Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Cima L, Lin S, Cecchini MJ. Sclerosing pneumocytoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungtumorsclerosingheman.html. Accessed December 25th, 2024.
Definition / general
- Pulmonary pneumocytoma are indolent tumors with a characteristic dual population of cuboidal surface cells resembling type II pneumocytes and stromal round cells
- Displays varying amounts of hemangiomatous, papillary, sclerotic and solid histologic patterns
Essential features
- First described by Liebow and Hubbell in 1956 as sclerosing hemangioma (Cancer 1956;9:53)
- Renamed pulmonary sclerosing pneumocytoma and categorized into adenoma in WHO 4th edition
- Characteristic bland cytologic features with multiple growth patterns and a dual cell population of surface and round cells that can be highlighted by immunohistochemistry
- Difficult to establish diagnosis on small biopsies, cytology or frozen sections and permanent sections are preferred
Terminology
- Obsolete term (not recommended): pulmonary sclerosing hemangioma
ICD coding
- ICD-O: 8832/0 - sclerosing pneumocytoma
- ICD-11: 2F0Z & XH7436 - benign neoplasms of respiratory and intrathoracic organs, unspecified & sclerosing pneumocytoma
Epidemiology
- Most frequent
- F > M (3.5:1)
- Wide age range, incidence higher in middle age (mean: 46 years) (Am J Surg Pathol 2000;24:906)
- Predilection for Eastern Asian population; rare among individuals of European descent
