Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Vazzano J, Chen W. Intrahepatic cholangiocarcinoma (small and large duct types). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/livertumorcholangiocarcinoma.html. Accessed January 5th, 2025.
Definition / general
- Intrahepatic cholangiocarcinoma (iCCA) is a malignant intrahepatic epithelial neoplasm with biliary differentiation, arising in the liver periphery / proximal to the left and right hepatic ducts (WHO 2019)
- iCCA classification systems (Surg Pathol Clin 2018;11:403, Nat Rev Gastroenterol Hepatol 2020;17:557):
- Histologic features
- Small duct type versus large duct type (2 types have morphologic and molecular but not prognostic differences) (Am J Surg Pathol 2018;42:1334)
- Macroscopic growth pattern
- Mass forming
- Periductal infiltrating
- Intraductal
- Mixed growth patterns
- Cell of origin
- Stem cells residing in the canals of Hering
- Stem cells within peribiliary glands
- Histologic features
Essential features
- Nonencapsulated, white-tan and firm intrahepatic mass
- Glandular malignancy with various degrees of atypia and differentiation, infiltrating a dense fibrous stroma
- Exclusion of metastatic adenocarcinoma by review of the patient's clinical history and application of an appropriate immunohistochemistry panel
- Large duct type iCCA: often hilar masses, obstructive cholestasis; share risk factors with extrahepatic bile duct adenocarcinomas (primary sclerosing cholangitis [PSC], liver fluke infection)
- Small duct type iCCA: peripheral, larger liver masses; shares risk factors with hepatocellular carcinomas (viral hepatitis, nonbiliary cirrhosis)
Terminology
- Also known as peripheral cholangiocarcinoma and intrahepatic bile duct carcinoma
