Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Peripheral smear images | Positive stains | Negative stains | Flow cytometry description | Flow cytometry images | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Amoth H, Perry AM. APL with PML::RARA. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/leukemiaapl.html. Accessed March 27th, 2025.
Definition / general
- Acute myeloid leukemia (AML) with predominance of abnormal promyelocytes and fusion of the promyelocytic leukemia (PML) gene with the retinoic acid receptor alpha (RARA) gene
Essential features
- Acute myeloid leukemia with predominance of abnormal promyelocytes, characterized by t(15;17)(q24.1;q21.2) and leading to PML::RARA fusion
- Young to middle aged adults are most commonly affected
- 2 morphologic variants are hypergranular and microgranular
- Early diagnosis and treatment are imperative due to the high risk of coagulopathy and disseminated intravascular coagulation (DIC)
- Standard treatment includes all trans retinoic acid (ATRA) plus arsenic trioxide (ATO), with or without chemotherapy; excellent prognosis and high cure rates
- Reference: Arch Pathol Lab Med 2015;139:1308
Terminology
- Acute promyelocytic leukemia with PML::RARA fusion (World Health Organization Classification, 5th Edition)
- Acute promyelocytic leukemia (APL) with t(15;17)(q24.1;q21.2) / PML::RARA (2022 International Consensus Classification)
- Formerly called AML M3 by the French American British classification
ICD coding
- ICD-O: 9866/3 - acute promyelocytic leukemia with PML::RARA
- ICD-11: 2A60.0 & XH1A50 - acute myeloid leukemia with recurrent genetic abnormalities & acute promyelocytic leukemia, t(15;17)(q22;q11-12)
Epidemiology
- Relatively uncommon acute myeloid leukemia subtype
- Young and middle aged adults are most commonly affected (20 - 59 years)
