Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Monroe H, El Naili R. Small cell neuroendocrine carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/bladdersmallcell.html. Accessed April 1st, 2025.
Definition / general
- High grade neuroendocrine neoplasm characterized by small to medium sized cells with high N:C ratio and indistinct nucleoli arising from urothelium of urinary bladder, renal pelvis and ureters
Essential features
- Histologically characterized by small to medium sized cells with scant cytoplasm, large nuclei with absent or inconspicuous nucleoli, finely granular salt and pepper chromatin, nuclear molding, Azzopardi phenomenon (crush artifact), abundant mitoses and often pervasive necrosis
- In the genitourinary tract, small cell neuroendocrine carcinoma most commonly arises from the bladder and less commonly from the renal pelvis and ureters
- Usually shows mixed histology; a concomitant urothelial carcinoma, adenocarcinoma or other carcinomatous / sarcomatous component(s) is common
- Typically reaches advanced clinical stage characterized by muscularis propria invasion, lymphovascular invasion or distant metastases by time of diagnosis and is thus associated with a poor prognosis
- Treatment regimens are variable but surgical resection with platinum agent based neoadjuvant or adjuvant chemotherapy is most commonly implemented
Terminology
- Small cell neuroendocrine carcinoma (preferred terminology)
- Small cell carcinoma
- Oat cell carcinoma (no longer in use)
ICD coding
- ICD-10
- C65 - malignant neoplasm of renal pelvis
- C66 - malignant neoplasm of ureter
- C67 - malignant neoplasm of bladder
- C67.1 - malignant neoplasm of trigone of bladder
- C67.2 - malignant neoplasm of lateral wall of bladder
- C67.3 - malignant neoplasm of anterior wall of bladder
- C67.4 - malignant neoplasm of posterior wall of bladder
- C67.5 - malignant neoplasm of bladder neck
- C67.6 - malignant neoplasm of ureteric orifice
- C67.7 - malignant neoplasm of urachus
- C67.8 - malignant neoplasm of overlapping sites of bladder
- C67.9 - malignant neoplasm of bladder, unspecified
Epidemiology
- Small cell neuroendocrine carcinomas account for < 0.5% of all genitourinary malignancies (Urol Case Rep 2019;29:101099)
- Accounts for < 1% of bladder malignancies but is the most common neuroendocrine tumor of the bladder with ~150 cases reported (Hum Pathol 2018;79:57, Clujul Med 2017;90:13)
- Most common neuroendocrine tumor of the bladder with ~150 cases reported (Hum Pathol 2018;79:57, Clujul Med 2017;90:13)
