Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Peripheral smear images | Positive stains | Flow cytometry description | Flow cytometry images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Cite this page: Choudhuri J, Shi Y. Chronic myeloid leukemia (CML), BCR::ABL1 positive. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/myeloproliferativecml.html. Accessed December 22nd, 2024.
Definition / general
- Chronic myeloid leukemia (CML), BCR-ABL1 positive, is a myeloproliferative neoplasm (MPN) characterized by clonal granulocytic proliferation
- Arises in a pluripotent stem cell with t(9;22)(q34.1;q11.2) chromosomal translocation and formation of the Philadelphia (Ph) chromosome, containing the BCR-ABL1 fusion gene (Swerdlow: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 2017)
Essential features
- Occurs in 3 different phases (Am J Hematol 2018;93:442)
- Chronic phase (CP)
- Accelerated phase (AP)
- Blast phase (BP)
- Natural progress: most cases progress from chronic phase to blast phase in 3 - 5 years
- Relatively rare in pediatric patients
- Children and young adults tend to have a more aggressive clinical presentation than older adults, more frequent in accelerated or blast phase (Blood 2012;119:1821)
Terminology
- Chronic myelogenous leukemia, BCR-ABL1 positive
- Chronic granulocytic leukemia, BCR-ABL1 positive
- Chronic myelogenous leukemia, Philadelphia chromosome positive (Ph+)
- Chronic myelogenous leukemia, t(9;22)(q34;q11)
- Chronic granulocytic leukemia, Philadelphia chromosome positive (Ph+)
- Chronic granulocytic leukemia, (9;22)(q34;q11)
- Chronic granulocytic leukemia, BCR-ABL1
ICD coding
- ICD-10: C92.1 - Chronic myeloid leukemia, BCR-ABL1 positive
Epidemiology
- Incidence: 10 - 20 cases per million/year
- M:F = 1.2 ~ 1.7:1 (Ann Hematol 2015;94:S241)
- Median age: about 65 years; pediatric cases rare
Sites
- Chronic phase: blood, bone marrow, spleen and liver
- Blast phase: spleen, liver, lymph nodes, skin and soft tissue are the most common extramedullary sites of involvement
