Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Chen-Yost HI, Konopka K. Bronchiectasis. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungnontumorbronchiectasis.html. Accessed April 1st, 2025.
Definition / general
- Permanent dilation of bronchi and bronchioles caused by the destruction of mucosal and elastic tissues; often caused by or associated with chronic necrotizing infection of bronchi and bronchioles
Essential features
- Gross pathology shows markedly distended peripheral bronchi, often with mucoid impaction and possible mucosal ulcerations
- Histology shows a range of findings in the acute setting that include luminal dilation, mucostasis, acute inflammation and inflammatory debris
- Usual respiratory type epithelium may be ulcerated and replaced by granulation tissue or squamous metaplasia
- Chronic inflammation may be variably dense and include prominent lymphoid follicles and granulomas
- Chronic findings include loss of smooth muscle, cartilage and elastic fibers with replacement fibrosis
- Can mimic squamous cell carcinoma on frozen section; on histology, need to distinguish from other lung diseases, such as chronic obstructive pulmonary disease (COPD), emphysema, cystic fibrosis and asthma
ICD coding
Epidemiology
- About 350,000 to 500,000 adults in the United States have bronchiectasis (Chron Respir Dis 2017:14:377)
- Increased risk of noncystic fibrosis bronchiectasis with extremes of ages (< 5 years, adults > 75 years)
- Associated with underserved populations
- Increased incidence of childhood bronchiectasis in Maori, Pacific Islanders of New Zealand, Australian aboriginal and Alaskan native children (Expert Rev Respir Med 2017;11:517)
Sites
- Bronchi walls in lungs
Pathophysiology
- Obstruction (i.e., due to tumor, foreign body, inspissated mucus) (Int J Chron Obstruct Pulmon Dis 2009;4:411)
- Causes resorption of air distal to the obstruction
- Causes atelectasis and accumulation of intraluminal secretions
- Nonobstructive bronchiectasis (i.e., due to pneumonia and atelectasis) (Int J Chron Obstruct Pulmon Dis 2009;4:411)
- Increased negative intrapleural pressure creates a force on bronchial walls, leading to dilation
- Infectious bronchiectasis (N Engl J Med 2002;346:1383)
- Enhanced cellular and mediator responses lead to destruction of tissue
- Biopsies have shown infiltration by neutrophils and T lymphocytes
- Chemoattractants have been detected (IL8, TNFα, prostanoids)
Etiology
- Idiopathic (Paediatr Respir Rev 2011;12:104)
- Congenital
- Cystic fibrosis: most common cause in children
- Primary ciliary dyskinesia and other mucociliary disorders
- Immune deficiencies (hypogammaglobulinemia, MHC1 deficiency, TAP1 deficiency, IFNγ receptor deficiency)
- Alpha-1 antitrypsin deficiency
- Williams-Campbell syndrome
- Infection
- Mycobacterial infection: most common cause globally
- Viral infection
- Allergic bronchopulmonary aspergillosis
- Postinfection sequelae (pneumonia, measles, whooping cough): most common, known noncystic fibrosis cause in adults
- Autoimmune
- Rheumatoid arthritis
- Other causes
- Chronic obstructive pulmonary disease / smoking
- Aspiration
- Extremes of age
- Immune dysfunction: HIV
- Foreign body / obstruction (i.e., tumor)
- Malnutrition
Diagrams / tables
Clinical features
- Cough and chronic sputum production (Thorax 2019;74:1)
- Sputum is typically mucoid to mucopurulent, thick
- Can be bloody due to erosive airway damage
- Dyspnea and wheezing
- Pleuritic chest pain
Diagnosis
- Physical exam (Thorax 2019;74:1)
- Abnormal breath sounds: crackles, wheezing, rhonchi
- Digital clubbing
- CT scan to differentiate between bronchiectasis and COPD (can overlap)
Laboratory
- CBC with differential
- Elevated blood platelets (> 400 x 109/L in stable state) are associated with increased mortality (Ann Am Thorac Soc 2021;18:1316)
- Depends on cause of bronchiectasis
- If infectious: sputum culture positive for infectious etiology
- If congenital: positive sweat chloride test, positive for genetic mutations, etc.
