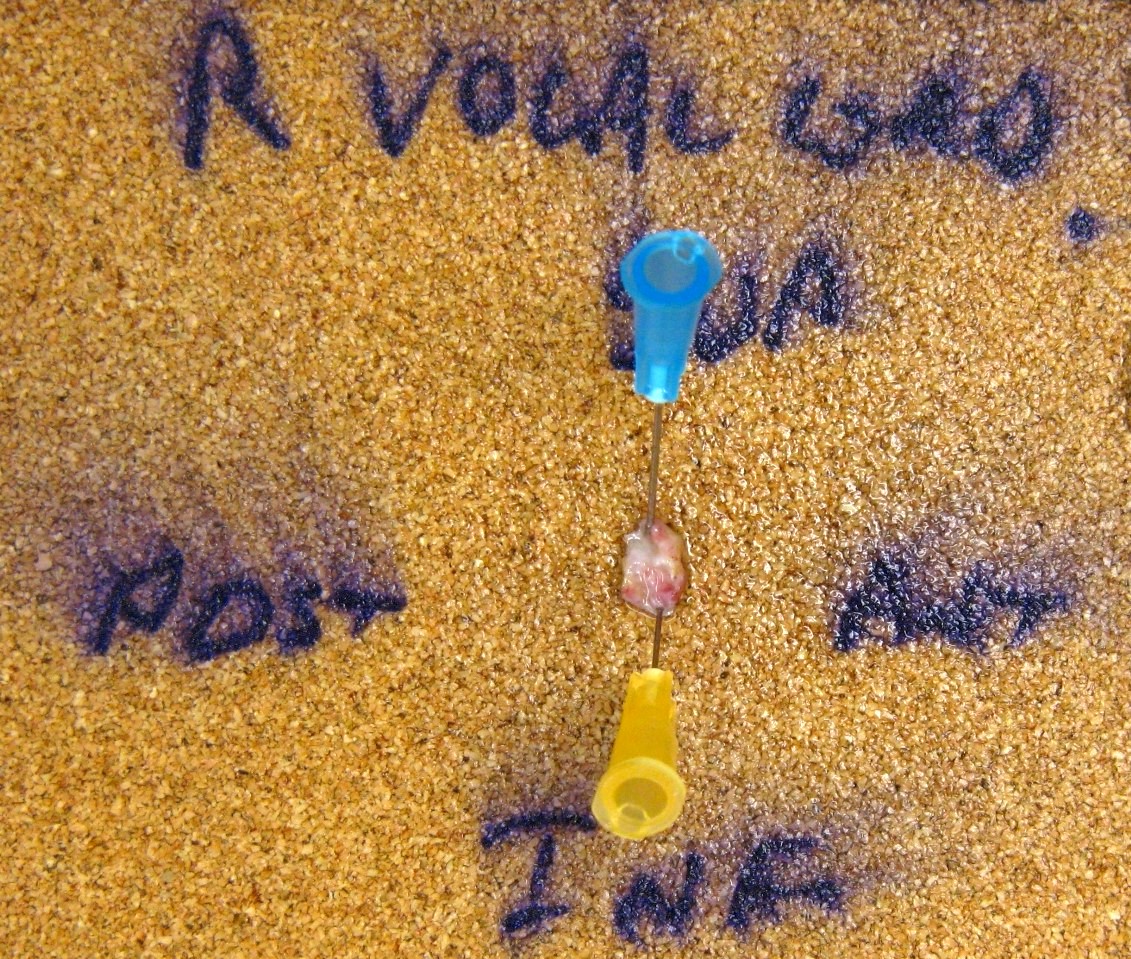Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Brown J, Gupta R. Squamous cell carcinoma of larynx. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/larynxscc.html. Accessed April 2nd, 2025.
Definition / general
- Malignant proliferation of squamous epithelial cells
- Most common primary cancer of the larynx (CA Cancer J Clin 2017;67:31)
Essential features
- Malignant proliferation of squamous epithelial cells
- Most common primary cancer of the larynx (CA Cancer J Clin 2017;67:31)
- Strong association with smoking and alcohol use
- Recognized subtypes are conventional, verrucous, basaloid, papillary, lymphoepithelial, adenosquamous and spindle cell
Terminology
- Squamous cell carcinoma (SCC) of the larynx has several historical names; these are currently not recommended and are included below for historical interest only as these reflect the evolution of surgical pathology
- Epidermoid carcinoma
- Ackerman tumor
- Lane tumor, pseudosarcoma sarcomatoid carcinoma, carcinosarcoma
ICD coding
- Conventional squamous cell carcinoma
- ICD-O
- ICD-11
- 2B6C.0 - squamous cell carcinoma of piriform sinus
- 2B6D.0 - squamous cell carcinoma of hypopharynx and variants
- 2C23.10 - squamous cell carcinoma of larynx, glottis
- 2C23.20 - squamous cell carcinoma of larynx, supraglottis
- 2C23.30 - squamous cell carcinoma of larynx, subglottis
- 2C24.1 - squamous cell carcinoma of trachea
- Nonconventional subtypes
- Adenosquamous carcinoma
- ICD-11
- 2B6C.Y & XH7873 - other specified malignant neoplasms of piriform sinus & adenosquamous carcinoma
- 2B6D.Y & XH7873 - other specified malignant neoplasms of hypopharynx & adenosquamous carcinoma
- 2C23.1Y & XH7873 - other specified malignant neoplasms of larynx, glottis & adenosquamous carcinoma
- 2C23.2Y & XH7873 - other specified malignant neoplasms of larynx, supraglottis & adenosquamous carcinoma
- 2C23.3Y & XH7873 - other specified malignant neoplasms of larynx, subglottis & adenosquamous carcinoma
- 2C24.Y & XH7873 - other specified malignant neoplasms of trachea & adenosquamous carcinoma
- ICD-11
Epidemiology
- Second most common malignancy of the aerodigestive tract after lung cancer
- 200,000 cases globally and 123,000 deaths in 2019 (Lancet Respir Med 2021;9:1030)
- Estimated 12,650 new laryngeal cancer cases in the U.S. in 2024; 3,880 deaths (CA Cancer J Clin 2024;74:12)
- ~80% occur in males; strong male predominance is not explained by other risk factors (CA Cancer J Clin 2024;74:12, Cancer 2022;128:3531)
- Typically presents in the seventh decade
- More common in rural areas than urban, with no difference in staged matched survival (Laryngoscope 2018;128:1874)
- Strong association with smoking and alcohol use, with compounding combined effects
