Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Alsugair Z, Benzerdjeb N. Neuroendocrine neoplasm. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/larynxne.html. Accessed April 2nd, 2025.
Definition / general
- Neuroendocrine neoplasms are composed of neuroendocrine epithelial neoplasms that arise in the larynx and are either well differentiated or poorly differentiated (high grade)
Essential features
- Laryngeal neuroendocrine neoplasms are divided into 3 categories: well differentiated neuroendocrine neoplasms, poorly differentiated neuroendocrine carcinomas and mixed neuroendocrine - nonneuroendocrine neoplasm
- Well differentiated neuroendocrine neoplasm with immunohistochemical evidence of neuroendocrine differentiation and positivity for at least 1 keratin
- Ki67 proliferation index
- No abnormal p53 staining and no loss of Rb (in selected cases with Ki67 > 20%)
- Poorly differentiated neuroendocrine carcinomas
- Small cell neuroendocrine carcinoma
- High grade carcinoma
- Cell size smaller than diameter of 3 lymphocytes
- Prominent apoptotic bodies and necrosis
- Large cell neuroendocrine carcinoma
- High grade malignancy carcinoma
- Cell size larger than diameter of 3 lymphocytes
- Peripheral palisading, rosette formation or comedo pattern necrosis
- Small cell neuroendocrine carcinoma
- Well differentiated neuroendocrine neoplasm with immunohistochemical evidence of neuroendocrine differentiation and positivity for at least 1 keratin
Terminology
- Neuroendocrine neoplasms (NEN) are subclassified into 2 subtypes
- Well differentiated neuroendocrine neoplasms (NET)
- Well differentiated neuroendocrine tumor, grade 1
