Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Immunohistochemical panels | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Cite this page: Miller TI, Tretiakova M. Plasmacytoid. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/kidneytumormalignanturothelialcarcinomasubtypesplasma.html. Accessed March 30th, 2025.
Definition / general
- Aggressive variant of urothelial carcinoma (UC) characterized by single infiltrating cells with eccentrically placed nuclei and abundant eosinophilic cytoplasm similar to plasmacytes or resembling signet ring cells due to intracytoplasmic vacuoles
Essential features
- Uncommon histologic variant of UC (~1 - 3%) with plasmacytoid morphology
- Discohesive infiltrating tumor cells can spread extensively along tissue planes and peritoneal surfaces
- Plasmacytoid tumor cells are often positive for CD138, which can result in a misdiagnosis for a plasma cell neoplasm; must use other immunohistochemistry markers to differentiate
- Often has loss of membranous E-cadherin due to mutations in CDH1, similar to lobular breast carcinoma
- More likely to be diagnosed at an advanced stage compared with conventional UC resulting in a poorer prognosis
Terminology
- PUC (plasmacytoid urothelial carcinoma)
- Plasmacytoid variant (PCV)
- Signet ring cell variant
ICD coding
- ICD-O: 8120/3 - transitional cell carcinoma, NOS
Epidemiology
- Same as conventional urothelial carcinoma: typically older men (median of 58 - 68 years) with smoking history association (Arch Pathol Lab Med 2019;143:1562)
