Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Hereditary renal cell tumors | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Treatment | Handling and staging guide | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | IHC panels | Electron microscopy description | Molecular / cytogenetics description | Videos | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Popescu MC, Tretiakova M. Renal cell carcinoma overview. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/kidneytumormalignantrcc.html. Accessed March 28th, 2025.
Definition / general
- Adult renal cell carcinoma (RCC) is a malignant epithelial neoplasm arising from renal tubular epithelium
Essential features
- Sixteenth most common cause of death from cancer worldwide
- Clear cell RCC represents ~65 - 70% of adult renal carcinomas
- Second and third most common RCC subtypes are papillary (10 - 15%) and chromophobe RCC (5%)
- See subtypes: Kidney tumor
Terminology
- Historic synonyms (obsolete): nephrocellular carcinoma, Grawitz tumor, hypernephroma (due to perceived origin from adrenal gland)
ICD coding
- ICD-O: see Kidney tumor subtypes
- ICD-10: C64 - malignant neoplasm of kidney, except renal pelvis
- ICD-11: 2C90 - malignant neoplasms of kidney, except renal pelvis
Epidemiology
- Incidence: worldwide as of 2020, it is the seventh most common cancer in men (271,249 new cases) and the tenth most common in women (160,039 new cases)
- M:F = ~2:1; some subtypes are more common in women (e.g., eosinophilic solid and cystic RCC)
- Patients are usually > 50 years old; peak incidence between the ages of 60 and 70 years
