Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: de Lima Guido LP, Amador C. Nodal T follicular helper cell lymphoma, NOS. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lymphomaPTCLTFH.html. Accessed April 1st, 2025.
Definition / general
- Mature T cell lymphoma with a T follicular helper (TFH) phenotype, defined by the expression of CD4 and at least 2 (ideally 3) TFH markers, primarily involving lymph nodes
- Must not fulfill the diagnostic criteria for other defined mature T cell lymphoma entities, including other nodal TFH lymphomas (Leukemia 2022;36:1720, Blood 2016;127:2375)
Essential features
- Aggressive, mature T cell lymphoma
- Expression of CD4 and at least 2, preferably 3, T follicular helper markers (such as PD-1, CD10, BCL6, CXCL13 and ICOS)
- Part of a spectrum of T cell lymphomas with TFH phenotype, which also includes nodal TFH cell lymphoma, angioimmunoblastic type (nTFHL-AI), formerly angioimmunoblastic T cell lymphoma and nodal TFH cell lymphoma, follicular type (nTFHL-F), formerly known as follicular T cell lymphoma
- Other mature T cell lymphomas must be excluded
- Frequent mutations in RHOA and epigenetic regulators such as TET2 and DNMT3A
Terminology
- Current terminology
- Nodal T follicular helper (TFH) cell lymphoma, not otherwise specified in the 5th edition of the World Health Organization Classification of Hematolymphoid Tumors: Lymphoid Neoplasms (WHO HAEM5) (Leukemia 2022;36:1720)
- Follicular helper T cell lymphoma, NOS in the International Consensus Classification of Mature Lymphoid Neoplasms (ICC 2022) (Mod Pathol 2022;35:306)
- Historical names
- These cases were classified initially within peripheral T cell lymphoma, not otherwise specified (PTCL, NOS) in the 4th edition of the WHO classification (WHO 2008)
- Posteriorly renamed as nodal PTCL with a TFH phenotype in the revised 4th edition of the WHO classification (WHO 2017), where it was grouped with angioimmunoblastic T cell lymphoma (AITL) and T follicular lymphoma under the provisional umbrella category of nodal lymphomas of TFH origin (Blood 2016;127:2375)
ICD coding
Epidemiology
- Primarily found in men > 60 years old
- True incidence of nTFHL, NOS is unknown
- ~40% of neoplasms once designated as PTCL, NOS express TFH markers (Mod Pathol 2022;35:306, Haematologica 2017;102:e148)
Sites
- nTFHL, NOS is characterized by disseminated disease similar to nTFHL-AI (Leukemia 2022;36:1720)
- Nearly all cases involve lymph nodes
Pathophysiology