- Gradual decline in incidence corresponds with decreasing smoking rates
Sites
- Classified based on location in relationship to glottis as glottic, subglottic, supraglottic and tracheal
- Most tumors are glottic
- Glottis subsites include true vocal cords, anterior commissure and posterior commissure
- 30% are supraglottic
- Supraglottic subsites include suprahyoid epiglottis, aryepiglottic folds (laryngeal aspect), infrahyoid epiglottis, arytenoids and ventricular bands (or false cords)
- 1.5% are subglottic (JAMA Otolaryngol Head Neck Surg 2023;149:34)
- Primary tracheal SCC is rare
Pathophysiology
- Epithelial dysplasia is accepted as a precursor lesion
- Epithelial dysplasia can be graded using 2 tiered (low grade dysplasia / squamous intraepithelial lesion [SIL] versus high grade dysplasia / squamous intraepithelial lesion) or 3 tiered systems (also includes carcinoma in situ); by definition, the basement membrane is preserved (Head Neck Pathol 2020;14:1046)
- Chromosomal instability in precursor lesions has been associated with a significantly increased rate of progression to invasive cancer (Pathology 2014;46:216)
- Several driver gene mutations have been implicated in oncogenesis, including p53, cyclin D1, p16 (CDKN2a / INK4a, independent of human papillomavirus [HPV]), p14 (ARF), FHIT, RASSF1A, EGFR and RB1 (J Clin Pathol 2006;59:445, N Engl J Med 2007;357:2552)
- Significantly higher rate of smoking related mutations in laryngeal SCC compared to other head and neck SCCs (Sci Rep 2019;9:19256)
Etiology
- Smoking is strongly associated with laryngeal SCC development (Eur Arch Otorhinolaryngol 2017;274:1617)
- Heavy alcohol use is an established risk factor
- Combined smoking and alcohol use have a multiplicative effect on risk
- Gastroesophageal reflux disease (GERD) is a possible risk factor for laryngeal but not hypopharyngeal SCC (Am J Otolaryngol 2020;41:102653)
- HPV is found in a minority of cases, with recent studies finding 11.6% and 13% of cases positive (J Pathol Transl Med 2020;54:411, Oral Oncol 2019;98:20)
- Unlike oropharyngeal SCC, the role of HPV in the carcinogenesis of laryngeal SCC has not been established (Head Neck 2011;33:581, Nat Rev Dis Primers 2020;6:92)
- Unlike lymphoepithelial carcinoma of the nasopharynx, lymphoepithelial carcinoma of the larynx does not appear to be associated with Epstein-Barr virus (EBV) (Mod Pathol 2019;32:621)
Clinical features
- Most common presentation is hoarseness with or without sore throat
- Larger lesions can present with dysphagia or airway obstruction
- Women are more likely to present with supraglottic tumors (Arch Otolaryngol Head Neck Surg 1991;117:774)
- Clinical staging includes endoscopic visualization of the larynx, palpation of cervical nodes, cranial nerve examination and endoscopic assessment of vocal cord mobility (Amin: AJCC Cancer Staging Manual, 8th Edition, 2017)