- ~70% of new cases occur in countries with high socioeconomic development
- Estimated lifetime prevalence of 2.3% for men and 1.3% for women in the U.S. (JAMA 2024;332:1001)
- RCC incidence varies widely from region to region with the highest rates (as of 2018) in Belarus (16.8 cases per 100.000 population), followed by other Central and Eastern European countries
- High incidence rates of RCC are also found in the U.S., with an estimated ~81,800 new cases in 2023 and over 14,000 deaths from RCC each year (CA Cancer J Clin 2017;67:7, JAMA 2024;332:1001)
Sites
- Kidney (based in cortex or medulla, depending on subtype)
- RCC arising in extrarenal locations with no identifiable renal primary is a rare phenomenon that has been described (Histopathology 2022;81:635)
Pathophysiology
- Various molecular pathways, depending on Kidney tumor subtype
Etiology
- Risk factors: obesity, smoking, hypertension, acquired cystic kidney disease due to end stage renal disease, occupational exposure to trichloroethylene, heavy metals and industrial solvents, prior chemotherapy (JAMA 2024;332:1001)
- Genetic susceptibility is estimated to account for 2 - 4%
Hereditary renal cell tumors
- Von Hippel-Lindau (VHL) syndrome
- Autosomal dominant, due to germline mutation of VHL tumor suppressor gene on chromosome 3p25
- Associated renal lesions: clear cell renal cell carcinoma, benign or atypical renal cysts and numerous microscopic nodules of clear cells (Semin Diagn Pathol 2024;41:20)
- RCC in VHL syndrome can be multifocal or bilateral in 50% (eMedicine: Von Hippel-Lindau Syndrome Imaging [Accessed 7 November 2024])
