Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Li H, Schulte JJ. Tuberculosis. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungnontumorTB.html. Accessed March 31st, 2025.
Definition / general
- Infectious disease caused by Mycobacterium tuberculosis
- Disease can be manifested as primary, secondary and miliary tuberculosis
Essential features
- Infectious disease caused by M. tuberculosis
- Hallmark is necrotizing granulomatous inflammation, composed of central necrotic zone surrounded by epithelioid histiocytes and Langhans type giant cells
- Presence of M. tuberculosis should be confirmed by microbiologic cultures or nucleic acid amplification testing (NAAT)
Terminology
- TB
- Mycobacterium tuberculosis (MTB)
ICD coding
Epidemiology
- Globally, an estimated 10 million people developed active tuberculosis (TB) disease in 2019, with 1.4 million TB deaths (Int J Infect Dis 2021;113:S7)
- 8 countries accounted for 66% of the total number of global cases of TB: India (26%), Indonesia (8.5%), China (8.4%), the Philippines (6.0%), Pakistan (5.7%), Nigeria (4.4%), Bangladesh (3.6%) and South Africa (3.6%) (Int J Infect Dis 2021;113:S7)
- Risk factors:
- Immunosuppression, including HIV / AIDS, chronic immunosuppressive therapy or an inborn immunodeficiency (Cureus 2021;13:e19852, Emerg Microbes Infect 2016;5:e10)
- Occupational: mining, construction work, pneumoconiosis (silicosis) (J Clin Tuberc Other Mycobact Dis 2021;23:100218)
Sites
- Most commonly affects the respiratory system but other systems can be involved in disseminated disease (gastrointestinal [GI] system, lymphoreticular system, skin, central nervous system, musculoskeletal system, reproductive system and liver) (J Family Community Med 2019;26:83)
