Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Lin S, Cecchini MJ. Atypical adenomatous hyperplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungtumorbronchAAH.html. Accessed March 31st, 2025.
Definition / general
- Atypical adenomatous hyperplasia (AAH) is a small, localized proliferation of atypical pneumocytes (usually ≤ 5 mm) that line intact alveolar spaces
- AAH is recognized as a precursor lesion to invasive adenocarcinoma (Cancer Metastasis Rev 2010;29:15)
Essential features
- Small, localized lesion (usually ≤ 5 mm)
- Discrete from alveolar parenchyma with proliferation of atypical pneumocytes
- Forms a discontinuous monolayer of cells
- Surrounding parenchyma should be devoid of substantial inflammation or fibrosis
Terminology
- Obsolete terms (not recommended): atypical alveolar epithelial hyperplasia, atypical bronchoalveolar cell hyperplasia, atypical alveolar cell hyperplasia, bronchoalveolar cell adenoma, bronchial adenoma
ICD coding
- ICD-O: 8250/0 - atypical adenomatous hyperplasia
- ICD-11: 2F0Z & XH5QL3 - benign neoplasms of unspecified respiratory and intrathoracic organs & atypical adenomatous hyperplasia
Epidemiology
- Commonly seen in lung resections as an incidental finding
- In some series, AAH has been reported in ≤ 30.2% of female patients undergoing a resection for adenocarcinoma (Br J Cancer 2000;83:632)
- In autopsy series, AAH has been reported in 2 - 4% of cases without cancer (Mod Pathol 1997;10:469, Lung Cancer 2000;29:125)
- F > M (Br J Cancer 2000;83:632)
- Associated CYP19A1 polymorphisms have been reported (Carcinogenesis 2010;31:1794)
Sites
- More common in the upper lobes (Thorax 2001;56:302)
Pathophysiology
- Conceptualized as the earliest morphologically recognizable lesion in the proposed stepwise progression of lung adenocarcinoma (Int J Mol Sci 2018;19:1259)
Etiology
- Unknown; some series suggest that AAH is not correlated with smoking (Thorax 2001;56:302)
