Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Kruse E, Husain AN. Pleuropulmonary blastoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungtumorPPB.html. Accessed April 2nd, 2025.
Definition / general
- Pleuropulmonary blastoma (PPB) is a rare malignant tumor of mesenchymal origin that arises in the lungs, pleura or mediastinum of children (J Clin Med 2023;12:1918)
- International registry was established in 1987 (The International Pleuropulmonary Blastoma / DICER1 Registry [Accessed 4 June 2024])
Essential features
- There are 4 morphologic subtypes of PPB: I, IR, II and III
- Type I: cystic tumor lined by benign nonneoplastic epithelial cells with a varying amount of malignant mesenchymal cells in the wall (no solid component) (Cancer 2023;129:600)
- Type IR (R stands for regressed or regressing): similar to the cystic type I tumor but the population of malignant mesenchymal cells is not found; only benign nonneoplastic epithelium forming cysts with some immature mesenchyme in the wall (Cancer 2023;129:600)
- Type II: combination of multicystic tumor and solid sarcomatous tumor (Children (Basel) 2019;6:86)
- Type III: entirely solid sarcomatous tumor without cystic foci; can be necrotic or calcified (Children (Basel) 2019;6:86)
Terminology
- Older terms not currently used (StatPearls: Pleuropulmonary Blastoma [Accessed 4 June 2024])
- Pediatric pulmonary blastoma
- Pneumoblastoma
- Pulmonary rhabdomyosarcoma
- Rhabdomyosarcoma in lung cyst
ICD coding
- ICD-10
- C34.1 - malignant neoplasm of upper lobe, bronchus or lung
- C34.2 - malignant neoplasm of middle lobe, bronchus or lung
- C34.3 - malignant neoplasm of lower lobe, bronchus or lung
- C34.8 - malignant neoplasm of overlapping sites of bronchus and lung
- C34.9 - malignant neoplasm of unspecified part of bronchus or lung
- ICD-11: 2C25.Y & XH2FY9 - other specified malignant neoplasms of bronchus or lung & pleuropulmonary blastoma
Epidemiology
- Primarily occurs in children under 4; median age of diagnosis differs with morphologic classification
- Type I: median 7 months (Cancer 2023;129:600)
- Type IR: median 31.2 months (Cancer 2023;129:600)
- Type II: median 35 months (Children (Basel) 2019;6:86)
- Type III: median 41 months (Children (Basel) 2019;6:86)
Sites
- Primarily arises from peripheral lung and pleura
- Metastasis is more common in type II and III PPB (Children (Basel) 2019;6:86)
- Common site for metastasis: brain (most common), liver, bone, lymph nodes, kidney, pancreas and adrenal glands (Children (Basel) 2019;6:86)
Pathophysiology
- Proliferation of malignant mesenchymal cells with nonneoplastic epithelial components (Clin Cancer Res 2018;24:2251)
- Evidence shows that PPB type I can progress to type II and then to type III but does not have to (Clin Cancer Res 2018;24:2251, Cancer 2015;121:276)
Etiology
- Germline loss of function mutations of DICER1 have been found in familial PPB and somatic mutations have been identified in others (Mod Pathol 2022;35:4)
- DICER1 is involved in microRNA production, which helps regulate gene expression
- With a DICER1 mutation, the dicer protein is unable to help with microRNA production, leading to abnormal gene expression (Mod Pathol 2022;35:4)
- Currently estimated that 70% of patients with PPB have a DICER1 mutation (Mod Pathol 2022;35:4)
Clinical features
- Often nonspecific cough, chest pain, dyspnea or abdominal pain (J Clin Med 2023;12:1918)
- Can present with pneumothorax or pleural effusion (J Clin Med 2023;12:1918)
- In individuals with DICER1 mutations, can see features such as macrocephaly, renal structural anomalies, retinal abnormalities, dental abnormalities and the GLOW syndrome (global developmental delay, lung cysts, overgrowth and Wilms tumor) (Mod Pathol 2022;35:4)
- DICER1 mutations have been associated with several extrapulmonary neoplasms, such as Sertoli-Leydig cell tumor of the ovary, Wilms tumor, cystic nephroma, renal sarcoma, nasal chondromesenchymal hamartoma, nodular hyperplasia and carcinoma of the thyroid gland, pituitary blastoma, pineoblastoma and others (Clin Cancer Res 2018;24:2251)
Diagnosis
- Chest radiograph, CT or MRI can aid in diagnosis (Children (Basel) 2019;6:86)
- Histological analysis via biopsy or resection is gold standard for diagnosis
Radiology description
- Radiograph: unilateral whiteout of lung with mediastinal shift away from whiteout (Radiopaedia: Pleuropulmonary Blastoma [Accessed 4 June 2024])
- CT scan (Children (Basel) 2019;6:86, Radiopaedia: Pleuropulmonary Blastoma [Accessed 4 June 2024])
- Type I: unilateral cystic to multicystic lesion in the thorax
- Type II: unilateral mass consisting of cystic (often air filled; can be fluid filled) components as well as solid foci
