Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Monroe H, El Naili R. Biliary adenofibroma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/livertumorbiliaryadenofibroma.html. Accessed December 23rd, 2024.
Definition / general
- Premalignant hepatic neoplasm of bile duct origin with epithelial and myofibroblastic stromal components
Essential features
- Benign neoplasm defined histologically by an epithelial component comprised of tubuloacinar and cystically dilated glands and a stromal component comprised of myofibroblast type cells in a background of extensive fibrosis (Patholog Res Int 2010;2010:504584)
- May undergo malignant transformation to cholangiocarcinoma or rarely biliary adenosarcoma (Case Rep Surg 2022;2022:5280884, Hum Pathol 2018;73:108)
- Primary differential includes von Meyenburg complex, bile duct adenoma, simple biliary cysts, biliary cystadenoma, peritoneal inclusion cysts and cholangiocarcinoma (Patholog Res Int 2010;2010:504584, ACG Case Rep J 2018;5:e72)
- Rare: ~35 reported cases
Terminology
- Biliary adenosarcoma may be applicable if the stromal component is malignant (Hum Pathol 2018;73:108)
ICD coding
- ICD-O: 9013/0 - adenofibroma, NOS
- ICD-11: 2E92.7 & XH91Y8 - benign neoplasm of liver or intrahepatic bile ducts & adenofibroma, NOS
Epidemiology
- Rare: ~35 reported cases
- Median age: ~57 years (BMC Med Imaging 2022;22:47)
- No predilection for men or women (Asian J Surg 2023;46:2229)
Sites
- Liver; slight predilection for right lobe
Pathophysiology
- Thought to be a primary epithelial neoplasm with secondarily induced stroma (Am J Surg Pathol 2003;27:693)