- Immunoglobulin quantitation
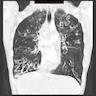
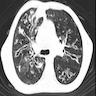
Radiology description
- Xray (Radiopaedia: Bronchiectasis [Accessed 25 August 2022]):
- Tram track opacities
- Air fluid levels
- Increase in bronchovascular markings
- CT:
- Tram track sign: thickened nontapering bronchial walls
- Signet ring sign: dilated bronchus with pulmonary artery branch seen in cross section looks like a signet ring; bronchus and artery should be the same size
- String of pearls sign
- Bunch of grapes sign: closely apposed dilated bronchi appear to look like a bunch of grapes
- Bronchus visualized within 1 cm of pleural surface
- Lack of tapering
- Increased bronchoarterial ratio
- Bronchial wall thickening
- Mucoid impaction, air trapping
Prognostic factors
- Bronchiectasis severity index
- Endorsed by the Thoracic Society of Australia and New Zealand
- Uses clinical, radiological and microbiological features to predict morbidity and mortality in noncystic fibrosis bronchiectasis
- Point system on a scale from 0 - 9
- 0 - 4: mild bronchiectasis
- 5 - 8: moderate bronchiectasis
- 9+: severe bronchiectasis
- Calculator: University of Dundee: Bronchiectasis Prediction Tools [Accessed 25 August 2022]
- FACED score (Open Respir Med J 2015;9:46)