ICD coding
- ICD-10: C22.1 - intrahepatic bile duct carcinoma
Epidemiology
- In the United States, the average annual incidence is 1.6 per 100,000/year (Cancer Control 2017;24:1073274817729245)
- 15% of primary liver malignancies worldwide
- Second most common malignancy arising from the liver
- 3% of all cases of gastrointestinal cancer
- ~10% of all bile duct carcinomas
- More common in East Asia, due to endemic liver fluke infection
- Most patients are aged between 55 and 75 years (Cancer Lett 2016;379:198)
- M > F (Cancer Lett 2016;379:198)
Pathophysiology
- Chronic inflammation of the intrahepatic bile ducts with sustained IL6-STAT3 signaling (Nat Rev Gastroenterol Hepatol 2020;17:557)
- IDH, EPHA2 and BAP1 mutations and FGFR2 fusions in iCCA; in contrast to PRKACA and PRKACB fusions, ELF3 and ARID1B mutations in extrahepatic tumors (Nat Genet 2015;47:1003)
- TP53 and SMAD4 mutations
- CpG island hypermethylation are more frequent in liver fluke associated cholangiocarcinoma
Etiology
- Common underlying risk factor: chronic inflammation of the biliary epithelium and bile stasis (Liver Int 2019;39:19)
- Small duct type iCCA:
- Nonbiliary cirrhosis: independent risk factor (Cancer Control 2017;24:1073274817729245)
- Alcoholic steatohepatitis / nonalcoholic steatohepatitis (NASH)
- Hepatitis B / hepatitis C (Cancer Control 2017;24:1073274817729245)
- Large duct type iCCA:
- Primary sclerosing cholangitis: 5 - 20% lifetime risk of developing cholangiocarcinoma (Cancer Control 2017;24:1073274817729245)
- Hepatobiliary parasites: Opisthorchis viverrini, Clonorchis sinensis
- Hepatolithiasis: more common in Southeast Asia and associated with an increased risk of 6 - 50 times
