Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Flow cytometry description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Guo X, Wang Y. Monomorphic epitheliotropic intestinal. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lymphomameitl.html. Accessed April 1st, 2025.
Definition / general
- Monomorphic epitheliotropic intestinal T cell lymphoma (MEITL), previously known as type II enteropathy associated T cell lymphoma, is a primary intestinal mature T cell lymphoma that is considered to arise from intestinal intraepithelial T lymphocytes, is usually positive for CD8 and CD56 and lacks association with celiac disease
Essential features
- Aggressive primary T cell lymphoma with a poor clinical outcome, with an average of 6 - 10 months overall survival (Leukemia 2013;27:1688)
- Histologically, it is monomorphic small to medium sized T cells expressing CD3, CD8, CD56 and cytotoxic markers (TIA1 or granzyme B)
- Predominately expresses gamma / delta T cell receptors
- Usually has complex cytogenetic abnormalities; mutations in STAT5B, SETD2, JAK3, GNAI2 and CREBBP are common; aberrant overexpression of SYK has been described (Blood Adv 2020;4:4769)
Terminology
- Previously known as type II enteropathy associated T cell lymphoma (EATL) or enteropathy associated T cell lymphoma type II
ICD coding
- ICD-10: C86.2 - enteropathy type (intestinal) T cell lymphoma
Epidemiology
- Rare aggressive disease with a frequency of < 5% among primary gastrointestinal malignant lymphomas (Am J Surg Pathol 2011;35:1557)
- Predominantly occurs in Asian and Hispanic / indigenous origin; rare in Western population (Mod Pathol 2011;24:983)
- Median age in the sixth decade