- Proposed origin from TFH cells (a T cell subset essential for germinal center reactions and B cell specialization)
- nTFH, NOS has a gene expression profile more comparable to nTFH-AI than PTCL, NOS (Blood 2016;127:2375, Haematologica 2017;102:e148)
- Similarly, the mutational landscape resembles that of nTFH-AI
- Physiologically, RHOA mediates migration and polarity of T cells
- RHOA also functions in thymocyte development and activation of pre-T cell receptor (pre-TCR) signaling in thymocytes
- G17V RHOA protein is considered to be a loss of function mutant on RhoA signaling pathway
- Physiologic functions of RHOA are presumably abrogated in the G17V mutant, as the protein cannot be converted to the active GTP bound form
- Its mutation may drive naive CD4+ T cells to differentiate to TFH cells and facilitate neoplasia
- TET2 and DNMT3A are considered early mutations in TFH cells (Leukemia 2018;32:694)
Clinical features
- Lymphadenopathy with typical advanced stage disease at presentation
- Similar bone marrow involvement to nTFHL-AI and PTCL, NOS (Blood 2021;137:2161)
Diagnosis
- Must not fulfill the required histopathological criteria for nTFHL, angioimmunoblastic type (nTFHL-AI) such as extrafollicular follicular dendritic cell expansion or high endothelial venule hyperplasia or nTFHL, follicular type (nTFHL-F), which displays a follicular growth pattern