- Well differentiated neuroendocrine tumor, grade 2
- Well differentiated neuroendocrine tumor, grade 3
- Poorly differentiated neuroendocrine carcinomas (NEC)
- Small cell neuroendocrine carcinoma
- Large cell neuroendocrine carcinoma
- Well differentiated neuroendocrine neoplasms (NET)
- Mixed neuroendocrine - nonneuroendocrine neoplasm
ICD coding
Epidemiology
- Second most common group of neoplasms of the larynx after squamous cell carcinomas (Head Neck 2009;31:1634)
Sites
- Most frequent site: the supraglottic larynx (Head Neck Pathol 2022;16:375, Cancer 1988;62:2658)
- Most frequent type: grade 2 NET (Head Neck Pathol 2022;16:375, Cancer 1988;62:2658)
Pathophysiology
- Unknown
Etiology
- For NET: unclear
- Involvement of tobacco use (Adv Anat Pathol 2019;26:246)
- No well established association with human papillomavirus (HPV) (Eur Arch Otorhinolaryngol 2013;270:719)
- For NEC
- Tobacco use (Cancer Treat Rev 2019;78:42)
- No association with HPV (Am J Surg Pathol 2016;40:471)
Diagrams / tables
Table 1: WHO 2022 epithelial neuroendocrine neoplasms of the upper aerodigestive tract and salivary glands (Head Neck Pathol 2022;16:123)
| Neuroendocrine neoplasm | Tumor category | Diagnostic criteria |
| Well differentiated neuroendocrine neoplasm (neuroendocrine tumor, NET) | Well differentiated neuroendocrine tumor, grade 1 (NET, G1) | No necrosis and < 2 mitoses/2 mm²
Ki67 < 20% |
| Well differentiated neuroendocrine tumor, grade 2 (NET, G2) | Necrosis or 2 - 10 mitoses/2 mm²
Ki67 < 20% | |
| Well differentiated neuroendocrine tumor, grade 3 (NET, G3) | > 10 mitoses/2 mm²
Ki67 > 20% Absence of NEC cytomorphology | |
