Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Gagné A, Joubert P. Diffuse idiopathic pulmonary neuroendocrine hyperplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungtumordipnech.html. Accessed April 2nd, 2025.
Definition / general
- Generalized proliferation of pulmonary neuroendocrine cells that can be limited to the respiratory mucosa or invade locally to form tumorlets and carcinoid tumors
Essential features
- Diffuse idiopathic pulmonary neuroendocrine hyperplasia (DIPNECH) is considered a preinvasive lesion that may progress into tumorlets or carcinoid tumors (usually typical carcinoid)
- Histologic features comprise a generalized intramucosal proliferation of pulmonary neuroendocrine cells in monolayers or small groups that can penetrate through the bronchial basement membrane to form tumorlets
- Diagnostic criteria are still debated; the latest WHO edition separates diffuse idiopathic pulmonary neuroendocrine hyperplasia into a pathological diagnosis based on histologic features and a clinical diagnosis
- Some authors consider it a syndrome with a distinctive clinical presentation, typical radiological findings and a pathological demonstration
- Must be differentiated from secondary DIPNECH, which is a localized neuroendocrine proliferation secondary to another chronic lung disease
- Symptomatic patients present with insidious onset of a nonproductive chronic cough with dyspnea and wheezing that mimics asthma, chronic obstructive pulmonary disease or gastroesophageal reflux