- Accounts for 5 - 8% of acute myeloid leukemia cases in younger patients
- Higher incidence in Latin American population (Best Pract Res Clin Haematol 2003;16:357, Cancer 2012;118:5811)
- M ≈ F
Sites
- Peripheral blood and bone marrow
- Extramedullary involvement is rarely seen
Pathophysiology
- Characterized by balanced translocation t(15;17)(q24.1;q21.2) leading to PML::RARA fusion and a PML::RARA oncoprotein
- PML::RARA oncoprotein suppresses gene expression by recruitment of a number of transcriptional repressors, which leads to block in differentiation and malignant transformation of myeloid cells (PLoS One 2014;9:e104906)
- Secondary chromosomal and genetic abnormalities contribute to the phenotype
Etiology
- Unknown in majority of cases
- Prior exposure to chemotherapy or radiotherapy in some cases (Cancer 2015;121:2393, Clin Lymphoma Myeloma Leuk 2022;22:e7)
Clinical features
- Leukopenia is a common presenting sign
- Leukocytosis in the microgranular variant
- Symptoms of pancytopenia are fatigue, weakness, infection, bleeding
- High risk of coagulopathy and disseminated intravascular coagulation, especially in microgranular acute promyelocytic leukemia (Arch Pathol Lab Med 2015;139:1308, Blood Cancer J 2021;11:123)
- Early diagnosis and treatment are imperative
Diagnosis
- Established by detection of t(15;17) / PML::RARA fusion by RT-PCR, conventional cytogenetics or FISH
- RT-PCR is the most sensitive method of detection, as the translocation can be cryptic by karyotype and FISH
- Blast count can be < 20%
- Reference: Arch Pathol Lab Med 2015;139:1308
Laboratory
- Complete blood count showing pancytopenia
- Leukocytosis in microgranular acute promyelocytic leukemia
- Prolonged prothrombin time (PT) or activated partial thromboplastin clotting time (aPTT)
- Elevated D dimer and fibrin degradation products