- < 50 cases each have been reported of ureteric and renal pelvic origin (Urol Case Rep 2019;29:101099)
- Median age across all sites: 67 - 70 years (Hum Pathol 2018;79:57, J Med Case Rep 2019;13:71, Onco Targets Ther 2017;10:4105)
- Generally exhibits predilection for males across all sites (Hum Pathol 2018;79:57, Clujul Med 2017;90:13)
- As with urothelial carcinoma and squamous cell carcinoma, exhibits well documented association with smoking tobacco use (Hum Pathol 2018;79:57)
Sites
- Bladder (Hum Pathol 2018;79:57)
- Lateral wall (37.0%)
- Posterior wall (24.7%)
- Trigone / neck (14.8%)
- Anterior wall (13.6%)
- Dome (6.17%)
- Diverticulum (3.7%)
- Urachal remnant origin reported in 1 case (Clujul Med 2017;90:13)
- Kidney
- Generally medullary due to proximity with the renal pelvis (ISRN Urol 2011;2011:786505)
- Ureter (Onco Targets Ther 2017;10:4105)
- Lower segment is most common
- Right > left
Pathophysiology
- Transdifferentiation / dedifferentiation of urothelial carcinoma (iScience 2020;23:101201, Clin Cancer Res 2018;24:1965)
- BRCA1 / BRCA2, TP53 and RB1 with or without APOBEC mutations create high mutation rate and predisposition to malignancy in basal subtype conventional urothelial carcinoma
- Urothelial to neural plasticity and activation of epithelial - mesenchymal transition
- Urothelial to neural plasticity: downregulation of genes responsible for urothelial differentiation and upregulation of genes responsible for neural / neuroendocrine differentiation
- Activation of epithelial mesenchymal transition: downregulation of p53 and p63 pathways in addition to loss of epithelial adhesion molecules (E-cadherin, claudin1, tight junction protein 1)
- Overexpression of ADORA2A (adenosine receptor 2A) and loss of expression of immunostimulatory genes leads to immune desert phenotype
- Other theories regarding pathogenesis
- Multipotent / pluripotent stem cell in urothelium differentiates into small cell neuroendocrine carcinoma and other common bladder carcinomas (i.e., shares same clonal cell of origin as other bladder carcinomas) (Hum Pathol 2018;79:57)
- Malignant transformation of endemic Kulchitsky or enterochromaffin cells in urothelial mucosa or submucosa (Hum Pathol 2018;79:57)
Etiology
- Smoking
- Other proposed etiologies in bladder (Mol Clin Oncol 2018;9:335)
- Chronic cystitis
- Bladder calculi
- History of cystoplasty
Clinical features
- Painless gross or microscopic hematuria is most common
- Flank or back pain (due to hydronephrosis)
- Weight loss