ICD coding
Epidemiology
- Description of the disease based on a few case series (approximately 200 reported cases) (Hum Pathol 2015;46:176, Eur Respir J 2016;47:1829)
- Exact prevalence unknown, since it is a rare underrecognized disease
- Can be found in 5.4% of lung resections for typical or atypical carcinoid (Eur J Cardiothorac Surg 2004;26:165)
- F:M, > 10:1 (Eur Respir J 2016;47:1829, Am J Respir Crit Care Med 2011;184:8)
- Mean age: 58 years (Lung 2016;194:243)
- Can be found in patients with multiple endocrine neoplasia type 1 (MEN1) (Thorax 2007;62:248)
Sites
- Proliferation of neuroendocrine cells arise from terminal bronchioles
Pathophysiology
- Not well described
- Idiopathic by definition
- Secondary DIPNECH is caused by other chronic lung diseases
- Considered a preneoplastic proliferation as neuroendocrine cells can cross the basement membrane to form tumorlets and carcinoid tumors (Histopathology 2011;59:751)
- Clinical symptoms are thought to be caused by the production of peptides by the neuroendocrine cells, which leads to a peribronchiolar fibrosis and an obstructive respiratory defect (Chest 2015;147:415)
Etiology
- Unknown
- Mostly nonsmoker patients (Chest 2015;147:415, Clin Imaging 2015;39:243)
Clinical features
- Symptomatic patients present with insidious onset of a nonproductive chronic cough with dyspnea and wheezing that mimics asthma, chronic obstructive pulmonary disease or gastroesophageal reflux (Eur Respir J 2016;47:1829, Chest 2015;147:415, Am J Respir Crit Care Med 2011;184:8)