- Uses a 0 - 7 point scale to determine the severity of bronchiectasis in noncystic fibrosis patients
- FACED stands for different metrics
- F: forced expiratory volume in 1 second (FEV1) (≥ 50% = 0 points, < 50% = 2 points)
- A: age (< 70 years = 0 points, ≥ 70 years = 2 points)
- C: chronic colonization (no Pseudomonas = 0 points, presence of Pseudomonas = 1 point)
- E: extension (1 - 2 lobes affected = 0 points, > 2 lobes affected= 1 point)
- D: dyspnea; modified Medical Research Council scale (mMRC) (0 - 2 = 0 points, 3 - 4 = 1 point)
Case reports
- 42 year old man, a gardener with recurrent respiratory infections (Hosp Pract (Off Ed) 1985;20:97)
- 49 year old man who developed bronchiectasis after bronchial thermoplasty (J Thorac Dis 2018;10:E721)
- 52 year old man who developed COVID-19 associated bronchiectasis (Cureus 2021;13:e15051)
Treatment
- Nonpharmacologic therapies (UpToDate: Bronchiectasis in Adults [Accessed 25 August 2022]):
- Avoid lung irritant
- Physiotherapy to clear airways
- Mucolytics
- Airway hydration
- Pulmonary rehabilitation for severe cases
- Antibiotics to prevent exacerbations:
- Macrolides: especially for Pseudomonas aeruginosa
- Other pharmacologic treatments:
- Bronchodilators
- NSAIDs
- Glucocorticoids