Sites
- Bladder
- Renal pelvis
- Ureter (primary PUC rare) (Am J Case Rep 2018;19:158)
Pathophysiology
- Carcinoma in situ plasmacytoid variant exists but not thought of as obligate precursor to invasive PUC (Am J Surg Pathol 2019;43:1638)
Etiology
- Similar to conventional UC, there is an association with smoking (Arch Pathol Lab Med 2019;143:1562)
Clinical features
- 1 - 3% of all UC variants (Eur Urol Focus 2020;6:653)
- 1.9% incidence of 633 UC diagnoses (BMC Urol 2020;20:72)
- Predilection for peritoneal carcinomatosis and spread along fascial planes (J Urol 2020;204:215)
- 33% of PUC cases in 1 series had peritoneal spread at presentation (J Urol 2012;187:852)
- Lack of desmoplastic reaction makes it challenging to assess tissue involvement (Eur Urol Focus 2020;6:653)
- Cystoscopy may only show mucosal induration / thickening with no well defined mass (J Urol 2008;180:1923)
- May be linitis plastica-like
- More likely to be diagnosed at an advanced stage compared with conventional UC (Arch Pathol Lab Med 2019;143:1562)
- May present with gross or microscopic hematuria, dysuria, frequency, urgency, abdominal pain or hydronephrosis (Arch Pathol Lab Med 2019;143:1562, Am J Surg Pathol 2008;32:752, Am J Clin Pathol 2017;147:500, Int J Clin Exp Pathol 2012;5:601)
Diagnosis
- Clinical symptoms prompt cystoscopy, which then results in a transurethral biopsy or resection of tumor
