Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Cima L, Lin S, Cecchini M. Granulomatosis with polyangiitis (GPA). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungnontumorgranulomatosiswithpolyangiitis.html. Accessed April 1st, 2025.
Definition / general
- Granulomatosis with polyangiitis (GPA) is a systemic autoimmune vasculitis syndrome commonly involving the lower respiratory tract, the upper respiratory tract and the kidney
- Characterized by a necrotizing vasculitis and a systemic granulomatous inflammatory process which replaces the involved tissues
Essential features
- Classic GPA clinicopathological triad
- Vascular, respiratory and renal involvement
- Histopathological features
- Necrotizing vasculitis of small to medium sized arteries and veins
- Eosinophilic palisading granulomas
- Glomerulonephritis
- Association with antineutrophil cytoplasmic antibodies (ANCA) positivity
- C-ANCA is more specific for GPA
Terminology
- Obsolete term (not recommended): Wegener granulomatosis (WG), name changed due to Wegener's Nazi association in the 1930s (Lancet 2006;367:1362)
ICD coding
- ICD-11: 4A44.A1 - granulomatosis with polyangiitis
Epidemiology
- Incidence
- M = F (slight male predominance)
- Mean age: 50 years (Int J Immunopathol Pharmacol 2016;29:151)
- Rare in children and young adults
- Caucasian population of European descent is most commonly affected
- Risk factors
- Infectious, environmental and drug induced triggers
- First degree relatives with GPA
- HLA-DPB1*0401 variant as genetic risk factor (Ann Rheum Dis 2011;70:707)
- Thyroid disease: Graves disease and Hashimoto thyroiditis (Arthritis Rheum 2003;48:2299)