- Decreased fibrinogen (Blood Cancer J 2021;11:123)
Prognostic factors
- White blood cell (WBC) and platelet count at presentation are useful prognostic factors for segregating patients into low, intermediate and high risk categories
- Low risk: WBC < 10,000/mL and platelets > 40,000/mL
- Intermediate risk: WBC < 10,000/mL and platelets < 40,000/mL
- High risk: WBC > 10,000/mL (Blood Cancer J 2021;11:123)
- With current treatment regimens, cure rates are > 90% (N Engl J Med 2013;369:111, J Clin Oncol 2017;35:605, Blood 2017;129:1275)
Case reports
- 37 and 60 year old women with acute promyelocytic leukemia (Leuk Res Rep 2022;18:100353)
- 42 year old woman with acute promyelocytic leukemia with myelofibrosis (Medicine (Baltimore) 2021;100:e24567)
- 62 year old man with ZBTB16::RARA positive atypical promyelocytic leukemia (Medicina (Kaunas) 2022;58:520)
- 67 year old man with extramedullary acute promyelocytic leukemia (Front Oncol 2022;12:886436)
- 71 year old man with acute promyelocytic leukemia and chronic lymphocytic leukemia (Hematol Oncol Stem Cell Ther 2019;12:161)
Treatment
- All trans retinoic acid plus arsenic trioxide is currently the standard of care for low and intermediate risk acute promyelocytic leukemia patients
- In high risk patients, chemotherapy is added to all trans retinoic acid and arsenic trioxide
- If arsenic trioxide is not available, combination of all trans retinoic acid and chemotherapy is used for all risk categories (Blood Cancer J 2021;11:123)
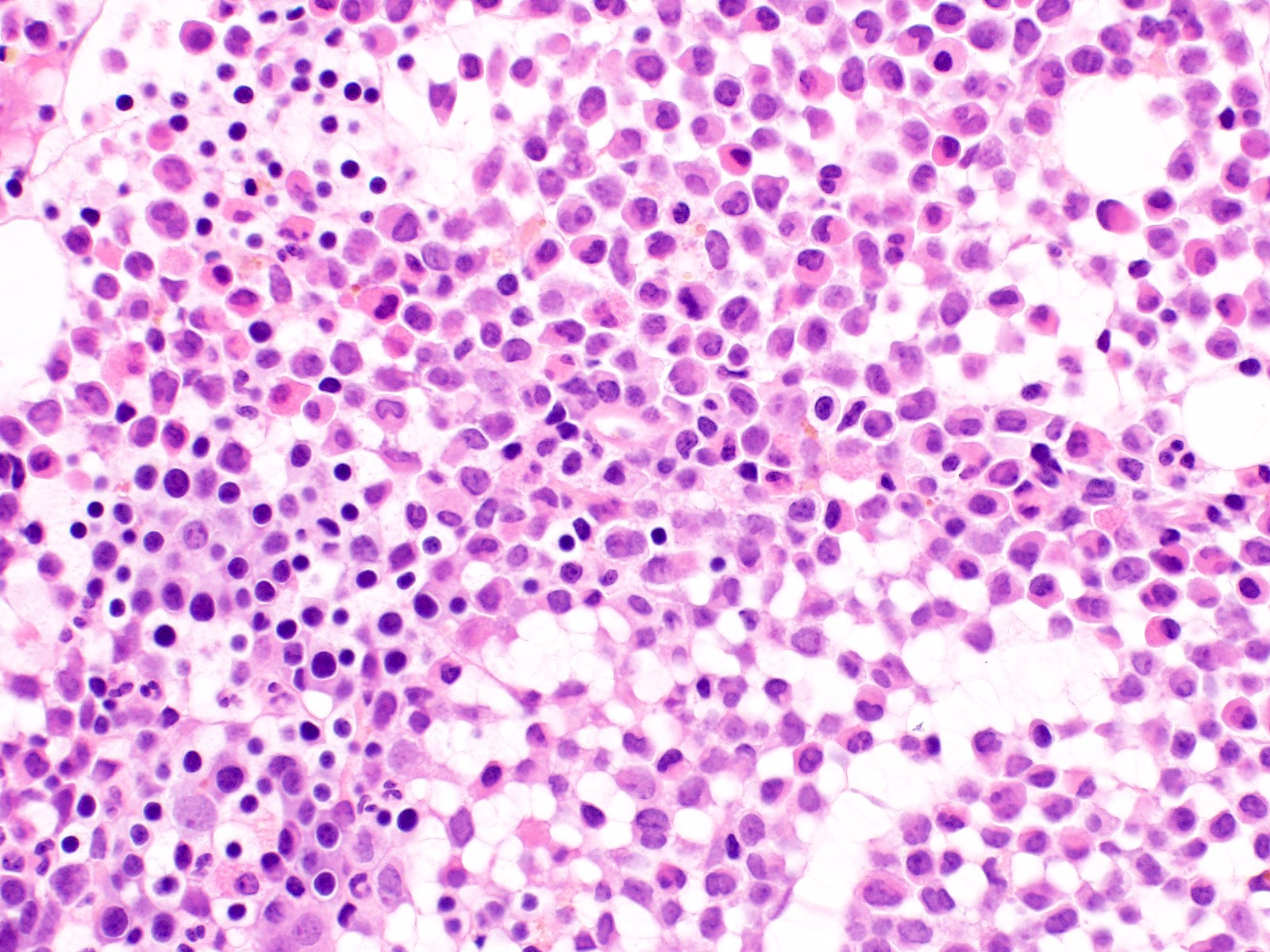
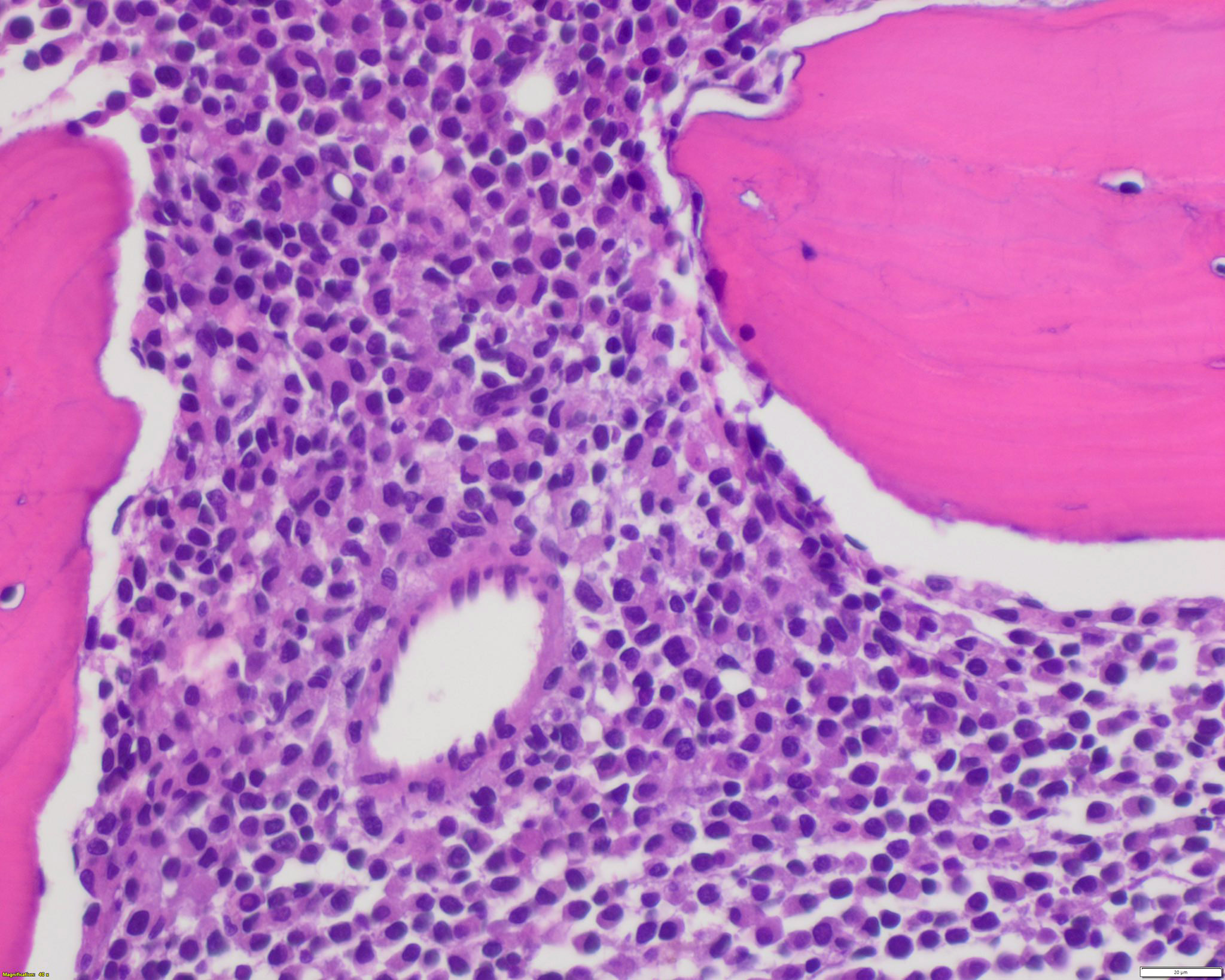
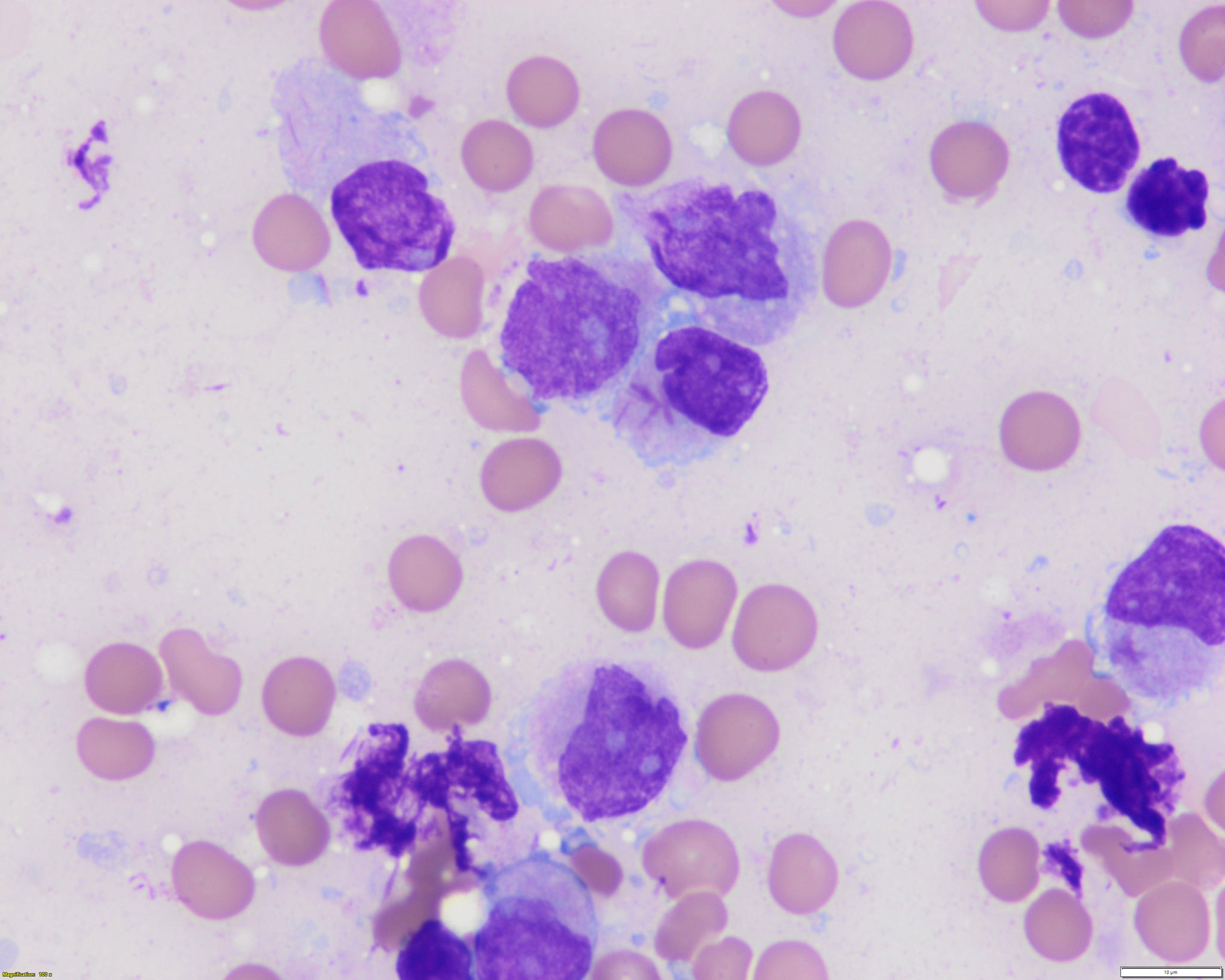
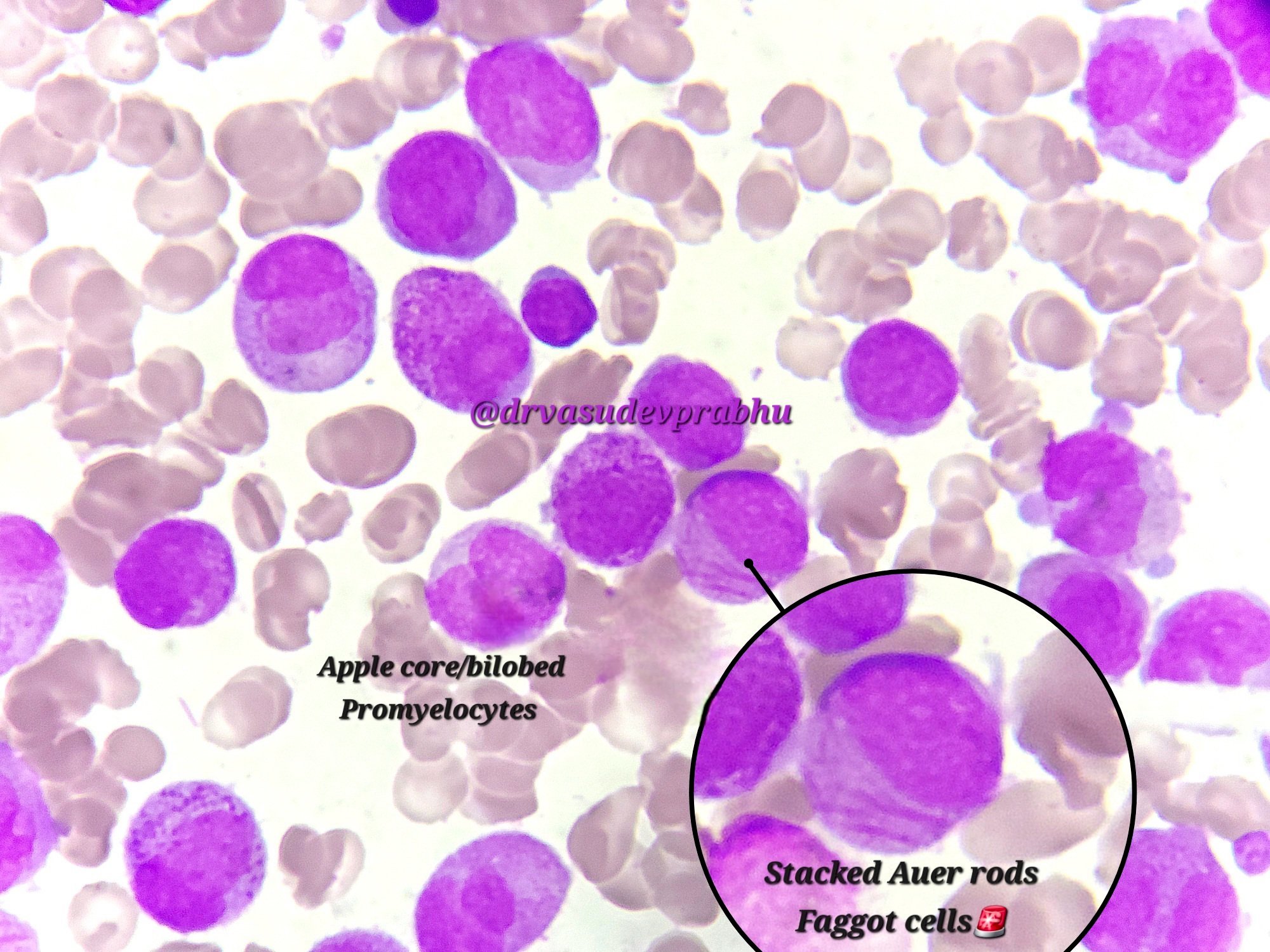
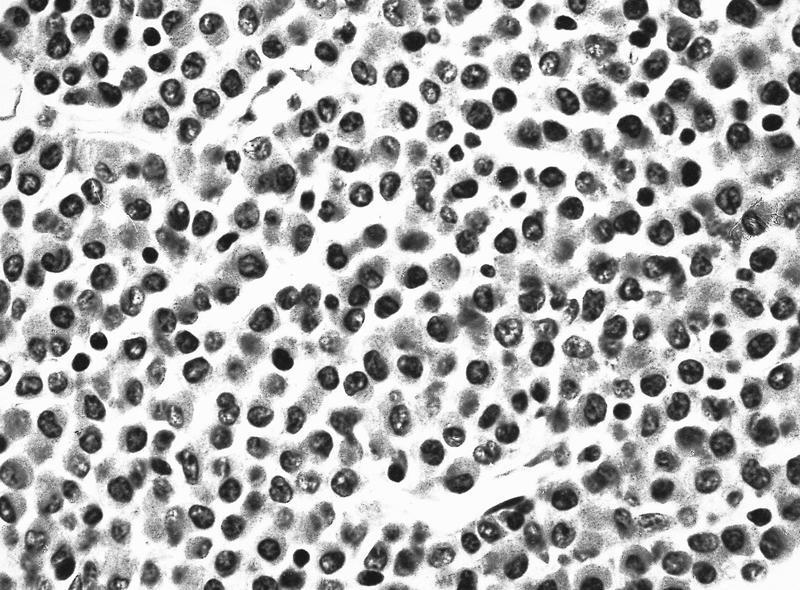
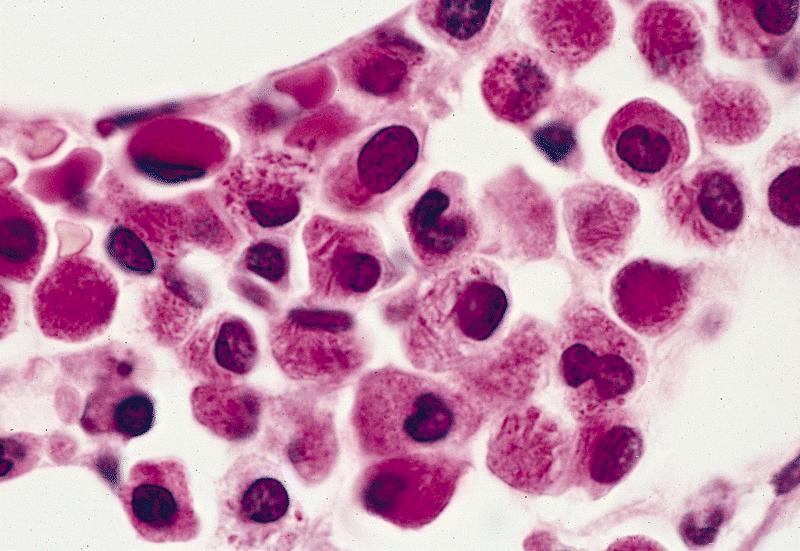
Microscopic (histologic) description
- Bone marrow hypercellular
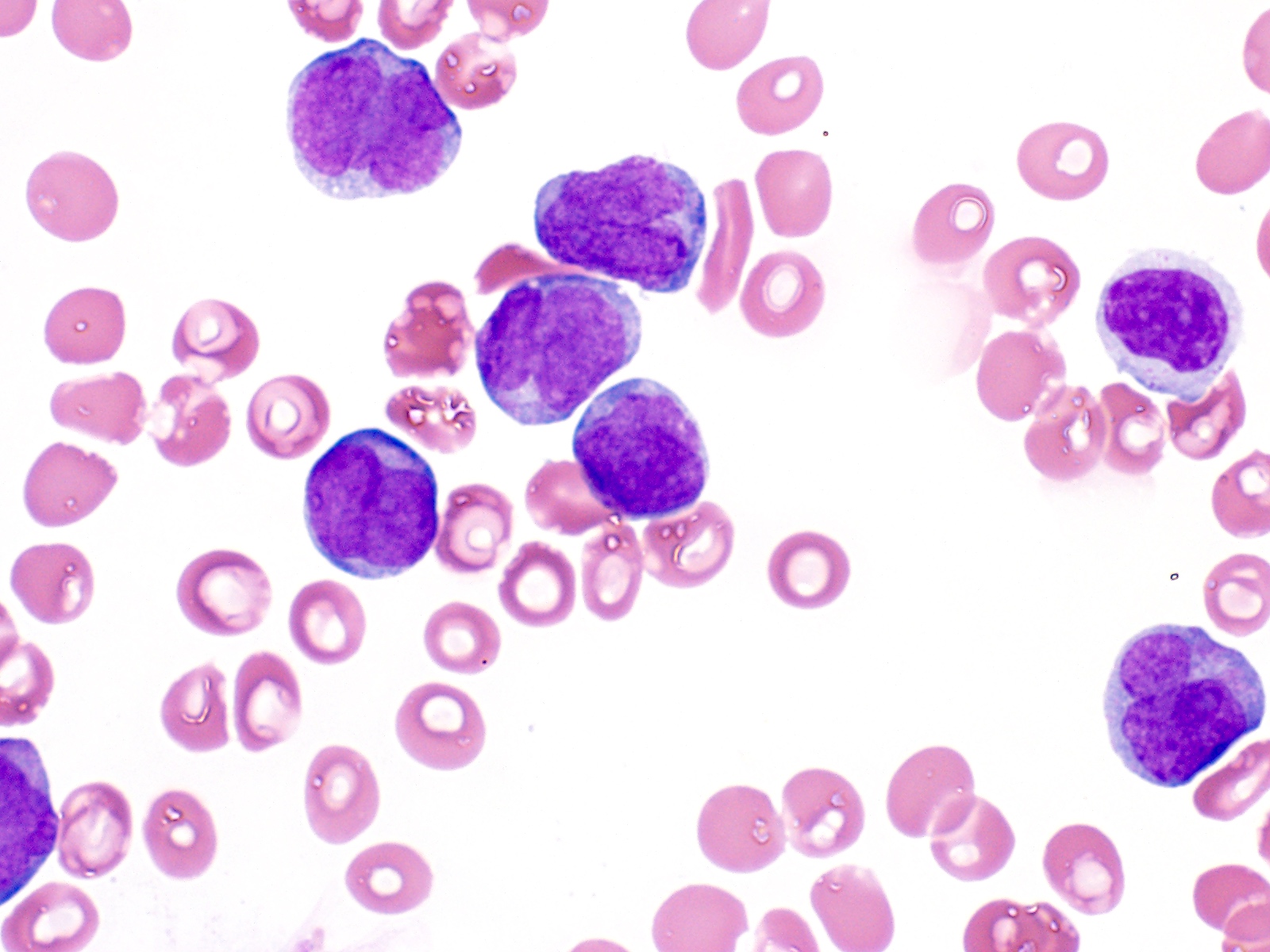