Clinical features
- AAH is usually undetectable by imaging techniques and is typically found incidentally in surgical pathology specimens
- Larger lesions may be present on high resolution CT imaging (Eur Radiol 2001;11:811)
Diagnosis
- Typically diagnosed in lung resection specimens (often incidentally)
- Usually not diagnosed on small core needle or transbronchial biopsies since the lesion should be examined in its entirety
Laboratory
- Not typically assessed in AAH
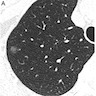
Radiology description
- Present as faint, not solid, nodules on CT scans (J Thorac Dis 2018;10:S790)
Prognostic factors
- Patients are cured upon resection
Case reports
- 12 year old girl presenting with incidental AAH upon bulla surgical resection (Korean J Thorac Cardiovasc Surg 2016;49:141)
- 50 year old woman with 3 ground glass nodules showing coexistence of atypical adenomatous hyperplasia, minimally invasive adenocarcinoma and invasive adenocarcinoma (Thorac Cancer 2021;12:693)
- 73 year old woman with AAH nodule progressing to semisolid lesion (Cureus 2019;11:e6079)
Treatment
- CT surveillance; frequency and duration dependent on size of nodule (JAMA 2022;327:264)
- Patients are cured with resection
Gross description
- Gray, tan-yellow, ill defined area
- Can be multifocal
- Localization: peripheral lung parenchyma, near pleura
- Size: measures ≤ 5 mm
Frozen section description
- Diagnosis is not typically made on frozen section, given the subtle changes associated with this lesion
- Some cases with subtle atypia on frozen section, may turn out to be classified as AAH based on permanent sections
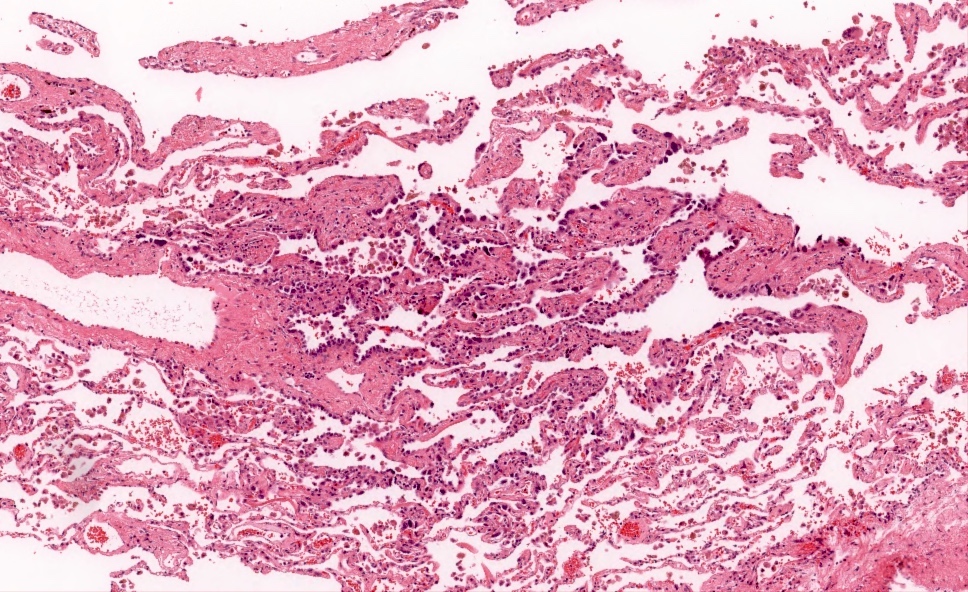
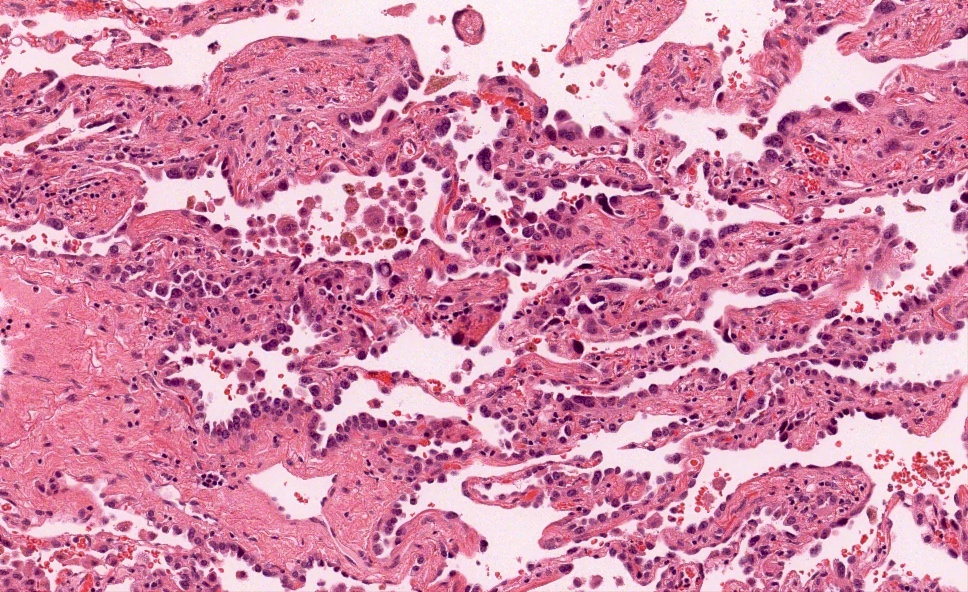
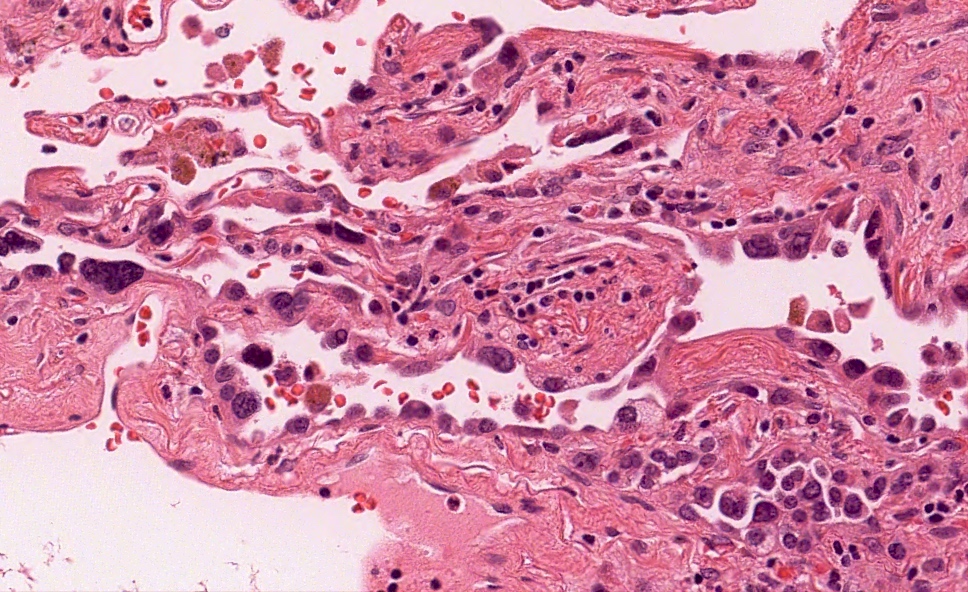
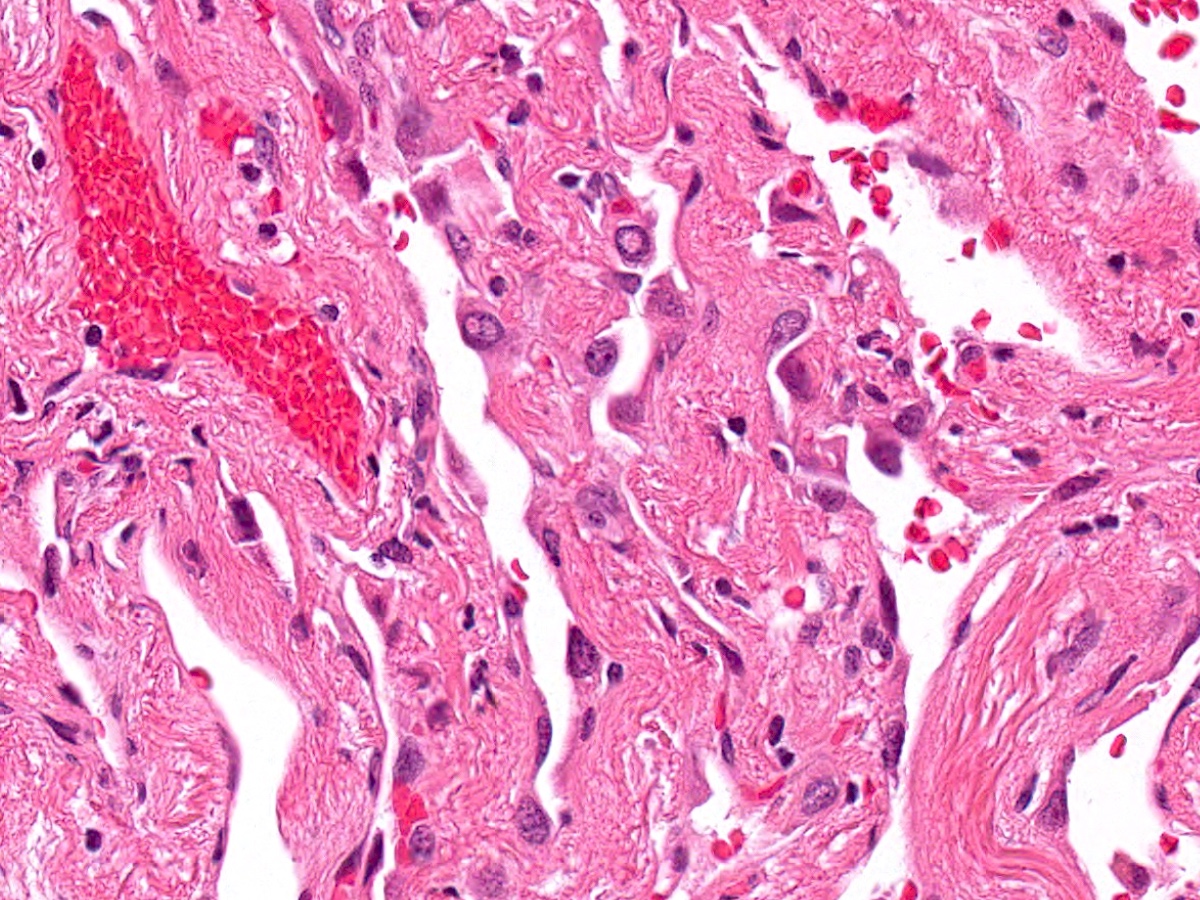
Microscopic (histologic) description
- Proliferation of atypical pneumocytes