- Imaging may show focal mass in bladder wall or diffuse bladder wall thickening, which results in cystoscopy with biopsy
Laboratory
- Hematuria
- Elevated serum CA19-9 reported in a metastatic case (Am J Case Rep 2020;21:e923130)
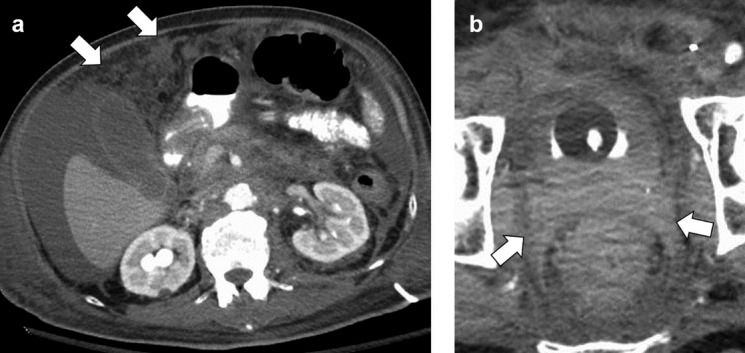
Radiology description
- On CT / MRI, PUC case series demonstrated focal bladder mass in 68% of cases and diffuse mural thickening in 32% of cases (Can Urol Assoc J 2017;11:E50)
- On CT / MRI, imaging specific to PUC compared with conventional UC may be discontinuous thick sheets of tissue extending along fascial pelvic planes (Can Urol Assoc J 2017;11:E50)
Radiology images
Prognostic factors
- Overall uniformly poor prognosis (Eur Urol Focus 2020;6:653, J Urol 2020;204:1129)
- Compared with conventional UC:
- More likely to have pT3 stage or greater and lymph node metastasis (J Urol 2020;204:215, Urol Oncol 2014;32:833, Bladder Cancer 2020;6:71, Eur Urol Focus 2019;5:104, Urol J 2020;17:607)
- More likely to have positive surgical margins after cystectomy (40.5%) (Hum Pathol 2019;90:27, Eur Urol Focus 2019;5:104)
- Worse survival outcomes but no significant difference in cancer specific mortality, although approached close to significant in 1 literature review (J Urol 2020;204:215)
- Largest series (98 cases), median overall survival 3.8 years compared with 8 years in matched conventional UC controls (Eur Urol Focus 2019;5:104)