| Poorly differentiated neuroendocrine neoplasm (neuroendocrine carcinoma, NEC) | Small cell neuroendocrine carcinoma | > 10 mitoses/2 mm²
Ki67 > 20% (often > 70%) Small cell NEC cytomorphology |
| Large cell neuroendocrine carcinoma | > 10 mitoses/2 mm²
Ki67 > 20% (often > 50%) Large cell NEC cytomorphology |
Diagnosis
- Diagnosis is based on tissue examination findings of typical histologic and immunohistochemical features
Radiology description
- Structural imaging studies by CT and MRI: delineate extent and tumor location of NENs (Front Endocrinol (Lausanne) 2020;11:397)
Prognostic factors
- Adverse prognostic factors include
- Small cell neuroendocrine carcinoma (Cancer Treat Rev 2019;78:42)
- Stage IV disease (Head Neck 2015;37:707, Cancer Treat Rev 2019;78:42)
- Neck metastases (Head Neck 2015;37:707)
- 5 year survival rate
- Well differentiated neuroendocrine neoplasms: variable prognosis and based on the presence of distant metastasis and lymph node metastases (Head Neck 2015;37:707)
- Poorly differentiated neuroendocrine carcinomas: 5 - 20% (Ann Otol Rhinol Laryngol 2016;125:464, Laryngoscope 2017;127:1785)
- Regardless of site, lower stage tumors are associated with longer survival (Laryngoscope 2017;127:1785)
- Usually supraglottic
- Heavy smokers
- No association with HPV
Case reports
- 56 year old woman presented with painful subcutaneous skin lesions that were diagnosed as metastatic carcinoma at an outside facility; diagnosed as large cell neuroendocrine carcinoma (J Cutan Pathol 2018;45:229)
- 59 year old man presented to a community based hospital complaining of a left sided neck mass that has been present for ~1 month; diagnosed as small cell neuroendocrine carcinoma (Ear Nose Throat J 2022;101:NP96)