Sites
- Systemic vasculitis may manifest in a classic triad involving
- Head and neck region (upper respiratory tract)
- Lower respiratory tract
- Kidney
- Can also occur in a combination of 1 or 2 of the classic sites, along with additional sites
- Skin, joints, middle ear, eye and nervous system
- Solitary lung involvement can occur and is difficult to diagnose for the pathologist
- Reference: Autoimmun Rev 2014;13:1121
Pathophysiology
- Exact pathophysiology is unclear and pathogenesis is likely multifactorial
Etiology
- Exact mechanism is unclear
- Thought to be a result of exposure to infectious, environmental or drug induced triggers in patients with predisposing genetic background
- Recent discoveries suggest excessive activation of neutrophils that form neutrophil extracellular traps (NETs) (Front Immunol 2019;10:2617)
- Excessive NET formation is involved in ANCA mediated vascular injury and production of ANCA themselves
- Viscous cycle of NET formation and ANCA production is thought to drive GPA pathogenesis
Clinical features
- Typically manifest in upper / lower respiratory tract and kidney; pulmonary symptoms / signs in the absence of upper respiratory symptoms / signs are unusual
- Most common presenting symptoms / signs (local): rhinorrhea, purulent / bloody nasal discharge, oral or nasal ulcers, sinus pain, cough, hemoptysis and chest pain
- Most common presenting symptoms / signs (systemic): polyarthralgias, myalgias, fever, malaise, weight loss
- Reference: Arthritis Rheum 1990;33:1101
Diagnosis
- Clinical setting: corresponding sites of pathology, symptoms, radiological findings and patient demographics