Pathophysiology
- Infection begins when M. tuberculosis enters lungs via inhalation, reaches the alveolar space and encounters resident alveolar macrophages
- If alveolar macrophages do not eliminate the bacteria, M. tuberculosis invades the lung interstitial tissue, either by direct infection of alveolar epithelium or as a result of the infected alveolar macrophage migrating to the interstitium
- Dendritic or other inflammatory cells transport M. tuberculosis to pulmonary lymph nodes for T cell priming; this yields recruitment of immune cells to form a granuloma
- Granuloma may contain infection (latent TB)
- Bacteria replicate within the growing granuloma; if the granuloma fails to contain the infection, bacteria may disseminate to other organs and can enter the bloodstream, leading to reentry and release into the respiratory tract; the infected host is now infectious and symptomatic (active TB disease)
- Primary TB is defined as an infection caused by MTB in a previously uninfected host; secondary TB usually occurs due to reactivation of latent TB after initial primary infection; miliary TB is the disseminated form with hematogenous spread of MTB to the lungs and other organs
- Selected reviews on the pathophysiology of M. tuberculosis infection: Nat Rev Dis Primers 2016;2:16076, FEMS Microbiol Rev 2019;43:341
Etiology
- Mycobacterium tuberculosis (M. tuberculosis)
- M. tuberculosis complex comprises the following subgroups: M. tuberculosis, M. africanum, M. canettii, M. bovis, M. caprae, M. pinnipedii, M. microti and M. mungi (J Bacteriol 2006;188:4271, Proc Natl Acad Sci U S A 2002;99:3684, Emerg Infect Dis 2010;16:1296)
- Multidrug resistant (MDR) strains exist
Clinical features
- Chronic and persistent cough (often productive), weight loss, fever, night sweats and hemoptysis (J Biomed Sci 2020 Jun;27:74)
- History of latent TB or exposure to infected individual may be identified
Diagnosis
- Based on a combination of exposure history, clinical symptoms, radiologic and laboratory findings (Nat Rev Dis Primers 2016;2:16076)
- Diagnosis can be made with lung or lymph node biopsy, surgical resection specimen, cytology specimen and autopsy specimen, although lung biopsy does not have a routine role in diagnosis
Laboratory
- Active TB disease
- Imaging techniques (chest Xrays and PET CT)
- Sputum smears (AFB or Ziehl-Neelsen staining)
- Cultures
- Molecular tests (nuclear amplification and gene based tests)
- Latent TB infection
- Tuberculin skin testing (Mantoux test with purified protein derivative [PPD])
- Interferon release assays (IGRA)
- Selected references with discussions on diagnostic techniques: Nat Rev Dis Primers 2016;2:16076, J Biomed Sci 2020 Jun;27:74, N Engl J Med 2021;385:2271
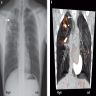
Radiology description
- Often identified on chest Xray or CT
- Commonly presents as cavitary lesion in upper lobe in infected immunocompetent hosts
- Immunocompromised patients can show lower lobe disease with adenopathy and pleural effusion
- Review of radiographic findings: Insights Imaging 2022;13:3
Radiology images
Prognostic factors
- Immune status of host