- Originates from interlobular or larger bile ducts per D10 and 1F6 staining (Am J Surg Pathol 2003;27:693)
- Stromal component spindle cells are myofibroblastic per immunostains
- May represent neoplastic transformation of von Meyenburg complexes (Am J Surg Pathol 2003;27:693)
- Malignant transformation may be associated with accumulation of oncogenic and tumor suppressor mutations such as TP53, KIT and NRAS (Surg Case Rep 2019;5:104, Case Rep Surg 2022;2022:5280884)
Etiology
- Precise etiology unknown at this time
Clinical features
- Majority of cases present with dull, intermittent epigastric pain
- Rarely presents as a palpable epigastric mass (Case Rep Surg 2022;2022:5280884, AJR Am J Roentgenol 2002;179:280)
- Postprandial nausea / vomiting, weight loss or changes in appetite (Hepatology 2017;65:380, Case Reports Hepatol 2012;2012:793963)
- Other cases are usually asymptomatic and discovered incidentally
- May undergo transformation to intrahepatic cholangiocarcinoma or rarely adenosarcoma (Case Rep Surg 2022;2022:5280884, Hum Pathol 2018;73:108)
- Von Meyenburg complexes may be present in background of tumor (BMC Med Imaging 2022;22:47)
- May rarely present in the background of other primary biliary neoplasms such as mucinous cystic neoplasm (ACG Case Rep J 2018;5:e72)
Diagnosis
- Ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI) used to screen for lesion
- Histology is gold standard
- WHO essential criteria
- Solid microcystic epithelial neoplasm
- Microcystic and tubuloacinar glandular structures lined by nonmucinous biliary epithelium
