Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Cite this page: Bowden R, Montero-Fernández MA. Transplantation / rejection. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungnontumorlungtransp.html. Accessed December 12th, 2024.
Definition / general
- Lung transplant is the treatment for end stage lung nontumoral diseases
- Lung allograft rejection is a significant cause of acute graft dysfunction and eventually chronic graft failure (Arch Pathol Lab Med 2017;141:437)
Essential features
- Lung allograft rejection is a significant cause of acute graft dysfunction and eventually chronic graft failure
- Rejection of allograft is through a cellular mediated response or antibody mediated response
- Acute cellular rejection resolves in 80 - 90% with immunosuppression
- Chronic lung allograft dysfunction is the main cause of mortality in 50% of lung transplant recipients after 5 years
Terminology
- Acute cellular rejection (ACR)
- Chronic lung allograft dysfunction (CLAD): irreversible graft loss, 20% decrease in forced expiratory volume in 1 second (FEV1)
- Bronchiolitis obliterans syndrome (BOS): 85% of chronic lung allograft dysfunction cases
- Obliterative bronchiolitis (OB): clinical bronchiolitis obliterans syndrome
- Restrictive allograft syndrome (RAS [rCLAD]): 15% of chronic lung allograft dysfunction cases
ICD coding
Epidemiology
- 17.1 - 64% incidence of acute cellular rejection in the first year post-lung transplant (J Thorac Dis 2019;11:S1732)
- Acute rejection is responsible for 3.6% of deaths among lung transplant recipients in the first 30 days (J Thorac Dis 2017;9:5440)