- 2 cytomorphologic variants of acute promyelocytic leukemia are hypergranular (classic) and microgranular (hypogranular)
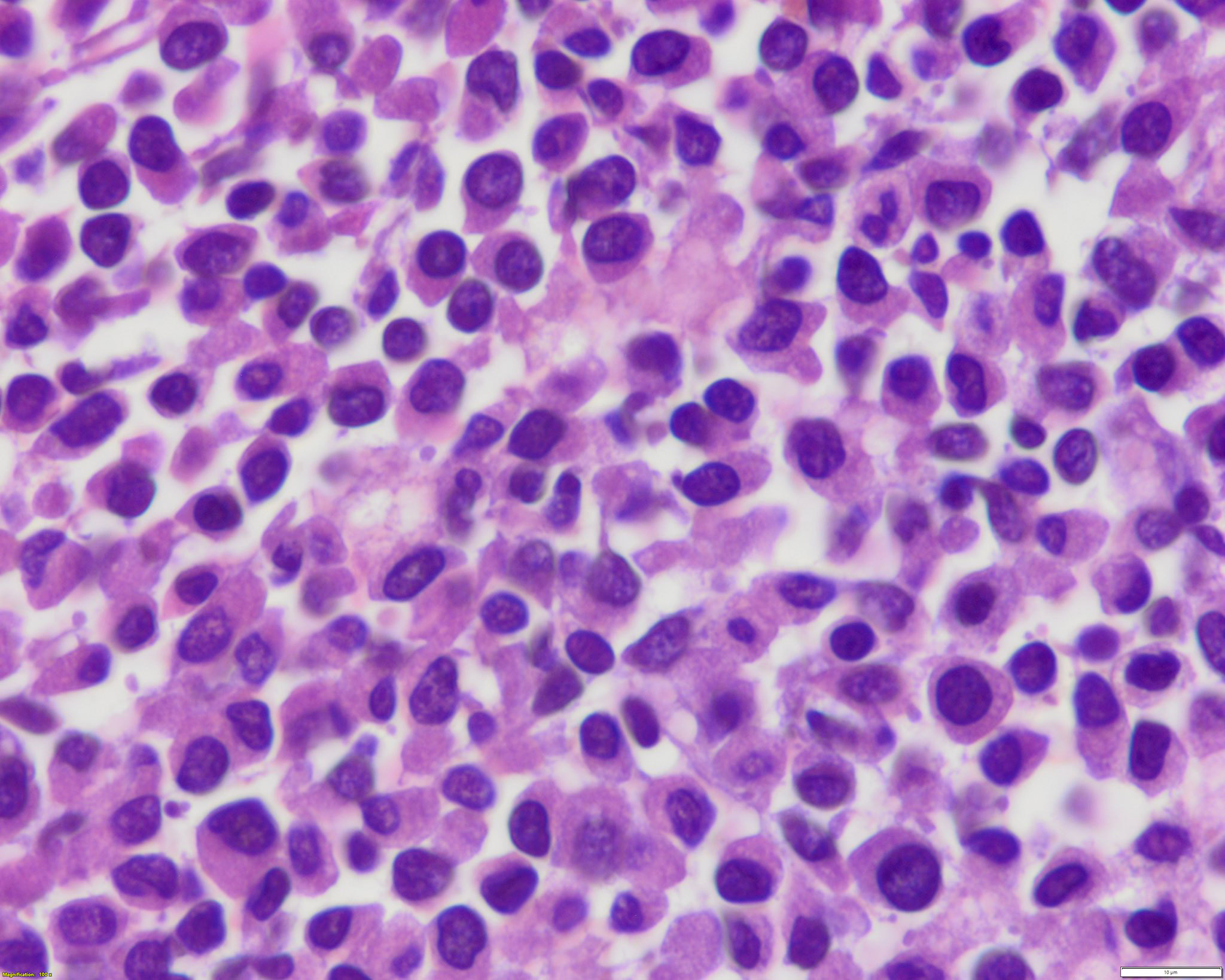
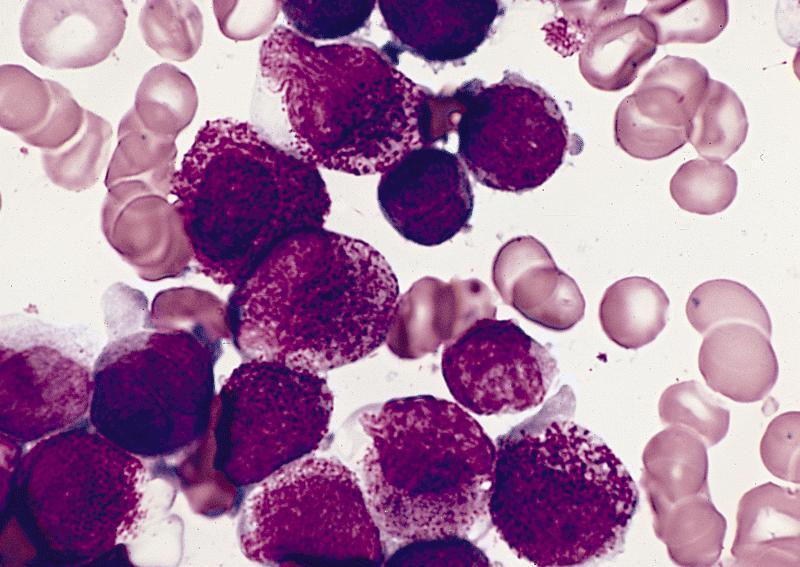
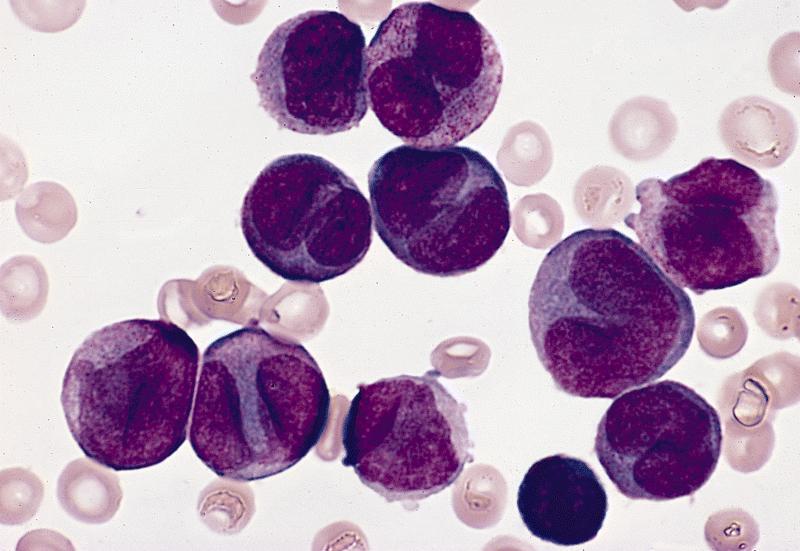
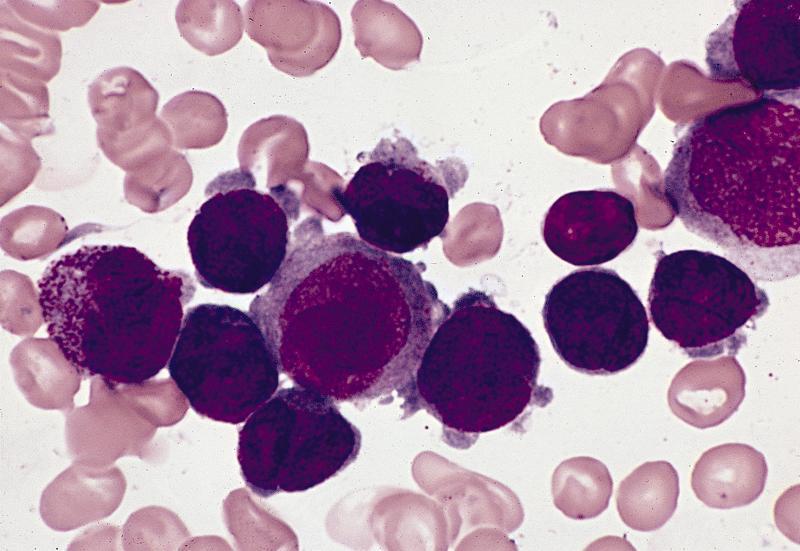
- Hypergranular acute promyelocytic leukemia
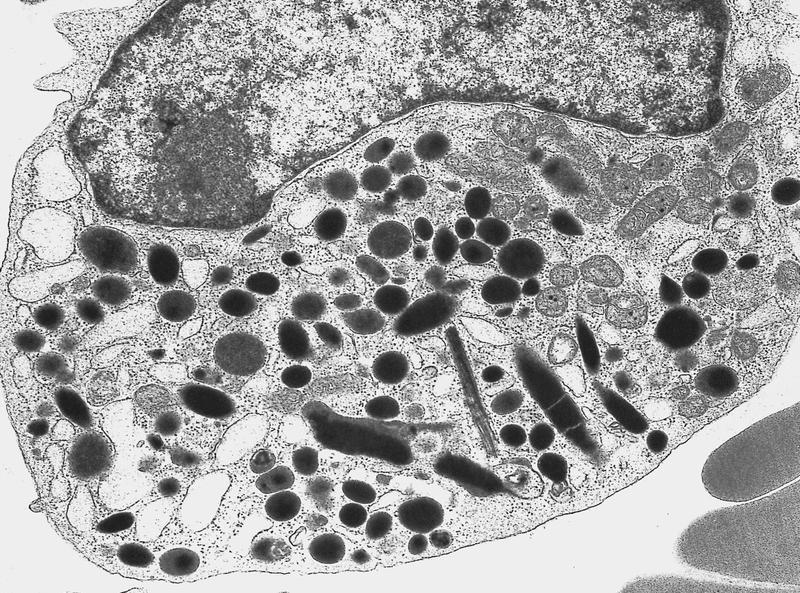
- Abnormal promyelocytes vary in size and shape, frequently with kidney shaped or bilobed nuclei
- Cytoplasm packed with large granules, staining bright pink, red or purple
- Auer rods, frequently in bundles
- Myeloblasts with Auer rods can be seen
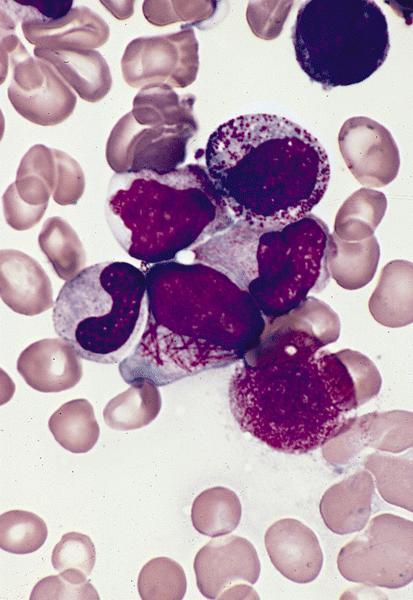
- Microgranular acute promyelocytic leukemia
- Predominantly bilobed nuclei
- Hypogranular appearing cytoplasm due to submicroscopic size of azurophilic granules
- Most cases have some abnormal promyelocytes with prominent granules or Auer rods (Arch Pathol Lab Med 2015;139:1308)
- ZBTB16::RARA t(11;17) acute promyelocytic leukemia has some characteristic morphologic features (Blood 2000;96:1287)
- More regular nucleus, not bilobed; occasionally more condensed nuclear chromatin compared with classic acute promyelocytic leukemia
- Coarse cytoplasmic granules or less often, fine or no granules
- No bundles of Auer rods
- Increased number of hypogranular pelgeroid neutrophils
Microscopic (histologic) images
Contributed by Anamarija M. Perry, M.D., @sanamloghavi on Twitter, @DrVasudevPrabhu on Twitter and AFIP
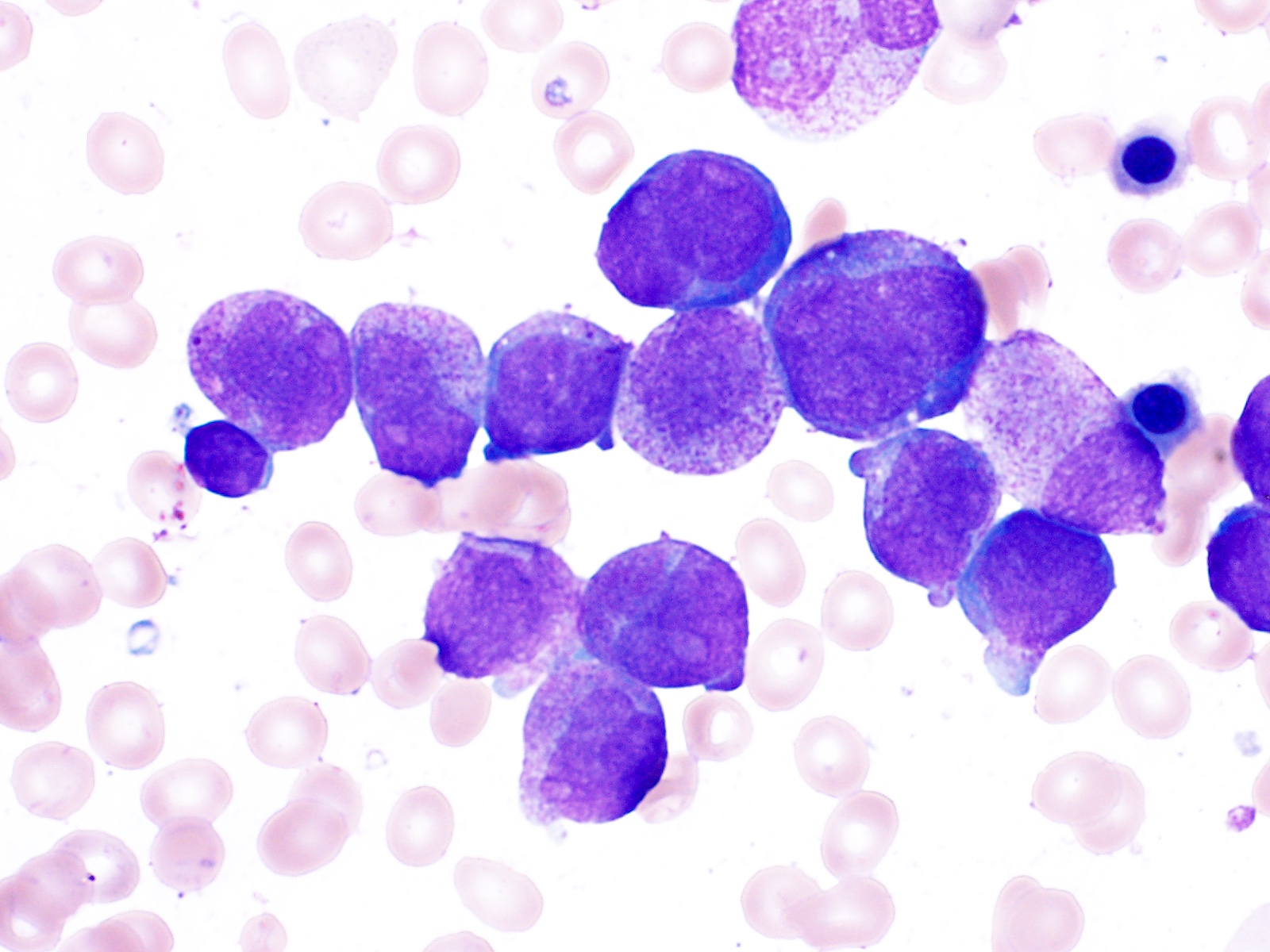
Peripheral smear description
- In classic variant, pancytopenia is frequently seen with occasional circulating abnormal promyelocytes
- In microgranular acute promyelocytic leukemia, leukocytosis with numerous abnormal promyelocytes
Positive stains
Negative stains
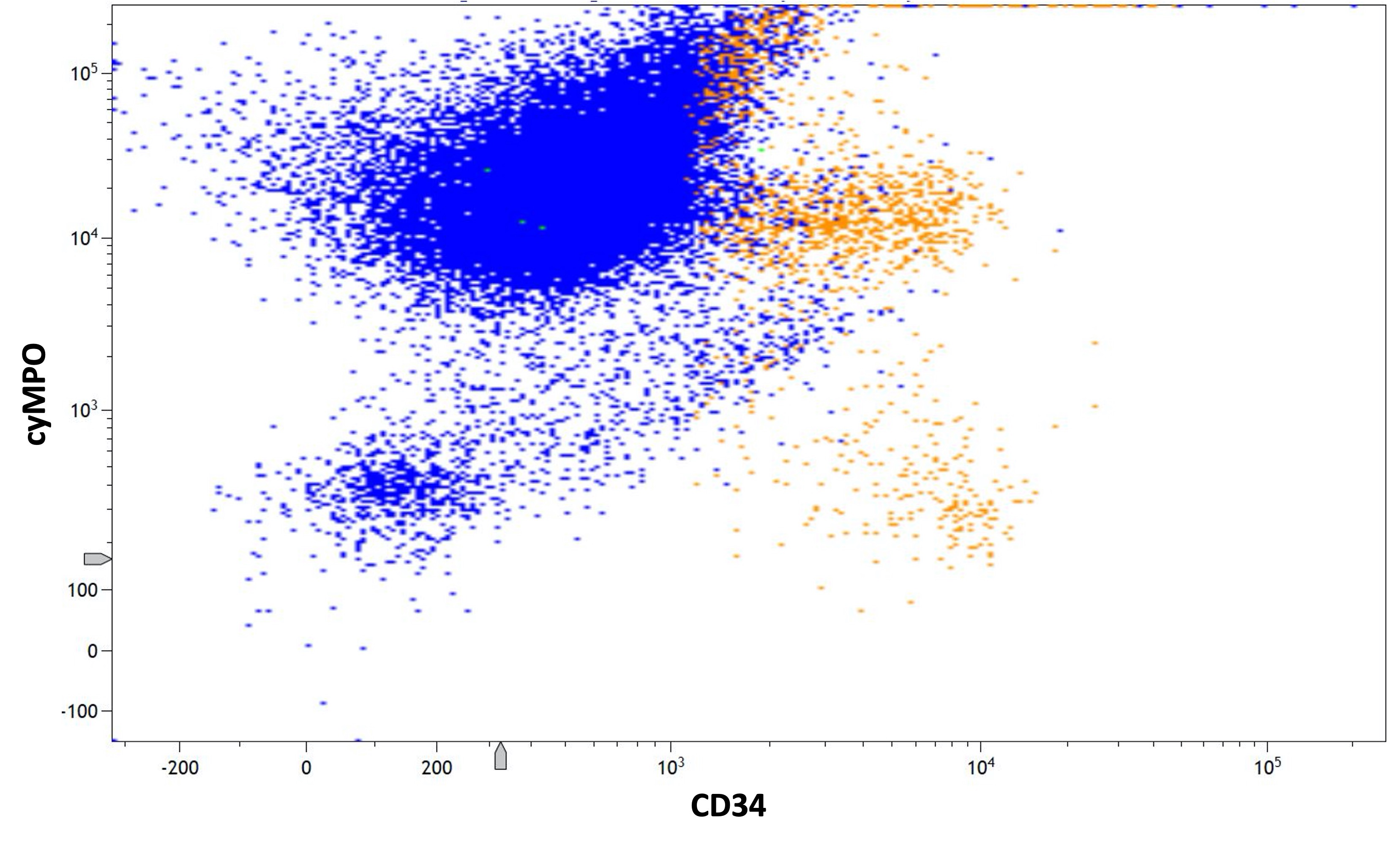
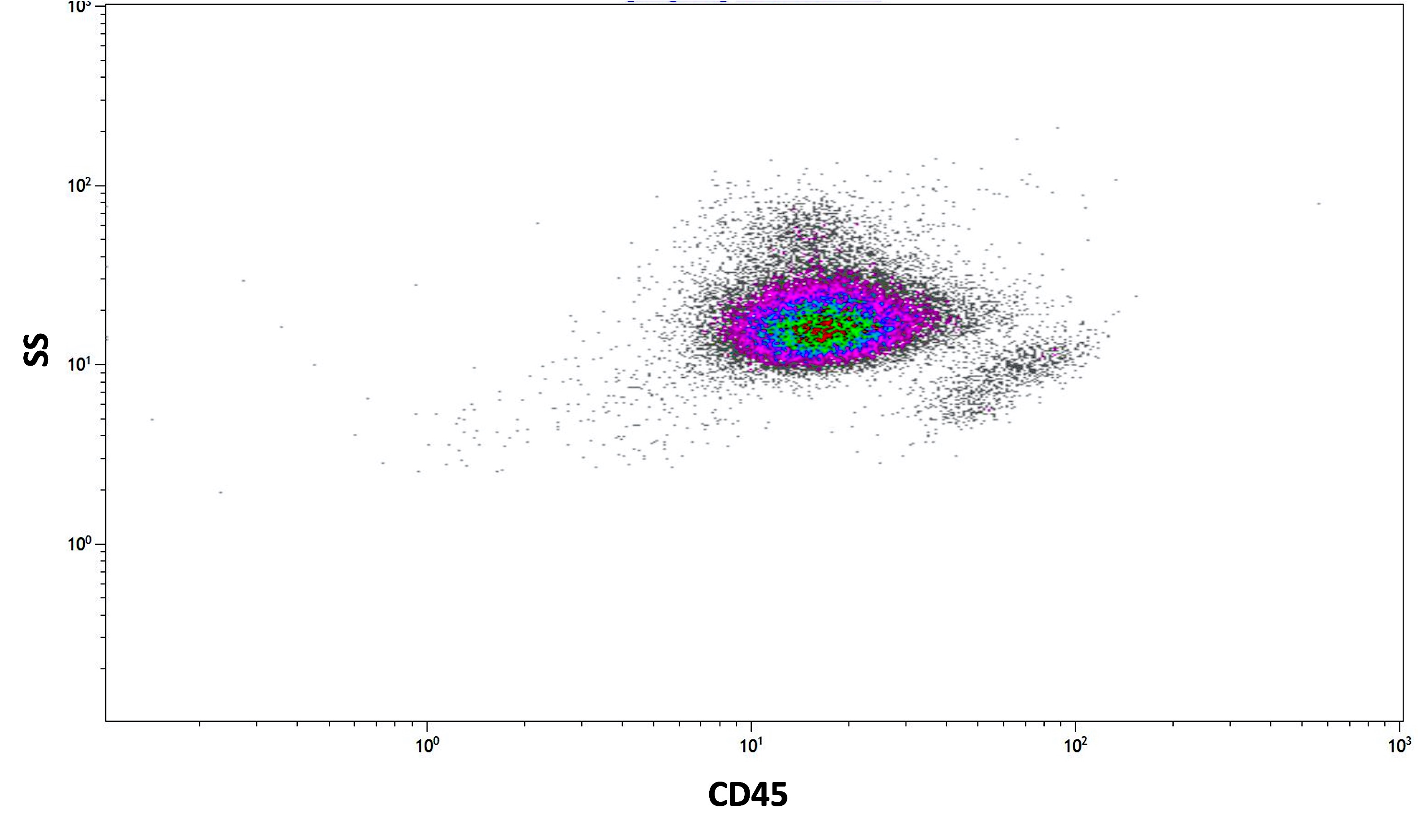