- Series of 46 cases: 36% 5 year survival compared with 57% in conventional UC (Urology 2017;102:143)
- Reduced survival is likely due to advanced stage at presentation and not because histologic variant is independently a worse prognostic factor (Eur Urol Focus 2019;5:104, Urology 2017;102:143, Bladder Cancer 2020;6:71)
- Desmoplastic PUC subvariant slightly worse overall survival compared with classic and pleomorphic subvariants (Hum Pathol 2019;90:27)
Case reports
- 33 year old woman presenting with macroscopic hematuria and a solid lesion (Urol J 2019;16:86)
- 53 year old man with plasmacytoid urothelial carcinoma metastatic to the duodenum (Case Rep Pathol 2017;2017:5209059)
- 57 year old man presenting with renal colic and a solid lesion (Urol J 2019;16:86)
- 60 year old man with a small bowel obstruction and an obstructive ureteric mass on CT scan (Am J Case Rep 2018;19:158)
- 69 year old woman with a history of bilateral hydronephrosis, presenting with weight loss and vaginal spotting (Case of the Month #510)
- 71 year old man with incidental plasmacytoid bladder cancer causing bilateral ureteral obstruction, hydroureteronephrosis and renal failure (Urol Case Rep 2020;33:101415)
Treatment
- More likely to receive neoadjuvant chemotherapy compared with conventional UC because it is discovered at a higher stage (J Urol 2020;204:215)
- Literature review found overall pathological complete response to neoadjuvant chemotherapy to be 21% (J Urol 2020;204:215)