Diagnosis
- Clinical evaluation of vocal cord mobility / function is crucial for identifying a lesion, staging, prognosis and determining treatment modalities (Oral Oncol 2024;152:106744)
- Diagnosis requires histological assessment, with biopsy during direct laryngoscopy being the most common method to obtain tissue from the primary tumor
- Fine needle aspiration (FNA) cytology of suspected nodal disease is also commonly employed as part of diagnostic workup
Radiology description
- Computed tomography (CT): asymmetrical enhancing tissue mass that may be exophytic or infiltrative
- Magnetic resonance imaging (MRI) is limited by motion artifact and is typically used to assess for cartilage involvement (T4a) when CT is equivocal (Hodler: Diseases of the Brain, Head and Neck, Spine 2020-2023 - Diagnostic Imaging, 1st Edition, 2020)
Prognostic factors
- 5 year survival rate is (Laryngoscope Investig Otolaryngol 2020;5:74)
- 77.4% for localized disease
- 44.7% for patients with regional nodal involvement
- 33.3% for those with distant disease
- Prognosis is dependent on anatomical location and stage
- Glottic tumors tend to present at an earlier stage and have a better prognosis; this is primarily due to poor lymphatic supply and lower incidence of regional node involvement
- Supraglottic tumors are more likely to have nodal disease at presentation (Laryngoscope Investig Otolaryngol 2020;5:74)
- Subglottic tumors have a poor prognosis (Braz J Otorhinolaryngol 2022;88:S70)
- Prognosis depends upon histologic subtypes
- Verrucous carcinoma does not metastasize and has a better prognosis than conventional SCC, with an overall survival rate of 80.3% (Otolaryngol Head Neck Surg 2017;156:38)
- Papillary SCC has a better prognosis than conventional SCC, with a 5 year disease specific survival rate of 83.1% (Otolaryngol Head Neck Surg 2015;153:54)
- Spindle cell SCC has a comparable prognosis to conventional SCC and generally presents at an early stage (Am J Surg Pathol 2002;26:153)
- Overall disease specific 5 year survival rate is 74.1% (Laryngoscope 2015;125:2709)
- Basaloid SCC has traditionally been considered a more aggressive subtype, with a poorer prognosis compared to conventional SCC in the larynx (JAMA Otolaryngol Head Neck Surg 2013;139:1306)
- Adenosquamous carcinoma is considered more aggressive than conventional laryngeal SCC
- Prognosis also depends upon the presence or absence of adverse histopathologic factors
- Japanese study comparing early stage SCC subtypes found equivalent survival rates for conventional, verrucous, papillary and spindle cell, with adenosquamous having a worse prognosis (Acta Otolaryngol 2023;143:70)