Flow cytometry description
- Hypergranular acute promyelocytic leukemia
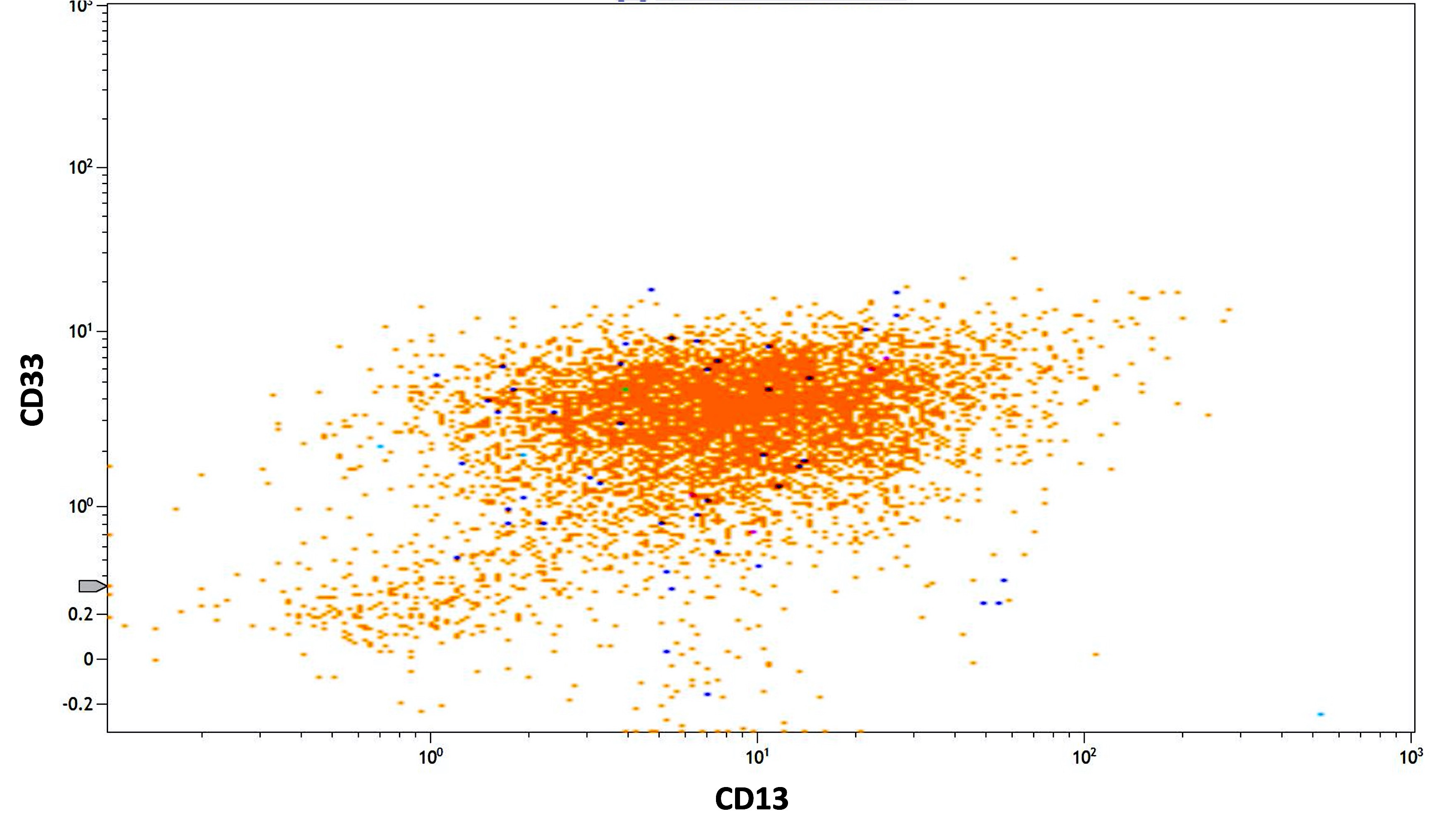
- Abnormal promyelocytes with high forward and side scatter
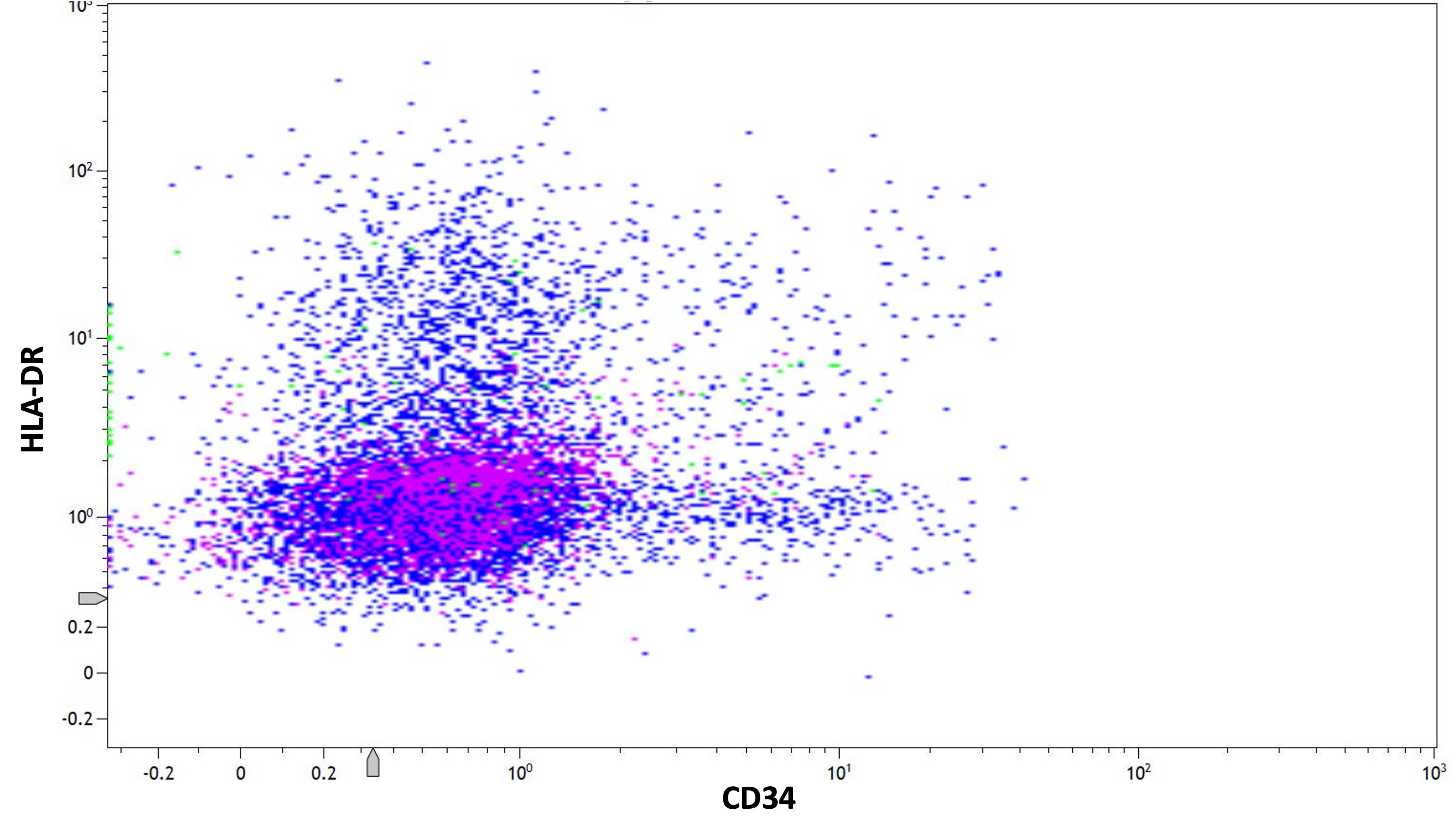
- Low or absent expression of CD34 and HLA-DR
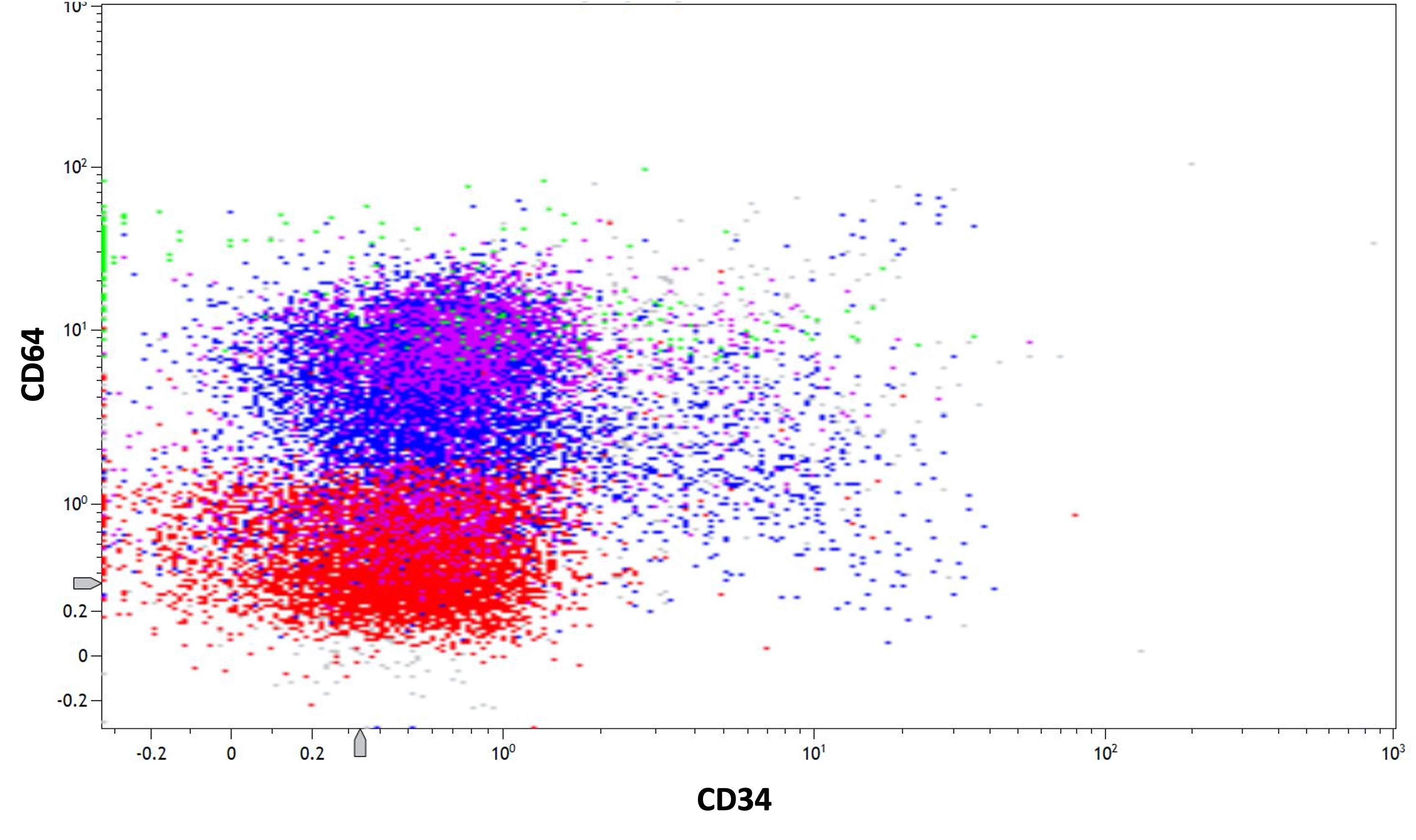
- Positive for CD13, CD33, CD64, CD117 and cytoplasmic myeloperoxidase
- Negative for CD11a, CD11b, CD15, CD18, CD65, CD66b and CD66c
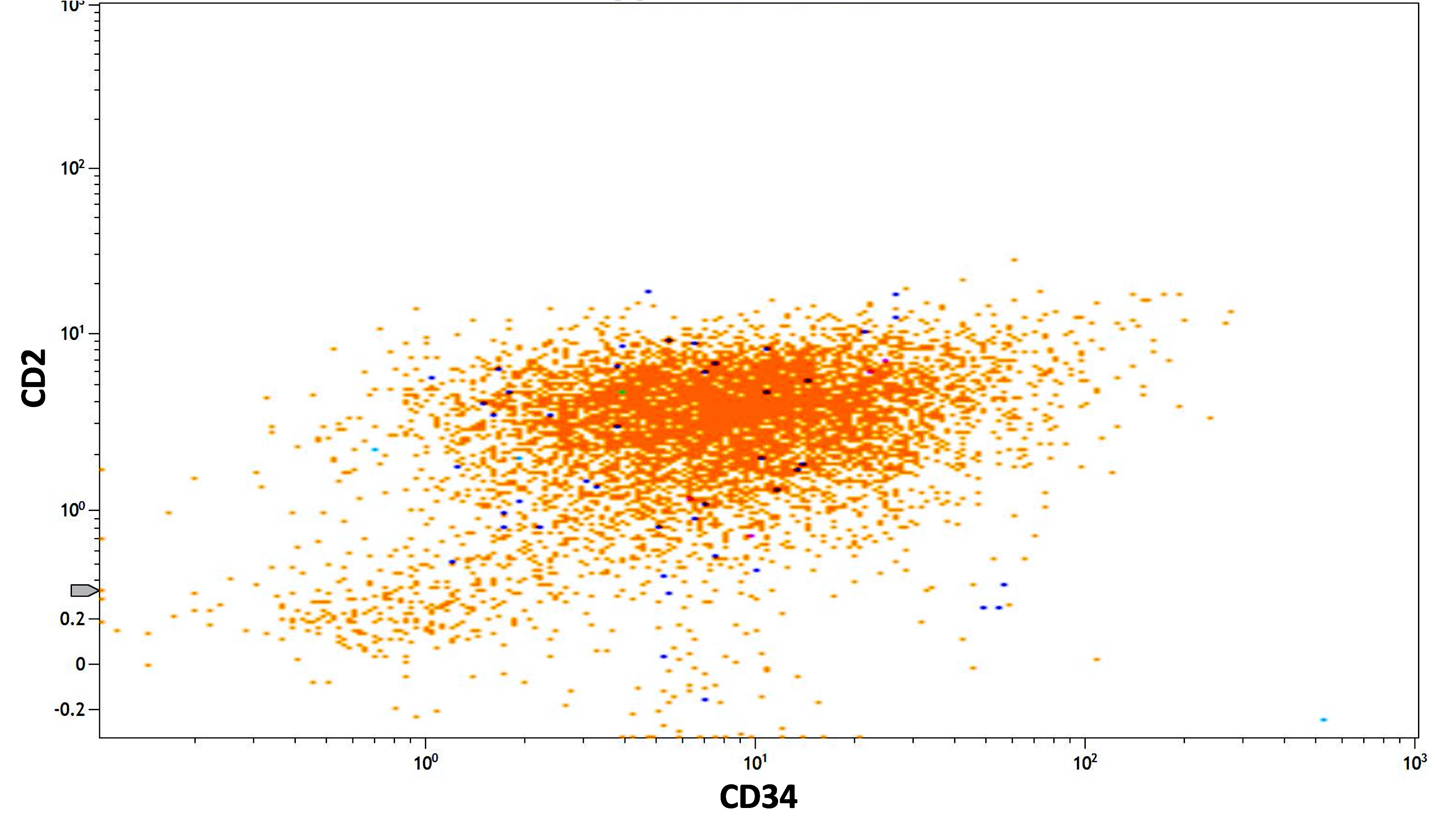
- CD56 seen in 10% of cases; CD2 rarely expressed, frequently with CD34 and CD56
- Lymphoid markers (e.g., CD5, CD7, CD19, CD22, cytoplasmic CD3) and TdT rarely expressed (Best Pract Res Clin Haematol 2003;16:369, Haematologica 1999;84:405, J Clin Oncol 2000;18:1295, Blood 2006;108:3484, Cytometry B Clin Cytom 2022;102:283)
- Microgranular acute promyelocytic leukemia
- Abnormal promyelocytes have lower side scatter and are located in the blast gate on the CD45 versus side scatter plot
- Similar phenotype to hypergranular acute promyelocytic leukemia with more common expression of CD2 and CD34
- Expression of CD2 in acute promyelocytic leukemia is associated with FLT3 internal tandem duplication (ITD) mutation (Am J Hematol 2001;67:34, Pol J Pathol 2012;63:8, Cytometry B Clin Cytom 2022;102:283)
Flow cytometry images
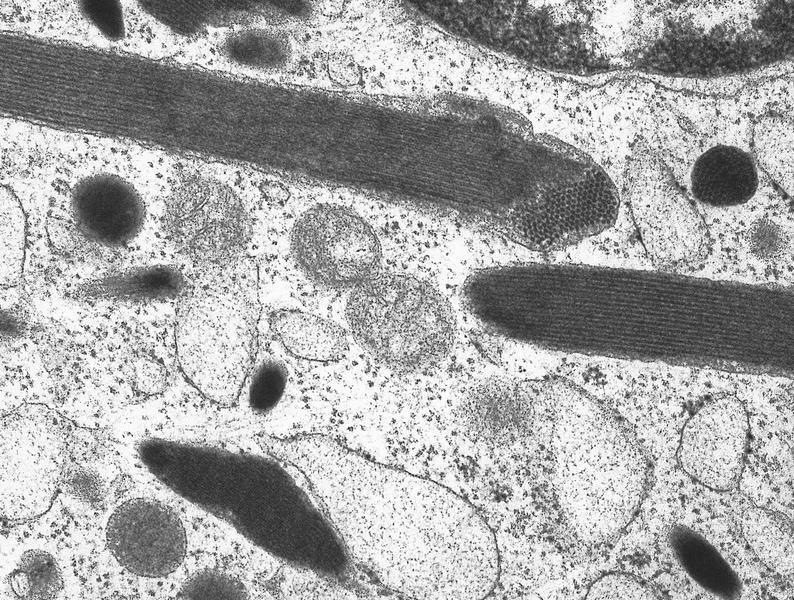
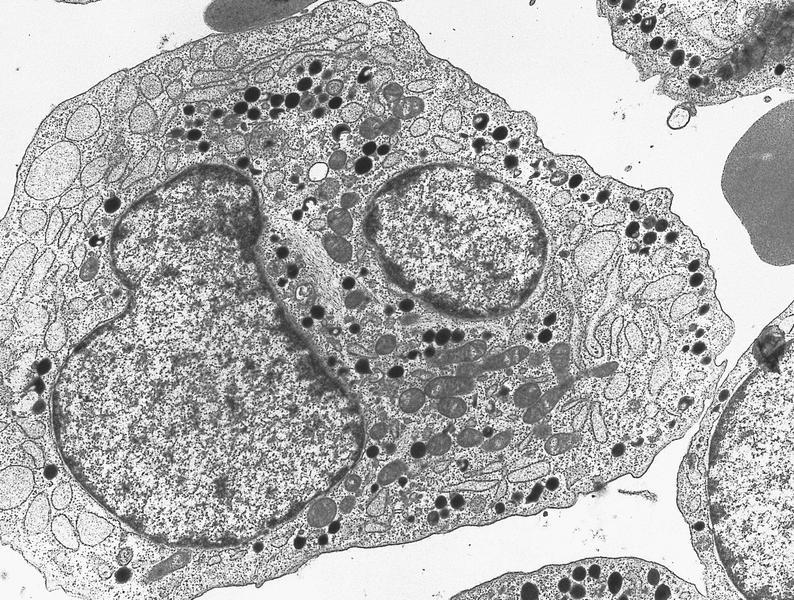
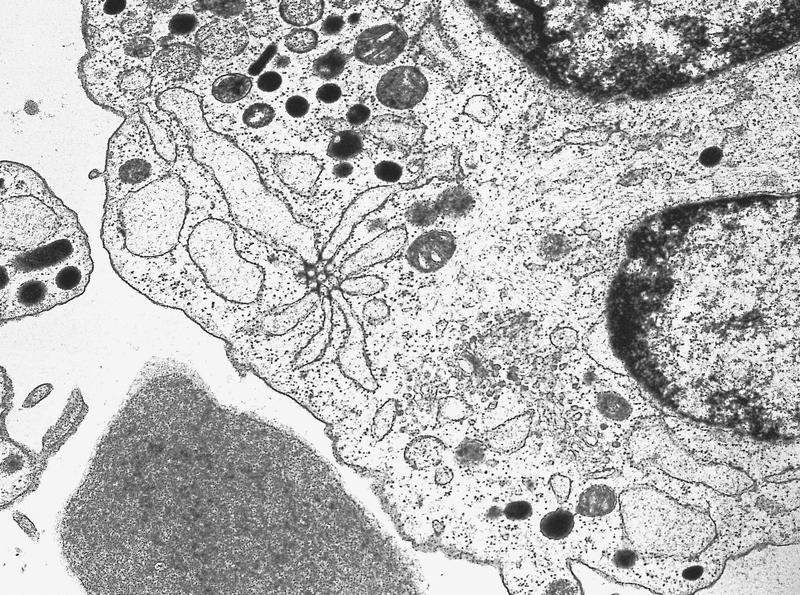
Electron microscopy images
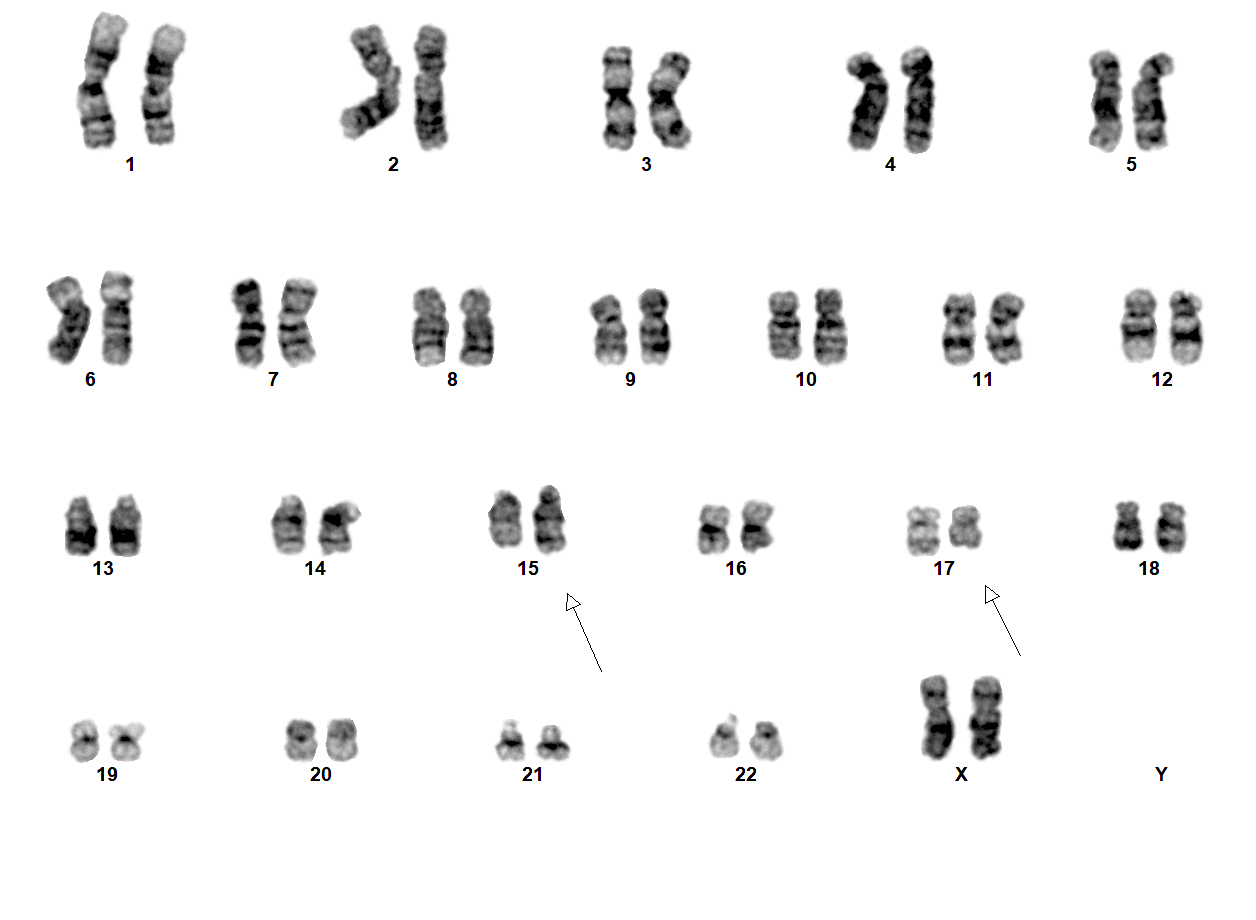
Molecular / cytogenetics description
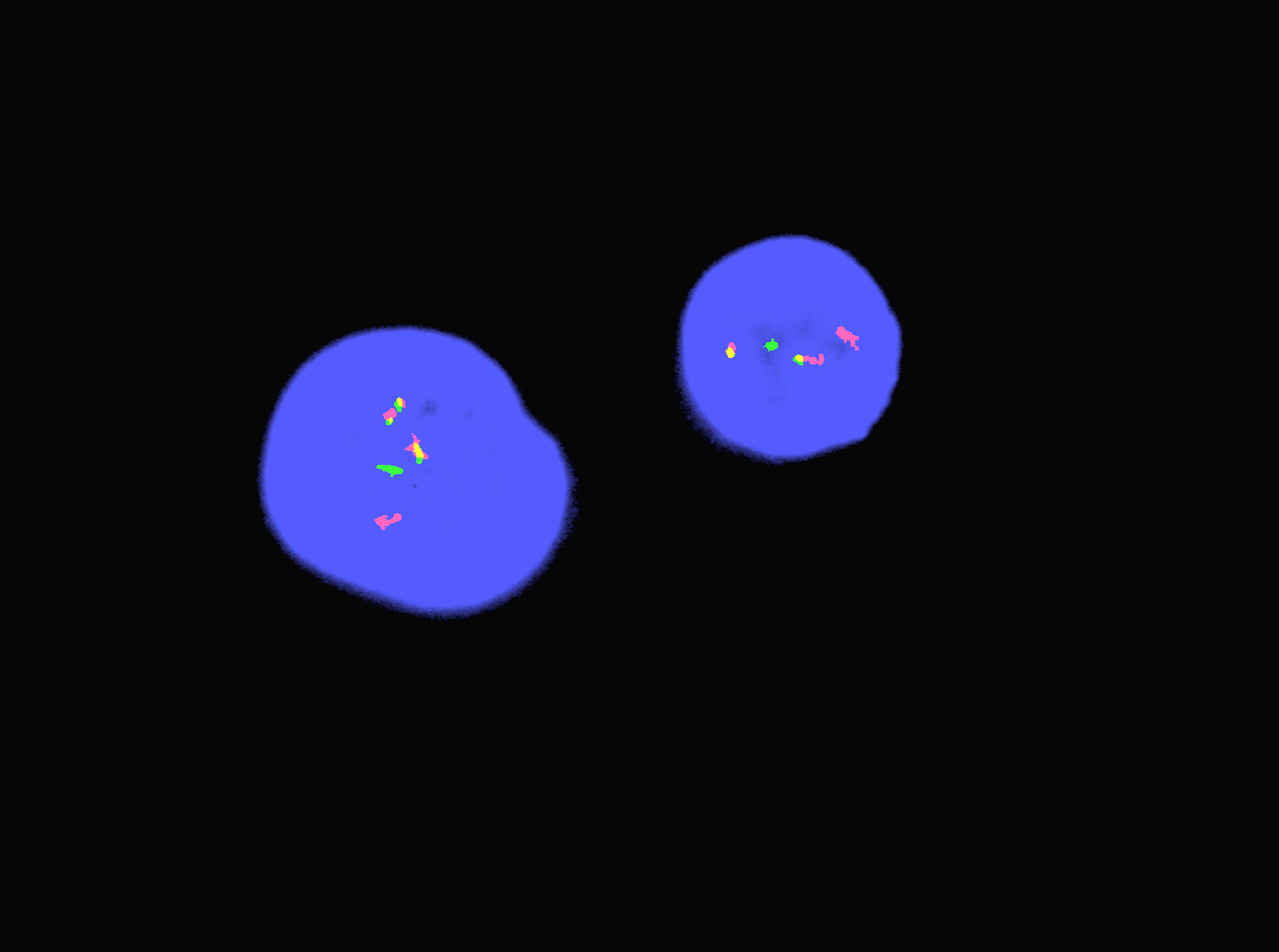
- t(15;17)(q24.1;q21.2) leading to PML::RARA fusion
- PML::RARA can be detected in 90 - 95% of cases (Nature 1990;347:558)
- 3 PML::RARA transcript isoforms (depending on the PML breakpoint location on chromosome 15): long (in intron 6, Bcr1), variant (in exon 6, Bcr2) and short (in intron 3, Bcr3) (Cancers (Basel) 2020;12:624)