- Mean duration of symptoms before diagnosis: 10 years
- May be asymptomatic and an incidental finding during investigation for another disease (Lung 2016;194:243)
- Associated with tumorlets (67% of cases) and lung carcinoids, mostly typical carcinoids (40% of cases) (Am J Respir Crit Care Med 2011;184:8)
- Can also be found in association with lung adenocarcinoma (Pathol Res Pract 2013;209:578, J Med Case Rep 2008;2:21)
Diagnosis
- Lung function tests may be normal or show an obstructive or a mixed obstructive and restrictive respiratory defect (Lung 2016;194:243, Lung 2018;196:577)
- Diagnostic criteria are still debated
- WHO classification of tumors and some authors use only microscopic findings to define (Hum Pathol 2015;46:176)
- Some authors consider DIPNECH a syndrome with a distinctive clinical presentation, typical radiological findings and a pathological demonstration; Carr et al. proposed diagnostic criteria but a minimum set of findings to confirm diagnosis has yet to be established (N Engl J Med 1992;327:1285, Clin Radiol 2015;70:317, Am J Surg Pathol 2018;42:646, Chest 2015;147:415)
Laboratory
- Serum chromogranin A may be elevated (Chest 2015;147:415)
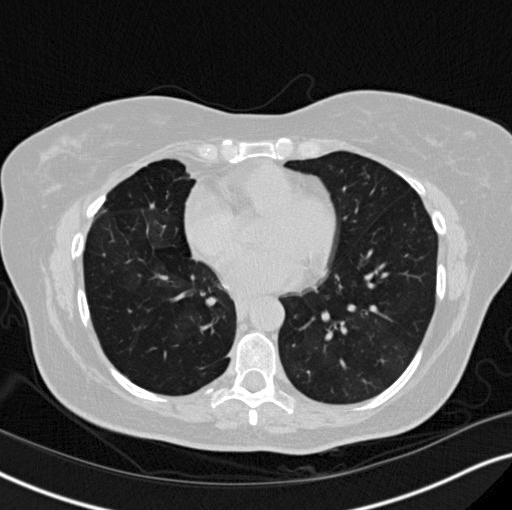
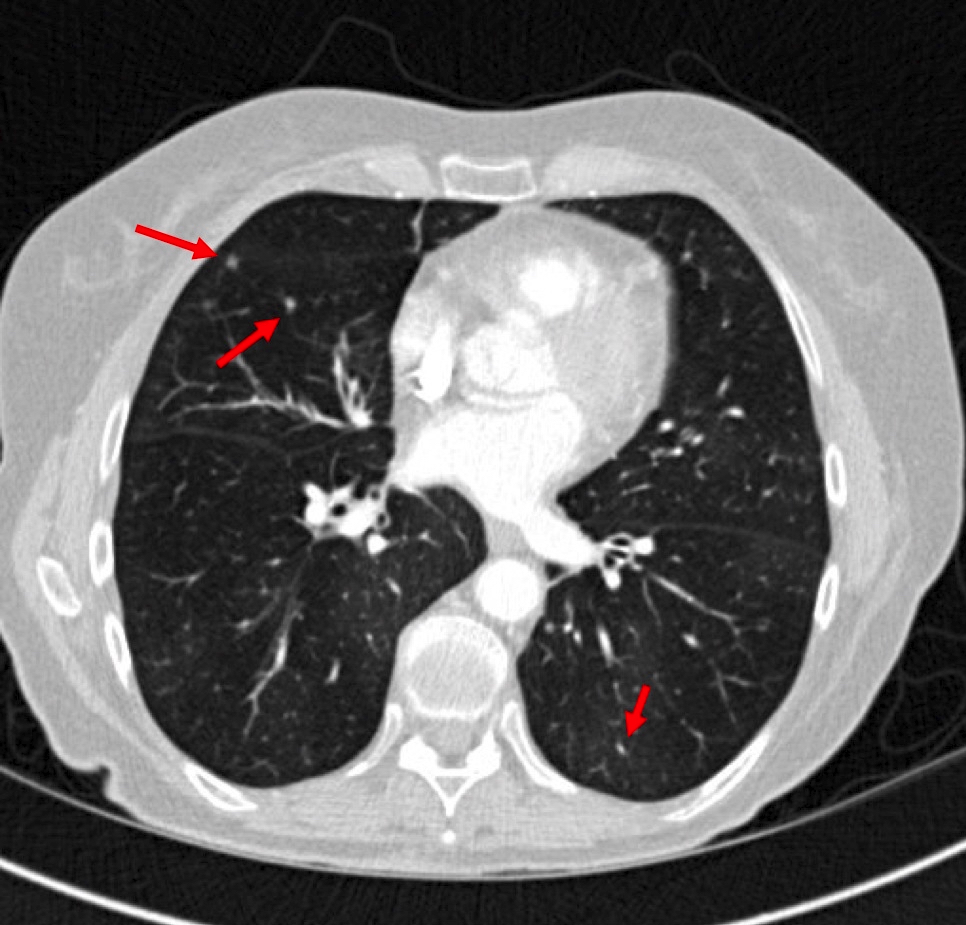
Radiology description
- CT scan:
- Bilateral mosaic perfusion with air trapping is the most important feature (Clin Radiol 2015;70:317, Clin Imaging 2015;39:243)
- Better evaluated on expiratory CT scan