- Caroli disease: a congenital disorder characterized by multifocal, segmental dilatation of large intrahepatic bile ducts
- Choledochal cysts (types I and IV)
- Multiple bile duct hamartomas (von Meyenburg complexes) (Eur J Gastroenterol Hepatol 2009;21:580)
- Thorotrast: a colloidal suspension of thorium dioxide that was used as an intravascular contrast agent until the 1950s, with an increased risk of 300 times (Jarnagin: Blumgart's Surgery of the Liver, Biliary Tract and Pancreas, Volume 1, 6th Edition, 2016)
Clinical features
- Asymptomatic in early stages (Nat Rev Clin Oncol 2018;15:95)
- Nonspecific symptoms in late stages, such as abdominal pain, malaise, fever, night sweats and weight loss / cachexia
- Jaundice / biliary obstruction are uncommon in iCCA, compared with extrahepatic cholangiocarcinoma
Diagnosis
- Clinicopathologic diagnosis by correlation of radiological, laboratory and surgical pathology findings
- Broad investigation of hepatic pathology and other malignancies should be conducted
- Colon cancer risk / screening history
- Alcohol consumption history
- Travel history (liver flukes)
- Viral hepatitis risk and serologies
- Autoimmune hepatitis serologies
- Iron studies (hemochromatosis), copper studies (Wilson disease)
- BMI (NASH)
- Exposure to hepatotoxins (thorotrast, aflatoxin)
- Labs
- CA 19-9, CEA, AFP
- In patients with PSC with or without ulcerative colitis, development of CCA can be predicted with CA 19-9 (a proposed cutoff level of 130 U/mL carries a sensitivity and specificity of 78.6% and 98.5% respectively
- Reference: Surg Oncol Clin N Am 2019;28:587, Surg Pathol Clin 2018;11:403
Laboratory
- Elevated serum CA 19-9
- Elevated serum CA 125
- Variably elevated serum alkaline phosphatase and bilirubin
- Reference: J Coll Physicians Surg Pak 2019;29:962