- Generally in never smokers (World J Surg Oncol 2022;20:140)
Sites
- Typically solitary and peripheral masses, in lower lobes of lung
- Rarely multifocal endobronchial masses or localized in hilum, visceral pleura or mediastinum
Pathophysiology
- Round and surface cells have been demonstrated to be clonal (Mod Pathol 2007;20:1208)
- Alterations in AKT1 are present in most cases (Proc Natl Acad Sci U S A 2016;113:10672)
Etiology
- Tumor is likely derived from primitive respiratory epithelium (Am J Surg Pathol 2000;24:906, J Clin Pathol 2020;73:531)
- Exact underlying mechanism is unknown
Clinical features
- Typically asymptomatic and incidental findings by routine radiological CT / Xray scans
- Patients can present with symptoms based on localization and tumor size
- Nonspecific respiratory symptoms: cough, sputum, hemoptysis and chest pain
- Cases with lymph node metastasis have been reported, however, this does not adversely affect prognosis (Arch Pathol Lab Med 2003;127:321)
Diagnosis
- Histological diagnosis supported by clinical and radiological CT / Xray findings and corresponding ancillary immunohistochemical testing
- Preoperative diagnosis: solitary pulmonary nodule, challenging histological diagnosis on small biopsies, cytology, cell block
- Intraoperative diagnosis: malignancy cannot be ruled out, challenging histological diagnosis on frozen sections