- Positive ELISA serum C-ANCA test or less commonly P-ANCA test
- Biopsy or resection shows characteristic histologic findings consistent with GPA
- Special stains and cultures exclude infections
- Reference: Arthritis Rheum 1990;33:1101
Laboratory
- ELISA test for serum antineutrophil cytoplasmic antibodies (ANCA) serotypes
- Cytoplasmic (C) [PR3]-ANCA (proteinase 3); highly specific for GPA
- Perinuclear (P) [MPO]-ANCA (myeloperoxidase); less specific for GPA
- C-ANCA found in 90% with active generalized disease and 60% with limited disease
- Positive test in an appropriate clinical setting supports GPA diagnosis
- Negative test does not exclude the diagnosis, especially with characteristic histopathology
- ANCA positivity strongly related to relapse in GPA with renal involvement (J Am Soc Nephrol 2015;26:537)
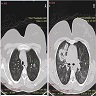
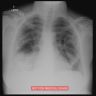
Radiology description
- Upper respiratory tract involvement: sinonasal mucosal thickening with bony / cartilaginous erosion
- Lower respiratory tract involvement: 4 patterns of involvement (Chest 1990;97:906, J Thorac Imaging 1988;3:33, Clin Radiol 1982;33:545)
- Nodules with or without cavitation, often around bronchovascular bundles or in a subpleural distribution
- Pulmonary hemorrhage, which can occur with nodules but in some cases the consolidation associated with the hemorrhage predominates
- Reticulonodular pattern can be the first pattern and is often asymptomatic
- Peripheral wedge-like consolidation
- Renal involvement: typically a hypovascular mass with unclear margins; in some cases, opacity of the perirenal fat and para-aortic lymph nodes can be seen