- Supporting fibroblastic / myofibroblastic stroma
- WHO essential criteria
Laboratory
- Unremarkable liver function tests (LFTs)
- CA 19-9 elevated in a minority of cases (Case Rep Surg 2022;2022:5280884, Taehan Yongsang Uihakhoe Chi 2021;82:721)
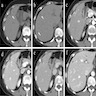
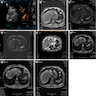
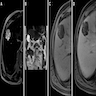
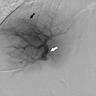
Radiology description
- Ultrasound
- Rarely demonstrates a hypoechoic or hyperechoic mass (BMC Med Imaging 2022;22:47)
- Contrast enhanced: early enhancement (World J Clin Cases 2022;10:1366)
- Generally not ideal for complex cystic lesions
- CT
- Solitary, hypodense lobulated solid cystic mass with well defined margins, internal septation and capsular retraction (BMC Med Imaging 2022;22:47)
- MRI (BMC Med Imaging 2022;22:47, Korean J Gastroenterol 2019;74:356)
- Generally considered most reliable screening method
- Solitary, subcapsular, multilocular, solid cystic mass
- T1 weighted: low intensity
- T2 weighted: high intensity; well circumscribed, lobular mass with no intrahepatic bile duct communication
- Contrast enhanced: delayed enhancement
- Features favoring malignancy (BMC Med Imaging 2022;22:47)
- Multiple lesions
- Poorly defined margins
- Unilocular
- Marked enhancement in arterial phase and washout in venous phase
- Peripheral edematous halo
- Diffusion weighted imaging (DWI): restricted diffusion
- Portal venography (BJR Case Rep 2015;1:20150100)
- Portal vein branches moderately displaced with faintly enhancing mass in background