- After 5 years, chronic lung allograft dysfunction is the main cause of mortality in 50% of lung transplant recipients (J Thorac Dis 2017;9:2684)
Sites
- Respiratory system
Pathophysiology
- Immune response to the allograft is characterized by the following processes
- Hyperacute rejection
- Due to preformed donor specific antibodies, which activate the complement system and platelet aggregation leading to vascular thrombosis and graft necrosis (J Heart Lung Transplant 2016;35:397)
- Acute cellular rejection
- Recipient T lymphocytes recognize donor human leukocyte antigen (HLAs) or other antigens
- Pathways: direct (donor dendritic cells presenting major histocompatibility complex directly to recipient T cells) and indirect (recipient dendritic cells presenting alloantigen from donor antigen presenting cells to T cells) (J Thorac Dis 2017;9:5440)
- Antibody mediated rejection
- Donor specific antibodies produced by B cells and plasma cells, target foreign HLA on the donor capillary endothelial cells
- HLA donor specific antibodies cause persistent insult to the graft epithelium, resulting in epithelial ischemia and antibodies to self antigens such as K-α 1 tubulin (Kα1T) and collagen V (Col V0) (Arch Pathol Lab Med 2017;141:437)
- Hyperacute rejection
Etiology
- Rejection of allograft through cellular mediated response or antibody mediated response (Arch Pathol Lab Med 2017;141:437)
Clinical features
- Shortness of breath
- Pulmonary edema
- Diffuse alveolar hemorrhage (J Thorac Dis 2019;11:S1732)
- Fever
- Cough
- Decline in pulmonary function tests
Diagnosis
- Bronchoscopy