Radiology images
Prognostic factors
- Can be fatal without treatment
- Excellent prognosis with treatment; 5 year survival is > 80%
- Increased incidence of infection with higher disease burden, long term exposure to glucocorticoids and kidney involvement
- Reference: Nat Rev Dis Primers 2020;6:71
Case reports
- 15 year old girl with granulomatosis with polyangiitis with cardiac involvement (AME Case Rep 2022;7:8)
- 16 year old girl with granulomatosis with polyangiitis masquerading as tuberculosis (Pan Afr Med 2021;38:285)
- 25 year old man with granulomatosis with polyangiitis presented with pulmonary, cutaneous and neurological manifestations (Cureus 2022;14:e31753)
- 25 year old woman with granulomatosis with polyangiitis diagnosed during the postpartum period (Matern Health Neonatol Perinatol 2023;9:2)
- 25 year old woman with granulomatosis with polyangiitis presented with thrombotic vasculopathy (Cureus 2023;15:e34479)
- 32 year old woman with granulomatosis with polyangiitis related pancolitis and stricturing small bowel disease (Case Rep Gastroenterol 2023;17:155)
- 36 year old man with granulomatosis with polyangiitis presented with recurrent myocardial infarction (Clin Exp Emerg Med 2023;10:246)
- 44 year old woman with granulomatosis with polyangiitis presented with oral involvement (J Dent Sci 2023;18:451)
- 48 year old woman with granulomatosis with polyangiitis presented with acute pancreatitis (ACG Case Rep J 2023;10:e00986)
- Man in his 60s with granulomatosis with polyangiitis presented with multiple renal masses (Radiol Case Rep 2023;18:1292)
- 65 year old man with obstructive pneumonia progressing to hypertrophic pachymeningitis (Medicine (Baltimore) 2021;100:e24028)