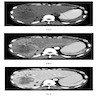
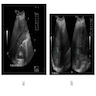
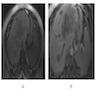
Radiology description
- Usually single, large, homogeneous mass with irregular margins on CT scan
- No pathognomonic CT / MRI features: may show progressive delayed enhancement, peripheral rim enhancement, subcapsular retraction
- Reference: Radiology 2018;288:7
Prognostic factors
- Serum CEA, CA 19-9, tumor diameter and number, lymph node metastasis, vascular invasion and direct invasion and local extrahepatic metastasis are important prognostic factors (J Clin Oncol 2013;31:1188)
- Periductal infiltration and AJCC 8th edition pT stage (Am J Surg Pathol 2018;42:1334)
Case reports
- 38 year old pregnant woman with 1 week history of headache, nausea, vomiting and right upper abdominal pain (Case Reports Hepatol 2018;2018:6939747)
- 45 year old Thai man with 3 month history of right upper abdominal pain (Case Rep Surg 2018;2018:3862575)
- 58 year old woman with abdominal pain and jaundice (Medicine (Baltimore) 2018;97:e11015)
- 67 year old woman with chest pain and a lung nodule (Am J Case Rep 2018;19:35)
- 70 year old man with mass forming tumor with portal vein and bile duct thrombus (Int J Surg Case Rep 2017;40:13)
Treatment
- Surgical resection of the tumor is the potential curative therapy
- Frequently inoperable, often recur after surgery
- First line treatment for locally advanced or metastatic disease is gemcitabine / cisplatin
- Molecular testing recommended by National Comprehensive Cancer Network (NCCN) for unresectable and metastatic tumors (> 50% of NGS tested subjects have potentially actionable mutations)