- For early stage glottic cancers, positive margins do not affect local control and surgical margins of 1 - 2 mm are considered adequate (Eur Arch Otorhinolaryngol 2018;275:2333, Curr Oncol Rep 2020;22:82)
- Patients with advanced cancers with involved margins have a significantly worse prognosis than those with close or clear margins (Curr Oncol Rep 2020;22:82)
- Proportion of involved nodes is an important prognostic factor (J Otolaryngol Head Neck Surg 2020;49:31)
- HPV status has not been shown to affect prognosis (Cureus 2018;10:e2234)
- Occult metastasis occurs in ~20% of laryngeal SCCs and is more common in supraglottic cases, with a greater risk at advanced stage (Ann Otol Rhinol Laryngol 2021;130:67)
- Staging (see Staging-larynx)
- Specific tumor staging criteria (pT) for each site: supraglottis, glottis and subglottis
- pT stage relates to the extent of involvement of adjacent structures
- pN stage is dependent on the number of involved nodes (single versus multiple), their site (ipsilateral versus contralateral), the size of the deposit (cutoffs 3 cm and 6 cm) and the presence of extranodal extension
Case reports
- 55 year old man with 2 month history of hoarseness (lymphoepithelial carcinoma) (Front Surg 2022;9:851481)
- 65 year old man who presented with hoarseness and dyspnea (Int J Surg Case Rep 2023;111:108791)
- 72 year old man with 2 month history of progressive worsening dysphonia (Rare Tumors 2024;16:20363613241242705)
Treatment
- Treatment goals include local control of cancer and the preservation of laryngeal function
- Surgery, chemotherapy, radiotherapy or a combination of these
- Definitive radiotherapy with or without chemotherapy can be considered for low stage or low volume glottic tumors (Cancer Control 2016;23:208)
- Total laryngectomy is used for definitive treatment and salvage therapy
- Neck dissection is usually carried out with laryngectomy
Gross description
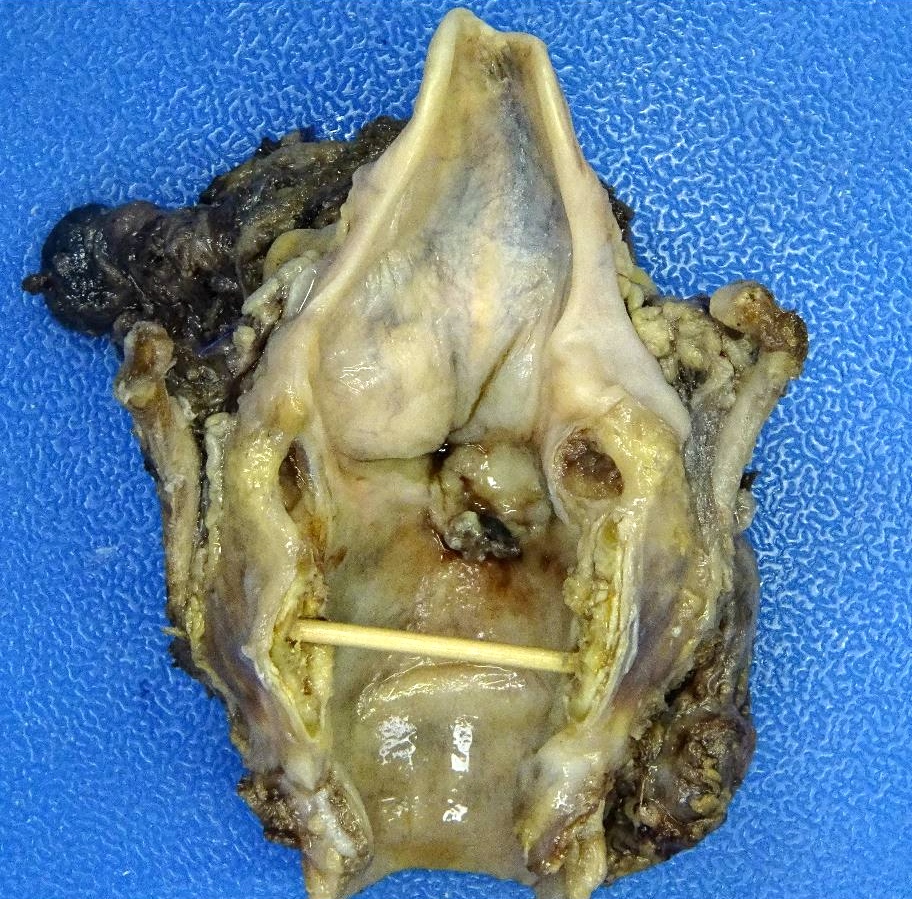
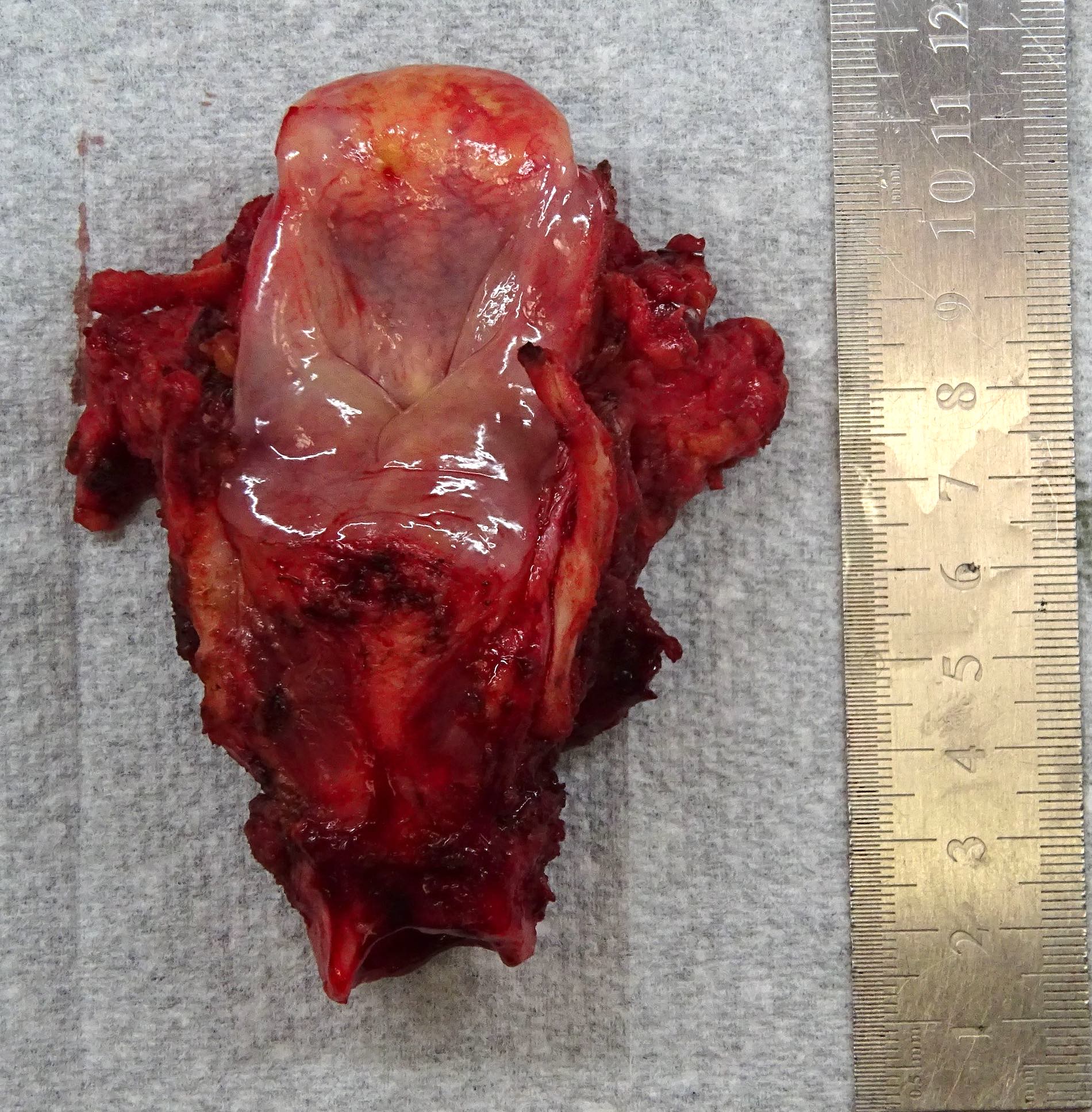
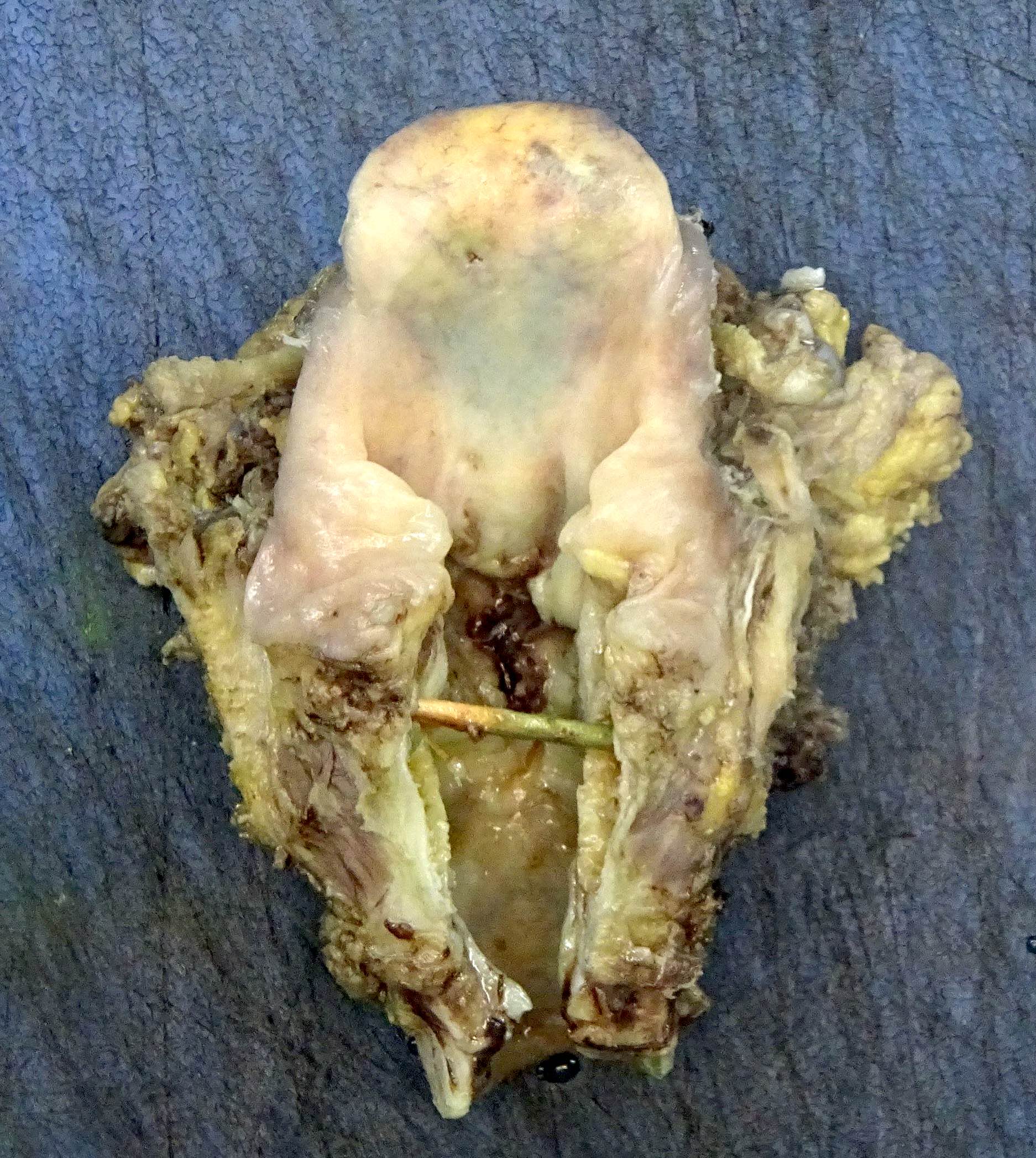
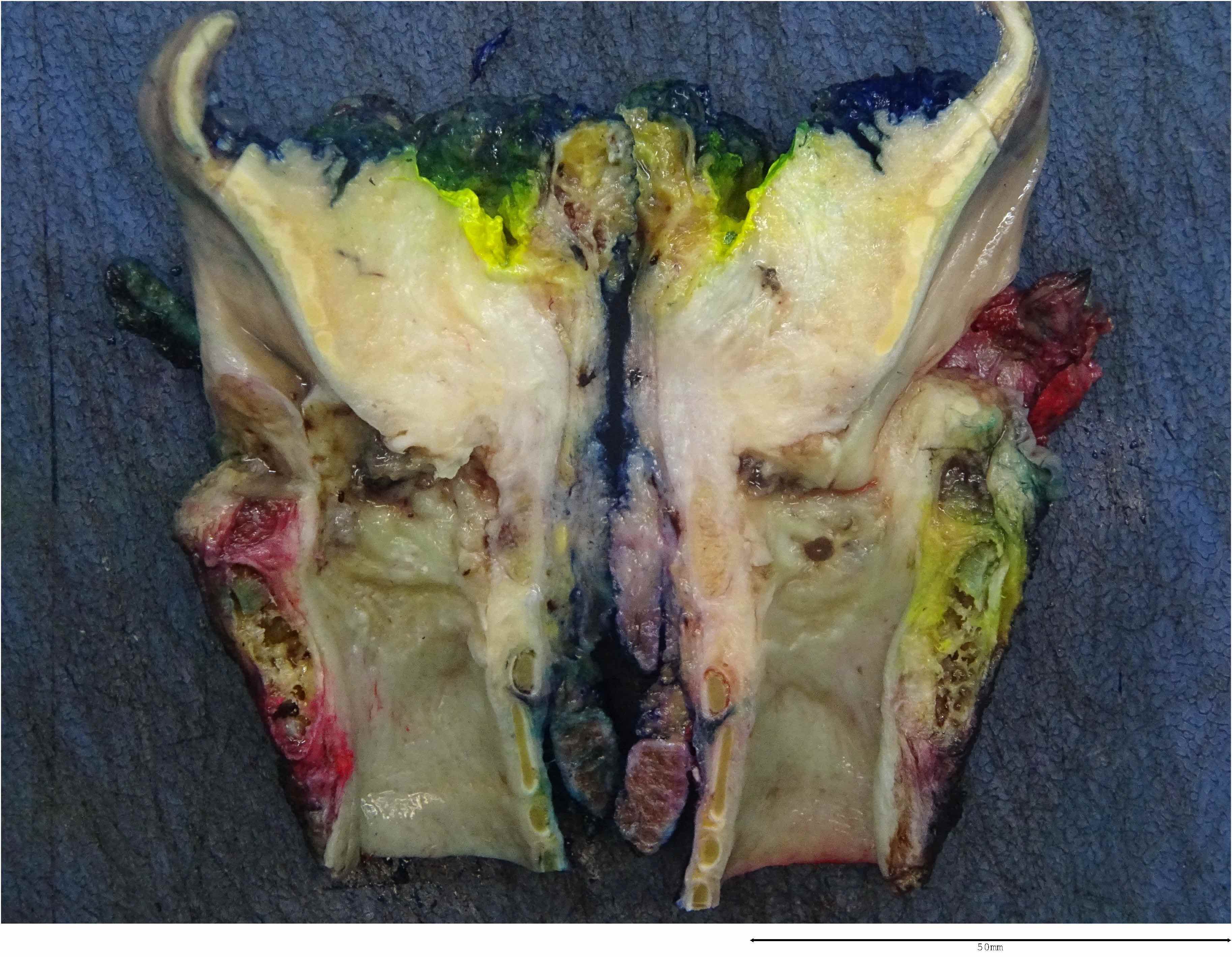
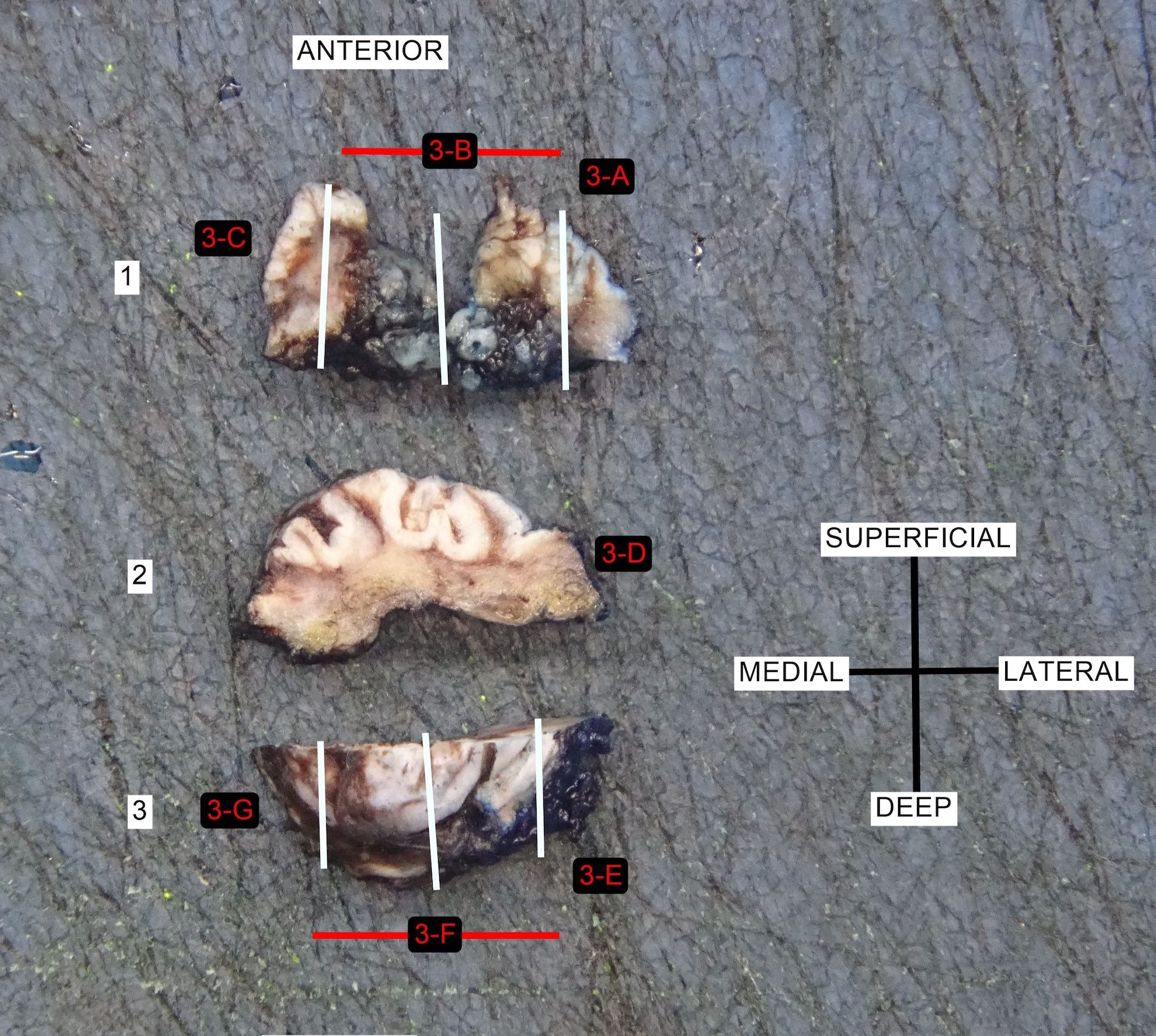
- Conventional SCC is firm, white and presents as exophytic masses or with endophytic growth patterns; ulceration is usually present
- Verrucous SCC is typically exophytic with a hyperkeratotic and friable, ragged surface (Head Neck Pathol 2011;5:23)
- Papillary SCC has a papillary appearance macroscopically and is usually friable and soft, with frond-like extension into the lumen (Head Neck Pathol 2011;5:23)
- Spindle cell SCC is usually a polypoid mass macroscopically
- Basaloid SCC, lymphoepithelial carcinoma and adenosquamous carcinoma have a similar appearance to conventional SCC and may have necrosis
- Higher stage tumors can invade adjacent sites, making classification of primary tumor site or subsite difficult