- Bronchoalveolar lavage (BAL): cytological and microbiological analysis
- Transbronchial biopsy forceps and cryobiopsy (Ann Thorac Surg 2019;108:1052)
- Clinical
- Forced expiratory volume in 1 second (FEV1), 10% reduction from baseline for > 48 hours (J Thorac Dis 2017;9:5440)
Laboratory
- Eosinophilia, neutrophilia, leukocytosis
- Levels of donor specific antibodies detected in serum associated with increased risk of antibody mediated rejection and chronic lung allograft dysfunction (Transplantation 2017;101:1215)
- Increased circulating cell free DNA detected by next generation sequencing (NGS) (Proc Natl Acad Sci U S A 2015;112:13336, JHLT 2021;40:822)
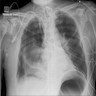
Radiology description
- Chest Xray
- Ground glass opacities, pulmonary edema and pleural effusions
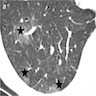
- High resolution CT
- Ground glass opacities with interlobular septal thickening, without consolidation and atelectasis (J Thorac Dis 2017;9:5440)
Radiology images
Prognostic factors
- Hyperacute rejection has high mortality (J Heart Lung Transplant 2016;35:397)
- Acute rejection resolves in 80 - 90% with immunosuppression
- Chronic lung allograft dysfunction is a major cause of death after 6 months posttransplant
- Immunosuppressant therapy causes secondary infections (CMV, EBV) and posttransplant lymphoproliferative disorder (Respiration 2015;90:451)
- Posttransplant lymphoproliferative disorder
- Majority occur within the first year posttransplant (> 85%)
- Risk factors include immunosuppressive burden and the oncogenic nature of the Epstein-Barr virus (EBV) (World J Transplant 2020;10:29)