- Secondary to a disturbed airflow caused by the proliferation of tumor cells within small airways and constrictive bronchiolitis
- Multiple bronchiolocentric parenchymal nodules of a solid or ground glass appearance (Clin Radiol 2015;70:317)
- Represents aggregates of neuroendocrine cells, tumorlets or carcinoid tumors
- Bilateral mosaic perfusion with air trapping is the most important feature (Clin Radiol 2015;70:317, Clin Imaging 2015;39:243)
Radiology images
Prognostic factors
- Poorly understood
- Prognosis is mostly linked to the progressive worsening of pulmonary function (Chest 2015;147:415)
- Progression can be rapid (within 2 years) or over a long period
- Presence of constrictive bronchiolitis on histology is not a prognostic factor
Case reports
- 39 year old woman with a history of chronic cough (BMJ Case Rep 2018;11:e226203)
- 55 year old woman with a coexisting thymoma and Crohn's disease (Ann Thorac Med 2019;14:161)
- Woman in her 60s with a history of unexplained dry cough with metastatic carcinoid tumors to the liver has radiological findings compatible with diffuse idiopathic pulmonary neuroendocrine hyperplasia (BMJ Case Rep 2019;12:e228536)
- 77 year old woman with an atypical carcinoid (J Thorac Dis 2017;9:E774)
- 4 patients with DIPNECH (Medicine (Baltimore) 2018;97:e13806)
Treatment
- Guidelines for treatment do not exist and no treatment has been shown to be superior (Lung 2015;193:659)