- Occasional cases have cryptic t(15;17)(q24.1;q21.2) or complex rearrangements involving an additional chromosome (Leukemia 2016;30:1672)
- Deletion 7q and trisomy 8 are the most common frequent additional cytogenetic alterations (Cancers (Basel) 2020;12:624)
- Variant RARA translocations (Hematol Oncol Stem Cell Ther 2020;13:189, Cancers (Basel) 2020;12:967, Arch Pathol Lab Med 2015;139:1308, Front Oncol 2022;12:871590)
- Detected in ~5% of cases, involve RARA gene at 17q21.2 and different partners
- Should be diagnosed as acute promyelocytic leukemia with variant RARA translocations
- Partner genes
- ZBTB16 at 11q23 has a poor response to all trans retinoic acid and arsenic trioxide, characteristic morphology (see Microscopic (histologic) description)
- NUMA1 at 11q13
- NPM1 at 5q35
- STAT5B at 17q21 has a poor response to all trans retinoic acid and arsenic trioxide
- PRKAR1A at 17q24
- FIP1L1 at 4q12
- BCOR at Xp11
- OBFC2A at 2q32
- TBLR1 at 3q26
- GTF2I at 7q11
- IRF2BP2 at 1q42
- FNDC3B at 3q26
- STAT3 at 17q21
- HNRNPC at 14q11
- NUP98
- TNRC18
- Most common somatic mutations include FLT3 (including FLT3 ITD and FLT3 p.D835), WT1, NRAS, KRAS, USP9X, ARID1A and EED (Leukemia 2016;30:1672, Leukemia 2019;33:1387)
Molecular / cytogenetics images
Sample pathology report
- Bone marrow, core biopsy, touch imprints, aspirate clot, aspirate smears and peripheral blood smears:
- Acute promyelocytic leukemia with PML::RARA
- Adequacy: The core biopsy represents ~7 mm of evaluable bone marrow. The aspirate materials are adequate.
- Cellularity: Hypercellular, ~90% on average.
- Erythroid elements: Decreased quantity. Full spectrum of maturation. No dysplasia.