- Rarely, dysuria, change in urinary frequency or recurrent urinary tract infections (UTI)
- Rarely, abdominal mass (Clin Case Rep 2022;10:e6156, World J Clin Cases 2022;10:5884)
- Rare paraneoplastic syndromes
- Hyponatremia due to antidiuretic hormone (ADH) secretion in renal pelvic small cell neuroendocrine carcinoma (J Med Case Rep 2019;13:71)
- Cushing syndrome due to adrenocorticotropic hormone (ACTH) secretion in bladder small cell neuroendocrine carcinoma (ISRN Urol 2011;2011:786505)
- Hypophosphatemia in bladder small cell neuroendocrine carcinoma (ISRN Urol 2011;2011:786505)
- References: Urol Case Rep 2019;27:100995, Urol Case Rep 2019;29:101099
Diagnosis
- Urinalysis
- Urine / bladder washing cytology (Oncol Lett 2014;7:369)
- Cystoscopy
- Typically shows large nodular, polypoid or sessile mass with superficial necrosis (Clujul Med 2017;90:13, Anticancer Res 2017;37:4529)
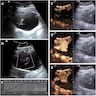
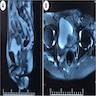
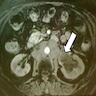
- Imaging (Clujul Med 2017;90:13)
- Ultrasound (US): usually first line and may identify hydronephrosis
- Computerized tomography (CT): identifies masses and tumoral extension
- Magnetic resonance imaging (MRI): used to identify extravesical or extranephroureteric extension and distant metastases
- Gadolinium enhanced MRI: may identify brain metastases
- 99mTc MDP bone scan may identify bone metastases
- Transurethral resection of the bladder (TURBT) or resection for confirmation
Laboratory
- Anemia
- Urinalysis: hematuria (Urol Case Rep 2019;27:100995)
- Renal function tests in nephroureteric carcinomas
- Elevated serum creatinine (Urol Case Rep 2021;40:101923, Medicine (Baltimore) 2018;97:e11113)
- Decreased glomerular filtration rate (GFR) (Medicine (Baltimore) 2018;97:e11113)
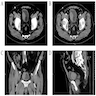
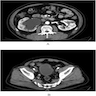
Radiology description
- Generally similar to conventional urothelial carcinoma
- Ultrasound: may present as hypoechoic mass with hyperemia per color doppler flow imaging (BMC Cancer 2017;17:746)
- CT: enhancing polypoid intraluminal mass (Korean J Radiol 2003;4:130)
- May contain nonenhancing necrotic or cystic regions
- Calcifications may be present
- MRI
- Hypointense signal on T1 and heterogeneous, mixed signal on T2 (Korean J Radiol 2003;4:130, ISRN Urol 2011;2011:786505)
- Superior to CT for detection of extravesical / nephroureteric extension and distant metastases (Korean J Radiol 2003;4:130)
Radiology images
Prognostic factors
- Dismal prognosis across all sites in patients with metastases (Clin Genitourin Cancer 2022;20:431)