- Desirable clonal TCR gene rearrangement or mutation involving RHOA p.G17V
- Staging performed according to the Lugano modification of the Ann Arbor staging system (J Clin Oncol 2014;32:3059)
Laboratory
- nTFH, NOS has been linked to Coombs positive hemolytic anemia and polyclonal hypergammaglobulinemia but not as strongly as nTFH-AI (Blood 2021;137:2161)
Prognostic factors
- Similar overall survival (OS) and progression free survival (PFS) to nTFHL-AI but better than PTCL, NOS, based on limited data (Haematologica 2017;102:e148)
- Male gender, an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or higher and thrombocytopenia are related to poor OS (Haematologica 2017;102:e148)
Case reports
- 72 year old man with sore throat and bilateral tonsillar enlargement (Virchows Arch 2023;483:349)
- 77 year old man with bone marrow involvement by lymphoma (Virchows Arch 2023;483:349)
Treatment
- Cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) or CHOP-like chemotherapy is used in most patients
- Ifosfamide, mesna, carboplatin, etoposide (ICE) gemcitabine based chemotherapy is less commonly used
- Epigenetic modifiers, such as histone deacetylase inhibitors (HDACi) and DNA methyltransferase inhibitors, have shown a better treatment response rate than other PTCLs in the relapsed setting (Blood 2018;132:2305, Blood Adv 2020;4:4640, Am J Surg Pathol 2019;43:1282)
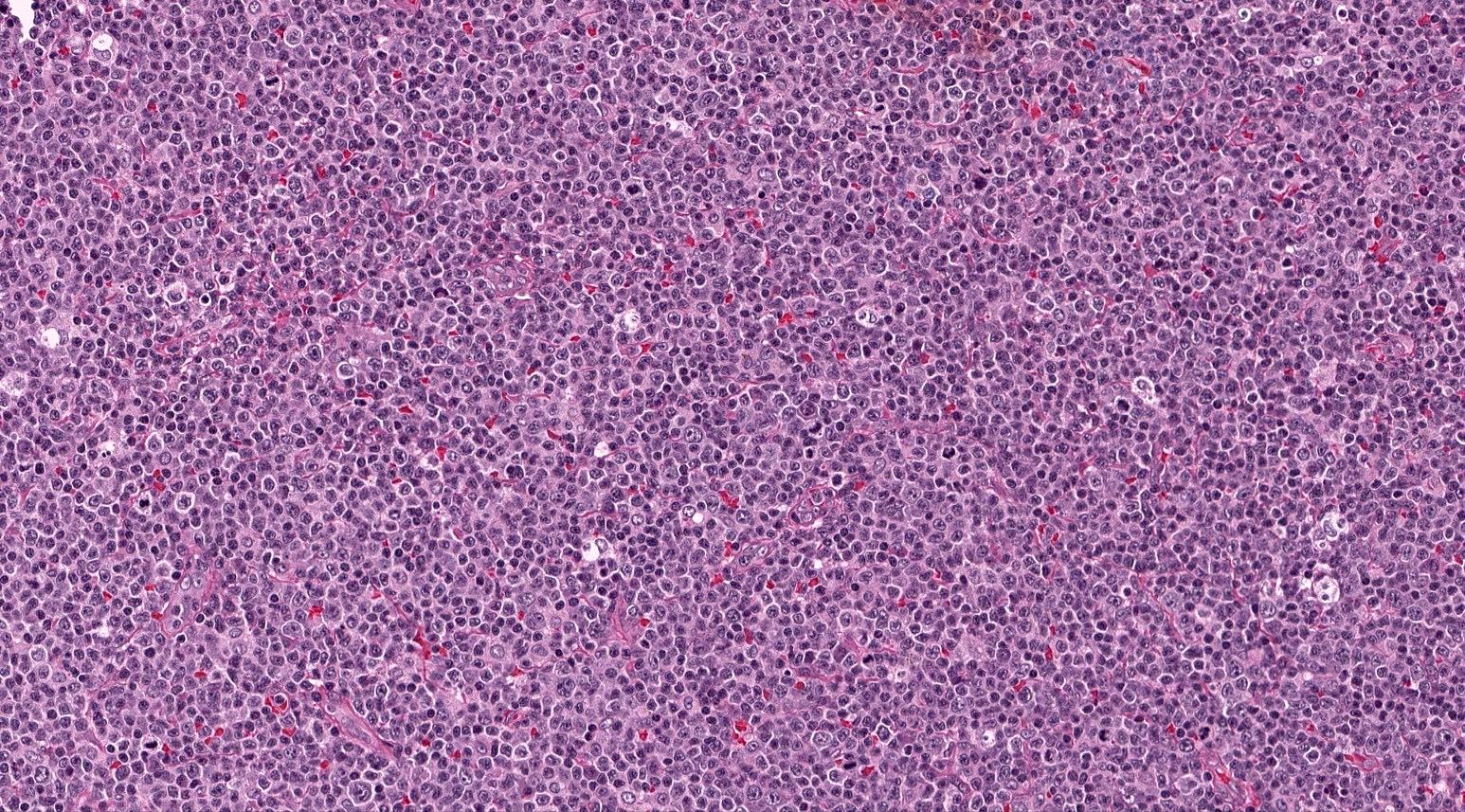
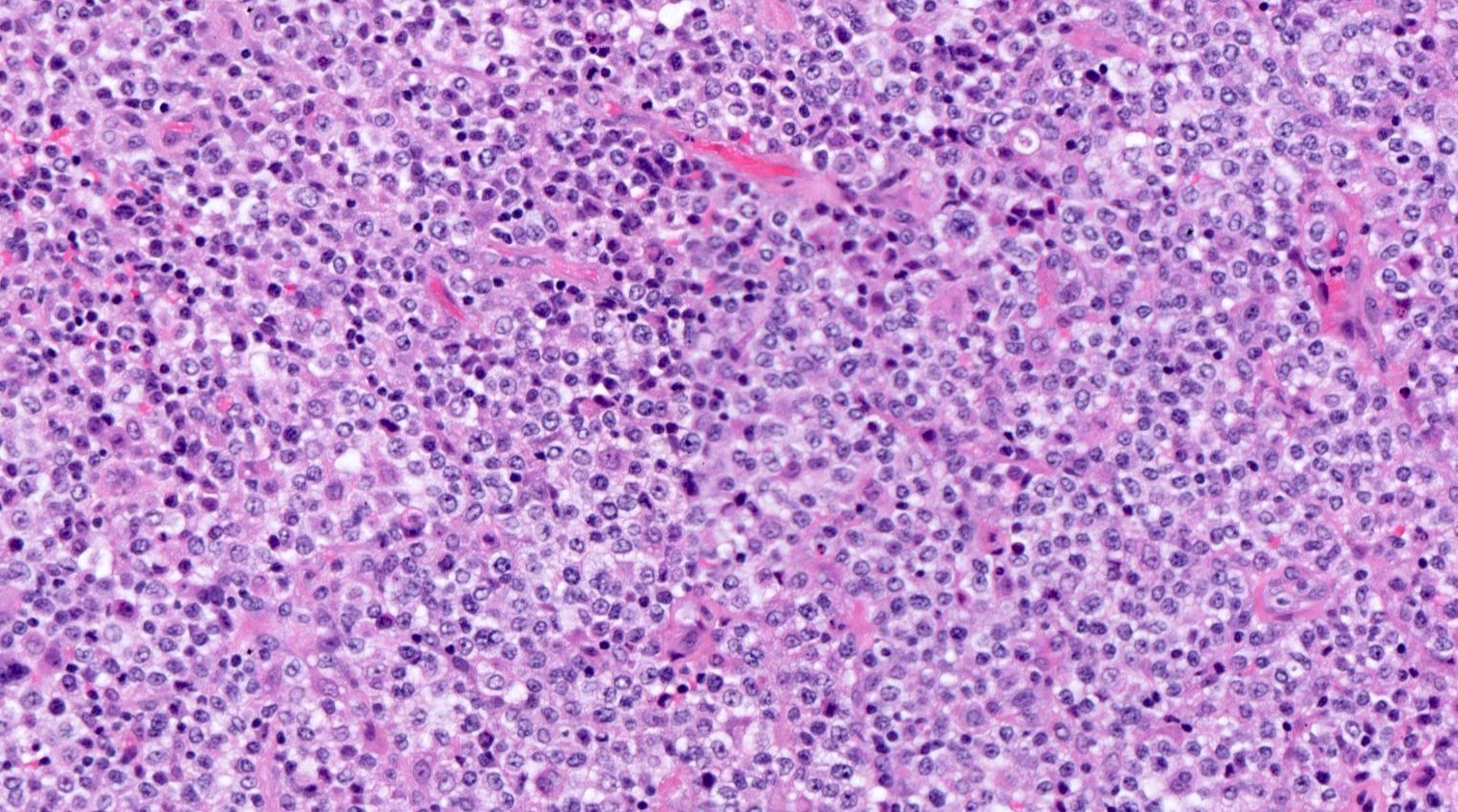
Microscopic (histologic) description
- Lymph node architecture is effaced by a tumor cell rich infiltration of varying sized lymphoid cells (typically medium to large sized)
- 2 recognized patterns: diffuse infiltration or proliferation around follicles in a paracortical T zone infiltration
- Lack of a prominent polymorphic inflammatory background, high endothelial venule hyperplasia or follicular dendritic cell meshwork proliferation; however, some of these features typical of nTFH-AI may be present (Am J Surg Pathol 2016;40:1249)
- Secondary B cell proliferations, unlike nTFH-AI, have not been thoroughly studied (Leukemia 2022;36:1720)
- Lymphoepithelioid (Lennert morphology) may be encountered (Am J Surg Pathol 2007;31:1695)
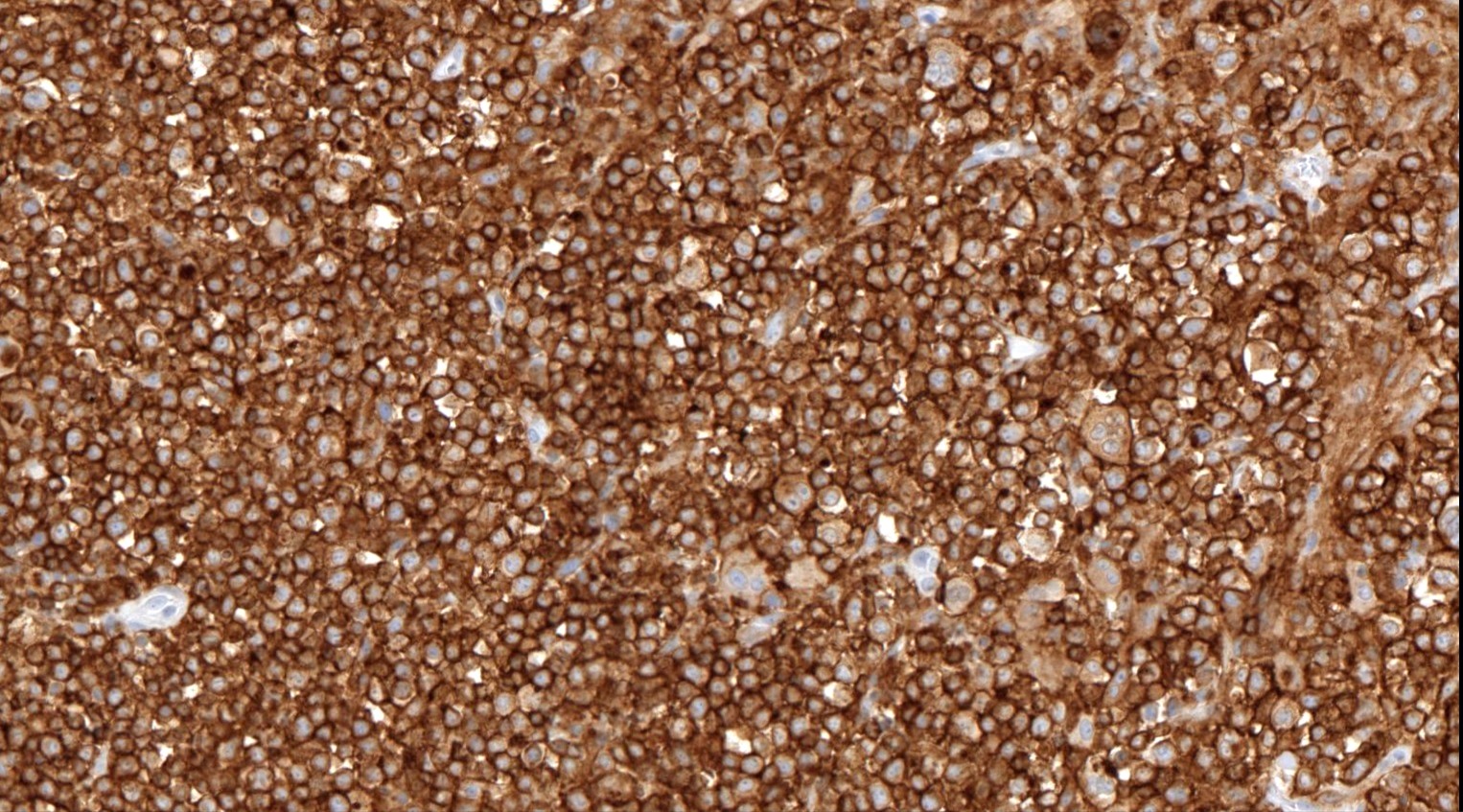
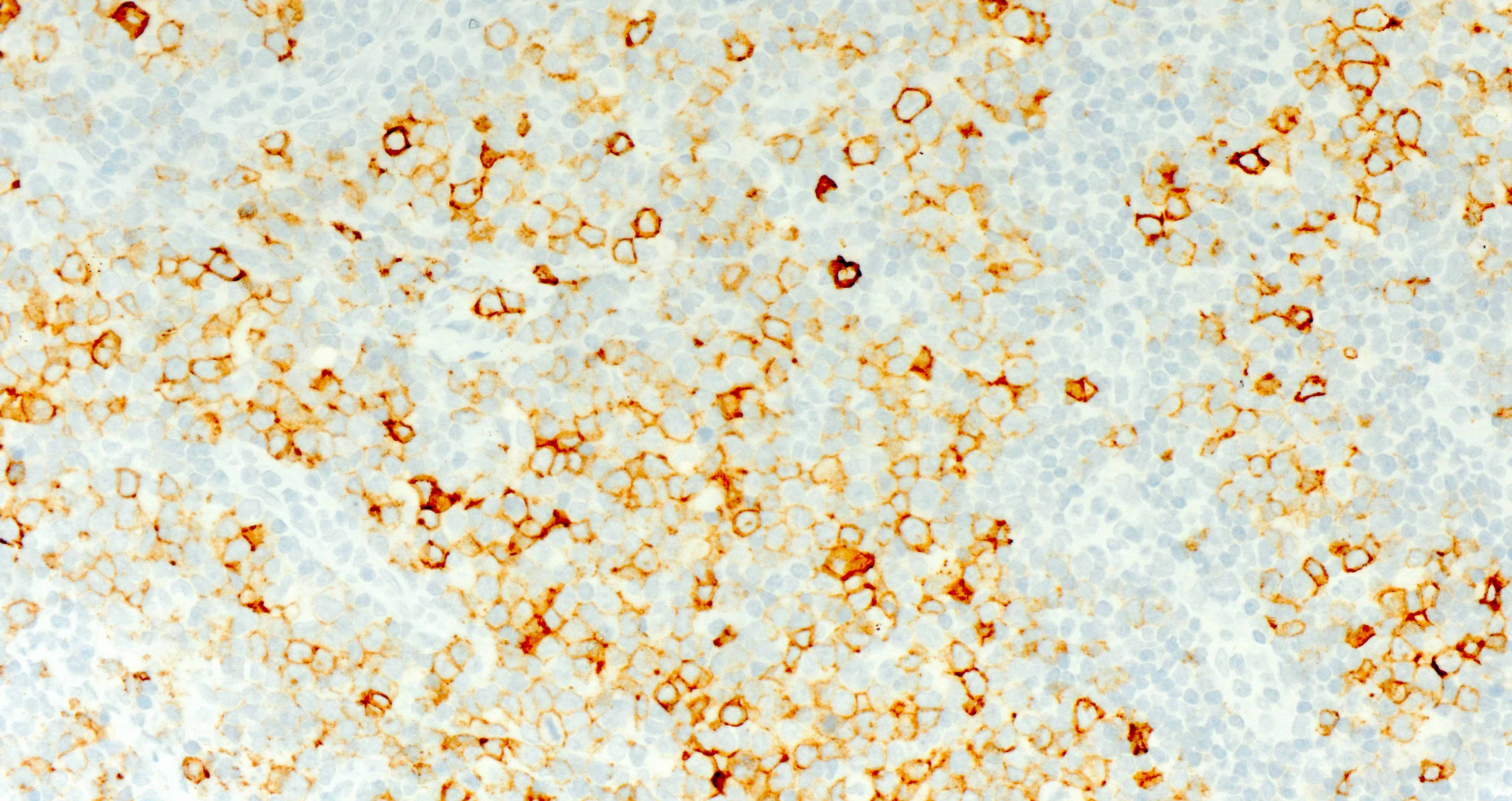
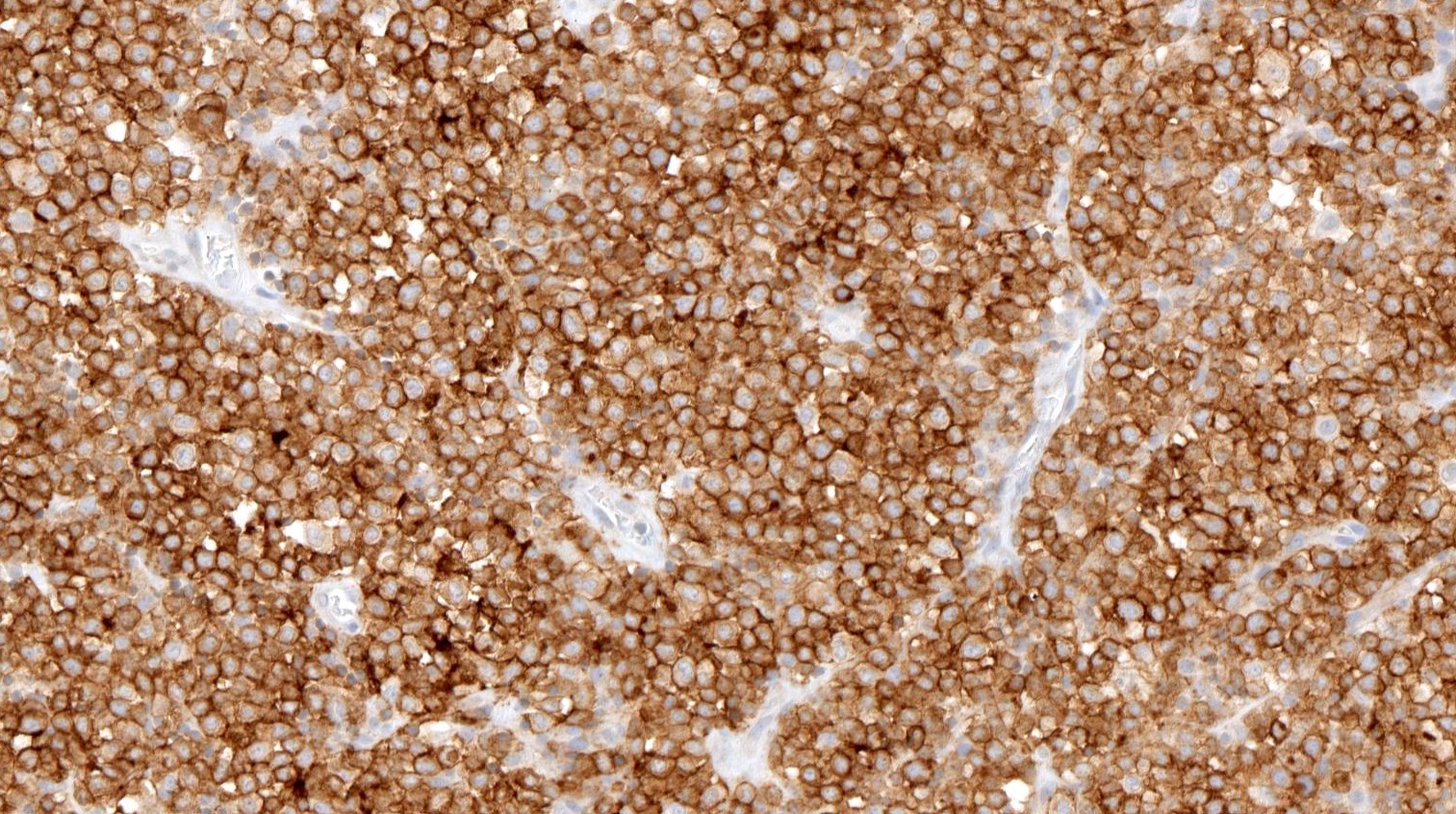
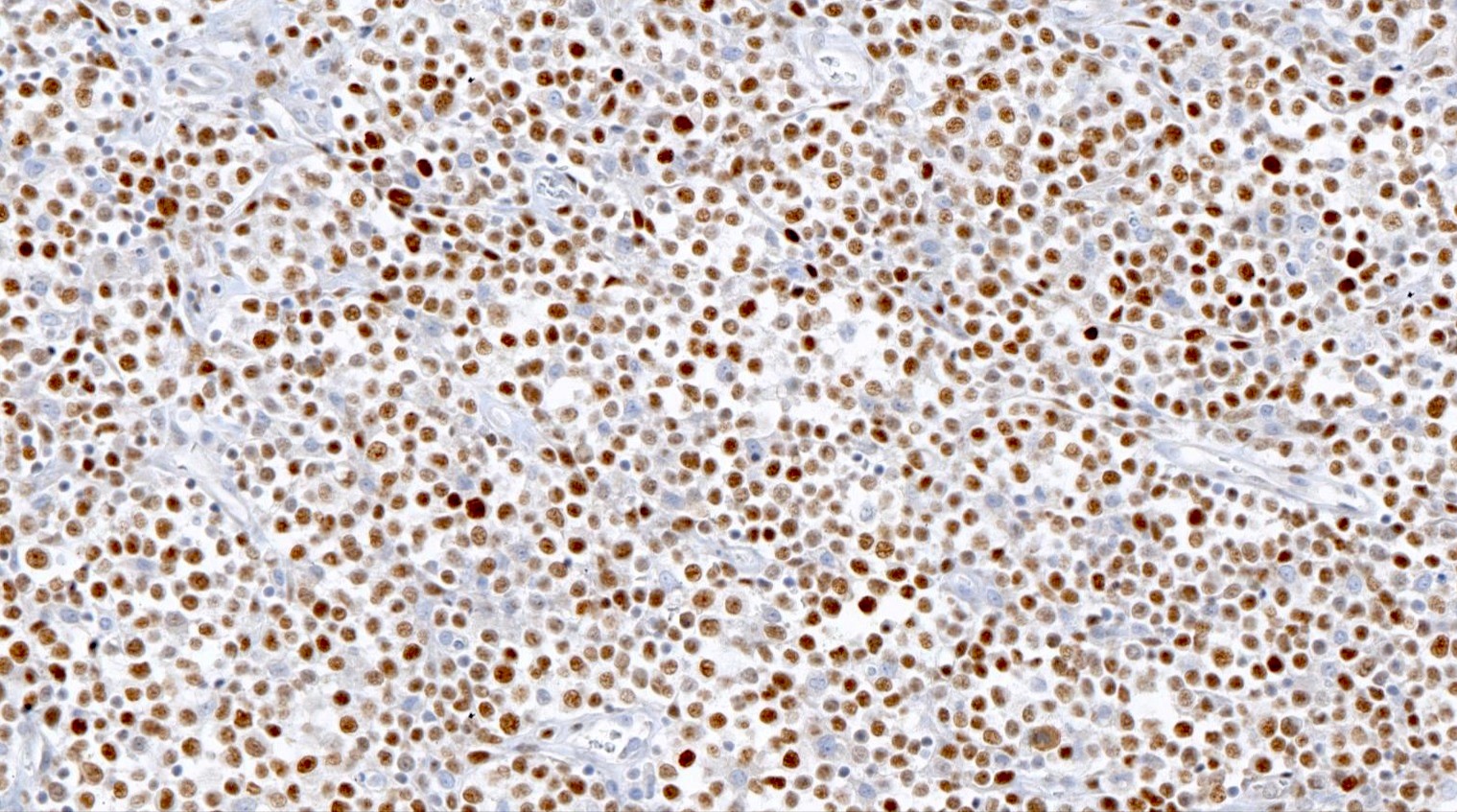
Microscopic (histologic) images
Contributed by Catalina Amador, M.D.