- HIV infection increases risk of TB infection (J Biomed Sci 2020 Jun;27:74)
- Diabetes mellitus increases risk for active TB infection (PLoS Med 2008;5:e152)
- Approximately 29% of people with TB infection are unidentified, leading to inadequate treatment, morbidity and mortality (Int J Infect Dis 2021;113:S7)
- Suboptimal treatment and multidrug resistant strains contribute to morbidity and mortality (Int J Infect Dis 2021;113:S7)
Case reports
- 8 year old girl with drug resistant miliary tuberculosis (N Engl J Med 2018;378:e10)
- 56 year old man with miliary tuberculosis and choroidal tubercles (N Engl J Med 2020;383:e78)
- 63 year old man with hemophagocytic lymphohistiocytosis (HLH) associated with disseminated tuberculosis (N Engl J Med 2020;382:1749)
- 67 year old man with coexistence of lung adenocarcinoma and pulmonary tuberculosis within a single lesion (Medicine (Baltimore) 2019;98:e17378)
- 72 year old man with advanced NSCLC developed acute pulmonary tuberculosis following anti-PD1 antibody treatment (J Thorac Oncol 2016;11:2238)
Treatment
- Latent TB infection
- 6 - 9 months of isoniazid, 3 - 4 months of isoniazid plus rifampicin, or 3 - 4 months of rifampicin alone (WHO: Guidelines on the Management of Latent Tuberculosis Infection [Accessed 23 February 2022])
- Active drug sensitive TB disease
- Minimum of 6 months of therapy with rifampicin, isoniazid, pyrazinamide and ethambutol during the first 2 months (the intensive phase of treatment), followed by rifampicin and isoniazid for 4 months (the continuation phase) (WHO: Treatment of Tuberculosis - Guidelines, 4th edition [Accessed 23 February 2022], Clin Infect Dis 2016;63:e147, Nat Rev Dis Primers 2016;2:16076)