- References: Goldblum: Rosai and Ackerman's Surgical Pathology, 11th Edition, 2017, Longacre: Mills and Sternberg's Diagnostic Surgical Pathology, 7th Edition, 2022
Gross images
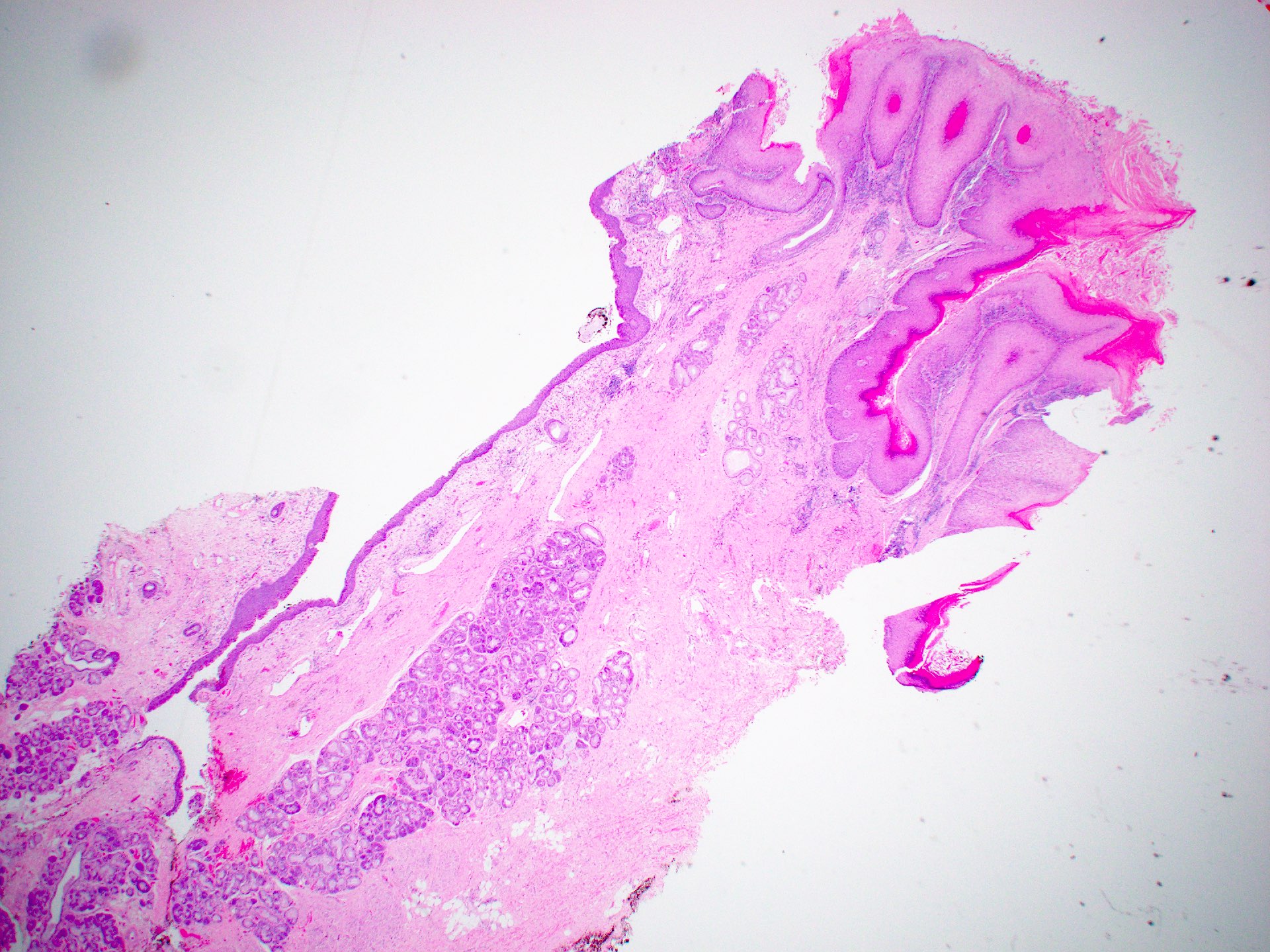
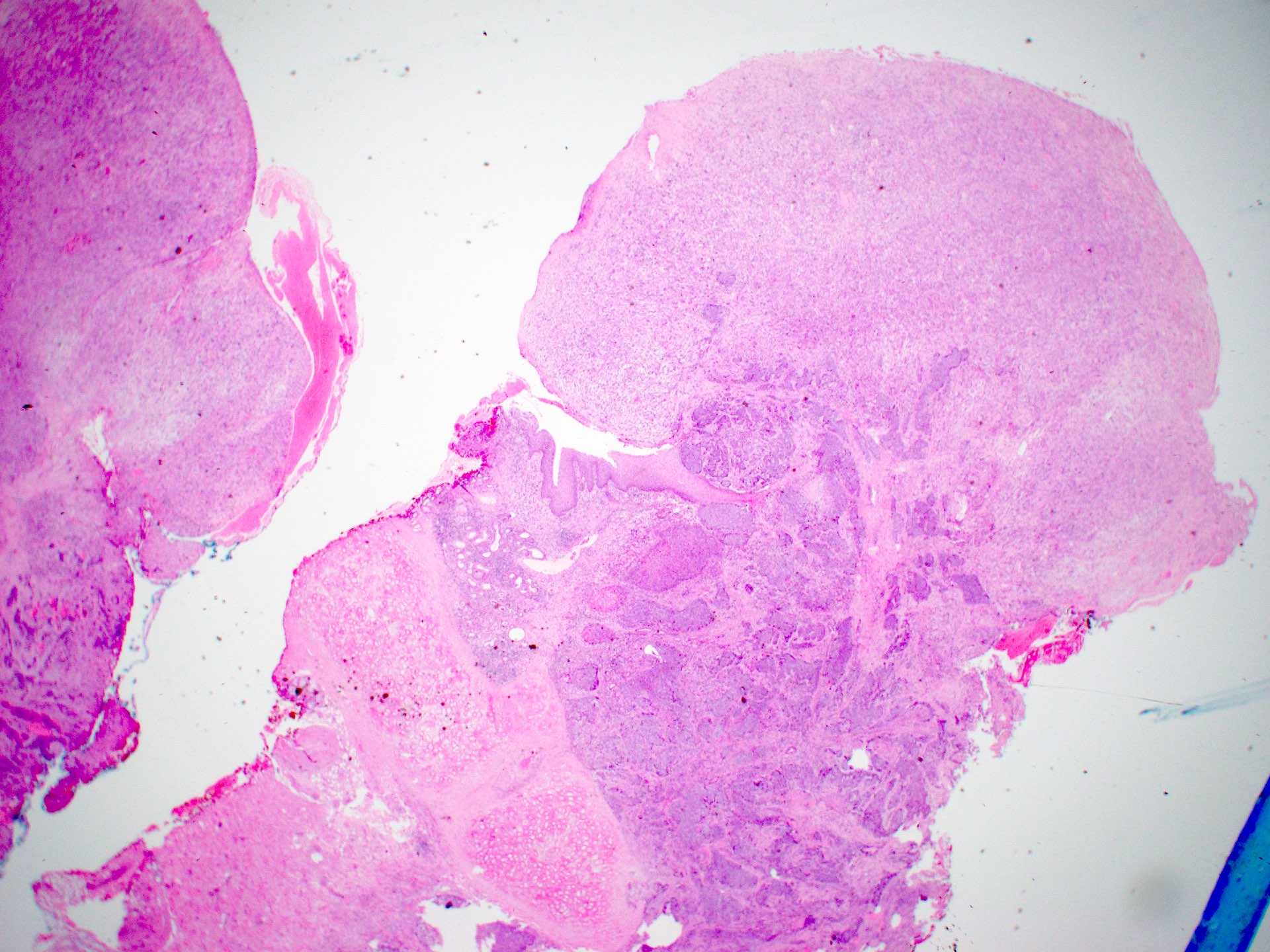
Microscopic (histologic) description
- In situ lesions (noninvasive) can be keratinizing or nonkeratinizing
Squamous cell carcinoma
- Conventional SCC
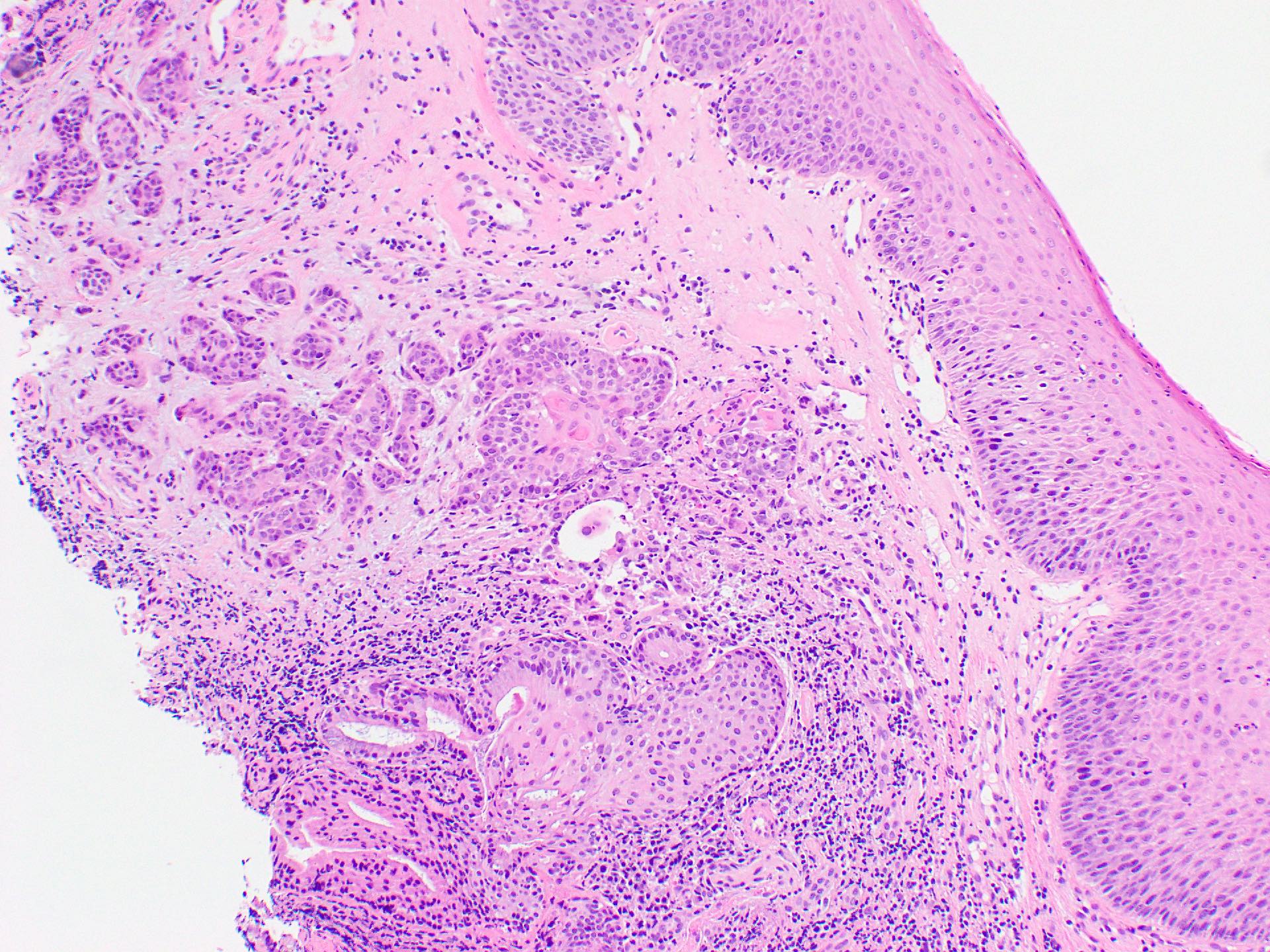
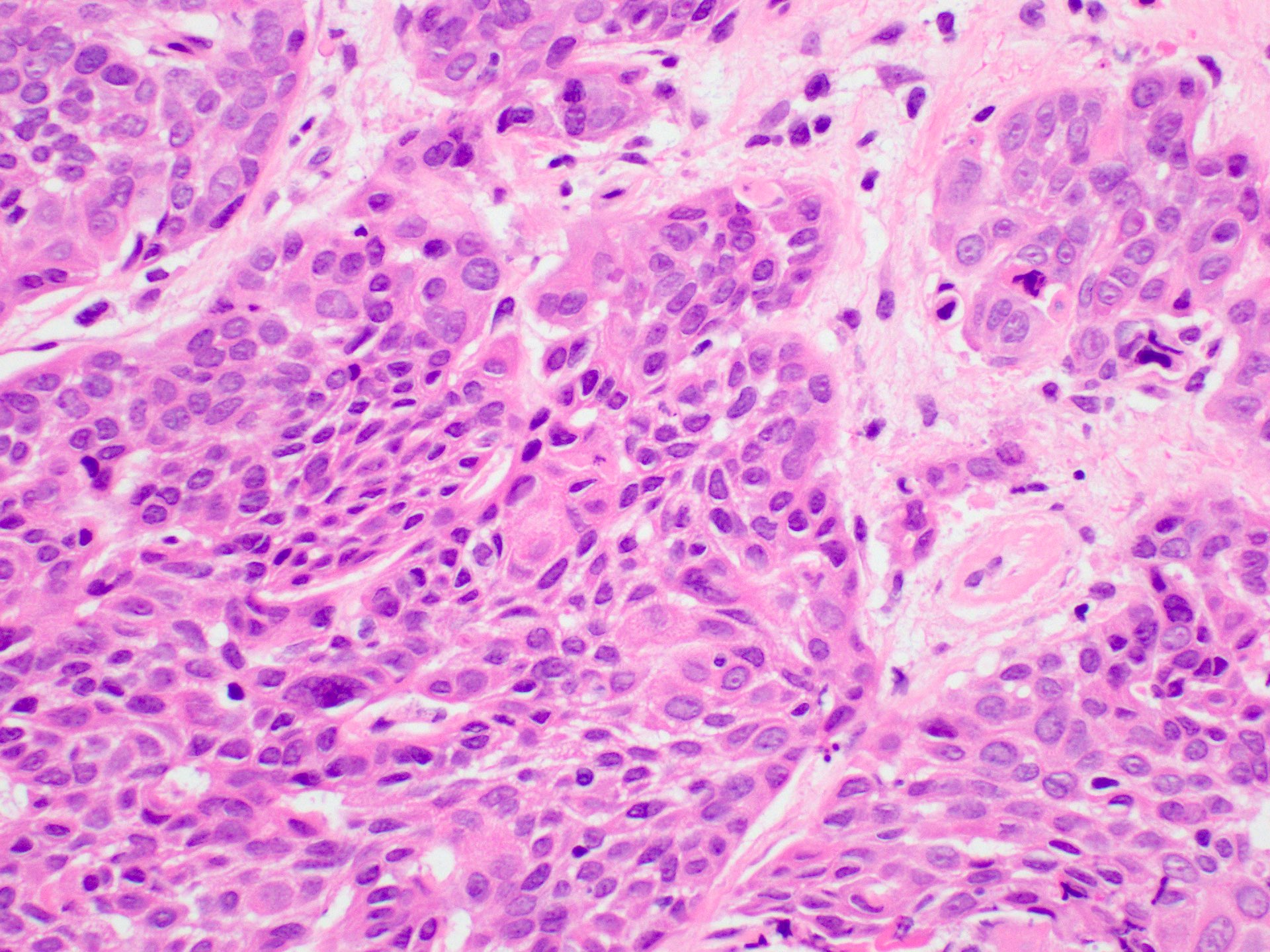
- Histologically, similar to conventional squamous cell carcinoma at other head and neck sites
- Typically consists of nests of squamous cells with or without keratin pearls and intercellular bridges
- Evidence of stromal invasion is required for diagnosis: desmoplastic stromal reaction is usually present
- Tumor grading is based on resemblance to normal squamous epithelium
- WHO diagnostic criteria
- Essential: a malignant epithelial neoplasm with evidence of squamous differentiation and stromal invasion
- Evidence for squamous differentiation: keratinization with keratin pearls or the presence of intercellular bridges; immunohistochemistry may be required for poorly differentiated tumors
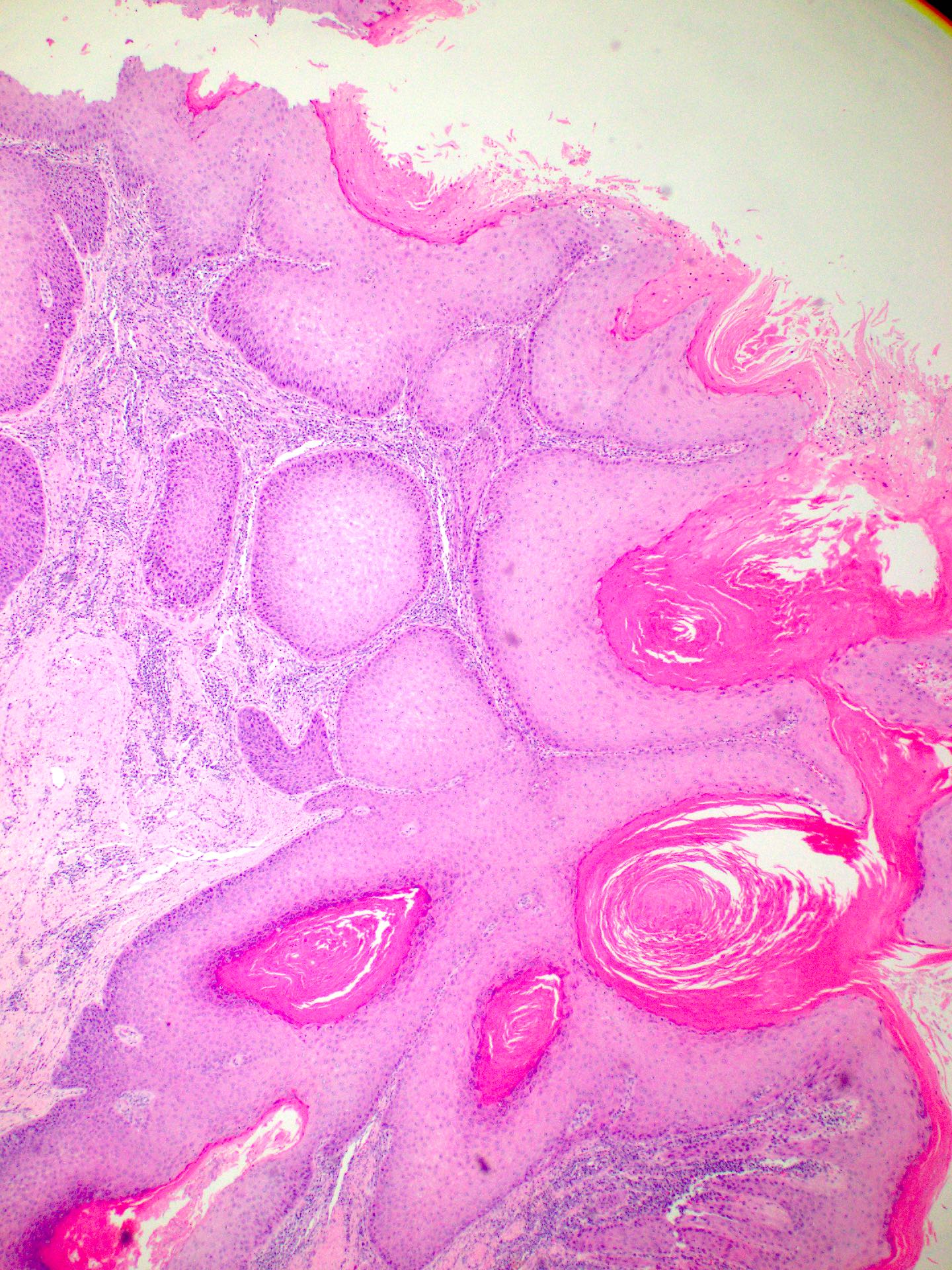
Verrucous (Head Neck Pathol 2011;5:23)
- Infoldings of well differentiated squamous epithelium with abundant surface keratinization
- Densely eosinophilic cytoplasm
- Low mitotic rate
- Pushing borders and generally absent desmoplastic stromal reaction
- Prominent lymphoplasmacytic infiltrate usually present
- Presence of any high grade dysplasia or a minor component of conventional SCC warrants a diagnosis of hybrid verrucous SCC or invasive well differentiated SCC with verrucous features (ICD-O: 8070/3)
- WHO diagnostic criteria
- Essential: acanthotic, hyperkeratotic and undulating squamous epithelium; bulbous keratotic crypts (elephant feet)
- Smooth interface with the stroma with pushing invasion below the level of the surrounding normal epithelium
- Cytologically bland squamous epithelium
- Mitoses limited to basal / parabasal layers
Papillary SCC
- Papillary architecture composed of branching fibrovascular cores lined by stratified squamous epithelium with or without keratinization
- Epithelium lining papillae show marked dysplasia, with little or no maturation and a high N:C ratio (Head Neck Pathol 2011;5:23)
- WHO diagnostic criteria
- Essential: exophytic growth composed predominantly of papillary fronds with fibrovascular cores covered by a nonkeratinizing malignant stratified squamous epithelium or in the keratinizing type, by high grade atypia
- Desirable: stromal infiltration
Basaloid
- Lesional cells resemble the basal layer of stratified squamous epithelium; tumor cells have basophilic chromatin and a high N:C ratio and are usually arranged in nests with peripheral palisading (Head Neck Pathol 2011;5:23)