Radiology images
Prognostic factors
- Benign form is associated with favorable prognosis and no risk of recurrence
- Malignant form has risk of local recurrence and distant metastasis
- Recurrence with pulmonary metastasis has been reported (AJR Am J Roentgenol 2002;179:280)
Case reports
- 30 year old woman with an upper abdominal mass between liver and duodenum (Case Rep Gastroenterol 2022;16:535)
- 44 year old asymptomatic woman with a history of chronic hepatitis B and right liver mass (Taehan Yongsang Uihakhoe Chi 2021;82:721)
- 63 year old man with epigastric pain and palpable upper abdominal mass (Case Rep Surg 2022;2022:5280884)
Treatment
- Partial liver resection or hepatectomy is usually curative for benign and malignant forms
- Lobectomy recommended if large or malignant (Pathol Res Pract 2016;212:468)
- Preoperative portal vein embolization (BJR Case Rep 2015;1:20150100)
- Robotic enucleation shows promising results (BMJ Case Rep 2021;14:e242737)
- Value of chemoradiation is not known in a setting of malignancy
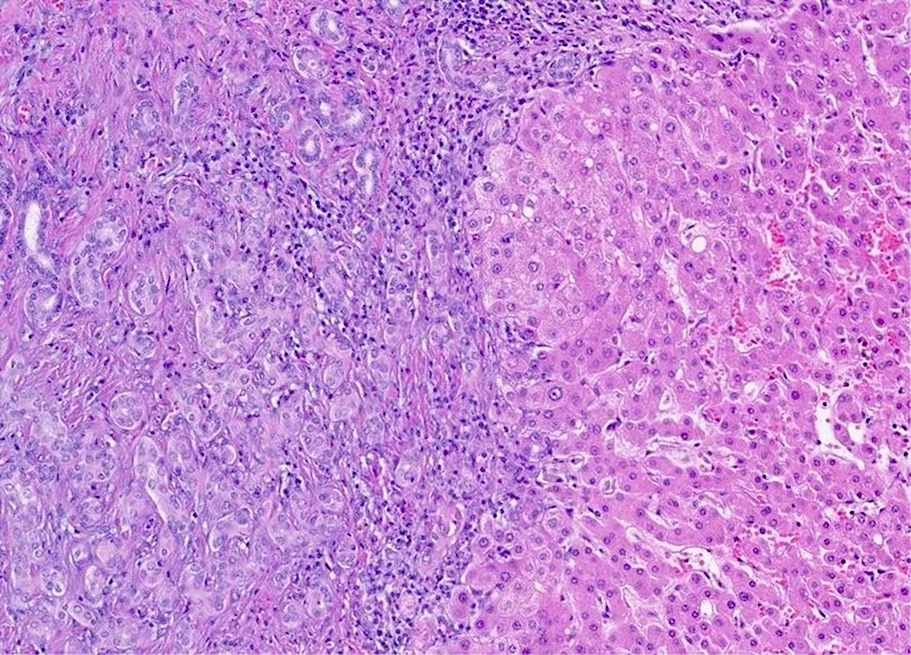
Gross description
- Well circumscribed, unencapsulated, solid cystic mass measuring 5 to 16 cm (BMC Med Imaging 2022;22:47, Patholog Res Int 2010;2010:504584)
- White-purple or white-tan, spongy cut surface
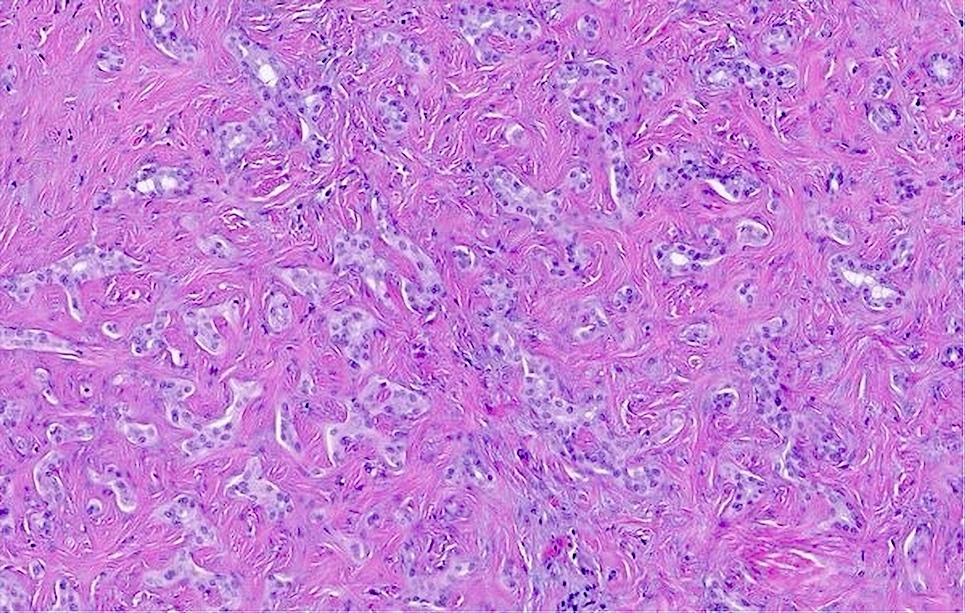
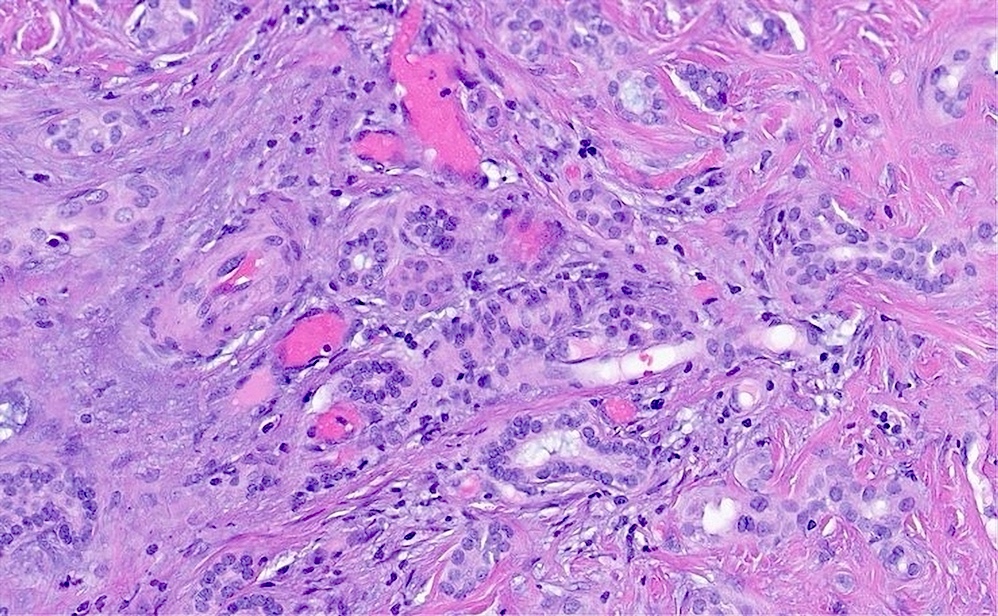
Microscopic (histologic) description
- Benign (Patholog Res Int 2010;2010:504584)
- Complex nonmucinous, tubuloacinar glandular structures
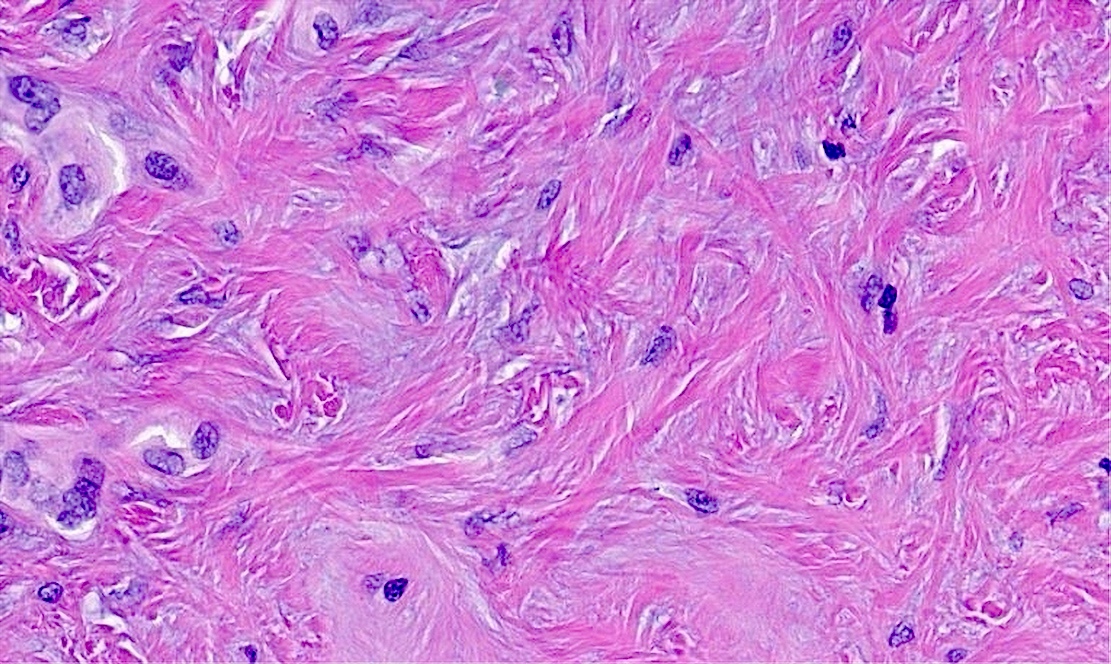