- 65 year old man, a smoker, and 63 year old man, a former smoker, presented with well differentiated neuroendocrine tumor (grade moderately differentiated neuroendocrine carcinoma) causing death 13 and 33 months after diagnosis (Arch Pathol Lab Med 1992;116:253)
Treatment
- G1 NET: local resection in the sense of either open or transoral endoscopic laser surgical partial laryngectomy is recommended (Laryngorhinootologie 2021;100:S1)
- G2 NET: radical surgical resection, elective neck dissection and radiotherapy (Laryngorhinootologie 2021;100:S1)
- G3 NET: not yet standardized because of lack of data
- NEC regardless of subtype: radiotherapy and chemotherapy (Head Neck 2015;37:707)
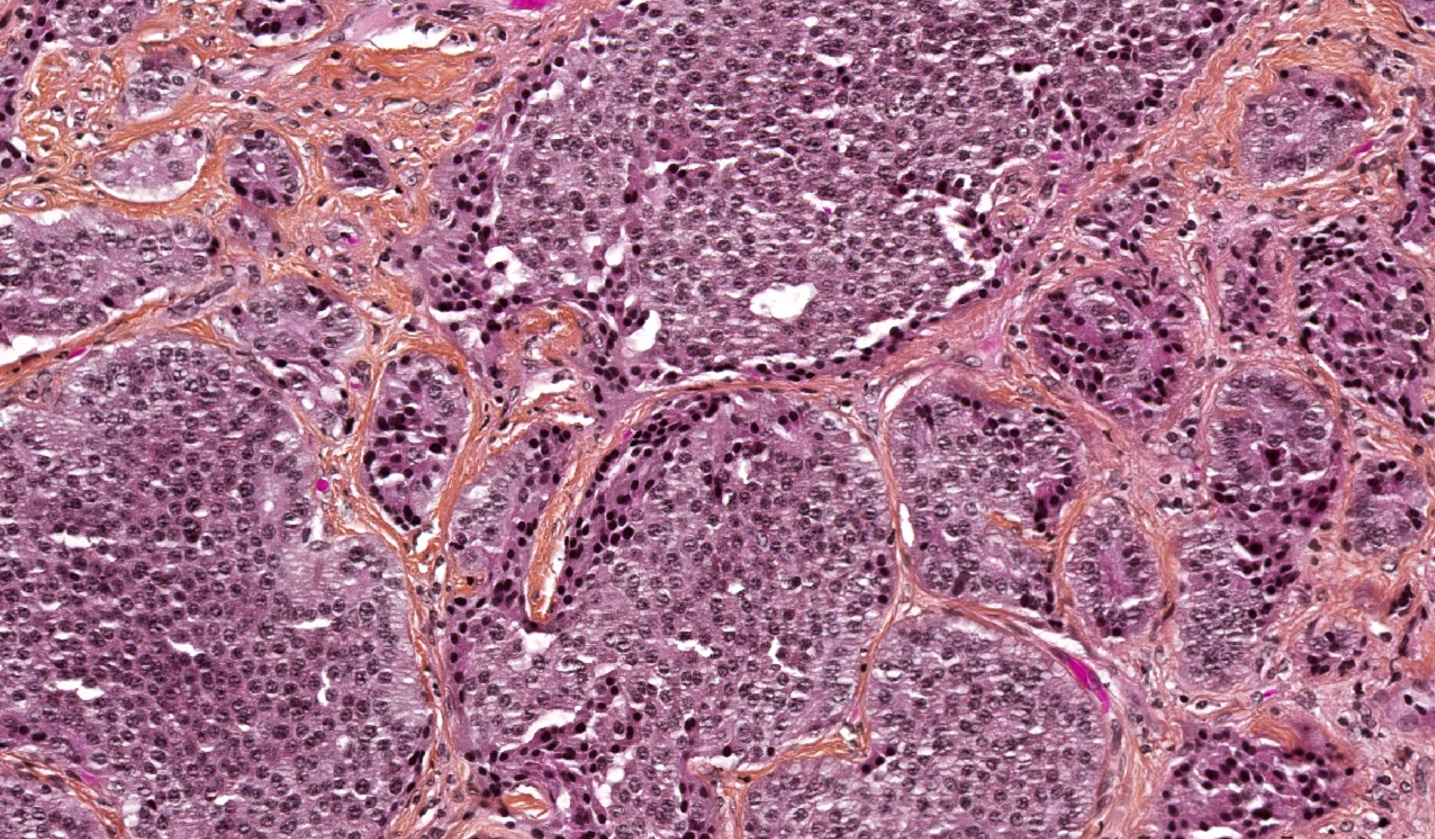
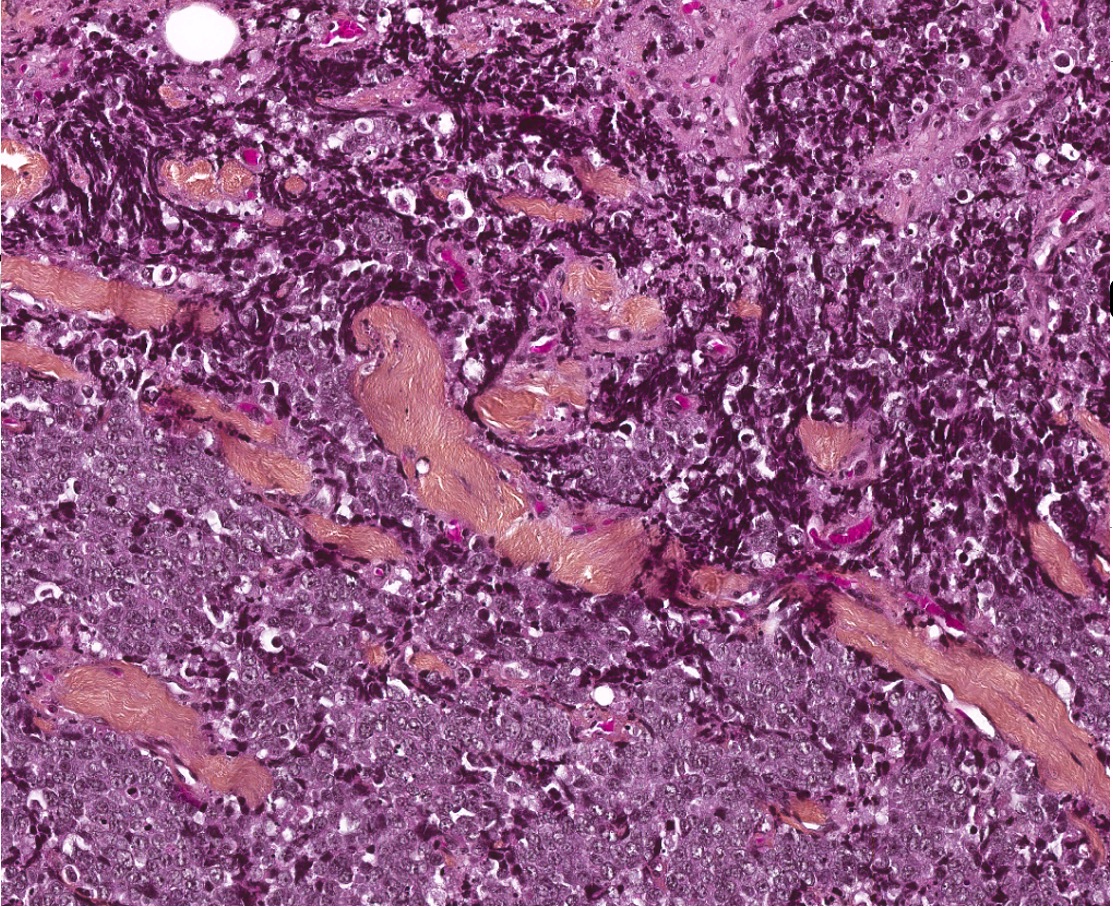
Microscopic (histologic) description
- Well differentiated neuroendocrine tumor (Head Neck Pathol 2022;16:123)
- Growth patterns: organoid (zellballen), trabecular, ribbon, solid, sheet-like (diffuse noncohesive) and individual cells
- Classical cytomorphology of tumor cells: uniform / monotonous round to oval nuclei, dispersed (salt and pepper) nuclear chromatin and ample granular pale eosinophilic cytoplasm often without distinct cell borders
- Uncommon cytomorphology of tumor cells: plasmacytoid, clear, oncocytic and rhabdoid (Head Neck Pathol 2022;16:375)
- Mild to focally moderate nuclear pleomorphism
- Necrosis, mitotic count and Ki67 proliferation index
- Grade 1: no necrosis and < 2 mitoses/2 mm² / Ki67 < 20%
- Grade 2: necrosis (often punctate [individual cell] and less commonly, coagulative [confluent foci]) or 2 - 10 mitoses/2 mm² / Ki67 < 20%