- Clear cell RCC in VHL syndrome may show branching tubulopapillary growth and apical polarization of nuclei resembling clear cell papillary renal cell tumor but shows characteristic molecular and immunohistochemical profile of clear cell RCC
- Other associated lesions: hemangioblastomas of cerebellum and retina, pancreatic or liver cysts, clear cell tumors of other sites, papillary cystadenoma of epididymis, pheochromocytoma
- Hereditary papillary renal cell carcinoma (PRCC) syndrome
- Autosomal dominant, due to germline activating mutations of MET proto-oncogene on chromosome 7q31 with nearly 100% penetrance
- Patients typically have a positive family history of RCC
- Multifocal and bilateral papillary renal cell carcinoma (formerly type I)
- Papillary adenomas may also be numerous
- No association with other, extrarenal lesions
- Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome
- Autosomal dominant, due to pathogenic germline mutations of FH gene on chromosome 1q42 encoding fumarate hydratase (enzyme of Krebs cycle)
- Usually multiple cutaneous or uterine leiomyomas at a younger age
- Associated renal tumors are classified as a separate entity in the current WHO 2022, termed FH deficient RCC, with distinctive morphology showing a combination of architectural patterns with at least focal papillary growth and large nuclei with a very prominent, inclusion-like eosinophilic nucleoli surrounded by perinucleolar haloes
- Rare, reported cases of testicular Leydig cell tumors (Med Rep 2024;5:100057)