- Ongoing clinical trials of targeted therapy: immune checkpoint inhibitors (anti-PD1, anti-PDL1, anti-CTLA4 antibodies), IDH1 inhibitors, AKT / PI3K / mTOR inhibitors, FGFR inhibitors and anti-EGFR and MEK inhibitors
- Reference: Surg Oncol Clin N Am 2019;28:587
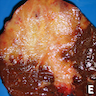
Gross description
- Usually large, nonencapsulated, well demarcated, firm (due to desmoplastic reaction), white-tan to gray and nodular intrahepatic mass
- More frequent in the right lobe of the liver
- Satellite nodules are present in 30%
- Calcification is common
- Noncirrhotic background liver in most cases
- Grossly classified into 3 growth patterns (J Gastroenterol 2013;48:647):
- Mass forming: hepatic parenchymal solid mass
- Periductal infiltrating: infiltrates along the portal tracts, causing bile duct strictures
- Intraductal growth: papillary or polypoid growth inside a dilated bile duct
Frozen section description
- Margin status may be assessed via frozen section
- Invasive foci of cholangiocarcinoma are infiltrating glands found as direct stromal invasion, vessel invasion or perineural invasion
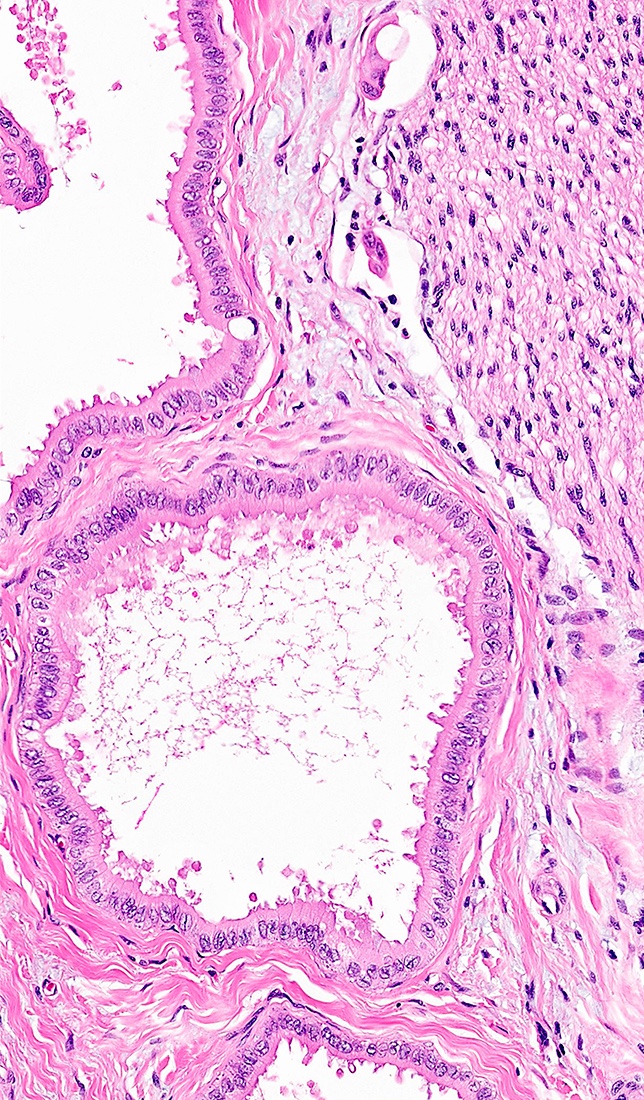
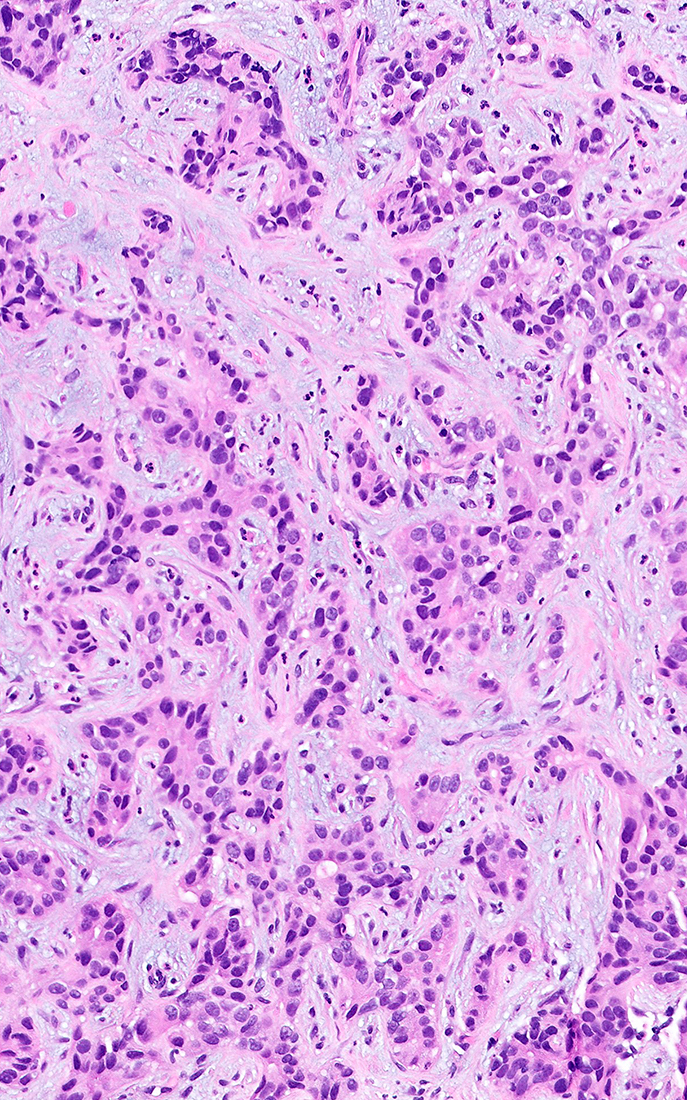
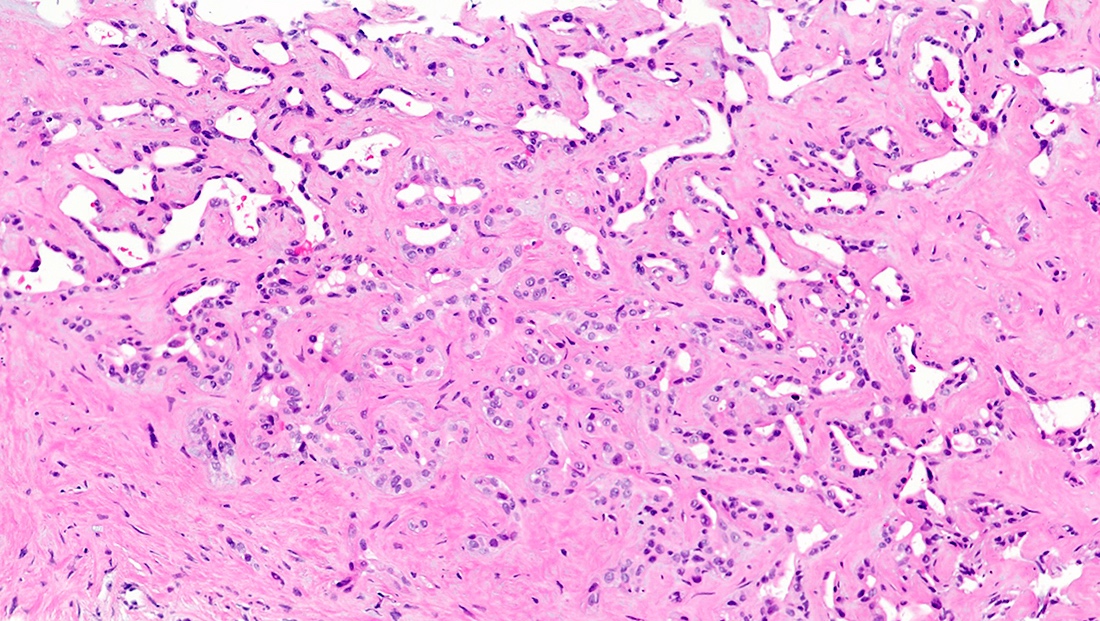
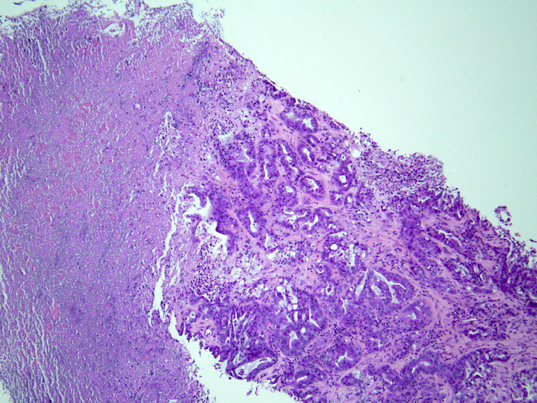
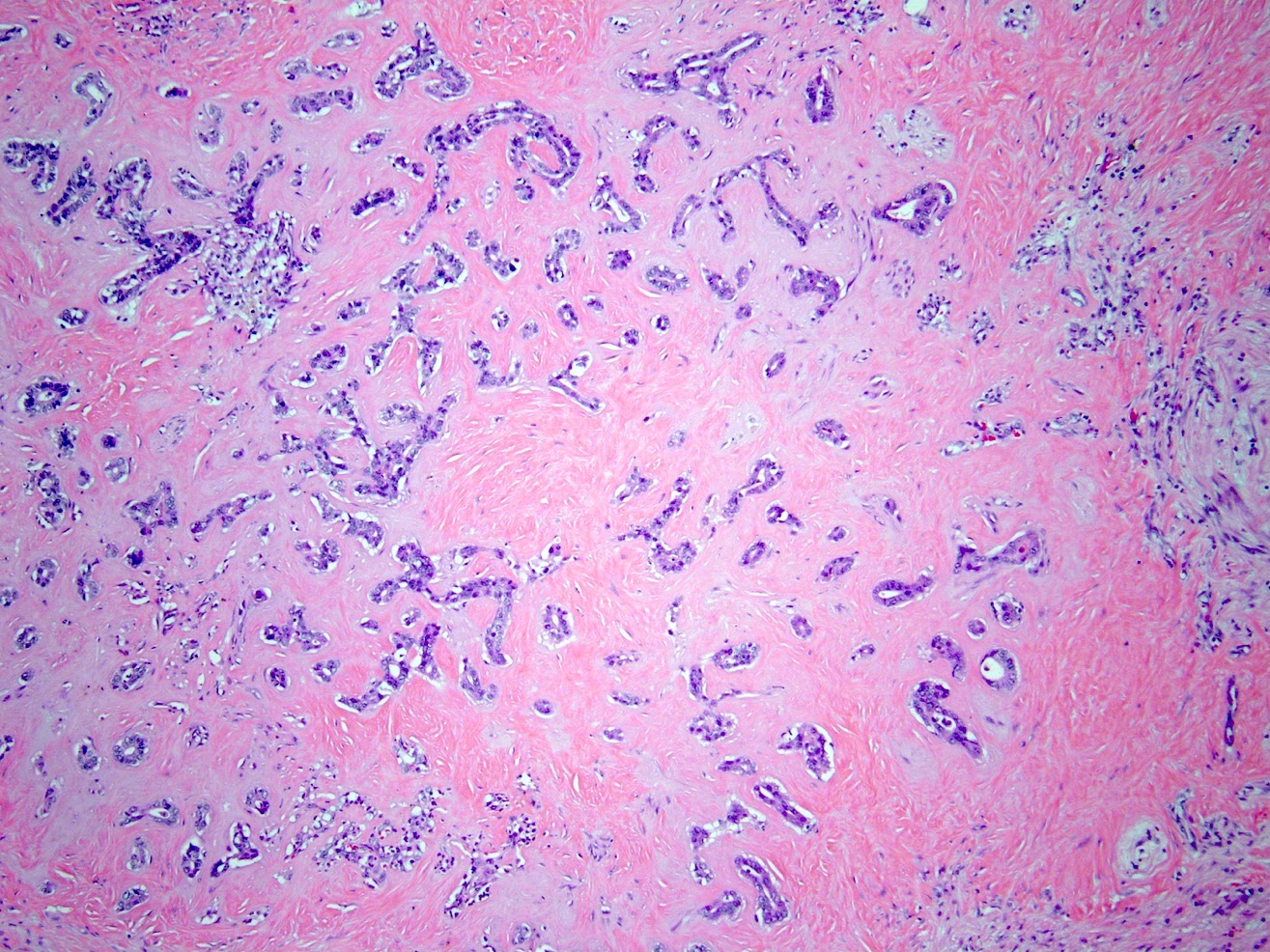
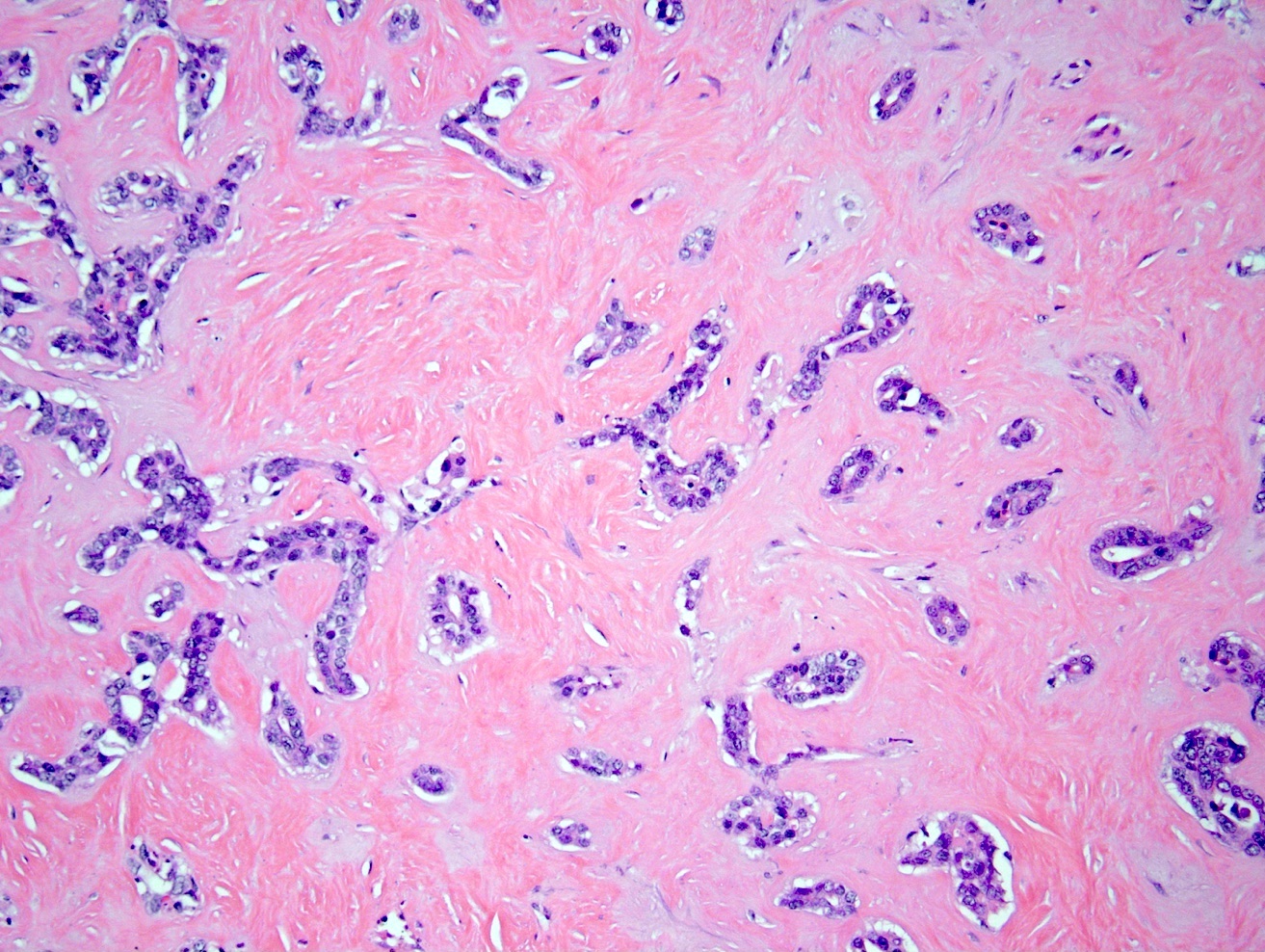
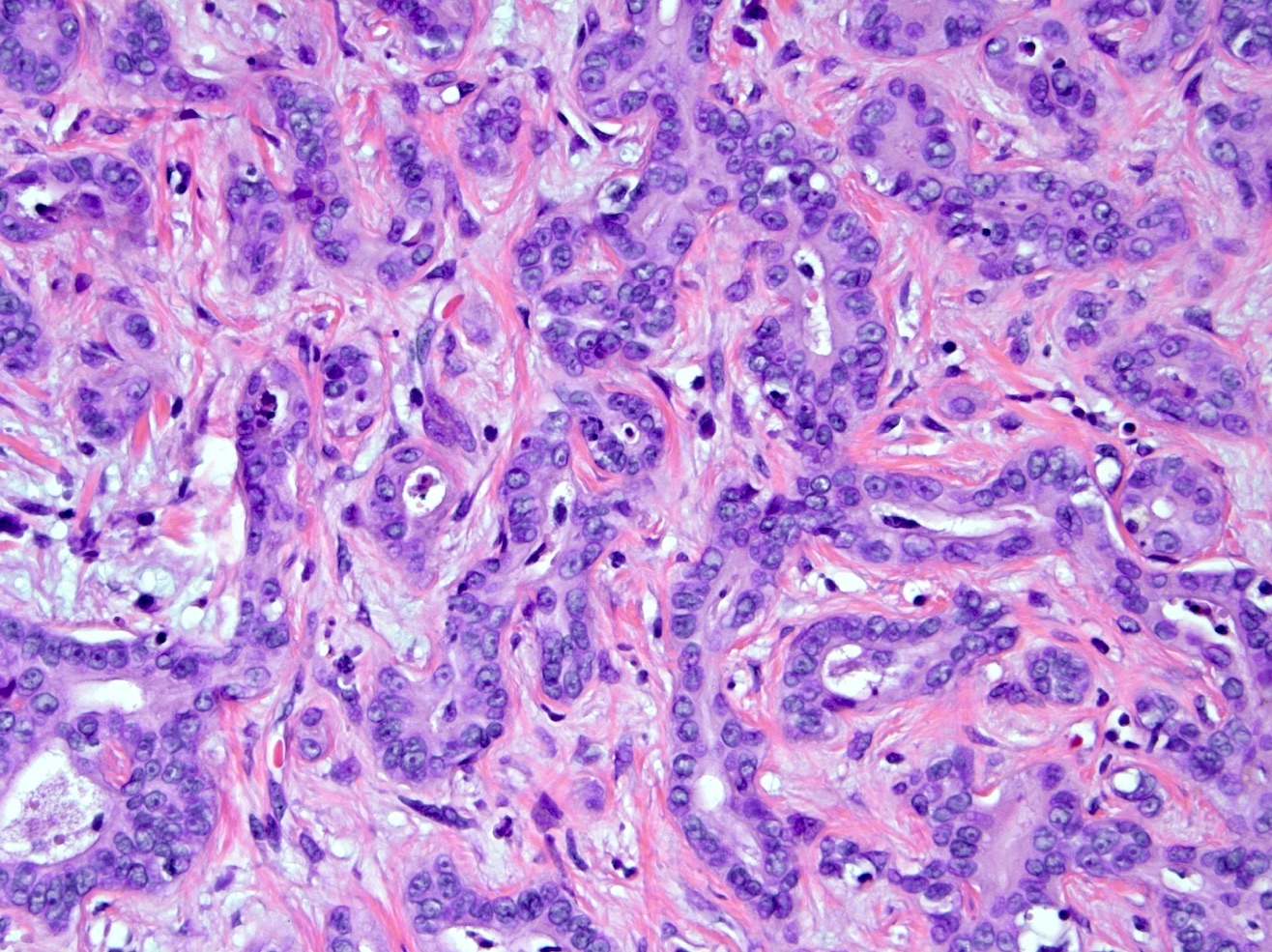
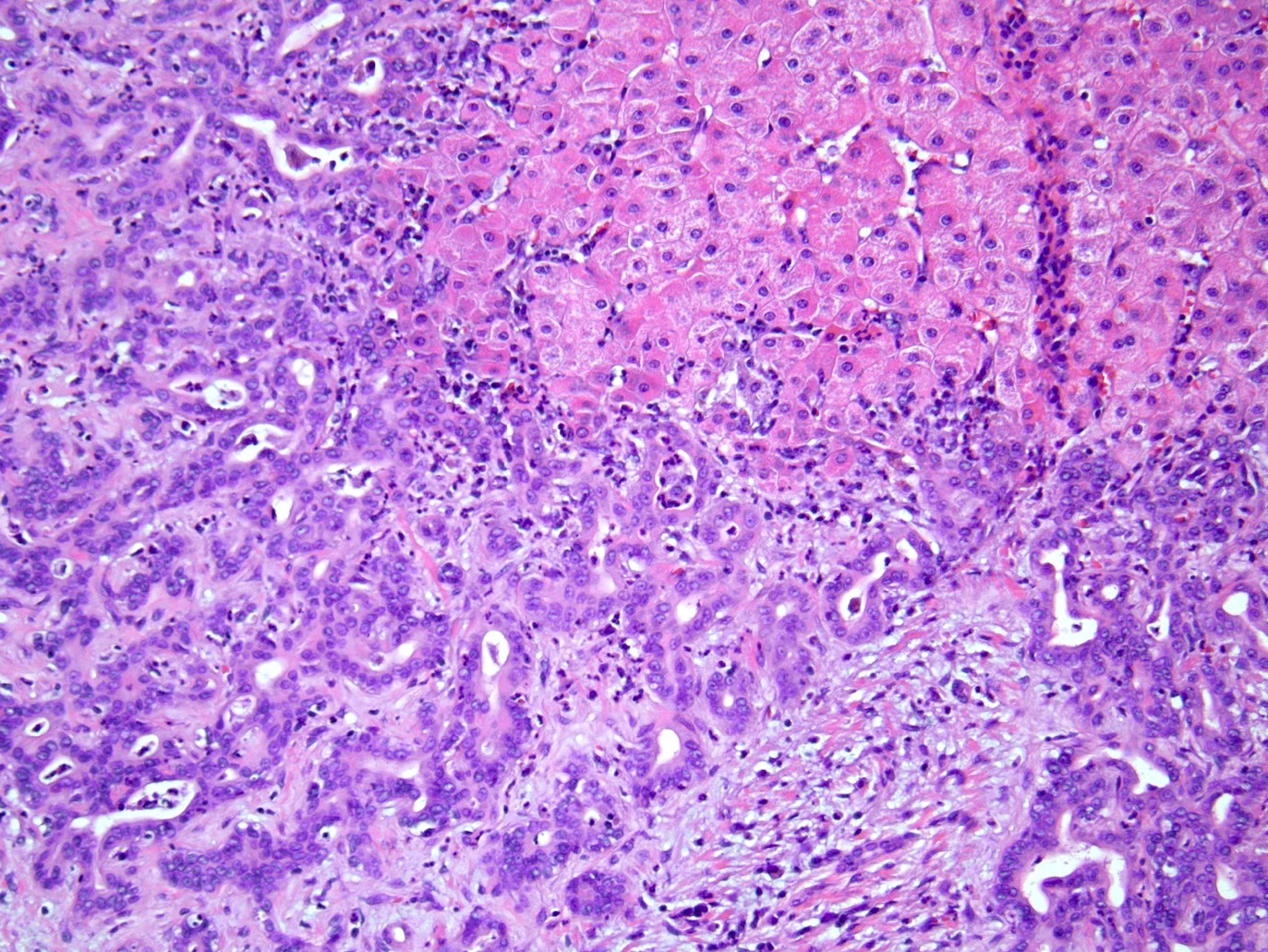
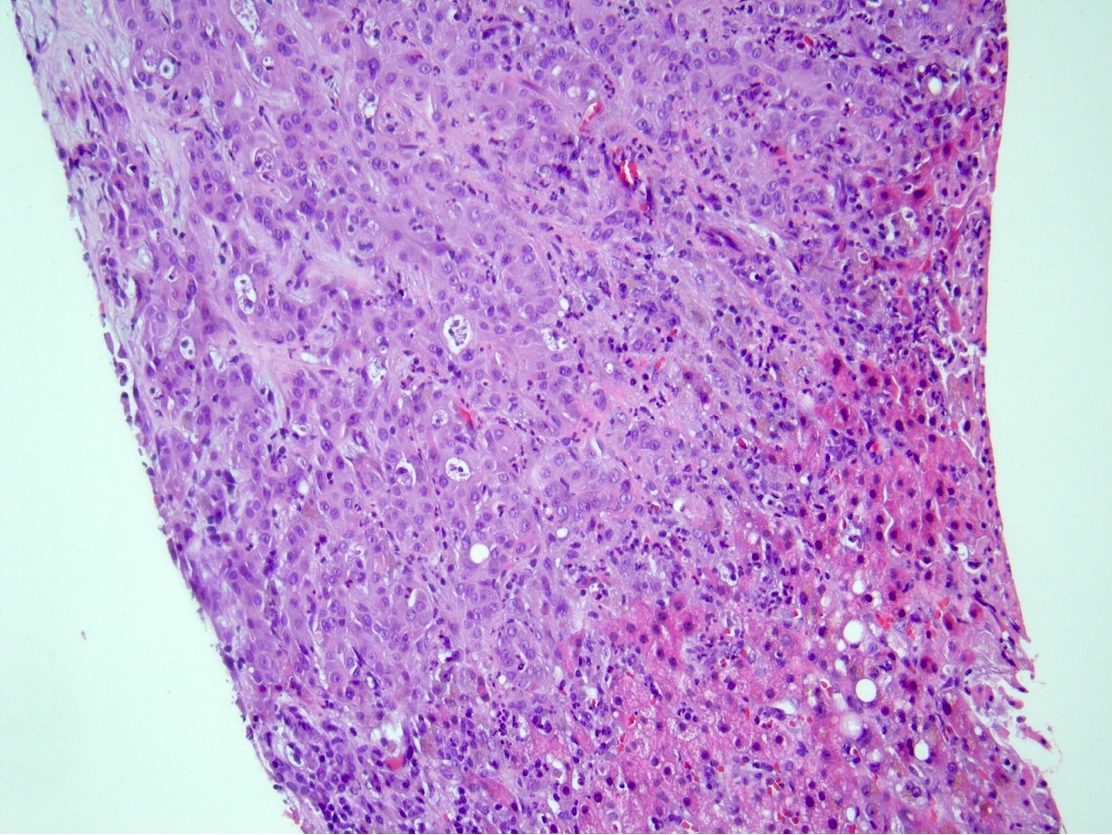
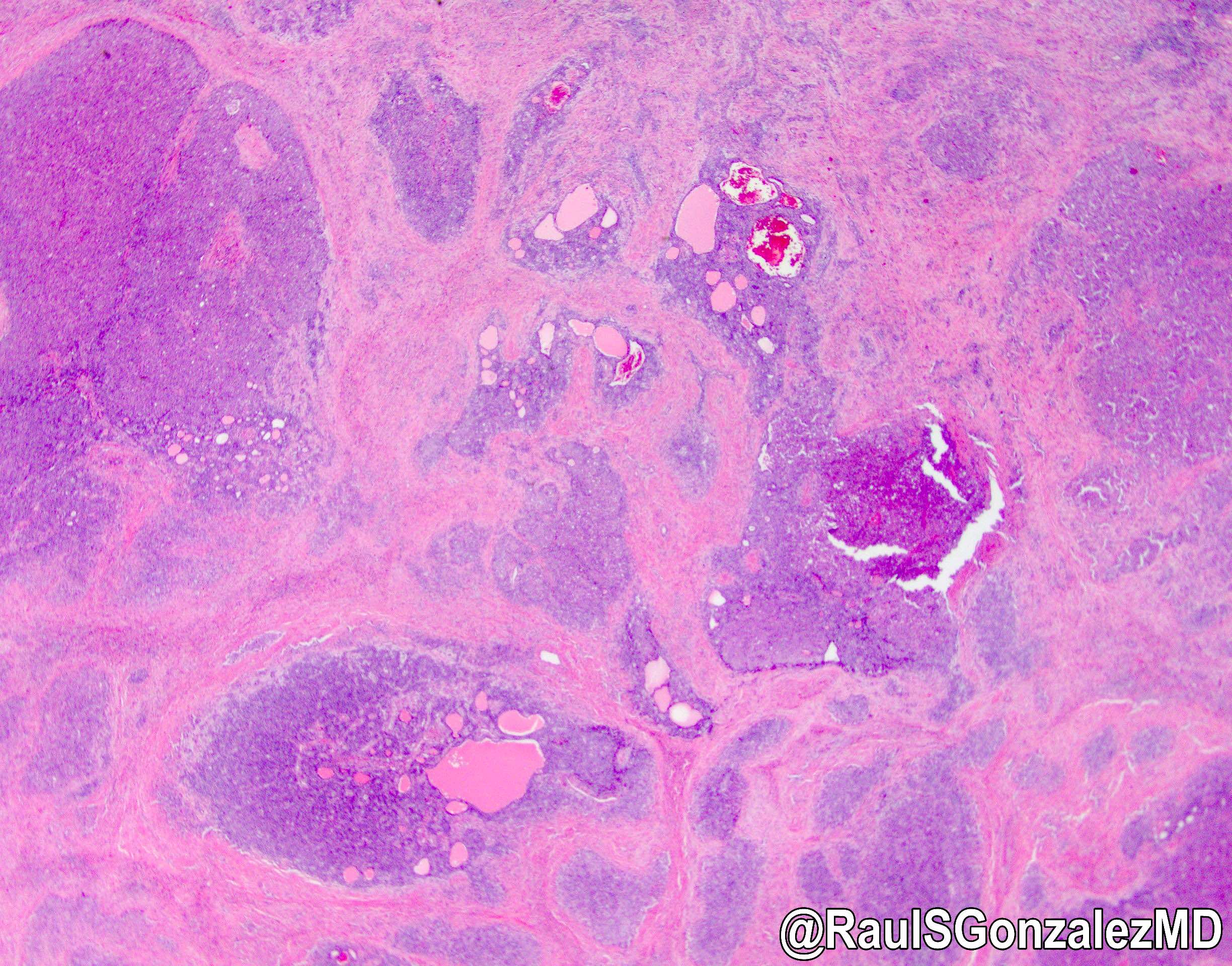
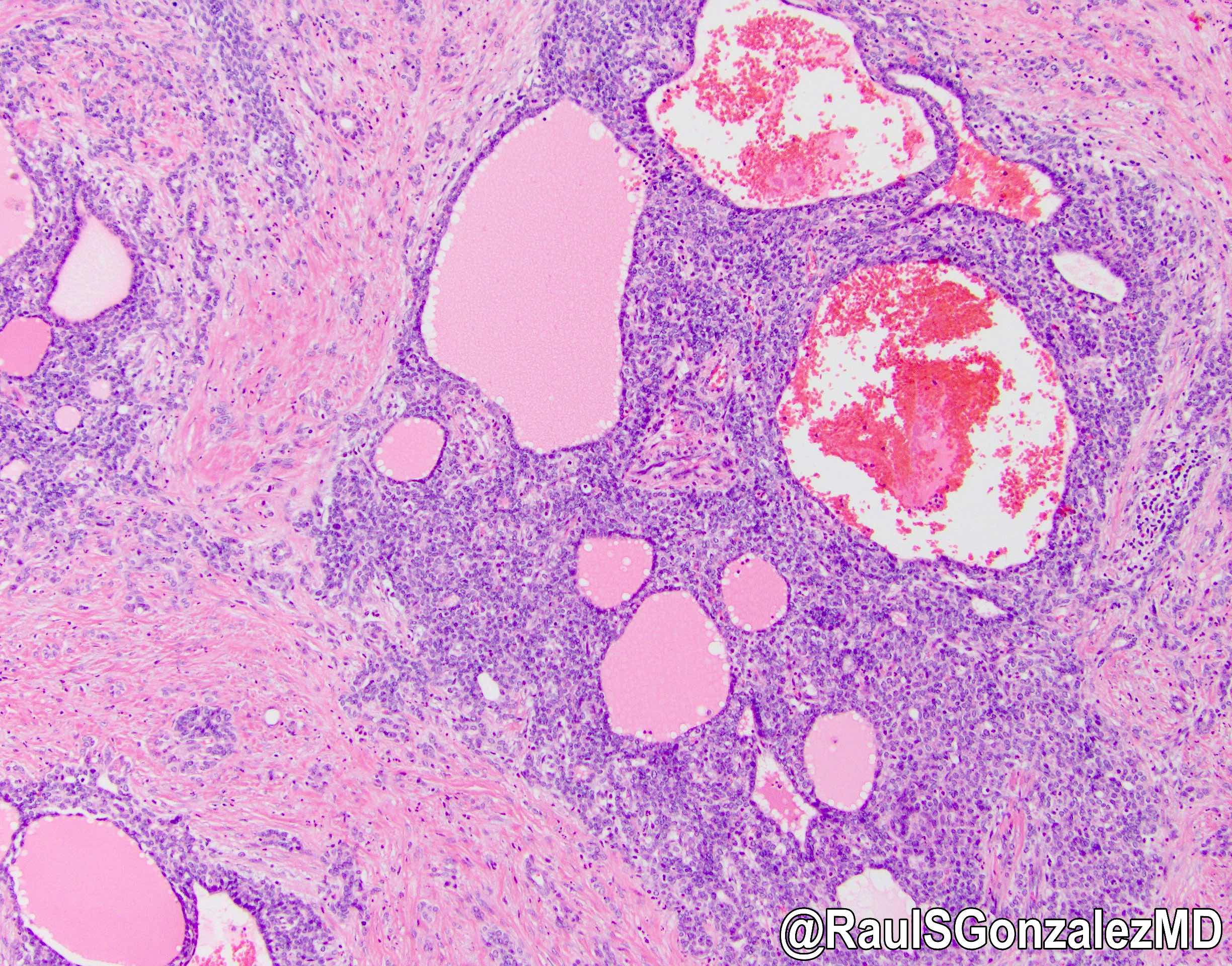
Microscopic (histologic) description
- iCCA consists of infiltrating well formed or cribriform glands in an abundant fibrous stroma (Liver Int 2019;39:7, Surg Pathol Clin 2018;11:403)