- Treatment efficacy and progress are usually monitored with repeat sputum smears, cultures and chest Xrays
- Drug resistant strains of TB are an emerging problem
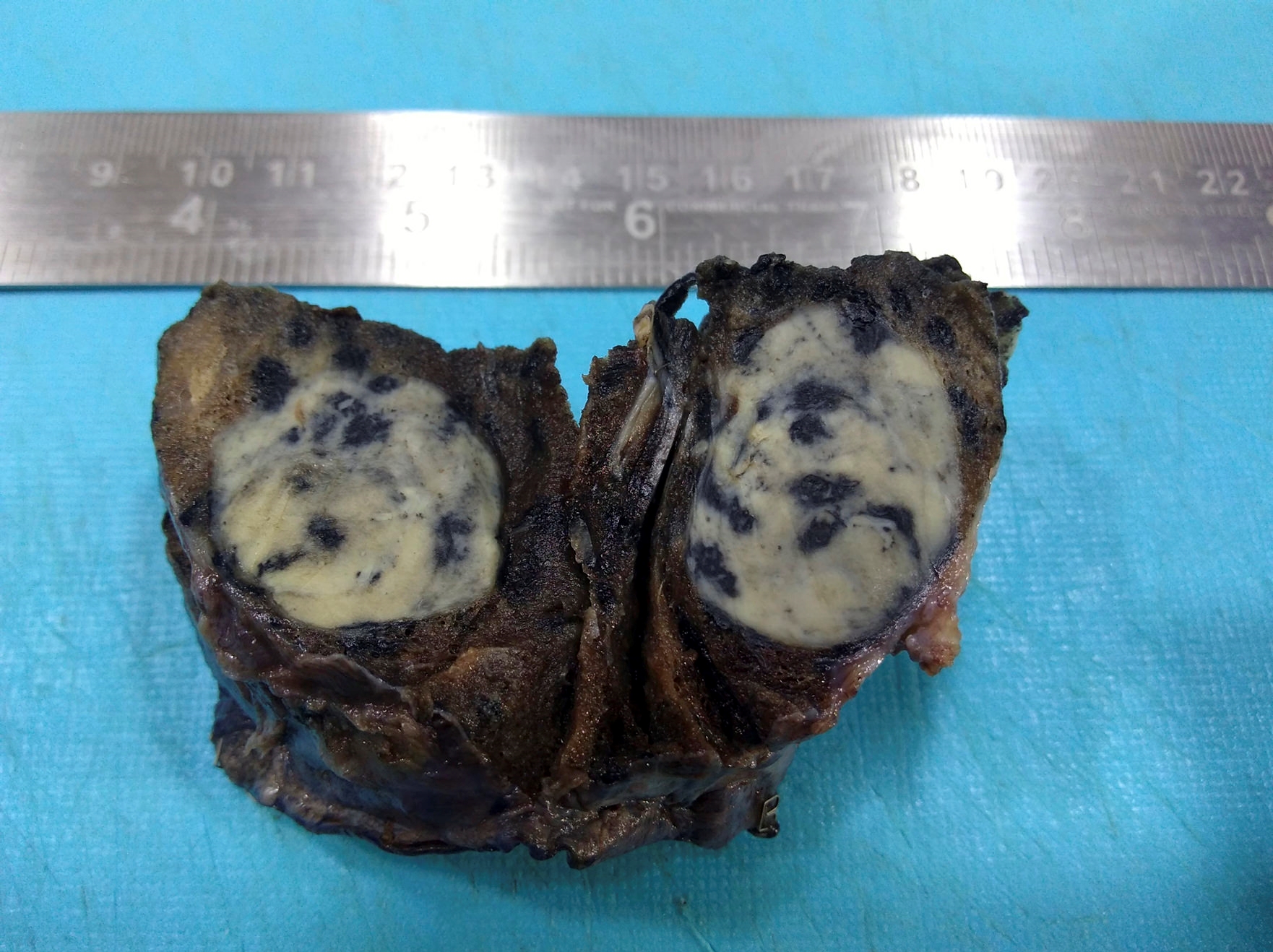
Gross description
- Cavitary disease
- Tuberculomas: localized conglomerates of necrotizing granulomatous infection
- Classically described findings:
- Ghon focus: subpleural, often upper lobe nodule
- Ghon complex: Ghon focus plus lymphatic or hilar lymph node involvement
- Caseous necrosis (cheese-like, tan to white grumous material)
- Can cause empyema (chronic or active infection of the pleural space), resulting in pus accumulation in pleural cavity
- Bronchiectasis (irreversible dilatation of the bronchi and bronchioles)
- References: Semin Diagn Pathol 2017;34:518, StatPearls: Ghon Complex [Accessed 23 May 2022]
Gross images
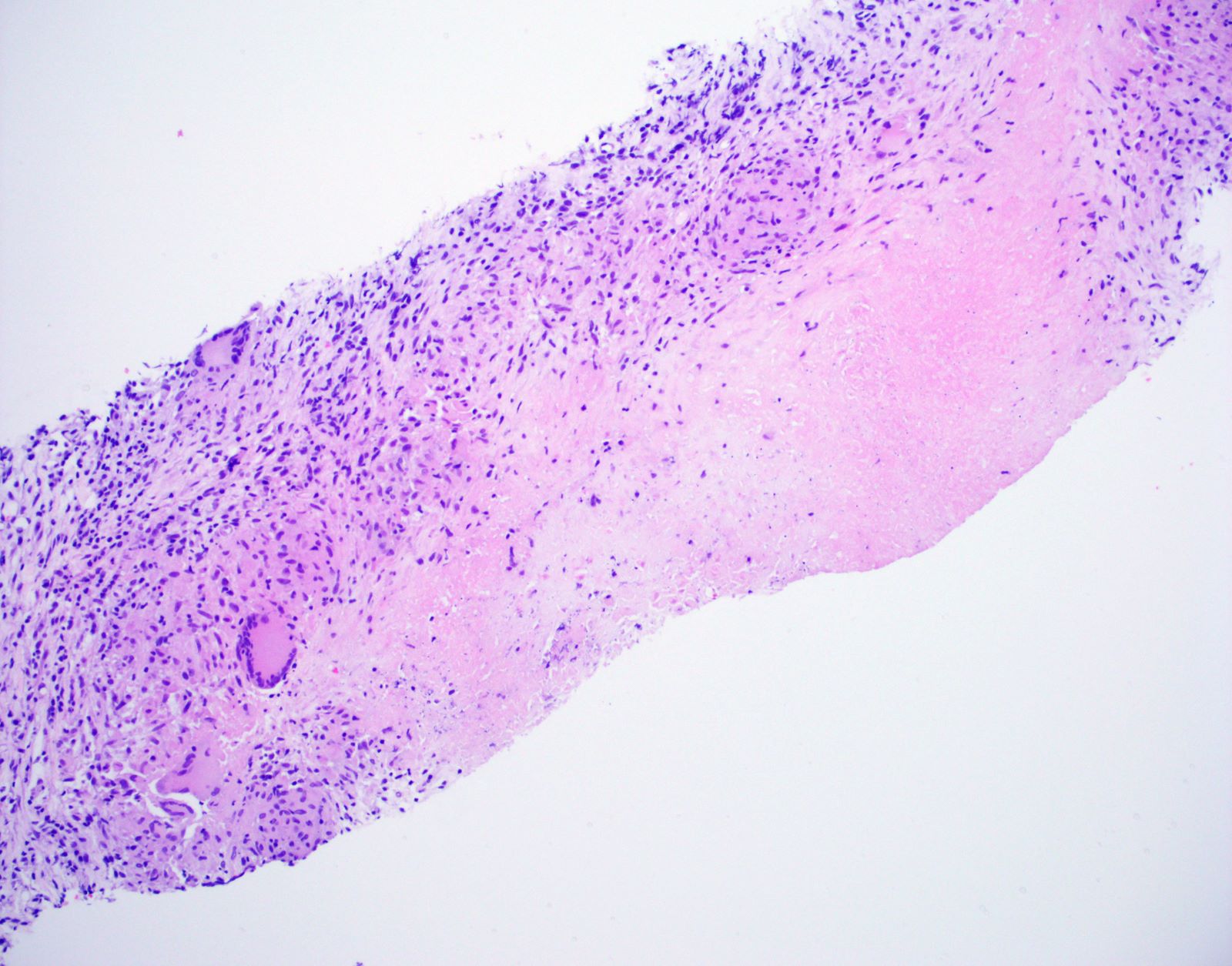
Microscopic (histologic) description
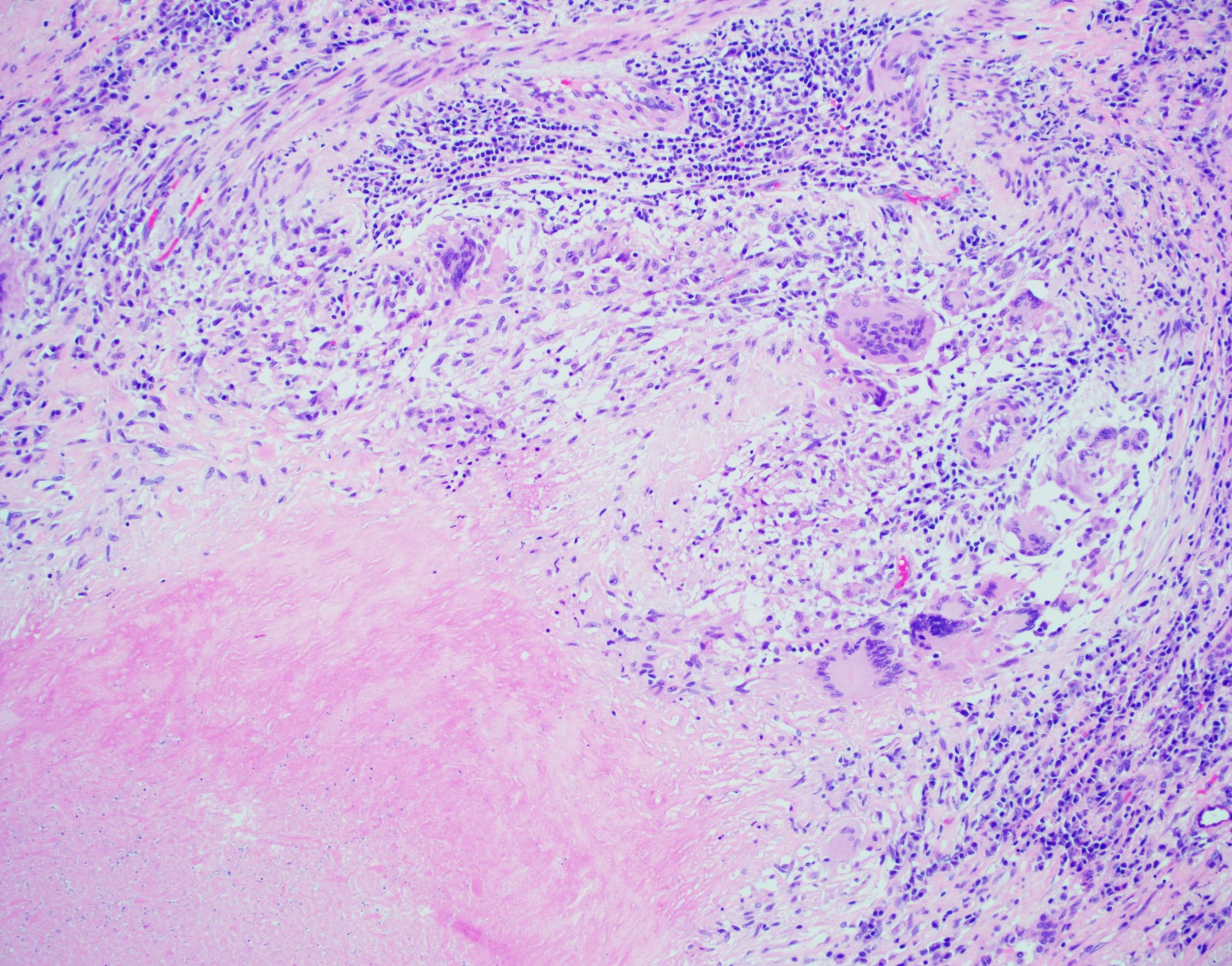
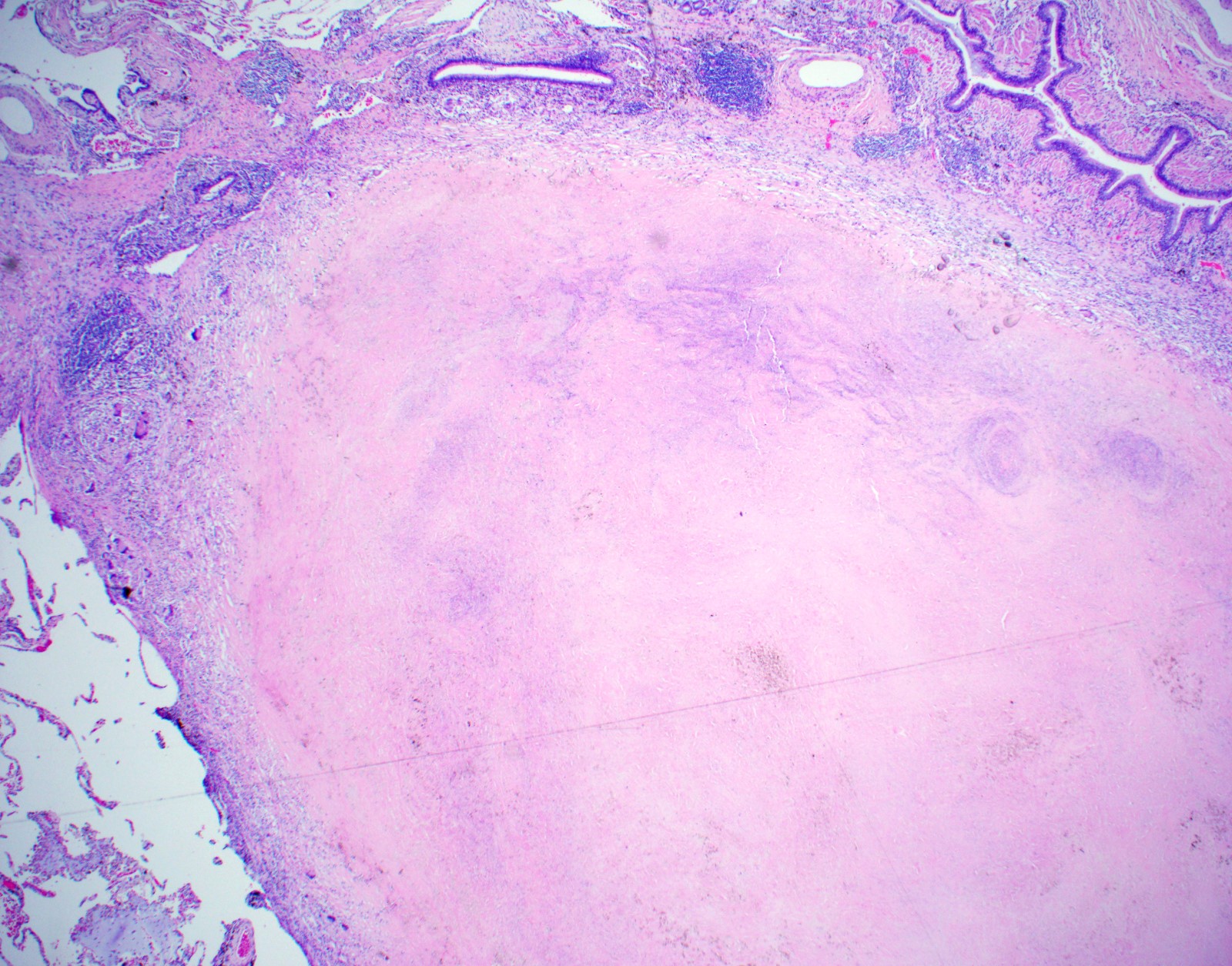
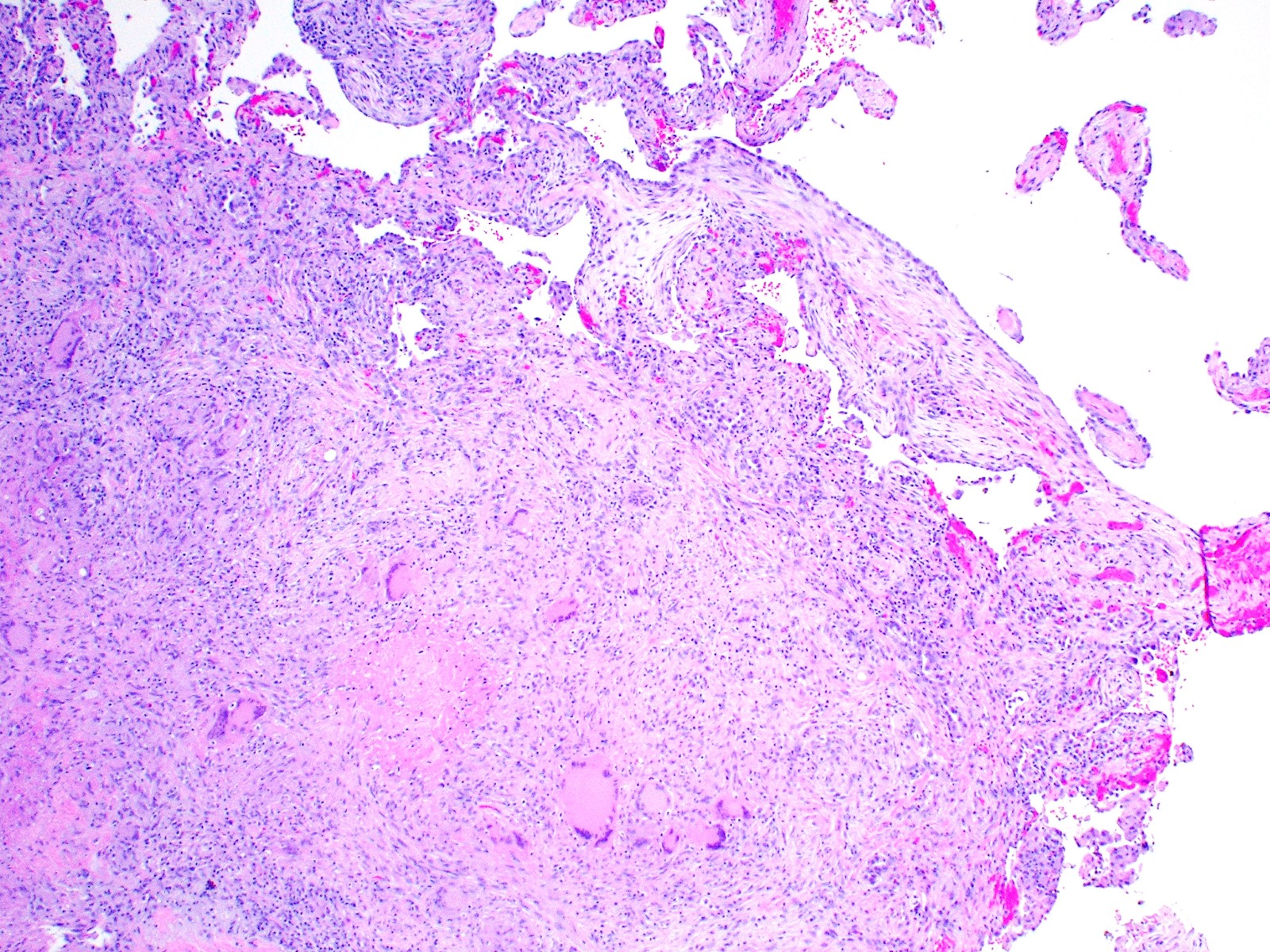
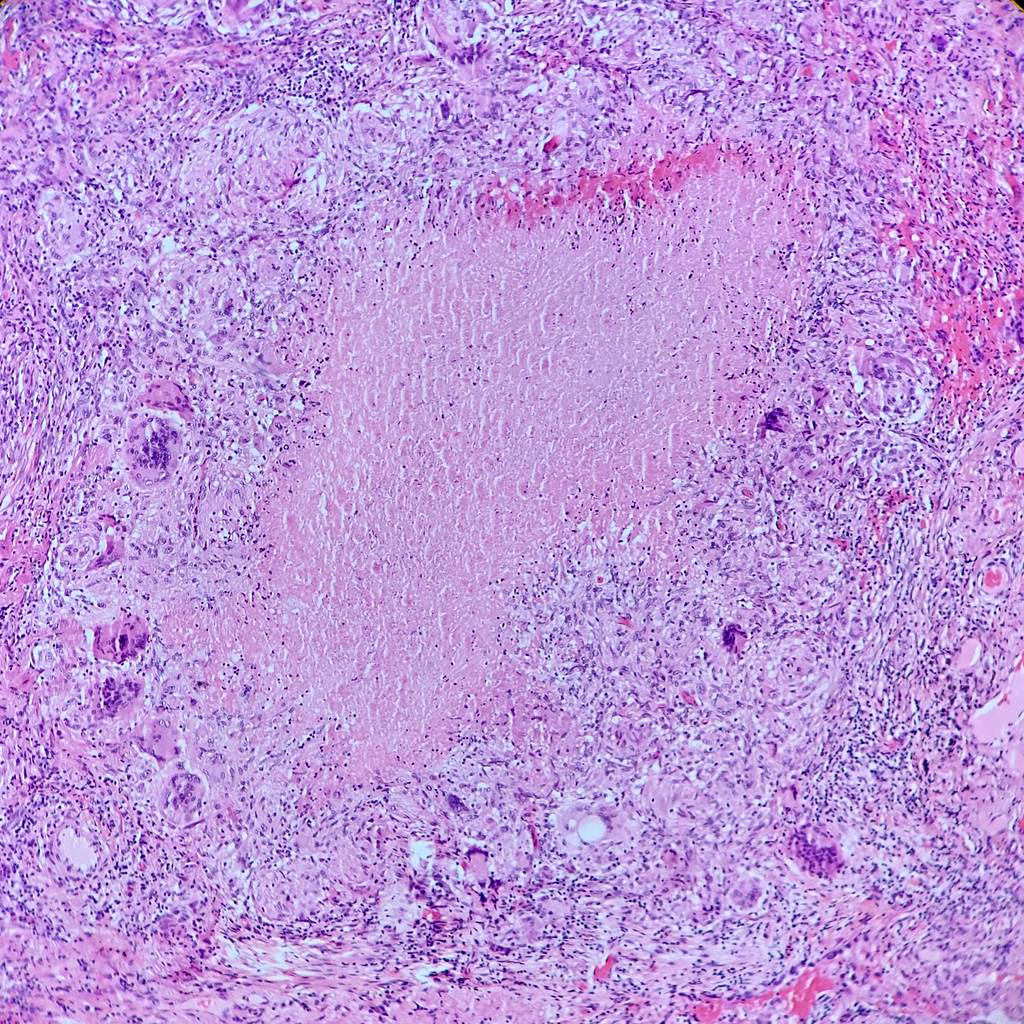
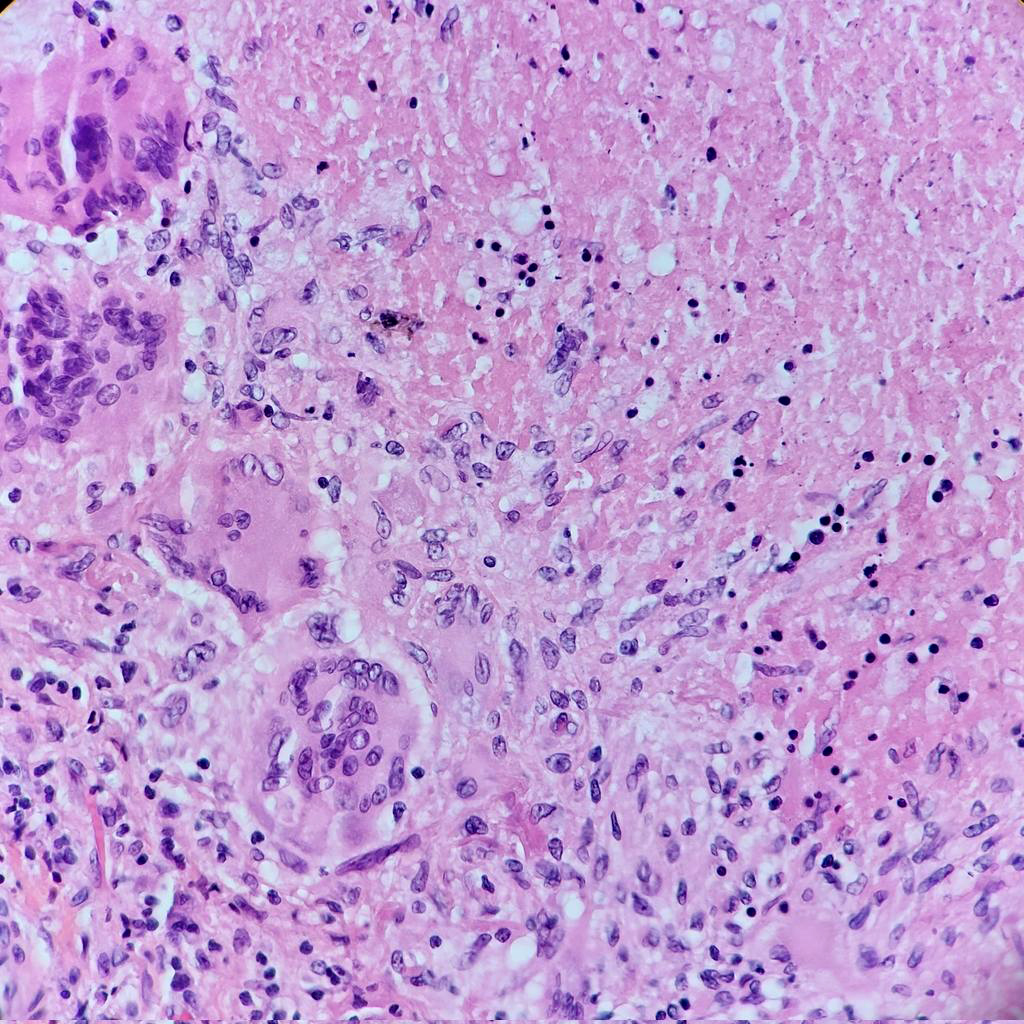
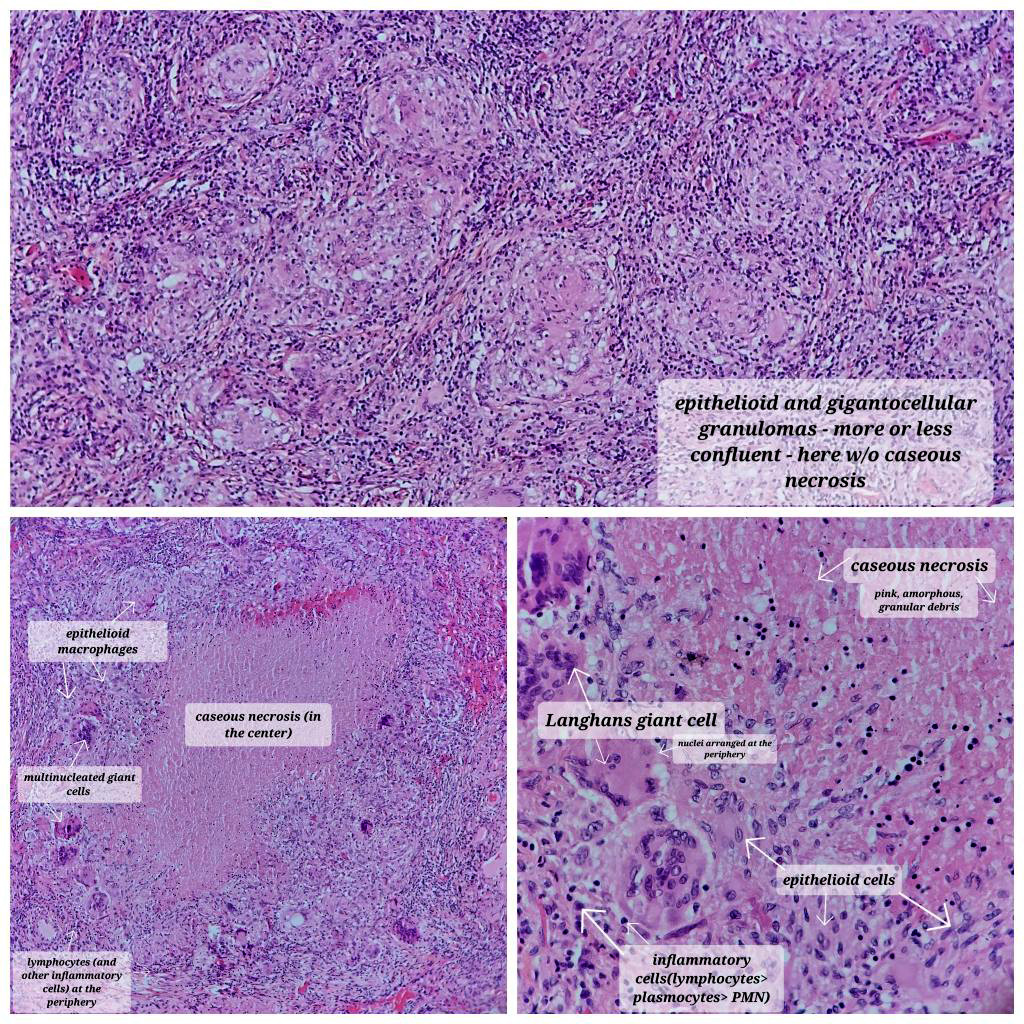
- Hallmark is necrotizing granulomatous inflammation, composed of central necrotic zone surrounded by epithelioid histiocytes with varied number of multinucleated giant cells and lymphocytes
- Multinucleated giant cells may contain Langhans type giant cells (nuclei arranged in a horseshoe shaped pattern at the periphery of the cell) but Langhans type giant cells are not specific for TB infection