- Bladder squamous cell carcinoma (SCC) without metastases (M0): generally chemosensitive with excellent long term survival following response to neoadjuvant chemotherapy (Clin Genitourin Cancer 2022;20:431)
- Bladder SCC with distant metastases (M1) has poor survival regardless of systemic chemotherapy (Clin Genitourin Cancer 2022;20:431)
- Bladder SCC: loss of function ERCC2 mutations associated with pathologic complete response to neoadjuvant chemotherapy (Clin Genitourin Cancer 2022;20:431)
- Generally comparable prognosis to stage matched urothelial carcinoma unless metastases are present, in which case the prognosis of small cell neuroendocrine carcinoma is significantly worse (Hum Pathol 2018;79:57)
- ~90% of bladder small cell neuroendocrine carcinomas have at least invasion of muscularis propria at diagnosis (Hum Pathol 2018;79:57)
- Recurrence rates are generally high across all sites (ISRN Urol 2011;2011:786505)
- Metastases to brain, bones, lung, liver and adrenal glands are most common (Pol J Pathol 2014;65:15)
- Median overall survival
- 8 - 15 months for renal pelvic small cell neuroendocrine carcinoma (J Med Case Rep 2019;13:71)
- 17 months for ureteric small cell neuroendocrine carcinoma (Onco Targets Ther 2017;10:4105)
- Adverse factors (Anticancer Res 2017;37:4529)
- Tumor size > 4 cm (Anticancer Res 2017;37:4529)
- Age > 65 years (ISRN Urol 2011;2011:786505)
- Vascular and perineural invasion (Anticancer Res 2017;37:4529)
- Distant metastases (Anticancer Res 2017;37:4529)
- Pure (nonmixed) histology (Hum Pathol 2018;79:57)
- Positive vimentin staining (extremely rare) (ISRN Urol 2011;2011:786505)
Case reports
- 50 year old man with smoking history who presents with chronic gross hematuria and palpable hypogastric mass due to bladder lesion (Clin Case Rep 2022;10:e6156)
- 60 year old man with chronic gross hematuria and dysuria due to bladder lesion (Int J Health Sci (Qassim) 2020;14:53)
- 68 year old man with gross hematuria, right flank pain and abdominal distention due to right renal lesion (World J Clin Cases 2022;10:5884)
- 76 year old man with congestive heart failure decompensation and incidental urinalysis positive for microscopic hematuria due to bladder lesion (Cureus 2020;12:e8609)
- 79 year old woman with gross hematuria and right back pain due to right ureter lesion (Medicine (Baltimore) 2018;97:e11113)
Treatment
- Surgery
- Bladder (Hum Pathol 2018;79:57)
- Radical cystectomy
- Partial cystectomy
- Transurethral resection