- Reference: World J Surg Oncol 2013;11:85
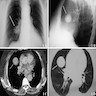
Radiology description
- Significant size variability, ranging from 1 to 8 cm in diameter, majority are < 3.5 cm
- Xray: solitary, well defined nodular lesion (oval to rounded shadow), may show calcification and crescent radiolucency at the periphery (air crescent sign)
- CT: well defined intraparenchymal nodular mass (often juxtapleural), may show calcification, air crescent sign and inhomogeneous enhancement
- Can occasionally present as lobulated, pure or mixed ground glass nodules or with pleural traction, mimicking malignant tumors
- Reference: Medicine (Baltimore) 2015;94:e498
Prognostic factors
- Diagnostic accuracy of sclerosing pneumocytoma is better in nodules > 1.0 cm in diameter
- Less invasive procedure is performed if sclerosing pneumocytoma can be diagnosed intraoperatively on frozen section
- Most sclerosing pneumocytomas behave in a benign fashion with an excellent prognosis
- Cases with recurrence are described (Thorac Cardiovasc Surg 2008;56:120)
- Cases with malignant transformation are described (BMC Cancer 2019;19:1154)
- Cases with lymph node metastasis are described (Am J Surg Pathol 2000;24:906, Jpn J Clin Oncol 1986;16:77, Ann Thorac Surg 2002;73:981, Arch Pathol Lab Med 2003;127:321, Yonsei Med J 2003;44:150, J Thorac Oncol 2016;11:1785, Histopathology 2020;77:718, Gen Thorac Cardiovasc Surg 2021;69:142)