- Treatment includes reduction of immunosuppressive therapy, rituximab, antivirals and chemotherapy
Case reports
- 48 year old man with seropositive HIV, desquamative interstitial pneumonia and successful lung transplantation (BMC Pulm Med 2018;18:162)
- 66 year old woman with everolimus induced pneumonitis in a double lung transplant (Oxf Med Case Reports 2018;2018:omy008)
- 66 year old woman who presented with Mycobacterium avium complex after double lung transplant (J Med Case Rep 2017;11:240)
- 68 year old man with shortness of breath, weight loss and abnormal chest CT (Chest 2018;153:e153)
Treatment
- Reserved for symptomatic grades of acute cellular rejection (> A2) and consists of intravenous methylprednisolone, tapering to oral
- Consider using tacrolimus if on cyclosporine or adding mammalian target of rapamycin (mTOR) inhibitor, such as everolimus (J Thorac Dis 2017;9:5440)
- Extracorporeal photophoresis stimulates and expands the number of peripheral regulatory T cells, stabilizing acute cellular rejection and reducing lung function decline in chronic lung allograft dysfunction / bronchiolitis obliterans syndrome (Clin Transplant 2017;31:e13041)
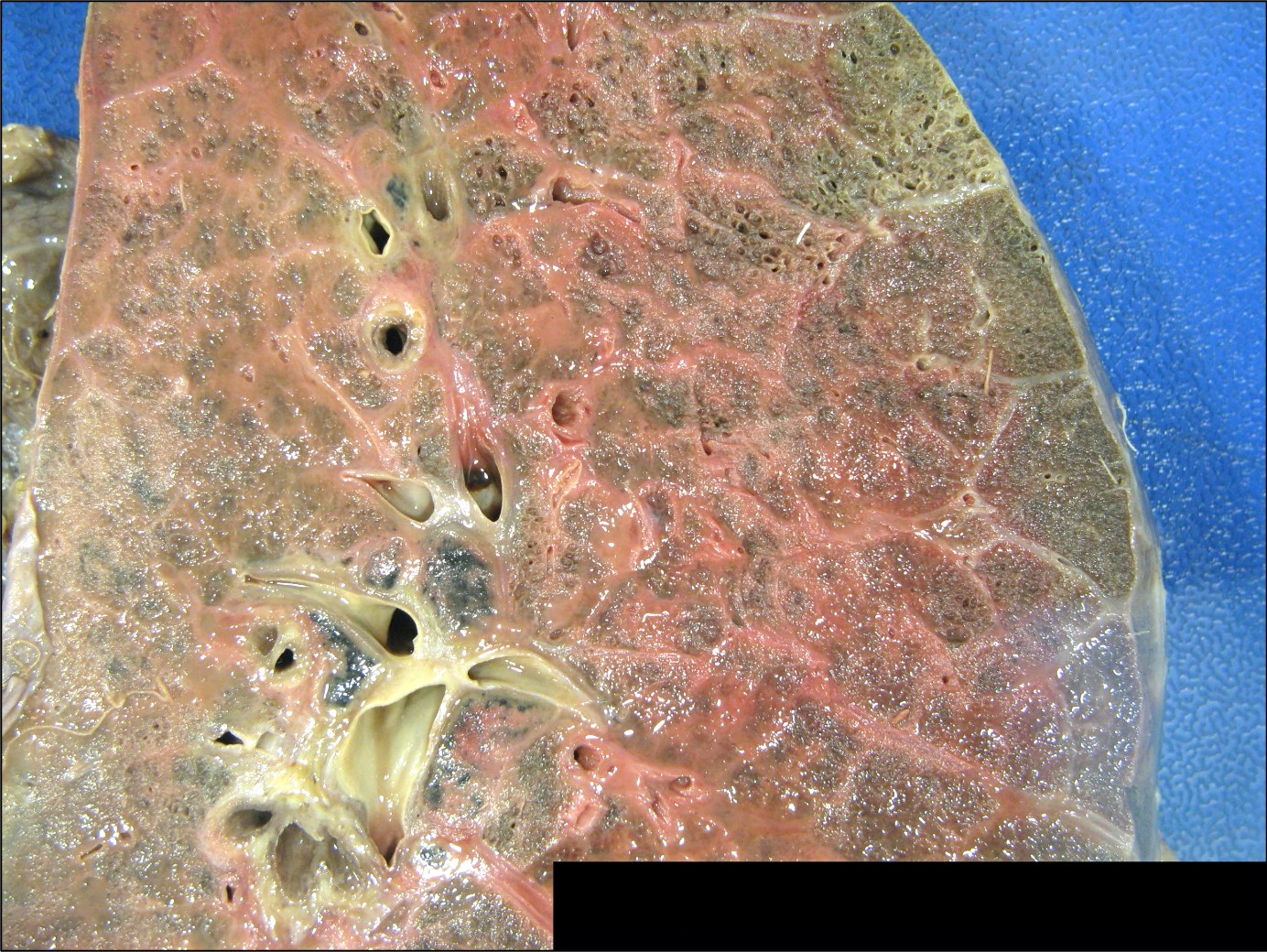
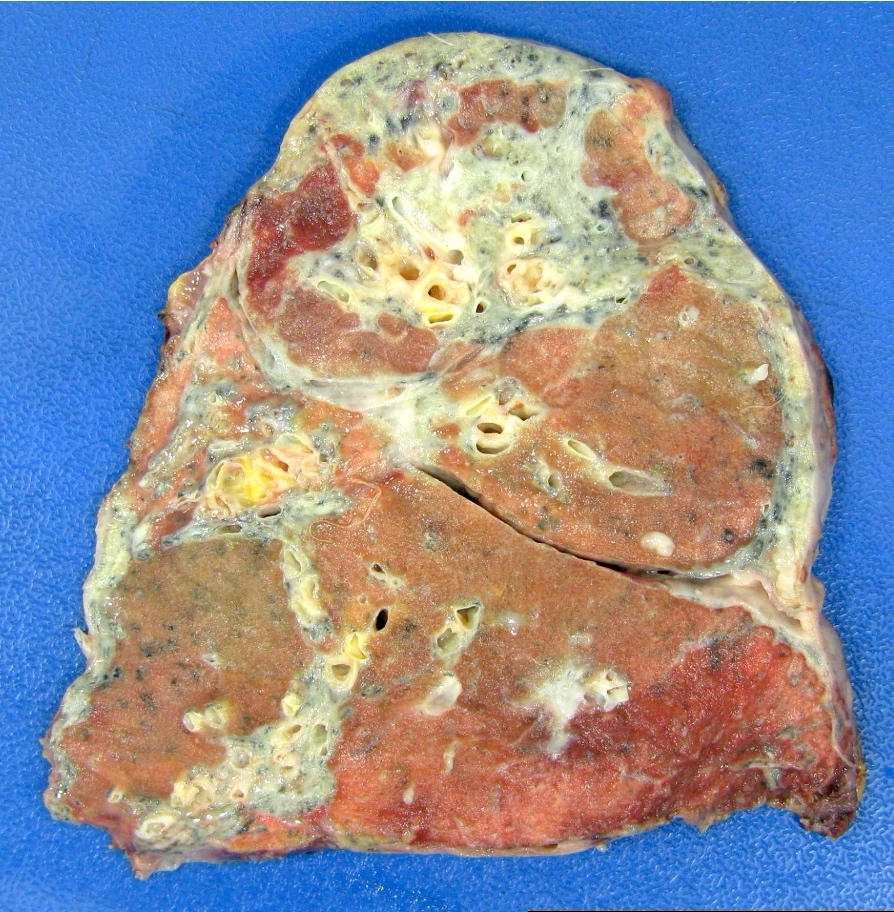
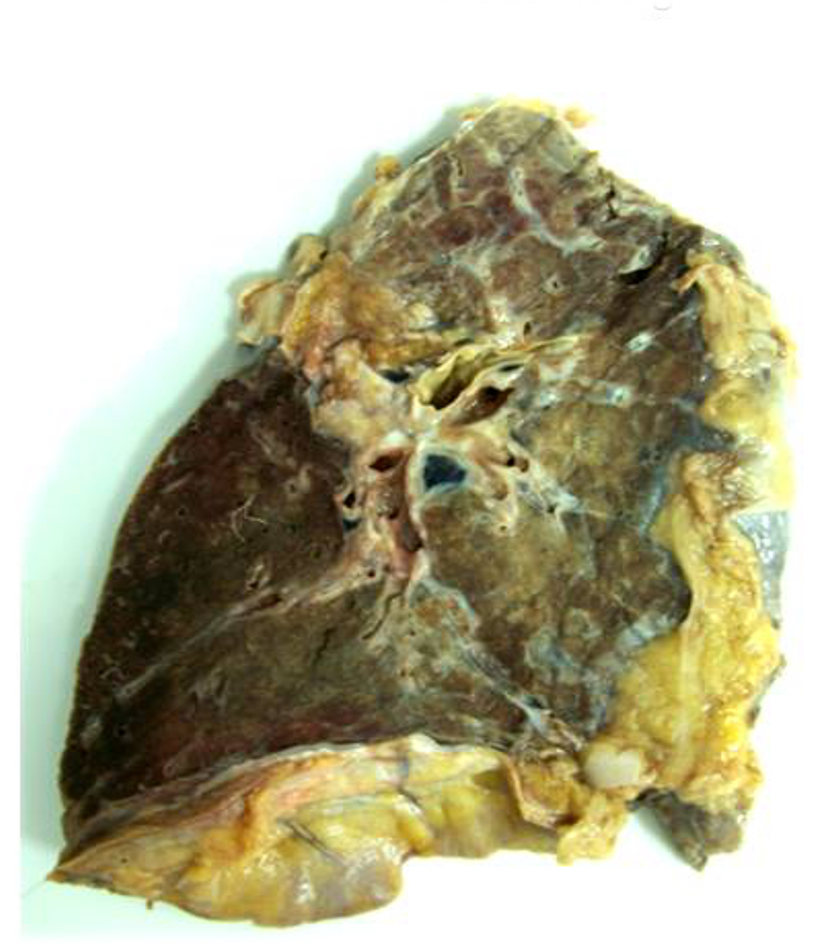
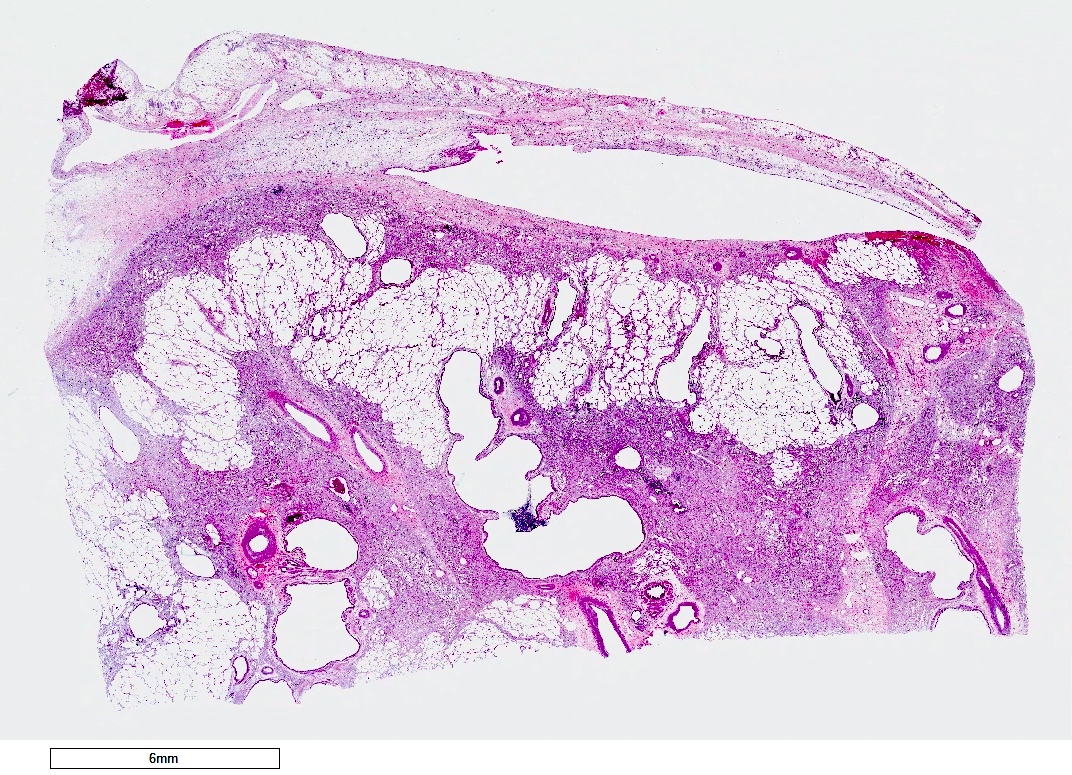
Gross description
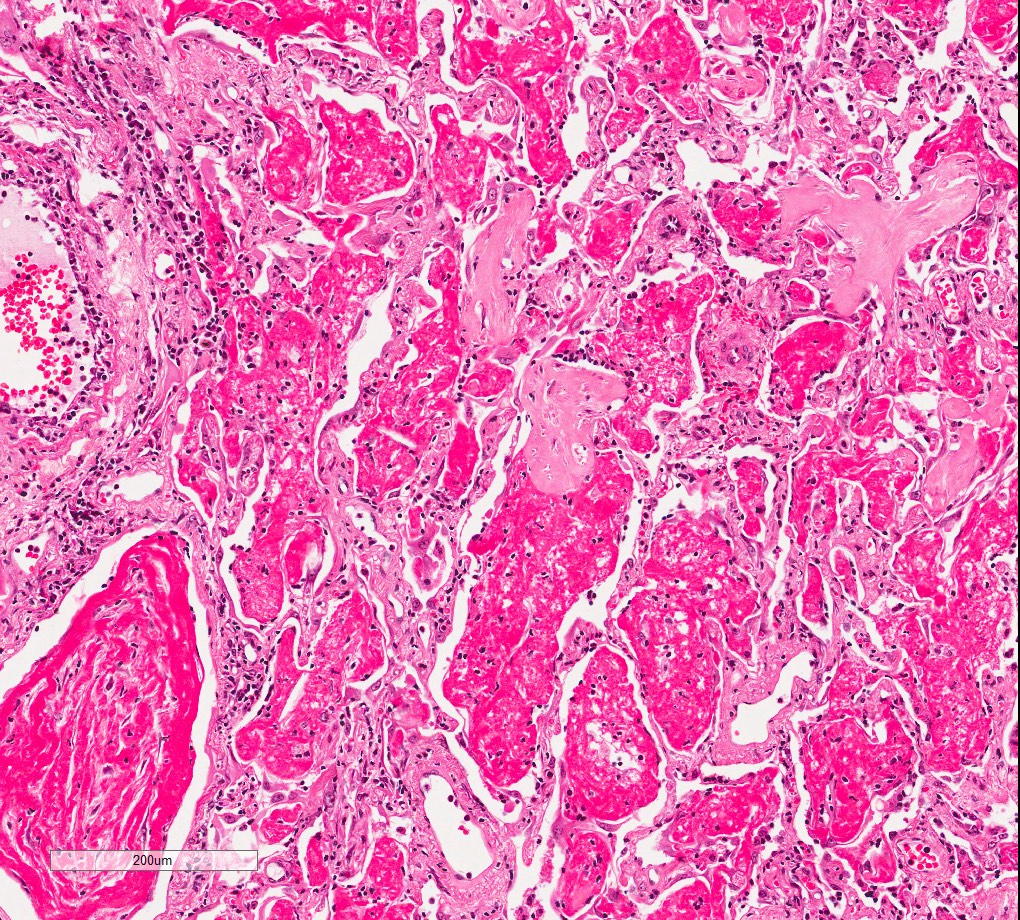
- Antibody mediated rejection: unfixed lungs are hyperemic and heavy
- Chronic rejection: hyperinflated lungs, with bronchiectasis, fibrosis of lung parenchyma (J Thorac Dis 2017;9:2684)
Gross images