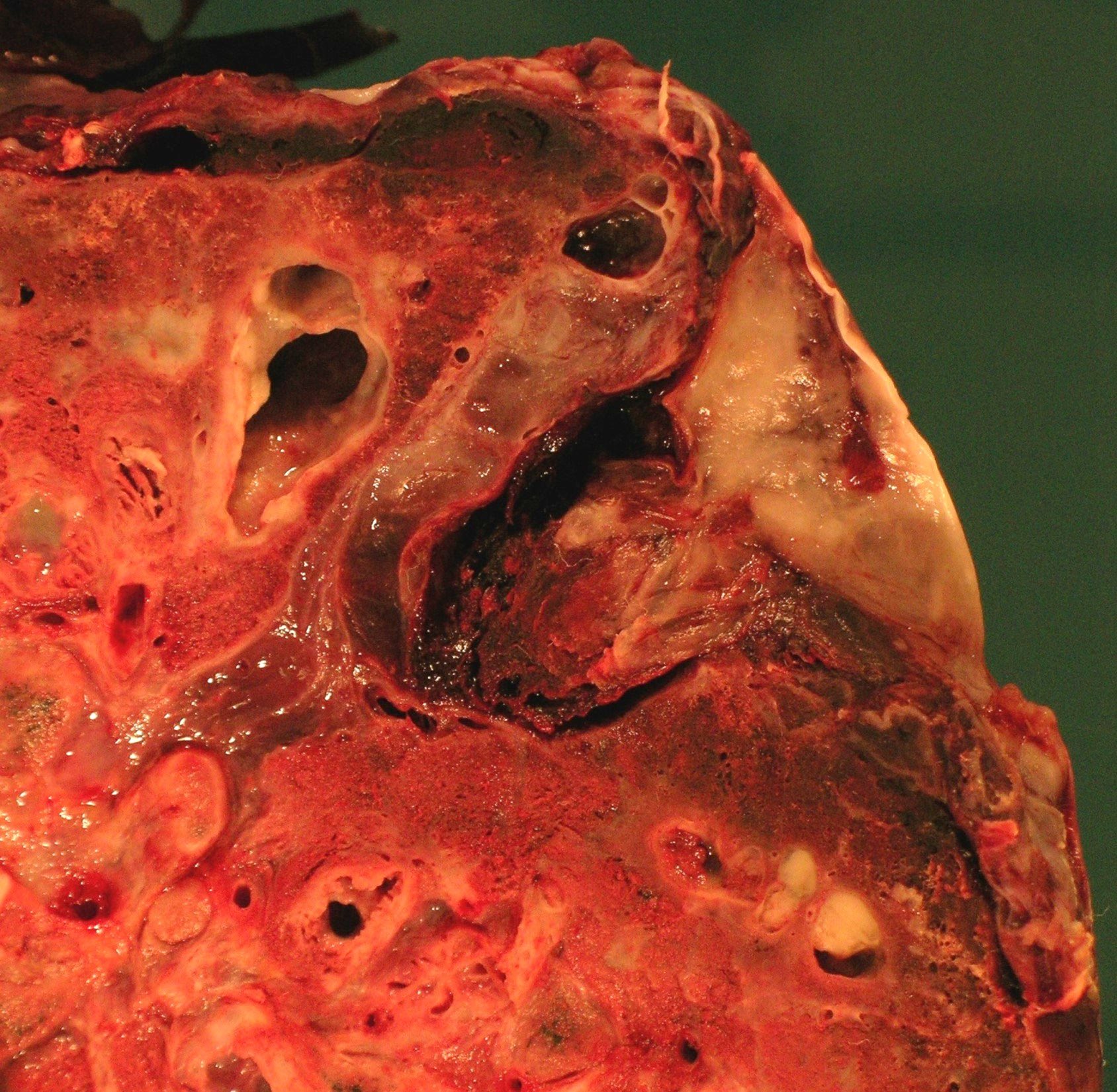
Gross description
- Markedly distended peripheral bronchi, usually in lower lobes; can be traced up to pleura (Zander: Pulmonary Pathology, 2nd Edition, 2017)
- Variety of shapes present: saccular, cystic, cylindrical
- Ulcerations of mucosal surfaces
- Irregularly thickened bronchial walls
- Honeycomb appearance on cut surface
- Can have thick mucoid or mucopurulent secretions present in the bronchi
Gross images
Frozen section description
- Can be challenging on frozen section to distinguish from carcinoma (Int J Clin Exp Pathol 2015;8:7961)
- Bronchial epithelial shedding
- Epithelial squamous metaplasia: can be confused for carcinoma in situ
- Bronchial mural destruction
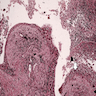
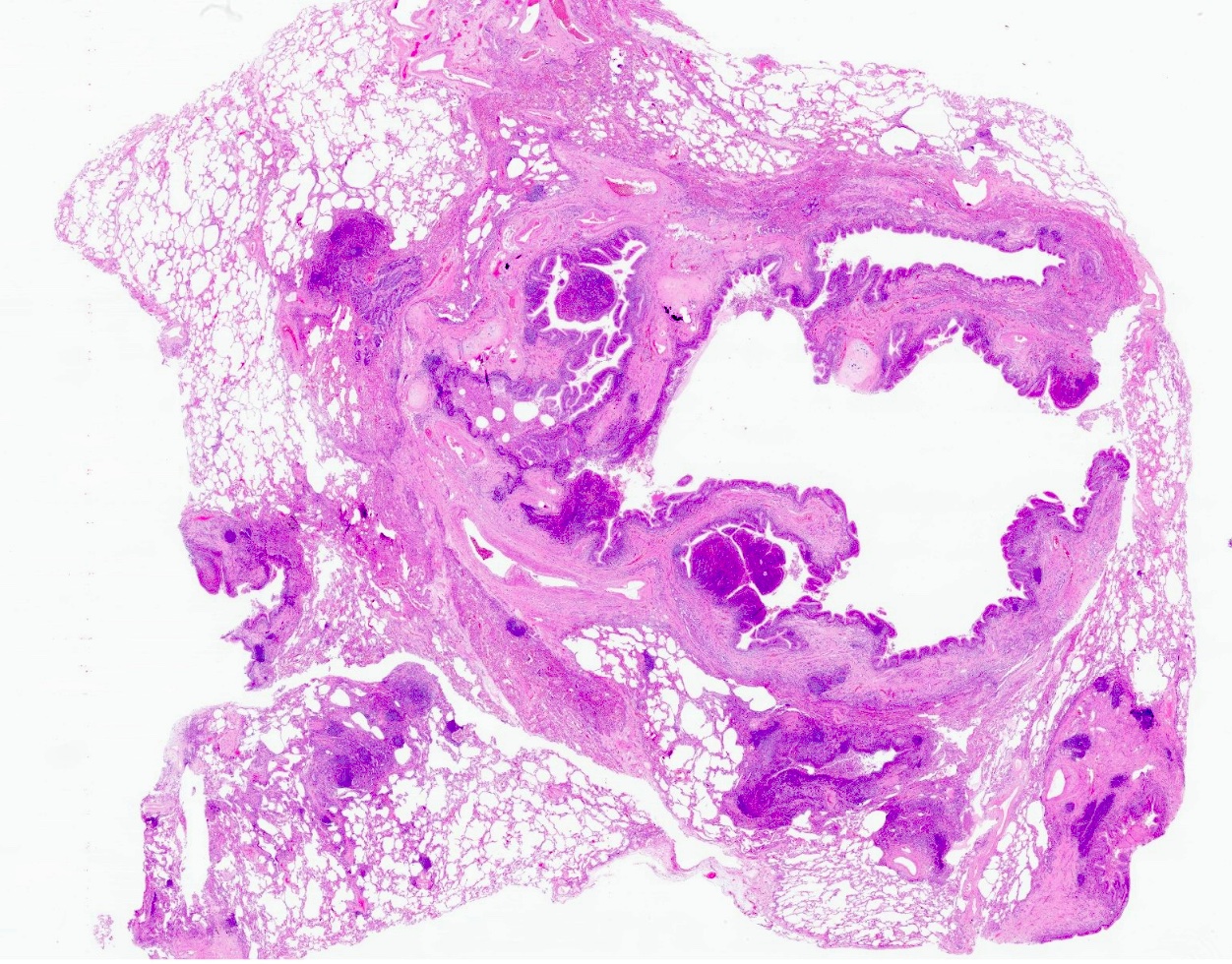
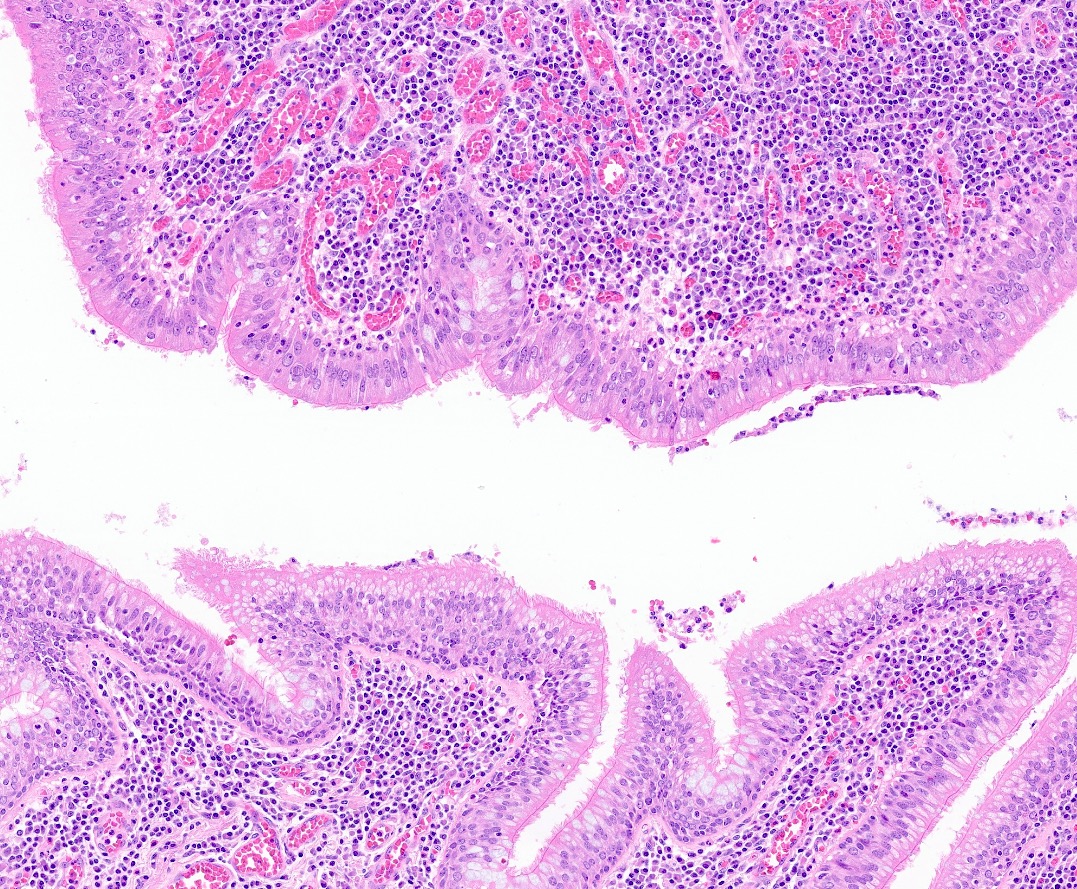
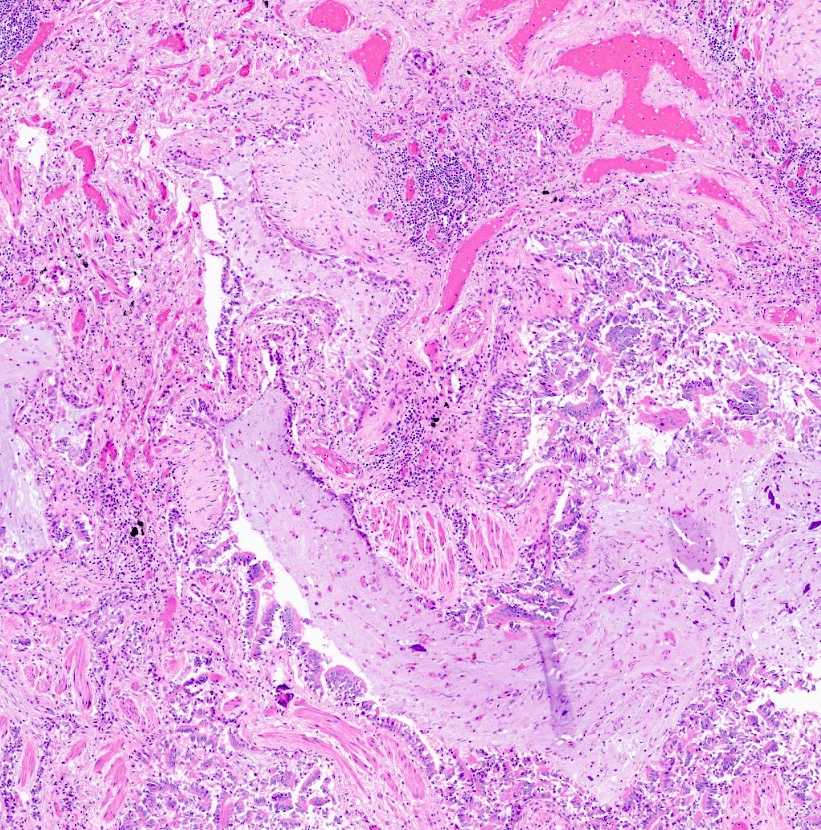
Microscopic (histologic) description
- Inflammatory cells along airways (Zander: Pulmonary Pathology, 2nd Edition, 2017)
- Prominent chronic inflammation with lymphoid follicles and germinal centers
- Varying degrees of neutrophilic inflammation
- Can have eosinophils depending on the cause or if the patient has superimposed diseases
- Epithelial ulceration
- Over time
- Loss of smooth muscle, elastic fibers, cartilage