- Malignant glands are lined by cells with varying degrees of atypia and pleomorphism (Liver Int 2019;39:7)
- Usually well differentiated adenocarcinoma with mild atypia, intracytoplasmic lumina and intraluminal cellular debris; however, focal atypia with marked pleomorphism can also be present (Liver Int 2019;39:7)
- Commonly infiltrates between hepatic parenchymal cords at the periphery of the tumor
- Multicentricity and perineural invasion are common
- Histologic variants include mucinous, signet ring cell, clear cell, lymphoepithelioma-like, thyroid follicular-like, adenosquamous and sarcomatoid
- Small duct type iCCA (Liver Int 2019;39:7)
- Peripheral hepatic parenchyma
- Mass forming
- Cuboidal cells forming small tubular or anastomosing glands
- Variable lymphovascular / perineural invasion
- CD56, N-cadherin, CRP positive
- Subtypes include cholangiolocarcinoma, iCCA with ductal plate malformation-like pattern
- Large duct type iCCA (Liver Int 2019;39:7)
- Proximal to liver hilum
- Periductal infiltrating or mass forming
- Tall columnar cells that form large glands within open lumina; usually contain mucin
- BilIN, IPNB, ITPN are precursors
- Positive lymphovascular / perineural invasion
- MUC5AC, MUC6, S100P and TFF1 positive
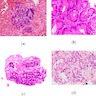
Microscopic (histologic) images
Cytology description
- Isolated clusters and sheets of cuboidal or columnar cells with various degrees of nuclear enlargement and pleomorphism
- Wide range of glandular differentiation on cell block; > 10 proliferating ductules on FNA is helpful to differentiate cholangiocarcinoma from metastatic adenocarcinomas (Diagn Cytopathol 2000;22:359)
Positive stains
- CK7 (~90%), CK19 (80 - 90%), CK17, CAM 5.2, MOC31, EMA, monoclonal CEA, polyclonal CEA (diffuse cytoplasmic pattern), claudin4, pVHL (Indian J Pathol Microbiol 2018;61:2)