Pathophysiology
- Reciprocal translocation t(9;22)(q34;q11) leads to the formation of the chimeric fusion gene BCR-ABL1, which is responsible for production of the oncoprotein BCR-ABL
- BCR-ABL protein has uncontrolled tyrosine kinase activity, making abnormal pluripotent hematopoietic progenitor cell initiate excessive production of all myeloid lineage cells (Am J Hematol 2020;95:691)
Etiology
- Underlying causes are largely unknown
- Radiation exposure has been indicated (Lancet Haematol 2018;5:e346)
Clinical features
- Most patients are diagnosed in the chronic phase
- Almost 50% of patients diagnosed in the United States are asymptomatic and diagnosed during a routine examination (Mayo Clin Proc 2015;90:1440)
- Insidious onset with leukocytosis, anemia, fatigue, weight loss, night sweats
- 50% of patients have splenomegaly with dragging sensation in left upper quadrant
- Rare patients present with marked thrombocytosis without leukocytosis, mimicking essential thrombocythemia
- 5% of patients present with accelerated or blast phase
- Accelerated or blast phase: fever, bone pain, bleeding
Diagnosis
- Chronic phase (CP) (Mayo Clin Proc 2015;90:1440):
- Leukocytosis (usually 12 - 1,000 x 10⁹/L, median: ~ 80 x 10⁹/L)
- < 2% blasts in blood
- < 5% blasts in bone marrow
- BCR-ABL1 positive by cytogenetics or molecular study
- Does not meet any of the following diagnostic criteria for accelerated or blast phase
- Accelerated phase (AP) is defined by the presence of ≥ 1 of the following criteria:
- 10 - 19% blasts in blood or marrow (Drug Des Devel Ther 2019;13:825)
- Persistent or increasing WBC > 10 x 10⁹/L, unresponsive to therapy
- Persistent platelet count > 1,000 x 10⁹/L, unresponsive to therapy
- Persistent platelet count < 100 x 10⁹/L, unrelated to therapy
- ≥ 20% basophils in blood
- Persistent or increasing splenomegaly, unresponsive to therapy
