Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Differential diagnosisCite this page: Wu R. Langerhans cell histiocytosis. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungtumorLCH.html. Accessed December 25th, 2024.
Definition / general
- Proliferative disease or disorder of Langerhans cells involving lung, highly associated with smoking
- Pulmonary Langerhans cell histiocytosis (PLCH) is thought to be distinct from systemic Langerhans cell histiocytosis (LCH)
- Most common pulmonary histiocytic lesion (Arch Pathol Lab Med 2008;132:1171)
Essential features
- Almost exclusively seen in young to middle aged smokers
- Stellate nodules centered around small airways, with early cellular phase and later fibrotic phase
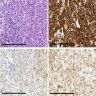
- Langerhans cells stain for CD1a, Langerin, and S100
Terminology
- Eosinophilic granuloma, Langerhans cell granulomatosis, histiocytosis X ("H-X")
- Multisystem diseases: Letterer-Siwi disease, Hand-Schüller-Christian disease, Hashimoto-Pritzker syndrome, where lung involvement is histologically indistinguishable from cellular phase of PLCH
Epidemiology
- Uncommon, encompassing 3 - 5% of lung biopsies for interstitial lung disease
- >90% of cases associated with smoking - considered a smoking related disease
- Usually ages 20 - 50 years
- More common in Caucasian than African Americans or Asians
Sites
- 50% of cases only involve lung
- 20% of those with multicentric disease (i.e. bone, skin, lymph nodes, spleen, CNS, pituitary, rarely thyroid and thymus) have lung involvement
Pathophysiology
- Debatable as reactive versus neoplastic, could arise from smoking related and immunomodulatory processes (Arch Pathol Lab Med 2016;140:230)
- PLCH may be a reactive process with a subset showing clonality, but extrapulmonary forms of Langerhans cell histiocytosis are thought to be neoplastic
- Unpredictable course, even with smoking cessation: some spontaneously resolve, others remain stable, some progress to honeycomb fibrosis
Etiology
- Disorder of cells with Langerhans cell phenotype
Clinical features
- Many patients asymptomatic
- Chronic cough and exertional dyspnea typical (Eur J Intern Med 2015;26:351)
- Rarely can present with hemoptysis
- Recurrent spontaneous pneumothorax in 15 - 25% of cases
- Pulmonary hypertension and vasculopathy frequent in advanced cases
- Diabetes insipidus in 15% of patients from pituitary involvement
- Generally restrictive features in early disease, obstructive features in late disease
- Secondary malignant and non-malignant neoplasms
Diagnosis
- Lung biopsy is necessary for a definitive diagnosis, although may not be required if imaging findings are highly characteristic (Orphanet J Rare Dis 2012;7:16)
- Transbronchial biopsy may be diagnostic; otherwise, requires surgical lung biopsy
Laboratory
- Normal peripheral eosinophil blood count
Radiology description
- Upper / middle lung predominance
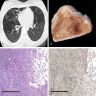
- X-ray: small nodules generally up to 1 cm and variably sized cysts
- High resolution CT: bronchiolocentric stellate nodules possibly with surrounding ground glass opacities, some with faint lucent cavity
- Advanced disease with cysts, emphysema adjacent to scarring, reticular and nodular opacities, honeycombing, can mimic IPF
- FDG-PET may show increased uptake, especially in early nodular disease
Prognostic factors
- 10 - 20% may progress to respiratory failure
- Median survival 12 years, 5 year survival 70%, 10 year survival 60%
- Worse prognosis in cases with pulmonary hypertension
- Worse prognosis for those with baseline poor respiratory function and those with concurrent neoplasms
- Worse prognosis with extremes of age, prolonged constitutional symptoms, multiorgan involvement, extensive cysts, honeycomb changes
Case reports
- 22 year old man with PLCH and tuberculosis (Int J Clin Exp Pathol 2015;8:2146)
- 40 year old woman with Stage IV diffuse large B cell lymphoma (Arch Pathol Lab Med 2002;126:747)
- 42 year old man with bilateral axillary ulcerated masses (Am J Med 2015;128:e7)
Treatment
- Smoking cessation most effective
- Corticosteroids +/- chemotherapeutic agents show mixed results
- Lung transplantation in advanced cases
Gross description
- Upper lobe predominance, fine nodular infiltrate, with nodules up to 1.5 cm
- Advanced disease with cysts, cavitary lesions, and honeycombing
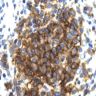
Microscopic (histologic) description
- Early cellular phase and older fibrotic lesions may be intermixed, but one pattern typically predominates
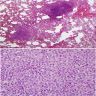
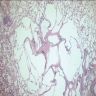
- Scattered, variably sized stellate nodules ("star fish-like" or "Medusa head-like") with interstitial scarring and aggregates of Langerhans cells
- Layered appearance of nodules, bronchiolocentric distribution with pleural/subpleural sparing
- Confluence of nodules → serpentine sheath around small airways
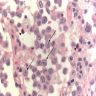
- Langerhans cells are relatively large with abundant, granular, mildly eosinophilic and indistinct cytoplasm, grooved nuclei with indented nuclear membranes / grooves, "crumpled tissue paper" nuclear outlines, pale basophilic nucleus, one or two small nucleoli
- Also prominent eosinophils, lymphocytes, plasma cells, activated macrophages, multinucleated giant cells
- Variably sized cystic spaces at periphery of nodules lacking lining cells, secondary to traction on surrounding alveolar walls or airways
- Frequent hemosiderin, necrosis, alveolar lining cell hyperplasia, pigmented alveolar macrophages ("smoker's macrophages")
- Variable vasculitis, patchy organization, other smoking related changes such as emphysema, respiratory bronchiolitis, desquamative interstitial pneumonia
- Older fibrotic lesions have fewer Langerhans cells and eosinophils, more fibrosis
- May show pulmonary hypertension
- Sarcomatous variant has significant atypia and mitotic figures
Microscopic (histologic) images
Cytology description
- Langerhans cells with abundant cytoplasm and grooved nuclei with background eosinophils and lymphocytes
- Bronchioalveolar lavage may show increased numbers (>5%) of CD1a+ Langerhans cells but test with low sensitivity (Respir Med 2012;106:1286)
Positive stains
Electron microscopy description
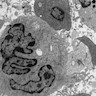
- Birbeck's granules (pentilaminar intracytoplasmic structures, tennis racket shaped)
Electron microscopy images
Molecular / cytogenetics description
- Subset of PLCH cases show BRAF V600E mutation (Am J Surg Pathol 2014;38:548)
- Mutations in other MAPK genes have been identified
Differential diagnosis
- Desquamative interstitial pneumonitis
- Eosinophilic pleuritis: no Langerhans cells, although mesothelial cells may appear similar
- Eosinophilic pneumonia
- Erdheim-Chester disease
- Hypersensitivity pneumonitis
- Lymphangioleiomyomatosis (LAM): HMB45 and actin positive
- Respiratory bronchiolitis