- Patients have been treated with somatostatin analogs / octreotide, sirolimus, everolimus, corticosteroids and transplantation (Lung 2018;196:577, Ann Intern Med 2018;169:197, Lung 2015;193:659)
Gross description
- Not visible macroscopically
Frozen section description
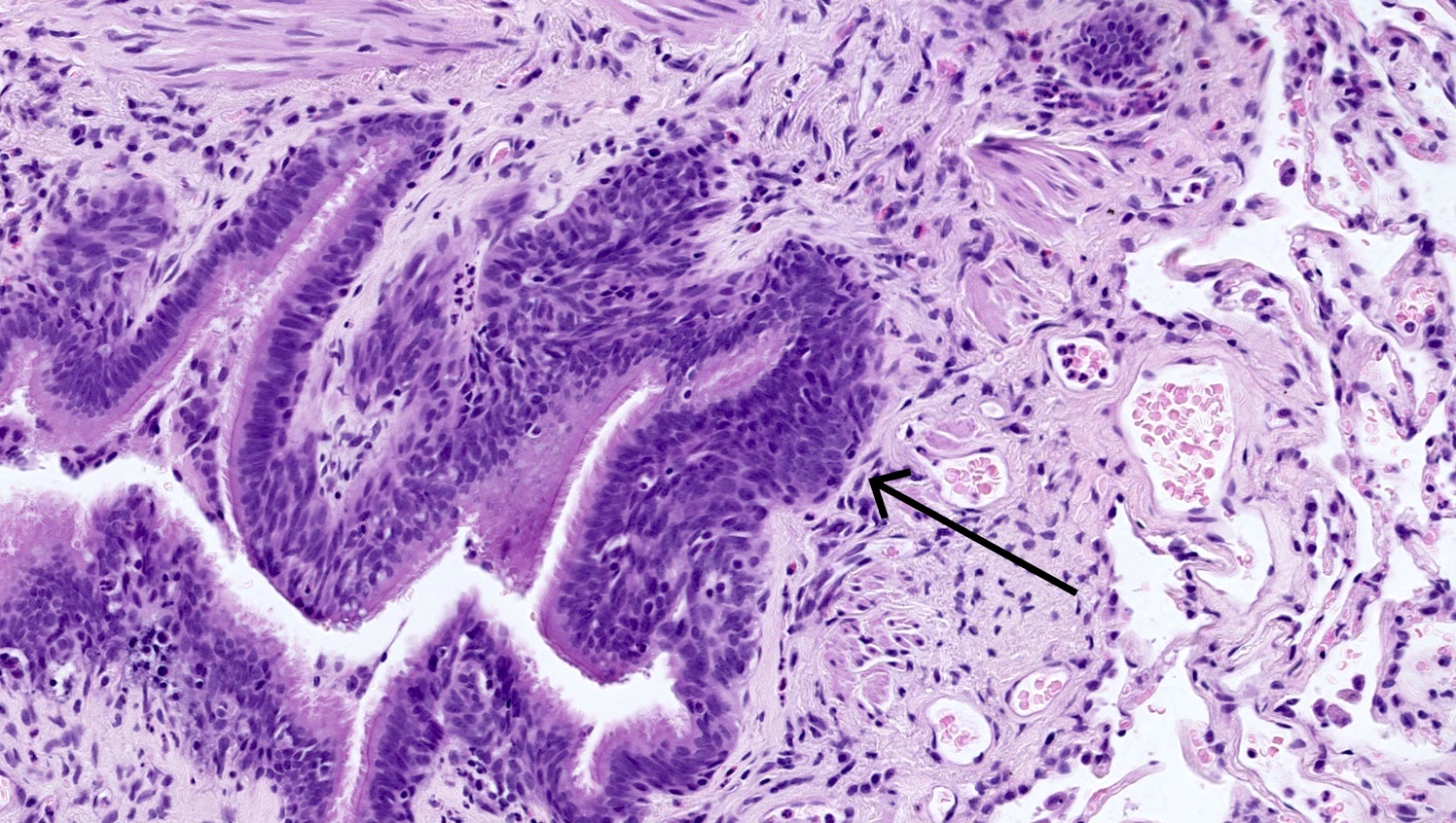
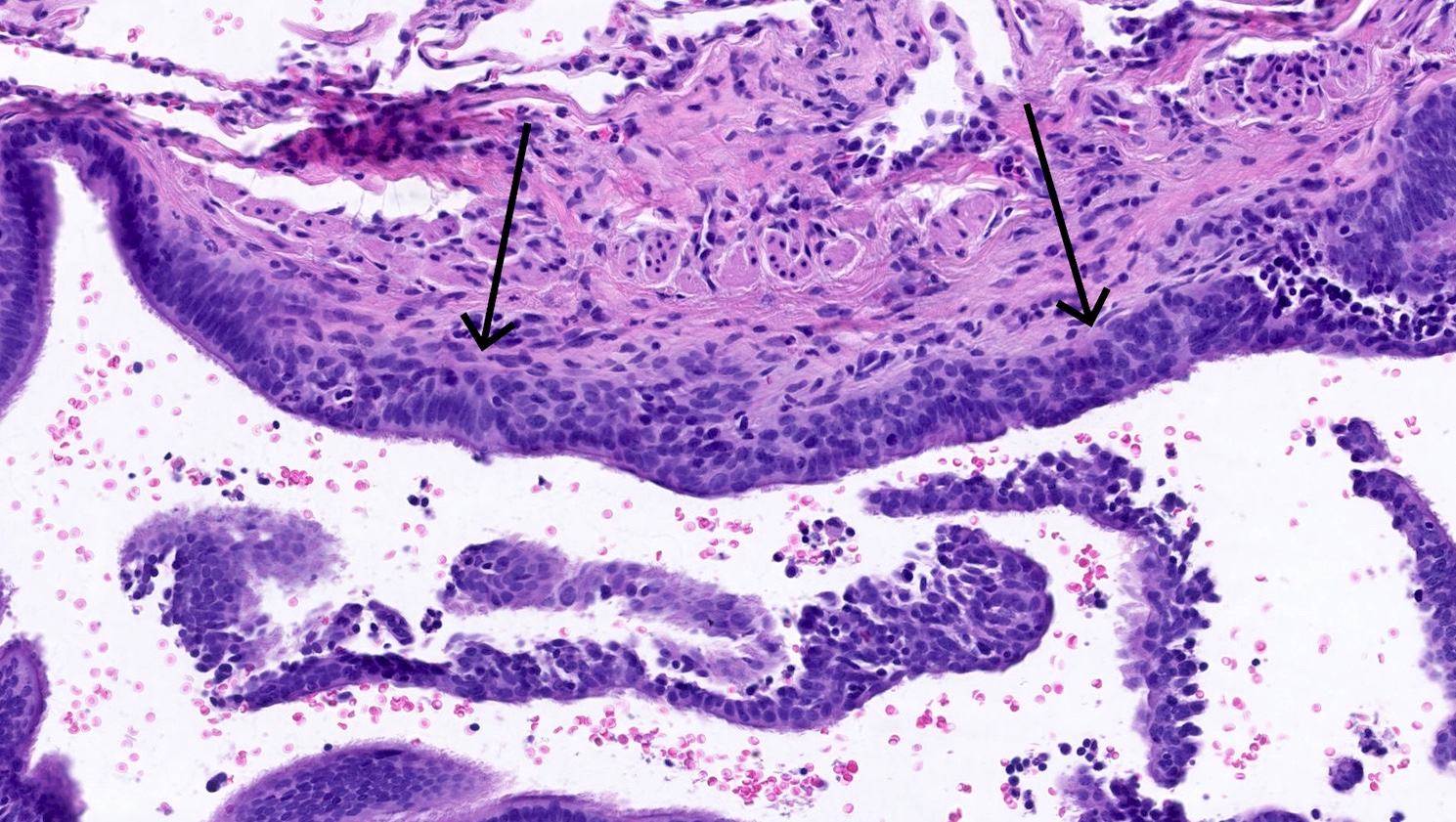
Microscopic (histologic) description
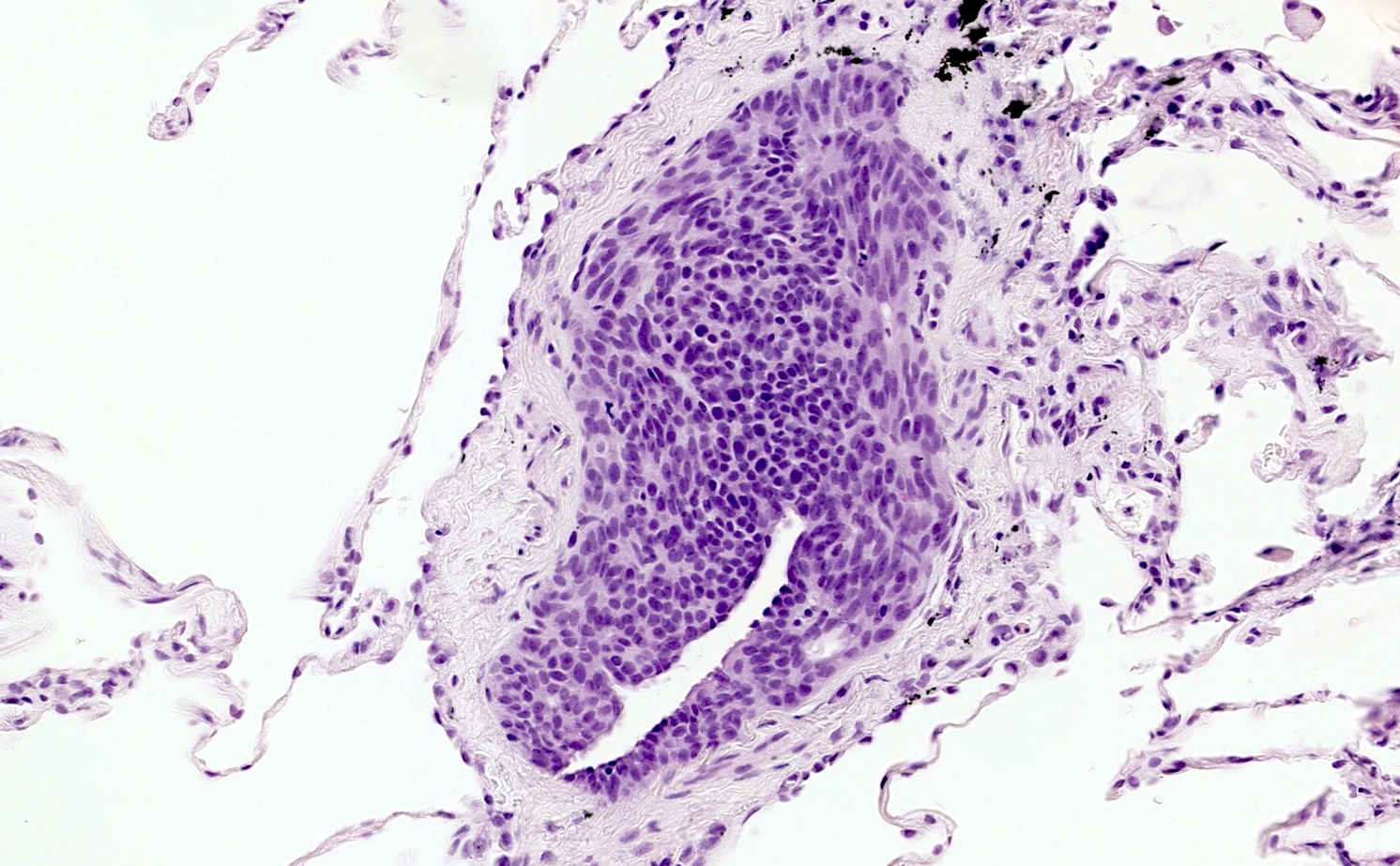
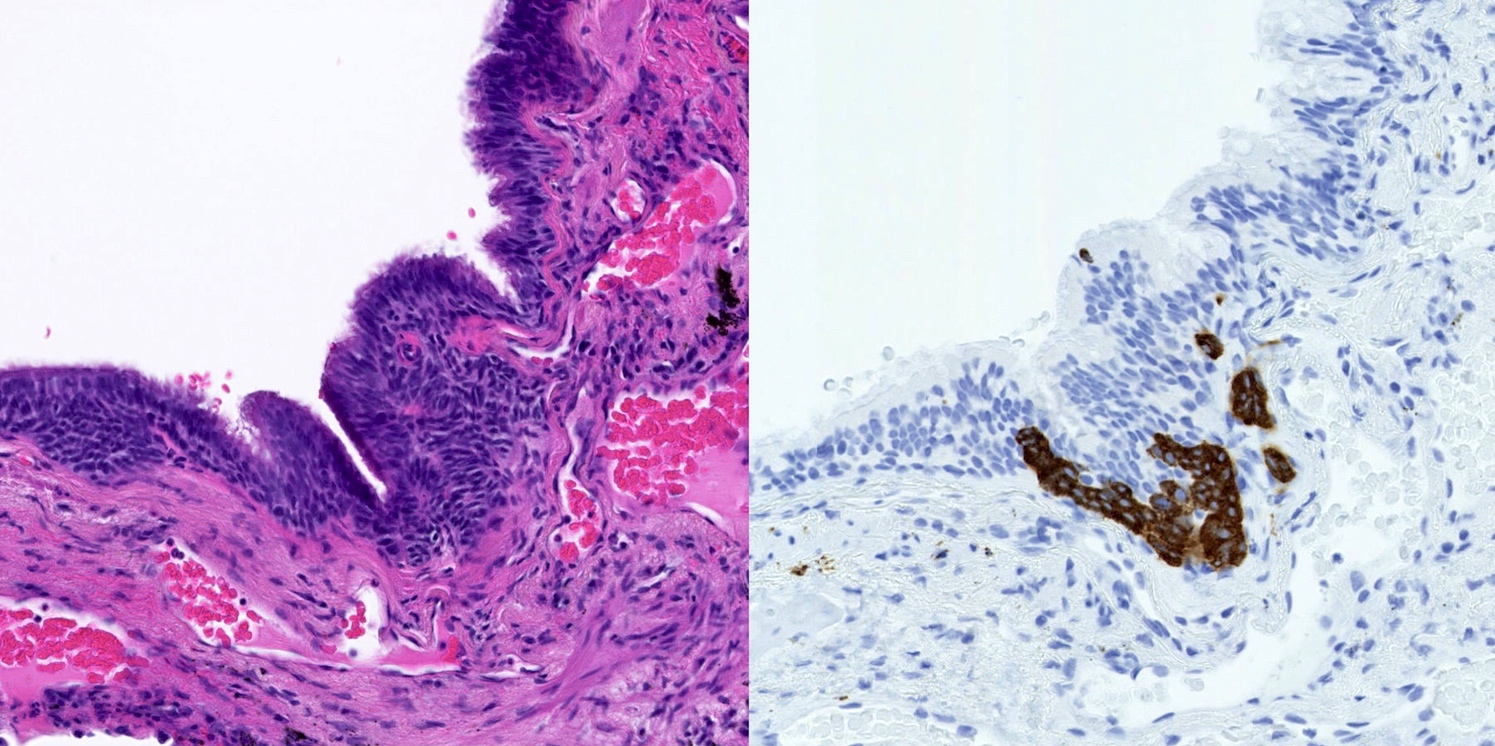
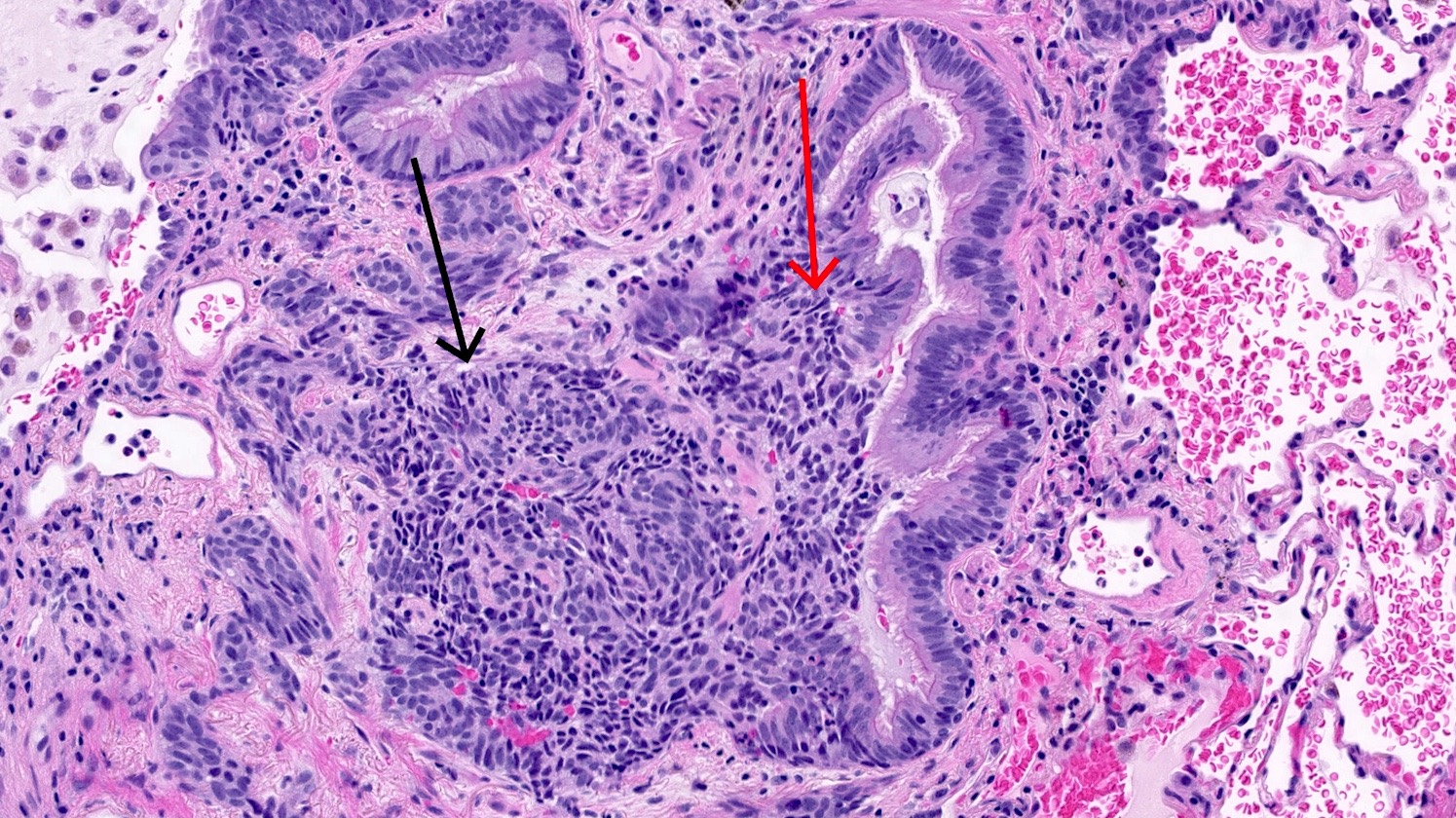
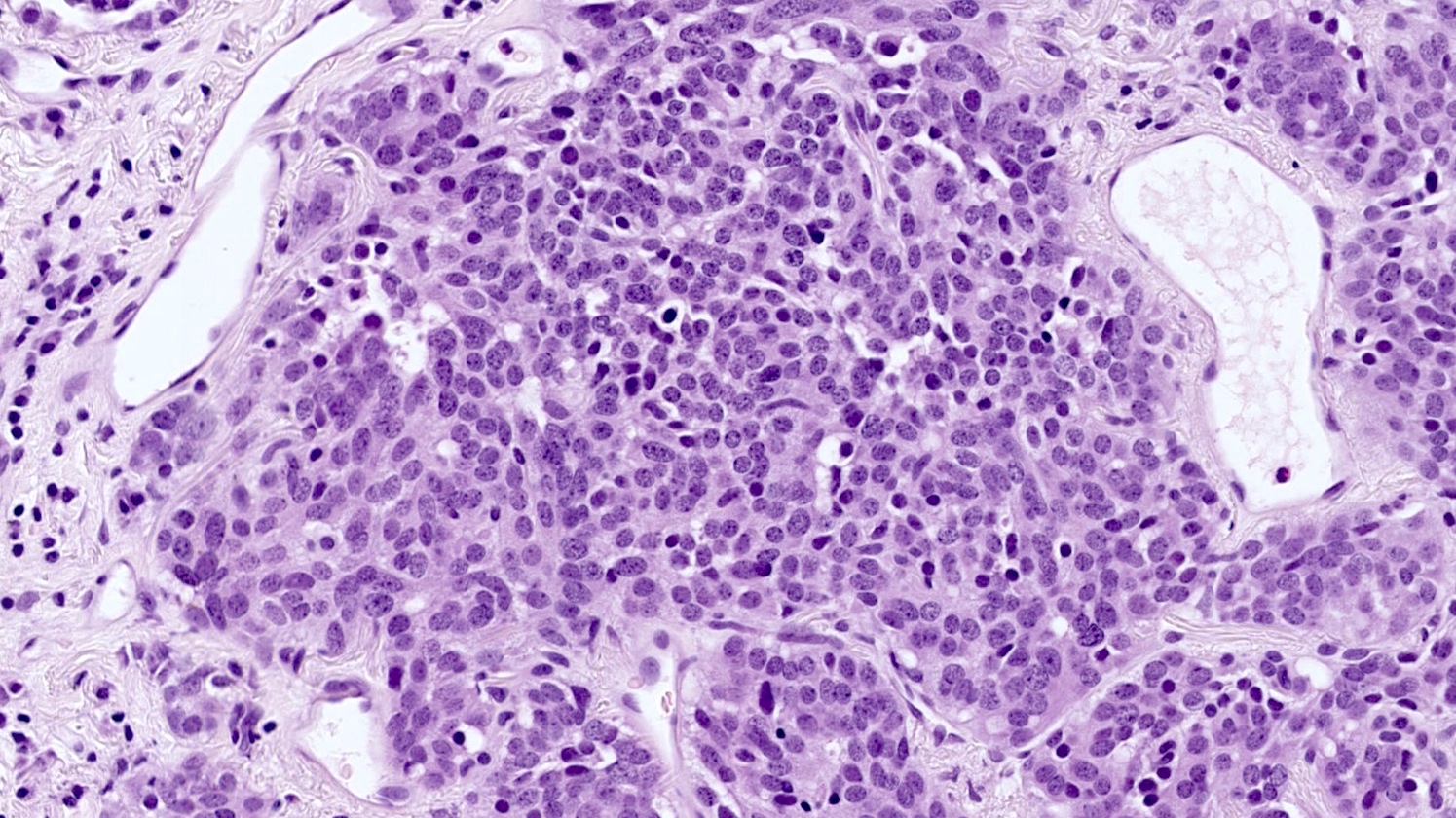
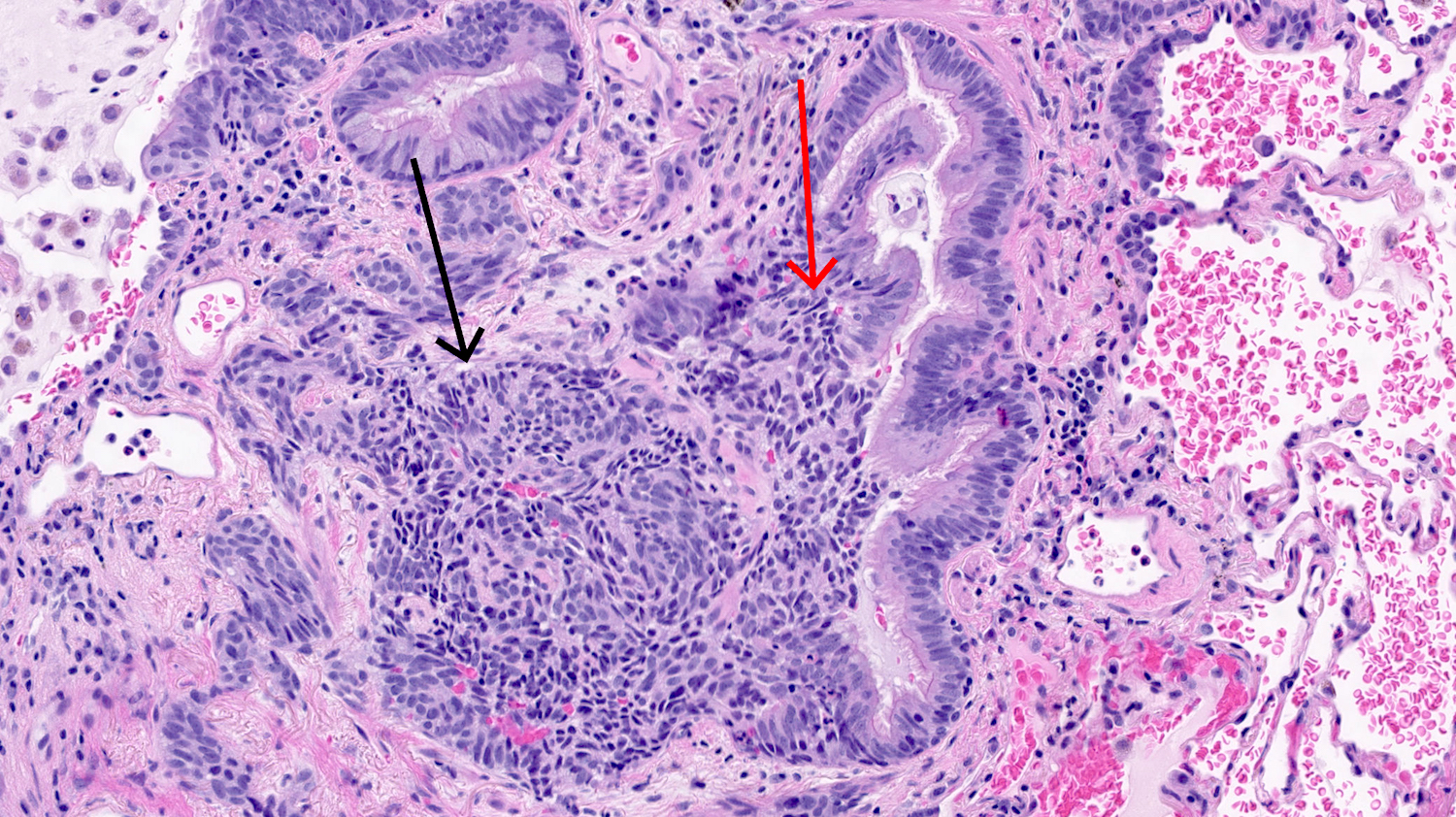
- Generalized intramucosal proliferation of pulmonary neuroendocrine cells forming monolayers or small groups that can protrude into bronchial lumen
- Cells do not cross the mucosal basal lamina
- Cells are round, oval or spindle shaped, have a moderate amount of eosinophilic cytoplasm and have round to oval nuclei with a salt and pepper chromatin
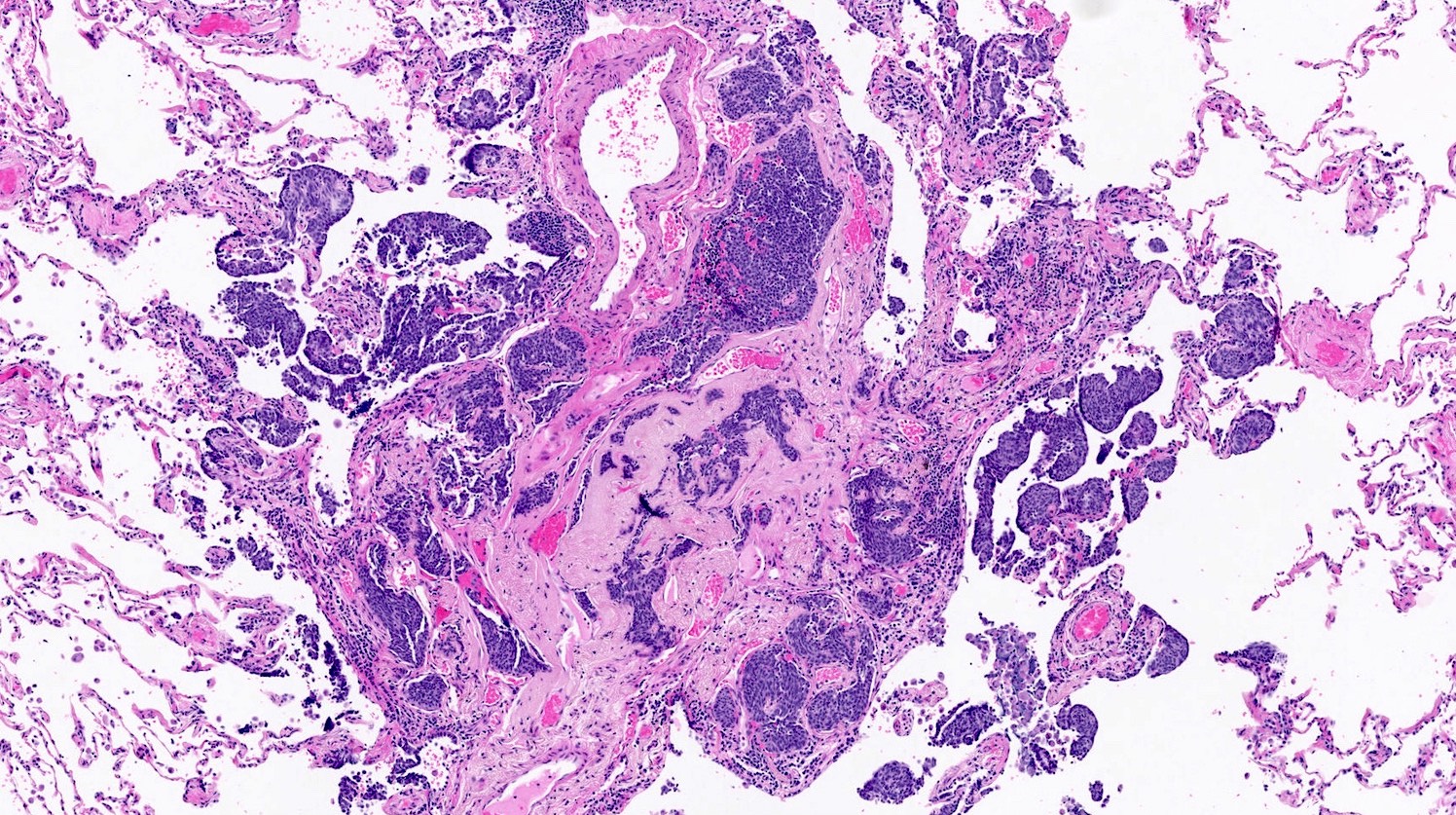
- If neuroendocrine cells cross the mucosal basal lamina = tumorlet
- Poorly defined nodules with infiltrative margins in a fibrotic stroma, usually found in relation to an airway
- Size < 5 mm with < 2 mitoses/2 mm² and absence of necrosis
- A minimum number of foci of proliferating neuroendocrine cells and tumorlets has been proposed but no consensus exists (Hum Pathol 2015;46:176):
- Presence of ≥ 5 neuroendocrine cells distributed in a linear fashion or in clusters within the basement membrane in ≥ 3 bronchioles
- Combined with an association to ≥ 3 tumorlets