- Type III: unilateral solid mass with homogeneous or heterogeneous enhancement
Radiology images
Prognostic factors
- 5 year overall survival varies based on type
- Type I: 98% (Cancer 2023;129:600)
- Type IR: 100% (Cancer 2023;129:600)
- Type II: 71% (Cancer 2015;121:276)
- Type III: 53% (Cancer 2015;121:276)
Case reports
- 2 year old with chest pain, difficulty breathing and low grade fever for past 2 months; 4 year old boy with difficulty in breathing and on and off fever for past 1 month; and 5 year old girl with difficulty breathing and cough for 3 months (Radiol Case Rep 2021;16:2862)
- 3 year old girl with nonimproving respiratory tract infections (Int J Surg Case Rep 2023;106:108261)
- 13 year old girl with nonproductive cough for 1 month (J Pediatr Surg Case Rep 2020;59:101482)
Treatment
- Type I: surgical resection; radiation is not used (Children (Basel) 2019;6:86)
- Adjuvant therapy is not typically utilized unless there is local invasion, tumor spill during operation or positive margins (Children (Basel) 2019;6:86)
- Type IR: surgical resection; radiation is not used (Children (Basel) 2019;6:86)
- Types II and III: surgical resection if possible and adjuvant chemotherapy (Children (Basel) 2019;6:86)
- Neoadjuvant therapy may be utilized if surgical resection is not possible or puts the patient at an unreasonable level of risk (Children (Basel) 2019;6:86)
- Radiation can be used if needed (Cancer 2015;121:276)
Gross description
- Type I: composed of thin walled, cystic structures
- Type II: composed of thin walled, cystic structures in addition to grossly visible solid foci in the cystic lesion (Mod Pathol 2022;35:4)
- Type III: composed of solid, noncystic tissue and may have areas of necrosis or calcification (Children (Basel) 2019;6:86)
Gross images
Microscopic (histologic) description
- Type I: thin walled cysts lined by benign respiratory epithelium with small malignant mesenchymal cells in the walls of the cysts (Cancer 2023;129:600)
- These primitive cells may have rhabdomyosarcomatous differentiation
- Type IR: thin walled cysts lined by benign respiratory epithelium without the malignant cell population mentioned above (Cancer 2023;129:600)
- Type II: areas of thin walled cysts lined by respiratory epithelium with areas of solid sheets of malignant mesenchymal cells that can appear as a spindled sarcoma, embryonal rhabdomyosarcoma or have no apparent differentiation (Children (Basel) 2019;6:86)
- Type III: heterogeneous solid tumor that can contain several different patterns (Children (Basel) 2019;6:86, Cancer 2015;121:276)
- Blastema-like area with hyperchromatic nuclei, high N:C ratio and mitotic bodies
- Larger malignant sarcoma cells in myxoid background
- Can have rhabdomyosarcomatoid, chrondrosarcomatoid or liposarcomatoid features (Cancer 1988;62:1516)
- Foci of anaplastic cells with pleomorphic nuclei, mitotic bodies and hyaline inclusions
- Some microscopic areas of benign nonneoplastic respiratory epithelium
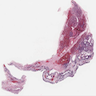
Microscopic (histologic) images
Contributed by Aliya N. Husain, M.D.
Cytology description
- Small ovoid cells with granular chromatin, inconspicuous nucleoli and scant eosinophilic cytoplasm with poorly defined cellular borders (Diagn Cytopathol 1997;16:336, Lung India 2015;32:281)
- Sheets of oval to spindle shaped cells with poor differentiation (Lung India 2015;32:281)
- Tumor cells with granular chromatin and a high N:C ratio (Lung India 2015;32:281)
- Oval to spindle shaped cells that can have perivascular arrangement (Lung India 2015;32:281)
Positive stains
- Vimentin (stains all differentiations), MSA (weak), CD56 (Mod Pathol 2022;35:4, Indian J Pathol Microbiol 2023;66:632, The International Pleuropulmonary Blastoma / DICER1 Registry [Accessed 4 June 2024])
- Depending on type of differentiation
- Rhabdomyosarcomatoid: desmin, myogenin, SMA (Indian J Pathol Microbiol 2023;66:632)
- Chondromatoid: S100 (Indian J Pathol Microbiol 2023;66:632)
Negative stains
- Cytokeratin, EMA: negative in malignant areas but positive in benign nonneoplastic epithelial regions (Indian J Pathol Microbiol 2023;66:632)
- NSE (can be weakly positive on rare occasions) (Indian J Pathol Microbiol 2023;66:632, The International Pleuropulmonary Blastoma / DICER1 Registry [Accessed 4 June 2024])
Molecular / cytogenetics description
- Patients with PPB should undergo testing for DICER1 mutation
Sample pathology report
- Right lung, lobectomy:
- Pleuropulmonary blastoma, type IR (see comment)
- Comment: The tumor is composed of a multicystic lesion with no solid component. It is lined by benign nonneoplastic epithelium and focally has small areas of immature mesenchymal cells in the wall of the cysts.