- Genetic testing is always warranted for these aggressive tumors, as purely somatic biallelic FH mutations may occur (rarely in the setting of FH deficient RCC but commonly in older patients with solitary FH deficient uterine leiomyomas)
- Birt-Hogg-Dubé (BHD) syndrome
- Autosomal dominant with incomplete penetrance
- Due to germline mutations in FLCN (BHD) gene on chromosome 17p12, which codes for folliculin protein
- Skin lesions: fibrofolliculomas, trichodiscomas, acrochordons
- Lung: cysts, spontaneous pneumothorax
- Renal tumors are multiple and bilateral in 15 - 30% of cases (WHO 2022): most commonly hybrid oncocytic / chromophobe tumor, followed by chromophobe RCC and oncocytoma, as well as renal oncocytosis (Semin Diagn Pathol 2024;41:119)
- Clear cell, papillary and RCC, NOS can also be seen
- Renal tumors of BHD syndrome are considered low risk tumors suitable for conservative therapy; however, patients develop chronic renal insufficiency due to tumor multifocality
- Tuberous sclerosis complex (TSC)
- Autosomal dominant syndrome with variable penetrance, due to mutations in TSC1 on chromosome 9q or TSC2 on chromosome 16p (GeneReviews: Tuberous Sclerosis Complex [Accessed 13 January 2025])
- Multisystem disorder with numerous manifestations, usually affecting the CNS
- Renal lesions: multiple, bilateral angiomyolipomas or epithelioid angiomyolipoma, renal cysts and variable, typically indolent renal tumors, including eosinophilic solid and cystic RCC, RCC with fibromyomatous stroma, hybrid oncocytic / chromophobe tumors and RCC NOS (Pediatr Nephrol 2021;36:1427, J Kidney Cancer VHL 2020;7:5)