- Same treatment for urothelial carcinoma of the same stage (Eur Urol Focus 2020;6:653)
- Wider resection is important because margins are more often positive (Eur Urol Focus 2020;6:653)
- Role of neoadjuvant therapy before cystectomy has been questioned for PUC
- In a study of 64 cases, neoadjuvant chemotherapy for PUC variant was not associated with improved overall survival compared with radical cystectomy alone (Bladder Cancer 2020;6:71)
- In study with 2 cases of plasmacytoid variant, no response to neoadjuvant chemotherapy (Clin Genitourin Cancer 2021 Jul 20 [Epub ahead of print])
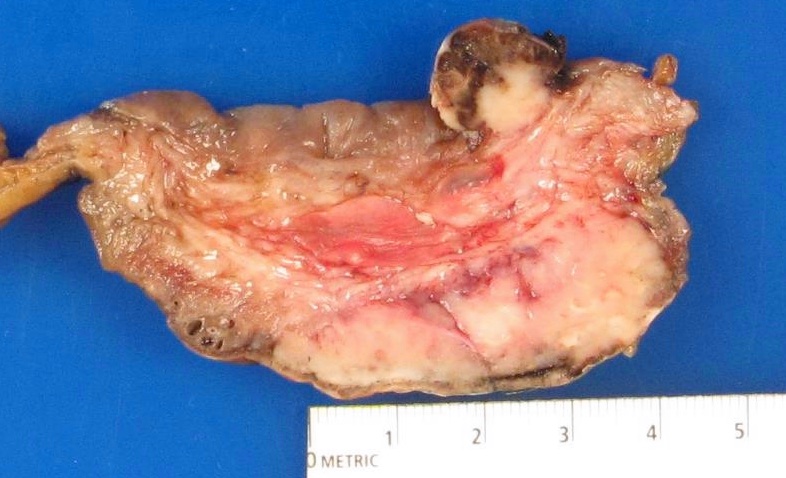
Gross description
- On cystectomy, primary single mass in bladder wall or multiple masses (Am J Surg Pathol 2008;32:752)
- May have linitis plastica appearance with diffusely thickened bladder wall (Arch Pathol Lab Med 2019;143:1562)
- On transurethral resection of bladder tumor: fragmented, irregular firm tissue (Am J Surg Pathol 2008;32:752)
Gross images
Frozen section description
- Special attention warranted during frozen section evaluation due to the frequent positive surgical margins in grossly negative areas along the ureters and paravesical soft tissue (Hum Pathol 2019;90:27, Urology 2014;83:1112, Histopathology 2019;74:77)
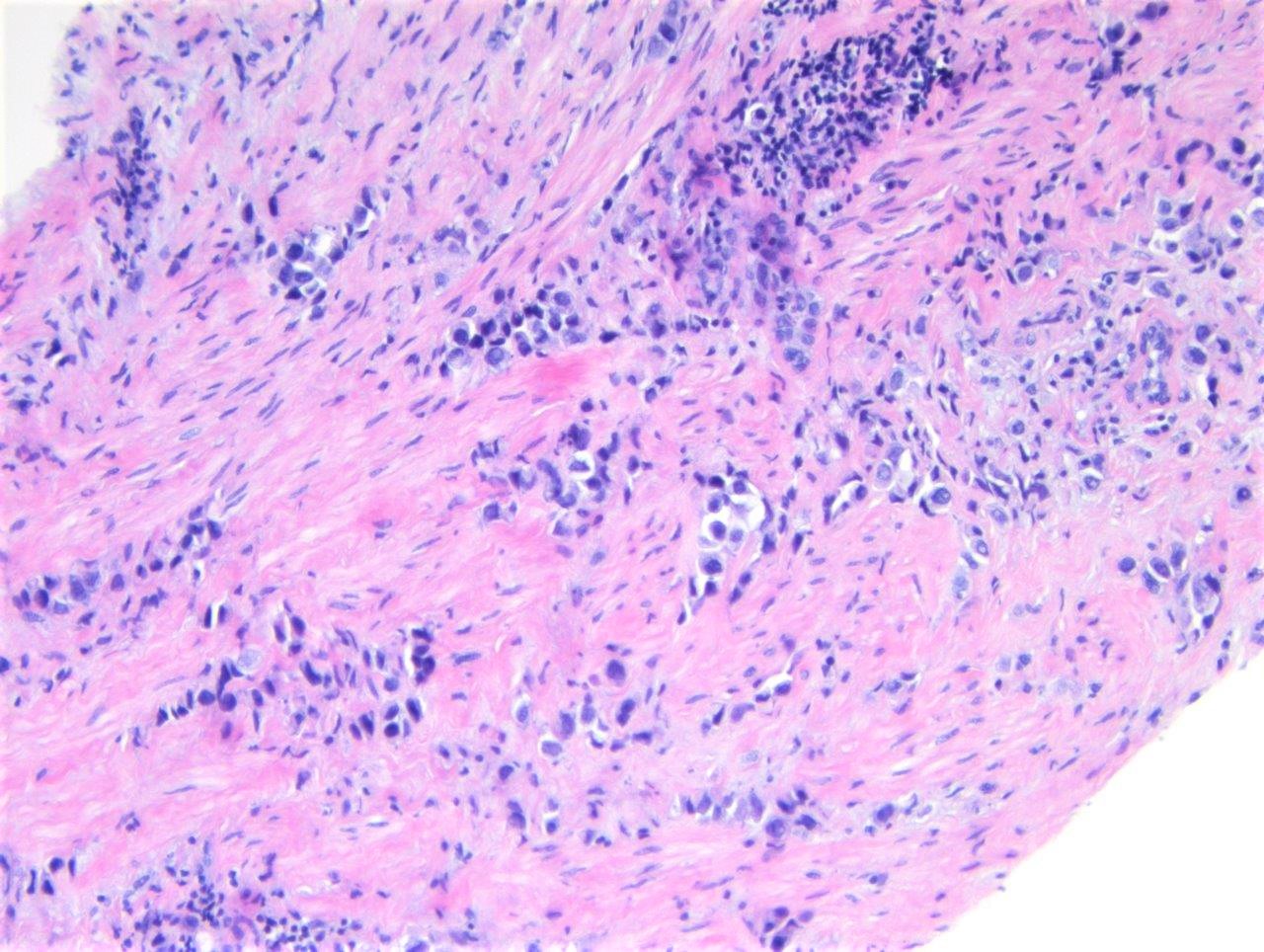
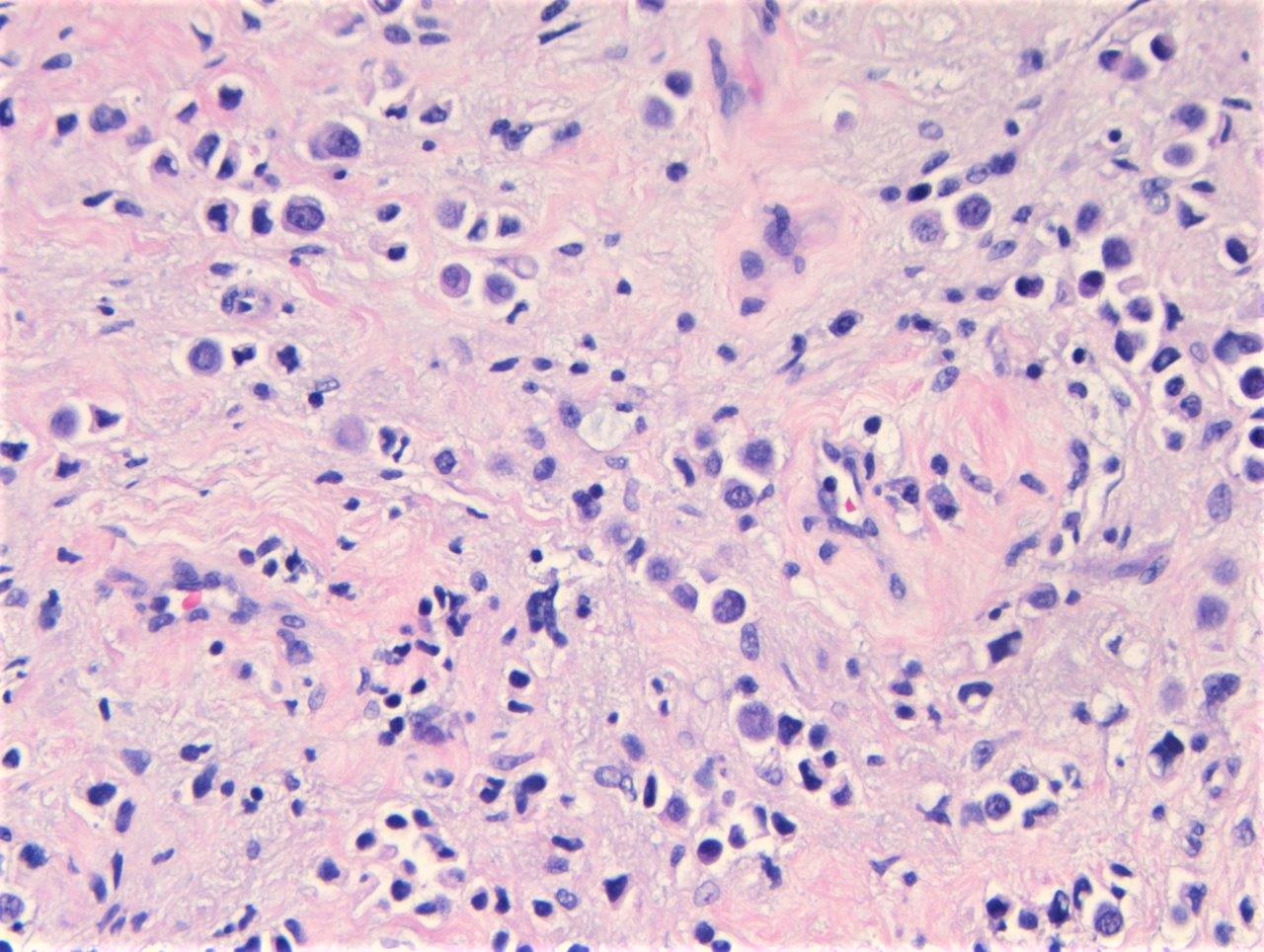
Microscopic (histologic) description
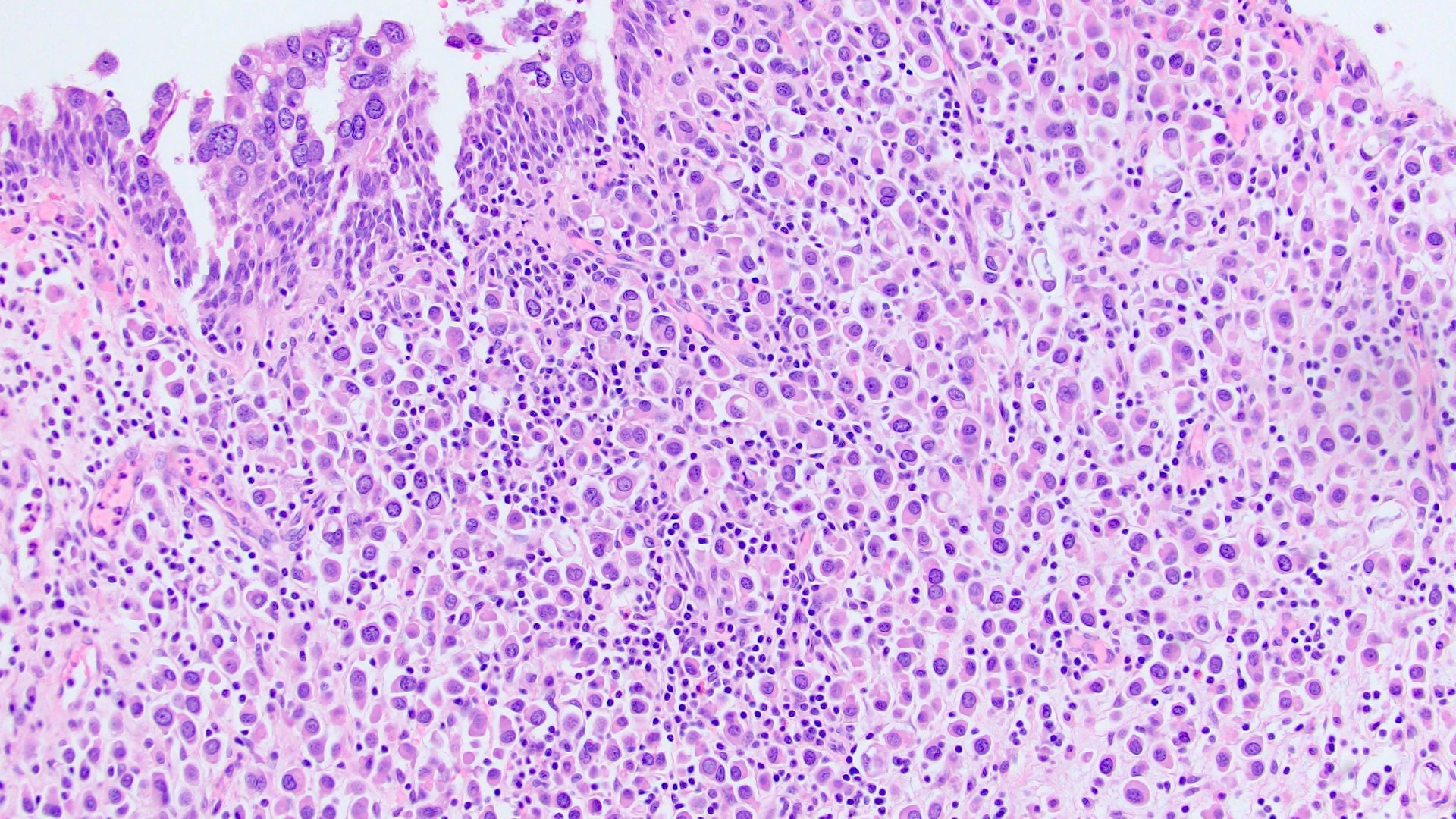
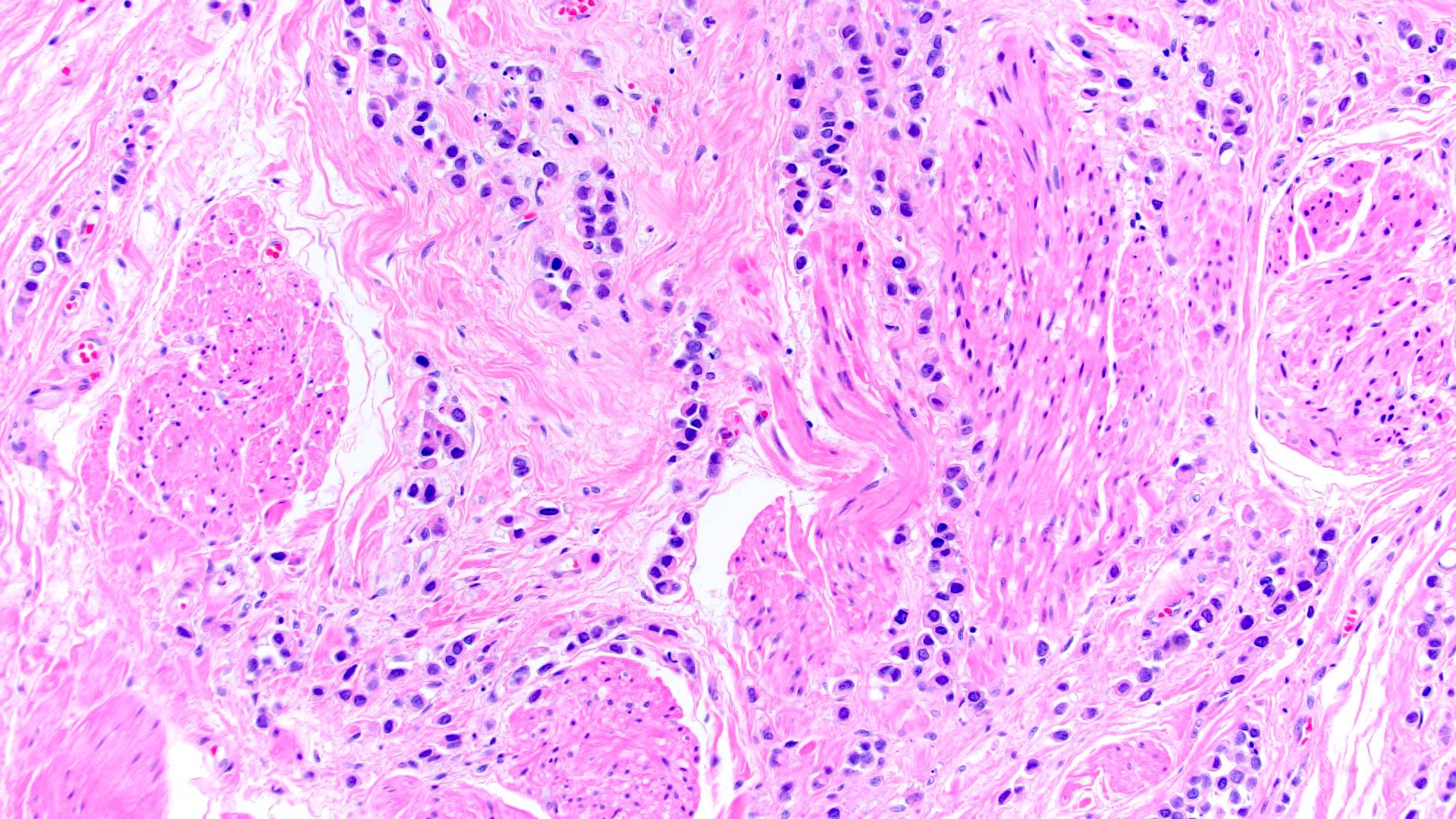