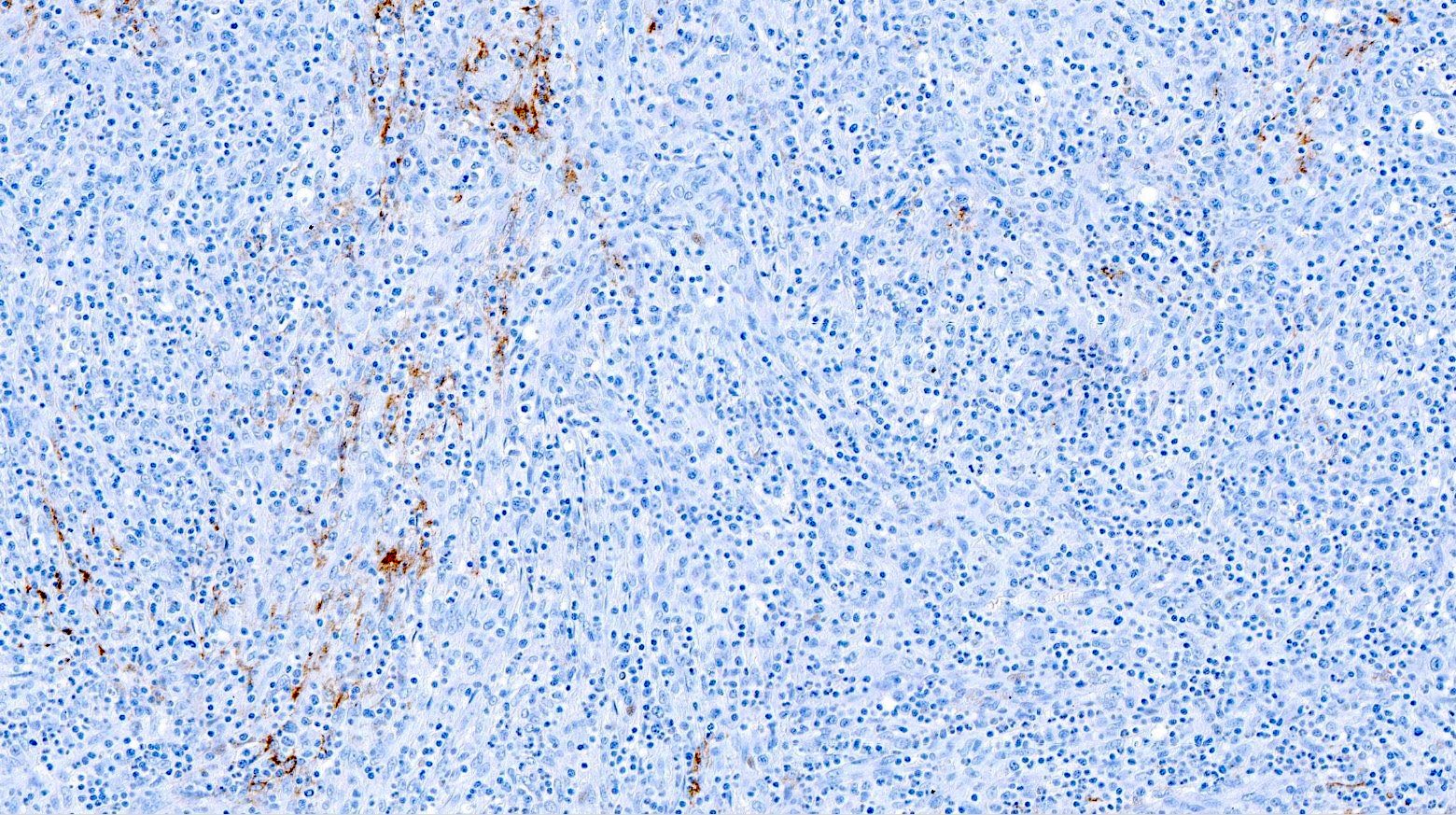
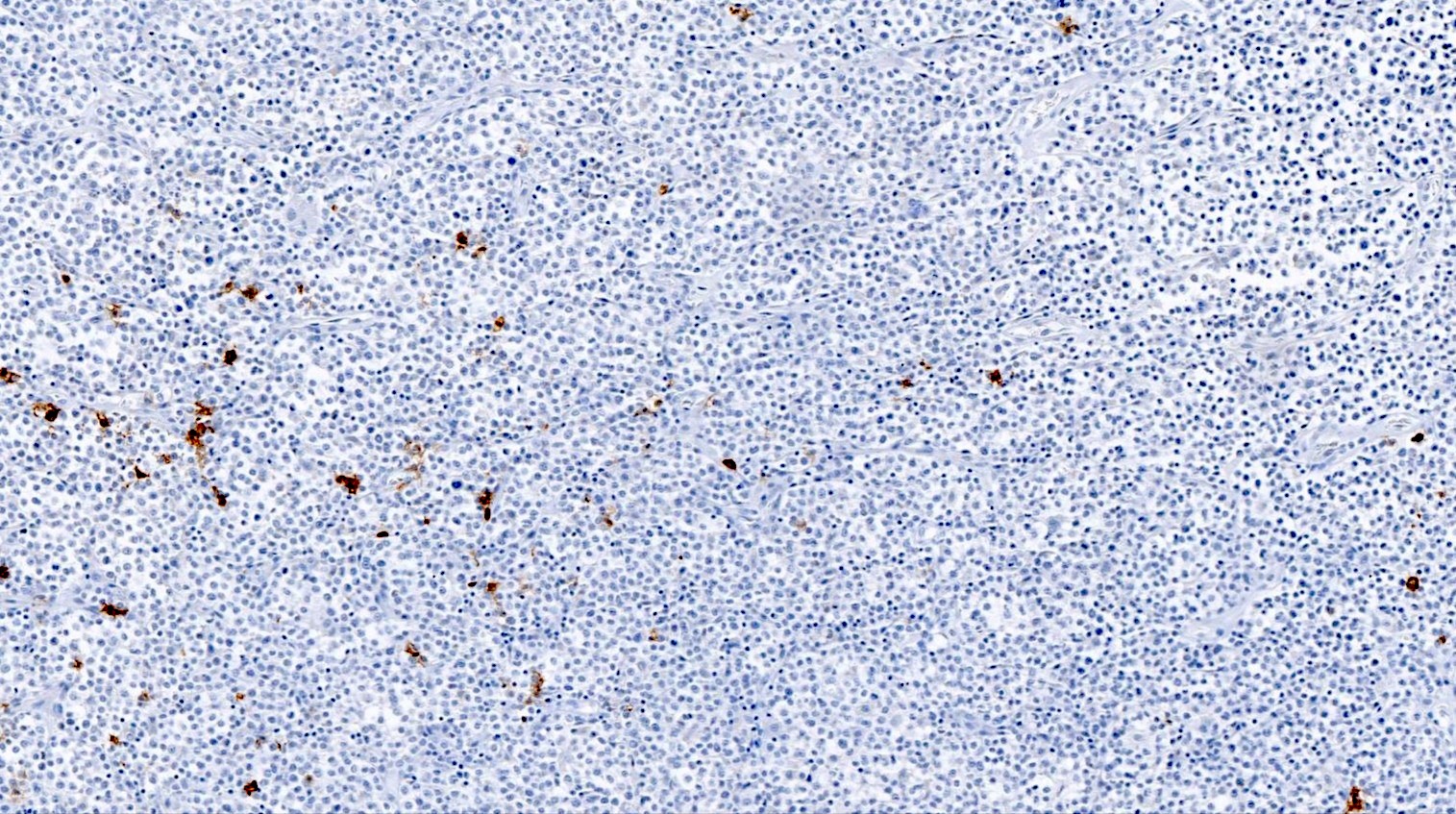
Positive stains
- CD4 and at least 2, preferably 3, T follicular helper markers (such as PD-1, CD10, BCL6, CXCL13 and ICOS)
- Pan-T cell antigens: CD2, CD3, CD5 and CD7
- SAP, CXCR5, cMAF and CD200 are other less commonly described TFH markers that are not commonly used (Hum Pathol 2023:131:47, Am J Surg Pathol 2011;35:76, Haematologica 2007;92:1059, Diagnostics (Basel) 2022;12:2001)
- None of the markers are completely specific for a TFH phenotype
- BCL6, CD10 and CXCL13 are more specific but less sensitive, whereas ICOS and PD-1 are more sensitive but less specific (Ann Diagn Pathol 2020;49:151623)
Negative stains
- CD21, CD23, CD35 or clusterin do not highlight expanded follicular dendritic cell meshworks
- CD20 and other pan-B cell markers
- Reference: Histopathology 2014;64:171
Molecular / cytogenetics description
- Clonal TCR gene rearrangement in most cases
- Similar gene expression profile to nTFH-AI and normal TFH cells (Blood 2007;109:4952)
- Mutational profiles of nPTCL-TFH overlap with nTFH-AI, except for IDH2, which is restricted to nTFH-AI
- Mutations in RHOA
- Mutations in G17V locus specific to TFH lymphomas
- Loss of RHOA GTPase activity
- Up to 60% frequency in nTFHL, NOS, similar to nTFH-AI (Blood 2021;137:2161)
- Mutations in epigenetic regulators
- Nonspecific for TFH lymphomas; seen in other T cell lymphomas and hematolymphoid neoplasms
- TET2
- Loss of function mutations
- 50 - 75% frequency in nTFH, NOS (comparable to 75% in nTFHL-F and 50 - 80% in nTFHL-AI)