- Kidney / ureter (Pol J Pathol 2014;65:15, Urol Case Rep 2019;29:101099)
- Radical nephroureterectomy
- Nephrectomy
- Segmental ureterectomy
- Bladder (Hum Pathol 2018;79:57)
- Chemotherapy
- Neoadjuvant chemotherapy has been shown to decrease clinical stage and improve overall survival time and rates (Hum Pathol 2018;79:57)
- Platinum agents with or without a topoisomerase inhibitor is generally preferred
- Cisplatin is most common
- Carboplatin may be used if patient is unable to tolerate myelosuppressive, nephrotoxic or neurotoxic side effects of cisplatin (Clujul Med 2017;90:13)
- Specific regimens for pure vs mixed carcinoma
- Pure: cisplatin + etoposide (Urol Case Rep 2021;40:101923)
- Mixed: MVAC (methotrexate, vinblastine, Adriamycin [doxorubicin] and cisplatin) (Urol Case Rep 2021;40:101923)
- Radiation therapy
- May be used for residual local postoperative disease (Urol Case Rep 2021;40:101923)
- Some efficacy has been shown for skull and bone metastases (Mol Clin Oncol 2018;9:335, World J Surg Oncol 2017;15:33)
- Immunotherapy
- Immune checkpoint inhibitors such as pembrolizumab have shown success in the treatment of recurrent bladder small cell neuroendocrine carcinoma (IJU Case Rep 2020;3:252)
- ADORA2A immune checkpoint mutation may be a plausible target of adenosine receptor 2A antagonists (iScience 2020;23:101201)
- Targeted / molecular therapy
- Tumor cells may demonstrate KIT or PDGFRA mutations, a theoretical target of imatinib mesylate and other tyrosine kinase inhibitors (Urol Case Rep 2019;27:100995, Urol Case Rep 2019;29:101099)
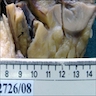
Gross description
- Firm tumor with grey outer surface harboring hemorrhagic foci and ill defined contours (Medicine (Baltimore) 2018;97:e11113)
- White, gray-white or gray-yellow cut surface (Pol J Pathol 2014;65:15)
- Necrosis and hemorrhage
- Perinephric fat extension common in nephroureteric carcinomas (Pol J Pathol 2014;65:15)
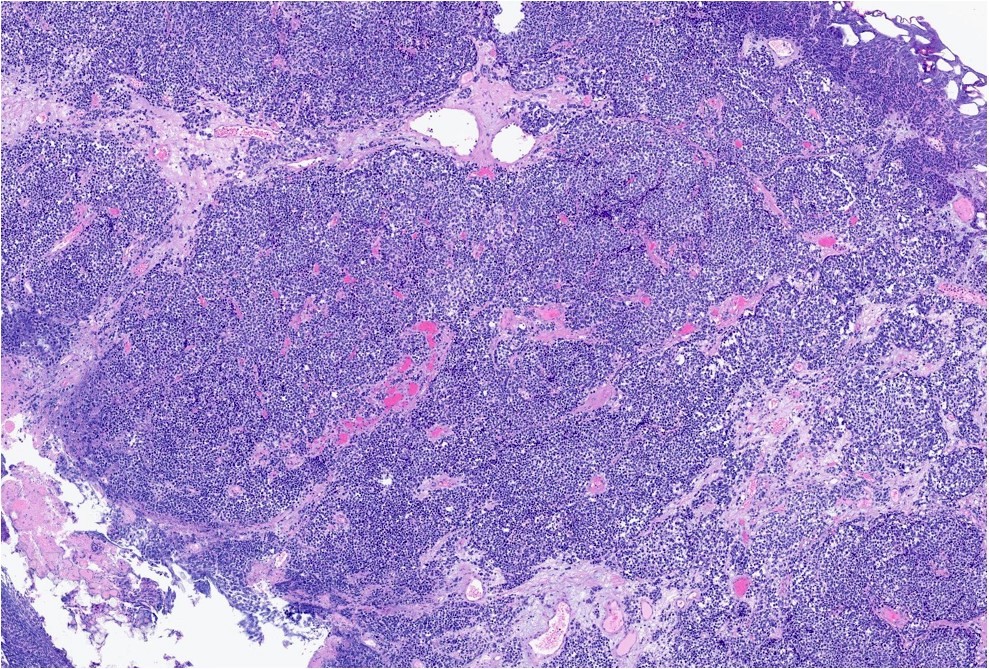
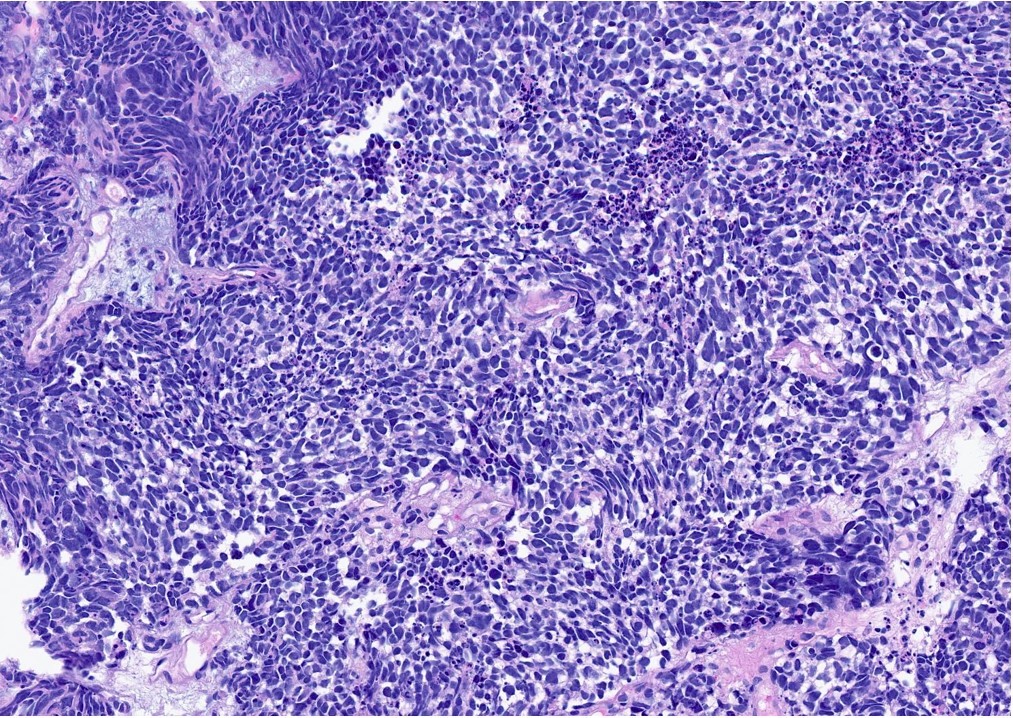
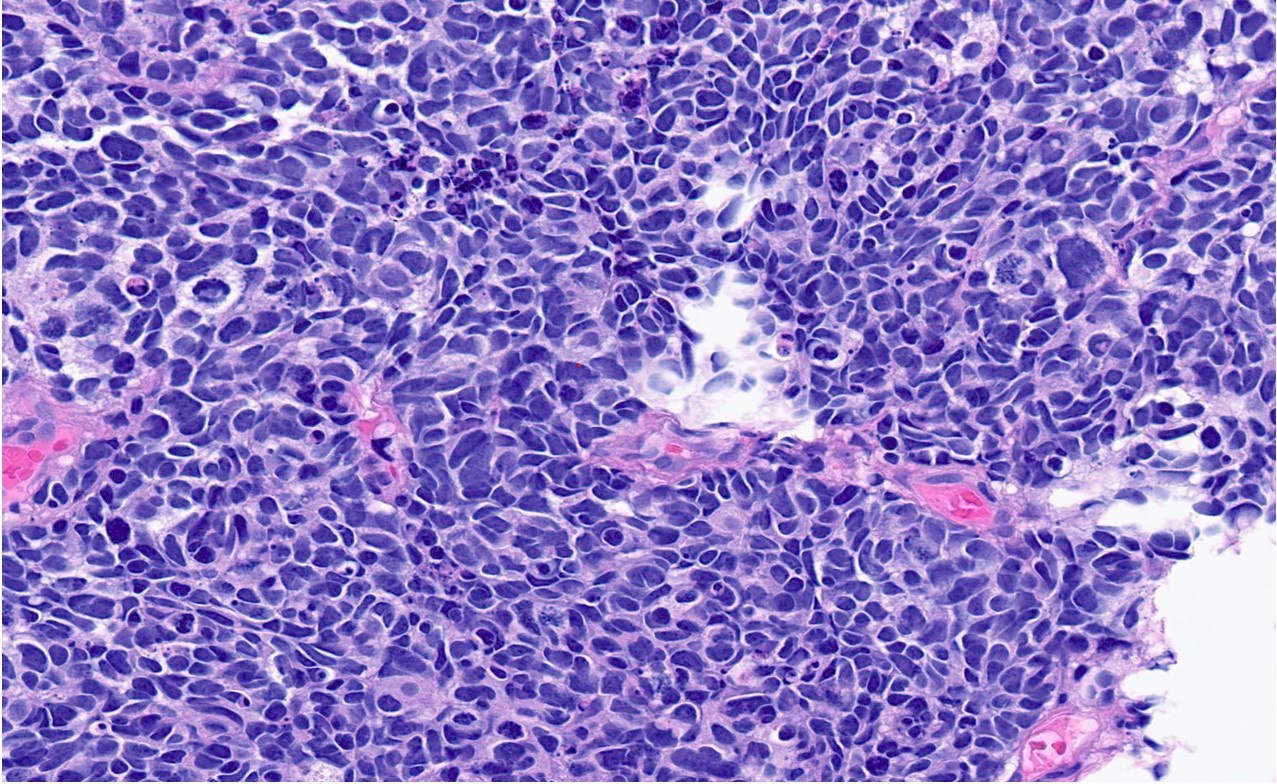
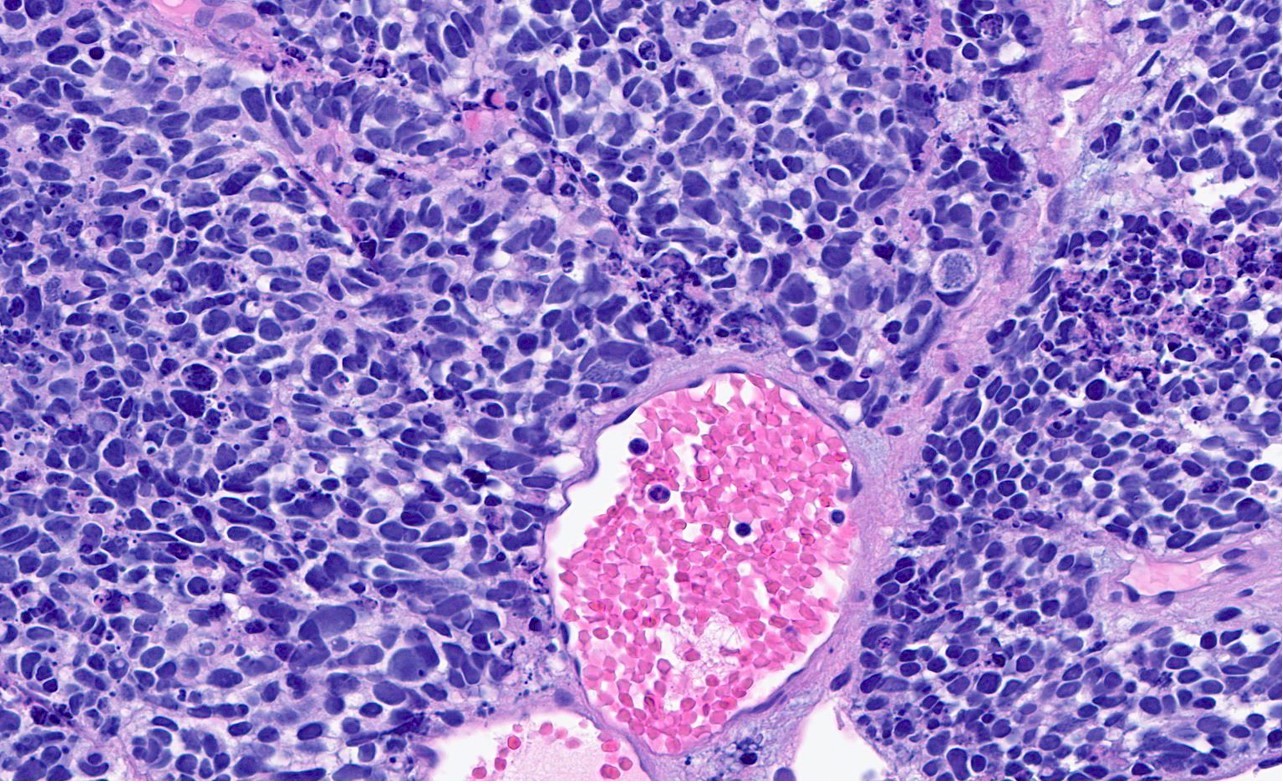
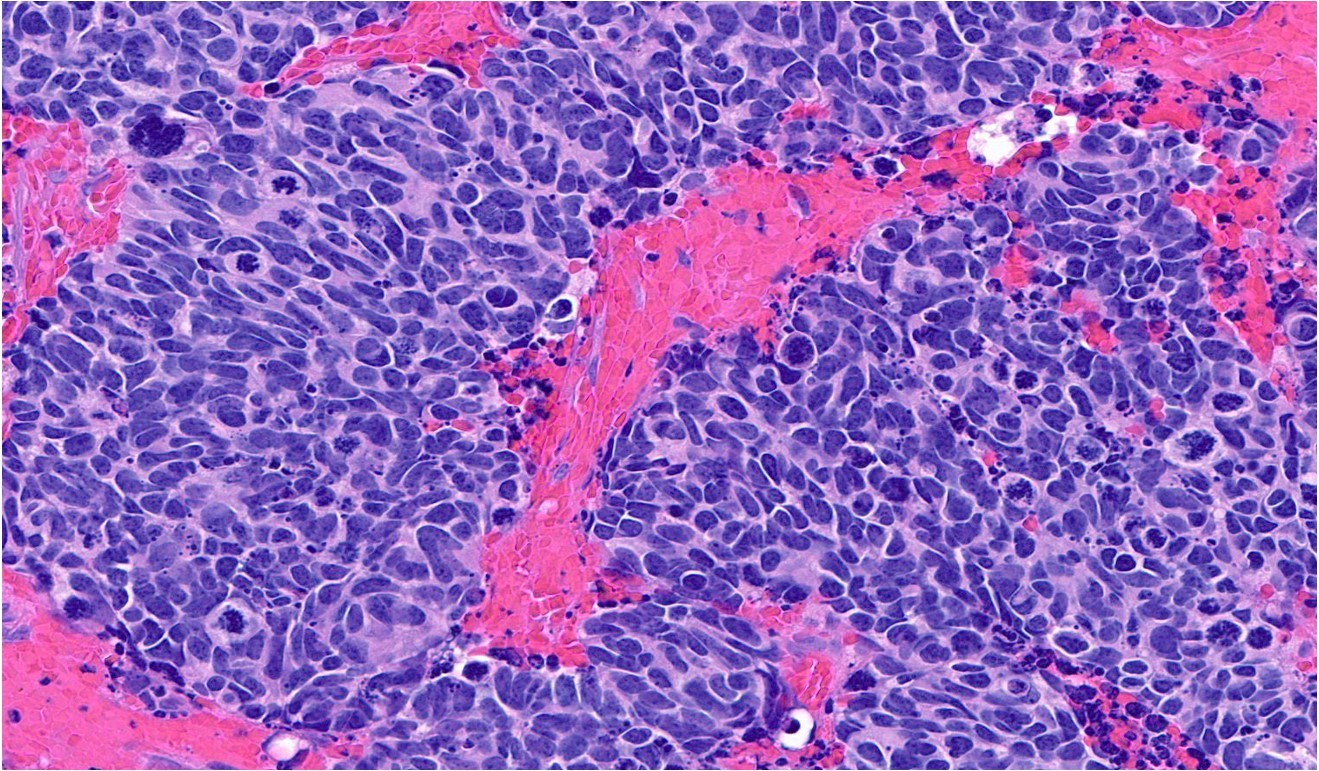
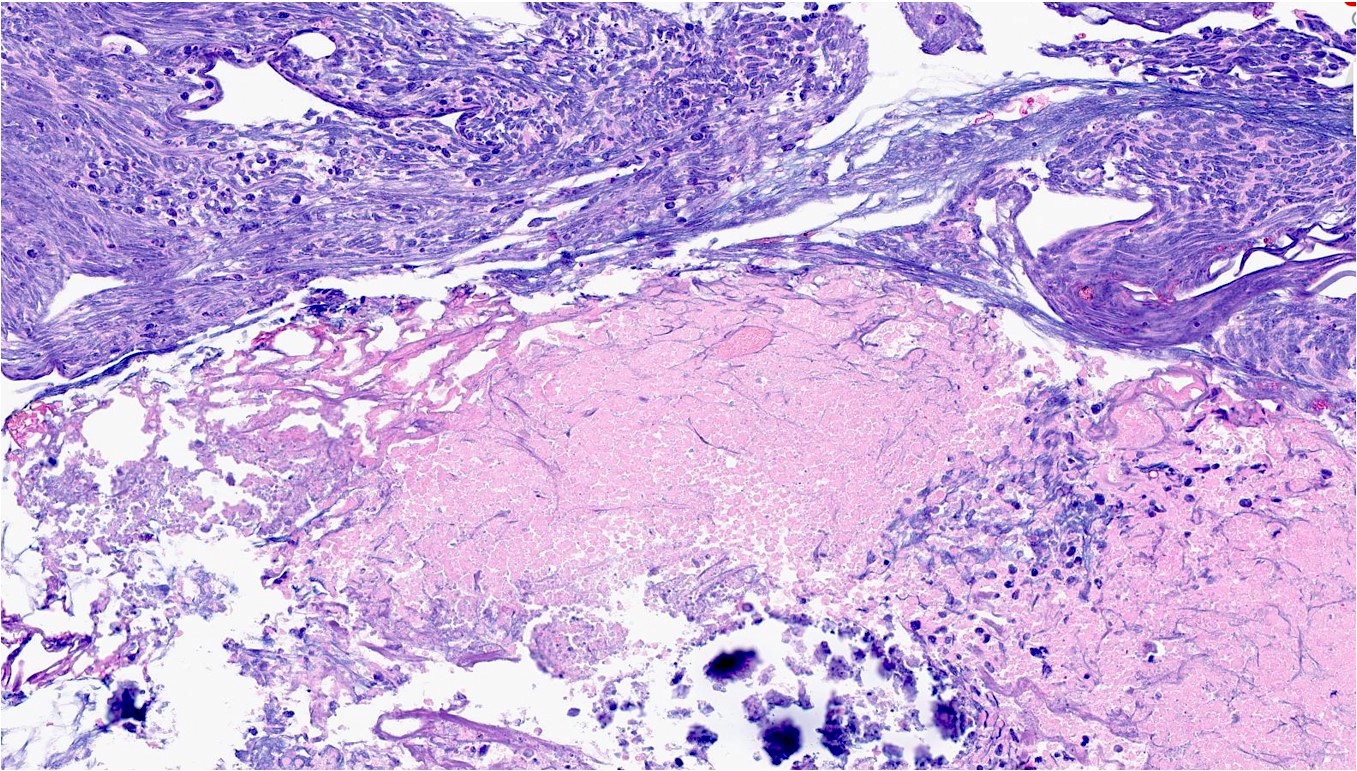
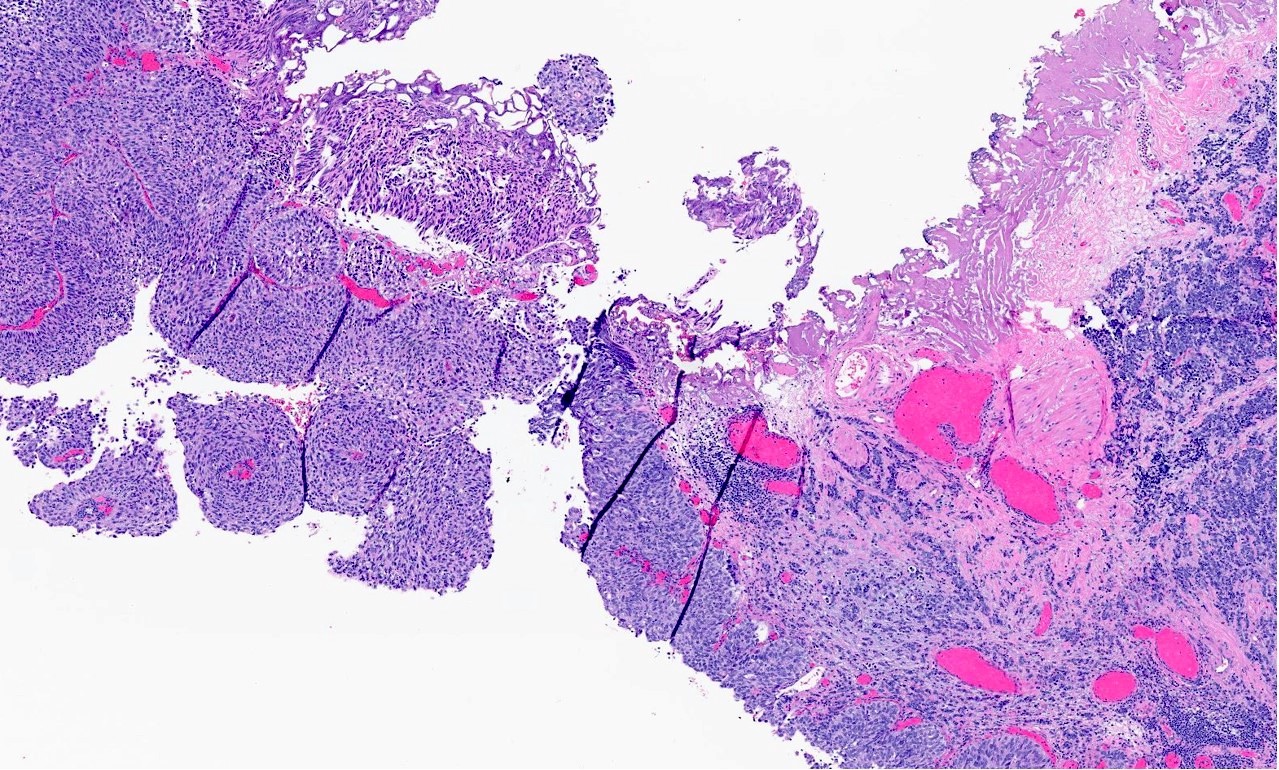
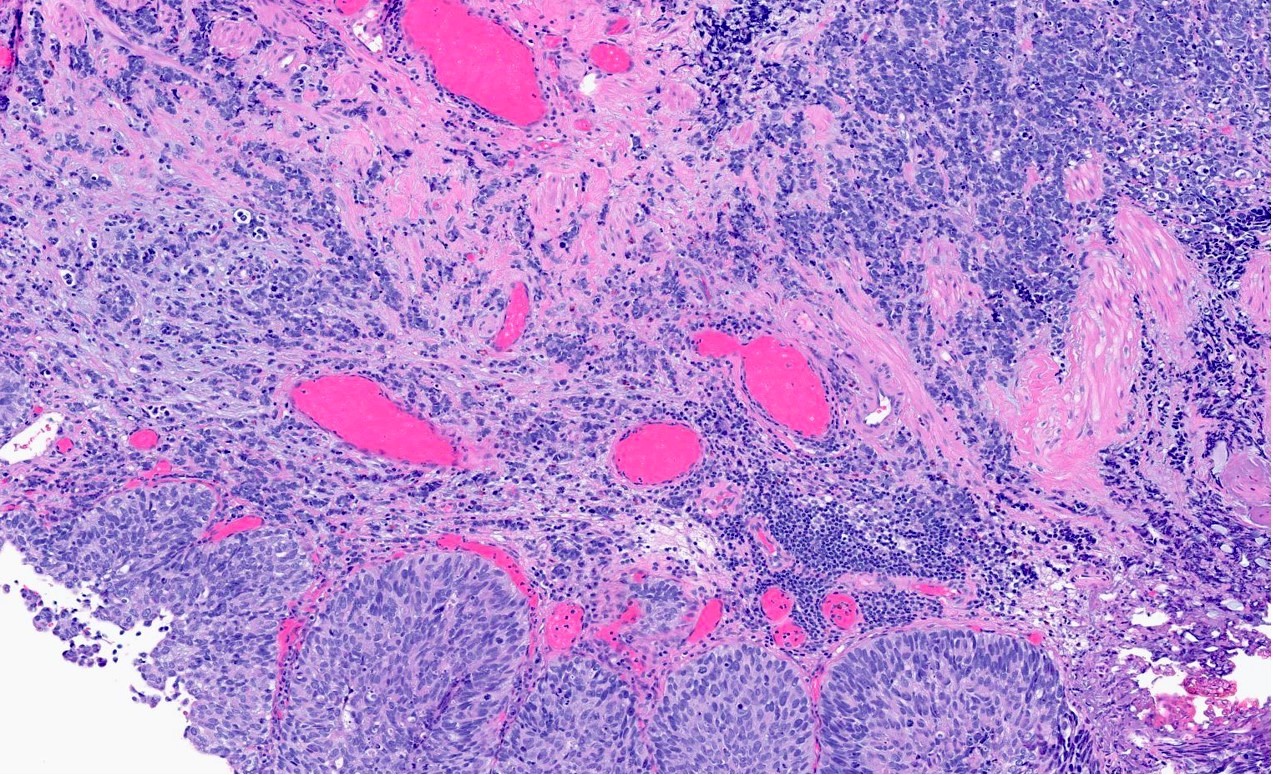
Microscopic (histologic) description
- General histologic features (Pol J Pathol 2014;65:15)
- Solid sheet-like, nested and trabecular architectures
- Small to medium sized cells with scant cytoplasm and large round to oval nuclei (i.e., high N:C ratio)
- Finely granular, stippled salt and pepper chromatin