- Cases with distal or pleural metastasis are described (Histopathology 2012;60:1162, Korean J Intern Med 2015;30:928, Surg Today 2011;41:258, Front Med (Lausanne) 2021;8:661032)
- Very rare cases with death are described (Front Med (Lausanne) 2021;8:661032)
Case reports
- 14 year old girl with carcinoid-like tumor characteristics on preoperative imaging (Indian J Thorac Cardiovasc Surg 2021;37:676)
- 23 year old woman with masquerading adenocarcinoma with coexisting BRAF and PTEN mutation (Cancer Treat Res Commun 2021;28:100429)
- 25 year old woman with pulmonary sclerosing pneumocytoma and mediastinal lymph node metastasis misdiagnosed on intraoperative frozen section as adenocarcinoma (Gen Thorac Cardiovasc Surg 2021;69:142)
- 25 year old man with large pulmonary sclerosing pneumocytoma with multiple foci and reported lymph node and extrapulmonary metastases (Front Med (Lausanne) 2021;8:661032)
- 43 year old woman with sclerosing pneumocytoma in highly unusual endobronchial location (Medicine (Baltimore) 2019;98:e15038)
- 64 year old woman with massive necrosis and vascular invasion (Oxf Med Case Reports 2019;2019:omz066)
Treatment
- Mainstay treatment is surgical resection
- Generally, sublobar wedge resection and lymph node dissection (with lymph node metastasis) is curative for peripheral small sized tumors (Korean J Thorac Cardiovasc Surg 2011;44:39)
- Limited resection is favored with preservation of lung function if sufficient resection margin can be achieved (World J Surg Oncol 2022;20:140)