- Grade 3: > 10 mitoses/2 mm² / Ki67 > 20% without NEC cytomorphology
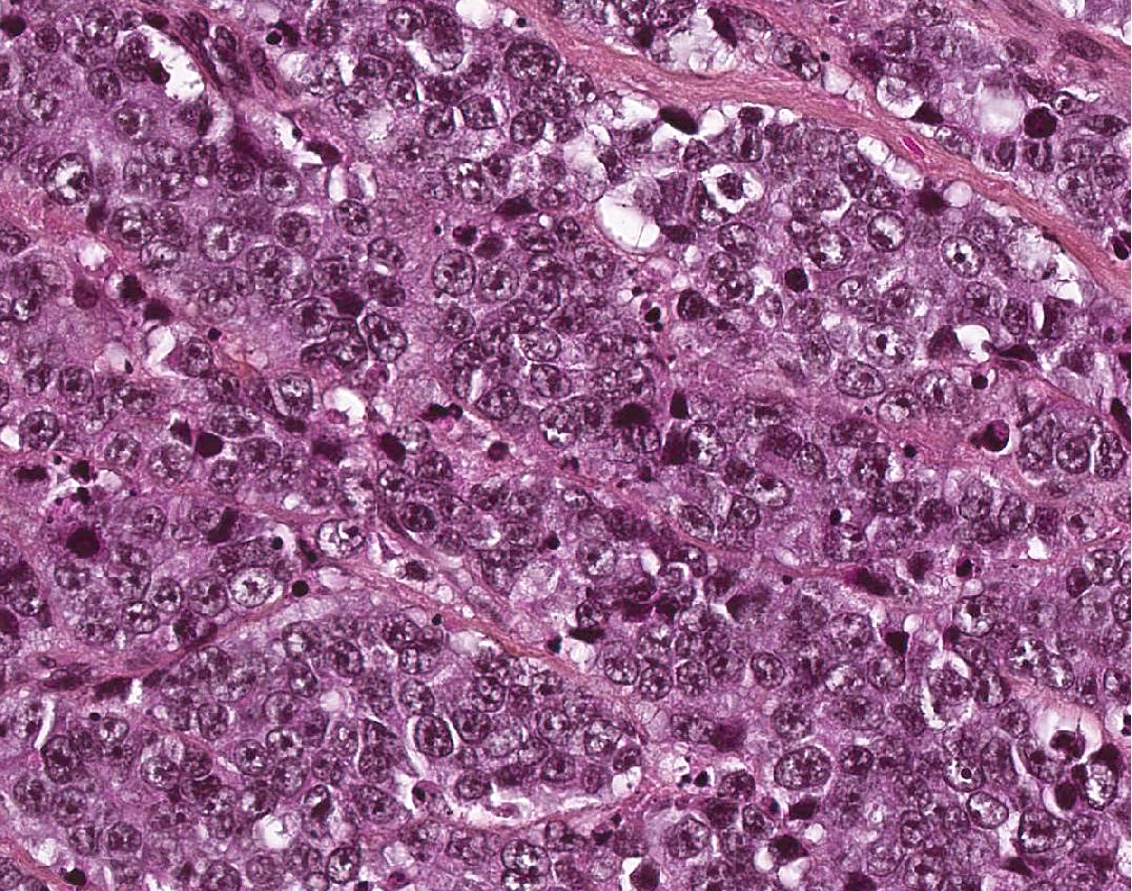
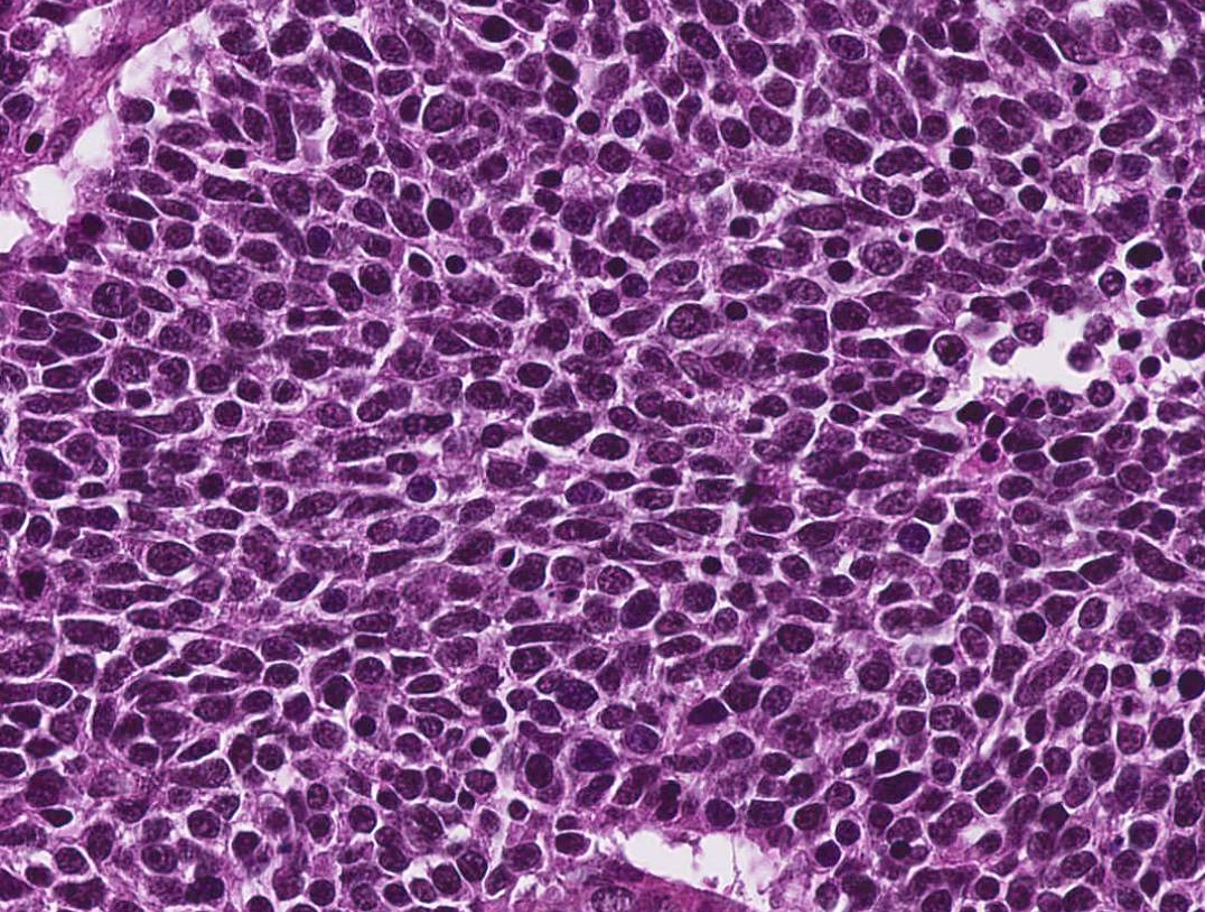
- Poorly differentiated neuroendocrine carcinomas (Head Neck Pathol 2022;16:123)
- Small cell neuroendocrine carcinoma
- Sheets, cords, nests, solid and diffuse (dyscohesive) but occasionally may be arranged in trabeculae
- May have peripheral nuclear palisading or rosettes
- Frequent necrosis in the form of apoptotic bodies or confluent (comedo type) foci
- Common: crush artifact with extravasated DNA coating blood vessels (Azzopardi phenomenon)
- Tumor cells are smaller than the diameter of 3 lymphocytes
- Hyperchromatic to finely granular or stippled appearing chromatin
- Inconspicuous nucleoli, scant cytoplasm and nuclear molding
- > 10 mitoses/2 mm²
- Ki67 > 20% (often > 70%)
- Large cell neuroendocrine carcinoma follows strict criteria
- Sheets, cords, nests, solid and diffuse (dyscohesive) but occasionally may be arranged in trabeculae
- May have peripheral nuclear palisading or rosettes
- Frequent necrosis in the form of apoptotic bodies or confluent (comedo type) foci
- Tumor cells are larger than the diameter of 3 lymphocytes with round to oval nuclei
- Vesicular chromatin
- Prominent nucleoli
- Abundant eosinophilic cytoplasm
- > 10 mitoses/2 mm²
- Ki67 > 20% (often > 50%)
- Small cell neuroendocrine carcinoma
- Mixed neuroendocrine - nonneuroendocrine neoplasm
- Rarely observed (J Laryngol Otol 2010;124:226)
- Typical cytomorphology of neuroendocrine neoplasm with the nonneuroendocrine components including squamous cell carcinoma or adenocarcinoma