- Mild to moderate atypia with increased cell size and nuclear to cytoplasmic ratio; nuclei are hyperchromatic and can have intranuclear eosinophilic inclusions
- Cells are present along intact alveolar spaces
- Atypical cells form a discontinuous monolayer of cells
- Mitotic activity is typically minimal
- Lesion is composed of a mixture of club cells and type II pneumocytes
- Club cells can be recognized as columnar cells with eosinophilic and cytoplasmic snouts
- Type II pneumocytes are cuboidal with finely vacuolated to clear cytoplasm
- Absence of significant parenchymal inflammation or fibrosis
Microscopic (histologic) images
Positive stains
- Positive for TTF1 but is not commonly required or recommended (Virchows Arch 1997;431:415)
Molecular / cytogenetics description
- Lesional cells have been shown to be clonal in nature
- Degree of copy number change and mutations has been shown in some studies to be less than in adenocarcinoma in situ (Nat Commun 2019;10:2978)
- AAH has been found to have driver mutations such as KRAS, EGFR and BRAF (J Thorac Oncol 2018;13:1776)
- Given that these lesions are cured with resection, molecular testing is not typically indicated in most clinical settings
Sample pathology report
- Right lung, middle lobe, wedge resection:
- Atypical adenomatous hyperplasia (AAH) (see comment)
- 3 mm in greatest dimension
- Comment: Atypical adenomatous hyperplasia (AAH) is a preinvasive lesion in the adenocarcinoma spectrum and is regarded as a precursor to adenocarcinoma in situ. No invasive or in situ malignancy is present.
Differential diagnosis
- Nonmucinous (adenocarcinoma in situ):
- Adenocarcinoma in situ (AIS) is typically > 5 mm
- More cellular cuboidal / columnar cells forming a continuous monolayer
- AIS typically has a sharper (abrupt) cutoff with the background lung parenchyma