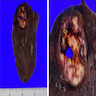
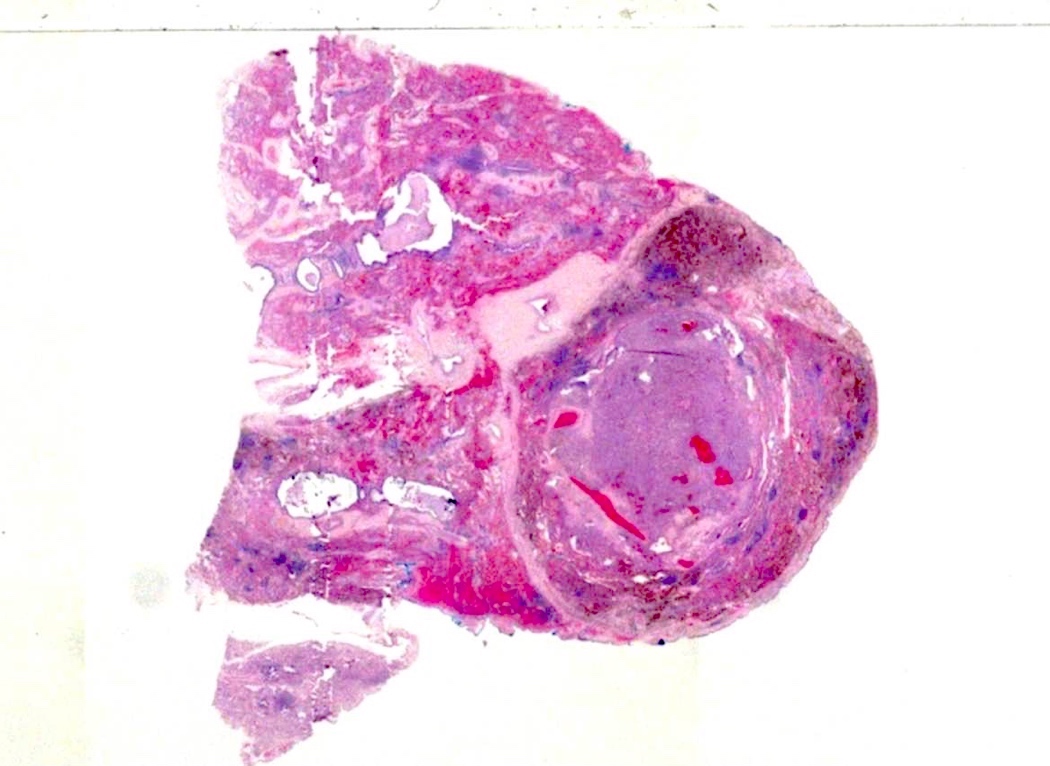
Gross description
- Well circumscribed mass, with varied gray-tan to yellow tumors
- Can also present dark red with hard texture (hemorrhagic)
- May find cystic degeneration and calcification
- Significant size variability, ranging from 1 to 8 cm in diameter, majority are < 3.5 cm
Frozen section description
- Key diagnostic clues intraoperatively
- Gross appearance is consistent with sclerosing pneumocytoma
- Recognition of the dual cell population
- Identification of different architectural patterns: papillary, sclerotic, solid and hemorrhagic
- Clinical history of a nonsmoker younger patient with a well circumscribed peripheral lesion
- Major diagnostic pitfalls: solid predominant pattern, hypercellularity, glandular spaces, desmoplasia-like sclerosis, cellular atypia, coagulative necrosis (Histopathology 2018;72:500)
Frozen section images
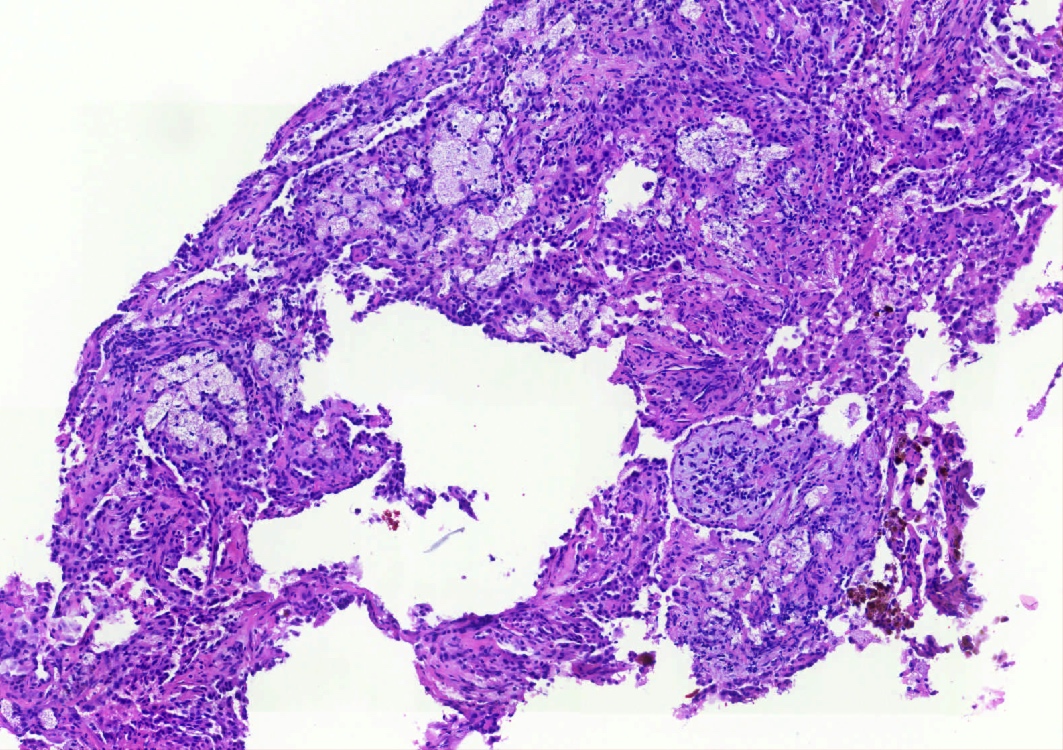
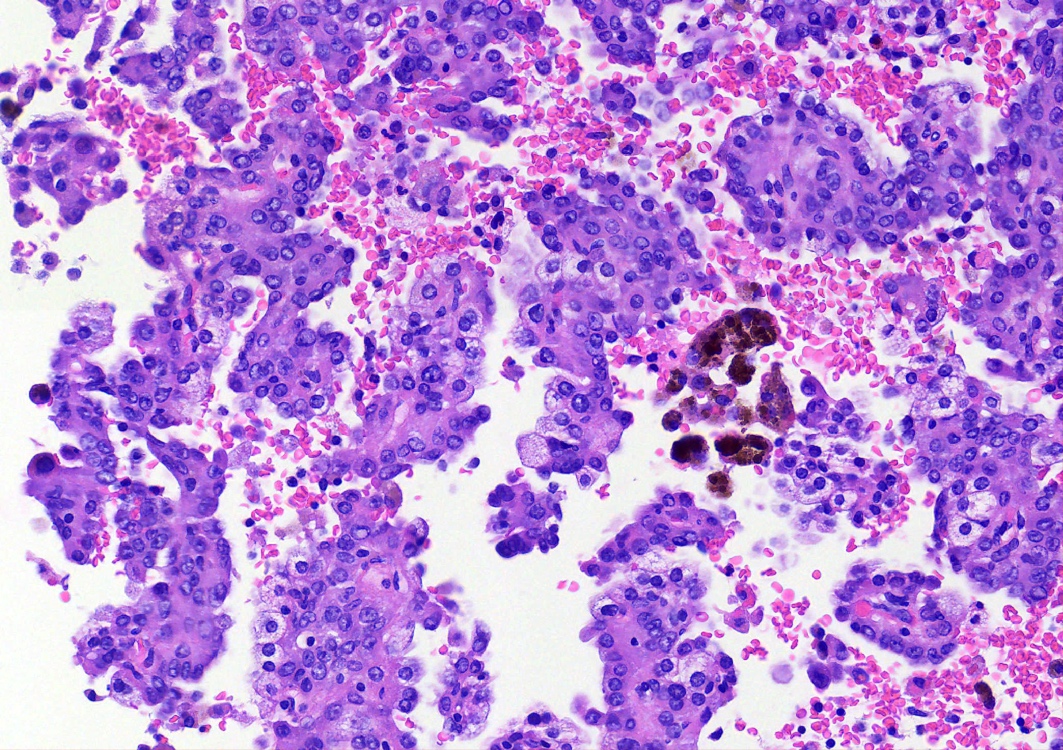
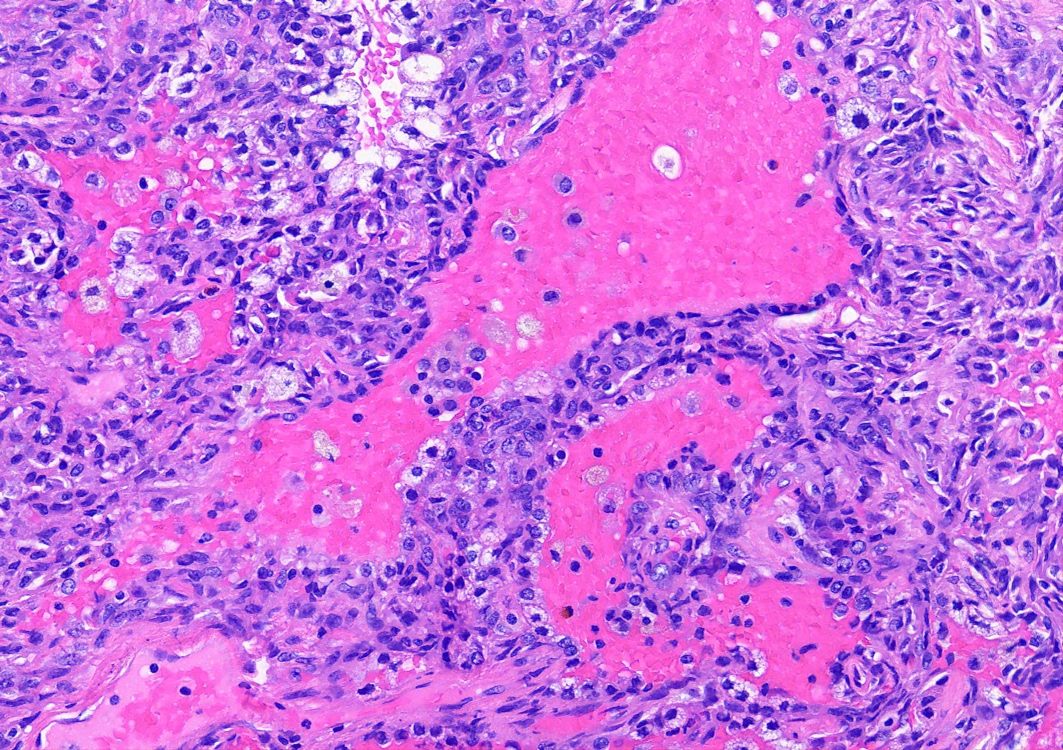
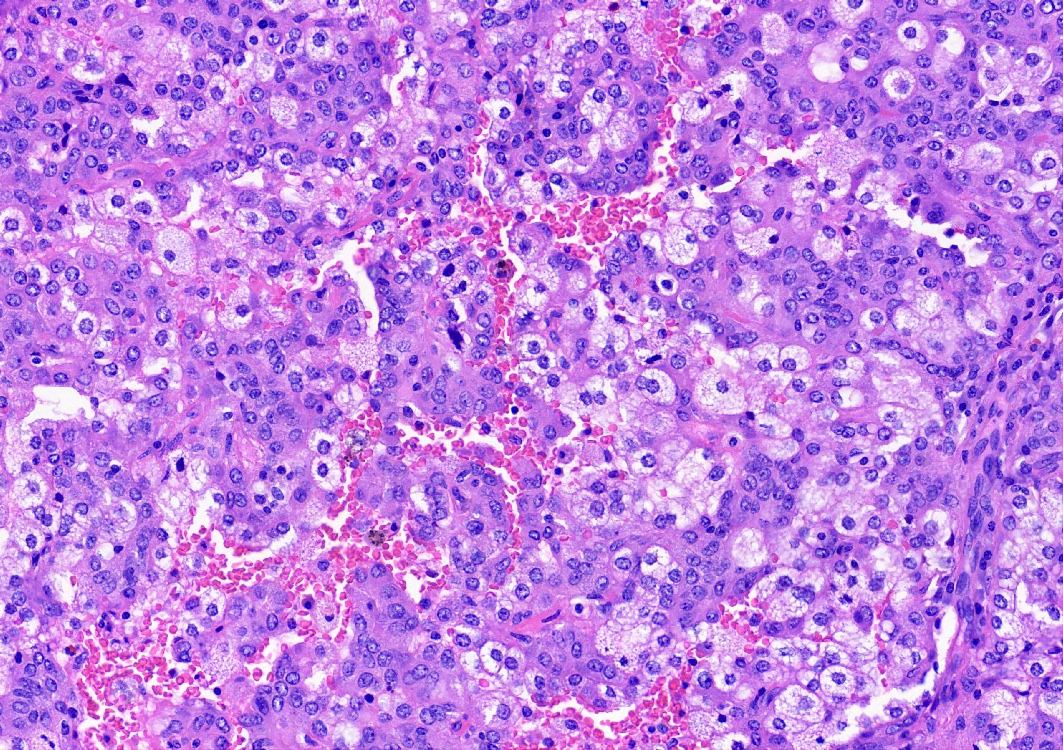
Microscopic (histologic) description
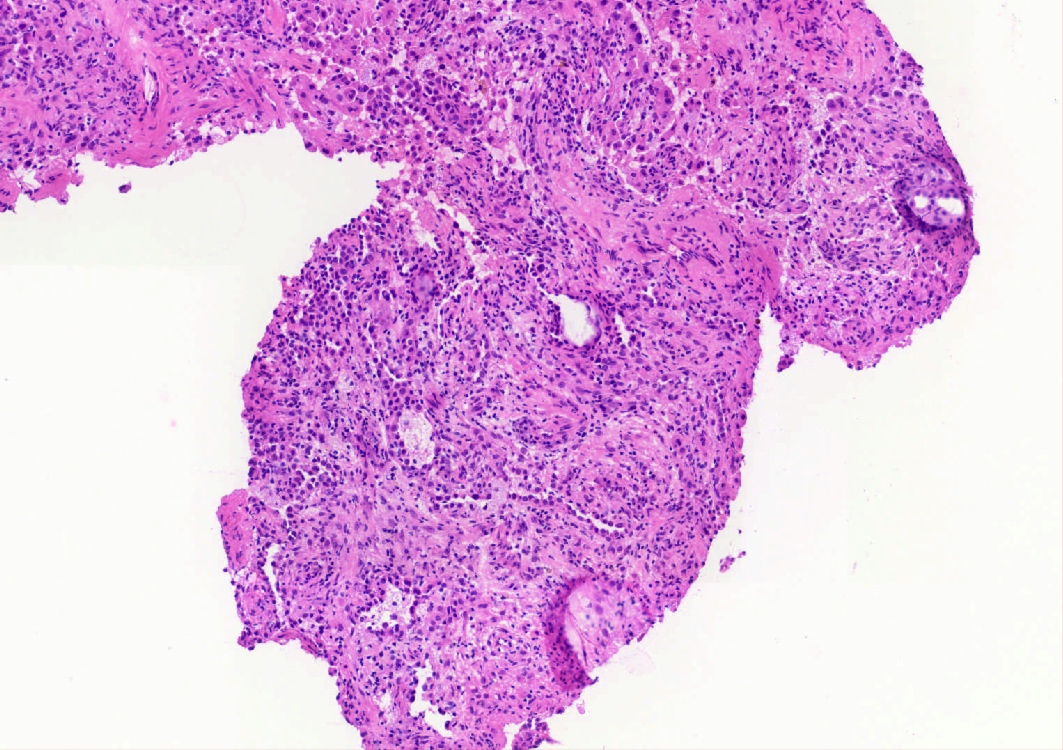
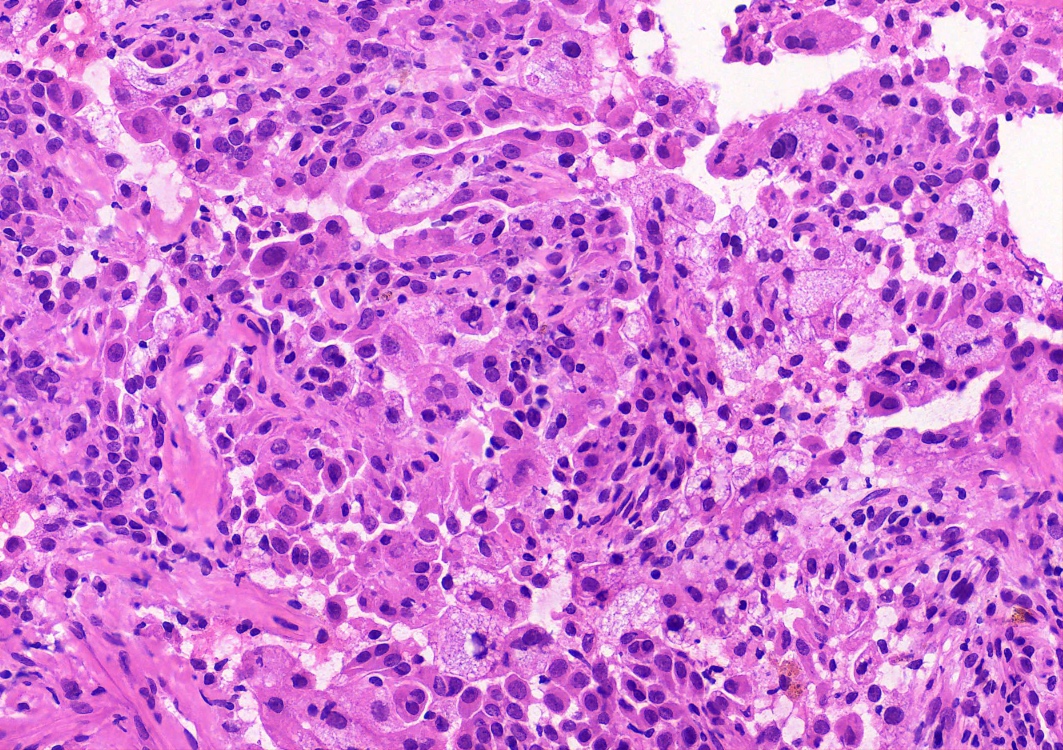
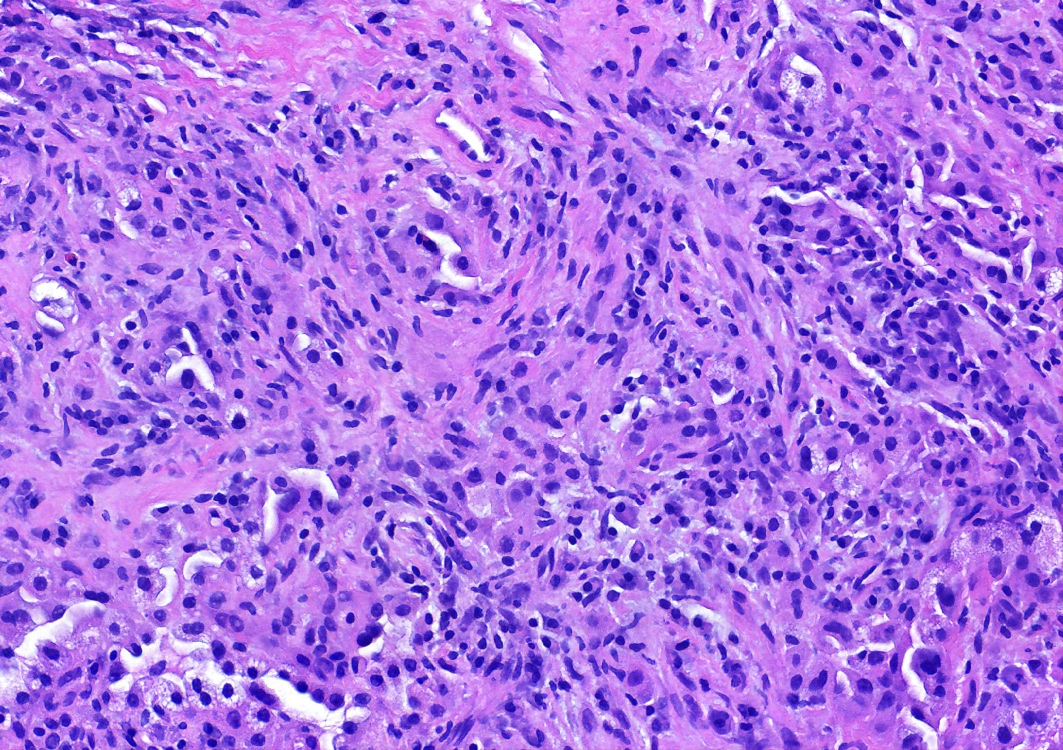
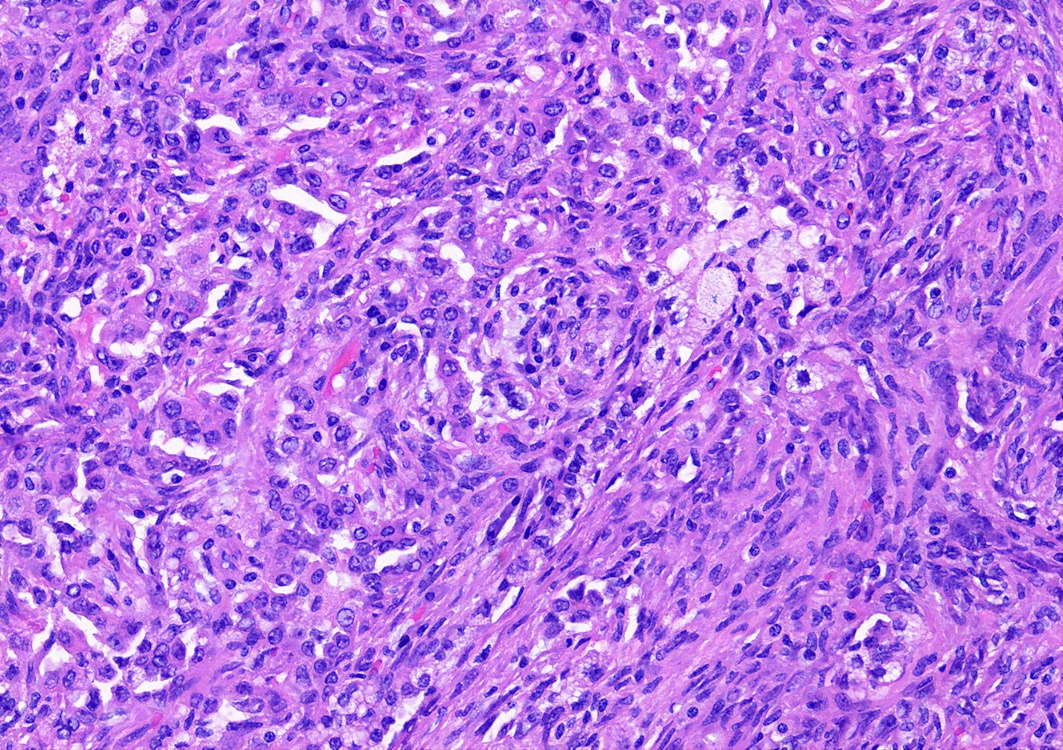
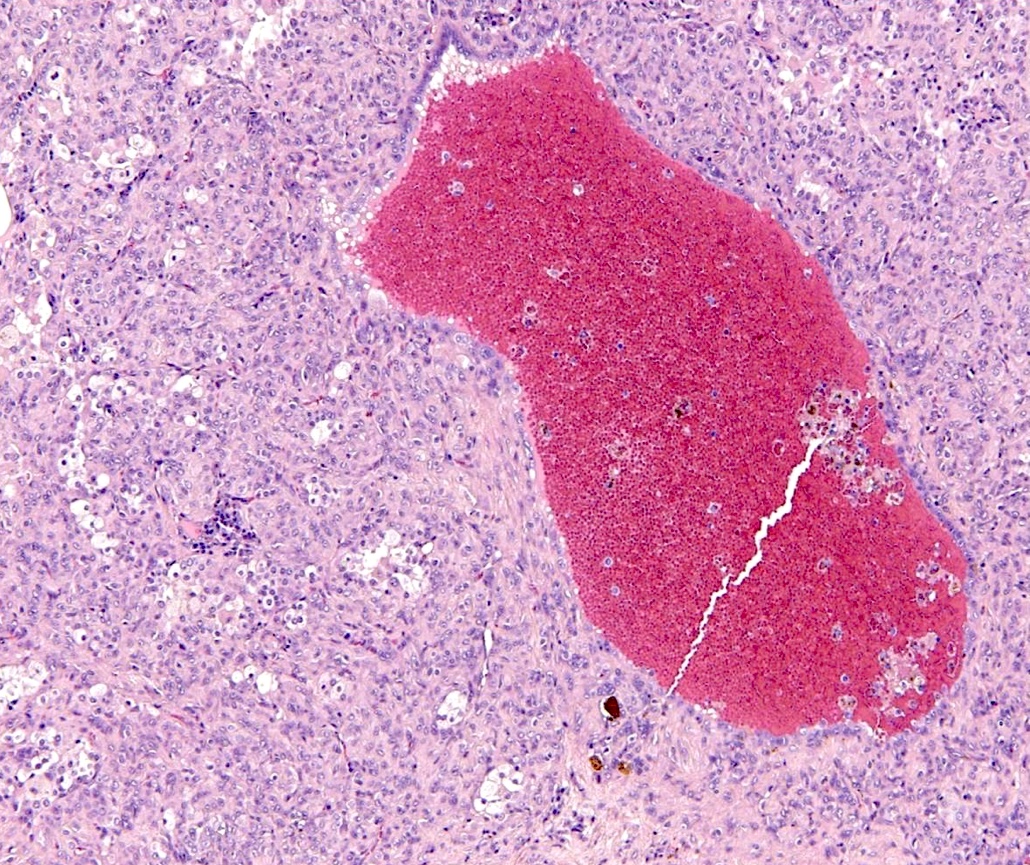
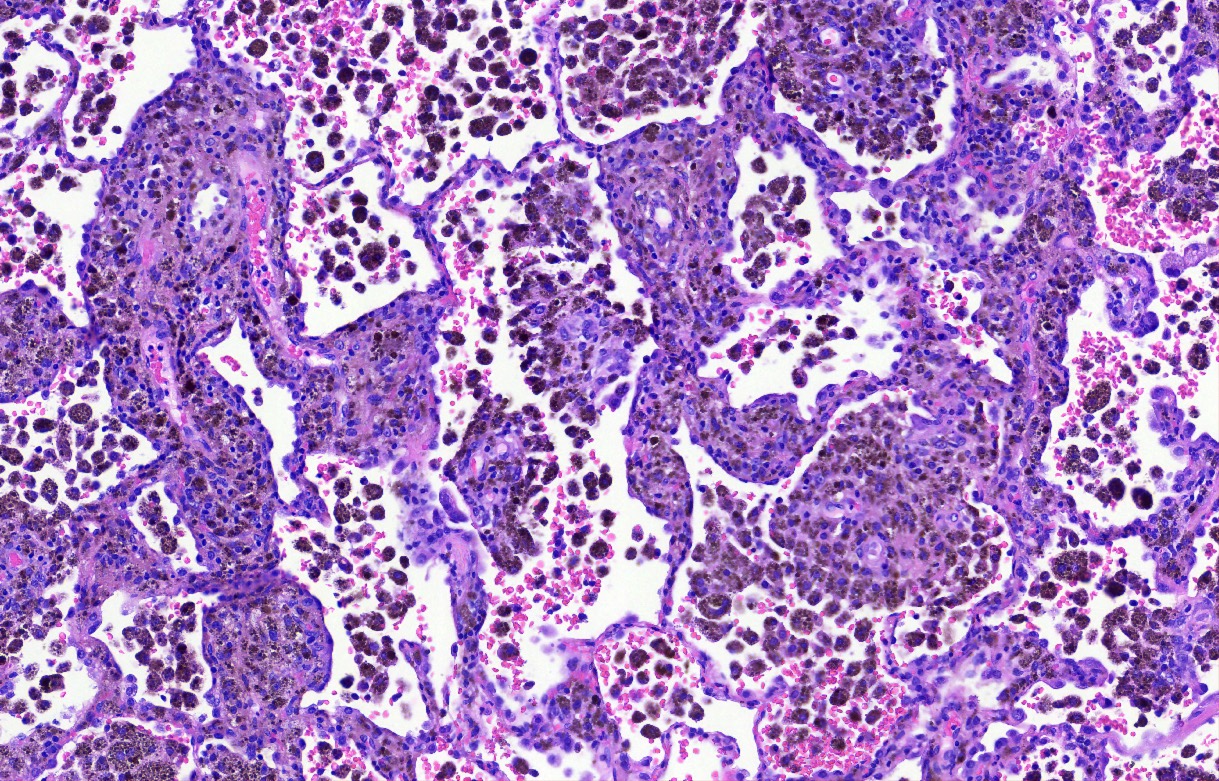