- Succinate dehydrogenase (SDH) deficient tumor syndromes
- Group of tumor predisposition syndromes due to germline inactivating mutations of SDH genes (SDHA, SDHB, SDHC, SDHD, SDHAF2)
- Endocrine lesions: pheochromocytoma / paraganglioma, pituitary neuroendocrine tumor (PitNET, formerly pituitary adenoma)
- GI tract lesions: SDH deficient gastrointestinal stromal tumor (GIST)
- Pulmonary lesions: chondroma
- Renal lesions (~14% penetrance): RCC subtype classified as a distinct entity in the current WHO 2022, termed SDH deficient RCC, with characteristic morphology showing ground glass cytoplasmic vacuoles with pale eosinophilic, flocculent material (Semin Diagn Pathol 2024;41:32)
- BAP1 tumor predisposition syndrome
- Autosomal dominant, due to heterozygous germline mutations in BAP1 tumor suppressor gene encoding BRCA associated protein 1
- Associated with uveal and cutaneous melanocytic tumors / atypical Spitz tumors, cutaneous melanoma, basal cell carcinoma, malignant mesotheliomas, meningiomas and other tumors
- Renal lesions: usually clear cell RCC with aggressive phenotype; isolated reports of non-clear cell tumors; may be multifocal (JAMA Netw Open 2021;4:e2132615)
- Other familial cancer syndromes associated with renal cell tumors
- Hyperparathyroidism jaw tumor syndrome
- Cowden or PTEN hamartoma syndrome (Am J Surg Pathol 2023;47:1001)
- Familial papillary thyroid carcinoma syndrome
- Constitutional balanced chromosome 3 translocation
- Lynch syndrome
Diagrams / tables
Clinical features
- Most common manifestation is gross or microscopic hematuria