- Albumin RNA ISH (~80%) but does not differentiate iCCA from hepatocellular carcinoma (Ann Surg Oncol 2015;22:S1609, Am J Surg Pathol 2018;42:1334)
- Glypican 3 and HepPar1 occasionally positive in peripherally located iCCA
Negative stains
- CK20, TTF1, napsin A, ER (Indian J Pathol Microbiol 2018;61:2, Arch Pathol Lab Med 2003;127:1591)
- Progesterone receptor (PR) can be positive in cholangiocarcinoma (31%) (Arch Pathol Lab Med 2003;127:1591)
- GATA3 rarely positive (3%), seen in poorly differentiated iCCA (Appl Immunohistochem Mol Morphol 2020;28:460)
Molecular / cytogenetics description
- Mutations in KRAS (22%), BRAF (7%), EGFR (2%), IDH1 / IDH2 (14%), MET, activation of mTOR, overexpression of cyclin D1, overexpression of p21, inactivating mutations of DPC4 / SMAD4 (13 - 15%) and TP53 (15%) (BMC Cancer 2019;19:185)
- Chromosomal gains of 1q, 5p, 7p, 8q, 17q and 20q and losses of 1p, 4q, 8p, 9p, 17p and 18q (BMC Cancer 2019;19:185)
Sample pathology report
- Right lobe of liver, partial hepatectomy:
- Moderately differentiated adenocarcinoma, 6.5 cm, consistent with intrahepatic cholangiocarcinoma, small duct type
- Resection margins are negative for carcinoma
- Lymphovascular invasion present
- Metastatic adenocarcinoma involving 1 of 3 lymph nodes
- Pathologic TNM stage (AJCC 8th edition): pT2N1
- Background liver with mild steatosis and steatohepatitis; no advanced fibrosis
Differential diagnosis
- Metastatic adenocarcinoma:
- Immunohistochemistry and clinical history are helpful to rule out metastasis
- Hepatocellular carcinoma:
- Combined hepatocellular cholangiocarcinoma:
- Histologic evidence of both hepatocellular and biliary differentiation by H&E morphology and supported by immunohistochemistry (WHO 2019)