- DNMT3A
- Loss of function mutations
- 7 - 10% frequency (compared with 25% in nTFHL-F and 20 - 40% in nTFHL-AI)
- Mutations in TCR signaling pathway genes
- Not specific, as they are also seen in other T cell lymphomas
- PLC gamma: up to 10% and FYN < 5%
- CD28 is absent as it seems restricted to nTFHL-AI (Blood Adv 2020;4:4640)
- Cytogenetic abnormalities
- Copy number gains in chromosomes 5 and 7 occur with a similar frequency to nTFH-AI (~10%) (Blood 2021;137:2161)
- Negative serologic or molecular evidence of HTLV-1
Sample pathology report
- Right inguinal lymph node, excisional biopsy:
- Lymph node with nodal T follicular helper cell lymphoma, not otherwise specified (WHO HAEM5) / follicular helper T cell lymphoma, not otherwise specified (see comment)
- Comment: The overall morphological features, including the expression of CD4 and 3 T follicular helper cell markers (ICOS, PD-1 and BCL6) by the neoplastic cells, support the diagnosis of a TFH lymphoma. The diagnosis of nTFH, NOS is favored over angioimmunoblastic T cell lymphoma due to the lack of polymorphism, expanded follicular dendritic cell meshworks and high endothelial venules. Given the patient's origin, adult T cell leukemia / lymphoma was also considered, given that these cases can express TFH markers. However, the serology study for HTLV-1 yielded negative results, excluding this possibility. T cell receptor gamma gene rearrangement studies were positive and the next generation sequencing panel revealed mutations in RHOA, TET2 and DNMT3, supporting the morphologic findings.
