Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Endoscopic description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Flow cytometry description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Cite this page: Aqil B. Indolent NK cell lymphoproliferative disease of the GI tract. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lymphomaindolentNKlymphoprolifGI.html. Accessed January 17th, 2025.
Definition / general
- Epstein-Barr virus (EBV) negative natural killer (NK) cell proliferation that involves the gastrointestinal (GI) tract and has an indolent clinical course
Essential features
- Indolent EBV negative NK cell involvement of superficial mucosa of the GI tract
- NK cell immunophenotype: CD2+, CD56+, CD7+, cCD3+, TIA1+ and granzyme B+
- Negative for clonal TRB or TRG gene rearrangements
Terminology
- NK cell enteropathy
ICD coding
- ICD-11: 2B2Y & XH2LK2 - other specified mature T cell or NK cell neoplasms & lymphoproliferative disorder, NOS
Epidemiology
- Occurs commonly in adults with age ranging from 30 to 80 years and presents equally between men and women (Blood 2011;117:1447, Blood 2010;116:5631)
Sites
- Stomach is the most commonly affected site and then small / large intestine (Blood 2011;117:1447, Blood 2019;134:986, Blood 2010;116:5631)
- Rare sites of involvement include regional lymph nodes, vagina and gallbladder (Am J Clin Pathol 2019;151:75, Am J Surg Pathol 2020;44:561, Virchows Arch 2021;478:1197)
Pathophysiology
- Immune response to antigenic stimulation is considered for indolent NK cell lymphoproliferative disorder (Blood 2011;117:1447, Blood 2010;116:5631)
- Gastric presentation of the disease is associated with Helicobacter pylori infection and responsive to anti-Helicobacter therapy (J Clin Exp Hematop 2022;62:114, Blood 2010;116:5631)
- Identification of somatic mutations support a neoplastic origin (Blood 2019;134:986)
Etiology
- Etiology is unknown
Clinical features
- Abdominal pain, constipation, diverticulosis and reflux (Blood 2011;117:1447, Blood 2019;134:986, Blood 2010;116:5631)
- No lymphadenopathy or organomegaly
- No history of inflammatory bowel disease or celiac disease
Diagnosis
- Superficial mucosal involvement of the GI tract by atypical small lymphoid cells (Blood 2011;117:1447, Blood 2019;134:986, Blood 2010;116:5631)