- M:F = 2:1 (Mod Pathol 2011;24:983)
Sites
- Small bowel, especially the jejunum, followed by ileum (Am J Hematol 2012;87:663)
Pathophysiology
- Neoplastic T cells are generally thought to be derived from the intestinal intraepithelial lymphocytes, with a phenotype of CD8+, CD56+ and megakaryocyte associated tyrosine kinase (MATK), which appears to have a pathogenetic role in MEITL (Leukemia 2013;27:1688)
- Hypothesized that altered T cell receptor (TCR) signaling contributes to the occurrence of MEITL
- Aberrant overexpression of SYK due to hypomethylation of the SYK promoter was reported, causing abnormal proliferation of the lymphoma cells (Mod Pathol 2018;31:505)
- Studies also showed that chromosome alterations may be pathogenetic for MEITL, including chromosomal copy number variations, gains of the chromosomal region 9q33-q34 and an amplification of chromosome locus 8q24, resulting in neoplastic cell proliferation (Mod Pathol 2015;28:1286)
- Characterized by activating mutations of the JAK-STAT pathway, with mutations of STAT5B being the most common
- Other commonly mutated genes include JAK3, GNAI2, CREBBP and SETD2, which are involved in the pathogenesis of MEITL (Nat Commun 2016;7:12602)
- Whole genome sequencing confirmed the previous studies of cytogenetic and molecular mutations involved in MEITL, including CREBBP, STAT5B, SETD2, GNAI2, JAK3 and AXSL3 (Blood Adv 2020;4:4769)
Etiology
- Unknown
Clinical features
- Usually nonspecific clinical symptoms, including a change in bowel habits, chronic abdominal pain (86%), chronic diarrhea (33%), weight loss and complications including intestinal obstruction / perforation (33%) and bleeding (17%); B symptoms are common (48%) (Ann Hematol 2019;98:2541)