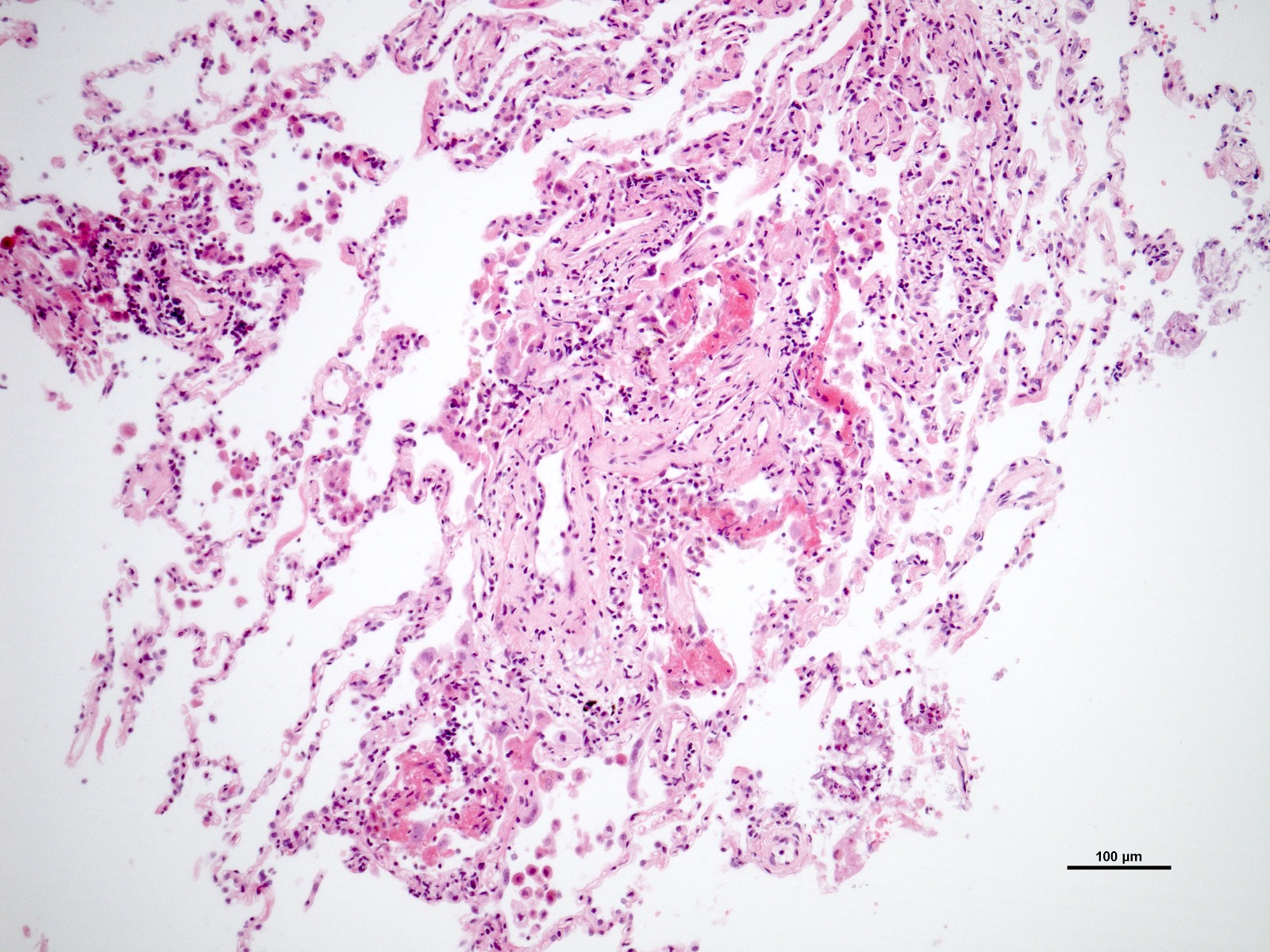
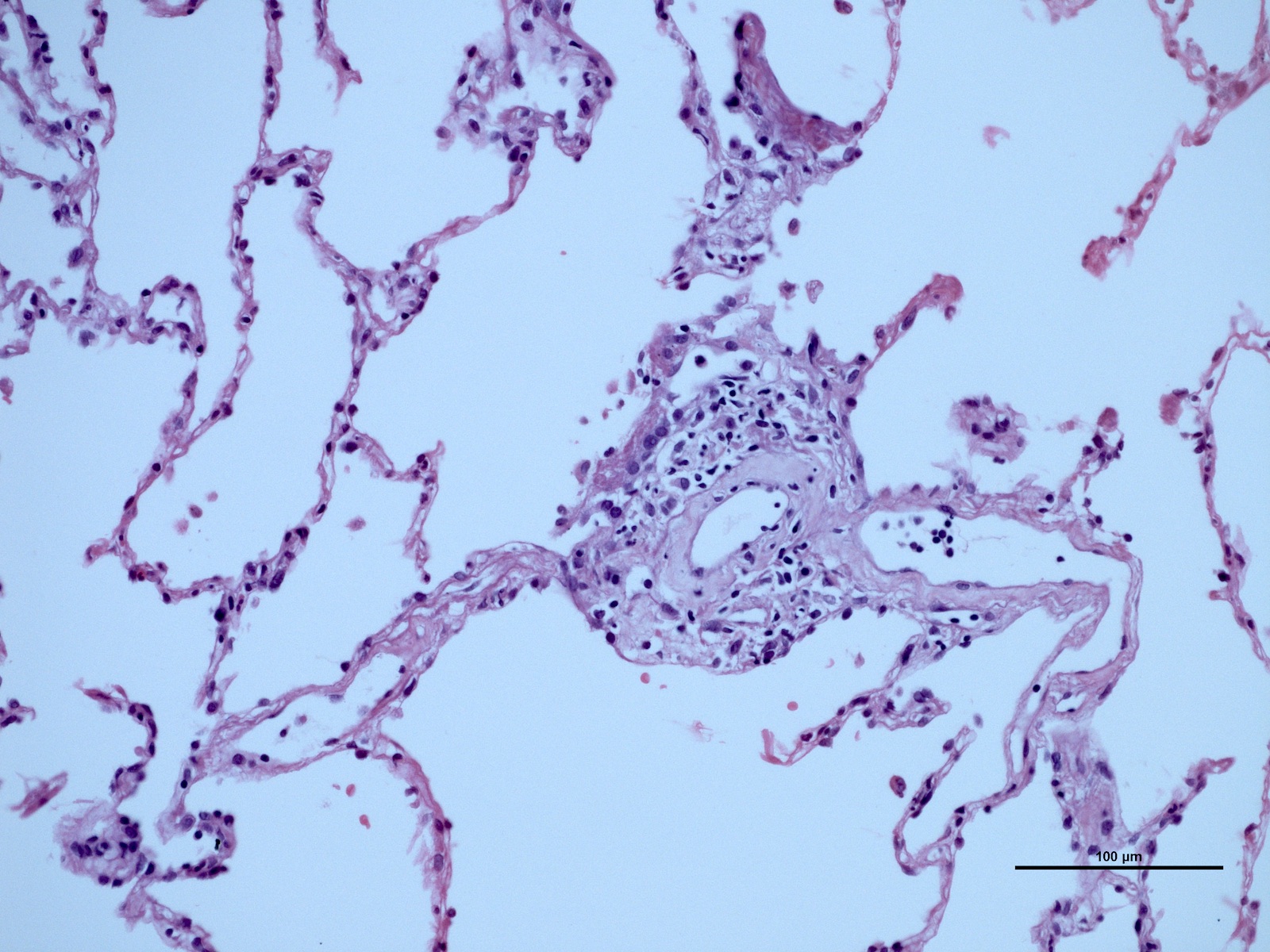
Microscopic (histologic) description
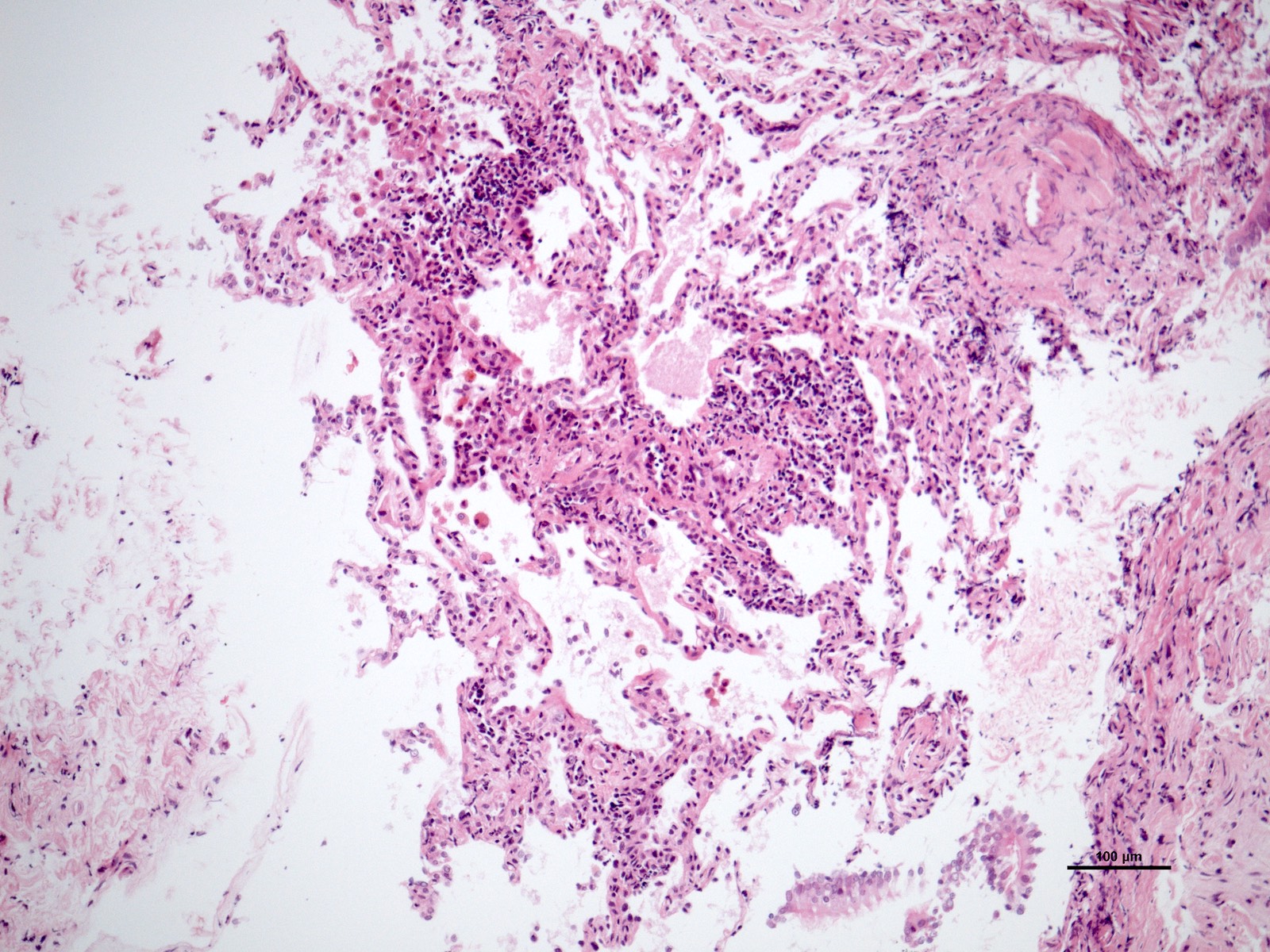
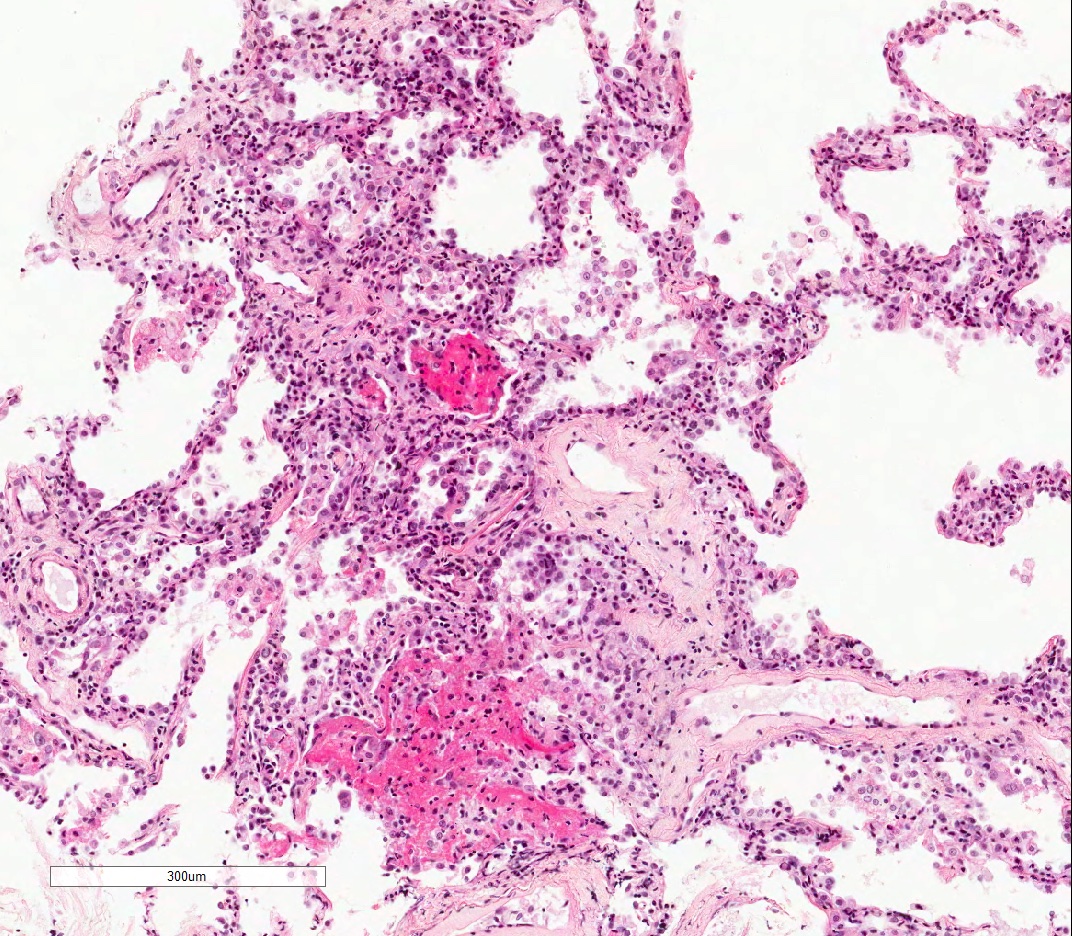
- Acute cellular rejection is characterized by infiltration of lymphocytes / monocytes around blood vessel endothelium (A grade) and airways / bronchioles (B grade) (J Thorac Dis 2017;9:5440)
- Classified by the International Society for Heart and Lung Transplantation (ISHLT) working party (see table below)
- Perivascular inflammation
- Minimal (A1): infrequent circumferential monocyte cuffing displaying a ring of 2 - 3 cell thick layers in the perivascular adventitia (venules and arterioles); no eosinophils or endotheliatis
- Mild (A2): more frequent circumferential monocyte cuffing with > 3 cell thick layer perivascular infiltrates; eosinophils and endothelialitis can be seen; no extension to the adjacent alveolar interstitium
- Moderate (A3): extension of perivascular inflammation into neighboring alveolar septa; neutrophils, eosinophils and endothelialitis are common findings
- Severe acute rejection (A4): diffuse inflammation, hemorrhage, parenchymal necrosis, necrotizing vasculitis and hyaline membrane formation (J Thorac Dis 2017;9:5440, Surgical Pathology 2020:13:119)
- Airway inflammation (grades B and E)
- Inflammation of noncartilaginous bronchioles (B) and large cartilaginous bronchi (E)
- Low grade (B1R E1): submucosal lymphocytic inflammation, without epithelial injury
- High grade (B2R E2): submucosal lymphocytic inflammation, with epithelial injury with or without ulceration (Surgical Pathology 2020:13:119)