- Classic clinical triad of costovertebral pain, palpable mass and hematuria is present in ~10% of patients
- Incidental RCC detection in 37 - 61% of cases due to increased use of imaging; historically, large (10 cm) or already metastatic at diagnosis (JAMA 2024;332:1001)
- Great mimicker due to associated paraneoplastic syndromes (20% of patients), including Cushing syndrome, gynecomastia, hypercalcemia, hypertension, leukemoid reaction, polycythemia, Stauffer syndrome (hepatomegaly with hepatic dysfunction), systemic amyloidosis, polyneuromyopathy
Diagnosis
- Diagnosis is usually established based on clinical findings and contrast enhanced computed tomography (CT) imaging
- Histologic confirmation on renal mass biopsy is indicated in the following situations (AUA: Renal Mass and Localized Renal Cancer Evaluation Management and Follow Up [Accessed 13 January 2025])
- Confirmation of renal primary versus metastasis when there is a known extrarenal primary
- Unresectable solid renal mass before initiation of systemic therapy
- In comorbid patients noneligible for surgery to further guide treatment and prognosis
- Clinically suspected mass forming infectious process that may be managed with antibiotics, such as pyelonephritis or abscess
- Before ablative procedures to ensure histologic diagnosis
- For tumors involving renal sinus to rule out urothelial carcinoma or lymphoma (JAMA 2024;332:1001)
- For reporting elements in the synoptic pathology report, see Features to report
Laboratory
- Kidney function tests may show elevated serum creatinine and decreased glomerular filtration rates (GFR) as a reflection of compromised renal function