- Endoscopically or grossly, the tumor is manifested with mucosal granular forming ulcers, nodules, plaques, diffuse mucosal thickening, strictures or large masses (single or multifocal)
- Patients usually do not manifest with malabsorption or celiac disease
Diagnosis
- Diagnosis requires clinicopathological correlation
- In patients without a history of celiac disease presenting with chronic abdominal pain, diarrhea and other related symptoms, abdominal imaging studies are warranted, including abdominal ultrasound, CT with or without contrast, MRI and endoscopy
- Endoscopy usually shows fine mucosal granularity and diffuse thickening of the mucosa with semicircular shallow ulcerations, while abdominal MRI or CT show thickening of bowel wall and mesenteric lymphadenopathy
- Tissue biopsy demonstrates monomorphic neoplastic lymphocytes with aberrant T cell phenotype, positive for CD3, CD8, CD56, TIA1 and T cell receptor gamma or delta
Laboratory
- Important to rule out celiac disease by demonstrating negativity for endomysial antibodies, HLA DQ2 or HLA DQ8
Radiology description
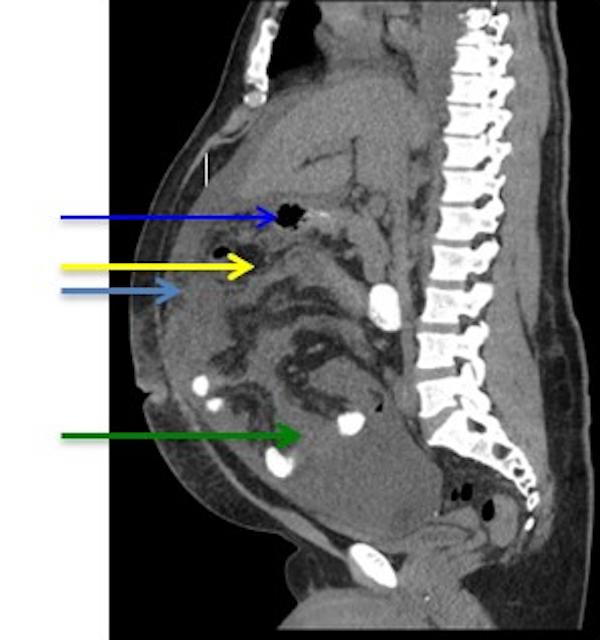
- Abdominal computed tomography (CT) sometimes can demonstrate a mass wrapping around the small bowel, with thickening of the intestinal wall or distension of the small bowel due to obstruction and ascites
- Free air under the diaphragm can be seen if perforated
- Mesenteric lymphadenopathy usually indicates neoplastic spreading (Hematology 2018;23:10)
Radiology images
Prognostic factors
- Overall, poor prognosis; estimated average survival is about 6 - 10 months and a median survival of 7 months due to lack of specific targeted therapy (Am J Hematol 2012;87:663)