- Solid and cribriform growth patterns are also common
- Tumors usually have a high mitotic rate and necrosis is frequently seen
- WHO diagnostic criteria
- Essential
- Tumor with prominent basaloid morphology
- Presence of squamous differentiation
- Absence of neuroendocrine or myoepithelial differentiation by histology or immunohistochemistry
- Desirable
- High grade histological features
- Myxoid to hyaline stromal alterations
- Essential
Adenosquamous
- Conventional SCC component and adenocarcinoma; may be juxtaposed or admixed
- Conventional SCC component may show keratinization
- Adenocarcinoma component composed of glands arranged in a tubular of cribriform configuration
- Necrosis is common
- Perineural and lymphovascular space invasion is frequently seen
- Special stains (e.g., PAS, Alcian blue) will highlight mucin in the adenocarcinoma component
- WHO diagnostic criteria
- Essential: biphasic tumor with squamous and glandular components that are distinctly recognized adjacent to each other on H&E examination
- Desirable
- Evidence of origin from surface epithelium (e.g., squamous dysplasia)
- Mucin production
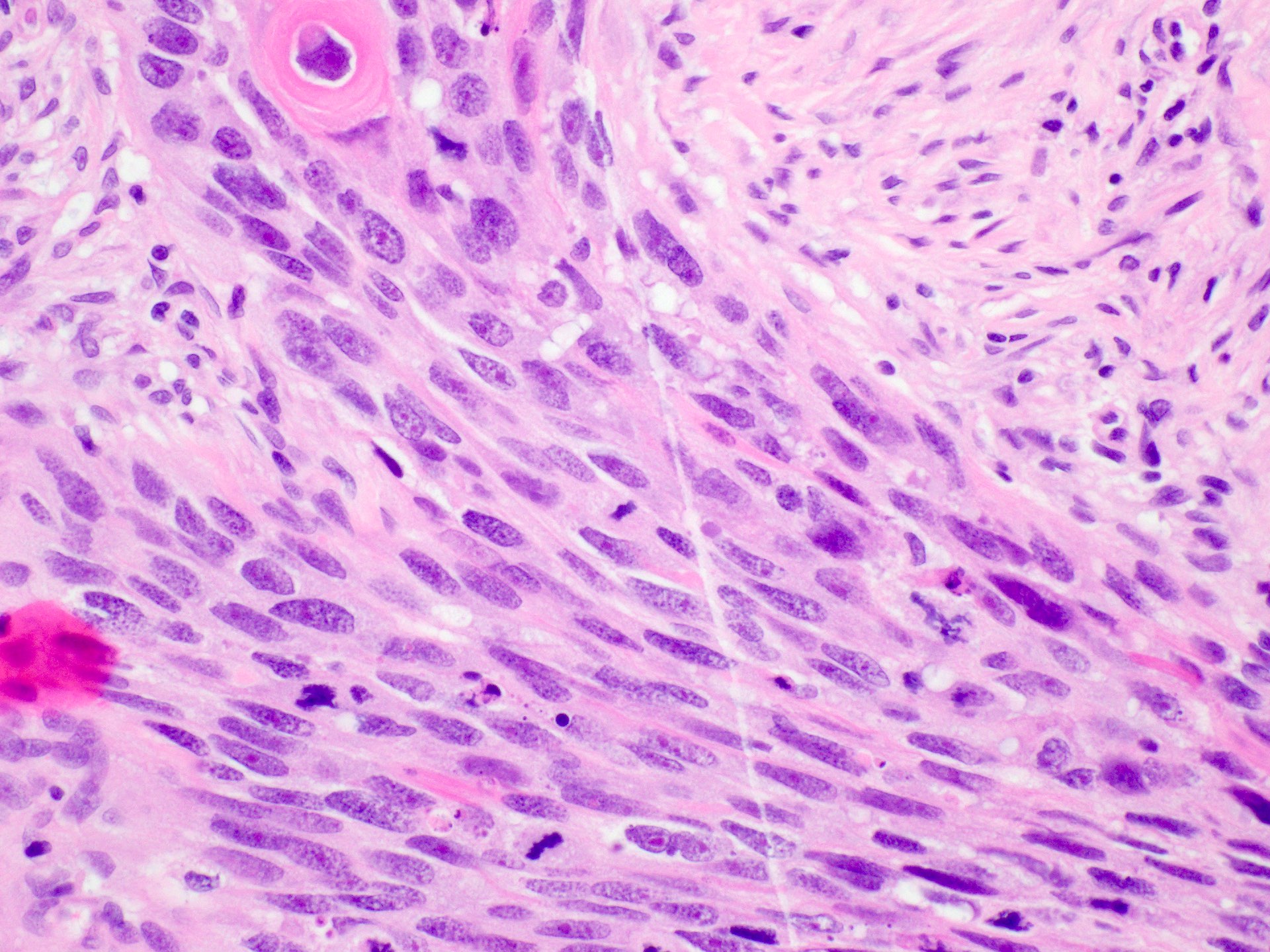
Spindle cell
- Spindle cells with overt features of malignancy such as pleomorphism, high mitotic rate, atypical mitoses
- May contain a conventional SCC component or intraepithelial dysplasia
- Heterologous differentiation may be present
- WHO diagnostic criteria
- Essential: a mucosa based carcinoma with a malignant spindle cell component
- Desirable
- Polypoid tumor
- Intraepithelial dysplasia
- Invasive conventional SCC
- Immunoreactivity for cytokeratin or p63 / p40 (in selected cases)
Lymphoepithelial
- Syncytial growth with a prominent lymphoplasmacytic infiltrate: may obscure tumor cells
- Solid or nested growth pattern
- Tumor cells usually have prominent nucleoli and a high mitotic rate
- Conventional SCC component may be present
- WHO diagnostic criteria
- Essential
- Tumor with syncytial growth
- Inflammatory infiltrate
- Vesicular chromatin
- Prominent nucleoli
- Desirable
- Positivity for pankeratin and squamous markers (e.g., p40, CK5/6)
- Exclusion of direct extension or metastasis
- Essential
Microscopic (histologic) images
Positive stains
- Cytokeratin
- p63 / p40
- Adenocarcinoma component of adenosquamous carcinoma is usually CK7 and CEA positive
- Majority (59%) of basaloid SCC: SOX10 (Head Neck Pathol 2019;13:543)
- p16 overexpression should not be interpreted as HPV infection (Br J Cancer 2015;112:1098)
Negative stains
- Synaptophysin, chromogranin, INSM1, SMA, S100, MelanA, HMB45, muscle specific actin and GFAP, CD45 (LCA), CD20, TTF1, napsin A
- Spindle cell SCC may be SMA (47%) and desmin (28%) positive (Virchows Arch 2021;479:729)