- Benign bile ductular reactions:
- Uniform growth pattern with lack of cytologic and architectural atypia and perineural invasion
- Epithelioid hemangioendothelioma:
- Bile duct adenoma:
- Small and well circumscribed, no or minimal cytologic atypia, lack of lymphovascular or perineural invasion
Additional references
- AJR Am J Roentgenol 2000;175:721, J Hepatobiliary Pancreat Sci 2015;22:101, Gut Liver 2017;11:13, Hepatobiliary Surg Nutr 2017;6:22, Hepatobiliary Pancreat Dis Int 2012;11:349, Onco Targets Ther 2017;10:1131, World J Gastrointest Oncol 2011;3:49, WHO Classification of Tumours Editorial Board: Digestive System Tumours, 5th Edition, 2019
Board review style question #1
Board review style answer #1
B. Cuboidal cells forming small tubular or anastomosing glands
Comment Here
Reference: Intrahepatic cholangiocarcinoma (small and large duct types)
Comment Here
Reference: Intrahepatic cholangiocarcinoma (small and large duct types)
Board review style question #2
Which of the following regarding intrahepatic cholangiocarcinoma (iCCA) risk factors is true?
- Alcoholic steatohepatitis / nonalcoholic steatohepatitis are risk factors for large duct type iCCA
- Biliary type cirrhosis is an independent risk factor for small duct type iCCA
- Chronic inflammation of the biliary epithelium is a common risk factor
- Primary sclerosing cholangitis is a risk factor for small duct type iCCA
Board review style answer #2
C. Chronic inflammation of the biliary epithelium is a common risk factor
Comment Here
Reference: Intrahepatic cholangiocarcinoma (small and large duct types)
Comment Here
Reference: Intrahepatic cholangiocarcinoma (small and large duct types)