- Presence of NK cell immunophenotype: CD2+, CD56+, CD7+, cCD3+, TIA1+ and granzyme B+
- Negative for EBER ISH (in situ hybridization for EBV encoded RNA)
- Absence of clonal TRB or TRG gene rearrangements
Radiology description
- Endoscopic examination shows multiple small lesions such as mucosal hemorrhage, target lesions or superficial bleeding ulcers (1 - 2 cm) (Blood 2011;117:1447)
- Extensive imaging workup (including CT scan, MRI, PET scan) shows no evidence of lymphadenopathy, organomegaly or masses (Blood 2011;117:1447)
Prognostic factors
- Most lesions regress spontaneously (Blood 2011;117:1447)
- Does not usually respond to treatment and tends to be persistent or recurrent with patients being symptomatic (Blood 2019;134:986)
- Majority of reported cases have demonstrated no disease progression (Blood 2011;117:1447)
Case reports
- 34 year old woman with a vaginal mass (Am J Surg Pathol 2020;44:561)
- 37 year old man underwent laparoscopic cholecystectomy for symptomatic biliary lithiasis (Virchows Arch 2021;478:1197)
- 58 year old woman who was found to have a 1 cm colonic polyp (Blood 2023;141:2033)
- 69 year old woman who had clinically long standing abdominal pain and recurrent mucosal ulcerations (Histopathology 2018;73:345)
Treatment
- Indolent NK cell lymphoproliferative disease is rare and usually regresses spontaneously
- Some patients have persistent / recurrent disease so the following treatment options have been attempted
- Systemic chemotherapy: gemcitabine, cisplatin, pemetrexed, etoposide and formostine (Virchows Arch 2021;478:1197, Korean J Pathol 2014;48:73, Ann Diagn Pathol 2011;15:370, Blood 2019;134:986)
- Bone marrow transplant (Blood 2011;117:1447)
- Surgery, such as gastrectomy (Blood 2010;116:5631)
- Radiation therapy (J Clin Exp Hematop 2020;60:7)
Endoscopic description
- Involvement of either single or multiple GI sites (esophagus, stomach, small intestine and colon) may be observed (Korean J Pathol 2014;48:73, Am J Surg Pathol 2006;30:539, Blood 2011;117:1447)
- Lesions can measure between 1 and 2 cm, with variable appearance; hyperemic foci, mucosal nodules, erythematous bullseye (target) lesions or superficial bleeding ulcers
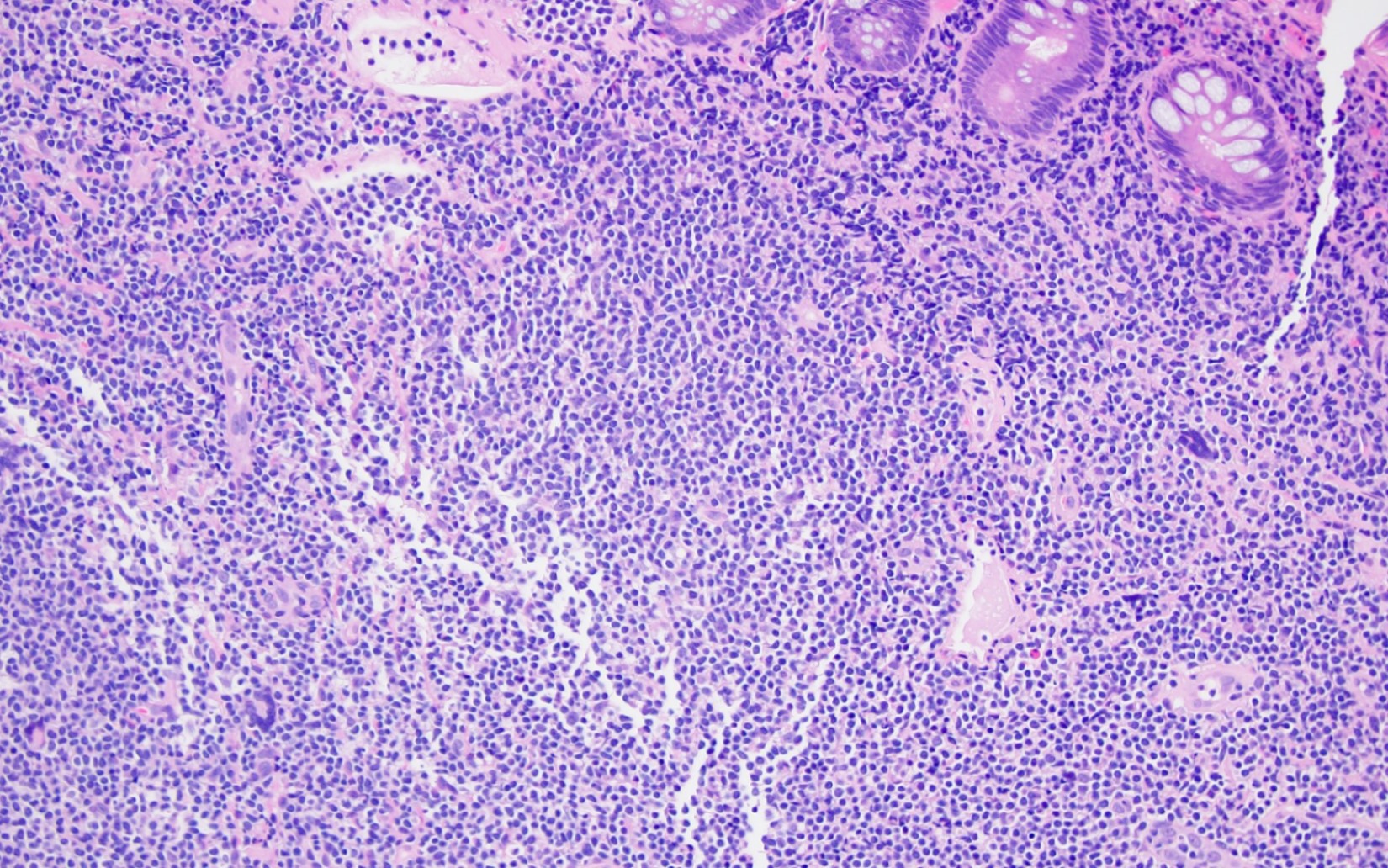
Microscopic (histologic) description