- Distinguishing between AIS and AAH can be challenging in some cases since there is overlap in morphology; however, this is of limited clinical relevance in most cases as both lesions are cured by resection
- Reactive pneumocyte hyperplasia:
- In reactive hyperplasia, the atypical pneumocytes tend to be more diffuse and do not form well defined nodular areas
- There is often a background of inflammation, fibrosis or features of acute lung injury seen admixed with the atypical cells
Board review style question #1
Which of the following histologic features seen in the image above, best supports the classification of this sample as atypical adenomatous hyperplasia (AAH) over adenocarcinoma in situ (AIS)?
- Absence of significant inflammation
- Architectural changes
- Discontinuous nature of the atypical cells
- Loss of polarity
- Mild to moderate atypia
Board review style answer #1
C. Discontinuous nature of the atypical cells. Discontinuous atypical cells are most often seen in small atypical pneumocyte proliferations that are best classified as AAH. Architectural changes are not typically seen in AAH or AIS. Absence of significant inflammation can be seen in AAH and AIS. Mild to moderate atypia and loss of polarity can be seen in both AAH and AIS.
Comment Here
Reference: Atypical adenomatous hyperplasia
Comment Here
Reference: Atypical adenomatous hyperplasia
Board review style question #2
How is atypical adenomatous hyperplasia typically identified / detected?
- After workup for hemoptysis
- As an incidental solid lesion on CT scan
- Based on PET scan
- Incidental finding in resected lung tissue
Board review style answer #2
D. Incidental finding in resected lung tissue. Atypical adenomatous hyperplasia is typically identified as an incidental finding in resected lung tissue. It is not easily appreciated based on imaging studies and patients are normally asymptomatic.
Comment Here
Reference: Atypical adenomatous hyperplasia
Comment Here
Reference: Atypical adenomatous hyperplasia