- Additional clonal chromosomal abnormalities in Philadelphia chromosome positive cells at diagnosis, including so called major route abnormalities (a second Ph chromosome, trisomy 8, isochromosome 17q, trisomy 19), complex karyotype and abnormalities of 3q26.2
- Large clusters or sheets of small, abnormal megakaryocytes associated with marked reticulin or collagen fibrosis may be considered presumptive evidence of accelerated phase, although these findings are usually associated with one or more of the criteria listed above
- Blast phase (BP):
- ≥ 20% blasts in marrow or blood or the presence of an extramedullary proliferation of blasts (Front Oncol 2019;9:1132)
- Approximately 70% have myeloid blasts (may include neutrophilic, monocytic, megakaryocytic, basophilic, eosinophilic or erythroid blasts), approximately 30% have lymphoid blasts (usually B lymphoblasts but T and NK blasts have been reported) (J Clin Invest 2010;120:2254)
Laboratory
- Chronic phase:
- Leukocytosis with predominantly neutrophils and myelocytes
- Basophilia and eosinophilia
- t(9;22)(q34;q11.2) by FISH, conventional cytogenetics or PCR
- Alkaline phosphatase decreased
- Reference: Mayo Clin Proc 2015;90:1440
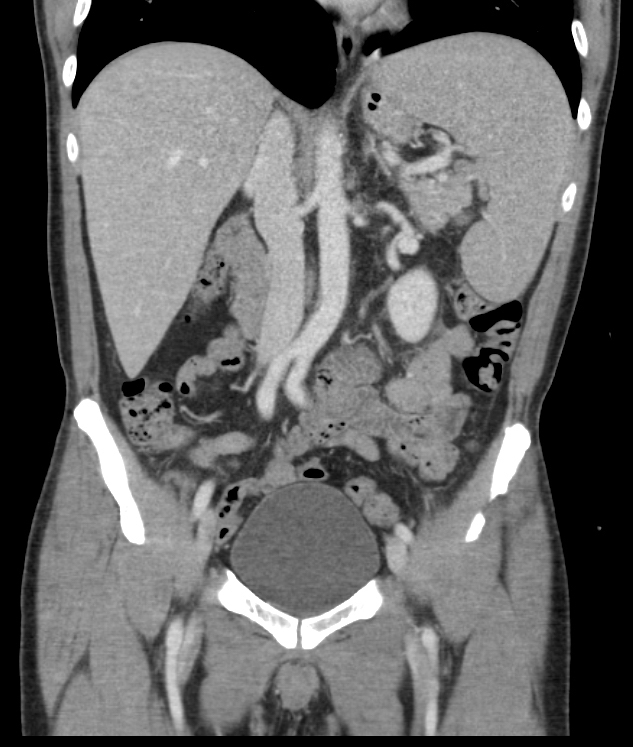
Radiology description
- Splenomegaly
Prognostic factors
- Most important prognostic indicator is response to tyrosine kinase inhibitors at the hematological, cytogenetic and molecular level (Cancer 2017;123:4391)
- 5 year progression free and overall survival rates: 80 - 95%
- Long term overall survival approaching that of aged matched controls
Case reports
- 11 year old boy with chronic myeloid leukemia possessing the unique combination of T lymphoblastic transformation and a subclone harboring inv(3)(q21q26.2) at diagnosis (Biomark Res 2016;4:14)
- 39 year old asymptomatic man with hematologically typical chronic phase chronic myeloid leukemia showed an unusual and complex translocation involving chromosomes 9, 11 and 22 (Arch Pathol Lab Med 2001;125:437)