- Microscopic description: The biopsy shows sections of lymph node with effaced architecture by an abnormal lymphoid infiltrate with a diffuse growth pattern. The neoplastic cells are composed of a spectrum of small, intermediate and large lymphoid cells with minimally associated inflammatory background. Immunohistochemistry shows that the neoplastic cells express CD3 and CD4, PD-1, BCL6 and ICOS and are negative for CD20, CD8, CD10, ALK and EBER in situ hybridization. CD30 is positive in 30% of the neoplastic cells. CD21 highlights the absence of expanded follicular dendritic cell meshworks. Ki67 proliferation is 50%. EBER in situ hybridization is negative.
Differential diagnosis
- Nodal TFH cell lymphoma, angioimmunoblastic type (nTFHL-AI) with a tumor cell rich pattern:
- Arborizing high endothelial venules (HEV) and enlarged follicular dendritic cell meshwork are consistently present
- Nodal TFH cell lymphoma, follicular type (nTFHL-F):
- TFH cell well defined nodules (follicular lymphoma-like pattern) or TFH cell small aggregates / clusters surrounded by numerous small IgD+ mantle zone B cells (progressive transformation of germinal center-like pattern)
- Peripheral T cell lymphoma, not otherwise specified (PTCL, NOS):
- TFH marker positivity can be seen; by definition, no more than 2 TFH markers can be expressed
- T cell histiocyte rich large B cell lymphoma:
- Background T cells can exhibit TFH markers but small B cells are uncommon and TCR gene rearrangements are negative, unlike nPTCL-TFH (Leukemia 2022;36:1720)
- Nodal marginal zone lymphoma with increased TFH cells:
- Associated TFH cell hyperplasia can mimic TFH lymphomas but TCR gene rearrangement is negative (Am J Surg Pathol 2020;44:657)
- Adult T cell leukemia / lymphoma:
- TFH marker expression has been reported but usually a different clinical presentation and positive for HTLV-1 serologic studies
Additional references
Board review style question #1
Which option describes characteristic features of nodal peripheral T cell lymphomas with T follicular helper phenotype?
- Increased expression of CD21 and CD23
- MYC and BCL2 gene rearrangements
- Polymorphic inflammatory background with expanded follicular dendritic cell meshwork
- Predominance in the young population
- Proliferation around follicles in a T zone infiltration pattern of the paracortex
Board review style answer #1
E. Proliferation around follicles in a T zone infiltration pattern of the paracortex. nTFHL, NOS characteristically presents with 2 recognized patterns: diffuse infiltration or proliferation around follicles in a paracortical T zone infiltration. Answer A is incorrect because CD21, CD23, CD35 or clusterin do not highlight expanded follicular dendritic cell meshworks. Answer B is incorrect because MYC and BCL2 gene rearrangements are characteristic of double hit high grade B cell lymphomas. Answer D is incorrect because this entity predominates in the elder population. Answer C is incorrect because this finding is characteristic of nodal TFH cell lymphoma, angioimmunoblastic type (nTFHL-AI).
Comment Here
Reference: Nodal PTCL with a TFH phenotype
Comment Here
Reference: Nodal PTCL with a TFH phenotype
Board review style question #2
Which option describes a typical feature of nodal peripheral T cell lymphomas with T follicular helper phenotype?
- Expression of CD8 and TdT
- Formation of well defined aggregates surrounded by numerous small IgD+ mantle zone B cells arranged into large irregular nodules
- Indolent progression with a low incidence of disseminated disease
- Lack of TCR gene rearrangements
- RHOA and TET2 gene mutations
Board review style answer #2
E. RHOA and TET2 gene mutations. Detection of RHOA p.G17V mutation is observed in up to 60% of nTFHL, NOS and is a useful adjunct, as it supports the diagnosis of a nTFHL over PTCL, NOS. TET2 loss of function mutations have a 50 - 75% frequency in nTFH, NOS, comparable to 75% in nTFHL-F and 50 - 80% in nTFHL-AI. Answer A is incorrect because CD8 and TdT are mature T cell lymphomas, hence TdT is characteristically negative. CD8 is typically not expressed in these tumors but the expression does not exclude diagnosis. Answer B is incorrect because these findings describe nodal TFH cell lymphoma, follicular type (nTFHL-F). Answer C is incorrect because these tumors typically present as advanced disease. Answer D is incorrect because clonal TCR gene rearrangement is detected in most cases.
Comment Here
Reference: Nodal PTCL with a TFH phenotype
Comment Here
Reference: Nodal PTCL with a TFH phenotype