- Absent or inconspicuous nucleoli
- Nuclear molding
- Azzopardi phenomenon (crush artifact)
- Brisk mitotic activity and atypical mitoses
- Extensive, often geographic necrosis
- Frequently coexists with other malignant cell populations
- Prevalence of additional histologic components (Hum Pathol 2018;79:57)
- Urothelial carcinoma (59%)
- Urothelial carcinoma in situ (48%)
- Adenocarcinoma (26%)
- Sarcomatoid carcinoma (7.4%)
- Micropapillary carcinoma (7.4%)
- Squamous cell carcinoma (5.6%)
- Plasmacytoid carcinoma (1.9%)
- Sarcomas (chondrosarcoma, leiomyosarcoma and rhabdomyosarcoma) have been described in rare case reports (Onco Targets Ther 2017;10:4105)
- Lymphoma described in 1 case report (Onco Targets Ther 2017;10:4105)
- May be triphasic or even tetraphasic in rare cases (World J Surg Oncol 2017;15:33)
- Prevalence of additional histologic components (Hum Pathol 2018;79:57)
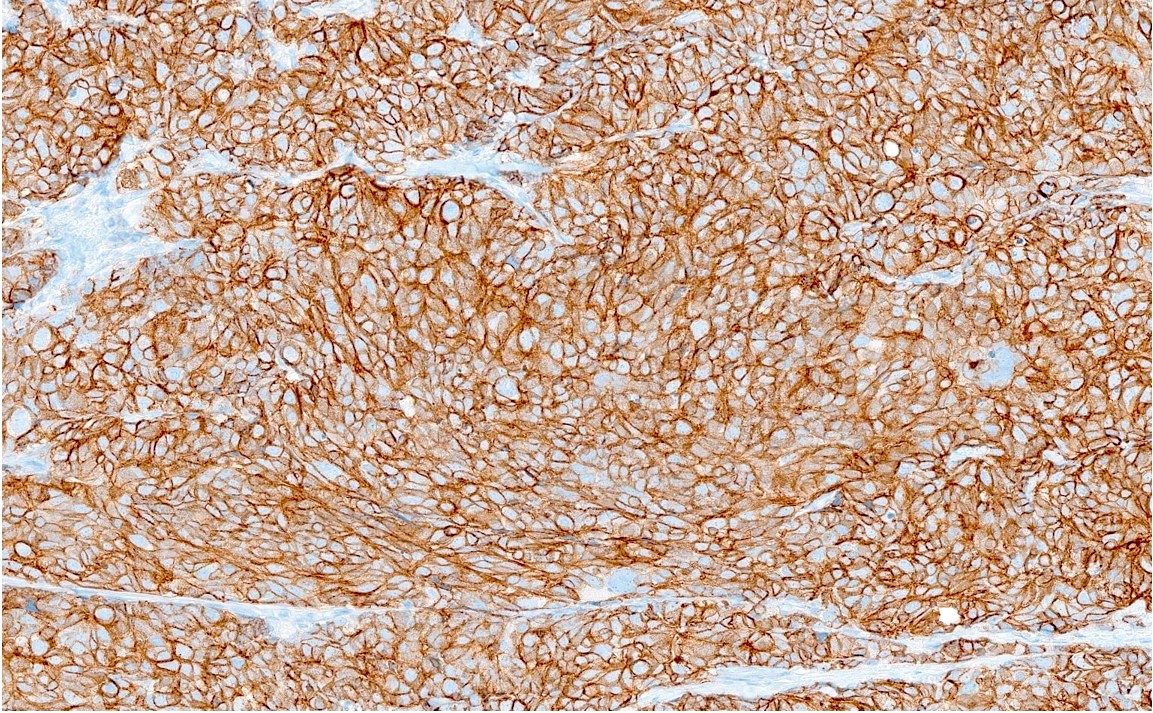
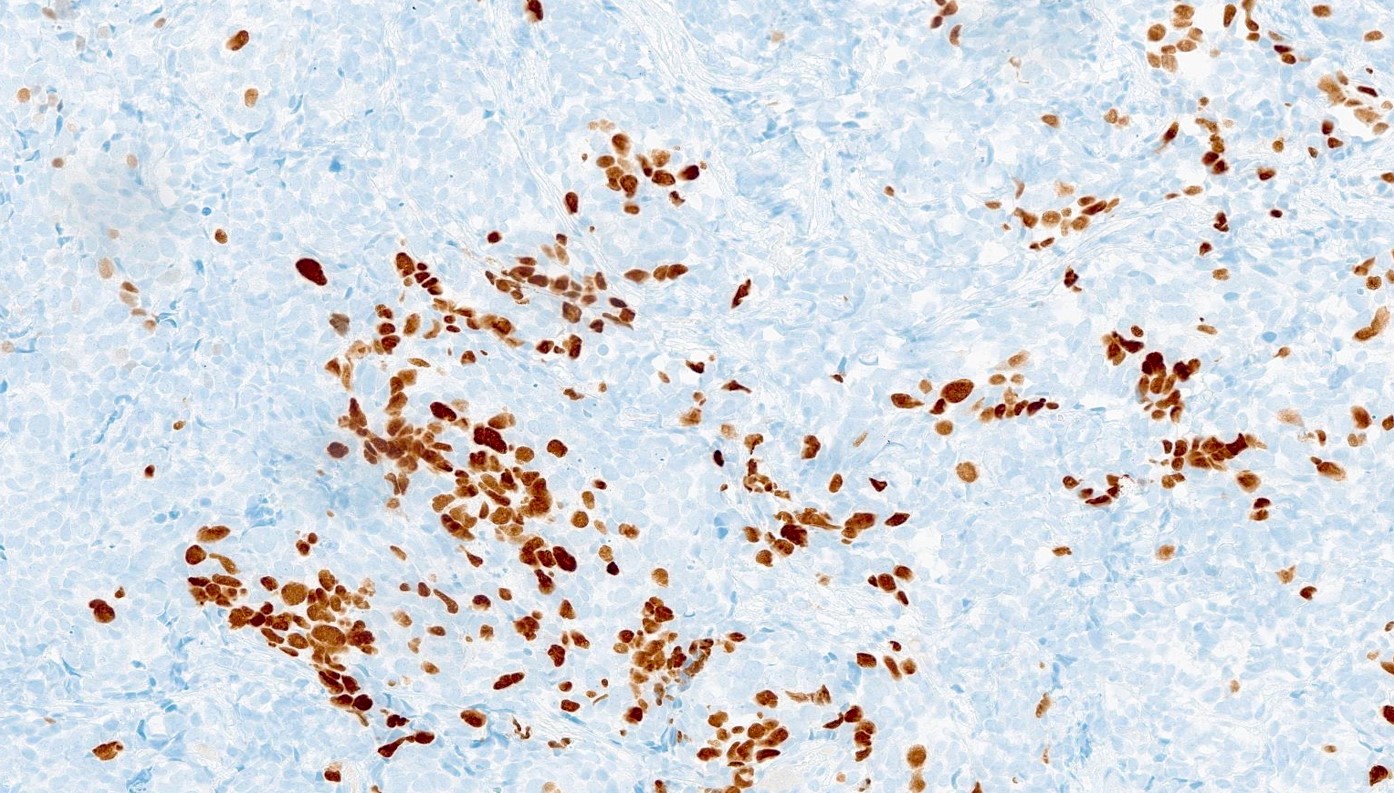
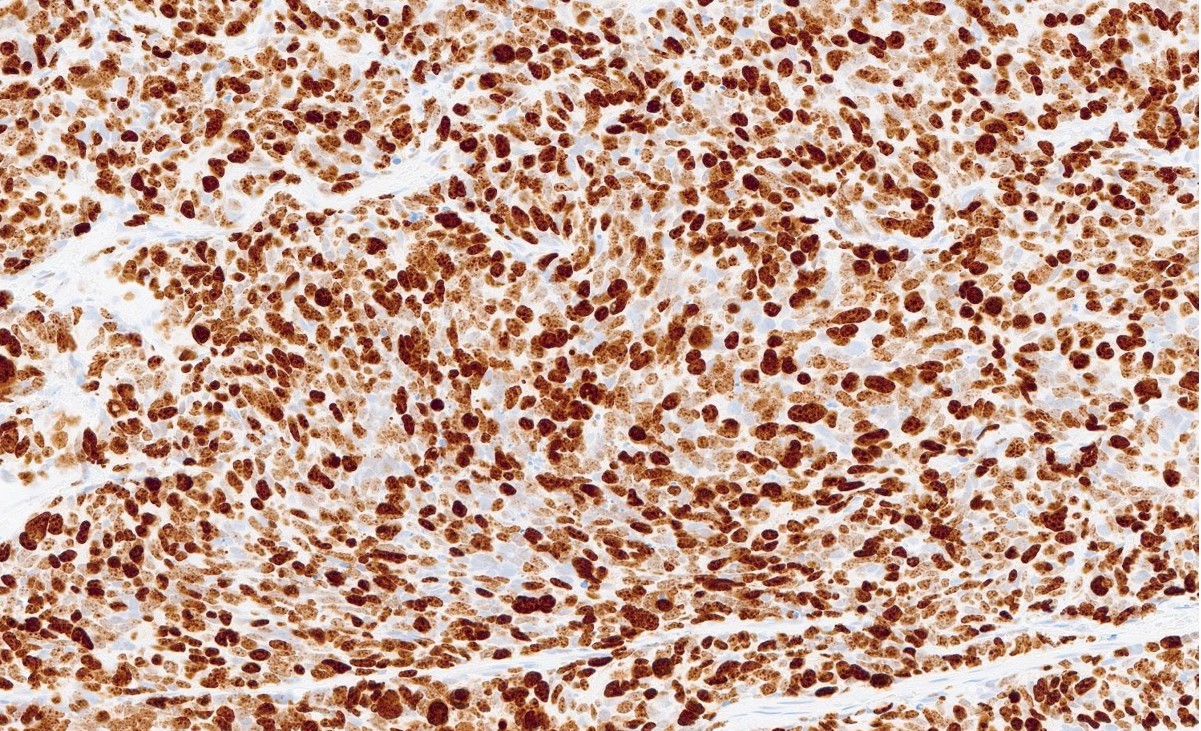
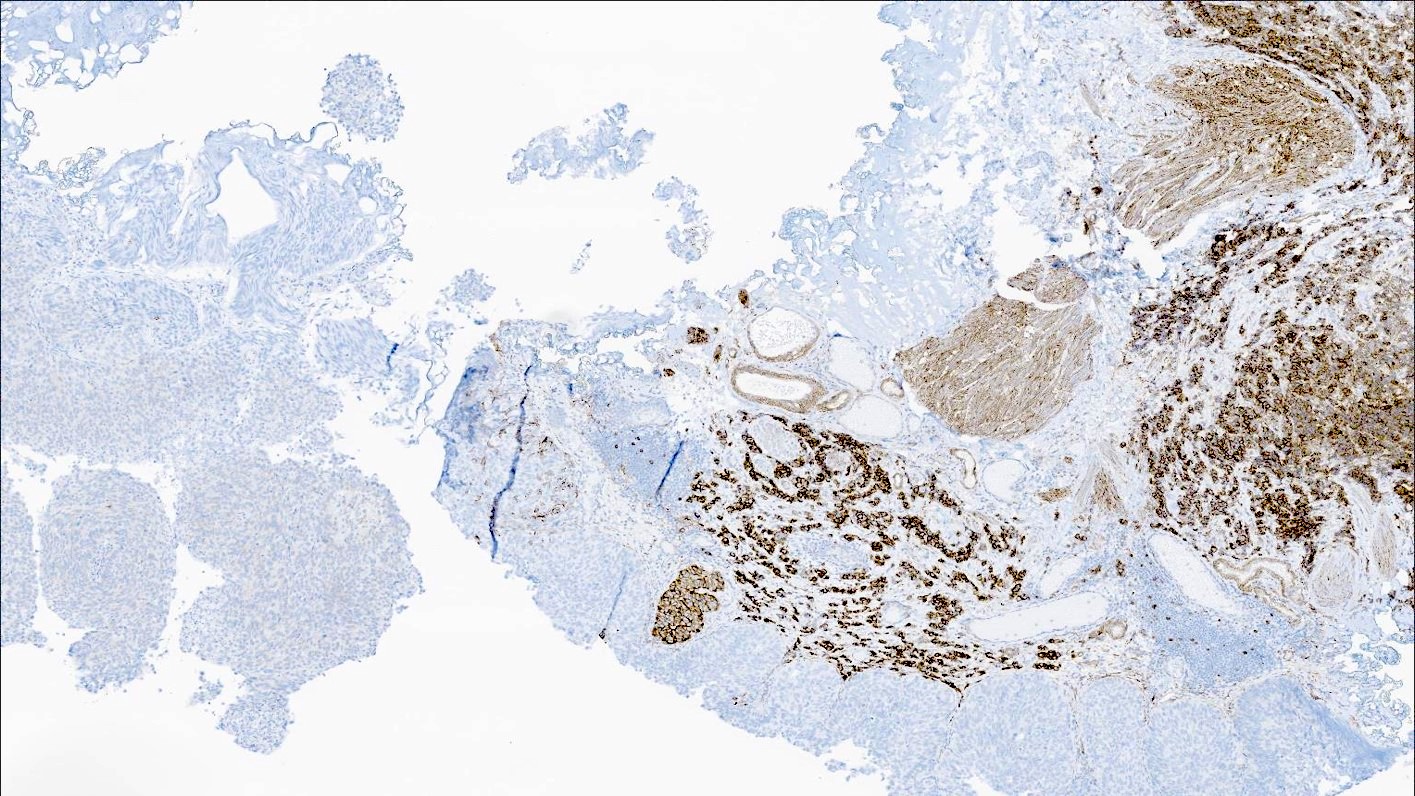
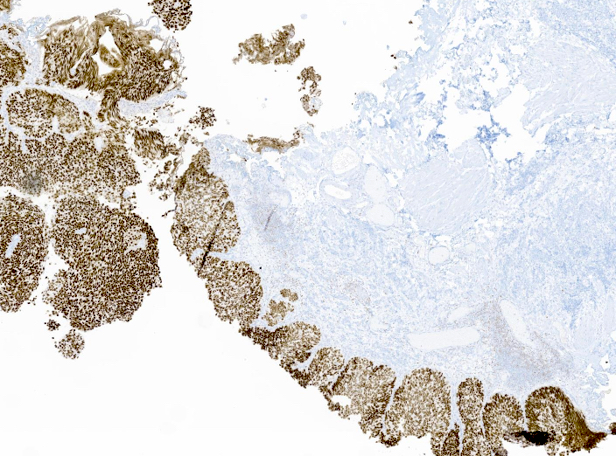
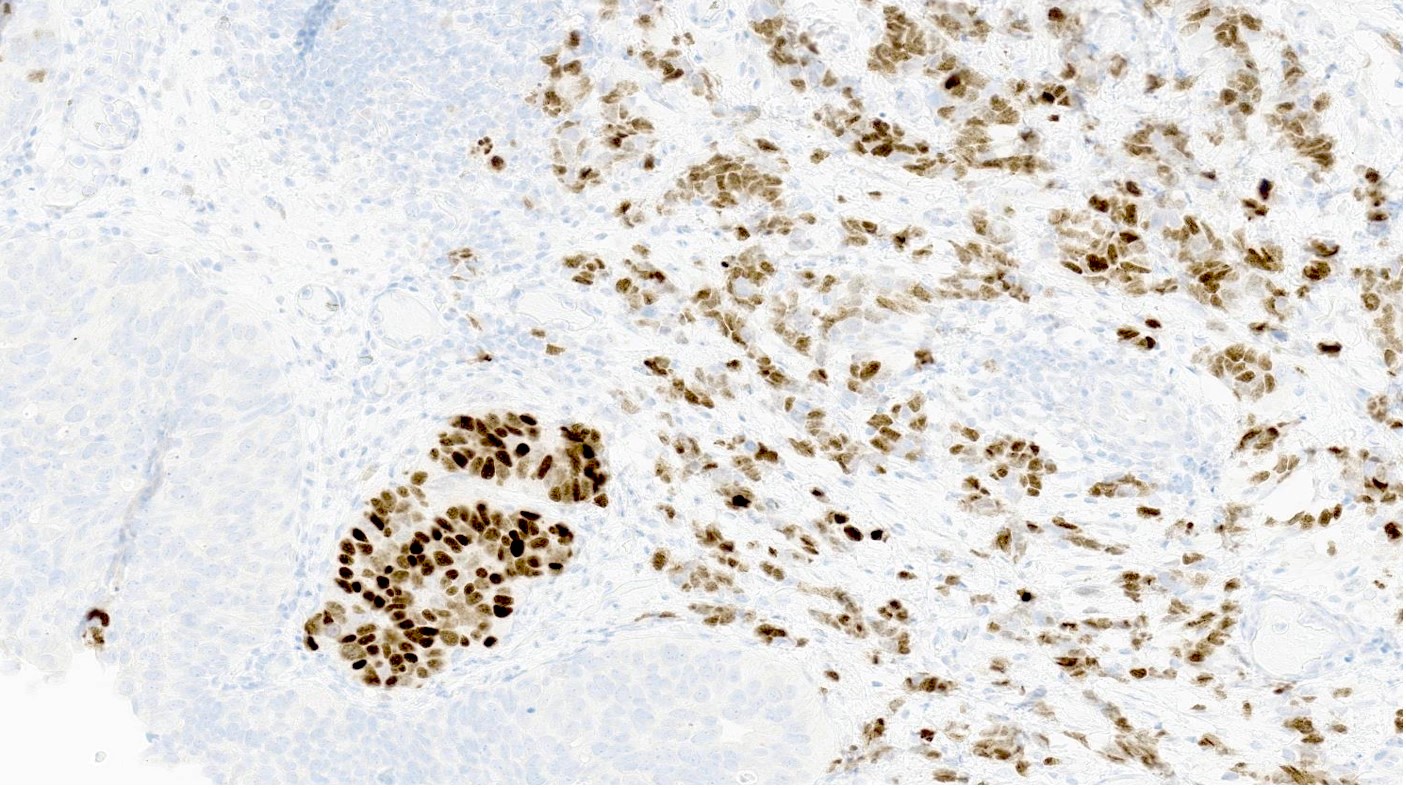
Microscopic (histologic) images
Contributed by Reima El Naili, M.D.
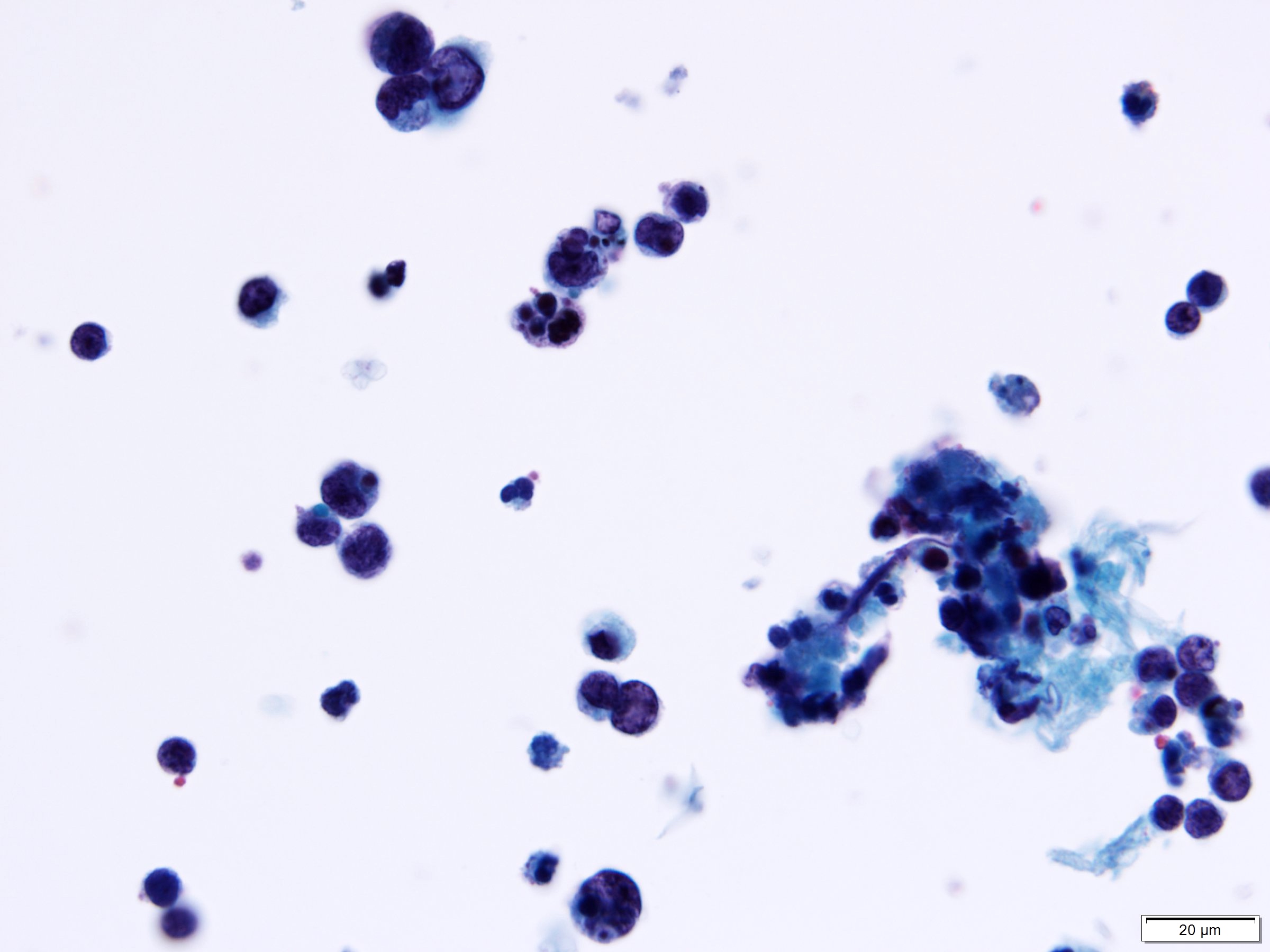
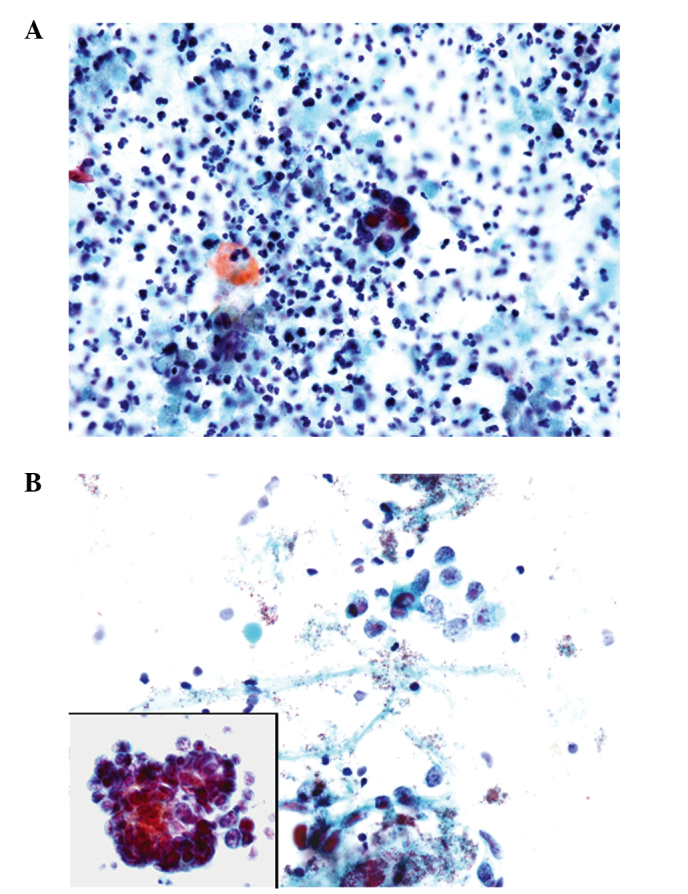
Cytology description
- Reciprocates histologic features, including small to medium cell size, scant cytoplasm, round to oval nuclei, salt and pepper chromatin and nuclear molding
- Cells are most commonly arranged into small clusters
- Variable abundance or paucity of tumor cells
- Inflammatory background primarily comprised of neutrophils
- Tumor diathesis may be present
- Limitation: additional histologic component, especially high grade urothelial carcinoma, may obfuscate the diagnosis
- Reference: Oncol Lett 2014;7:369
Cytology images
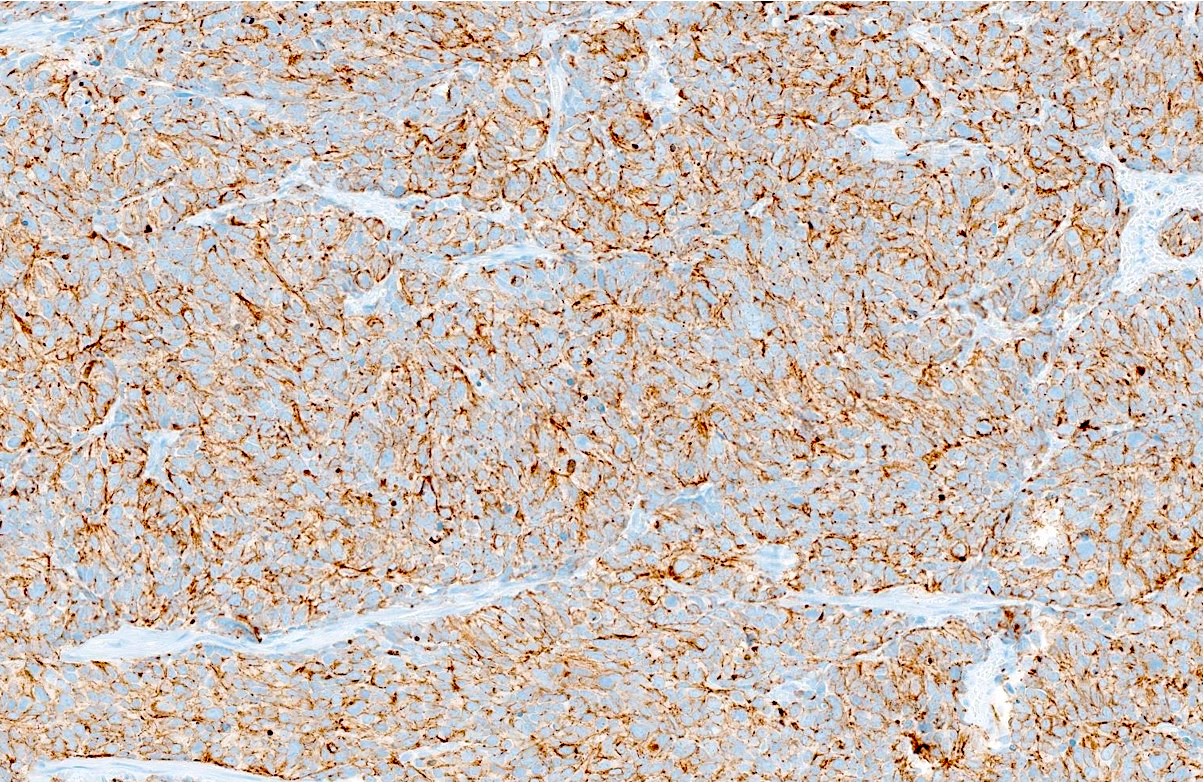
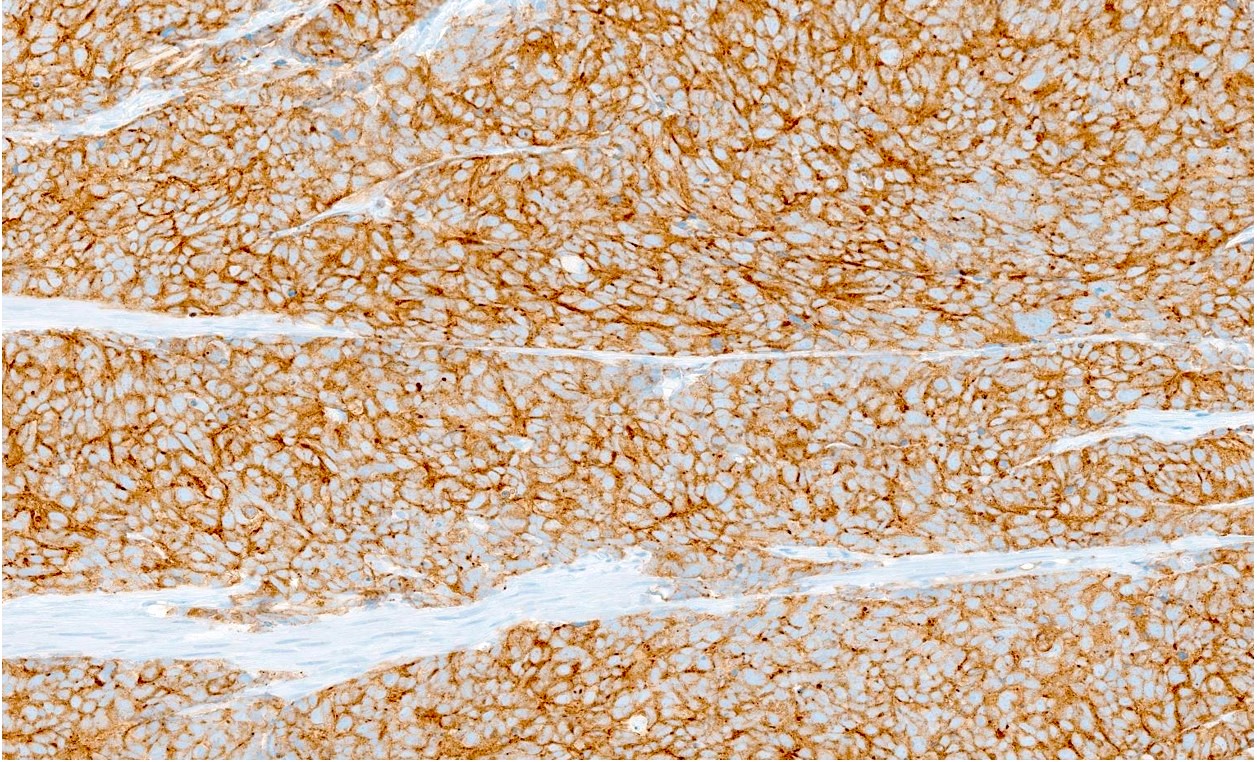
Positive stains
- Synaptophysin, chromogranin A, CD56, neuron specific enolase (NSE), INSM1
- NSE may be the most sensitive marker (World J Surg Oncol 2017;15:33)
- CAM 5.2 (100%), EMA (63%), CK AE1 / AE3: perinuclear dot-like staining is typical (World J Surg Oncol 2017;15:33)
- Ki67: 95 - 100%
Negative stains
- Aberrant loss of Rb (91.3%) (Hum Pathol 2018;79:57)
- Urothelial markers (CK20, GATA3, p63, uroplakin II) (Hum Pathol 2018;79:57)
- CD45, CD3, CD20
- Vimentin (Medicine (Baltimore) 2018;97:e11113)
- Extremely rare reports of positive staining as aforementioned (ISRN Urol 2011;2011:786505)