- Key feature is the presence of 2 cell types: cuboidal surface cells and round stromal cells
- Cuboidal surface cells resemble type II pneumocytes
- Round stromal cells have well defined border, with bland monotonous cells and fine chromatin
- 4 histologic growth patterns have been described
- Papillary: surface cells overlying hypercellular fibrovascular cores of round cells
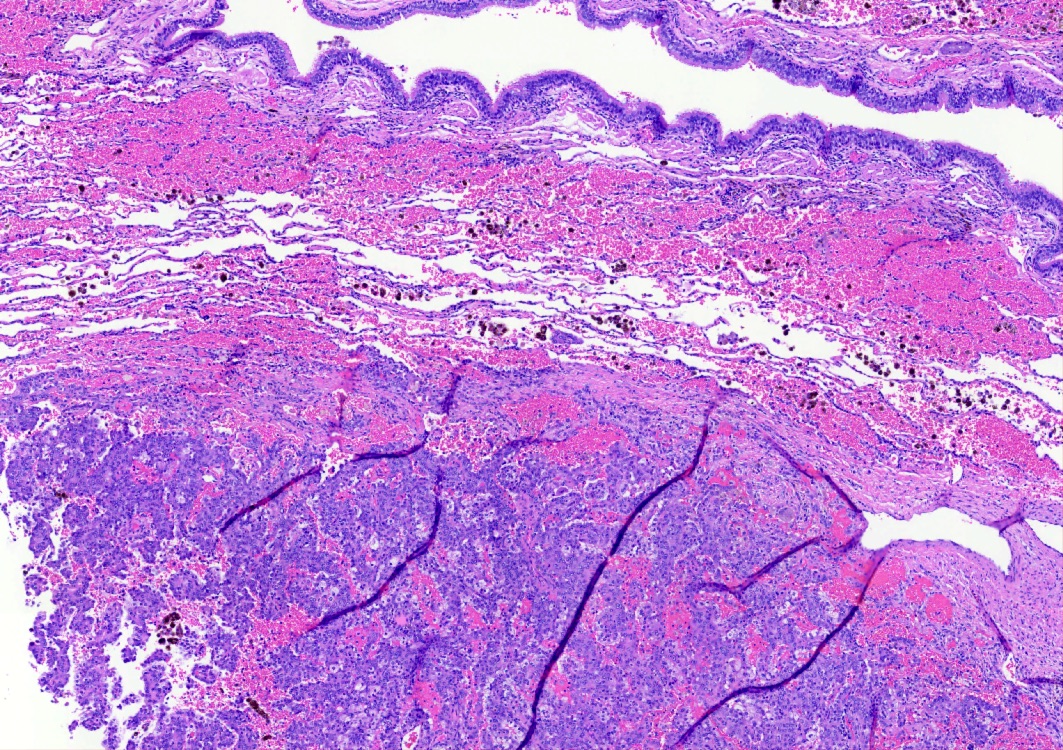
- Sclerotic: composed of hyalinized collagen, hemosiderin deposition in macrophages, cholesterol clefts and dystrophic calcification
- Solid: sheets of round cells with tubular / incomplete adenoid structures surrounded by surface cells
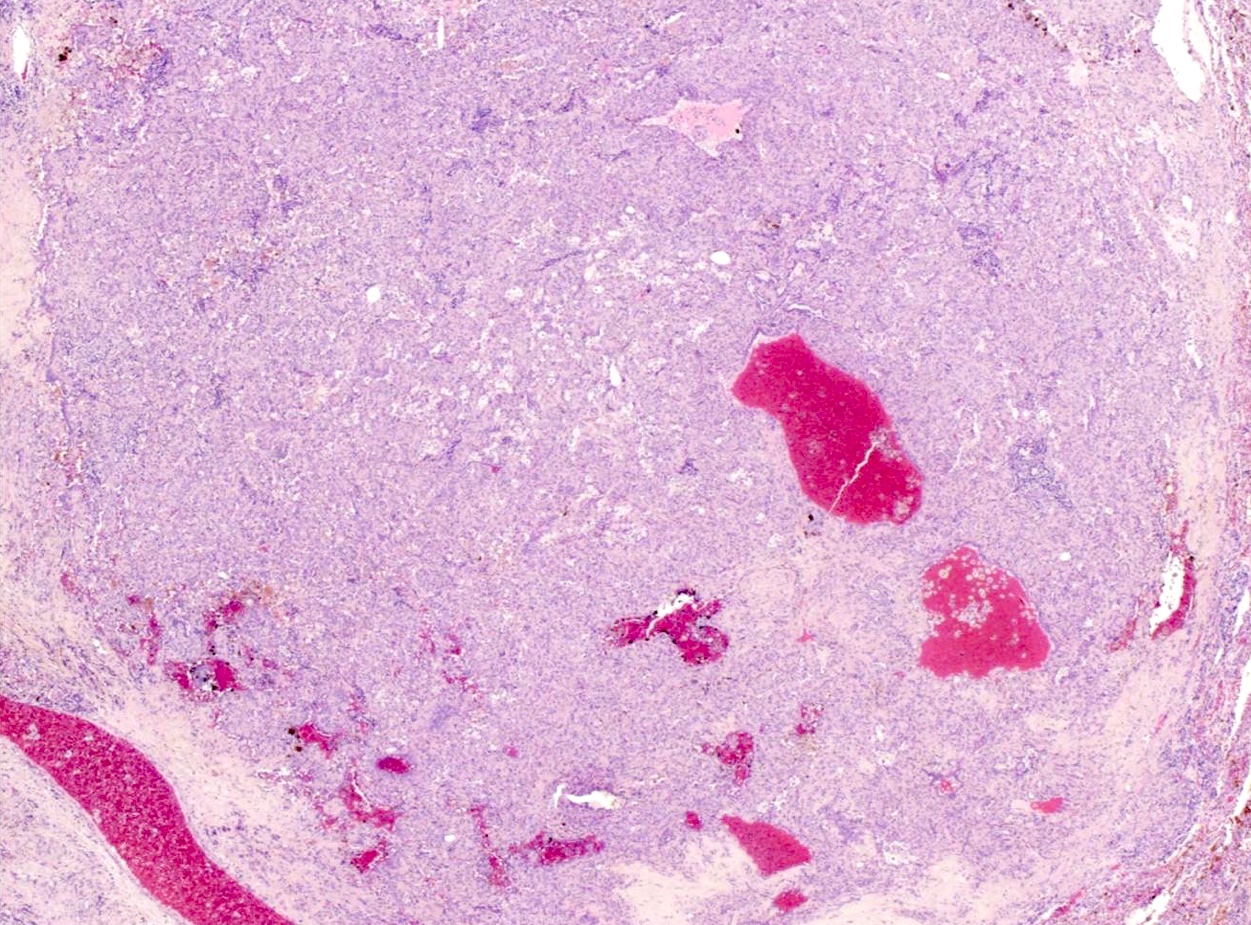
- Hemorrhagic: blood filled spaces lined by cuboidal epithelial cells
- Most tumors contain at least 3 of the 4 growth patterns in various proportions (Histopathology 2018;72:500)
Microscopic (histologic) images
Contributed by Matthew J. Cecchini, M.D., Ph.D. and Pooja Navale, M.D.
Cytology description
- Cytomorphological findings of sclerosing pneumocytoma overlap with lung adenocarcinoma (Cancer Cytopathol 2020;128:414)
- Most common cytological features (Diagn Cytopathol 2014;42:242)
- Moderately to highly cellular
- Papillary structures and large sheets
- Small to medium sized cells with moderate / abundant cytoplasm
- Bland round to oval nuclei with inconspicuous nucleoli
- Hemorrhagic background (fresh blood, siderophages, free hemosiderin pigment)