- Myeloid elements: Predominance of atypical promyelocytes in bone marrow. The neoplastic promyelocytes are large cells and most of them are heavily granulated. Many feature a lobulated nucleus and some have overlapping nuclear lobes. Rare cells with numerous (10 - 15) Auer rods are found in the bone marrow and blood smears. There is little granulocytic maturation beyond the promyelocyte stage.
- Megakaryocytes: Adequate number. No definite dysplasia.
- Ancillary studies: Fluorescence in situ hybridization analysis of the bone marrow aspirate is positive for PML::RARA gene fusion.
- Complete blood count:
- White blood cell (K/uL): 0.9
- Red blood cell (M/uL): 3.06
- Hemoglobin (g/dL): 10.1
- Hematocrit (%): 27.1
- Mean corpuscular volume (fl): 88.6
- Red cell distribution width (%): 14.5
- Platelet (K/uL): 106
- Peripheral blood: Absolute leukopenia with occasional leukemic promyelocytes noted. Slight red cell anisocytosis and moderate poikilocytosis with some ovalocytes but no schistocytes. A few nucleated red cells noted on scan. Slight to moderate thrombocytopenia with some variation in platelet size and occasional hypogranular forms.
Differential diagnosis
- Acute myeloid leukemia with monocytic differentiation:
- Can resemble the microgranular variant of acute promyelocytic leukemia with bilobed nuclei and apparent lack of granules
- In microgranular acute promyelocytic leukemia, usually some classic cells with coarse cytoplasmic granules or bundles of Auer rods are seen
- Other than CD64, acute promyelocytic leukemia is usually negative for monocytic markers (e.g., CD14 and CD36)
- Acute myeloid leukemia with mutated NPM1 (Hematol Oncol 2012;30:109, Cytometry B Clin Cytom 2022;102:283):
- Subset of these cases can have overlapping immunophenotypic features with microgranular acute promyelocytic leukemia
- CD2 and CD34 are commonly expressed in acute promyelocytic leukemia and usually negative in AML with mutated NPM1