- Expansion of lamina propria by atypical lymphoid infiltrate, which is medium to large in size with round, oval or irregular nuclei, fine chromatin, inconspicuous or rarely prominent nucleoli and moderate pale pink cytoplasm (Clin J Gastroenterol 2013;6:287, Korean J Pathol 2014;48:73, Ann Diagn Pathol 2011;15:370, Pathol Res Pract 2011;207:786, World J Gastroenterol 2012;18:2140, Am J Surg Pathol 2006;30:539)
- Paranuclear granules seen in some cases
- No significant intraepithelial lymphocytosis (epitheliotropism), villous atrophy and crypt hyperplasia noted
- Muscularis mucosae is usually intact
- Superficial hemorrhage is seen
- Glandular destruction is noted in florid cases
- No evidence of angiocentricity or angiodestruction (Clin J Gastroenterol 2013;6:287, Korean J Pathol 2014;48:73, Ann Diagn Pathol 2011;15:370, Pathol Res Pract 2011;207:786, World J Gastroenterol 2012;18:2140, Am J Surg Pathol 2006;30:539, Blood 2010;116:5631)
- Rim of small mature lymphocytes (mainly B cells) and a polymorphous infiltrate of eosinophils, plasma cells and histiocytes surround the atypical infiltrate at the base (Blood 2011;117:1447)
- Scattered reactive lymphoid follicles may be present in the adjacent mucosa
Microscopic (histologic) images
Positive stains
- Cytoplasmic CD3+, CD2+, CD7+, CD56+, CD43+ (Am J Clin Pathol 2019;151:75)
- Cytotoxic markers: TIA1+, granzyme B+
- Low proliferative index based on Ki67 (average ~25%) (Blood 2011;117:1447)
Negative stains
Flow cytometry description
Molecular / cytogenetics description
- No clonal T cell receptor (TCR) gene rearrangements have been identified (Blood 2011;117:1447, Blood 2010;116:5631, Blood 2019;134:986)
- JAK3 mutations (K563_C565del) in 3/10 patients are reported (Blood 2019;134:986)
- Other mutations detected include PTPRS, AURKB, AXL, ERBB4, IGF1R, PIK3CB, CUL3, CHEK2, RUNX1T1, CIC, SMARCB1 and SETD5 (Blood 2019;134:986)
Sample pathology report
- Colon, biopsy:
- Atypical T cell infiltrate, consistent with indolent NK cell lymphoproliferative disorder of gastrointestinal tract (see comment)
- Comment: The infiltrate of medium sized atypical cells is limited to the lamina propria and is positive for cCD3ε, CD2, CD7, CD56, TIA1 and negative for CD5, CD4, CD8, EBER ISH. TCR gene rearrangement is negative.