Positive stains
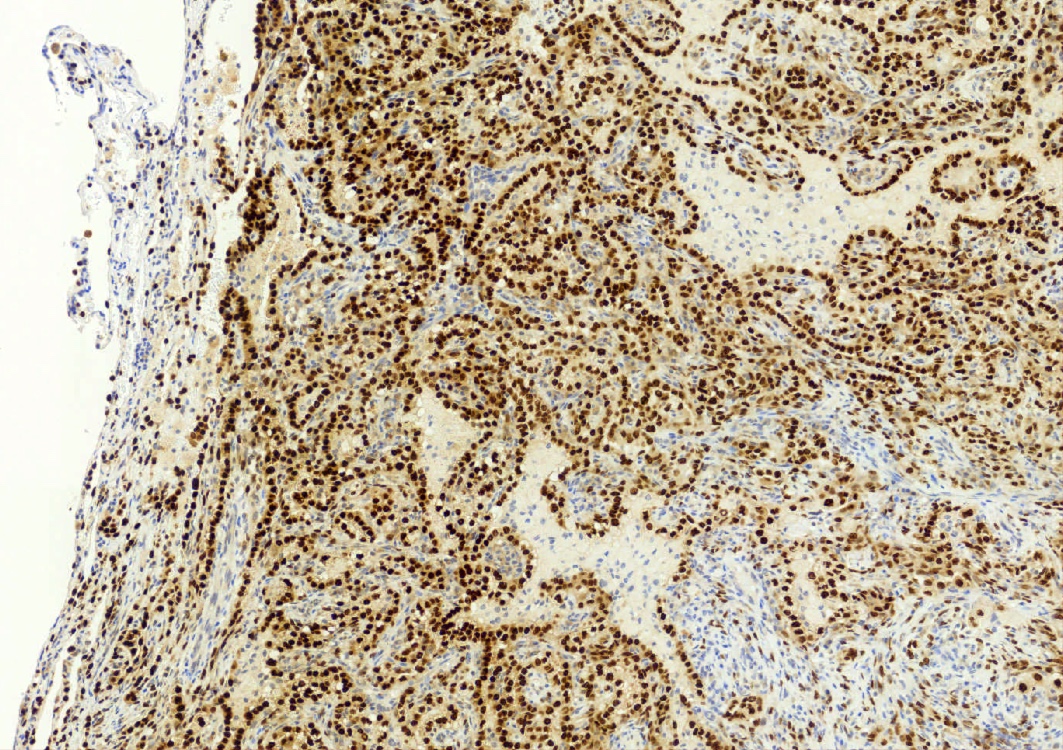
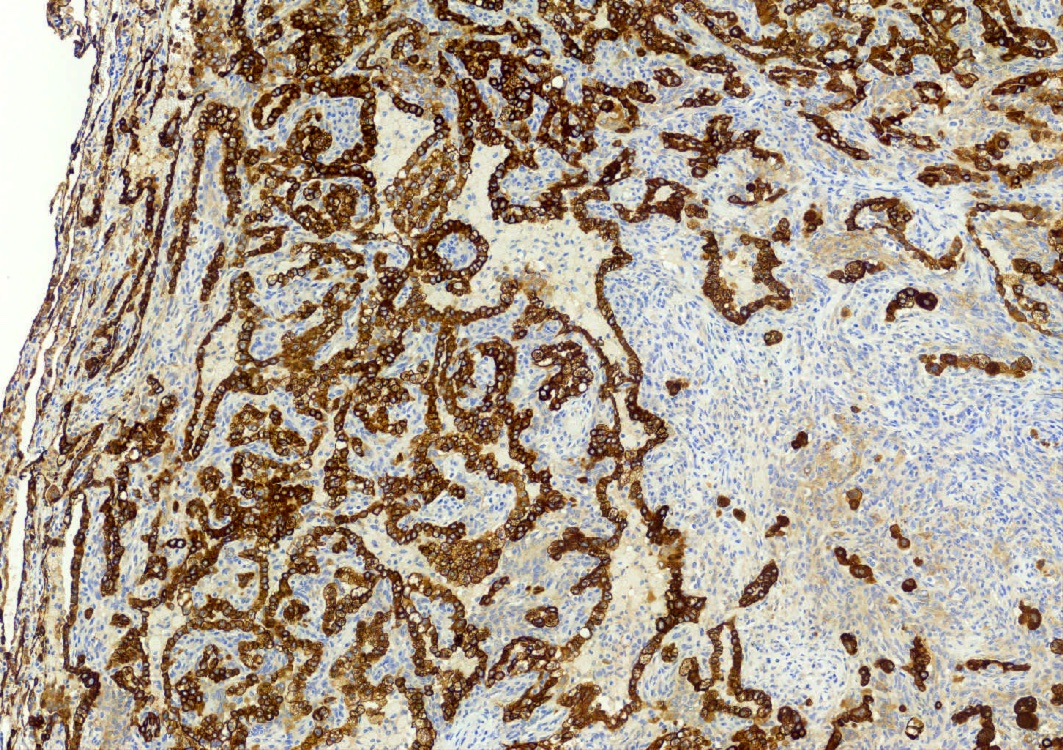
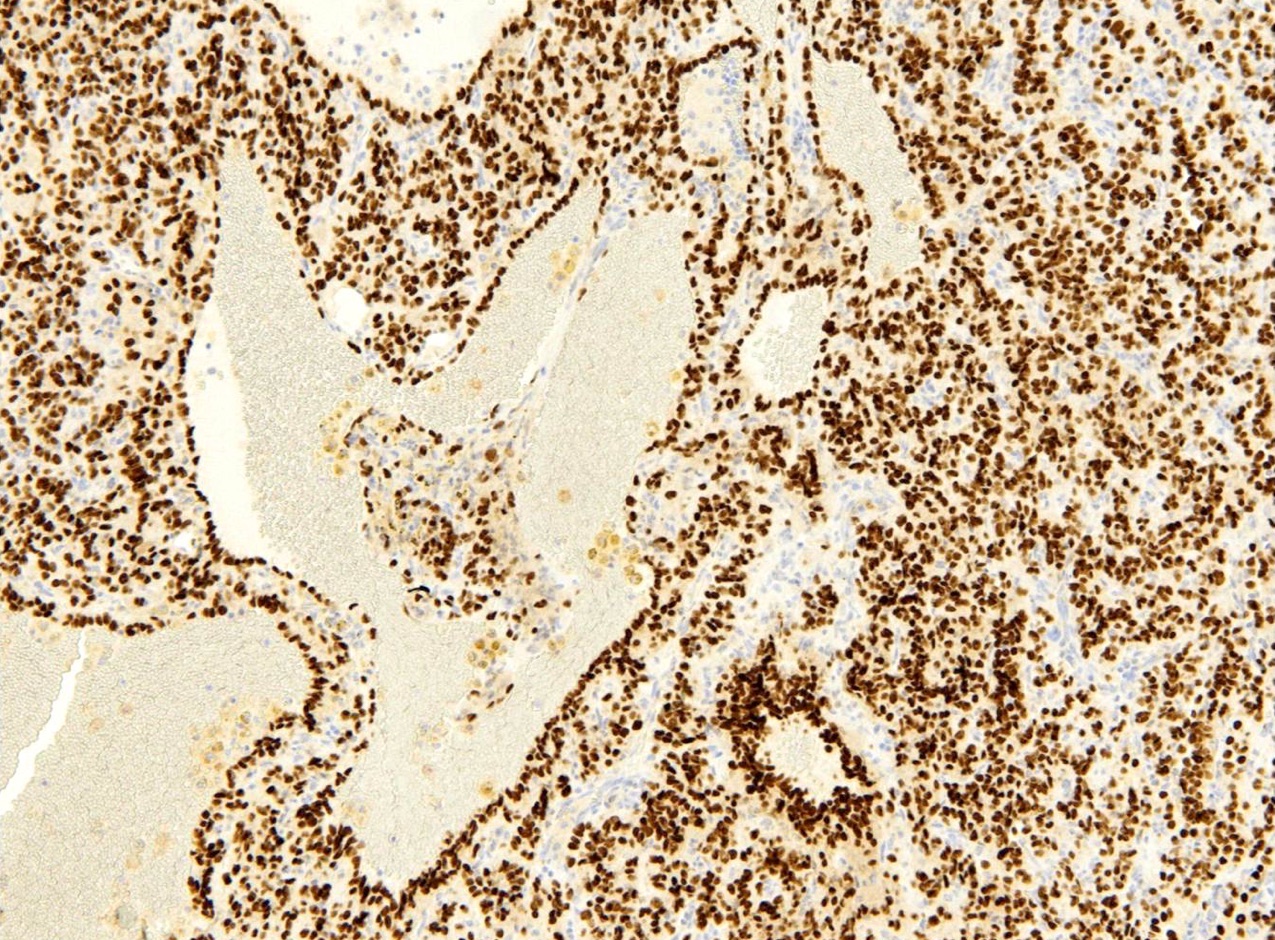
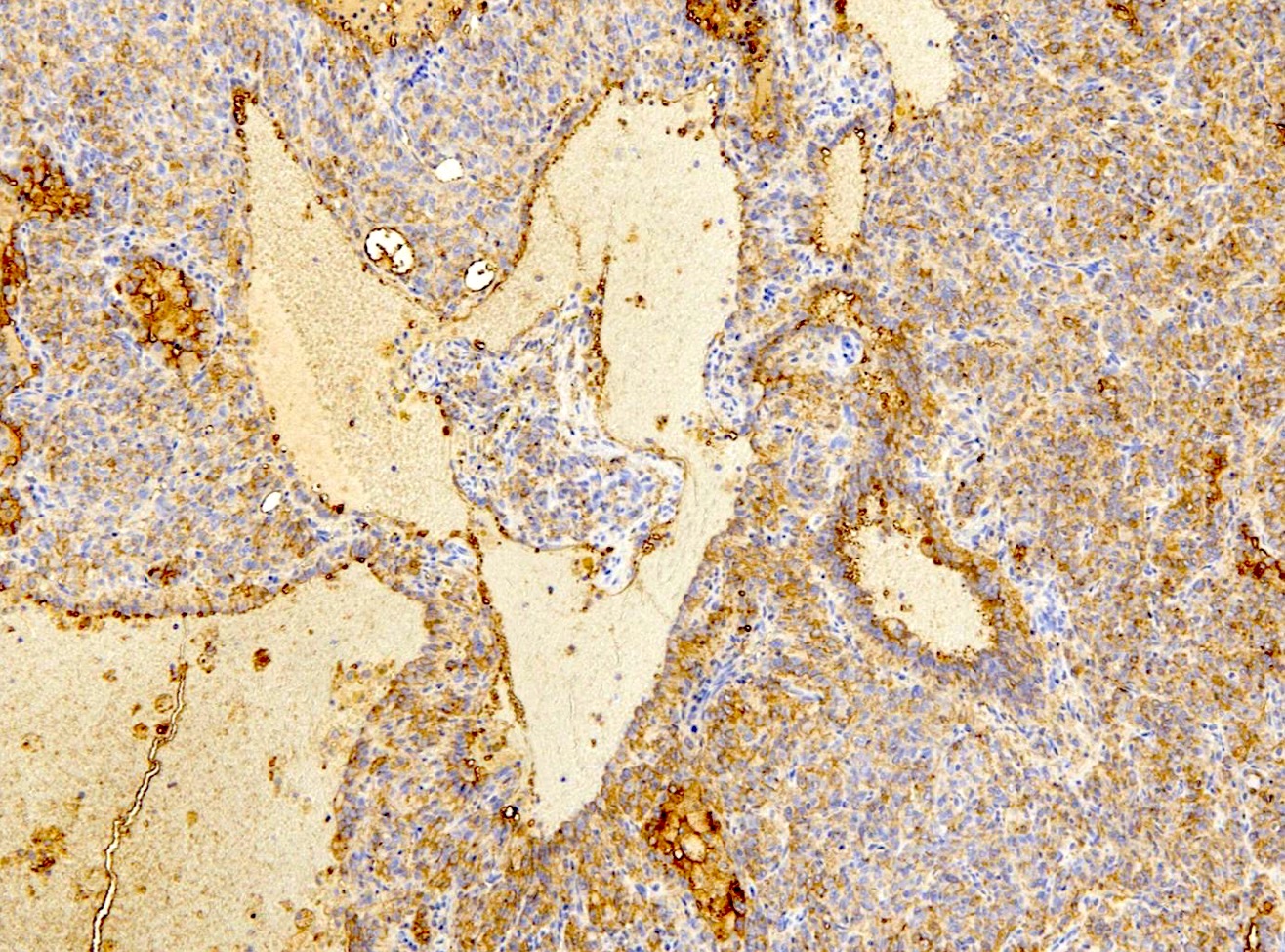
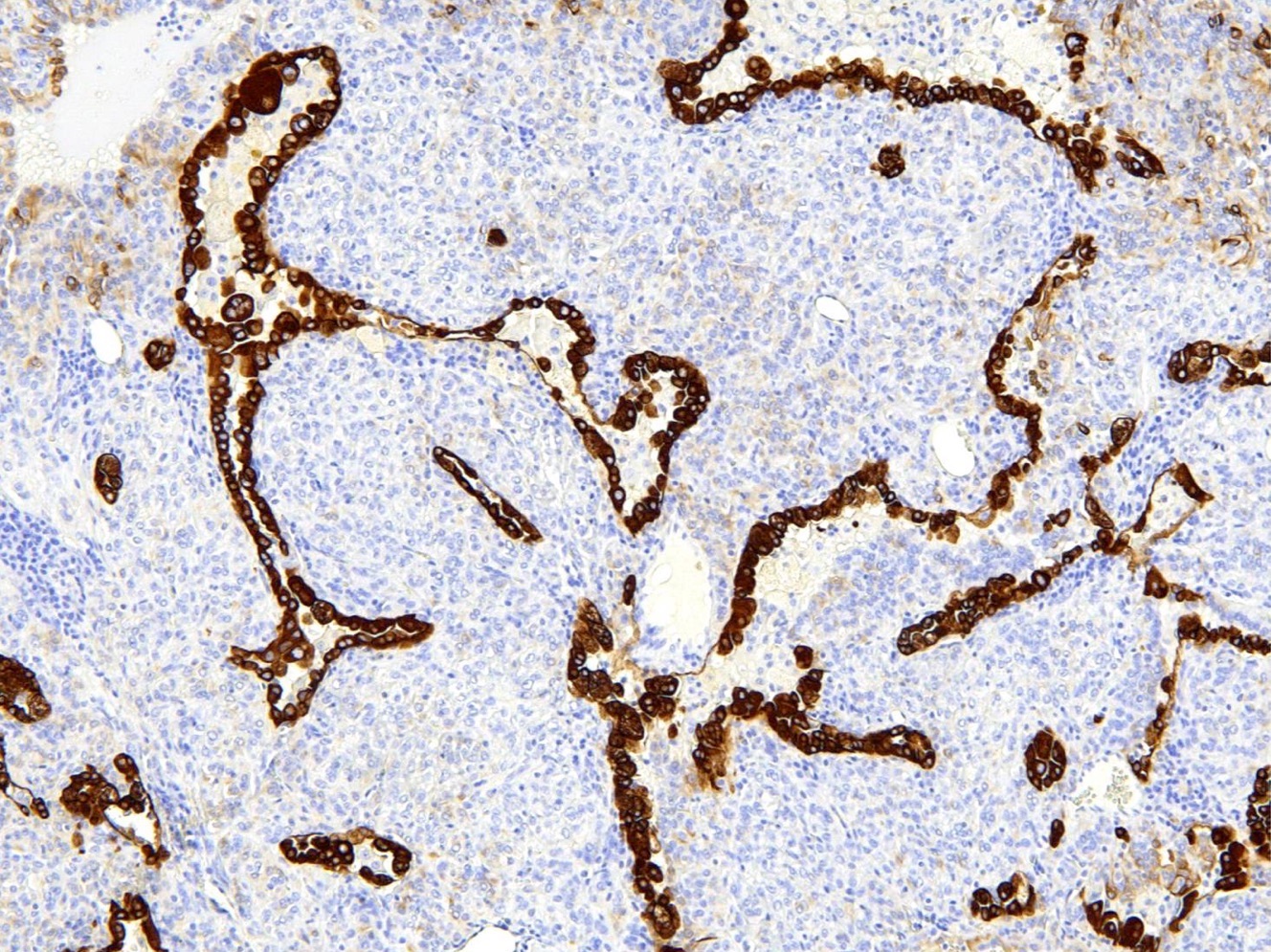
- EMA and TTF1: both surface and round cells stain strongly positive
- Pancytokeratin (AE1 / AE3), CAM5.2, CK7, Napsin A: stain surface cells diffusely but weakly or negative for round cells
- Reference: Am J Surg Pathol 2000;24:906
Negative stains
- Neuroendocrine markers: chromogranin A, synaptophysin
Molecular / cytogenetics description
- AKT1 mutations are the molecular hallmark of pulmonary sclerosing pneumocytoma (Mod Pathol 2020;33:391)
Videos
Sclerosing pneumocytoma in small biopsy
Sclerosing pneumocytoma in resection
Sample pathology report
- Right lung, lower lobe, wedge resection:
- Sclerosing pneumocytoma (see microscopic description)
- 1.5 cm in greatest dimension
- Tumor present 1.0 cm from nearest (parenchymal) margin
- 2 lymph nodes, negative for malignancy
- Sclerosing pneumocytoma (see microscopic description)
Differential diagnosis
- Adenocarcinoma (papillary):
- Malignant tumor with 1 (epithelial) cell population
- Neuroendocrine tumor (carcinoid tumor):
- Tumor with 1 cell population with expression of neuroendocrine markers by immunohistochemistry
Board review style question #1
What is the immunophenotype of the round cells in a sclerosing pneumocytoma?