- When expressed simultaneously, CD13 and CD64 are highly sensitive and specific for acute promyelocytic leukemia
Additional references
Board review style question #1
A 27 year old woman presents with fatigue of ~2 weeks' duration and pancytopenia with marked leukopenia. Bone marrow biopsy and aspiration were performed and representative morphology is shown in the image above. Which of the following studies should be done to confirm the diagnosis suspected on morphology?
- FISH for MYC rearrangement
- FISH for PML::RARA fusion
- Mutation analysis for FLT3
- Mutation analysis for NPM1
- RT-PCR for BCR::ABL1
Board review style answer #1
B. FISH for PML::RARA fusion. Bone marrow aspirate shows abnormal promyelocytes. These findings are suspicious for acute promyelocytic leukemia, however, a definitive diagnosis requires detection of PML::RARA fusion, which can be done with a rapid FISH study.
Comment Here
Reference: APL with PML::RARA
Comment Here
Reference: APL with PML::RARA
Board review style question #2
Which of the following immunophenotypes is characteristic for hypergranular acute promyelocytic leukemia?
- CD34+, CD13+, CD33+, MPO+, CD2-
- CD34+, CD13+, CD33+, MPO-, CD2-
- CD34+, HLA-DR-, CD13+, CD33+, CD64-
- CD34-, HLA-DR-, CD13+, CD33+, CD64+
- CD34-, HLA-DR+, CD13+, CD33+, CD64-
Board review style answer #2
D. CD34-, HLA-DR-, CD13+, CD33+, CD64+. Hypergranular variant of APL is characterized by low or absent expression of CD34 and HLA-DR, is usually brightly positive for CD33 and is positive for CD13 and cytoplasmic myeloperoxidase (MPO). CD64 is also frequently expressed. The other answers (A, B and C) can be eliminated since they show the phenotype of blasts that are CD34 positive or HLA-DR positive (answer E).
Comment Here
Reference: APL with PML::RARA
Comment Here
Reference: APL with PML::RARA