- Must be differentiated from secondary DIPNECH, which is a localized neuroendocrine proliferation associated to an underlying lung pathology
- Can be associated with constrictive bronchiolitis (44% of patients) (Chest 2015;147:415)
Microscopic (histologic) images
Positive stains
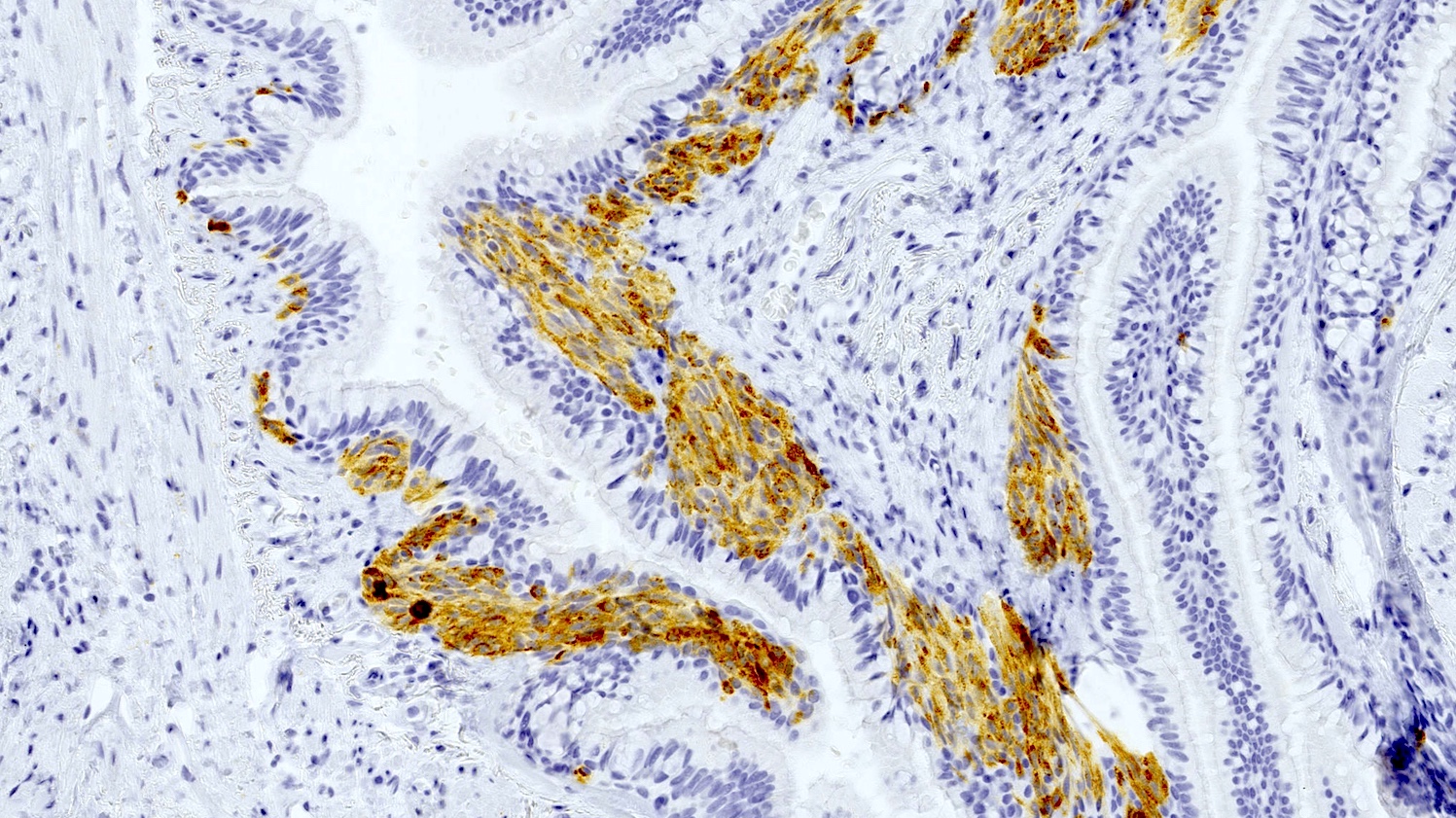
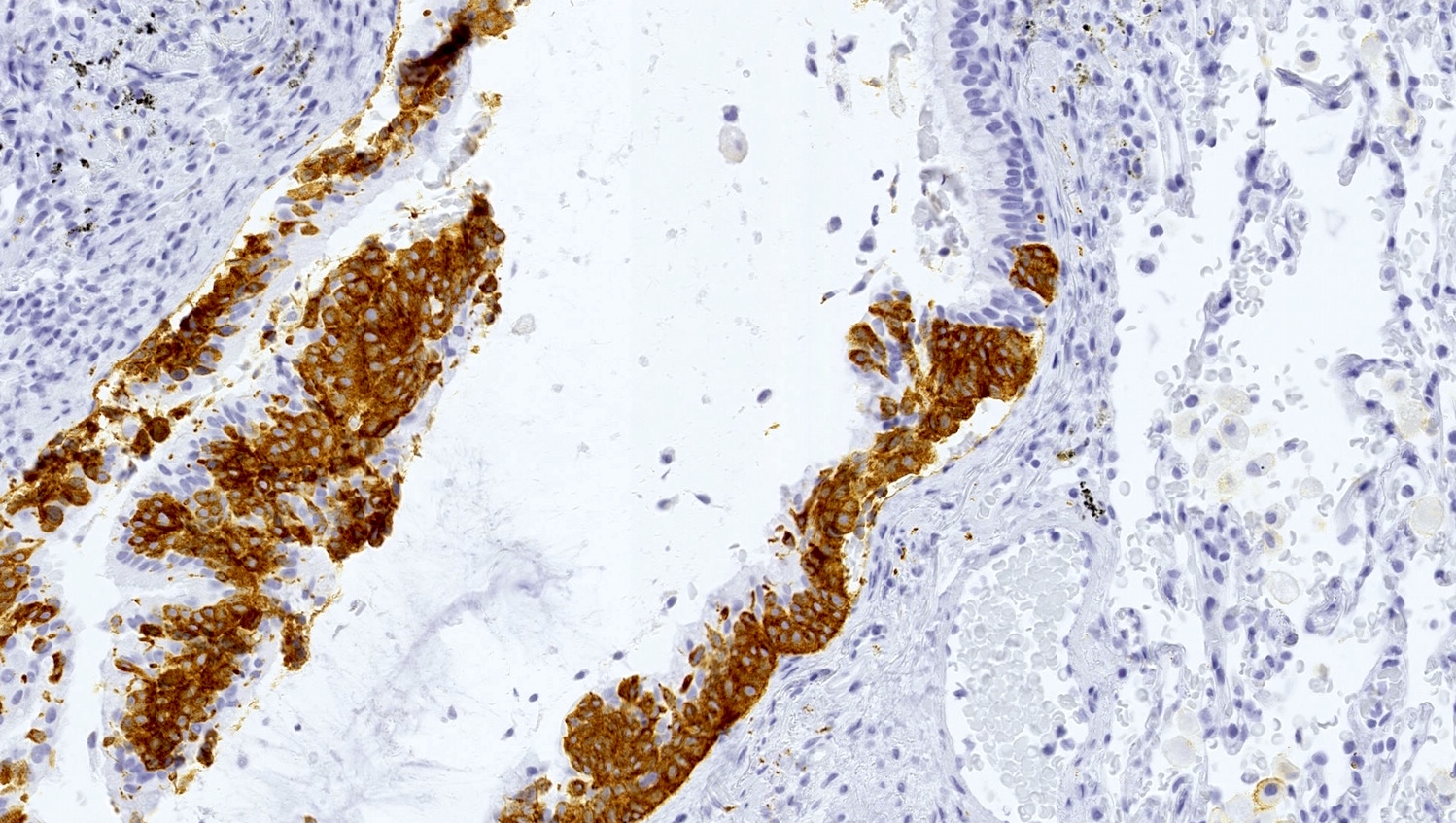
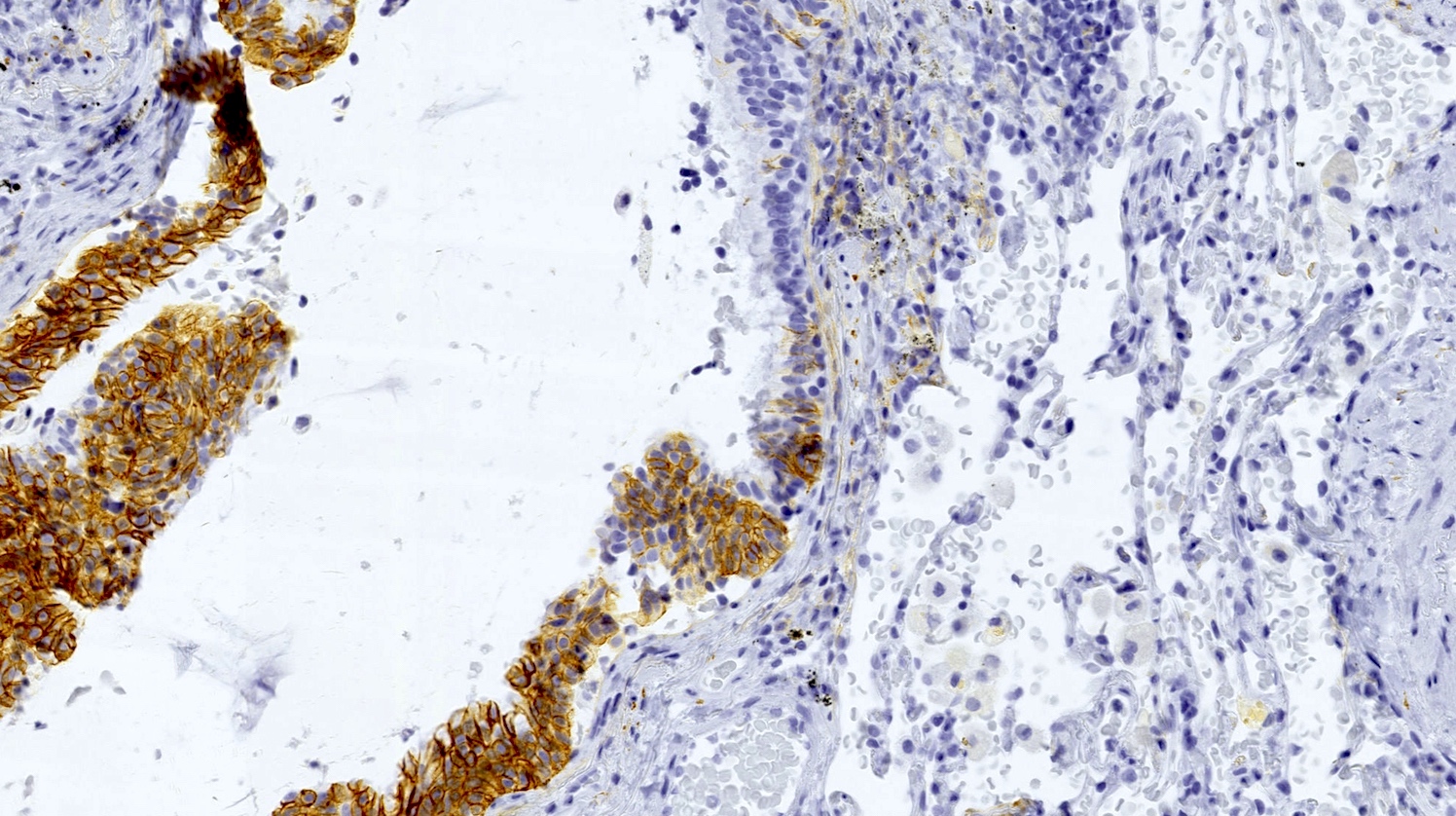
- Neuroendocrine markers (chromogranin A, synaptophysin, CD56) can be helpful to identify foci of hyperplasia (Histopathology 2011;59:751, Am J Surg Pathol 2018;42:646)
Molecular / cytogenetics description
- No specific molecular alteration described (Histopathology 2018;72:142)
- Screening of targetable genes like EGFR, KRAS, c-KIT, PDGFRs, c-MET and ALK did not show any alterations (Am J Surg Pathol 2018;42:646)
Sample pathology report
- Lung, left upper lobe, wedge resection:
- Multiple foci of neuroendocrine proliferation (neuroendocrine hyperplasia and neuroendocrine tumorlet) (see comment)
- Comment: The presence of foci of neuroendocrine proliferation raises the possibility of a diffuse idiopathic pulmonary neuroendocrine cells hyperplasia (DIPNECH). Correlation with clinical symptoms, pulmonary function tests and high resolution chest CT scan with an expiratory phase is recommended to confirm this diagnosis.
Differential diagnosis
- Meningothelial nodules:
- Perivascular localization instead of bronchiolar proliferation
- Neuroendocrine markers are negative but CD56 is positive (Am J Surg Pathol 2009;33:487)