- 52 year old woman with occurrence of chronic myeloid leukemia followed by CLL (Int J Hematol Oncol Stem Cell Res 2014;8:49)
- 68 year old woman with p190 BCR-ABL+ chronic myeloid leukemia and persistent moderate monocytosis (J Hematol 2018;7:120)
- 68 year old man with JAK2 V617F positive PV, evolving to BCR-ABL positive chronic myeloid leukemia (AJHO 2017;13:24)
Treatment
- Chronic phase: tyrosine kinase inhibitor (TKI), acting by competitive inhibition at the ATP binding site of BCR-ABL1 oncoprotein, resulting in inhibition of phosphorylation of proteins involved in cell signal transduction (Ulster Med J 2007;76:8)
- Tyrosine kinase inhibitor agents help achieve long term control of chronic myeloid leukemia in majority of patients (Leukemia 2020;34:966)
- Imatinib is the prototype drug and first approved tyrosine kinase inhibitor (N Engl J Med 2017;376:917)
- Before tyrosine kinase inhibitor era, interferon alpha and hydroxyurea were used to treat chronic myeloid leukemia (Leukemia 2013;27:803)
- Chronic myeloid leukemia resistant to imatinib can be treated by second generation tyrosine kinase inhibitor agents such as nilotinib, dasatinib and bosutinib (Clin Lymphoma Myeloma Leuk 2015;15:323)
- Complete hematological response (Blood Rev 2011;25:139):
- WBC < 10 x 10⁹/L
- Platelet count < 450 x 10⁹/L
- No immature granulocytes in the differential
- Spleen not palpable
- Treatment failure occurs most commonly due to compliance issues followed by BCR-ABL mutations altering drug binding
- Hematopoietic stem cell transplant is reserved for those rare patients who (Semin Hematol 2010;47:354):
- Present with blast phase
- Are resistant / intolerant to all tyrosine kinase inhibitors
- Progress to accelerated or blast phase while on tyrosine kinase inhibitor therapy
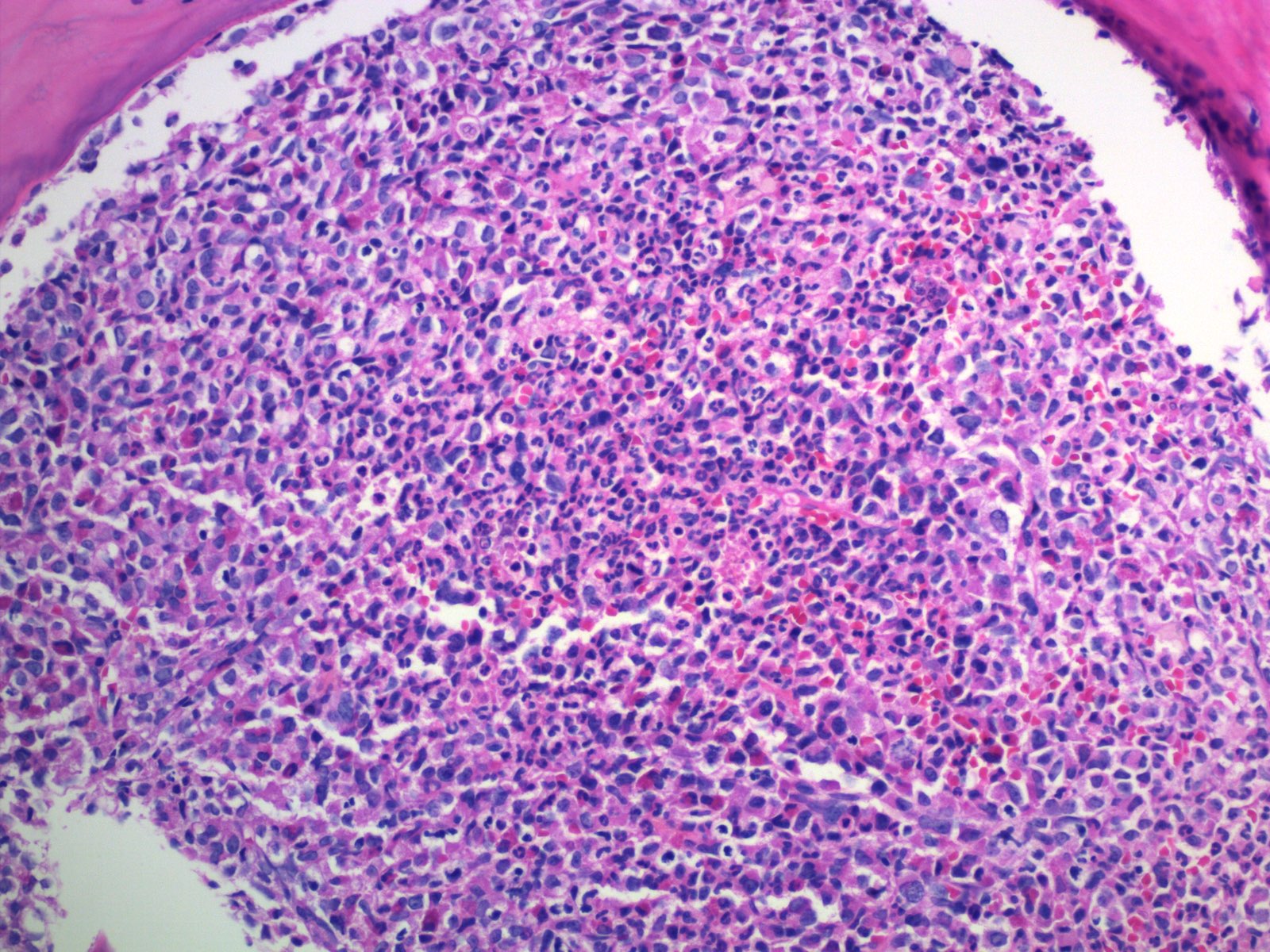
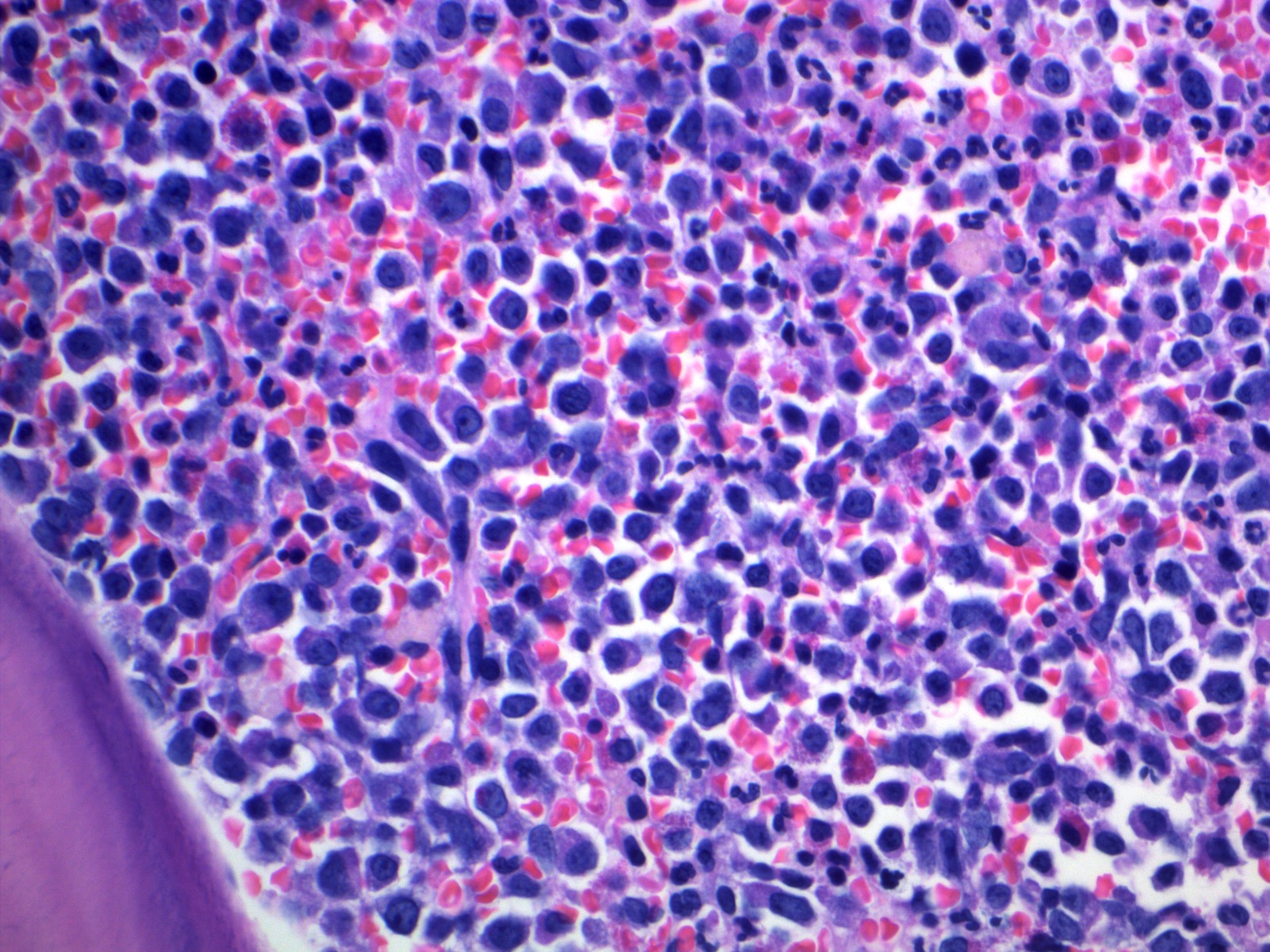
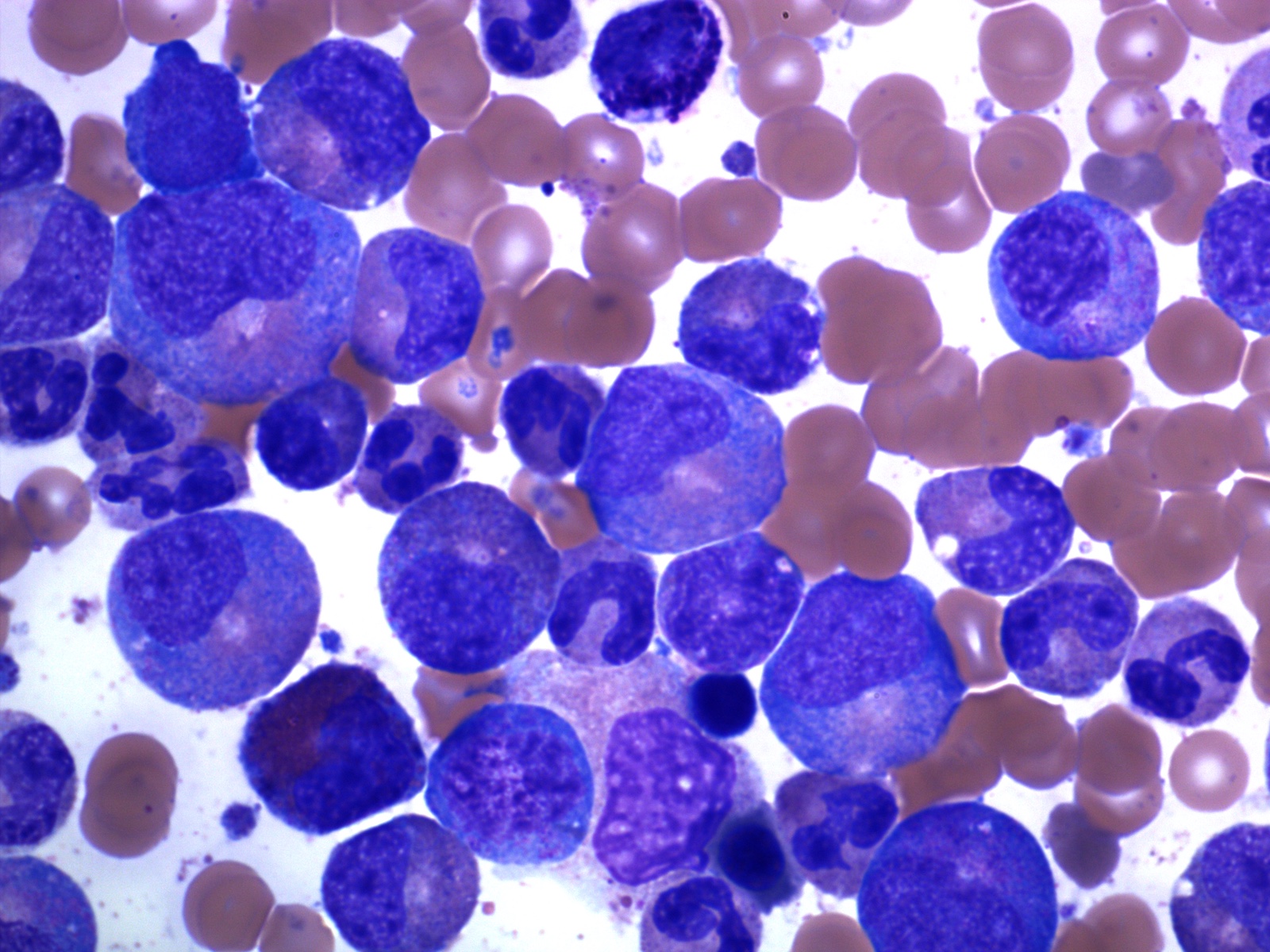
Microscopic (histologic) description
- Bone marrow (chronic phase):
- Hypercellular marrow with absolute myeloid hyperplasia and myelocyte bulge