Case reports
- 53 year old man with diffuse achy abdominal pain of 2 weeks duration (Cureus 2020;12:e10084)
- 64 year old man with chronic watery diarrhea and weight loss for 5 months (Intest Res 2017;15:546)
- 70 year old woman of Chinese ethnicity with a 4 week history of abdominal pain and persistent vomiting (Hematol Transfus Cell Ther 2020 Jun 6 [Epub ahead of print])
- 70 year old Hispanic woman with a history of diabetes mellitus type 2, presented with intermittent lower abdominal pain, nausea, vomiting and diarrhea for 14 months (Ecancermedicalscience 2017;11:771)
- 83 year old man with fever and diarrhea (J Clin Exp Hematop 2018;58:102)
Treatment
- Commonly used regimens include surgical tumor resection, CHOEP (cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone), CHOP14 and CHOP21 (cyclophosphamide, doxorubicin, vincristine, prednisone), dose adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin), hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone) / R-MTX-Ara-C (rituximab, methotrexate, cytarabine) and stem cell transplant (J Natl Compr Canc Netw 2016;14:1067)
Gross description
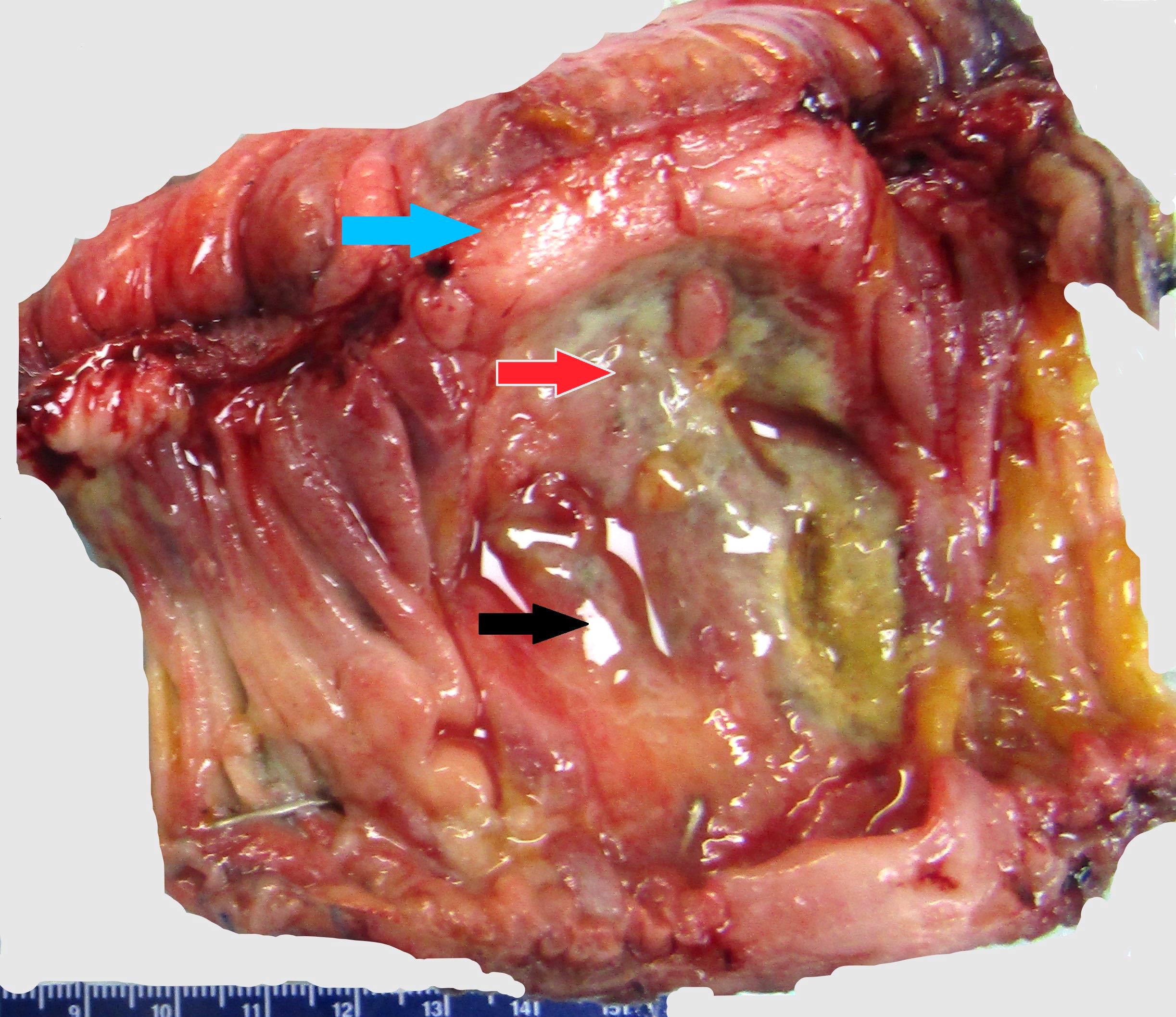
- Grossly, the findings vary depending the tumor stage, from edematous or granular mucosa, to diffuse thickening of the intestinal wall, to a mass with or without ulceration
- Mass can be sub centimeter to a few centimeters in size
Gross images
Microscopic (histologic) description
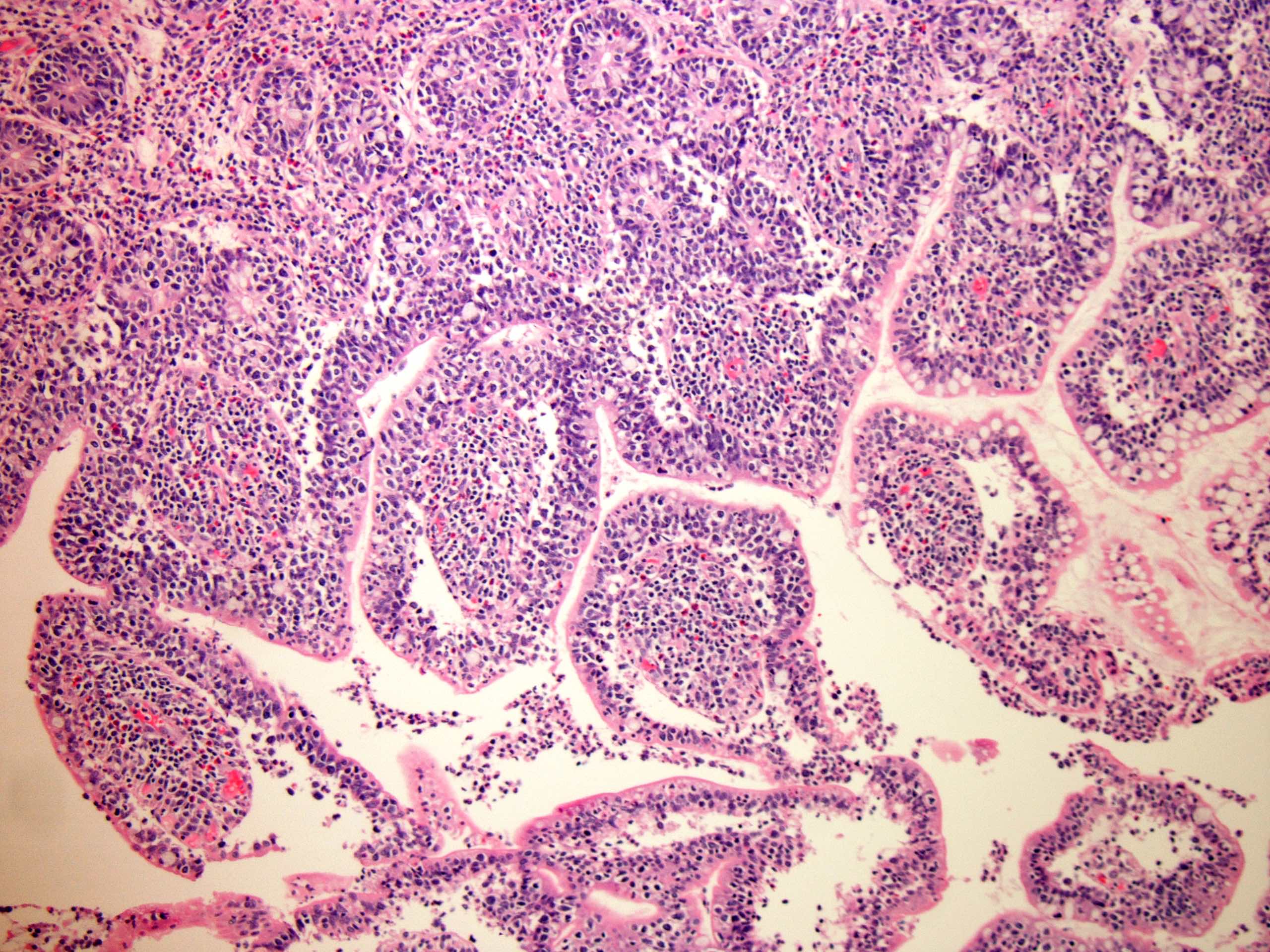
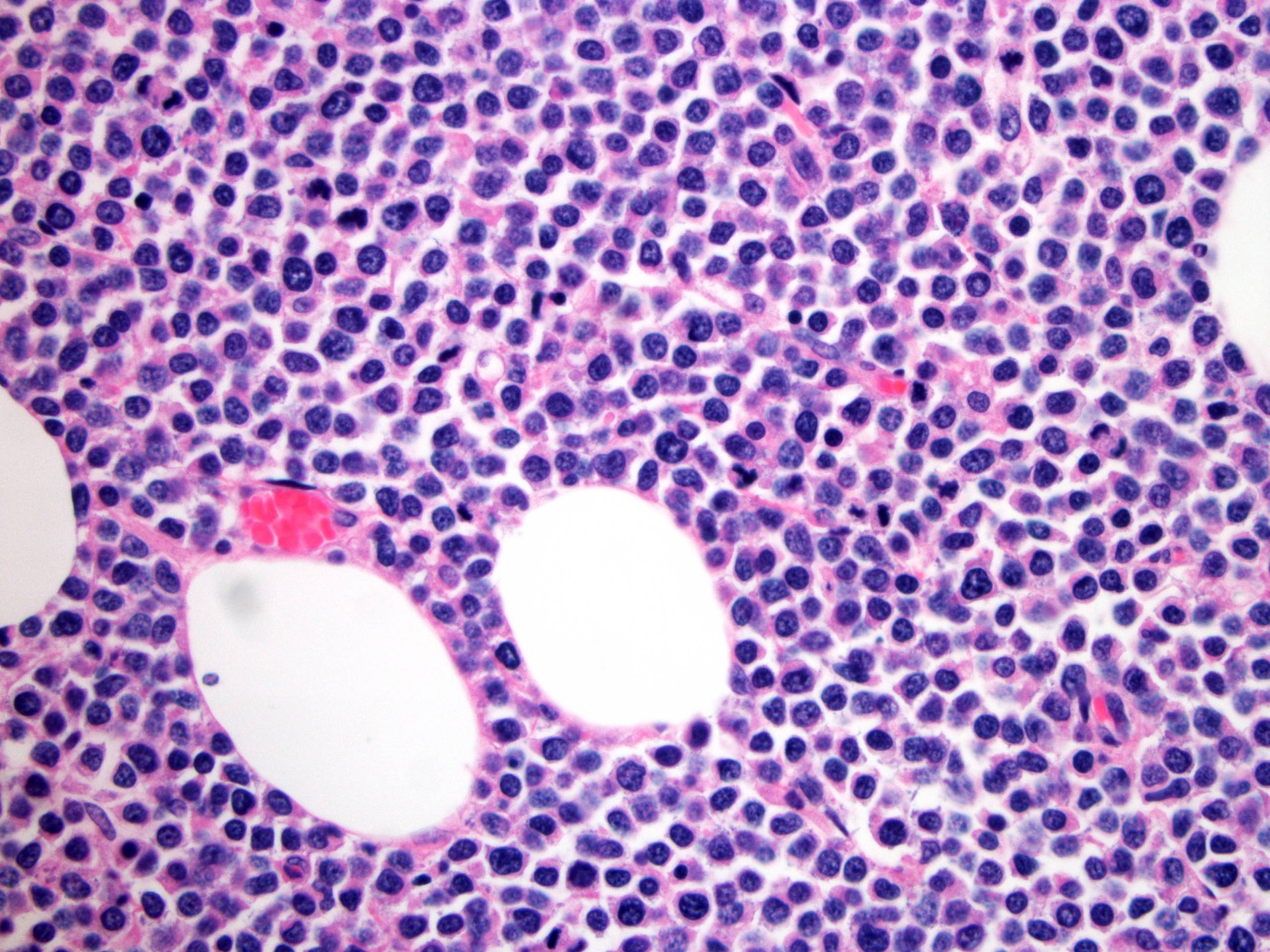
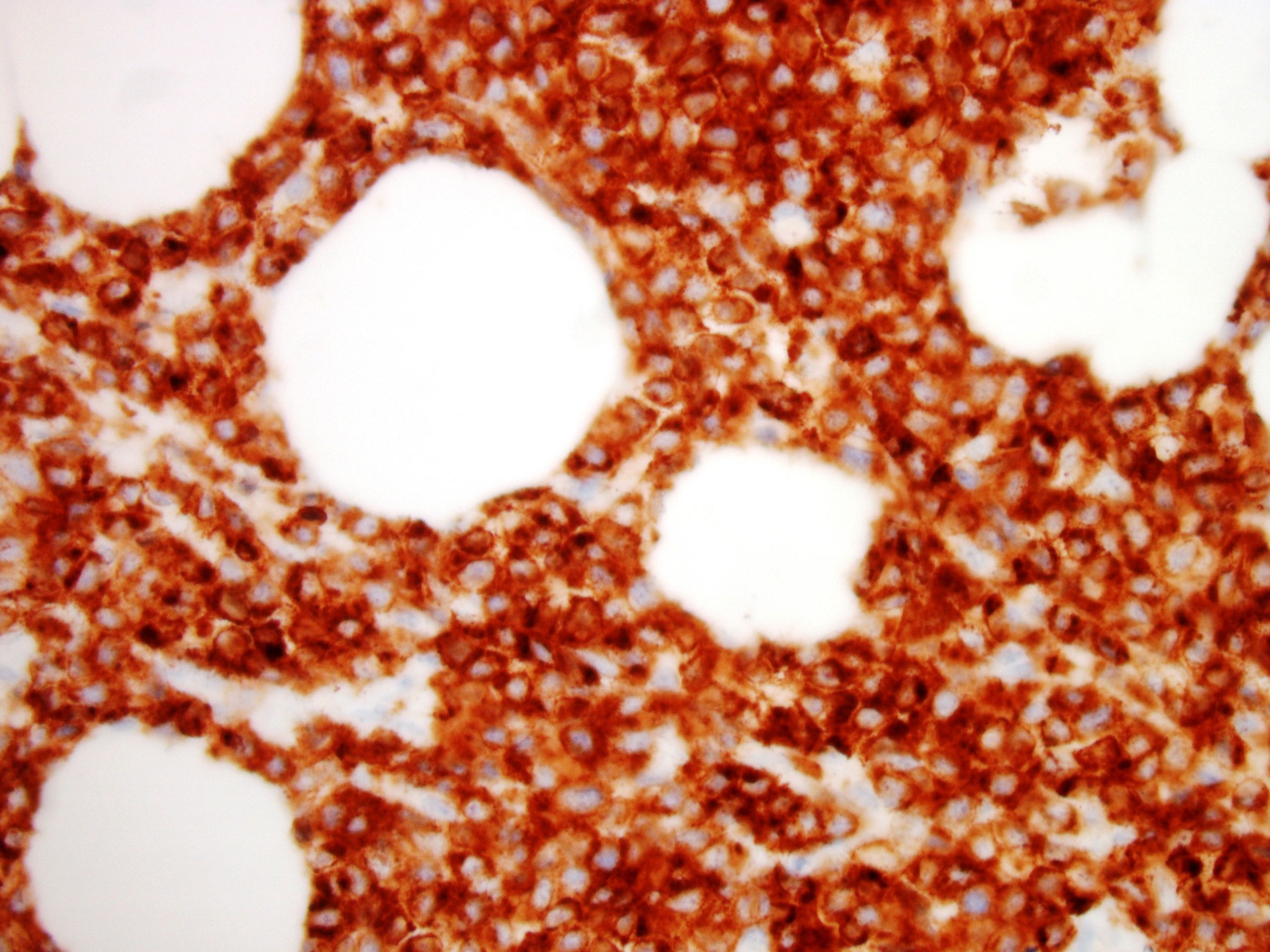
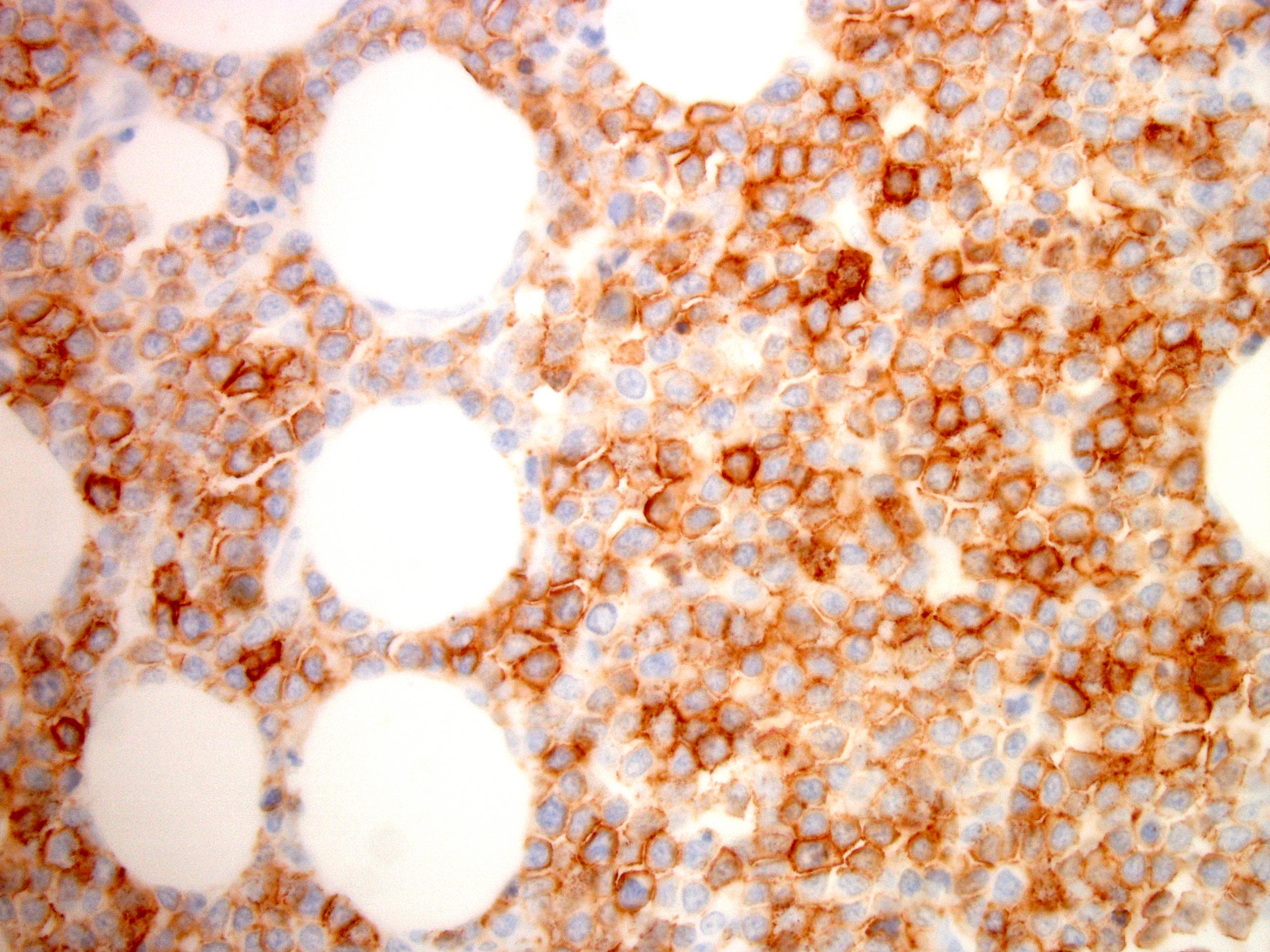