- Desmin, myogenin
- S100, HMB45
- CD99, WT1
- TTF1 (40% in bladder small cell neuroendocrine carcinoma) (Hum Pathol 2005;36:718)
- CK7 (47%)
- CK5/6 (36%) (Hum Pathol 2018;79:57)
Electron microscopy description
- Membrane bound, electron dense neurosecretory granules 150 - 250 mm in diameter (World J Surg Oncol 2017;15:33, Pol J Pathol 2014;65:15, ISRN Urol 2011;2011:786505)
- Intercellular junctions consistent with desmosomes may be present (Pol J Pathol 2014;65:15,ISRN Urol 2011;2011:786505)
Molecular / cytogenetics description
- RB1 biallelic inactivation, TP53 missense mutations and BRCA1 / BRCA2 mutations (Hum Pathol 2018;79:57, Clin Cancer Res 2018;24:1965)
- TERT promoter mutations highly specific for bladder small cell neuroendocrine carcinoma (Clin Cancer Res 2018;24:1965)
- BRD4, FSCN3, ISLR2, MAG, MAMDC2, PIK3CA and TAF1D mutations in bladder small cell neuroendocrine carcinoma (Clin Cancer Res 2018;24:1965, iScience 2020;23:101201)
- Copy number alterations are commonly present in bladder small cell neuroendocrine carcinoma; gain of chromosome 5p is most common (Clin Cancer Res 2018;24:1965)
- High tumor mutational burden (median: 10.7 mutations/MB) (Clin Cancer Res 2018;24:1965)
- Other features suggestive of common clonal origin and transdifferentiation / dedifferentiation of urothelial carcinoma and genitourinary small cell neuroendocrine carcinoma (Am J Pathol 2005;166:1533)
- Loss of heterozygosity of identical 1 - 5 loci in samples from both tumors
- Homogeneous nonrandom X chromosome inactivation patterns
- ERCC2 DNA repair gene loss of function mutation associated with potent neoadjuvant chemotherapy response among bladder SCCs (Clin Genitourin Cancer 2022;20:431)
Videos
Bladder small cell neuroendocrine carcinoma
Sample pathology report
- Bladder, lateral wall, transurethral resection:
- Small cell neuroendocrine carcinoma with invasion into muscularis propria (see comment)
- Comment: The tumor consists of cohesive, small blue cells with nuclear molding and extensive necrosis. The tumor cells are positive for CK AE1 / AE3 (moderate, dot-like), synaptophysin (strong, diffuse), chromogranin A (moderate, diffuse), INSM1 (strong, diffuse) and TTF1 (strong, diffuse). GATA3 is negative. Ki67 index is > 95%. Small cell neuroendocrine carcinoma of the urinary bladder is the primary consideration based on the anatomical site of the specimen and absence of evidence of pulmonary or other extrapulmonary site of origin. The findings above should be considered in the context of imaging and clinical history.