- Lining cells are cuboidal to flattened with central round to ovoid nuclei, amphophilic cytoplasm and no significant atypia
- Red blood cells or eosinophilic material may be present in lumina
- Rare papillary projections into lumina
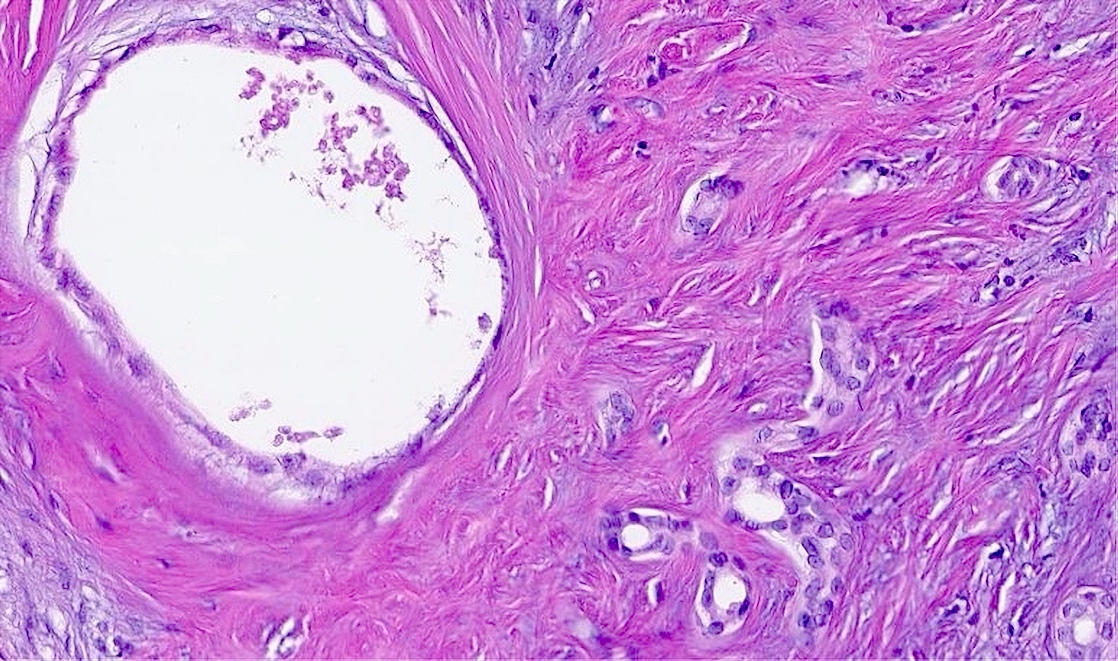
- Cystically dilated glands lined by a single layer of flat epithelial cells
- Moderately cellular fibrous stroma containing bland, myofibroblastic spindle cells
- Mild chronic inflammation characterized by scattered lymphocytes may be present
- Complex nonmucinous, tubuloacinar glandular structures
- Malignant
- Columnar cells with elongated hyperchromatic or vesicular nuclei and atypical mitoses (BMC Med Imaging 2022;22:47)
- Complex papillary, cribriform and back to back architecture (BMC Med Imaging 2022;22:47)
- Eosinophilic cytoplasm with apocrine features including snouts and decapitation secretions (BMC Med Imaging 2022;22:47)
- Fibrous stroma is less prominent or outright absent
- Capsular, lymphovascular or perineural invasion (BMC Med Imaging 2022;22:47)
- Osseous metaplasia has rarely been reported (Hum Pathol 2018;73:108)
Microscopic (histologic) images
Cytology description
- Cytologic features have not been described for this entity
- FNA may be avoided due to possible risk of seeding in setting of malignancy (BJR Case Rep 2015;1:20150100)
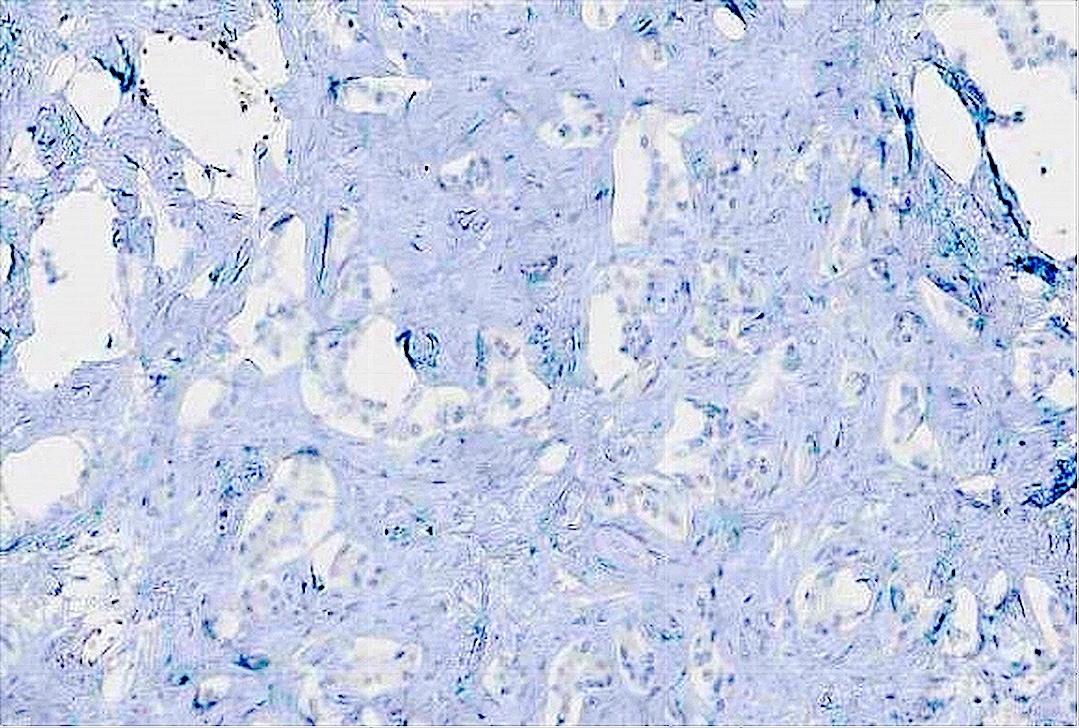
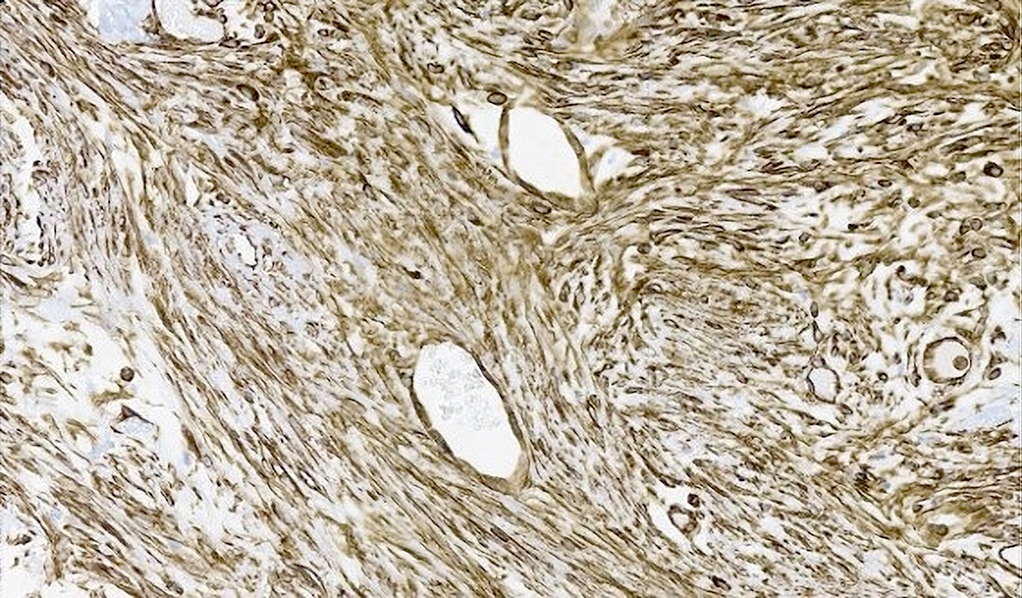
Positive stains
- Epithelium
- CK7, CK8/18, CK19
- CA 19-9, CEA, CAM5.2, MUC1 / EMA often positive
- D10 (supports interlobular duct origin) (Am J Surg Pathol 2003;27:693)