Microscopic (histologic) images
Positive stains
- Not recommended staining for nonspecific biomarkers (CD56, CD57, PGP9.5 and neuron specific enolase)
- Recommended neuroendocrine staining
- > 80% staining of tumor cells of synaptophysin (the most sensitive), chromogranin A (the most specific) (Cancer Cytopathol 2016;124:871)
- Or diffuse INSM1 (useful in case of synaptophysin+ and chromogranin A-)
- Keratins including CK7 / 8 and CAM5.2
- Paranuclear dot-like cytokeratin reactivity
- Ki67 proliferation index > 20% and often > 70%
- p53 and pRb: useful for biopsy (no aberrant expression for NET versus aberrant expression of p53 and loss of pRb for NEC)
- p16: can be positive regardless of HPV status
- Calcitonin in G2 NETs (Head Neck Pathol 2022;16:375)
- Reference: Head Neck Pathol 2022;16:123
Negative stains
- TTF1 (focally positive in 13%)
- p63 / p40 (if present, tend to be very focal, limited to scattered nuclei), smooth muscle actin, CD117
- CEA
- Reference: Mod Pathol 2003;16:1041
Electron microscopy description
- Presence of membrane bound, electron dense neurosecretory granules (Ear Nose Throat J 2012;91:E20)
Sample pathology report
- Supraglottic larynx, biopsy:
- Well differentiated neuroendocrine tumor, WHO grade 2 (see comment)
- Comment: The presence of increased mitotic activity (4 mitoses/2 mm²) on this biopsy combined with the classic morphology of a well differentiated neuroendocrine tumor and positivity of immunohistochemical stains for cytokeratin AE1 / AE3, synaptophysin, chromogranin and 10% for Ki67 proliferative index support the diagnosis of well differentiated neuroendocrine tumor WHO grade 2 (out of 3).