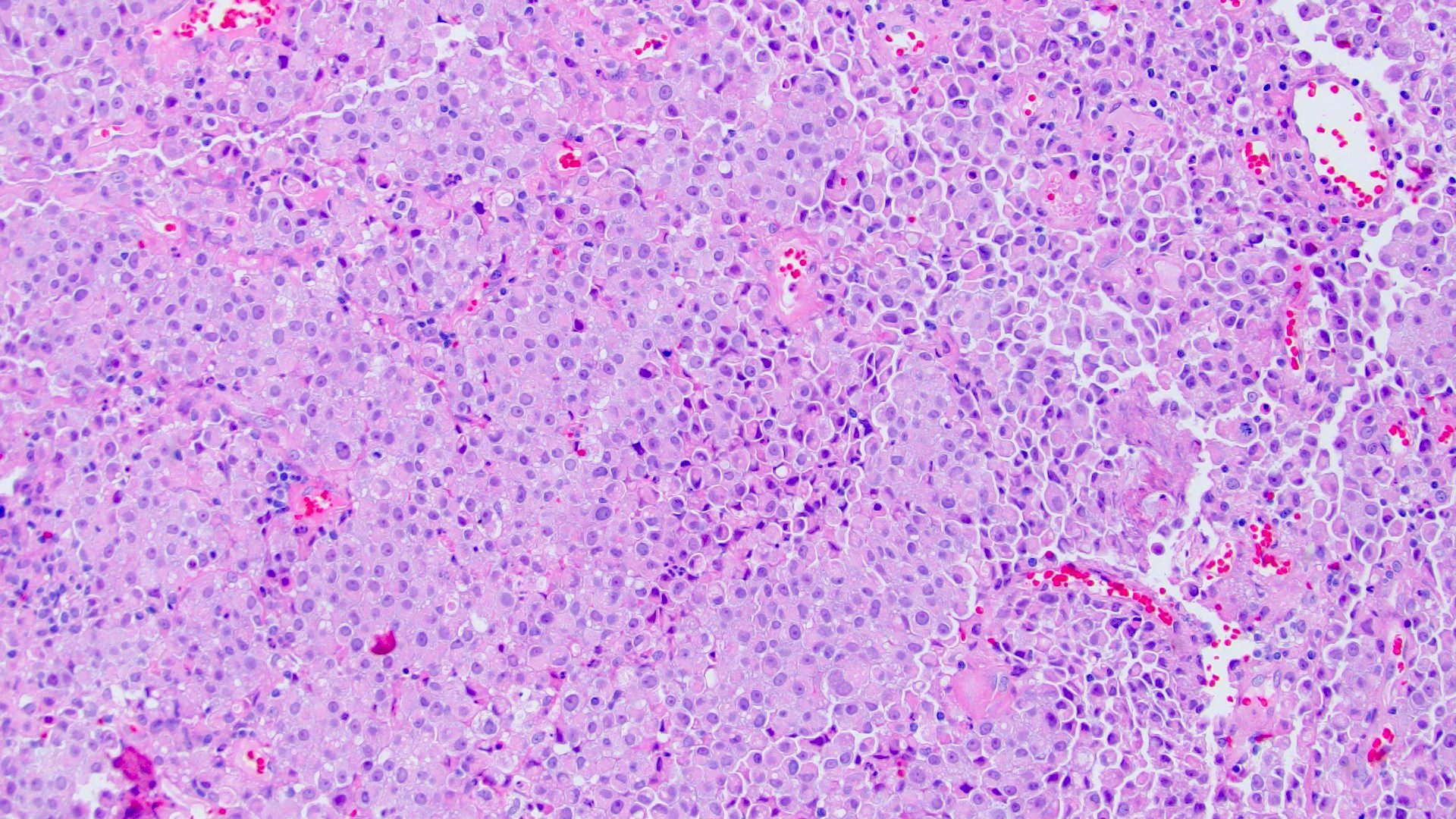
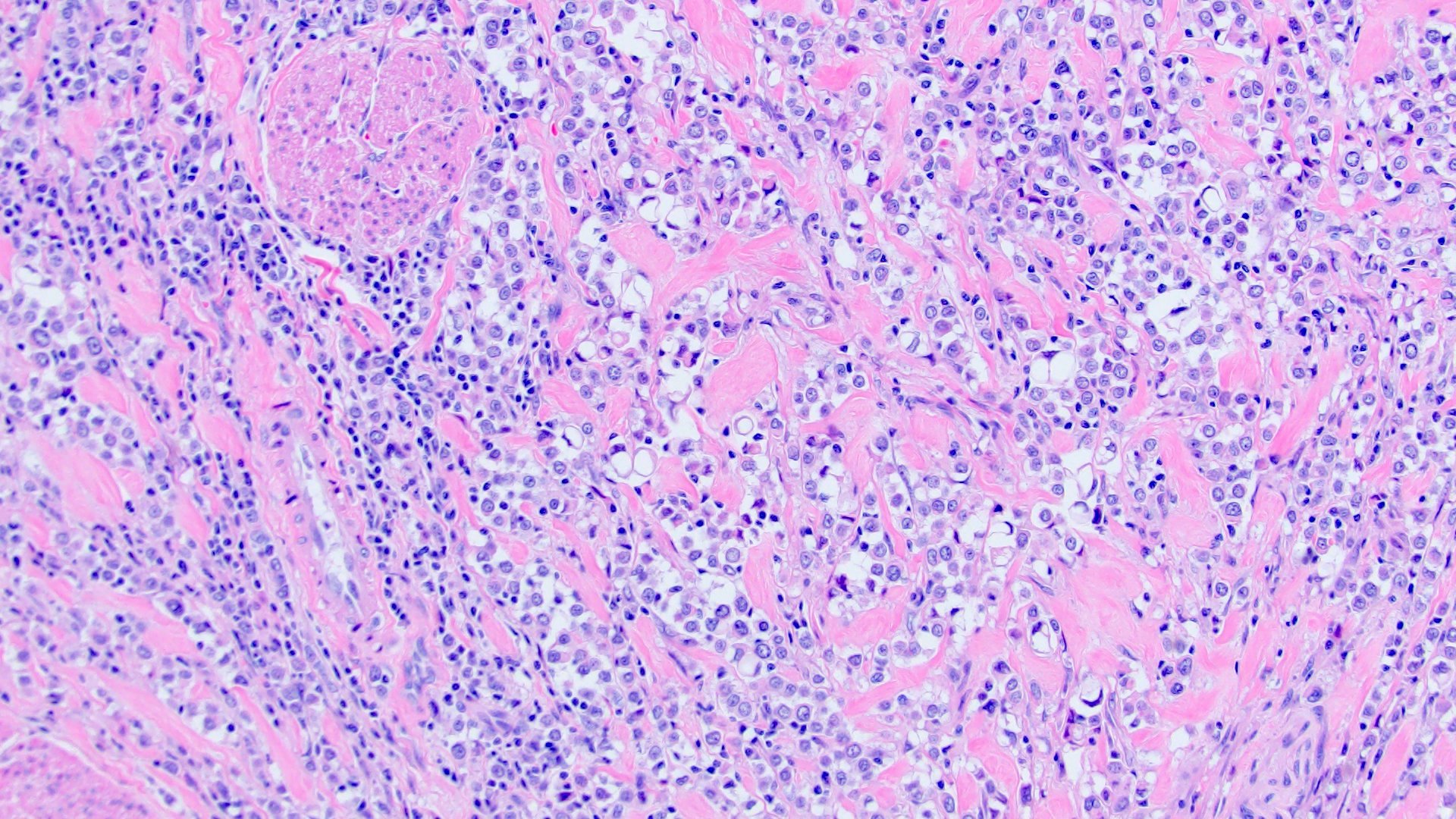
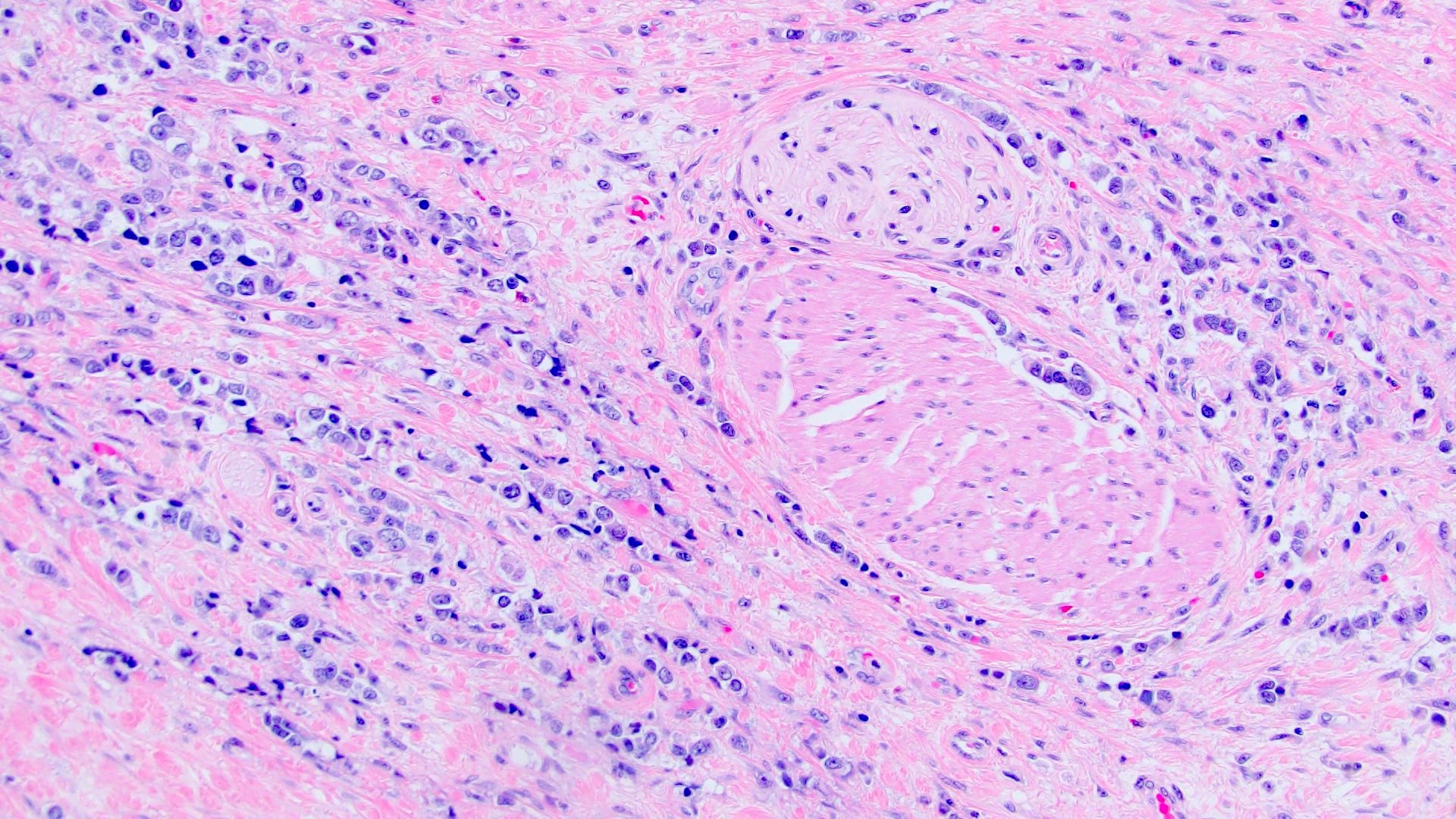
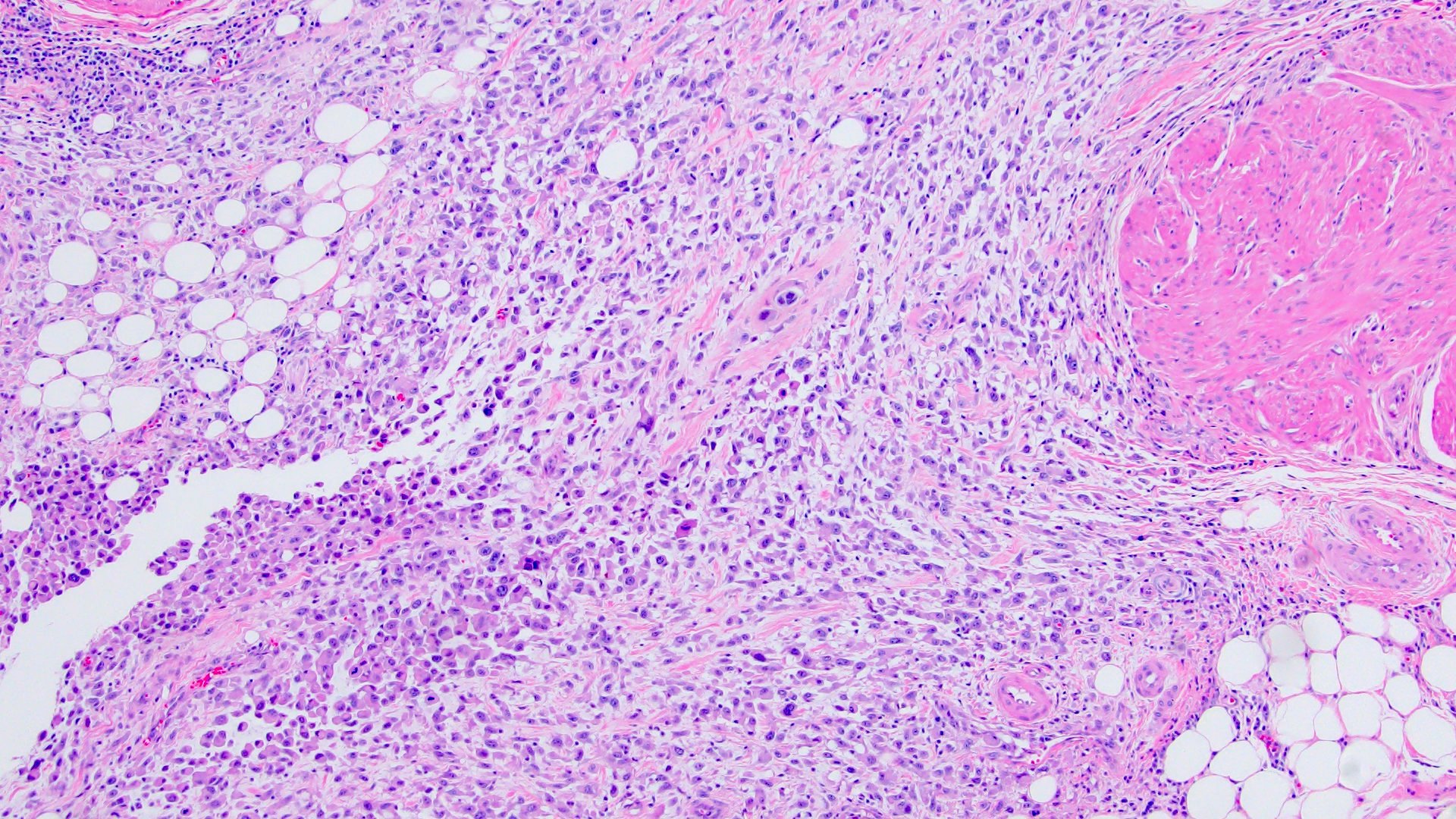
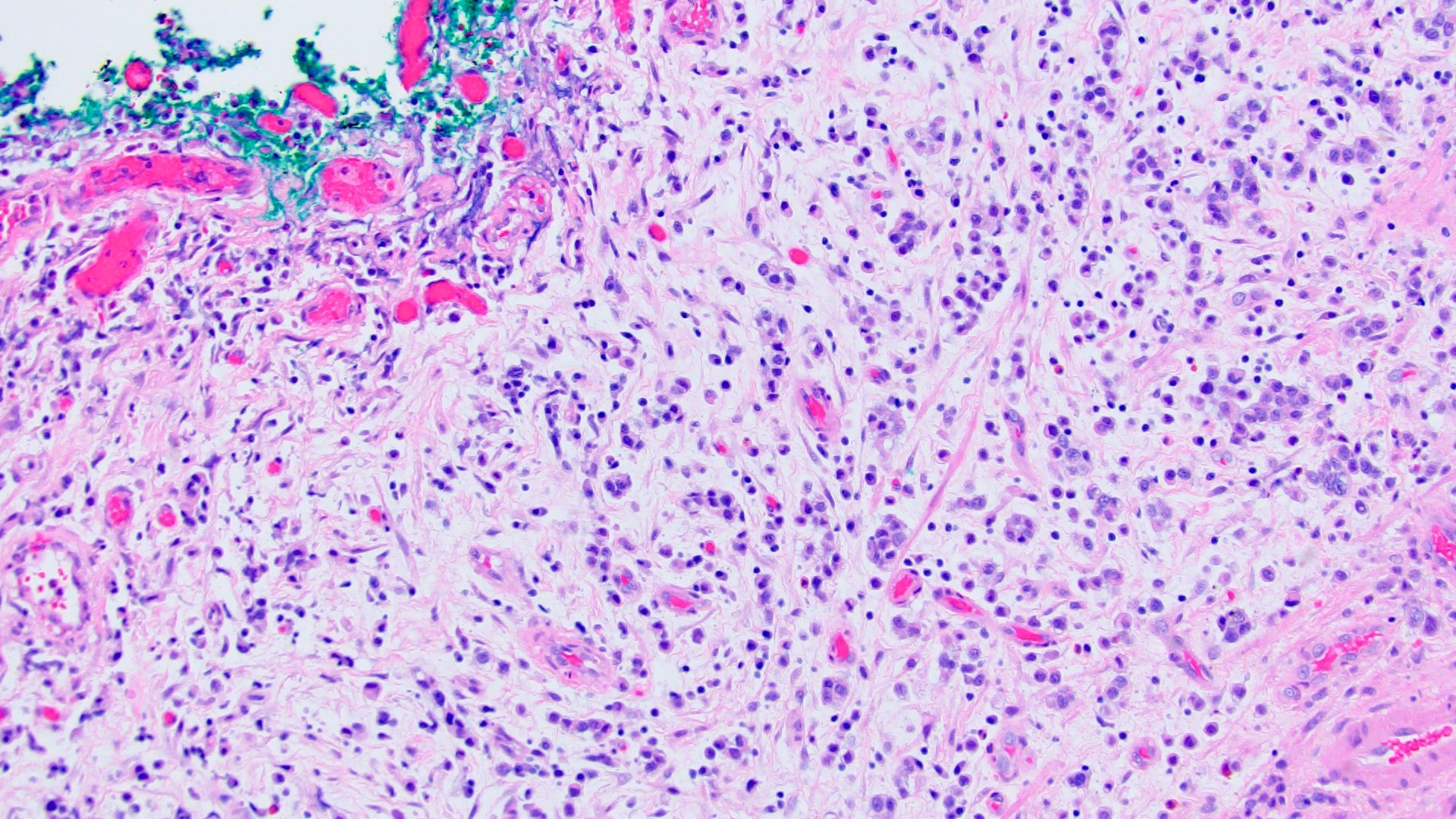
- Discohesive single cells with eccentrically placed nuclei and abundant eosinophilic cytoplasm
- Often deeply infiltrative but with minimal stromal reaction (Eur Urol Focus 2020;6:653)
- Further subclassified into classic, pleomorphic and desmoplastic subtypes:
- Classic: signet ring-like morphology, singly scattered and discohesive in loose aggregates forming cords (Hum Pathol 2019;90:27)
- Pleomorphic: similar to classic but with pleomorphic nuclei and more atypia; can be rhabdoid and bizarre appearing (Hum Pathol 2019;90:27)
- Desmoplastic: plasmacytoid neoplastic cells with a surrounding desmoplastic stromal response (Hum Pathol 2019;90:27)
- Associated with sarcomatoid variant in 31% of cases (Hum Pathol 2019;90:27)
- Often mixed with other histologic subtypes; in 1 case series, 53% of PUC had mixed histologic subtypes (Am J Clin Pathol 2017;147:500)
- Despite resemblance to signet ring carcinoma cells, notably lack extracellular mucin, contrasting to signet ring adenocarcinoma (Acta Cytol 1991;35:277, Am J Surg Pathol 1991;15:569)
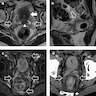
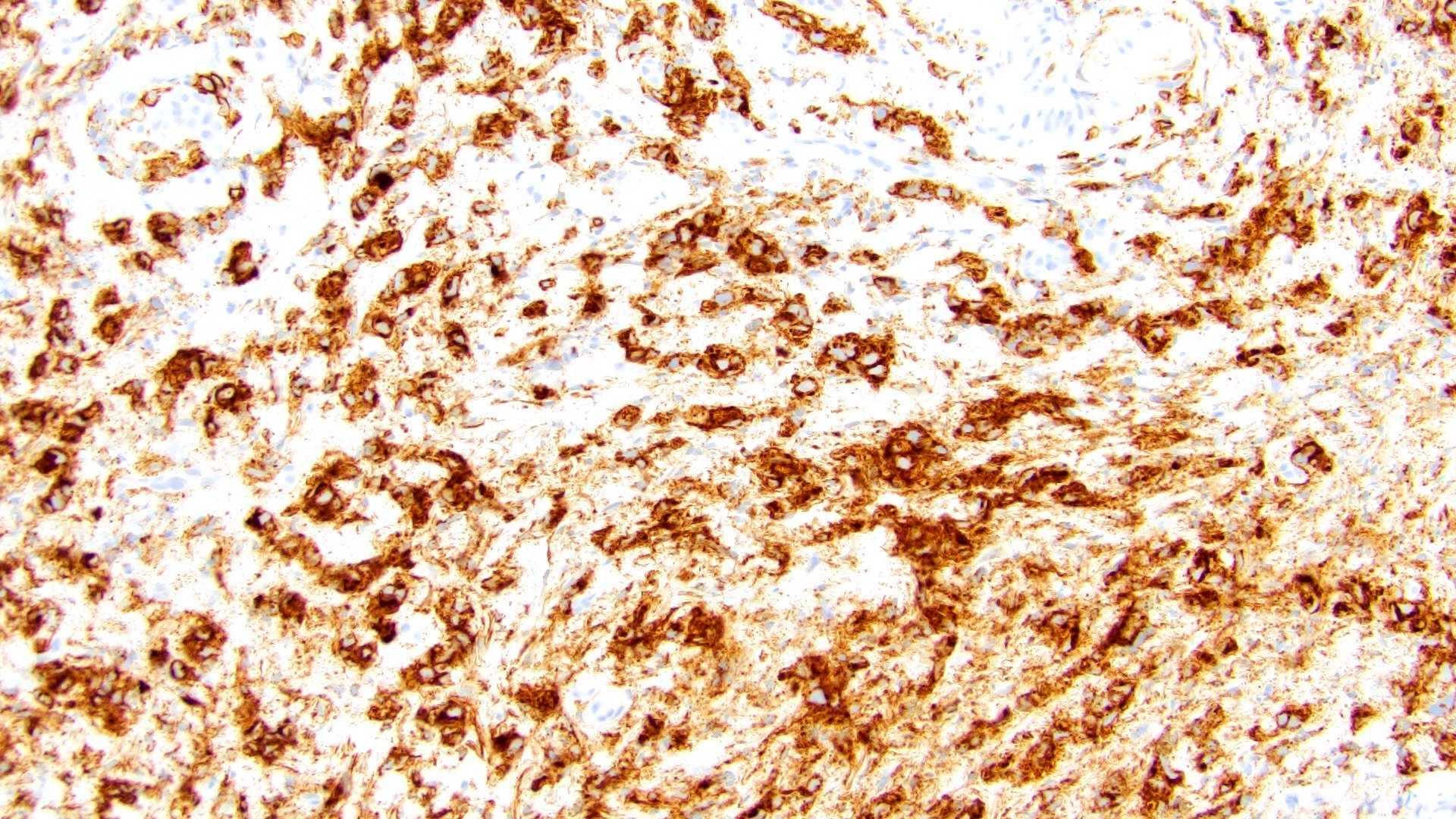
Microscopic (histologic) images
Contributed by Timothy Isaac Miller, M.D., M.A., Nicole K. Andeen, M.D. and Maria Tretiakova, M.D., Ph.D.