Additional references
Board review style question #1
A 55 year old woman with a long history of chronic cough and asthma undergoes an upper right lobectomy for a lung nodule. The final diagnosis is a typical carcinoid tumor. The adjacent lung parenchyma presents numerous lesions diffusely throughout the lobectomy as depicted in the image. Concerning this diagnosis, which of the following is true?
- Diagnostic criteria are well defined and combine histological, radiological and clinical findings
- EGFR mutations are frequently found
- Constrictive bronchiolitis can be found
- Most patients are men
Board review style answer #1
C. Constrictive bronchiolitis can be found. The diagnosis here is diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH). Diagnostic criteria for this entity are still debated (A). The WHO classification defines diffuse idiopathic pulmonary neuroendocrine cell hyperplasia with histologic features and some authors have proposed precise diagnostic criteria. However, other groups consider it a syndrome with a distinctive clinical presentation, typical radiological findings and a pathological demonstration. No specific molecular alterations have been described in diffuse idiopathic pulmonary neuroendocrine cell hyperplasia lesions (B). Mengoli et al. (Am J Surg Pathol 2018;42:646) tested a panel of targetable genes and have not found any specific molecular alterations. Concerning histologic findings, constrictive bronchiolitis can be found and is thought to be secondary to the production of peptides by the neuroendocrine cells (C). Finally, an overwhelming majority of patients with diffuse idiopathic pulmonary neuroendocrine cell hyperplasia are women (F:M, > 10:1) (D).
Comment Here
Reference: Diffuse idiopathic pulmonary neuroendocrine hyperplasia
Comment Here
Reference: Diffuse idiopathic pulmonary neuroendocrine hyperplasia
Board review style question #2
Regarding the histologic features of diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH), which of the following is true?
- There is a generalized intramucosal proliferation of pulmonary neuroendocrine cells
- There is a localized intramucosal proliferation of pulmonary neuroendocrine cells around bronchiectasis
- Tumorlets can have a size of > 5 mm
- A tumorlet is a nodule of neuroendocrine cells that is limited to the bronchial mucosa
Board review style answer #2
A. There is a generalized intramucosal proliferation of pulmonary neuroendocrine cells. A generalized intramucosal proliferation of pulmonary neuroendocrine cells (A) is found in diffuse idiopathic pulmonary neuroendocrine cell hyperplasia cases. It must be differentiated from localized pulmonary neuroendocrine cell proliferations that can be found associated to chronic lung diseases (B). Tumorlets are, by definition, < 5 mm (C) and cross the basal membrane of the mucosa (D).
Comment Here
Reference: Diffuse idiopathic pulmonary neuroendocrine hyperplasia
Comment Here
Reference: Diffuse idiopathic pulmonary neuroendocrine hyperplasia