- 66 year old woman with rare initial presentation of pancreatic disease (BMJ Case Rep 2021;14:e241033)
- 71 year old woman with spontaneously regressed granulomatosis with polyangiitis (Respir Investig 2021;59:372)
- 72 year old woman with ANCA negative granulomatosis with polyangiitis, complicated with peripheral neuropathy; the patient underwent remission induction with mepolizumab monotherapy (Intern Med 2023 Feb 1 [Epub ahead of print])
- 75 year old woman with refractory granulomatosis with polyangiitis complicated with IgG4 related disease (Intern Med 2023 Feb 22 [Epub ahead of print])
- 77 year old woman with ANCA negative granulomatosis with polyangiitis with multiple brain infarctions and endomyocarditis (Heliyon 2023;9:e12881)
Treatment
- Usually treated with corticosteroids and cyclophosphamide with excellent prognosis
- Recent addition of rituximab (monoclonal antibody) for poorly responding cases or relapses suggested (N Engl J Med 2010;363:221)
- Methotrexate serves as effective maintenance therapy for mild / limited GPA (Am J Med 2003;114:463)
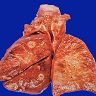
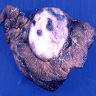
Gross description
- Multiple bilateral pulmonary nodules with frequent cavitation; rarely manifests as solitary lung lesion
Gross images
Frozen section description
- Necrotizing granulomatous inflammation, reactive changes in the background lung should not be interpreted as malignancy
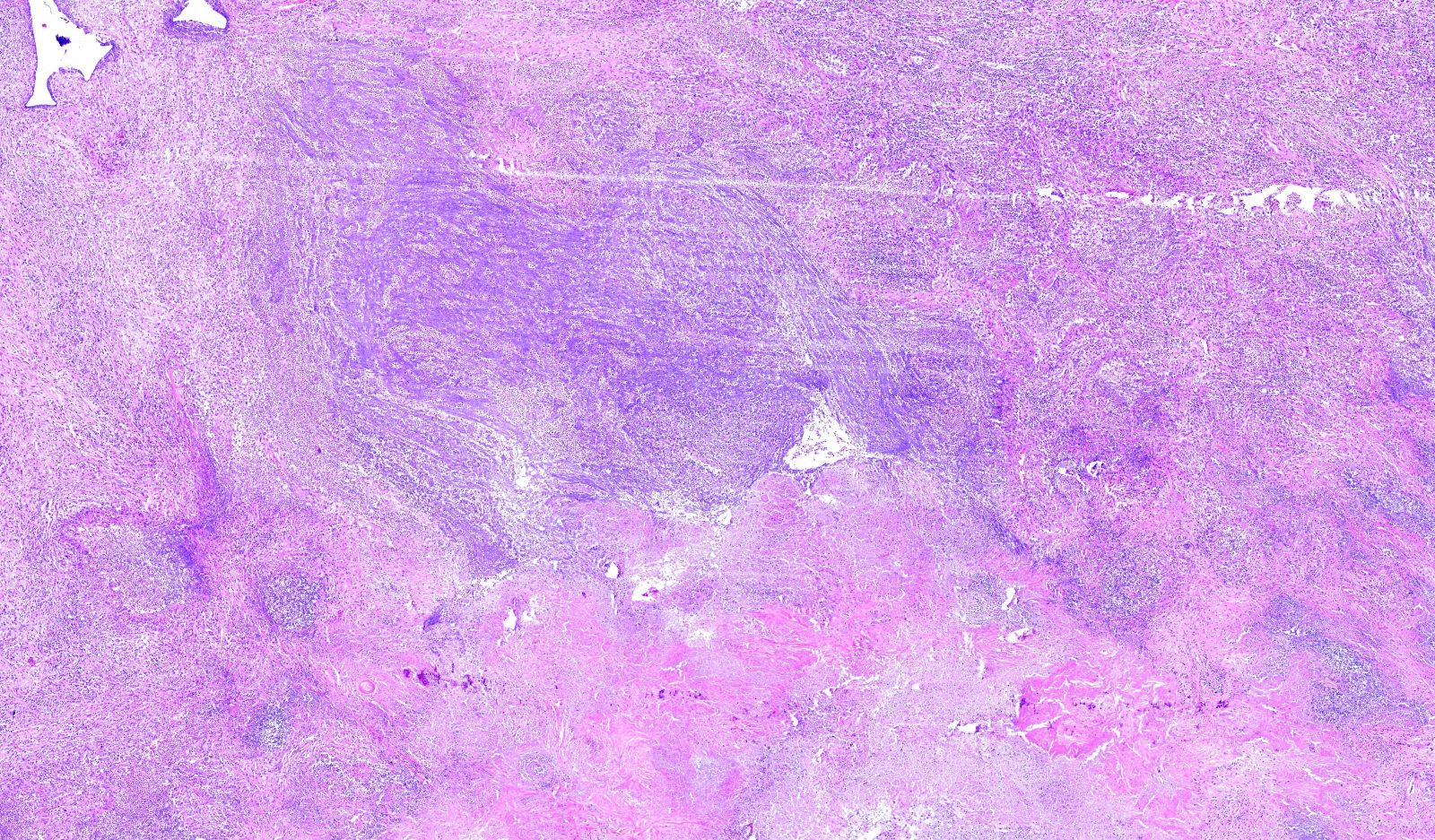
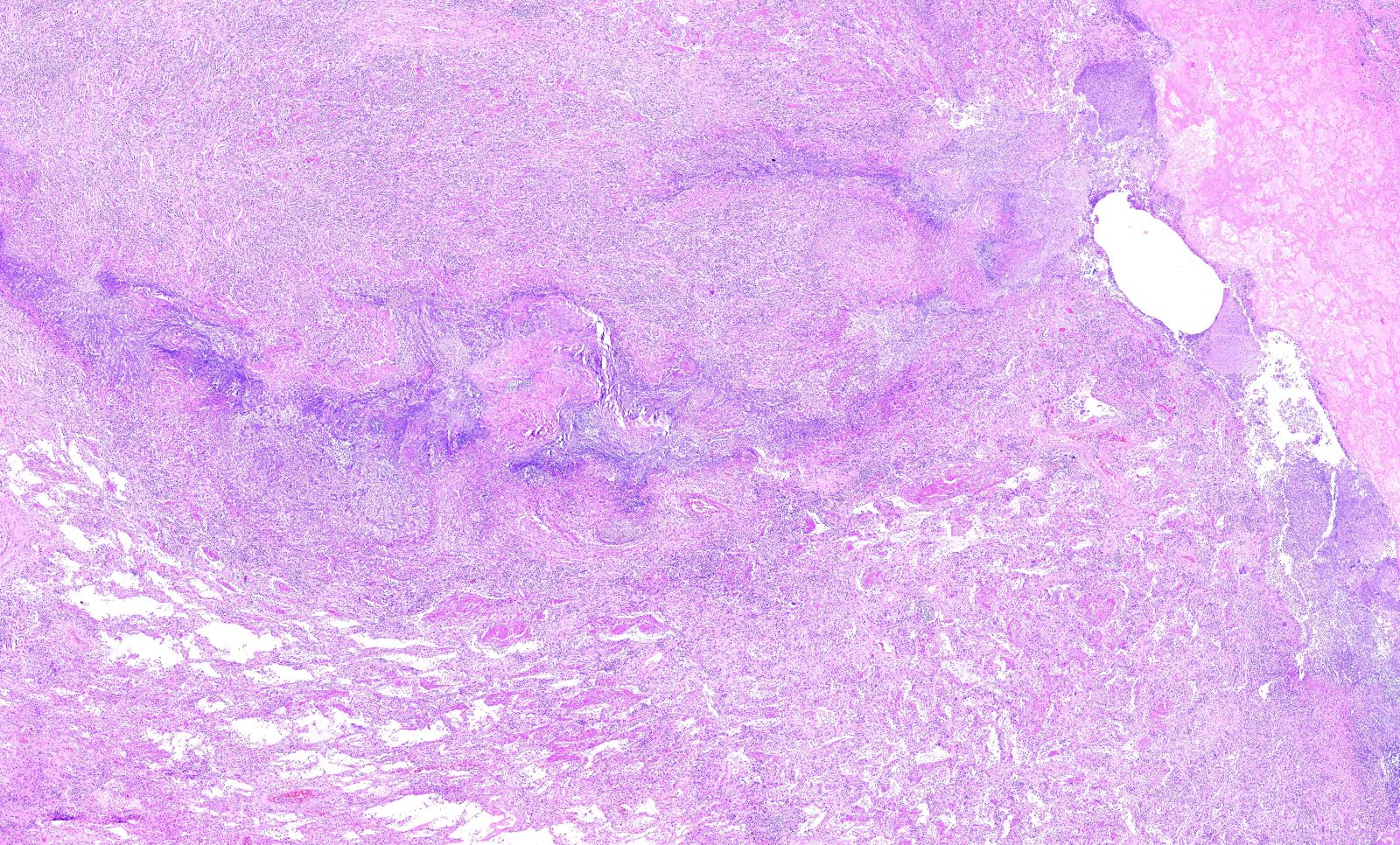
Microscopic (histologic) description
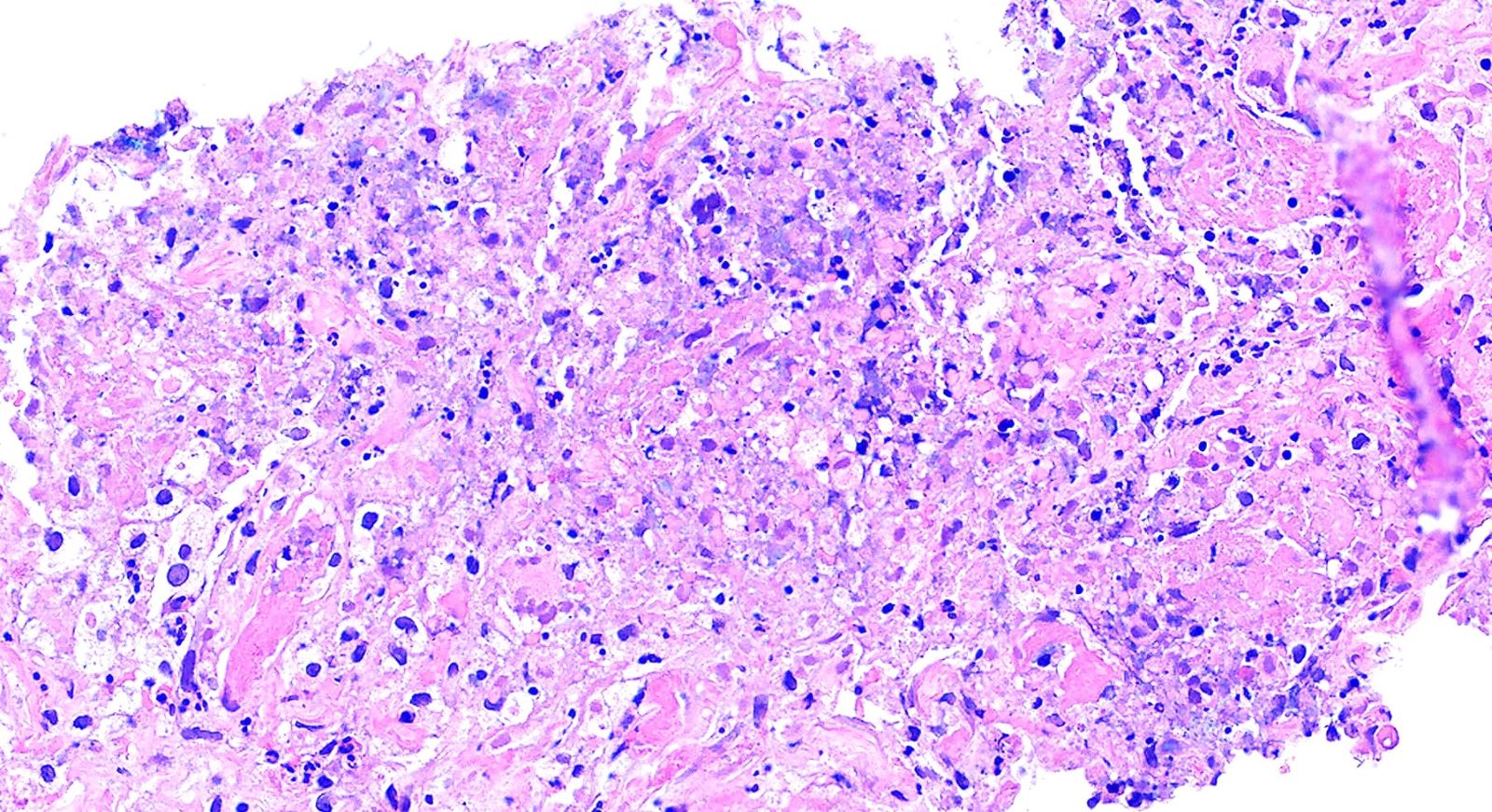