Differential diagnosis
- Embryonal rhabdomyosarcoma (Lung India 2015;32:281):
- Composed only of rhabdomyoblastic mesenchyme without chondroid differentiation
- Synovial sarcoma (Radiol Case Rep 2015;2:82):
- Metastasis of synovial sarcoma to the lung is much more common than a primary lung synovial sarcoma so history of synovial sarcoma elsewhere would favor this diagnosis
- Histologically divided into monophasic and biphasic
- Monophasic: composed entirely of spindle cells
- Biphasic: composed of spindle and epithelial cells
- Primary sarcoma:
- Lacks undifferentiated blastemic cells
- Pulmonary blastoma:
- In addition to the malignant mesenchyme, the epithelial lining is also malignant
- Seen in adults
- Metastatic Wilms tumor:
- Clinical history of primary Wilms tumor should be present
- Has fibroblastoid stroma with some epithelial components
- WT1 positive
- Fetal lung interstitial tumor (Children (Basel) 2023;10:828):
- Congenital pulmonary airway malformation (CPAM) (for types IR and I) (Children (Basel) 2019;6:86):
- PPB more likely if the patient has a family history of PPB (Cancer 2015;121:276)
- Prenatal detection is more common in CPAM (J Pediatr Surg 2016;51:33)
- Can contain clusters of mucinous cells (Mod Pathol 2022;35:1870)
- Negative for desmin or myogenin due to lack of malignant rhabdomyoblasts
Additional references
Board review style question #1
Which of the following is true regarding pleuropulmonary blastoma type IR?
- Can metastasize to the brain
- Composed of both solid and cystic foci
- Contains a population of malignant mesenchymal cells in addition to benign nonneoplastic epithelium
- Does not contain any malignant mesenchymal cells
- Often progresses to type III PPB
Board review style answer #1
D. Does not contain any malignant mesenchymal cells. The lack of malignant mesenchymal cells is what differentiates PPB type IR from types I - III. Answer E is incorrect because type IR does not progress to type III due to its lack of malignant mesenchymal cells. Answer C is incorrect because type IR does not have malignant mesenchymal cells, although it does have a benign nonneoplastic epithelium. Answer B is incorrect because type IR does not contain any solid foci; it is purely cystic. Answer A is incorrect because type IR does not have a malignant component and therefore does not metastasize.
Comment Here
Reference: Pleuropulmonary blastoma
Comment Here
Reference: Pleuropulmonary blastoma
Board review style question #2
A 2 year old patient with no past medical history presented to the clinic with a cough that persisted for 1 month. On CT scan, a solid tumor occupying the lung was seen. After resection, the tumor was sent to pathology where the diagnosis of PPB type III was made. It was noted that the tumor had rhabdomyosarcomatoid differentiation and no chondromatoid differentiation. A picture of the tumor with an immunostain is shown above. Which staining pattern would you expect to see in this tumor?
- Positive desmin and S100, negative vimentin
- Positive MSA and S100, negative SMA
- Positive SMA and desmin, negative vimentin
- Positive vimentin and desmin, negative S100
- Positive vimentin and S100, negative desmin
Board review style answer #2
D. Positive vimentin and desmin, negative S100. PPB is positive for vimentin and the rhabdomyosarcomatoid component will be positive for desmin. S100 will be negative in this tumor because there is no chondromatoid component. Answers A and E are incorrect because S100 would not be positive in a tumor without chondromatoid components; furthermore, we would expect desmin and vimentin to be positive in this tumor. Answer C is incorrect because we would expect vimentin to be positive in this tumor. Answer B is incorrect because S100 would not be positive in a tumor without chondromatoid components and we would expect SMA to be positive given the tumor's rhabdomyosarcomatoid component.
Comment Here
Reference: Pleuropulmonary blastoma
Comment Here
Reference: Pleuropulmonary blastoma