Differential diagnosis
- Paraganglioma:
- Cytokeratin negative
- SOX10, S100 positive for sustentacular cells around tumor nests (Hum Pathol 2020;103:72)
- GATA3 positive (Hum Pathol 2020;103:72)
- Medullary thyroid carcinoma (MTC):
- Immunostaining in favor of MTC: strong positivity and diffuse for cytokeratin, calcitonin and TTF1 (Mod Pathol 2004;17:631)
- Association with elevated calcitonin levels (Ther Adv Endocrinol Metab 2022;13:20420188221099344)
- Adenoid cystic carcinoma:
- Positivity of myoepithelial cells markers
- Strongly positive for MYB / MYBL1 or fusion of MYB::MYBL1
- CD117 positivity is not specific and could be seen in neuroendocrine carcinoma and adenoid cystic carcinoma (Mod Pathol 2008;21:876, Mod Pathol 2003;16:1041)
- Basaloid squamous cell carcinoma:
- Has different morphology with nuclear pleomorphism, hyperchromatic and peripheral palisading, often with central comedo type necrosis, without expression neuroendocrine markers (focally may be seen)
- Diffusely positive for p63 and high molecular weight cytokeratins
Board review style question #1
Which of the following diagnoses regarding neuroendocrine neoplasms of the larynx with 15 mitoses/2 mm² and Ki67 30% without necrosis is correct?
- Poorly differentiated neuroendocrine carcinomas
- Well differentiated neuroendocrine tumor, grade 1
- Well differentiated neuroendocrine tumor, grade 2
- Well differentiated neuroendocrine tumor, grade 3
Board review style answer #1
D. Well differentiated neuroendocrine tumor, grade 3 because the diagnostic criteria applied with NET cytomorphology with > 10 mitoses/2 mm² and Ki67 > 20% and most importantly, without NEC cytomorphology.
Comment Here
Reference: Neuroendocrine neoplasm
Comment Here
Reference: Neuroendocrine neoplasm
Board review style question #2
Which of the following statements regarding differential diagnosis of neuroendocrine neoplasms of the larynx is true?
- Adenoid cystic carcinoma is diffusely positive for p16
- Basaloid squamous cell carcinoma has the identical morphology as the poorly differentiated neuroendocrine carcinoma
- Calcitonin and TTF1 may be expressed in neuroendocrine neoplasms
- Paraganglioma could express cytokeratin
- Treatment of neuroendocrine carcinomas is different based on the subtype
Board review style answer #2
C. Calcitonin and TTF1 may be expressed in neuroendocrine neoplasms.
Adenoid cystic carcinoma is diffusely positive for p16, as this biomarker can be seen in NEC or adenoid cystic carcinoma (A).
Basaloid squamous cell carcinoma is distinguished from poorly differentiated neuroendocrine carcinoma by its diffuse squamous marker expression (e.g., p40, CK5/6) and lack of neuroendocrine marker (synaptophysin, chromogranin, INSM1) and TTF1 staining (B).
Paraganglioma could not express cytokeratin (D).
Treatment of neuroendocrine carcinomas is not different based on the subtype of small and large cell neuroendocrine carcinoma (E).
Comment Here
Reference: Neuroendocrine neoplasm
Comment Here
Reference: Neuroendocrine neoplasm