- Organisms are usually present within the central zone of necrosis, seen on special stains (in some cases)
- Nonnecrotizing granulomas can be present as well
- References: Semin Diagn Pathol 2017;34:518
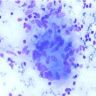
Microscopic (histologic) images
Contributed by Hui-Hua Li, M.D., Ph.D., Jefree J. Schulte, M.D. and Aliya N. Husain, M.D.
Contributed by @AnaPath10 on Twitter
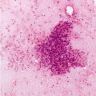
Cytology description
- Epithelioid granulomas, Langhans type giant cells, necrosis and acute or chronic inflammation
- Sources of sampling: aspirates from transbronchial lung biopsy, mediastinal lymph nodes and other smears
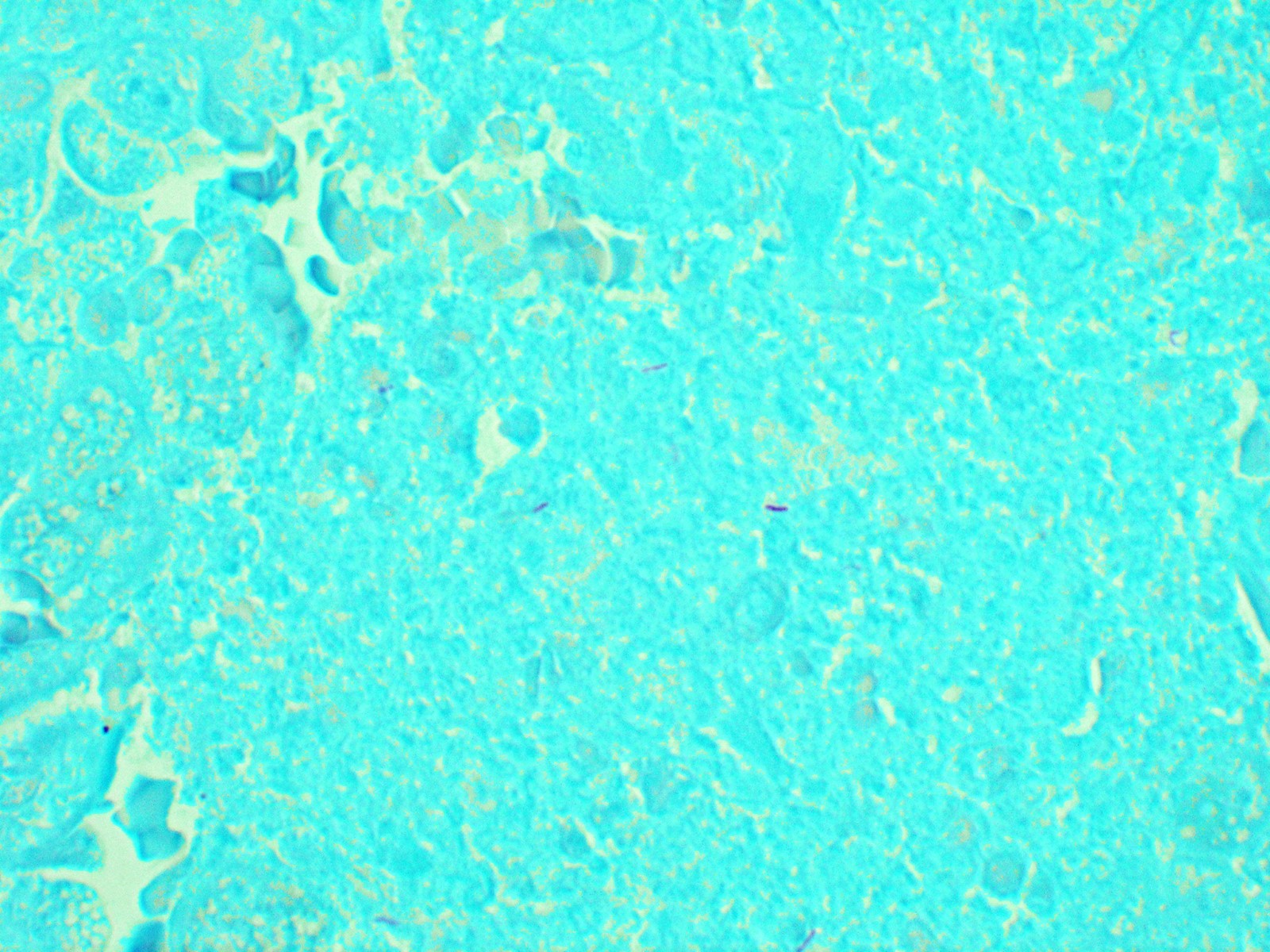
Positive stains
- Acid fast bacilli (AFB) / Ziehl-Neelsen (ZN) / fite; distinguishing M. tuberculosis from atypical mycobacteria can be very difficult based on the morphologic appearance of organism on AFB stain; correlation with culture is necessary
- Gomori methenamine silver (GMS) can occasionally highlight organisms
- References: Arch Pathol Lab Med 2010;134:667, Semin Diagn Pathol 2017;34:518
Videos
Case of pulmonary tuberculosis with granulomas
Histopathology of pulmonary tuberculosis
Sample pathology report
- Left lung, transbronchial biopsy:
- Necrotizing granulomatous inflammation with AFB positive bacilli (see comment)
- Comment: AFB histochemical stain highlights occasional AFB positive bacilli within necrotizing granulomas. GMS histochemical stain is negative for fungal elements. Correlation with microbial cultures is needed.
- Station 7 lymph node, fine needle aspiration:
- Necrotizing granulomatous inflammation with AFB positive bacilli (see comment)
- Comment: AFB histochemical stain highlights occasional AFB positive bacilli within necrotizing granulomas. GMS histochemical stain is negative for fungal elements. Correlation with microbial cultures is needed.
Differential diagnosis
- Nontuberculous mycobacterial disease / atypical mycobacterial infection:
- Including M. avium complex (MAC), M. kansasii, M. xenopi and M. abscessus
- Cannot be distinguished from tuberculosis based on gross or microscopic appearance
- Positive identification of the organism by culture or polymerase chain reaction (PCR) techniques is necessary for precise speciation
- Granulomatous fungal infections:
- Including histoplasmosis, blastomycosis, cryptococcosis and coccidioidomycosis
- Often presents as asymptomatic solitary pulmonary nodules
- Combination of necrotizing and nonnecrotizing granulomas with overlapping histologic features making it difficult to predict a specific pathogen based on the histologic findings alone
- Sarcoidosis:
- Noncaseating granuloma composed of epithelioid cells, giant cells and lymphocytes
- Well formed granulomas tend to be distributed along lymphatic pathways and may coalesce to form macroscopic nodules (nodular sarcoidosis)
- Granulomatosis with polyangiitis (Wegener):
- Combination of necrotizing granulomatous inflammation and necrotizing vasculitis targeting small to medium size vessels
Board review style question #1