Sample pathology report
- Larynx, laryngectomy:
- Moderately differentiated invasive squamous cell carcinoma of the glottis, 22 mm, margins clear by at least 5 mm
- No lymphovascular or perineural invasion
- 0/7 lymph nodes involved (see synoptic report)
Differential diagnosis
Well to moderately differentiated conventional SCC
Poorly differentiated conventional SCC
Papillary
Verrucous SCC
Basaloid SCC
Adenosquamous
Spindle SCC
Lymphoepithelial carcinoma
- Pseudoepitheliomatous hyperplasia:
- Overlying necrotizing sialometaplasia, granular cell tumor or infection
- Generally seen in the setting of inflammation; granulomas or giant cells may be present
- Identification of underlying causes
- Verrucous SCC:
- Broad tongues of invasion below the adjacent normal epithelium
- Prominent keratinization
- Lacks significant atypia
- Papillary SCC:
- Exophytic growth of papillary fronds with fibrovascular cores
Poorly differentiated conventional SCC
- Malignant melanoma:
- Lymphomas, particularly diffuse large B cell lymphoma:
- CD45 positive
- Cytokeratin and p40 / p63 negative
- Neuroendocrine neoplasms:
- Chromogranin, synaptophysin and INSM1 positive
- p40 / p63 negative
Papillary
- Verrucous SCC:
- Lacks significant cytological atypia and prominent keratinization
- Laryngeal papilloma:
- Lacks cytological atypia; lack of stromal infiltration (not required for papillary SCC diagnosis)
Verrucous SCC
- Verrucous hyperplasia:
- Histologically identical but lacks invasion
- Well differentiated conventional SCC:
- Irregular, infiltrative growth and more cytological atypia
Basaloid SCC
- Adenoid cystic carcinoma:
- No significant pleomorphism, no mitotic activity and no squamous differentiation
- Small cell carcinoma:
- Positive neuroendocrine markers
Adenosquamous
- Conventional SCC or basaloid SCC with entrapped nonneoplastic glands
- Lobular architecture and bland morphology of entrapped of glands
- Mucoepidermoid carcinoma (MEC):
- Lack of in situ component
- Lacks true squamous differentiation (instead has intermediate cells)
- Lacks MAML2 translocation (FISH)
Spindle SCC
- Stromal reaction in conventional SCC, especially following radiotherapy:
- Other sarcomas (e.g., chondrosarcoma):
- Lack of cytokeratin / p40 / p63 expression
- Lack of in situ or conventional component
- Inflammatory myofibroblastic tumor:
Lymphoepithelial carcinoma
- Large cell lymphomas (e.g., diffuse large B cell lymphoma):
- Lack of cytokeratin reactivity
- Positive for lymphoid markers (e.g., CD45)
- Metastatic / locally advanced nasopharyngeal carcinoma:
- Clinical correlation
- EBV positive
Additional references
Board review style question #1
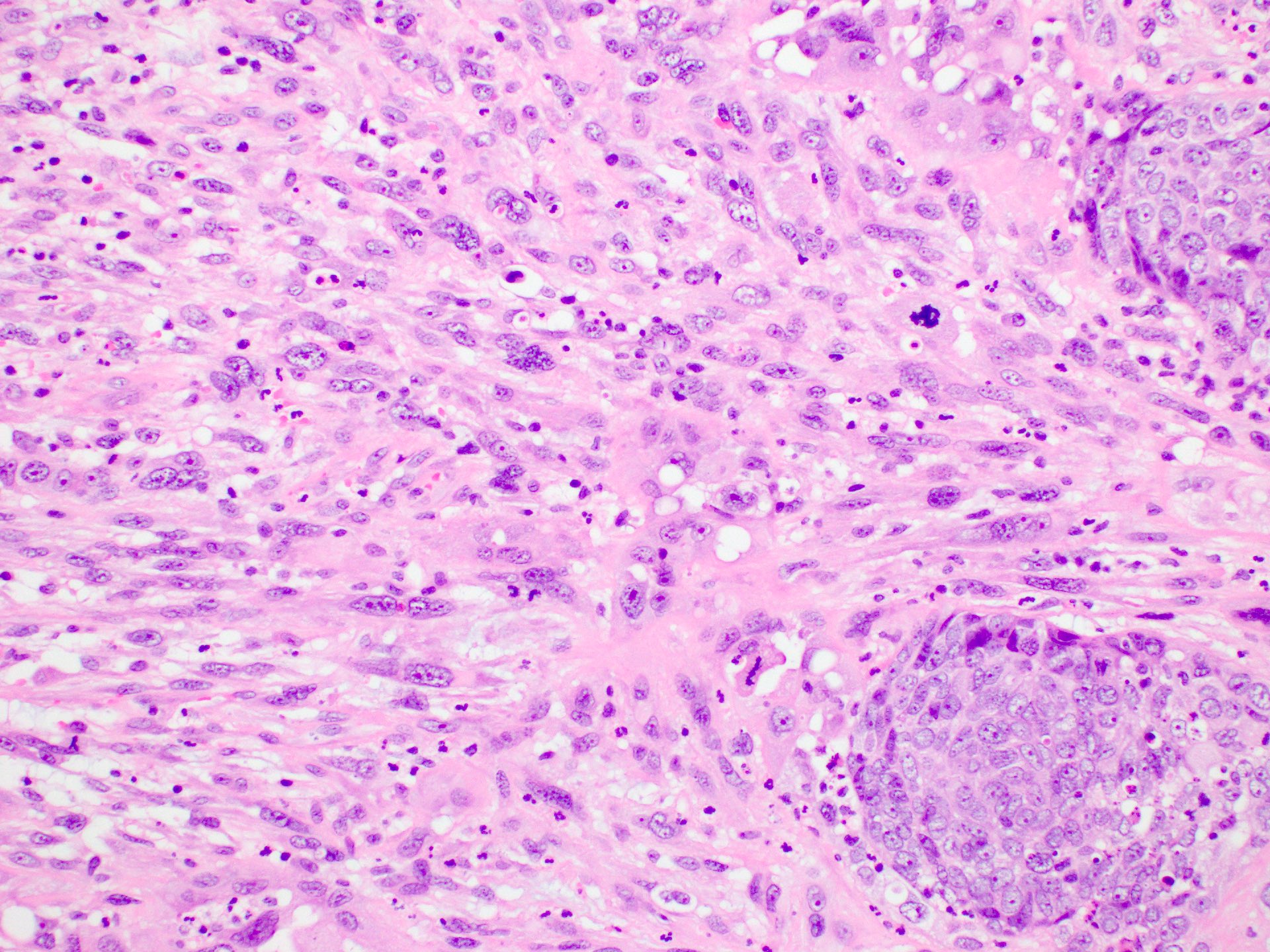
The image above shows an invasive lesion present on the larynx of a 52 year old man. Which of the following is true?
