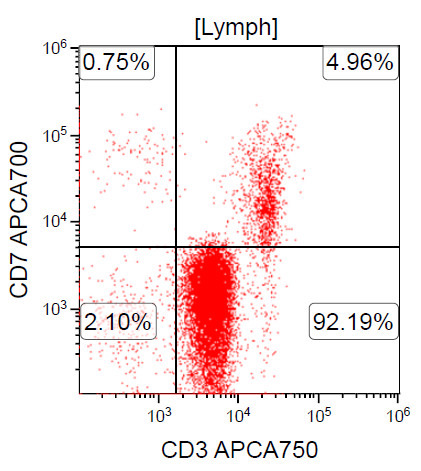
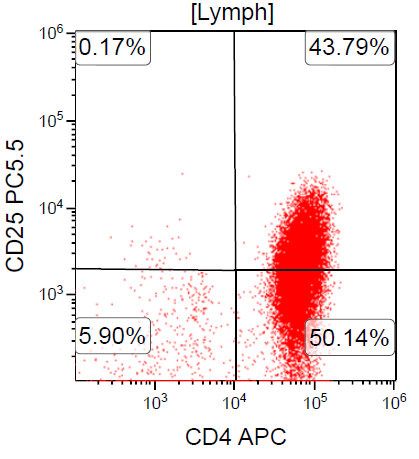
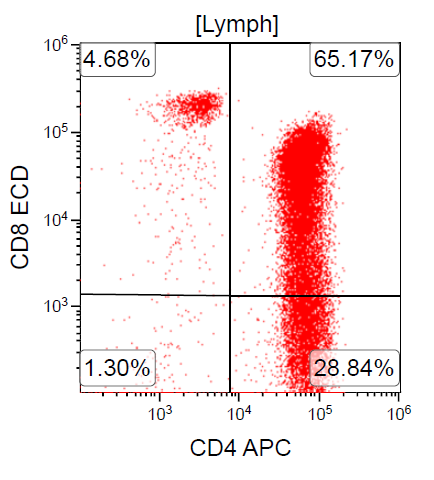
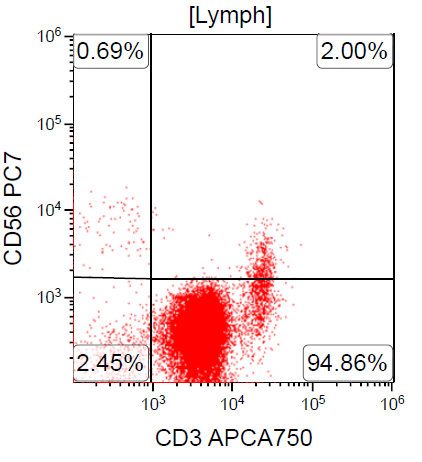
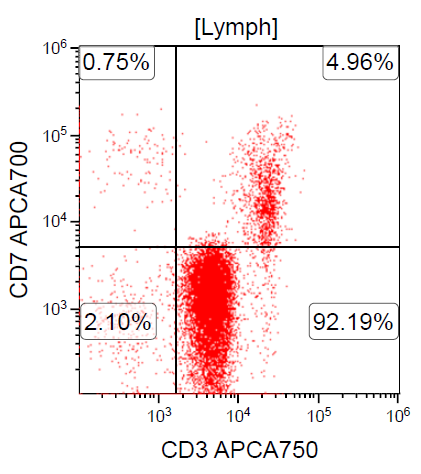
- ATLL is neoplasm of mature T cells, CD2+, CD3+, CD5+, CD45RO+, TCRαβ+, CD1a-, CD7-, CD10-, PD-1-
- ~ 90% of cases are CD4+ / CD8-
- Rare cases can be CD4- / CD8+ or CD4+ / CD8+
- Downregulation of CD7 and upregulation of CCR7 and CADM1 are more frequently observed in aggressive ATLL compared to indolent ATLL (Cancer Med 2017;6:298, Clin Cancer Res 2014;20:2851)
Flow cytometry images
Electron microscopy description
- Viral particles, 80 - 120 nm, are present in both cytoplasm and extracellular space
Molecular / cytogenetics description
- Clonal integration of HTLV1 genome in the neoplastic cells can be demonstrated with RNAscope (Mod Pathol 2021;34:51)
- PCR assays are positive for clonal T cell receptor gene rearrangement
- Quantitative HTLV1 levels
- CCR4 mutations in ~25% of cases (Nat Genet 2015;47:1304)
- RHOA mutations detected in ~15% of cases (Blood 2016;127:596)
- Tumor suppressor genes are inactivated either by mutation or epigenetic silencing
- Frequent gain of function alterations in TCR / NFκB signaling
- Activating mutations in PLCG1, PRKCB and CARD11 (Nat Genet 2015;47:1304)
- CTLA4::CD28 and ICOS::CD28 fusions and REL C terminal truncational changes (Nat Genet 2015;47:1304)
- Numerous complex chromosomal abnormalities are frequent, especially in acute and lymphomatous variants
- STAT3 mutations are more common in indolent subtypes
- Clonal chromosome abnormalities are frequent but not specific
Sample pathology report
- Lymph node, biopsy:
- Adult T cell leukemia / lymphoma (see comment)
- Comment: Diffuse infiltrate of atypical lymphocytes ranging from small and pleomorphic with condensed chromatin to large with open, vesicular chromatin are observed. Cells are positive for CD3, CD4, CD25 and weakly positive for CD30, as well as negative for CD7. Molecular testing confirms HTLV1 infection. Taken together with the patient's history of residence in an endemic region for HTLV1, these results strongly suggest a diagnosis of ATLL.