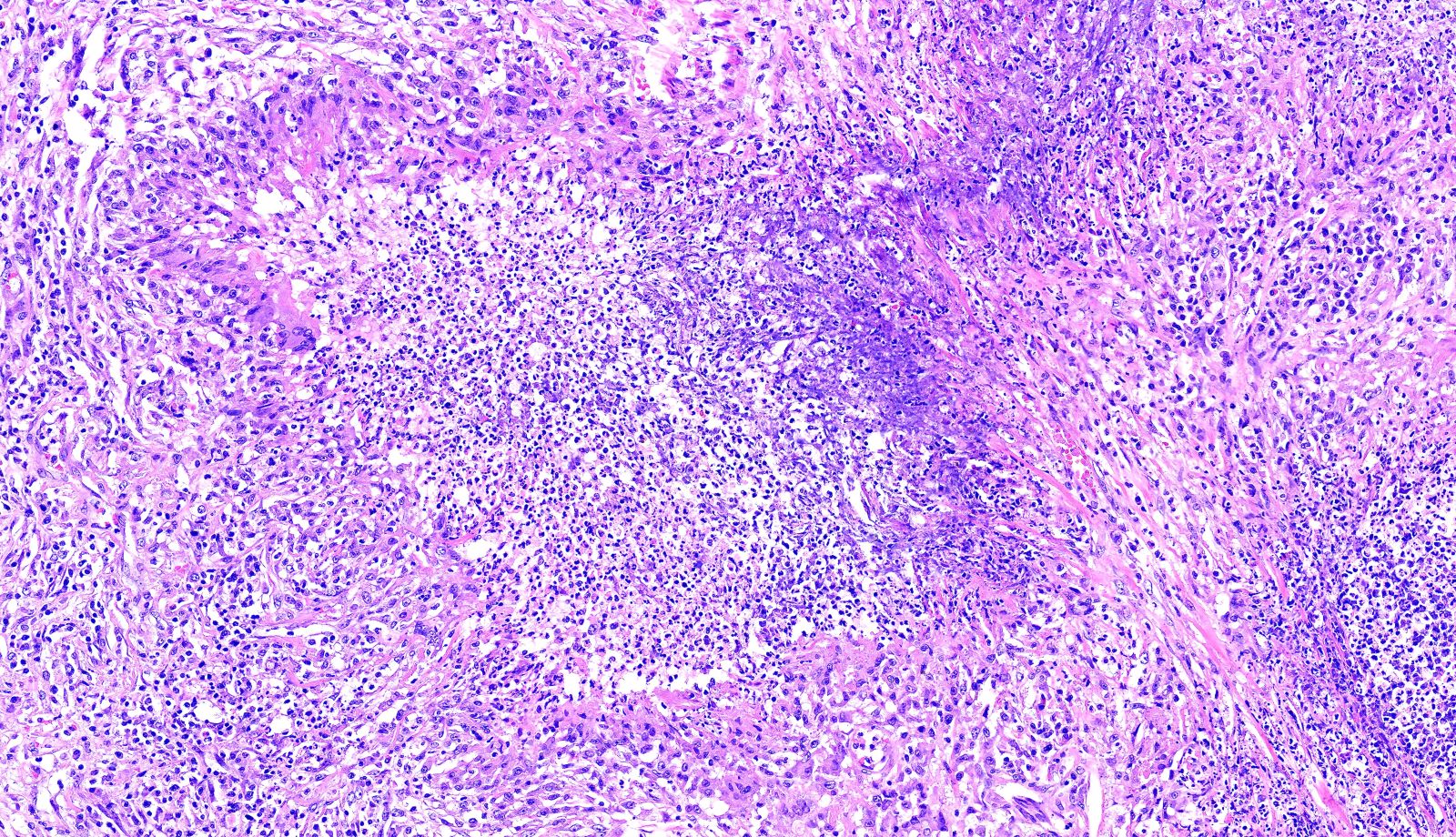
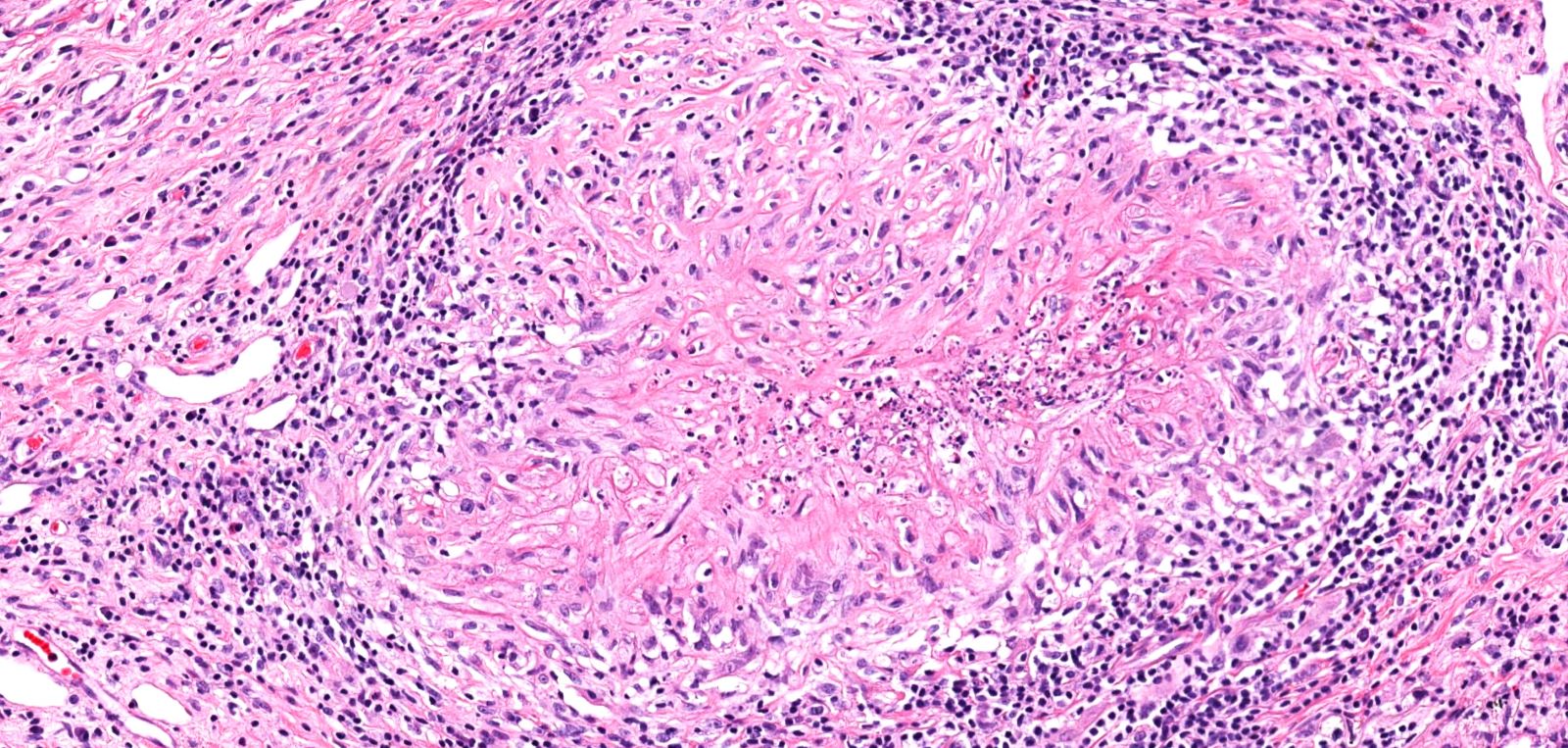
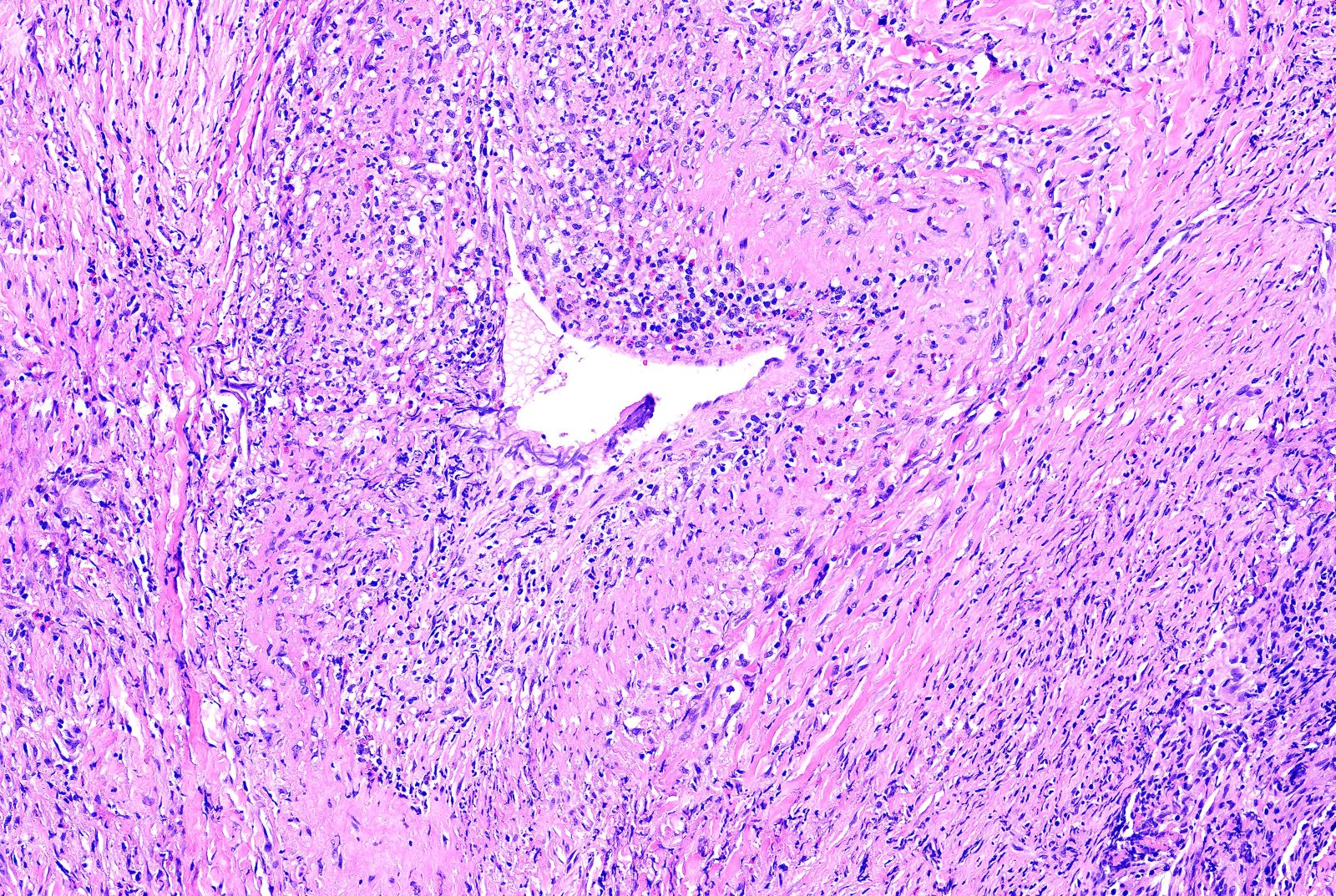
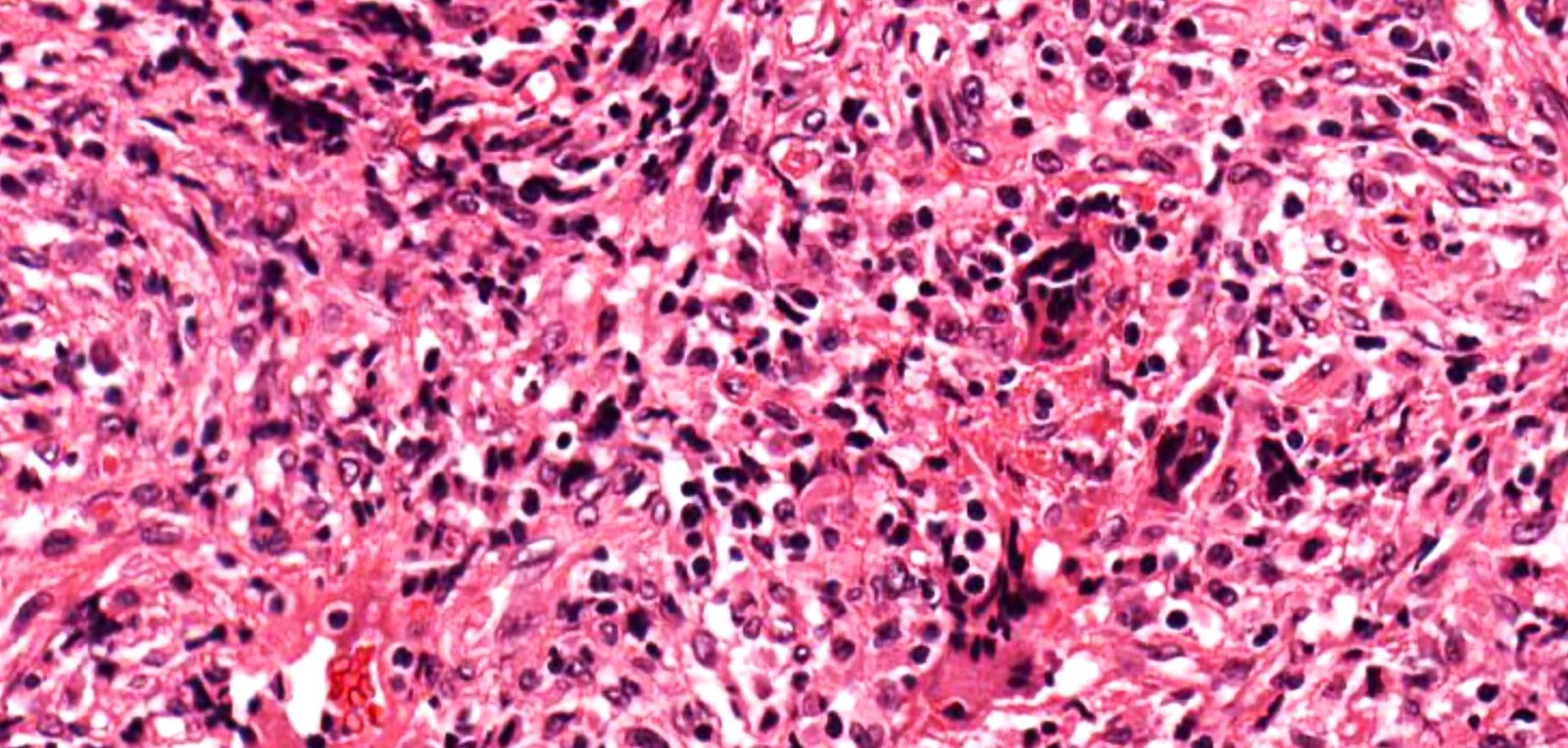
- Necrotizing granulomatous inflammation
- Deeply basophilic necrosis due to the presence of nuclear debris derived from necrosis and karyorrhexis; this type of blue necrosis is referred to as dirty necrosis
- Necrosis is often described as suppurative given the large number of neutrophils that are often present
- Necrosis is often described as geographic, which is best appreciated at low magnification and refers to the irregular contours of the necrosis that resemble the outlines of countries on a map (Am J Surg Pathol 1991;15:315)
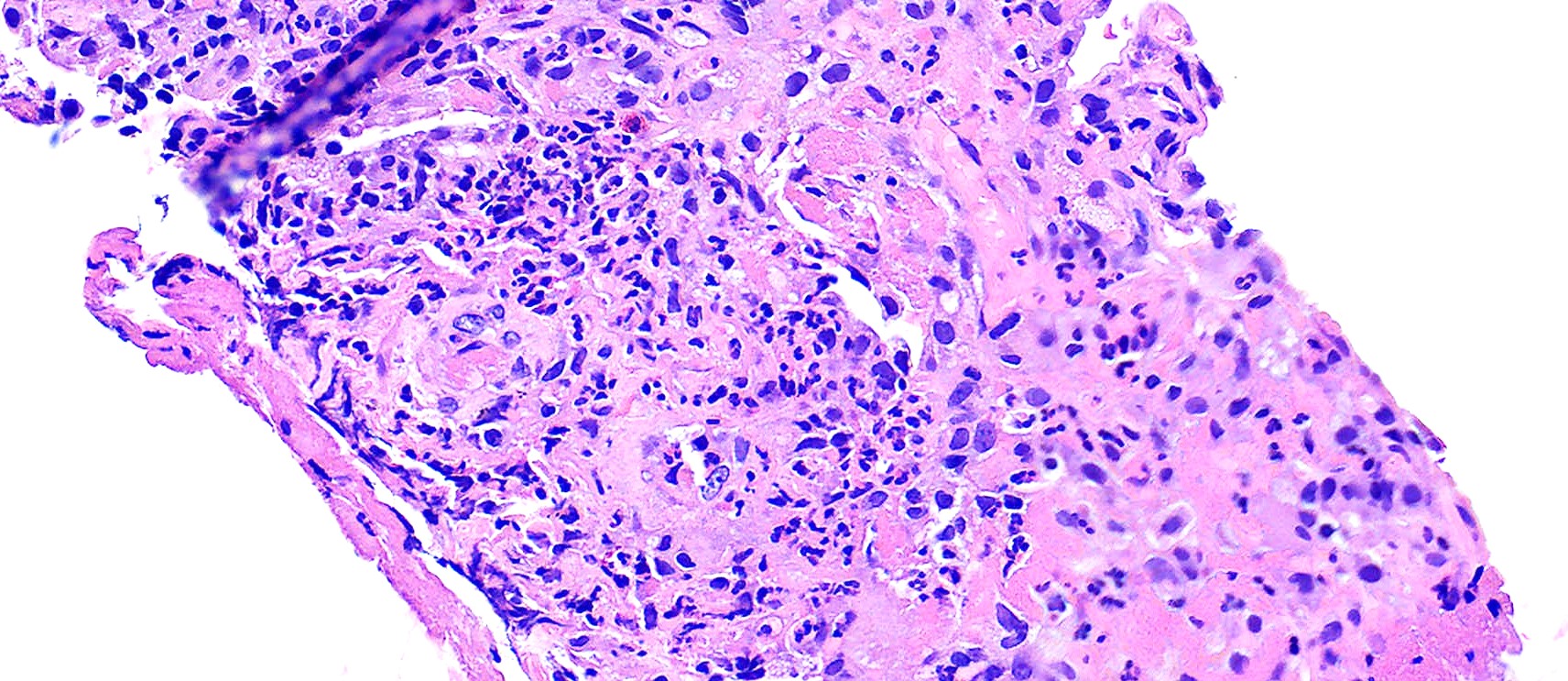
- Multinucleated giant cells in the granulomatous inflammation often have strikingly hyperchromatic nuclei
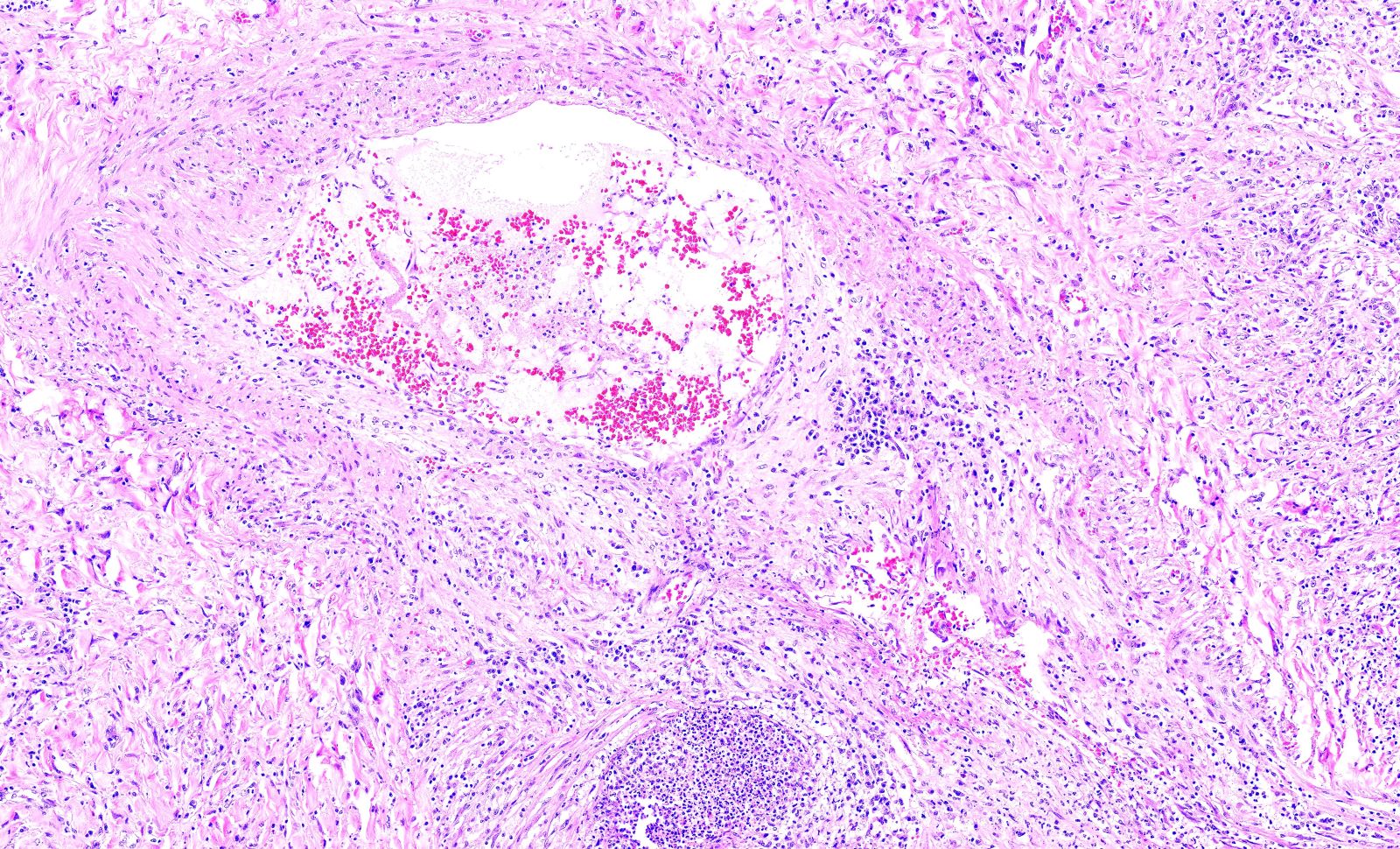
- Medium to small arteries and veins are heavily inflamed in GPA, the inflammatory cells being neutrophils, histiocytes and lymphocytes
- Necrotizing vasculitis is one of the diagnostic features and consists of necrosis of the vessel wall by an inflammatory infiltrate; this is best recognized when the vasculitis focally involves the vessel and the remainder of the vessel is intact (Hum Pathol 1988;19:1065)
- Elastic stains can help identify blood vessels; however, this may present a pitfall since completely necrotic blood vessels within zones of necrosis can be present that do not qualify for true necrotizing vasculitis
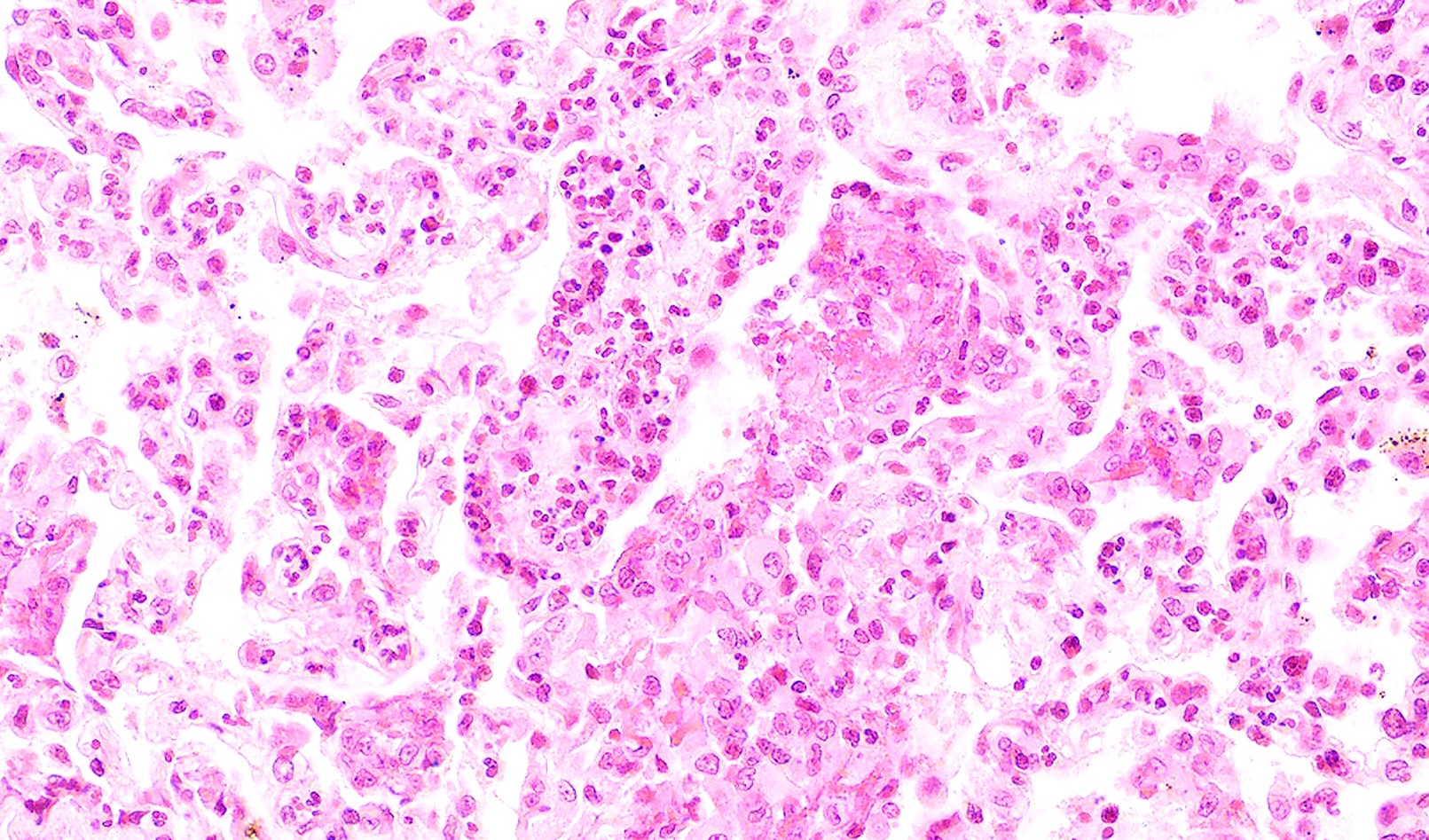
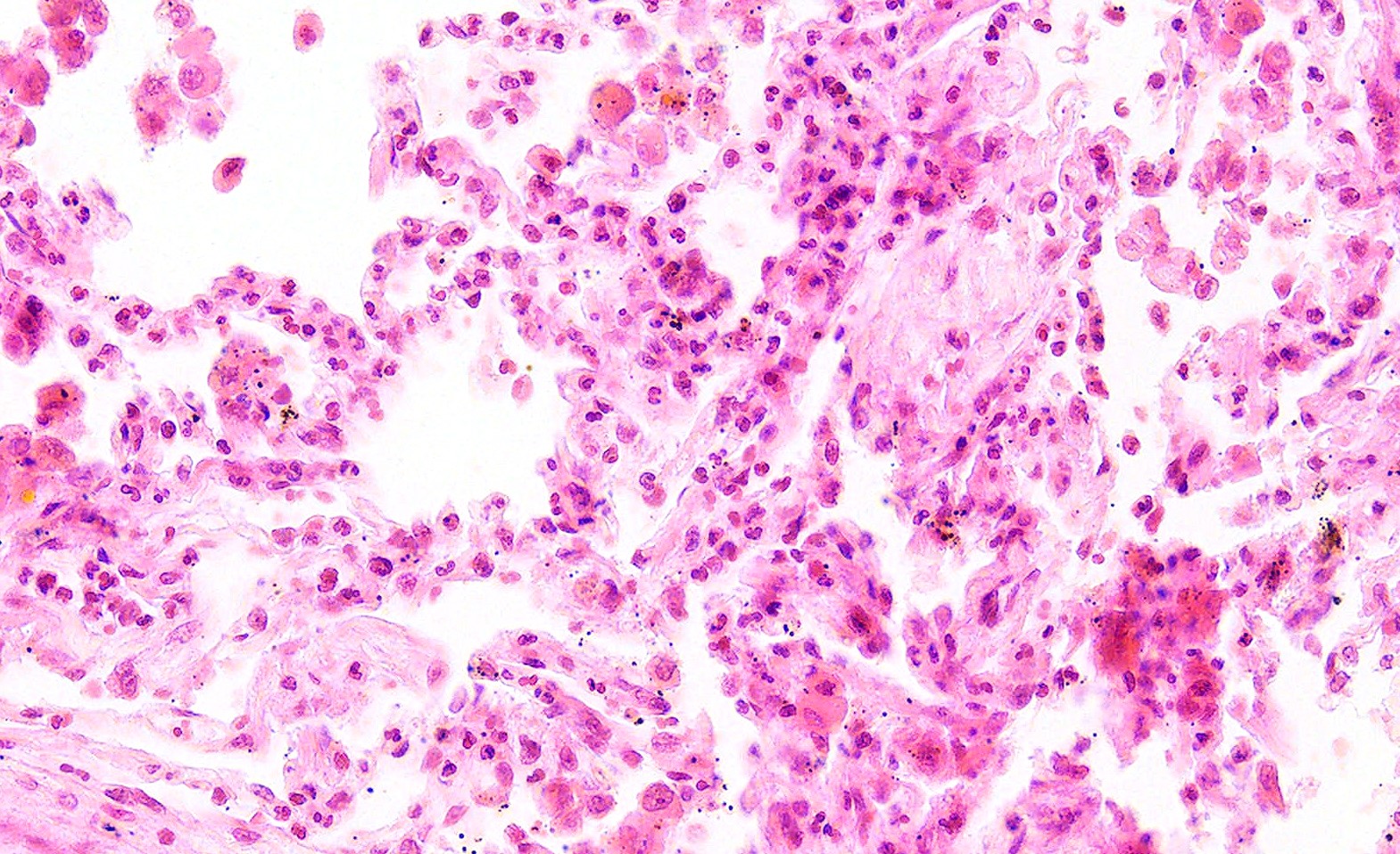