- Neoplastic lymphocytes are relatively monotonous, intermediate in size, with round or slightly irregular nuclear contours, dispersed chromatin, inconspicuous nucleoli and scant rim of pale cytoplasm
- Prominent epitheliotropism as well as transmural infiltrate are characteristic; only few inflammatory cells are noted in the background, unless associated with mucosal ulceration
- Reference: Cureus 2020;12:e10021
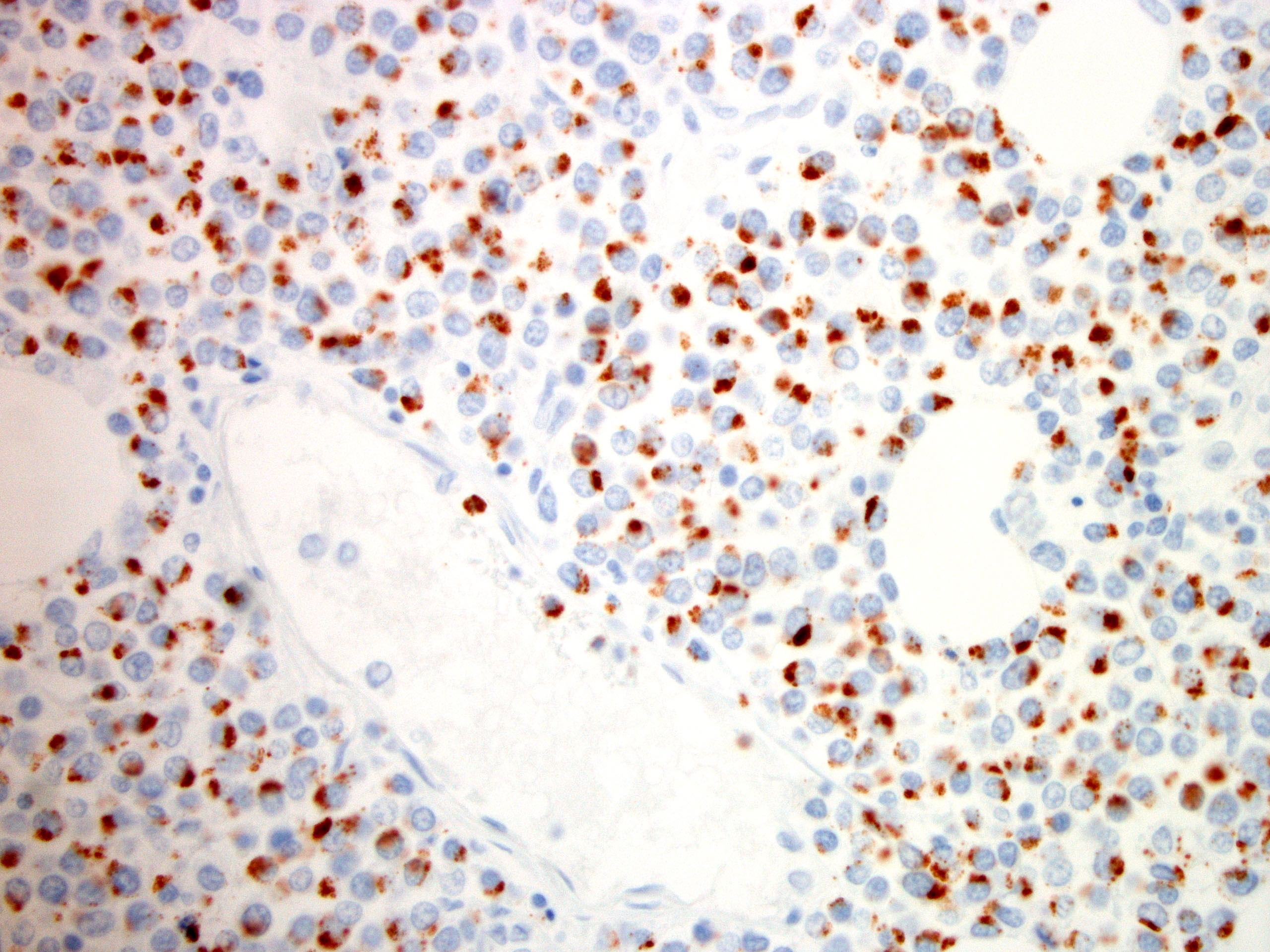
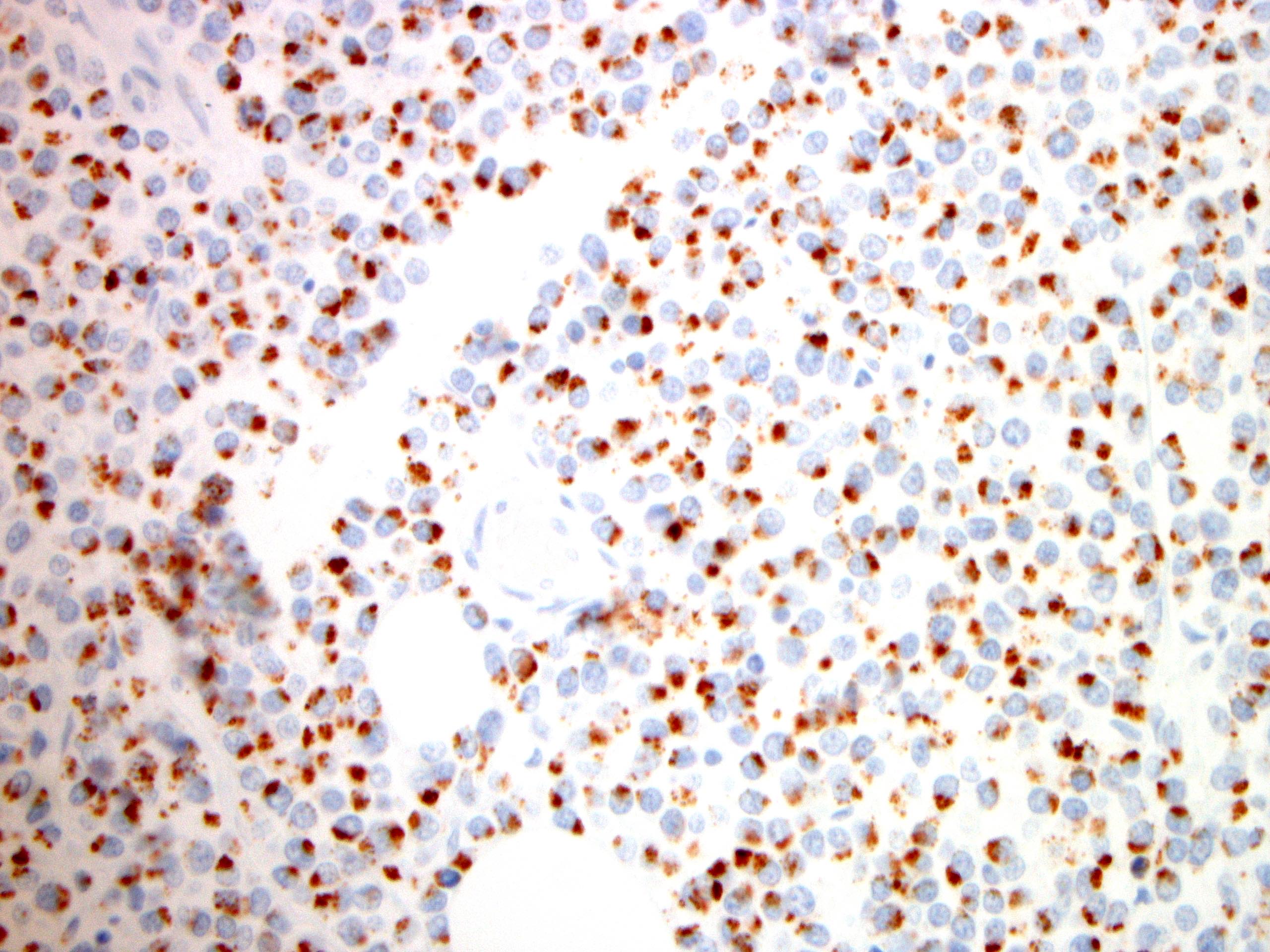
Microscopic (histologic) images
Positive stains
- CD3, CD8, CD56 and TIA1
- Other cytotoxic markers are variably expressed
- CD20 (approximately 20% of MEITLs aberrantly express CD20; however, other pan B cell markers are negative) (Pathology 2020;52:128)
Flow cytometry description
- Positive: surface CD3, CD56, granzyme B and TCRδγ
- Negative: CD4, CD8, surface TCRαβ, cytoplasmic perforin; a subset of cases are negative for TCRδγ and TCRαβ (silent TCR)
- Reference: J Clin Exp Hematop 2018;58:102
Molecular / cytogenetics description
- Extra signals for MYC at 8q24 are commonly seen (J Clin Exp Hematop 2018;58:102)
- Gains at 9q34.3 by FISH and copy number analysis were seen in > 75% of cases and other aberrations include gains at 1q32.3, 4p15.1, 5q34, 7q34, 8p11.23, 9q22.31, 9q33.2, 8q24 (MYC locus) and 12p13.31 and losses of 7p14.1 and 16q12.1 (Mod Pathol 2015;28:1286)
- 63% of cases had mutations in STAT5B when examined by whole exome sequencing (Leukemia 2016;30:1311)
- JAK3 and GNAI2 are also mutated in some cases, while SETD2 mutation was reported > 90% of cases (Nat Commun 2016;7:12602)
- Spleen tyrosine kinase (SYK) was overexpressed in MEITL, which was caused most likely by hypomethylation of the SYK promoter (Mod Pathol 2018;31:505)