- Replacement fibrosis
- Coexisting findings
- Organizing pneumonia
- Pulmonary hypertension
Microscopic (histologic) images
Virtual slides
Cytology description
- Seen in bronchial brushings and washings (Diagn Cytopathol 2008;36:13)
- Mixed inflammation
- Cellular debris
- Mucus
- Reactive bronchial cells
- Squamous metaplasia
Videos
Histopathology: lung bronchiectasis
Sample pathology report
- Bilateral lungs, explant:
- Severe acute and chronic bronchiolitis with patchy fibrosis, consistent with bronchiectasis. Acute bronchopneumonia involving the left lower lobe.
- Bilateral lungs, explant:
- Bronchiectasis with severe and necrotizing acute and chronic bronchitis and bronchiolitis consistent with cystic fibrosis.
Differential diagnosis
- Many things can mimic or be superimposed with bronchiectasis but key findings can differentiate other diseases from it
- Bronchitis:
- Increased goblet cells, thickened bronchial walls
- Asthma:
- More eosinophils, goblet cell hyperplasia and metaplasia, Charcot-Leyden crystals, smooth muscle hypertrophy
- Aspiration pneumonia:
- More neutrophils, abscesses, vegetable matter
- Tuberculosis:
- Necrotizing granulomas
- Emphysema:
- Gross findings with bullae
- Alveolar wall destruction versus bronchiole destruction
- Lymphoid malignancies:
- Monotonous lymphoid populations with abnormal germinal centers
- Bronchitis:
Additional references
Board review style question #1
Board review style answer #1
C. Cystic fibrosis. The histologic finding seen here is bronchiectasis. While the other answers can be associated with bronchiectasis, the most common cause in this question would be cystic fibrosis. Loeffler syndrome is not a congenital disease (associated with Ascaris infection).
Comment Here
Reference: Bronchiectasis
Comment Here
Reference: Bronchiectasis
Board review style question #2
Board review style answer #2
B. Bronchiectasis. The gross image of the explanted lung comes from a patient who had a lung transplant for cystic fibrosis disease. The bronchi walls are thickened, ulcerated, dilated and there is thick mucin present in the lumen. The dilation extends to the pleura. Emphysema would have more bullae. Asthma would also have mucin but also hyperinflation. Tuberculosis would most likely show granulomas, areas of caseating necrosis or miliary seeding. Interstitial fibrosis often shows honeycombing, prominent cobblestoning of the surface and may or may not have concurrent bronchiectasis.
Comment Here
Reference: Bronchiectasis
Comment Here
Reference: Bronchiectasis