- The patient is likely to be a past or current smoker
- The patient is likely to have a family history of laryngeal cancer
- The patient is likely to have had a high risk HPV infection in the past
- The patient is likely to have had an EBV infection in the past
- The patient is likely to have poorly controlled diabetes
Board review style answer #1
A. The patient is likely to be a past or current smoker. Laryngeal squamous cell carcinoma (SCC) is strongly associated with smoking. Answer C is incorrect because unlike mucosal SCC at other head and neck sites, laryngeal SCC is not generally associated with HPV infection. Answer D is incorrect because laryngeal SCC is not associated with EBV infection. Answer E is incorrect because laryngeal SCC is not associated with diabetes. Answer B is incorrect because most laryngeal SCC cases are sporadic.
Comment Here
Reference: Squamous cell carcinoma of larynx
Comment Here
Reference: Squamous cell carcinoma of larynx
Board review style question #2
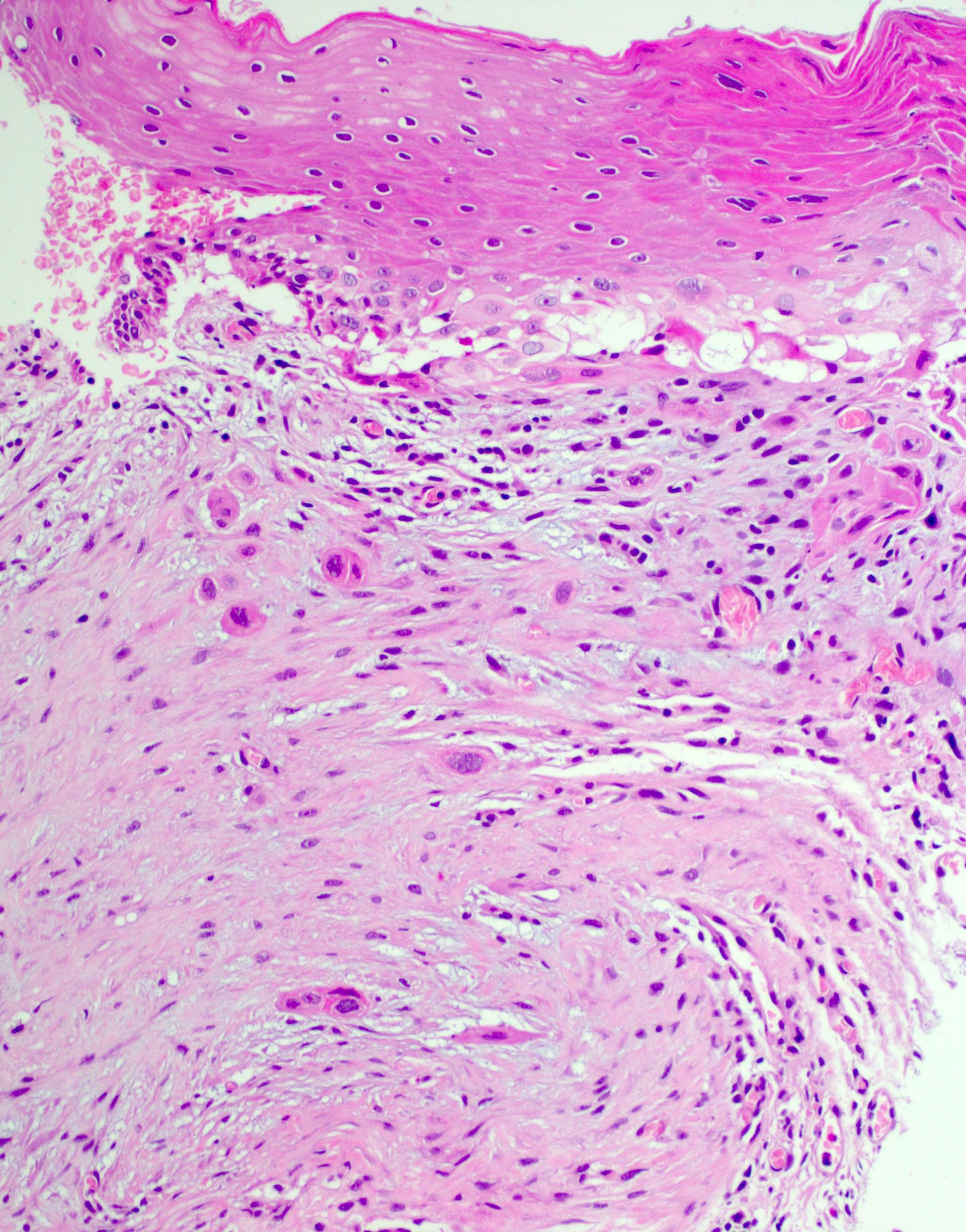
The image above shows an incisional biopsy of a suspicious lesion from a false vocal cord in a 62 year old man. The lesion is centered in the glottis and extends superiorly. Which of the following is true about regional node involvement in this case?
- Can be excluded due to the glottic location of the tumor
- Less likely as the micrograph shows HPV associated SCC
- Less likely due to the involvement of supraglottic structures
- More likely due to the involvement of supraglottic structures
- Unlikely as the micrograph shows the verrucous subtype
Board review style answer #2
D. More likely due to the involvement of supraglottic structures. The micrograph shows conventional invasive squamous cell carcinoma. We are told that the lesion is centered on the glottis, which has a poor lymphatic supply. Supraglottic structures, such as the false vocal cords, have better lymphatic supply and their involvement increases the risk of regional node involvement. Answer B is incorrect because HPV status has not been shown to affect the risk of regional node involvement in laryngeal squamous cell carcinoma. Answer C is incorrect because supraglottic structures have a better lymphatic supply than the glottis (on which the tumor is centered) and therefore increase the risk of regional node involvement. Answer E is incorrect because the micrograph shows conventional SCC with irregular infiltrative nests as opposed to the broad invasive front of verrucous SCC. Answer A is incorrect because regional lymph node involvement cannot be excluded on the basis of an incisional biopsy, regardless of the location.
Comment Here
Reference: Squamous cell carcinoma of larynx
Comment Here
Reference: Squamous cell carcinoma of larynx