A 72 year old woman who is HIV positive and has a significant medical history of diabetes mellitus presents to the ED with persistent cough, hemoptysis and chest pain. Imaging findings are concerning for infectious disease. A nodular lesion in the lung is biopsied (see image above). Special stains reveal AFB positive bacilli. Which of the following statements is true?
- Correlation with microbial cultures or PCR is required for definitive diagnosis
- Granulomatous changes would be expected to be observed near the lymphatics of the lung (lymphatic or lymphangitic distribution)
- These findings are consistent with sarcoidosis
- These findings are diagnostic of Mycobacterium tuberculosis
Board review style answer #1
A. Correlation with microbial cultures or PCR is required for definitive diagnosis
Comment Here
Reference: Tuberculosis
Comment Here
Reference: Tuberculosis
Board review style question #2
A 63 year old man is diagnosed with Mycobacterium tuberculosis infection following bronchoscopy, with smears showing acid fast bacilli and culture growing M. tuberculosis. Which of the following statements is true?
- If alveolar macrophages fail to eradicate M. tuberculosis, the resultant inflammatory response is typically characterized by eosinophil infiltration
- M. tuberculosis cannot grow outside of the lung parenchyma
- M. tuberculosis first infects type 1 pneumocytes and replicates within the cytoplasm of the pneumocyte
- Resident macrophages within the lungs are the primary cell that is infected, upon initial infection by M. tuberculosis
Board review style answer #2
D. Resident macrophages within the lungs are the primary cell that is infected, upon initial infection by M. tuberculosis
Comment Here
Reference: Tuberculosis
Comment Here
Reference: Tuberculosis