Contributed by Lisa Han, M.D. and Ricardo Lastra, M.D. (Case #510)
Cytology description
- Large, discohesive neoplastic cells with abundant cytoplasm (from 15 case series) (Diagn Cytopathol 2020;48:111)
- Eccentrically located and hyperchromatic nuclei
- Coarse chromatin and inconspicuous nucleoli
- Mostly case reports in literature:
- 2 cases (Cytopathology 2009;20:264)
- 1 case (Acta Cytol 2002;46:412)
- 1 case initially misdiagnosed as plasma cell neoplasm (Diagn Cytopathol 2013;41:369)
Positive stains
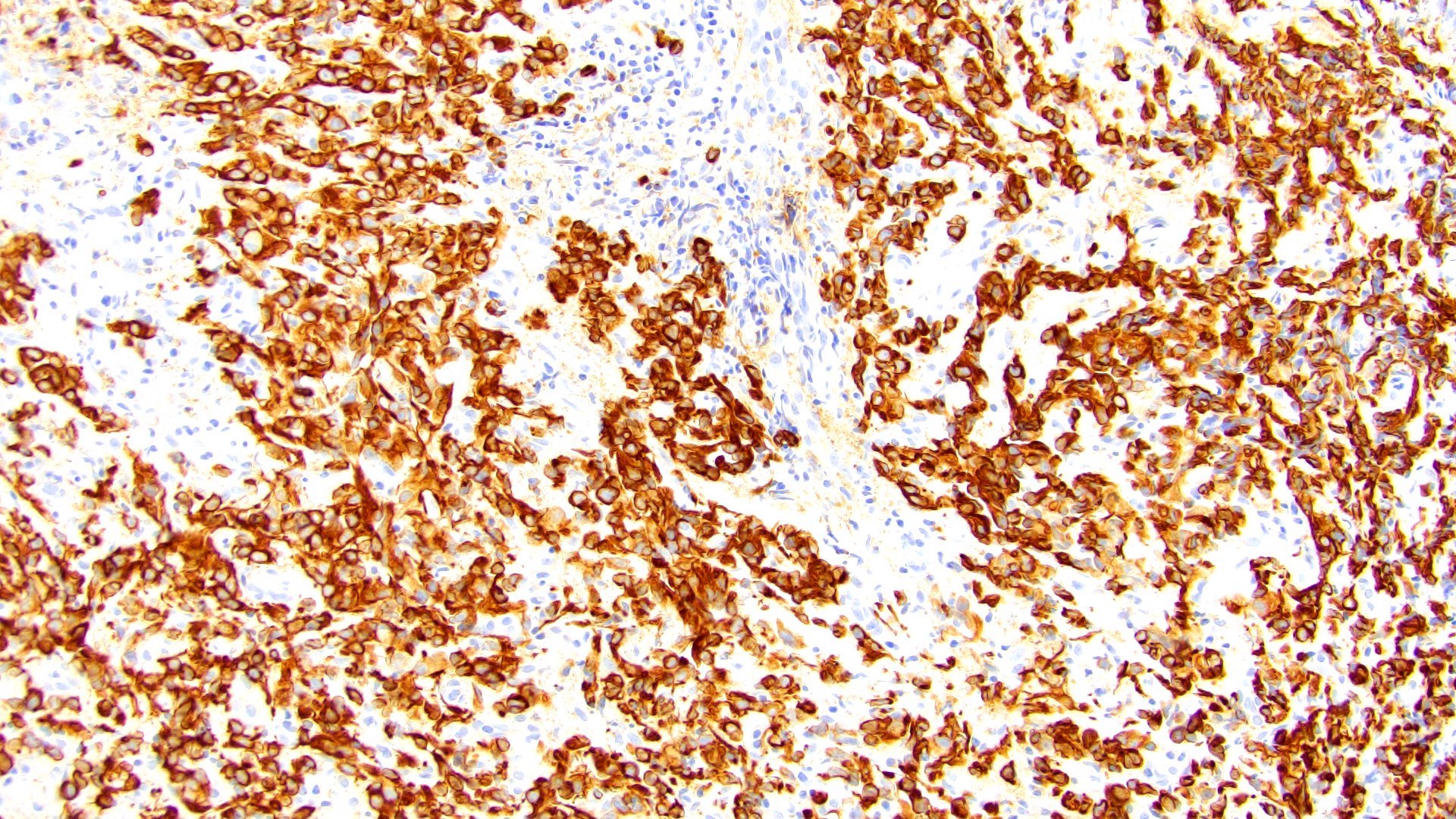
- Pancytokeratin
- GATA3 (80 - 96% sensitive) (Hum Pathol 2019;90:27, Am J Clin Pathol 2017;147:500, Arch Pathol Lab Med 2019;143:1562)
- CD138 (~83% sensitive) (Hum Pathol 2019;90:27, Int J Clin Exp Pathol 2012;5:601)
- CK7 (85 - 89%) (Hum Pathol 2019;90:27, Am J Clin Pathol 2017;147:500)
- CK20 (76 - 77%) (Hum Pathol 2019;90:27, Am J Clin Pathol 2017;147:500)
- Uroplakin II (33 - 69%) (Hum Pathol 2019;90:27, Am J Clin Pathol 2017;147:500, Am J Surg Pathol 2017;41:1570)
- Nectin4 (63%) (Appl Immunohistochem Mol Morphol 2021;29:619)
- p53 (53%) (Int J Cancer 2011;129:346)
- p63 (50%) (Hum Pathol 2014;45:1473)
- pCEA (49%) (Am J Surg Pathol 2017;41:1570)
- EMA (Int J Clin Exp Pathol 2012;5:601)
- Vimentin, CD38, ZEB1, N-cadherin, fibronectin (Diagn Pathol 2020;15:124, BMC Urol 2020;20:72)
- S100P (Hum Pathol 2014;45:1473)
Negative stains
- E-cadherin (57 - 87% loss of membranous staining) (Hum Pathol 2019;90:27, BMC Urol 2020;20:72, Am J Clin Pathol 2017;147:500, Hum Pathol 2020;102:54, Int J Cancer 2011;129:346)
- p120 (73% had loss of membranous staining) (Hum Pathol 2020;102:54)
- 94% concordance with E-cadherin loss (Hum Pathol 2020;102:54)
- ER, PR, mammoglobin, GCDFP-15 (76% loss) (Hum Pathol 2019;90:27, Am J Surg Pathol 2017;41:1570)
- Alcian blue / mucicarmine (Am J Surg Pathol 2008;32:752)
- CDX2 (Am J Surg Pathol 2017;41:1570)
- S100, CD45, synaptophysin, chromogranin (Am J Surg Pathol 2008;32:752, Int J Clin Exp Pathol 2012;5:601)
- MUM1 (Int J Cancer 2011;129:346)
Immunohistochemical panels
- More likely to be luminal subtype than basal subtype of UC (Urol Oncol 2021 Aug 21 [Epub ahead of print], Clinics (Sao Paulo) 2021;76:e2587)
- HER2 expression more likely than conventional UC (Urol Oncol 2021 Aug 21 [Epub ahead of print])
- Similar PDL1 expression on immune cells as conventional UC but less expression of PDL1 on neoplastic cells in PUC compared with conventional UC; similar infiltrating density of CD8+ T cells as conventional UC (Urol Oncol 2021 Aug 21 [Epub ahead of print])
Molecular / cytogenetics description
- Truncating mutation of CDH1 or CDH1 promoter hypermethylation, results in loss of E-cadherin expression (84%) (Nat Genet 2016;48:356)
- Not associated with germline CDH1 mutations
- CDH1 mutations not seen in the other histologic variants (Nat Genet 2016;48:356)
- FGFR3 mutation (60%) (Hum Pathol 2019;90:27)
- UroVysion bladder cancer:
- Deletion of chromosome 9p21 (60%) (Hum Pathol 2019;90:27)
- Genomic background otherwise similar to conventional urothelial carcinoma: frequent mutations in TP53, RB1, ARID1A, ERBB2 and PIK3CA (Nat Genet 2016;48:356, Surg Pathol Clin 2018;11:713, Am J Case Rep 2020;21:e923130)
- High aneuploidy (Int J Cancer 2011;129:346)
- TERT promoter mutations (predominantly g.1295228C > T) (Hum Pathol 2019;85:1)
Videos
Plasmacytoid variant histology in bladder cancer
Sample pathology report
- Bladder, transurethral resection:
- Urothelial carcinoma, high grade
- Histologic component: plasmacytoid (90%)
- Adjacent flat CIS: absent
- Angiolymphatic invasion: absent
- Muscularis propria: present, invaded by carcinoma
- pT: invasive of muscularis propria (pT2)
- Urothelial carcinoma, high grade
- Bladder, cystectomy:
- Invasive high grade urothelial carcinoma, plasmacytoid variant (see synoptic report)
Differential diagnosis
- Factors favoring plasmacytoid urothelial cell carcinoma (Am J Surg Pathol 2008;32:752):
- In situ urothelial carcinoma present
- Foci of cohesive growth
- Consistent immunohistochemical profile for urothelial carcinoma (GATA3, uroplakin II, CK7, CK20 positive)
- Other urothelial carcinoma histologic variants present
- Plasmacytoma:
- Positive for MUM1, CD45 / LCA
- Negative for GATA3, keratins, uroplakin II
- Cannot use CD138 to differentiate, as PUC is often positive for it
- Metastatic signet ring adenocarcinoma:
- Positive for extracellular mucin
- Negative for GATA3, uroplakin II (Am J Surg Pathol 2017;41:1570)
- Metastatic lobular breast carcinoma:
- Negative for uroplakin II (Am J Surg Pathol 2017;41:1570)
- Positive for ER