Sample pathology report
- Distal small intestine, resection:
- Monomorphic epitheliotropic intestinal T cell lymphoma (MEITL) with gamma / delta phenotype (see comment)
- No histomorphologic features of celiac disease in uninvolved adjacent nonneoplastic mucosa
- Comment: According to the clinical records, patient had no history of celiac disease. Microscopic examination revealed diffuse and dense monomorphic small to medium sized atypical T cells with transmural infiltration and notable absence of necrosis. The immunohistochemical stains demonstrated aberrant T cells positive for CD3, CD7, CD8, CD56, granzyme B, TIA1, TCR delta, T-bet and CD20 (subset). The aberrant cells were negative for CD4, CD2, CD5, CD30, CD57, GATA3, TCR beta, pSTAT3, CD79a and PAX5. Proliferation index (Ki67) was approximately 90%. EBV small RNA in situ hybridization (EBER) was negative. Overall, the clinical history, histomorphology and immunophenotype support the diagnosis of monomorphic epitheliotropic intestinal T cell lymphoma (MEITL).
Differential diagnosis
- Enteropathy associated T cell lymphoma (EATL):
- Intestinal T cell lymphoma, NOS:
- Phenotypically similar to other peripheral T cell lymphomas (PTCL), NOS with a predominance of CD4+; subset with cytotoxic T cells, subset with EBV and does not fulfill the criteria of EATL or MEITL (Naeim: Atlas of Hematopathology, 2nd Edition, 2018)
- Indolent T cell lymphoproliferative disorder of the gastrointestinal tract:
- Small monomorphic T cell nondestructive proliferation, predominately at lamina propria
- Muscularis mucosae and submucosa infiltration may be seen focally