- CDH17, CD56 (Surg Case Rep 2019;5:104)
- BAP1 retained if benign
- Stroma
- Ki67 index
- Benign: < 10%, < 1% (in stromal component)
- Malignant: 20 - 30% (BMC Med Imaging 2022;22:47)
Negative stains
- Epithelium
- Stroma
Electron microscopy description
- Electron microscopy findings have not been reported for this entity
Molecular / cytogenetics description
- Monosomy 22 in benign form (Cancer Genet Cytogenet 1997;93:183)
- Associated with benign mesenchymal neoplasms
- PCR / NGS
- CDKN2A loss of function mutation (J Gastrointest Oncol 2016;7:E107)
- TP53 and KIT mutations (Surg Case Rep 2019;5:104)
- NRAS mutation if malignant (Case Rep Surg 2022;2022:5280884)
- Array comparative genomic hybridization
- CCND1 and ERBB2 amplification (Case Rep Surg 2022;2022:5280884)
- Microsatellite stable (MSS) (Surg Case Rep 2019;5:104)
Sample pathology report
- Liver, left lateral segment, segmentectomy:
- Biliary adenofibroma (see comment)
- Comment: Histologic evaluation reveals benign appearing bile ducts without significant cytologic or architecture atypia. Immunohistochemical stains with adequate controls show the lesional cells to be positive for CK7 with low Ki67 and wild type staining pattern with p53. The lesional cells are negative for PAX8, TTF1, CK20, NKX3, HepPar1, arginase and glypican 3. BAP1 immunostain is retained. Overall, based on morphology, low Ki67 and BAP1 retention, a benign biliary lesion is favored. Due to the presence of extensive fibroblastic stroma, this entity may be further classified as biliary adenofibroma.