- Inflammation of noncartilaginous bronchioles (B) and large cartilaginous bronchi (E)
- Chronic vascular rejection (D0: absent; D1: present): fibrotic remodeling of veins and arteries
- Chronic rejection is characterized as present or absent
- Bronchiolitis obliterans (OB): partial or complete occlusion of bronchiole lumen, with patchy submucosal fibrosis, with or without inflammatory infiltrates
- Restrictive allograft syndrome (RAS): pleuroparenchymal fibroelastosis with or without bronchiolitis obliterans or diffuse alveolar damage
Revised working formulation for classification and grading of pulmonary allograft rejection
| A: Perivascular inflammation AX: Ungradable | B / E: Airway inflammation | C: Chronic airway rejection
D: Chronic vascular rejection |
| Grade 0: none | Grade 0: none | 0: absent |
| Grade 1: minimal | Grade 1R*: low grade | 1: present |
| Grade 2: mild | Grade 2R*: high grade | |
| Grade 3: moderate | BX / EX: ungradable | CX / DX: ungradable |
| Grade 4: severe |
- *R denotes new revised stage
- Reference: J Heart Lung Transplant 2007;26:1229
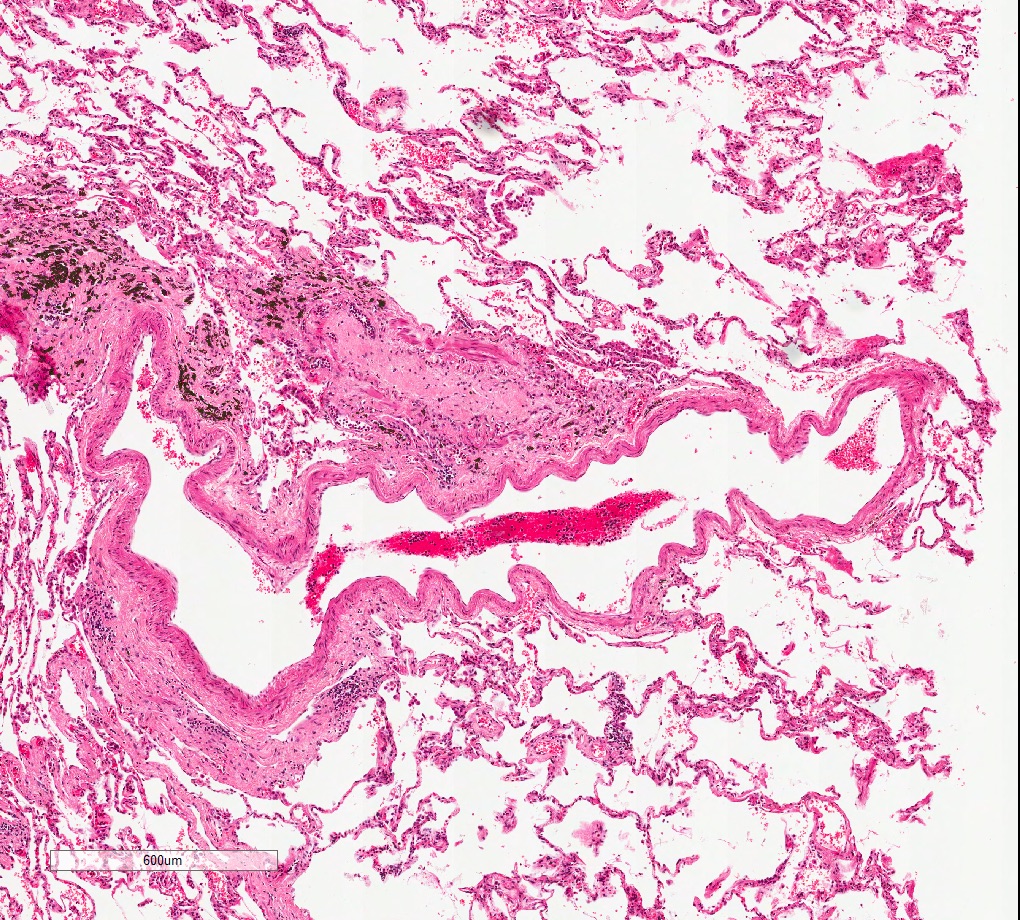
Microscopic (histologic) images
Contributed by M. Ángeles Montero-Fernández, M.D., Ph.D.
Cytology description
- Allograft rejection is associated with increased neutrophils and epithelial cells in bronchoalveolar lavage (Respir Res 2018;19:102)
Immunofluorescence description
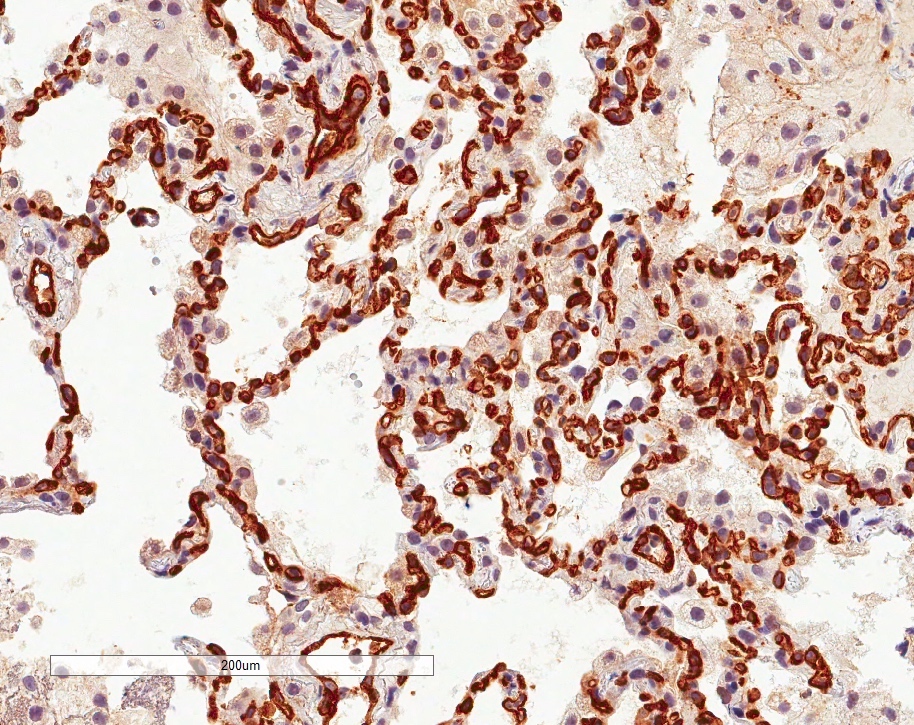
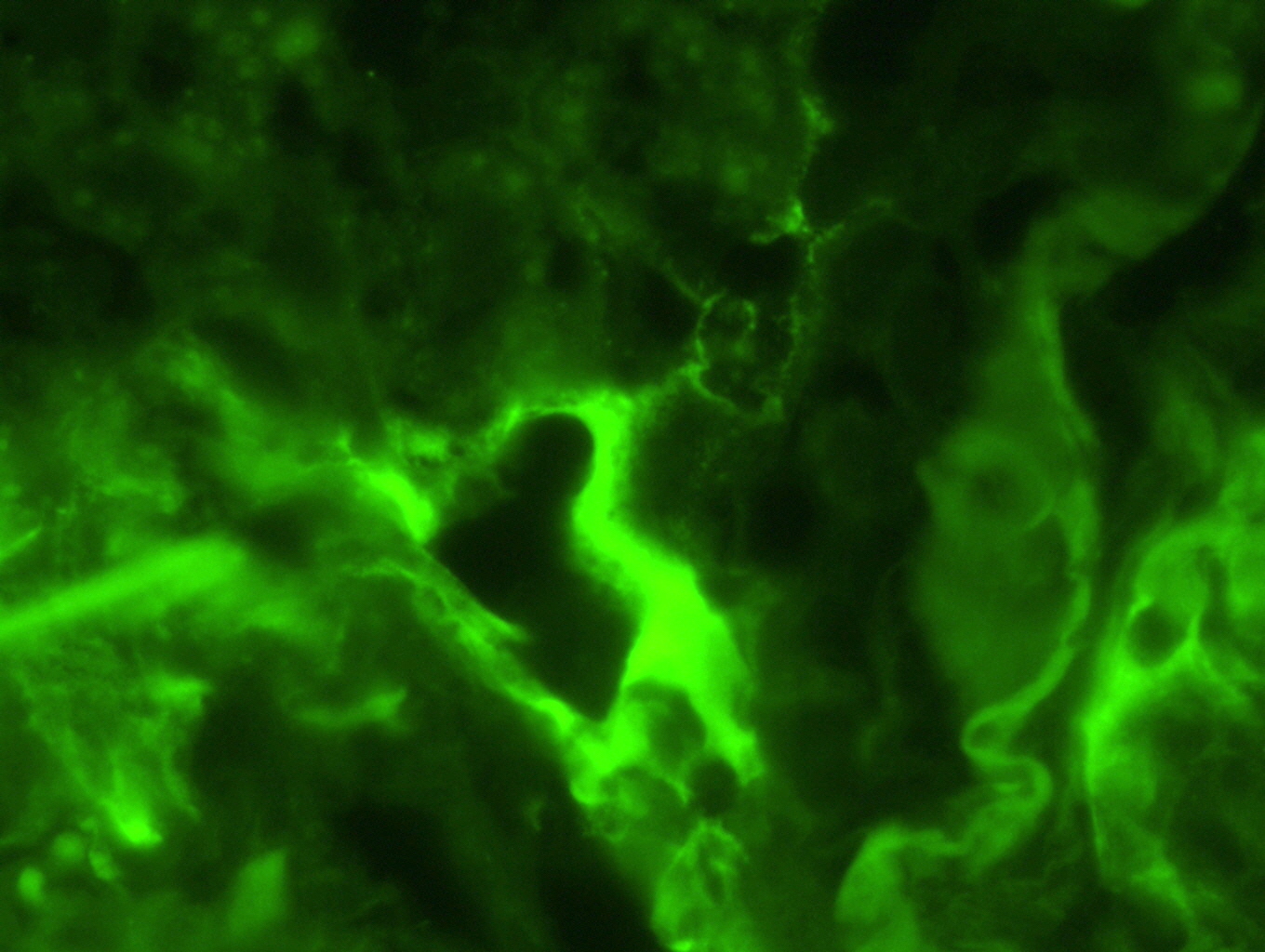
- In antibody mediated rejection, capillary C4d staining in alveolar tissue may support the presence of an antibody mediated process (Am J Transplant 2018;18:936)