Differential diagnosis
- Peripheral T cell lymphoma, not otherwise specified (PTCL, NOS):
- Accounts for 30% of patients with PTCL in Western countries
- Background of reactive cells, including eosinophils, plasma cells and histiocytes
- Negative serologic studies or lack of molecular evidence of HTLV1
- It is a diagnosis of exclusion
- Angioimmunoblastic T cell lymphoma (AITL):
- Anaplastic large cell lymphoma (ALCL):
- Sinusoidal distribution in partially involved lymph nodes
- Lymphoma cells are strongly CD30+ (ATLL with strong CD30 expression in 100% of lymphoma cells has been reported)
- Expression of cytotoxic granule associated proteins (can be seen in ATLL)
- Translocations involving ALK gene or ALK1 protein expression
- ALK- ALCL cases are difficult to differentiate from ATLL without HTLV1 testing
- Classic Hodgkin lymphoma (HL):
- Background T cells are immunophenotypically normal
- Lacks clonal T cell gene rearrangement
- Lacks malignant cells in peripheral blood; rare reports of skin lesions and hypercalcemia
- Cutaneous T cell lymphoma / mycosis fungoides (CTCL / MF), Sézary syndrome:
- T cell prolymphocytic leukemia (T PLL):
- Involves blood at presentation with increased white blood cells (WBC)
- Some nuclear irregularity but no flower cell morphology as in ATLL
- TCL1 gene rearrangements are present in the majority of cases
- Lack of anti-HTLV1 positive serology
- T lymphoblastic leukemia / lymphoma (T LBL):
- T cell large granular lymphocytic leukemia (LGL):
Additional references
Board review style question #1
Which of the following is the most common site of extranodal involvement in adult T cell leukemia / lymphoma (ATLL)?
- Central nervous system
- Heart
- Liver
- Skin
Board review style answer #1
D. Skin. Extranodal sites of ATLL include the skin, lungs, liver, gastrointestinal tract and central nervous system; however, the most common is skin (involved in > 50% of cases) as well as peripheral blood. Answers A and C are incorrect because the central nervous system and liver are potential extranodal sites but are less common than skin. Answer B is incorrect because extranodal spread to the heart, although possible, is extremely rare.