- Bladder, posterior wall, transurethral resection:
- Small cell neuroendocrine carcinoma mixed with high grade invasive urothelial carcinoma (see comment)
- Comment: Histologic evaluation shows small cell neuroendocrine carcinoma and adjacent high grade urothelial carcinoma. The small cell neuroendocrine carcinoma component is positive for synaptophysin, INSM1, CD56 and chromogranin while negative for GATA3. In contrast, the urothelial carcinoma component is positive for GATA3 while negative for the aforementioned neuroendocrine markers.
Differential diagnosis
- Large cell neuroendocrine carcinoma (Clujul Med 2017;90:13)
- Metastatic pulmonary small cell neuroendocrine carcinoma:
- Both entities may be positive for TTF1
- Lacks TERT promoter mutation
- Mixed histomorphology is rare
- Metastatic prostatic small cell neuroendocrine carcinoma (Hum Pathol 2018;79:57):
- Mixed histomorphology similar to small cell neuroendocrine carcinoma of urothelial origin may be present
- Often arises in a setting of concomitant prostatic adenocarcinoma
- PSA may be elevated
- TMPRSS2::ERG fusion may be present
- Lacks TERT promoter mutation
- Well differentiated neuroendocrine tumor (Hum Pathol 2018;79:57):
- In upper genitourinary tract, originates most commonly from kidneys
- Histologically characterized by well defined cords, trabeculae and ribbons containing columnar or cuboidal tumor cells
- Granular eosinophilic (oncocytic) cytoplasm is common
- Substantially lower mitotic count and Ki67 index, especially if grade < 3
- Necrosis is absent or less prominent
- Also positive for neuroendocrine markers
- Lymphoepithelioma-like carcinoma (Hum Pathol 2018;79:57):
- Derivation of urothelial carcinoma characterized by large pleomorphic nuclei and prominent nucleoli, often with abundant mitoses
- Cells are arranged into syncytial sheets with infiltration of epithelium by mixed inflammatory cells
- Frequently mixed with other malignant cell populations
- Positive for GATA3 and p63
- Negative for neuroendocrine markers
- Neuroblastoma (Pol J Pathol 2014;65:15):
- Most commonly located in adrenal gland
- Most common in kids
- Serum and urine catecholamine assays may be positive
- Differentiated type is characterized by Homer-Wright pseudorosettes containing central eosinophilic neurofilaments
- Undifferentiated type is characterized by small to medium sized cells with scant cytoplasm, salt and pepper chromatin and prominent nucleoli may be present
- Prominent nucleoli may be present
- ALK1 may be positive in up to 90% of cases
- May harbor N-MYC amplification
- Ewing sarcoma (Pol J Pathol 2014;65:15):
- Comprised of small round cells with sheet-like growth
- Dense fibrous tissue often surrounds islands of tumor cells
- Positive for CD99, FLI1 and NKX2.2
- Variably positive for neuroendocrine markers (NSE is most common [50%])
- Associated with pathognomonic EWSR1::FLI fusion
- Elevated erythrocyte sedimentation rate (ESR) may be present
- Pheochromocytoma / paraganglioma (Anticancer Res 2017;37:4529):
- Classic clinical triad of episodic headaches, sweating and tachycardia in addition to palpitations, anxiety, paroxysmal hypertension and syncope
- Serum and urine assays for catecholamine metabolites (e.g., VMA) may be positive
- Comprised of nests (zellballen) or solid sheets of large polygonal cells with granular, red-purple cytoplasm
- Chief cells are positive for neuroendocrine markers
- Sustentacular cells are positive for S100 and often GATA3
- Negative for cytokeratin stains
- VHL, RET, NF1 and SDHB mutations may be present
- Nephroblastoma / Wilms tumor (Pol J Pathol 2014;65:15):
- Lymphoma (Pol J Pathol 2014;65:15, Case Rep Hematol 2015;2015:934374):
- Desmoplastic small round cell tumor (Pol J Pathol 2014;65:15):
- Comprised of nests and trabeculae of small uniform round cells abounded by diffuse desmoplastic stroma
- Peripheral palisading may be observed
- Solid pattern lacks desmoplastic stroma and may appear nearly identical to small cell carcinoma
- Most common in kids and young adults
- Positive for WT1, CD99, desmin and vimentin
- EWSR1::WT1 fusion present
- Melanoma (Pol J Pathol 2014;65:15):
- Embryonal rhabdomyosarcoma (Pol J Pathol 2014;65:15):
- Most common in kids and infants
- Nuclear molding may be prominent
- Mixed hypercellular and hypocellular areas are usually present
- Typically harbors eosinophilic (rhabdoid) or clear cytoplasm
- May harbor striations consistent with rhabdoid differentiation
- Positive for desmin, myogenin, MyoD1 and vimentin
- Poorly differentiated synovial sarcoma (Pol J Pathol 2014;65:15, Pathol Oncol Res 2001;7:63):
Additional references
Board review style question #1
A 68 year old man with a 48 pack year smoking history presents with gross hematuria of 1 month's duration. Urinalysis reveals 9 red blood cells (RBCs) while a complete blood count (CBC) displays hemoglobin of 9.8 g/dL. An abdominopelvic ultrasound reveals a thickened lateral vesical wall while computerized tomography (CT) elucidates a 4.6 cm heterogeneous mass in the greatest dimension with possible extravesical extension into locoregional lymph nodes. Cystoscopy reveals a nodular mass with overlying necrosis. Due to suspicion of a bladder malignancy, a radical cystectomy is performed. An H&E stained image from this specimen is shown above. Immunohistochemistry is pertinent for positive staining with synaptophysin, CD56, NSE, INSM1 and CK AE1 / AE3, the latter of which exhibits a perinuclear dot-like pattern. Lesional cells are negative for GATA3, p63, CD99, WT1, HMB45, CD45, vimentin, desmin and NKX2.2. The Ki67 index exceeds 95%. What is the most likely diagnosis?
- Desmoplastic small round cell tumor
- Ewing sarcoma
- Large cell neuroendocrine carcinoma
- Pheochromocytoma
- Small cell neuroendocrine carcinoma
Board review style answer #1
E. Small cell neuroendocrine carcinoma. The high N:C ratio, nuclear molding, abundant mitoses and karyorrhectic debris on histology in tandem with the neuroendocrine immunohistochemical profile and history of smoking is most consistent with small cell neuroendocrine carcinoma. Answer A is incorrect because although the solid form of desmoplastic small round cell tumor may mimic the histomorphology of small cell carcinoma, the tumor cells in question are negative for CD99, desmin, vimentin and WT1. Answer B is incorrect because the tumor cells are negative for CD99 and NKX2.2. Answer C is incorrect because the tumor in question displays a high N:C ratio, stippled chromatin and inconspicuous nucleoli rather than the lower N:C ratio, vesicular chromatin and prominent nucleoli associated with large cell neuroendocrine carcinoma. Answer D is incorrect because the tumor cells do not exhibit granular reddish purple cytoplasm or GATA3 staining and are positive for cytokeratins; moreover, the patient does not display the characteristic symptoms of excess catecholamine secretion.
Comment Here
Reference: Small cell neuroendocrine carcinoma
Comment Here
Reference: Small cell neuroendocrine carcinoma
Board review style question #2
Which of the following is true of primary upper genitourinary tract small cell neuroendocrine carcinomas?
- History of smoking tobacco use is common
- Surgery with neoadjuvant or adjuvant cyclophosphamide-based chemotherapy is the mainstay of treatment
- TERT promoter mutations are specific for small cell neuroendocrine carcinomas of renal origin
- The nephroureter is the most common site of origin within this region
- TMPRSS2::ERG fusions are prevalent in small cell neuroendocrine carcinomas of bladder origin
Board review style answer #2
A. History of smoking tobacco use is common. As with other carcinomas from this region, smoking tobacco use is a common historical factor in patients with upper genitourinary tract small cell neuroendocrine carcinomas. Answer B is incorrect because platinum based agents, especially cisplatin, are used most often in tandem with surgical removal of tumors, not cyclophosphamide based regimens. Answer C is incorrect because TERT promoter mutations have only been reported in small cell neuroendocrine carcinomas of bladder origin. Answer D is incorrect as the majority of reported upper genitourinary tract small cell neuroendocrine carcinomas arise from the bladder rather than the kidney and ureters. Answer E is incorrect because TMPRSS2::ERG fusions have only been reported in small cell neuroendocrine carcinomas of prostatic origin.
Comment Here
Reference: Small cell neuroendocrine carcinoma
Comment Here
Reference: Small cell neuroendocrine carcinoma