- TTF1 negative, pancytokeratin negative
- TTF1 negative, pancytokeratin positive
- TTF1 positive, pancytokeratin negative
- TTF1 positive, pancytokeratin positive
Board review style answer #1
C. TTF1 positive, pancytokeratin negative. The round cells have a unique immunophenotype of TTF1 positive and cytokeratin negative, which is proposed to correspond to a primitive respiratory epithelium-like immunophenotype. Answer A is incorrect because the round cells have TTF1 expression.
Answer B is incorrect because the round cells are keratin negative and TTF1 positive. Answer D is incorrect because the round cells are keratin negative.
Comment Here
Reference: Sclerosing pneumocytoma
Comment Here
Reference: Sclerosing pneumocytoma
Board review style question #2
Board review style answer #2
A. Alveolar hemorrhage (hemosiderin deposition). These tumors are often associated with hemorrhage and the pigment typically relates to hemosiderin. This can be a clue to the diagnosis. Answer B is incorrect because cannabis pigment has a more coarse brown appearance than hemosiderin.
Answer C is incorrect because the smoking related pigment has a fine yellow-brown appearance with black specks.
Answer D is incorrect because melanin has a fine dusty appearance compared to hemosiderin. Melanin is not typically associated with sclerosing pneumocytoma.
Comment Here
Reference: Sclerosing pneumocytoma
Comment Here
Reference: Sclerosing pneumocytoma