- Myeloid to erythroid ratio ≥ 10:1
- Layer of immature granulocytes (5 - 10 cells in thickness) around the bone trabeculae (2 - 3 cells layer in normal marrow)
- No significant dysplasia
- Increased basophils and eosinophils (Intern Med 2011;50:501)
- Blasts < 5%
- Normal / slightly decreased megakaryocytes; 40 - 50% show moderate to marked megakaryocytes proliferation
- Megakaryocytes often smaller and hyposegmented with dwarf morphology
- Pseudo-Gaucher cells are common (Leukemia 1998;12:233)
- Bone marrow (accelerated phase):
- 10 - 19% blasts (Am Soc Clin Oncol Educ Book 2015;e381)
- Large clusters or sheets of small, abnormal megakaryocytes associated with marked reticulin or collagen fibrosis may be considered presumptive evidence of accelerated phase
- Bone marrow (blast phase):
- ≥ 20% blasts in marrow or blood or the presence of an extramedullary proliferation of blasts (Leuk Lymphoma 2014;55:1451)
- Approximately 70% have myeloid blasts (may include neutrophilic, monocytic, megakaryocytic, basophilic, eosinophilic or erythroid blasts) and approximately 30% have lymphoid blasts (usually B lymphoblasts but T and NK blasts have been reported) (J Clin Invest 2010;120:2254)
Microscopic (histologic) images
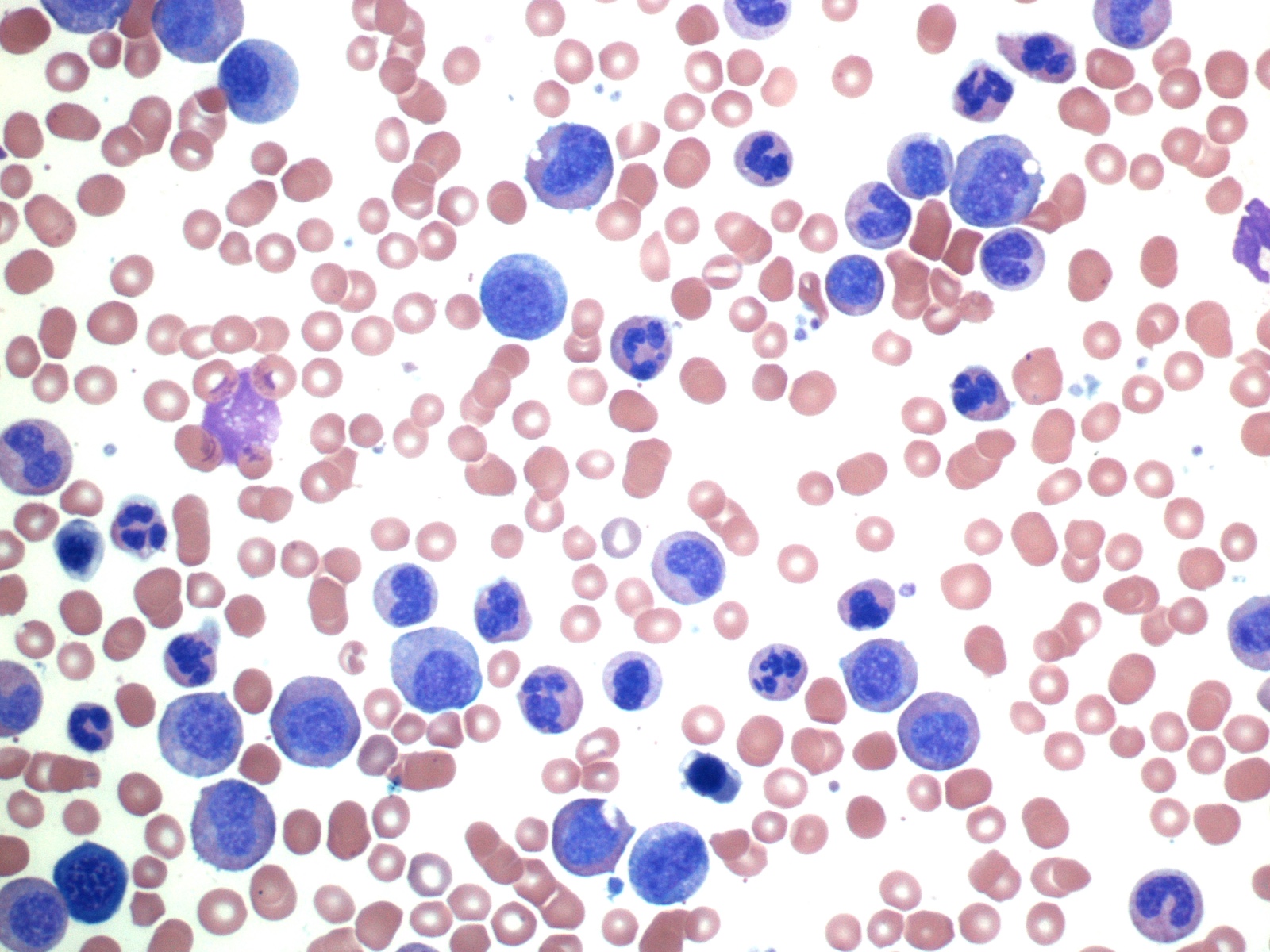
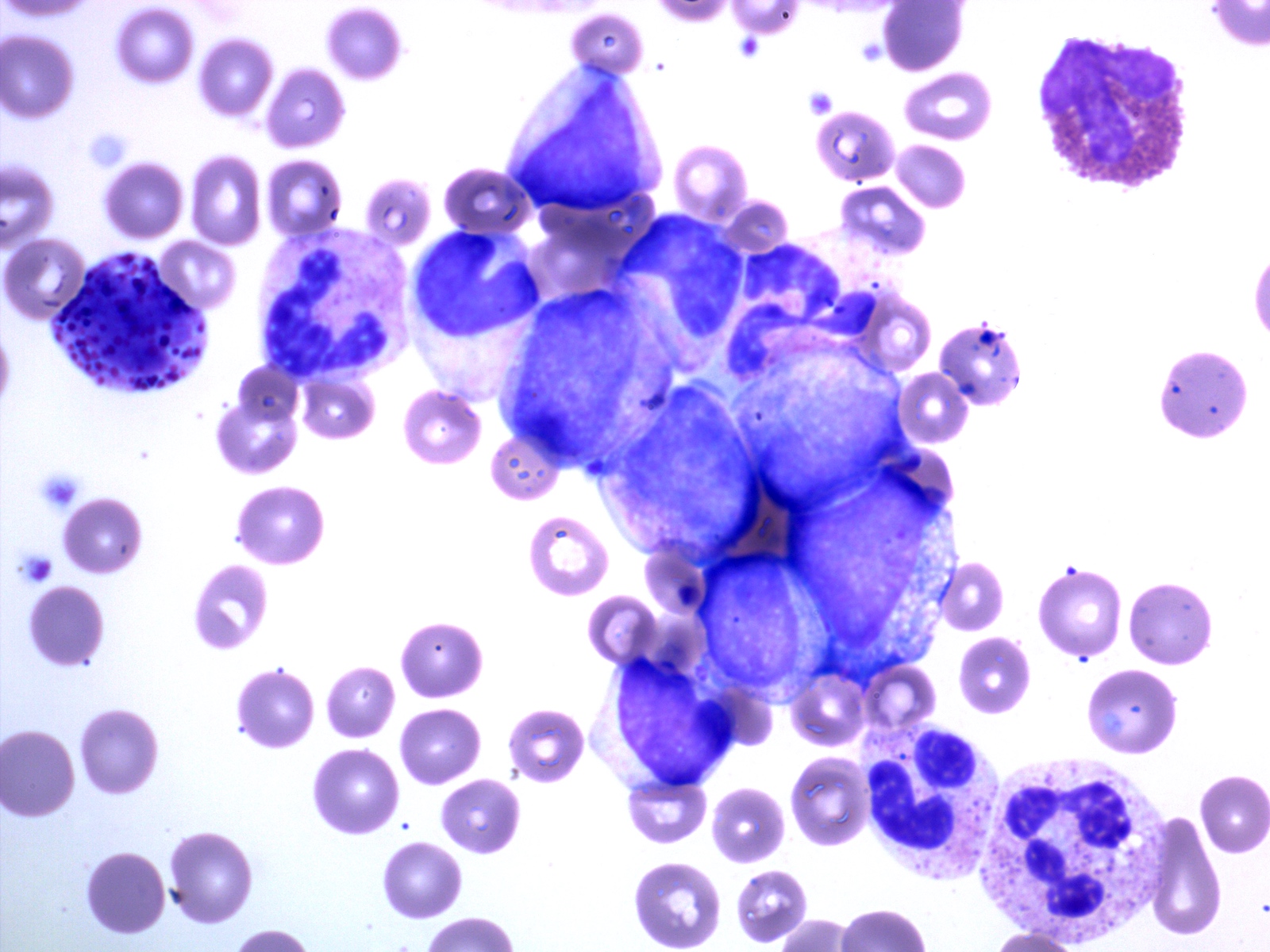
Peripheral smear description
- Chronic phase:
- Absolute leukocytosis (12 - 1,000 x 10⁹/L, median: ~80 x 10⁹/L)
- Left shifted with 2 peaks: myelocytes and segmented neutrophils
- Blasts < 2%
- No significant granulocytic dysplasia
- Absolute monocytosis may be present but are < 3%
- Absolute basophilia (100%) and eosinophilia (80 - 90%)
- Platelet counts are normal or thrombocytosis ≥ 1,000 x 10⁹/L
- Reference: Mayo Clin Proc 2015;90:1440
Positive stains
- Chronic phase:
- Myeloperoxidase, specific esterase (naphthol AS-D chloroacetate esterase)
- BB1 (basogranulin) stains basophils (Am J Clin Pathol 2006;125:273)
- Blast phase / accelerated phase:
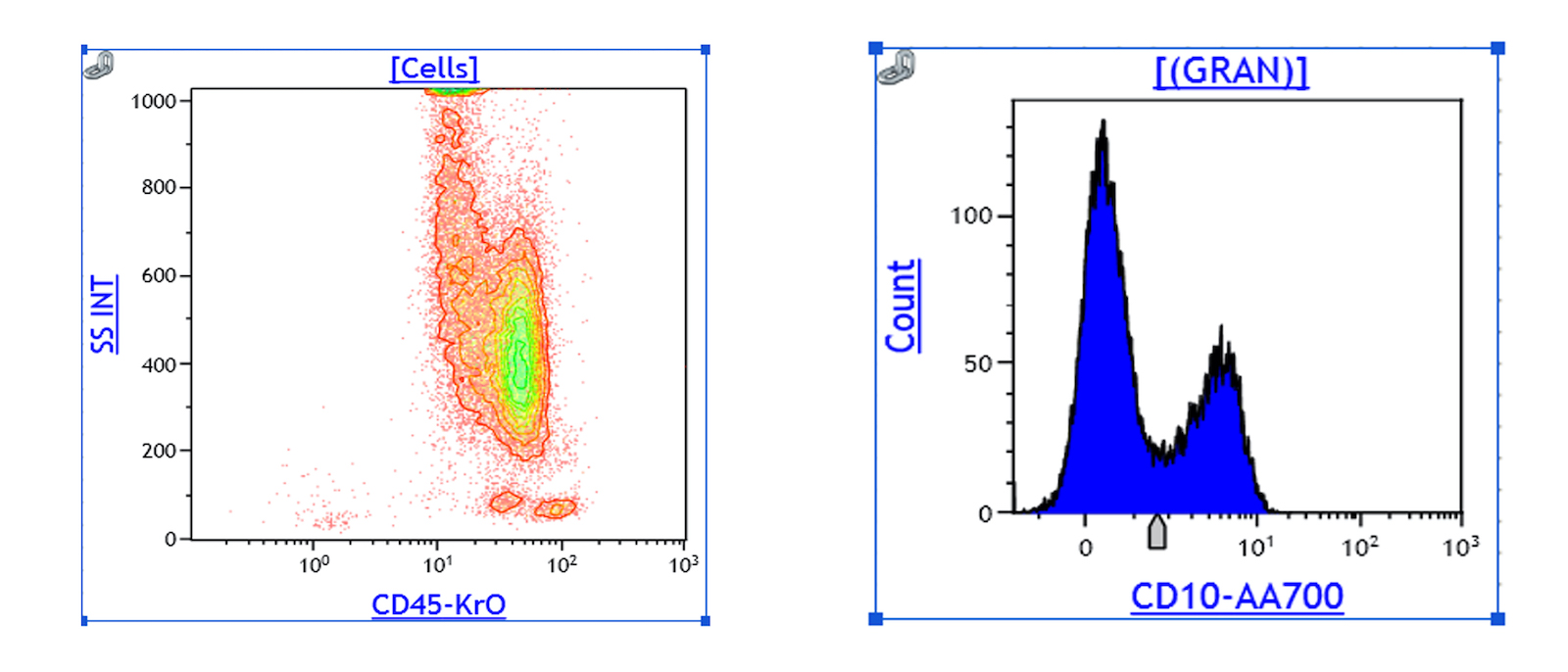
Flow cytometry description
- Peripheral blood (chronic phase) (Mayo Clin Proc 2015;90:1440):
- Left shifted leukocytosis
- No significant increase in blasts: < 2%
- Relative lymphopenia
- Myeloid lineage shows 2 peaks: correspond to myelocytes and segmented neutrophils respectively
- Accelerated phase: 10 - 19% blasts in blood or marrow
- Blast phase: ≥ 20% blasts in marrow or blood or the presence of an extramedullary proliferation of blasts (Front Oncol 2019;9:1132)
- Bona fide lymphoblasts in the peripheral blood or bone marrow (even if < 10%) should prompt immediate investigation for lymphoblastic transformation
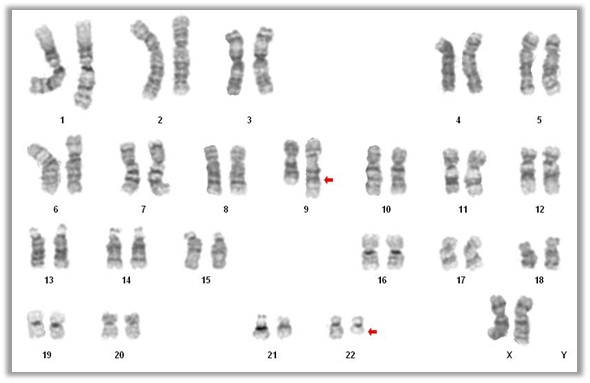
Molecular / cytogenetics description
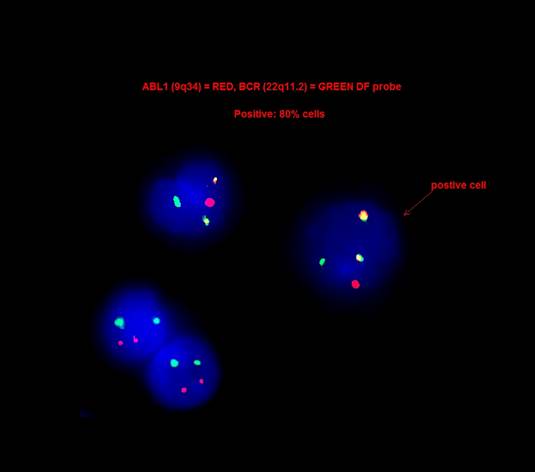
- 90 - 95% of chronic myeloid leukemia is characterized by t(9;22)(q34.1;q11.2) reciprocal translocation that results in the Ph chromosome, der(22)t(9;22) (Haematologica 2016;101:541)
- Translocation fuses the BCR gene on chromosome 22 with regions of ABL1 on chromosome 9 and generates the BCR-ABL1 fusion gene that characterizes leukemic cells (Am J Hematol 2018;93:442)
- 5 - 10% cases have either variant translocations that involve a third or even a fourth chromosome in addition to chromosomes 9 and 22 or a cryptic translocation of 9q34.1 and 22q11.2 that cannot be identified by routine cytogenetic analysis (Oncotarget 2017;8:29906)
- In these cases, the presence of BCR-ABL1 fusion gene can be detected by FISH or RT-PCR
- In most chronic myeloid leukemia cases the breakpoint cluster region is almost always in the major BCR (M-BCR), spanning exons 12 - 16 and form an abnormal fusion protein p210
- Rarely, the breakpoint in the BCR gene occurs in the p-BCR region, spanning exons 17 - 20 and form a larger fusion protein p230 (Haematologica 2001;86:320)
- Small amounts of P190 transcript with breaks in the minor breakpoint region, m-BCR (exons 1 - 2) can be detected in > 90% of patients with chronic myeloid leukemia due to alternative splicing of the BCR gene