- Phenotypically, they are CD3+, CD8+ and TIA1+ (Blood 2013;122:3599)
- Extranodal NK / T cell lymphoma:
- Can present primarily in intestinal sites, including the small bowel and colon
- Geographic necrosis and angiocentric / angiodestructive growth pattern of the tumor cells are common histological features
- Neoplastic cells are cytotoxic / NK cell phenotype, usually EBV+ (J Hematol Oncol 2013;6:86)
- Peripheral T cell lymphoma, NOS with intestinal spread:
- Usually CD3+, CD4+, CD7- (Ecancermedicalscience 2016;10:625)
- Mycosis fungoides with gastrointestinal involvement:
- Can be seen in patients with a long history of skin lesions and a diagnosis of mycosis fungoides
- Phenotypically, they are usually CD3+, CD4+, CD7- (J R Soc Med 1984;77:114)
Additional references
Board review style question #1
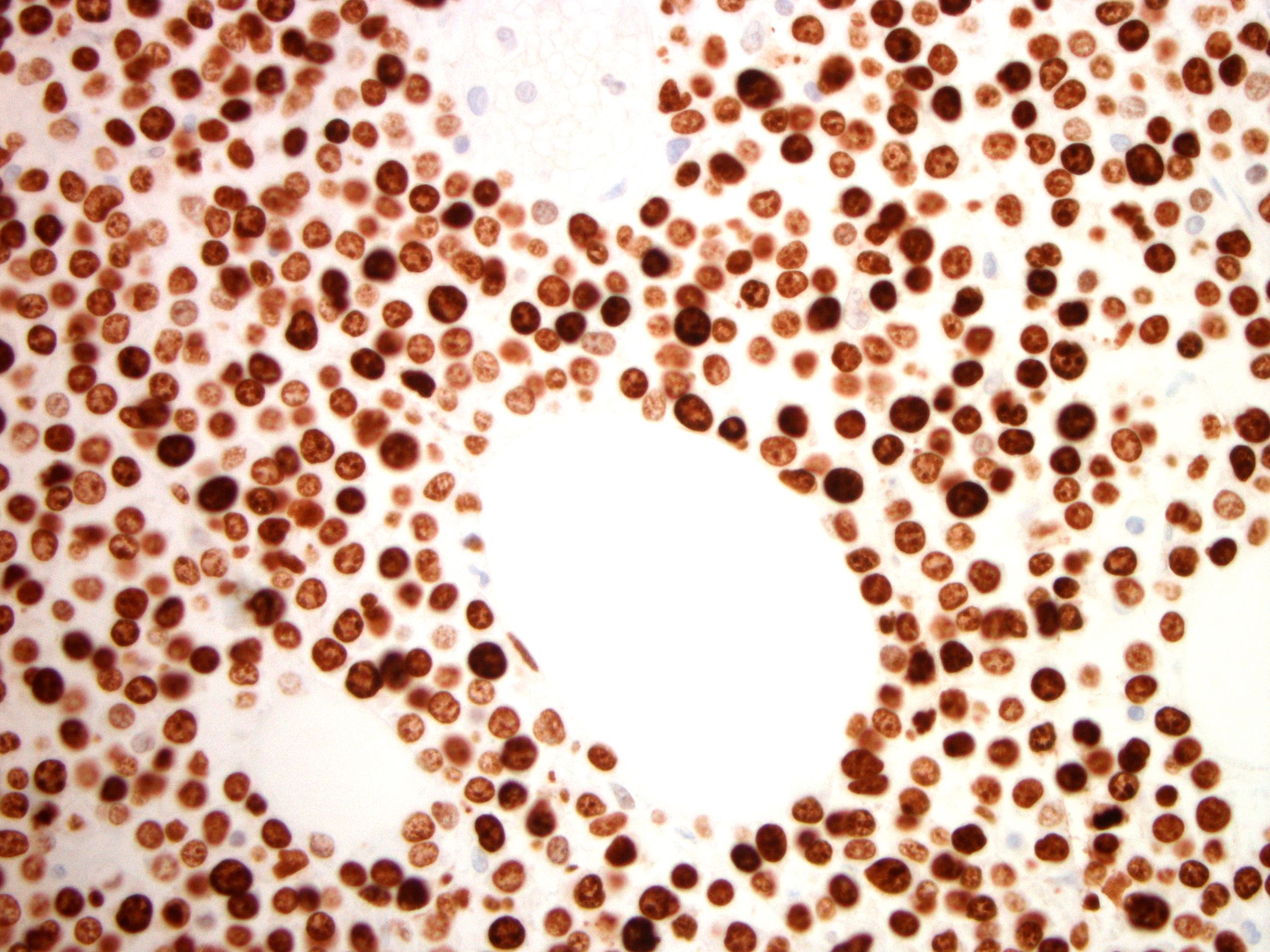
Which of the following best describes the features of the small intestine displayed in the above figure?
- Can be associated with celiac disease
- Commonly seen in Caucasian population
- Most common phenotype is CD3+, CD8+, CD56+ and TCRδγ+
- Monomorphic T cells in lymphoma with indolent clinical outcome
- Patients usually manifest symptoms of malabsorption
Board review style answer #1
C. Most common phenotype is CD3+, CD8+, CD56+ and TCRδγ+. The figure displays small intestine mucosa with a dense infiltrate of epithelium by monomorphic small to intermediate sized lymphocytes, consistent with monomorphic epitheliotropic T cell lymphoma (MEITL). This is a primary intestinal lymphoma that is distinguished from enteropathy associated T cell lymphoma (EATL) because it is not associated with celiac disease or malabsorption, it is more common in Asian populations and has an aggressive behavior with a poor outcome. Phenotypically, MEITL is usually CD3+, CD8+, CD56+ and TCRδγ+ and aberrantly expresses CD20 in 20% of patients.