Positive stains
- Proposed scoring threshold for positive C4d: diffuse staining (> 50%) of alveolar interstitial capillaries
Negative stains
- Proposed scoring threshold for negative C4d: focal staining (< 50%) of alveolar interstitial capillaries
Molecular / cytogenetics description
- MicroRNA has shown promising potential in distinguishing patients with acute cellular rejection from those without rejection (Transplant Direct 2015;1:e44)
- Donor derived cell free DNA increases with acute rejection and also correlates with lung function decline and histological rejection grading (JHLT 2021;40:822)
Videos
Indications, patient selection and outcomes
Sample pathology report
- Left lung, lower lobe, transbronchial biopsy:
- Mild acute cellular rejection, mild lymphocytic bronchiolitis and no evidence of antibody mediated rejection (A2 B1R C0, pAMR0) (see comment)
- Comment: There are 5 adequate pieces of lung, including 1 bronchiole and several vessels. 2 perivascular lymphocytic infiltrates that extend to the septa are noted. Occasional eosinophils and focal endotheliitis are noted. Bronchiolar submucosal lymphocytic infiltrate without epithelial injury is also seen. No bronchiolitis obliterans or capillaritis identified. Immunohistochemistry stain for C4d is negative.
Differential diagnosis
- Hyperacute (antibody mediated) rejection:
- Donor transmitted infection:
- Specific criteria of infection as granulomas, intra-alveolar neutrophils with cellular debris, viral inclusions, fungal structures
- Ischemia reperfusion injury (acute lung injury):