- Very rare chronic myeloid leukemia cases are associated with p190 only and frequently show monocytosis, mimicking chronic myelomonocytic leukemia (J Hematol 2018;7:120)
Molecular / cytogenetics images
Videos
Brief introduction of chronic myeloid leukemia
Chronic myeloid leukemia: pathogenesis, symptoms and treatment
Blast crisis in chronic myeloid leukemia
Chronic myeloid leukemia from Khan Academy
Treatment of chronic myeloid leukemia
Peripheral blood smear of chronic myeloid leukemia
Sample pathology report
- Bone marrow, left posterior iliac crest, core biopsy, clot section, aspirate smears and touch imprint:
- Hypercellular (90%) bone marrow with absolute myeloid hyperplasia and < 5% blasts (see comment)
- Comment: Flow cytometric analysis demonstrates 2.5% blasts. There are < 5% blasts by CD34 and CD117 immunohistochemical stains on the core biopsy. A CBC obtained on 03/29/2020 revealed leukocytosis (30.2 x 10⁹/L) and thrombocytosis (625 x 10⁹/L). FISH analysis from peripheral blood (03/29/2020): BCR-ABL1 dual color, dual fusion translocation DNA probe revealed a fusion of 9q34.1 (ABL1) and 22q11.2 (BCR) regions in 62 out of 100 cells (62%) interphase nuclei.
- Peripheral smear: Manual review of the peripheral blood shows left shifted leukocytosis with two bulges: myelocytes and segmented neutrophils. No definite morphologic dysplasia is seen. Rare (~1%) circulating blasts are noted. RBCs: mild normochromic, normocytic anemia. Platelets: moderate thrombocytosis with rare large platelets.
- Bone marrow biopsy: Quality: adequate. Cellularity: > 90%. Hematopoiesis: trilineage maturation with absolute myeloid hyperplasia and relative erythroid hypoplasia. Megakaryocytes are mildly to moderately increased. Most megakaryocytes are small and hyposegmented. Infiltrate: no apparent increase in blasts. Special stains: reticulin: mild diffuse increase in reticulin fibrosis. Trichrome: negative for collagen deposition.