Comment Here
Reference: Monomorphic epitheliotropic intestinal T cell lymphoma
Comment Here
Reference: Monomorphic epitheliotropic intestinal T cell lymphoma
Board review style question #2
Which of the following is helpful in the diagnosis of monomorphic epitheliotropic intestinal T cell lymphoma (MEITL), rather than enteropathy associated T cell lymphoma (EATL)?
- Expression of megakaryocyte associated tyrosine kinase (MATK) is downregulated in MEITL and upregulated in EATL
- Gains in chromosome 8q24 involving MYC are seen in a high proportion of cases of MEITL but not EATL
- HLA DQ2 or HLA DQ8 alleles are commonly seen in MEITL, not EATL
- T cell receptor expression in MEITL is usually alpha / beta more than gamma / delta, while in EATL, gamma / delta expression is more than alpha / beta expression
- T cell receptor expression is relatively the same in MEITL and EATL
Board review style answer #2
B. Gains in chromosome 8q24 involving MYC are seen in a high proportion of cases of MEITL but not EATL. The cytogenetic and molecular features associated with MEITL include gains of 8q24, overexpression of MATK and expression of TCRδγ. In contrast, EATL is characterized by expression of HLA DQ2 or HLA DQ8 alleles as well as the expression of TCRαΒ (Semin Diagn Pathol 2021 [Epub ahead of print]).
Comment Here
Reference: Monomorphic epitheliotropic intestinal T cell lymphoma
Comment Here
Reference: Monomorphic epitheliotropic intestinal T cell lymphoma