Differential diagnosis
- Inflammatory bowel disease:
- Crypt distortion may be present without cryptitis, along with crypt abscesses, reduced intraepithelial mucin, basal plasmacytosis, hypertrophic muscularis mucosae, submucosal fibrosis and panel cell hyperplasia (Odze: Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas, 4th Edition, 2022)
- Celiac disease:
- Indolent NK cell lymphoproliferative disease shows no significant infiltration of colonic crypt epithelium or surface epithelium by lymphoid cells
- While celiac disease shows villous atrophy, hyperplastic and elongated crypts and increase in plasma cells in lamina propria (Dig Liver Dis 2011;43:S385)
- Tissue transglutaminase antibodies (tTGA) present
- Improvement in symptoms with gluten free diet
- Enteropathy associated T cell lymphoma (EATL) (Blood 2011;118:148, Am J Surg Pathol 2011;35:1557, Best Pract Res Clin Gastroenterol 2010;24:43):
- EATL is aggressive disease and is associated with celiac disease
- Presents with multifocal involvement, extensive mucosal ulcerations and deep infiltration of the bowel wall
- While indolent NK cell lymphoproliferative disease in contrast presents as 1 or more shallow mucosal ulcers with associated erythema and regress spontaneously
- EATL is composed of medium to large sized pleomorphic cells with prominent nucleoli
- Indolent NK cell lymphoproliferative disease consists of medium sized cells with pale eosinophilic cytoplasm with granules
- EATL has necrosis with epitheliotropism, is of T cell lineage (CD4- / CD8-) and demonstrates TCR gene rearrangement
- High proliferative index, > 50% (Blood 2008 15;112:5103)
- JAK1 and STAT3 mutations are seen in EATL while JAK3, STAT5B and SETD2 mutations in MEITL (Nat Commun 2016;7:12602, Gut 2022;71:497, Leukemia 2016;30:1311)
- Monomorphic epitheliotropic intestinal T cell lymphoma (MEITL):
- Aggressive disease with deep GI tract involvement
- Composed of monomorphic small to medium sized lymphoid cells, shows epitheliotropism, is of T cell lineage (CD8+, CD56+) and demonstrates TCR gene rearrangement (Leukemia 2013;27:1688, Am J Surg Pathol 2011;35:1557)
- Indolent T cell lymphoproliferative disorder of the GI tract:
- Indolent disease with superficial involvement without necrosis and significant epitheliotropism (Hematol Oncol 2017;35:3, PLoS One 2013;8:e68343, Haematologica 2020;105:1895)
- Neoplastic cells are of T cell origin, CD4+ > CD8+ (Gut 1999;45:662, Clin Gastroenterol Hepatol 2014;12:599, PLoS One 2013;8:e68343)
- Expresses TCR, specially TCRαβ+
- Clonal TCR gene rearrangement present (Blood 2013;122:3599, PLoS One 2013;8:e68343)
- Extranodal NK / T cell lymphoma:
- Aggressive disease with deep GI tract infiltration
- Angiocentric and angiodestructive growth pattern, necrosis and association with EBV (EBER+) (Am J Surg Pathol 1994;18:938, Am J Surg Pathol 1993;17:392)
- May be NK cell (CD2+, cCD3ε+ and CD56+) or T cell lineage (CD5+, CD8+, TCRαβ+ or TCRγδ+, CD56-) (Am J Surg Pathol 2012;36:481, Am J Surg Pathol 2013;37:14, Am J Surg Pathol 2015;39:1)
- Negative for TCR gene rearrangement
Board review style question #1
A 48 year old man presented with abdominal pain and dyspepsia. The laboratory work up was negative for tTGA. CT was negative for lymphadenopathy and organomegaly. Colonoscopy was performed and showed superficial erosions and mucosal nodules. Biopsy was performed and showed the features seen in the images above. The molecular testing for TCR gene rearrangement was negative. What is the classic immunophenotype of this disease entity?
- CD2+, CD3+, CD4-, CD5-, CD8+, CD56+, EBER-
- CD2+, CD56+, CD7+, cCD3+, EBER-
- CD5+, CD4+, CD7+, TCRαβ+, EBER-
- CD5+, CD8+, TCRαβ+, cCD3ε+, CD56-, EBER+
Board review style answer #1
B. CD2+, CD56+, CD7+, cCD3+, EBER-. The disease entity is indolent NK cell lymphoproliferative disease of the GI tract, which has an NK cell phenotype but no EBV association.
Answer D is incorrect because this phenotype is for extranodal NK / T cell lymphoma (T cell lineage).
Answer C is incorrect because this phenotype is for indolent T cell lymphoproliferative disorder of GI tract.
Answer A is incorrect because this phenotype is for monomorphic epitheliotropic intestinal T cell lymphoma (MEITL).
Comment Here
Reference: Indolent NK cell lymphoproliferative disease of the GI tract
Comment Here
Reference: Indolent NK cell lymphoproliferative disease of the GI tract