- Usually up to 2 weeks after the transplant
- Intra-alveolar or membranous fibrin
- Type 2 pneumocyte hyperplasia, increase of interstitial neutrophils due to ischemia, organizing pneumonia
- Donor transmitted infection:
- Acute rejection:
- Infections (bacterial, viral or fungal):
- Neutrophils, necrosis and viral cytopathic effect
- Other causes of acute lung injury, like drug toxicity and aspiration (granulomas can be seen in both conditions), antibody mediated rejection, ischemia reperfusion injury and recurrence of primary lung disease
- Bronchus associated lymphoid tissue (BALT):
- Circumscribed lymphocytic aggregates that usually do not surround vessels, unlike acute cellular rejection (Arch Pathol Lab Med 2017;141:437)
- Infections (bacterial, viral or fungal):
- Chronic rejection:
- Nonspecific large airway fibrosis
- Constrictive bronchiolitis:
- Fibrotic bronchiolar stenosis nonrelated to transplant
- Organizing pneumonia:
- Nonspecific alveolar fibroblastic process
Board review style question #1
Board review style answer #1
C. Chronic lung allograft dysfunction. The main cause of mortality in lung transplant patients is chronic lung allograft dysfunction (CLAD), affecting 50% of transplant recipients after 5 years (J Thorac Dis 2017;9:2684). Answers A and B are incorrect because these conditions are treatable, although they may evolve to CLAD which may eventually cause graft failure and dead. Answer D is incorrect because hyperacute rejection is currently an infrequent complication.
Comment Here
Reference: Lung - Transplantation / rejection
Comment Here
Reference: Lung - Transplantation / rejection