- GPA can also present as diffuse alveolar hemorrhage due to the destruction of capillaries in the alveolar septa
- This produces an inflammatory infiltrate that is rich in neutrophils and karyorrhectic debris that produces a histologic picture similar to leukocytoclastic vasculitis of the skin
- This histologic finding is typically classified as capillaritis (Semin Respir Crit Care Med 2004;25:475)
- In these cases, the adjacent alveoli are filled with hemosiderin laden macrophages, red blood cells and neutrophils
Microscopic (histologic) images
Virtual slides
Positive stains
- Elastic stains can aid in the identification of areas of vasculitis
Negative stains
- Fungal stains (GMS and PASD) are negative for fungal organisms
- Acid fast stains (ZN) are negative for acid fast organisms
Videos
Granulomatosis with polyangiitis (pathophysiology, symptoms, treatment)
GPA symptoms, diagnosis, treatment
GPA review
GPA discussion at 5:01
Sample pathology report
- Lung, right, lower lobe, wedge biopsy:
- Necrotizing granulomatous inflammation and vasculitis, most consistent with granulomatosis with polyangiitis (GPA) (see comment)
- Comment: In this specimen, there is prominent granulomatous inflammation and vasculitis. Histochemical stains for fungal and acid fast organisms are negative. The features are most consistent with a diagnosis of granulomatosis with polyangiitis (GPA). Correlation with the clinical findings and serology is recommended for definitive classification.
Differential diagnosis
- Granulomatous infection:
- Cultures or special stains are positive
- Eosinophilic granulomatosis with polyangiitis:
- P-ANCA positive
- Eosinophil rich inflammation
- Microscopic polyangiitis:
- Absence of granulomatous inflammation
- Acute lupus pneumonitis:
- Nonspecific findings with alveolar wall damage, inflammation, edema and features of acute lung injury including hyaline membranes
- Idiopathic pulmonary hemosiderosis:
- Absence of vasculitis and granulomatous inflammation
Board review style question #1
Which of the following serology tests is the most specific for granulomatosis with polyangiitis?
- ANA
- C-ANCA
- CRP
- ESR
- P-ANCA
Board review style answer #1
B. C-ANCA. Cytoplasmic (C) [PR3]-ANCA (proteinase 3) is highly specific for GPA and found in 90% of cases with generalized disease. Answer A is incorrect because ANA detects antinuclear antibodies and is seen in systemic autoimmune disorders such as systemic lupus erythematosus. Answer C is incorrect because CRP stands for C reactive protein and is a test that measure general inflammation in the body and is not specific. Answer D is similarly incorrect because erythrocyte sedimentation rate (ESR) is a general nonspecific marker of inflammation. Answer E is incorrect because P-ANCA is more commonly seen in eosinophilic granulomatosis with polyangiitis.
Comment Here
Reference: Granulomatosis with polyangiitis (GPA)
Comment Here
Reference: Granulomatosis with polyangiitis (GPA)
Board review style question #2
Board review style answer #2
C. Capillaritis. Neutrophils and karyorrhectic debris is best classified as capillaritis. Answers A, B, D and E are incorrect because they should not have neutrophils or karyorrhectic debris in the interstitium.
Comment Here
Reference: Granulomatosis with polyangiitis (GPA)
Comment Here
Reference: Granulomatosis with polyangiitis (GPA)