- Cannot use GATA3 or loss of E-cadherin to differentiate
- Chronic inflammation:
- More dysplasia in PUC
- Polymorphous inflammatory infiltrate in chronic inflammation
- Lymphoma:
- Positive for CD45 / LCA
- Negative for GATA3, keratins, uroplakin II
- Melanoma:
- Neuroendocrine carcinoma:
- More cohesive growth
- Positive for chromogranin, synaptophysin
- Paraganglioma:
- More cohesive growth
- Sustentacular S100 staining
- Rhabdomyosarcoma:
Board review style question #1
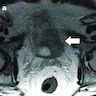
Which of the following is true regarding the histologic variant of urothelial carcinoma shown above?
- Neoplastic cells usually express CD138
- Mutations / loss of CDH1 are uncommon
- Patients are less likely to present at an advanced stage compared with conventional urothelial carcinoma
- Primary ureteral carcinoma of this variant is common
- Neoplastic cells are usually positive for mucin
Board review style answer #1
A. Neoplastic cells usually express CD138. The histologic variant of urothelial carcinoma shown above is the plasmacytoid variant, consisting of discohesive cells with eccentrically placed nuclei and abundant cytoplasm. The neoplastic cells are usually positive for CD138 (making choice A the correct answer). Choice B is false, as truncating CDH1 mutations are common, causing loss of E-cadherin. Choice C is false, as these patients are generally diagnosed at an advanced stage and thus, have a worse prognosis. Choice D is incorrect because the primary ureteral of the variant has rarely been reported. Choice E is false since the cells do not express mucin, making it possible to distinguish from metastatic signet ring cell adenocarcinoma. (Hum Pathol 2019;90:27, Int J Clin Exp Pathol 2012;5:601, Nat Genet 2016;48:356, Eur Urol Focus 2019;5:104, Urology 2017;102:143, Bladder Cancer 2020;6:71, Am J Case Rep 2018;19:158, Am J Surg Pathol 2008;32:752).
Comment Here
Reference: Plasmacytoid
Comment Here
Reference: Plasmacytoid
Board review style question #2
Which of the following immunoprofiles would be most helpful to differentiate primary plasmacytoid urothelial carcinoma of the bladder from metastatic lobular breast carcinoma?
- CK7, E-cadherin
- E-cadherin, uroplakin II
- GATA3, CK7
- GATA3, GCDFP-15
- Uroplakin II, ER
Board review style answer #2
E. Uroplakin II, ER. Membranous loss of E-cadherin will be seen in both lobular breast carcinoma and plasmacytoid UC. GATA3 and CK7 are usually expressed by both. GCDFP-15 can be positive in some cases of plasmacytoid UC (~25% in 1 study) and also positive in lobular breast carcinoma. ER is positive in most cases of lobular breast carcinoma and negative in plasmacytoid UC. Uroplakin II is negative in lobular breast carcinoma and may be positive in plasmacytoid UC. Overall, given these patterns, the immunoprofile most likely to discriminate between the 2 are uroplakin II and ER (choice E). (Breast Cancer Res 2015;17:12, Hum Pathol 2019;90:27, BMC Urol 2020;20:72, Ann Diagn Pathol 2015;19:6, Am J Surg Pathol 2017;41:1570, Cancers (Basel) 2021;13:3695, BMC Cancer 2014;14:546, Am J Clin Pathol 2017;147:500).
Comment Here
Reference: Plasmacytoid
Comment Here
Reference: Plasmacytoid
Board review style question #3
Which panel of immunohistochemical stains would be most helpful to distinguish plasmacytoid urothelial carcinoma from its mimickers at other primary sites?
- CD138, CK20, CK7, ER, PR
- CD138, CK20, CDX2, GCDFP-15
- p63, GATA3, E-cadherin
- GATA3, mammaglobin, ER, Uroplakin II
Board review style answer #3
D. GATA3, mammaglobin, ER, Uroplakin II. A panel including GATA3, mammaglobin, ER and uroplakin II would be helpful. GATA3, more so than p63, is positive in plasmacytoid urothelial carcinoma (PUC) and while it does not differentiate between breast and urothelial origin, is helpful in ruling out gastrointestinal metastases. Mammaglobin and ER are consistently negative in PUC, which helps differentiate PUC from breast metastasis, particularly lobular carcinoma, which is more often ER positive. UroplakinII is helpful as it is negative in nonurothelial mimickers. CD138 has been reported to be positive in PUC although it is nonspecific with expression reported in squamous cell carcinoma, normal urothelium and other epithelial and lymphoid malignancies (Int J Surg Pathol 2016;24:614). E-cadherin expression is typically abnormal in both PUC and breast lobular carcinoma, which includes complete absence of staining or aberrant cytoplasmic staining. Of note, E-cadherin staining was normal in 27% of PUC cases in a recent study (Hum Pathol 2020;102:54).
Comment Here
Reference: Plasmacytoid
Comment Here
Reference: Plasmacytoid