Comment here
Reference: ATLL
Comment here
Reference: ATLL
Board review style question #2
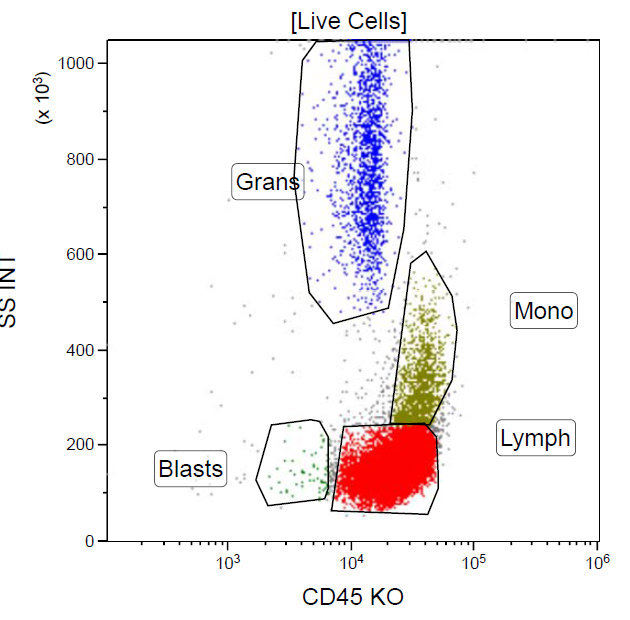
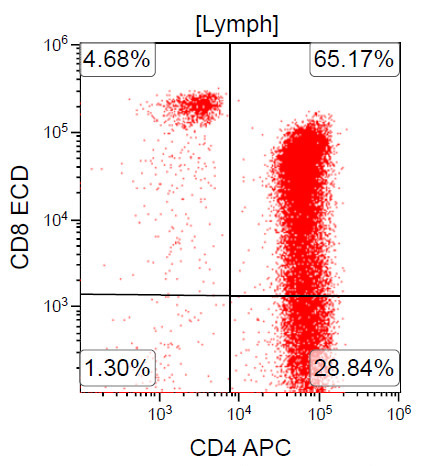
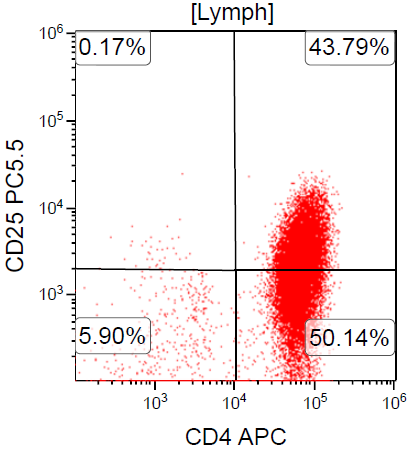
A 67 year old Haitian man with hepatitis B presents with fatigue and weakness. No other symptoms are present. Laboratory work up shows leukocytosis (55 x 109/L) and thrombocytopenia (100 x 109/L). Physical examination and imaging studies show massive splenomegaly with no lymphadenopathy. There was no hypercalcemia or increase in serum LDH. Peripheral smear review showed abnormal lymphocytosis with circulating lymphoma cells having mature nuclear features and highly lobulated nuclei. Flow cytometry performed in bone marrow aspirate is provided above. Serology for anti-HTLV1 antibody is positive. Which of the following is the most accurate statement?
- Dual positivity for CD4 and CD8 is uncommon in adult T cell leukemia / lymphoma
- Expression of CD25 is specific for adult T cell leukemia / lymphoma (ATLL) among T cell lymphomas / leukemias
- Patient has acute myeloid leukemia (AML) with maturation (NK / myeloid type)
- Patient has T cell large granular lymphocytic leukemia (LGL)
Board review style answer #2
A. Dual positivity for CD4 and CD8 is uncommon in adult T cell leukemia / lymphoma. 90% of cases are CD4+ / CD8-, with only rare cases showing dual positivity. Answer B is incorrect because although T cells coexpress CD25 in ATLL, it is not specific. Answer C is incorrect because acute myeloid leukemia with maturation (NK / myeloid type) would show positivity for myeloid antigens. Answer D is incorrect because T cell large granular lymphocytic leukemia would show a predominance of lymphocytes that demonstrate bright CD45 on flow cytometry.
Comment here
Reference: ATLL
Comment here
Reference: ATLL