- Bone marrow clot section: Quality: adequate. Cellularity: > 90% morphologic features are similar to those observed in the core biopsy.
- Bone marrow aspirate: Quality: adequate. Granulocytes: markedly increased with significant expansion of myelocytes. No definite morphologic dysplasia. Erythrocytes: relative erythroid hypoplasia with progressive maturation and no definite dysplasia. Megakaryocytes: mildly to moderately increased; most of them are small and hyposegmented. No sheets of megakaryocytes are present. Blasts: < 5% of nucleated cells. Iron: storage iron is adequate (2+ on a scale of 0 - 4). No ring sideroblasts are present.
Differential diagnosis
- Leukemoid reaction (Am J Hematol 2018;93:442):
- Underlying infection / inflammation
- Myelocyte bulge is absent
- Basophilia is absent
- Toxic granulation and cytoplasmic vacuoles might be present
- Alkaline phosphatase increased
- Chronic neutrophilic leukemia:
- Peripheral blood:
- Segmented neutrophils + band neutrophils ≥ 80%
- Neutrophil precursors (promyelocytes, myelocytes and metamyelocytes) < 10%
- Neutrophils might show toxic granulation and Dohle bodies
- No dysplasia
- Strong association with CSF3R mutation (Blood 2017;129:715)
- Peripheral blood:
- Atypical chronic myeloid leukemia, BCR-ABL1 negative (Hematology Am Soc Hematol Educ Program 2015;2015:264):
- Dysplastic neutrophils and their precursors
- Multilineage dysplasia is common
- No or minimal absolute basophilia, basophils < 2% of WBC
- No or minimal monocytosis, monocytes < 10% of WBC
- SETBP1 and ETNK1 mutations are common
- Chronic myelomonocytic leukemia (Am J Hematol 2018;93:824):
- Monocytes ≥ 10% of WBC
- Dysplasia involving ≥ 1 myeloid lineages
- Common mutations: TET2, SRSF2, ASXL1, SETBP1
- Chronic eosinophilic leukemia (Blood Cancer J 2018;8:15):
- Eosinophil count ≥ 1.5 x 10⁹/L
- Blasts < 20%
- A clonal cytogenetics or molecular genetic abnormality is present or blasts ≥ 2% in blood or blasts ≥ 5% in marrow
- Absence of BCR-ABL1 translocation
- Absence of rearrangement of PDGFRA, PDGFRB or FGFR1 and no PCM1-JAK2, ETV6-JAK2 or BCR-JAK2 fusion
- Other myeloproliferative neoplasms such as polycythemia vera, essential thrombocythemia, primary myelofibrosis, chronic neutrophilic leukemia, chronic myelomonocytic leukemia and BCR-ABL1 negative atypical chronic myeloid leukemia are excluded
- AML with inv(16)(p13.1q22), t(16;16)(p13.1;q22), t(8;21)(q22;q22.1) are excluded
- Essential thrombocythemia (Mayo Clin Proc 2015;90:1440):
- Platelet ≥ 450 x 10⁹/L
- Bone marrow biopsy shows significant megakaryocytic proliferation
- Megakaryocytes are large, hyperlobated
- No significant increase or left shifted granulopoiesis or erythropoiesis
- No basophilia
- JAK2, CALR or MPL mutation (+)
Board review style question #1
Which of the following is a criterion for the diagnosis of accelerated phase of chronic myeloid leukemia according to WHO 2017 criteria?
- 5 - 10% blasts in bone marrow or peripheral blood
- Persistent platelet count < 100,000 related to therapy
- ≥ 10% basophils in blood
- Persistent or increasing splenomegaly
- Additional clonal chromosomal abnormalities in Philadelphia chromosome positive cells at diagnosis, including so called major route abnormalities (a second Ph chromosome, trisomy 8, isochromosome 17q, trisomy 19), complex karyotype and abnormalities of 3q26.2
Board review style answer #1
E. Additional clonal chromosomal abnormalities in Philadelphia chromosome positive cells at diagnosis, including so called major route abnormalities (a second Ph chromosome, trisomy 8, isochromosome 17q, trisomy 19), complex karyotype and abnormalities of 3q26.2
Comment Here
Reference: Chronic myeloid leukemia (CML)
Comment Here
Reference: Chronic myeloid leukemia (CML)
Board review style question #2
A 15 year old boy with no significant medical history presented with worsening knee pain for 5 days. CBC: WBC: 229.4 x 10⁹/L, hemoglobin 12.1 g/dl, platelet 161 x 10⁹/L. Bone marrow biopsy showed > 90% cellular marrow with absolute myeloid hyperplasia without significant increase in blasts. FISH study showed BCR-ABL1 translocation. Which of the following statements is right?
- CML is very common in pediatric patients
- Children and young adults with CML tend to have a less aggressive clinical presentation than older adults
- Children and young adults with CML present with accelerated or blast phase more frequently
- Children and young adults tend to have higher complete cytogenetic response and molecular response rates compared with older adults
Board review style answer #2
C. Children and young adults with CML present with accelerated or blast phase more frequently
Comment Here
Reference: Chronic myeloid leukemia (CML)
Comment Here
Reference: Chronic myeloid leukemia (CML)
Board review style question #3
Which of the following statements about chronic myeloid leukemia is correct?
- Monocytosis rules out the possibility of chronic myeloid leukemia
- Thrombocytosis rules out the possibility of chronic myeloid leukemia
- Eosinophilia with basophilia is common in chronic myeloid leukemia
- Toxic granulation and Dohle bodies are common in chronic myeloid leukemia
- Alkaline phosphate is increased in chronic myeloid leukemia
Board review style answer #3
C. Eosinophilia with basophilia is common in chronic myeloid leukemia
Comment Here
Reference: Chronic myeloid leukemia (CML)
Comment Here
Reference: Chronic myeloid leukemia (CML)