Differential diagnosis
- Von Meyenburg complex (Patholog Res Int 2010;2010:504584):
- Usually presents as multiple, small (< 0.5 cm) cysts
- Irregular curvilinear ducts lined by cuboidal or flattened cells
- May exhibit dense fibrous stroma similar to adenofibroma
- Bile duct adenoma:
- Small (usually < 1 cm)
- Closely packed tubules with cuboidal lining and narrow lumina
- Cystic dilation is rarely present
- Fibrous stroma is less prominent
- Congenital biliary cyst / simple cyst:
- Usually multiple
- Strong association with autosomal dominant polycystic disease due to PKD1 or PKD2 mutations
- Serous cystadenoma:
- Often large (up to 15 cm)
- Small cysts lined by cuboidal or flattened cells with clear cytoplasm
- Mucinous cystic neoplasm (ACG Case Rep J 2018;5:e72):
- Cholangiocarcinoma:
- Infiltrating glands in fibrous stroma
- Neoplastic cells exhibit at least mild atypia
- May arise in background of chronic schistosomiasis or primary sclerosing cholangitis
- CA 19-9 and CA125 exhibit greater elevation
- Large, homogenous mass with irregular margins by imaging
- Albumin in situ hybridization (ISH) + (80%)
- Benign cystic mesothelioma / peritoneal inclusion cyst (Patholog Res Int 2010;2010:504584):
- Cysts lined by bland cuboidal or flattened mesothelial cells
- Prominent chronic inflammatory infiltrate usually present
- Presents as papillae protruding into cystic lumina
- Calretinin, HBME1, CK5/6, WT1+
- Hepatic endometrial cyst (Eur J Med Res 2015;20:48):
- Exclusively present in women
- Well circumscribed cystic lesion, often with thick overlying fibrous capsule
- Histology is characterized by at least 2 of the following
- Endometrial glands lined by Mullerian epithelium
- Endometrial type stroma
- Hemosiderin laden macrophages
- Epithelial and stroma cells positive for ER, PR and PAX2
- Stromal cells positive for CD10
- Intraductal papillary neoplasm of bile ducts (Proc (Bayl Univ Med Cent) 2019;32:124):
- Histologically characterized by intraductal papillary growth pattern with fibrovascular cores
- May present with pancreatobiliary, intestinal, gastric or oncocytic phenotypes
- Typically multifocal
- Frequently presents with high grade cytologic features
- May undergo malignant transformation into cholangiocarcinoma
- Hepatic foregut cyst (Diagn Pathol 2015;10:81):
- Small (< 4 cm) unilocular cysts
- Typically benign and asymptomatic
- Primarily found in segment 4 of the left lobe
- From deep to superficial, histologically characterized by inner ciliated pseudostratified columnar epithelium, loose lamina propria, smooth muscle band and outer fibrous capsule
- Epithelium may contain goblet cells or gastric metaplasia
Additional references
Board review style question #1
The image above was taken from the right hepatic lobe of a 54 year old woman who presented with dull right upper quadrant (RUQ) pain that began 2 weeks ago. Liver function tests were unremarkable. Per MRI, a large solid cystic mass was identified. Left lateral segmentectomy was pertinent for a single 15.3 cm solid cystic mass with white-purple, spongy cut surface. By immunostaining, a subset of cells were positive for CK7 and CK19 while negative for CK20, CDX2, calretinin and HBME1. The surrounding cells were positive for SMA and vimentin and negative for desmin, ER and PR. The Ki67 index was < 3% while p53 exhibited wild type staining in lesional cells. What is the patient's diagnosis?
- Bile duct adenoma
- Biliary adenofibroma
- Biliary cystadenoma
- Peritoneal inclusion cyst
- Von Meyenburg complex
Board review style answer #1
B. Biliary adenofibroma. The presence of neoplastic glands with biliary type epithelium in an abundantly fibrous stroma (per morphology and immunohistochemistry) in addition to the large size and solid cystic nature of the lesion favors biliary adenofibroma. The large size of this lesion is virtually unheard of among other biliary lesions such as bile duct adenomas and von Meyenburg complexes. Moreover, bile duct adenomas generally lack the overt fibrous stroma of this lesion while von Meyenburg complexes usually present as multiple lesions with irregular curvilinear glands by histology. Though biliary cystadenomas may also present as large cystic masses, the lesion in question does not harbor the clear cytoplasm of serous cystadenoma and lacks the mucin and ER / PR+ ovarian stroma of the mucinous type. Finally, the lesion lacks the papillary luminal infoldings and mesothelial immunohistochemical profile of peritoneal inclusion cysts.
Comment Here
Reference: Biliary adenofibroma
Comment Here
Reference: Biliary adenofibroma