- Anemia and hypercalcemia may be seen
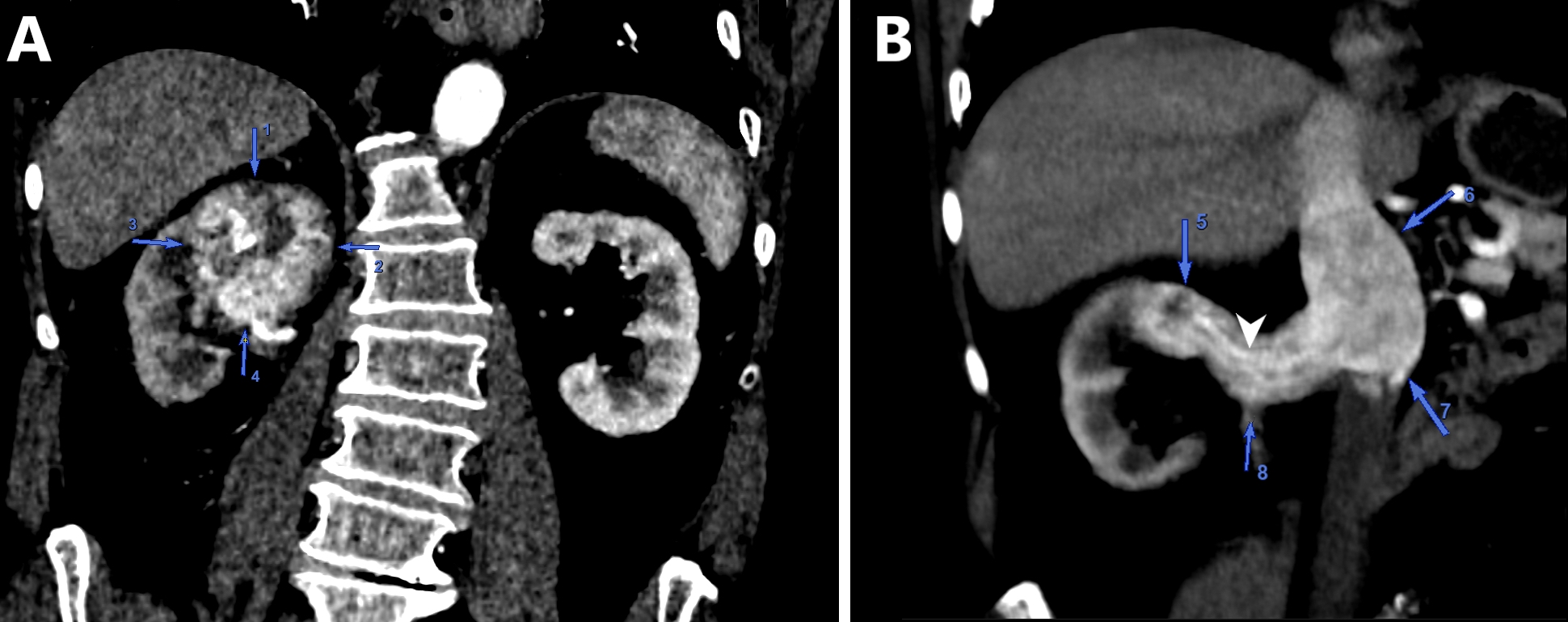
Radiology description
- Ultrasound
- Most frequently used for initial diagnosis of a renal mass and to assess the urinary tract
- Lower sensitivity and specificity for assessing malignancy and for staging than CT and magnetic resonance imaging (MRI)
- RCC may appear solid or cystic
- Can be hyper, iso or hypoechoic compared to noninvolved renal parenchyma
- Visualizing the tumor pseudocapsule as a hypoechoic halo is fairly specific but has low sensitivity (20%) (Radiopaedia: Renal Cell Carcinoma [Accessed 8 November 2024])
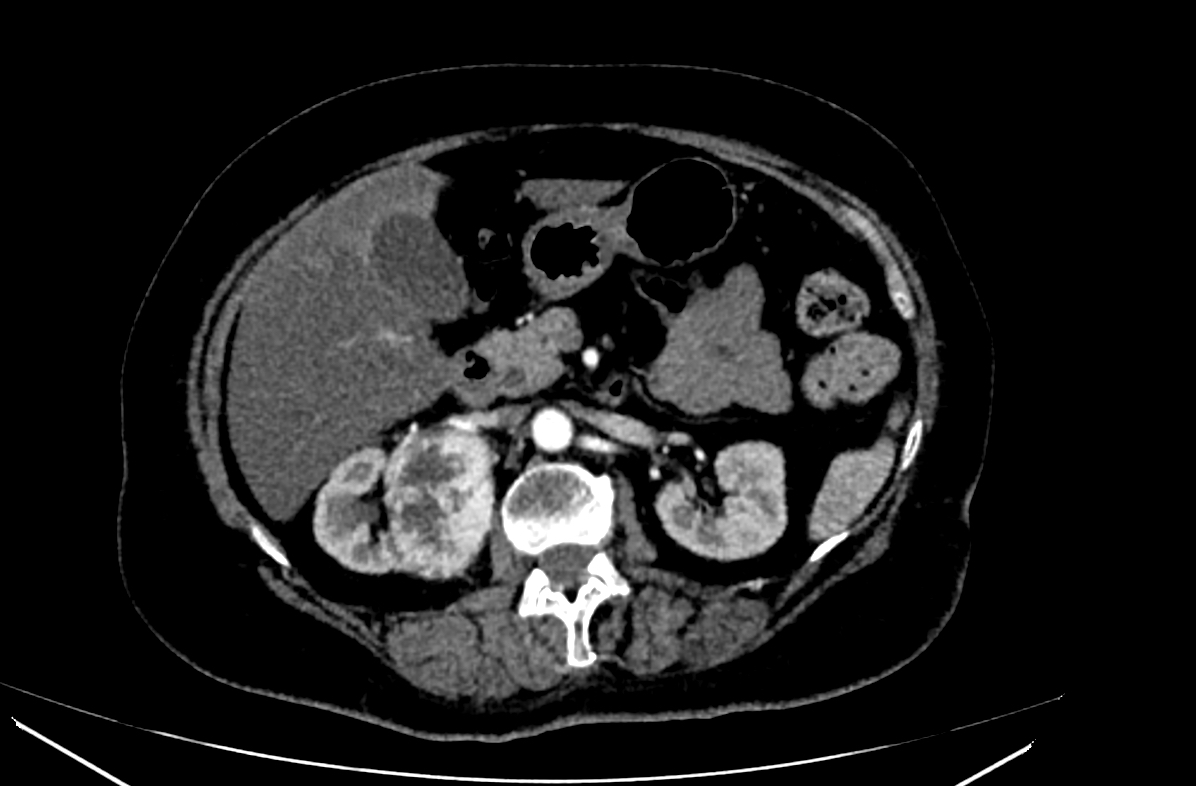
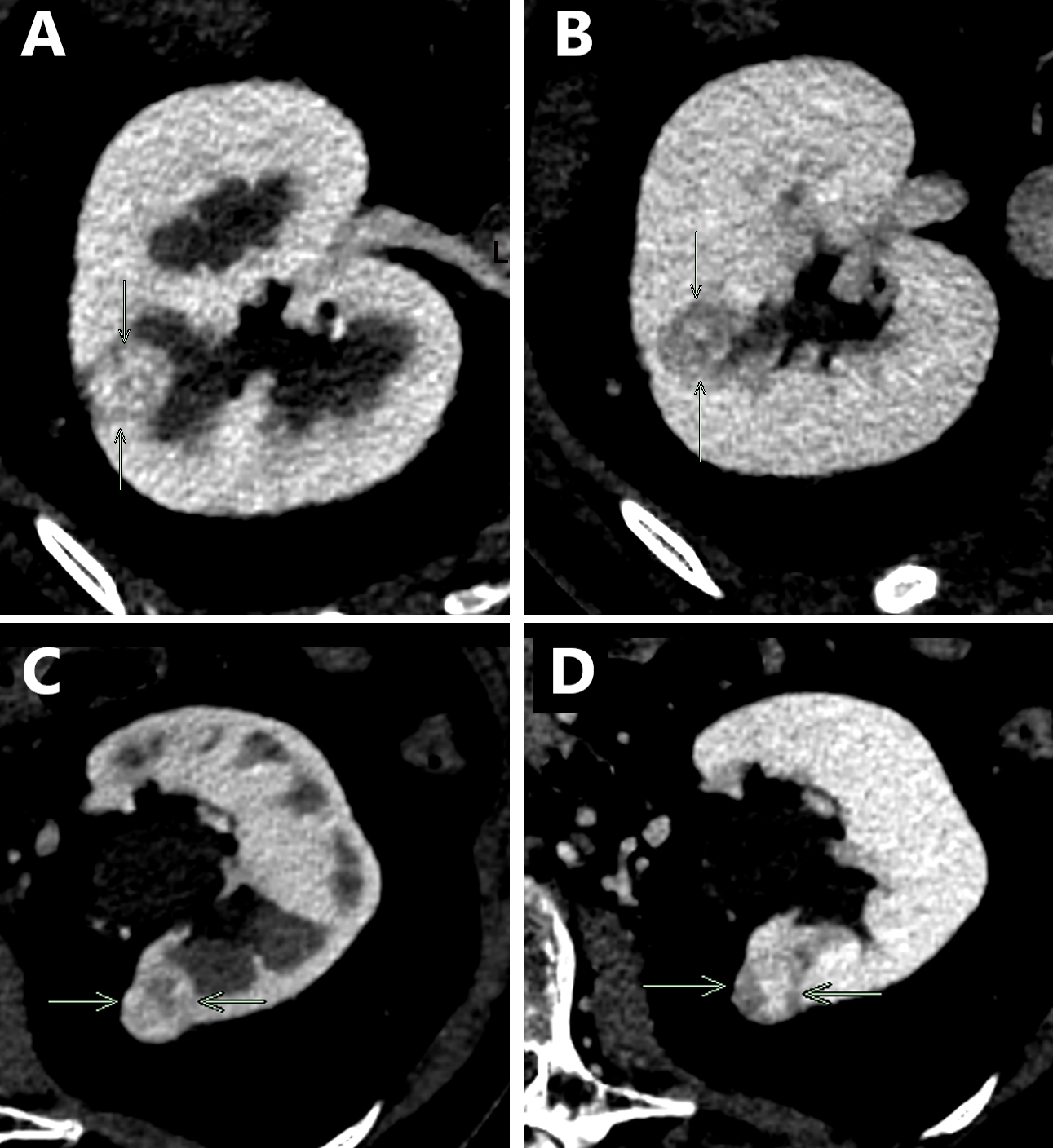
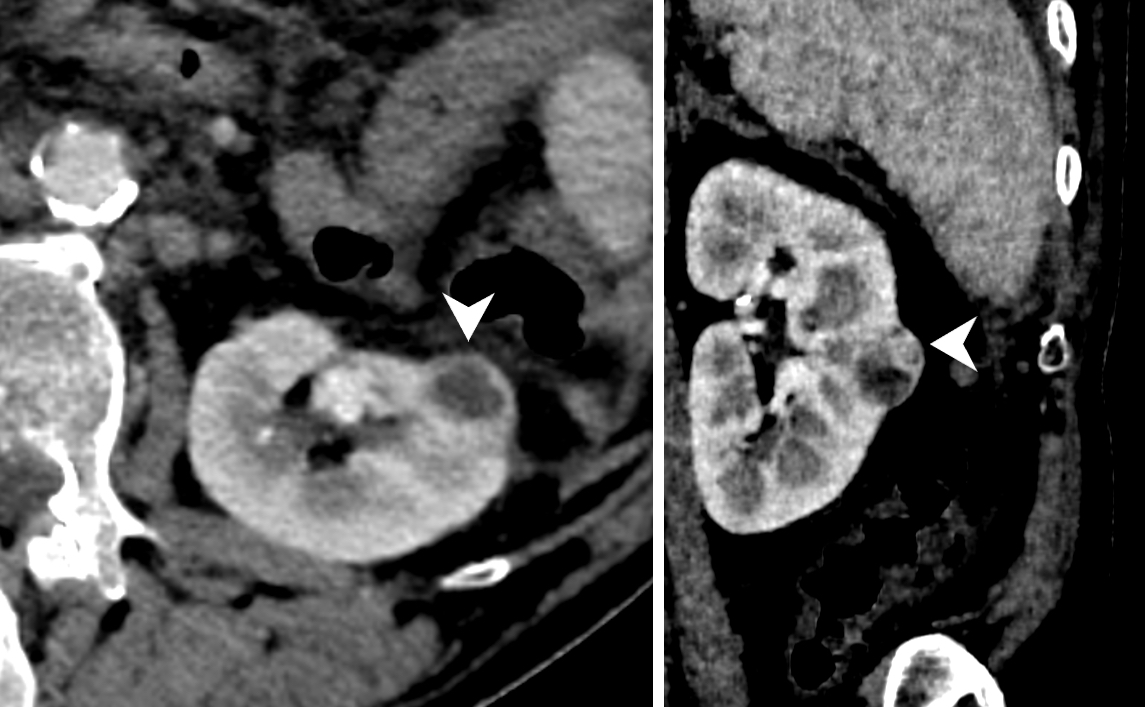
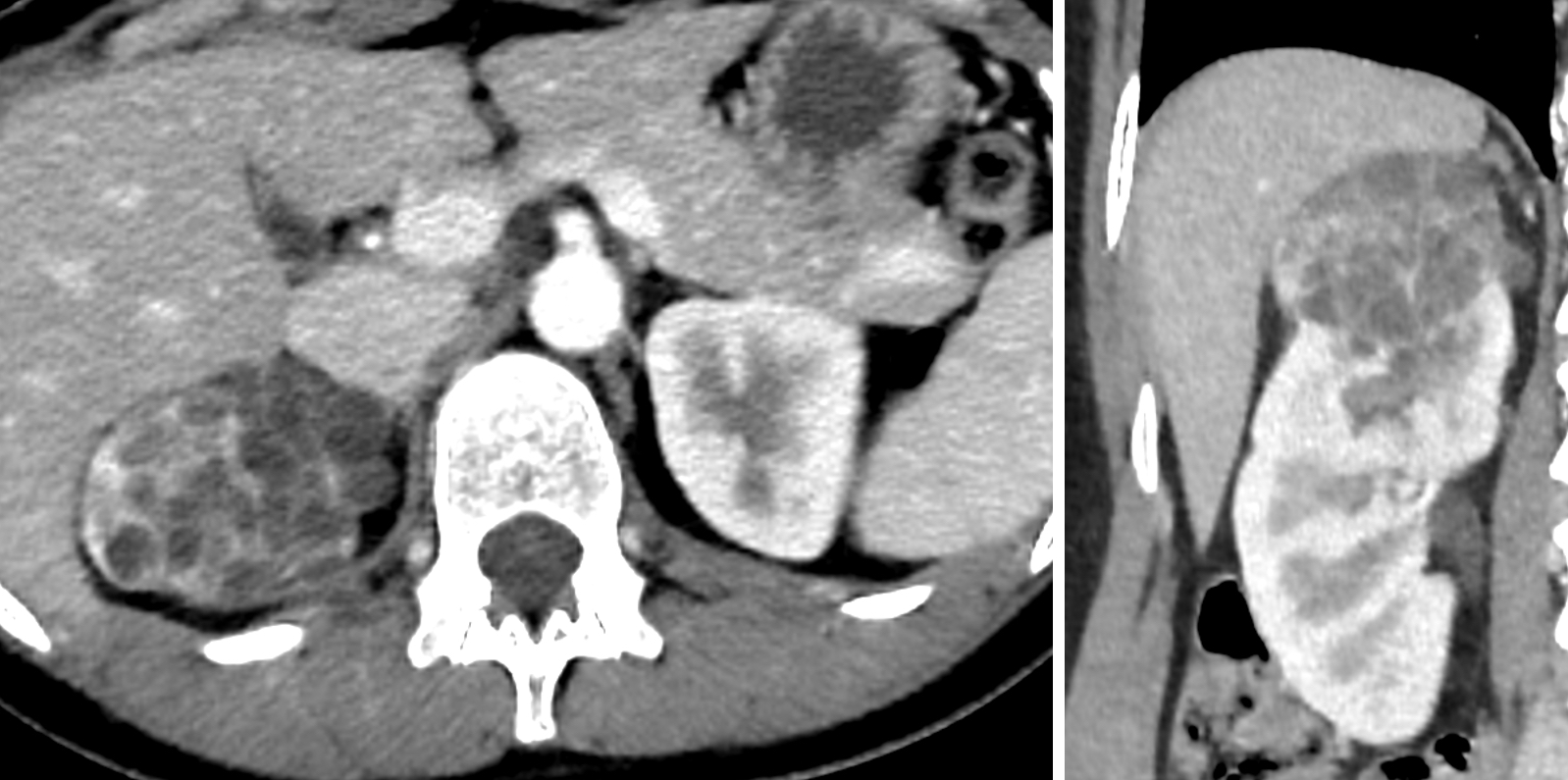
- CT
- Modality of choice for diagnosis and staging of RCC as well as for posttreatment follow up
- Dedicated renal CT protocols are used
- Can differentiate clear cell RCC from non-RCC subtypes with a specificity of up to 100% (Cureus 2021;13:e13231)
- RCC may appear solid or cystic, with irregular enhancement due to the presence of necrosis and can show peripheral or central calcifications
- Clear cell RCC typically demonstrates hypervascularity and enhancement similar to normal renal cortex as opposed to non-clear cell RCC subtypes (Cureus 2021;13:e13231)
- Bosniak classification (5 categories): helps predict risk of malignancy and guides clinical management for cystic renal masses based on contrast enhanced CT imaging (Cureus 2021;13:e13231)
- MRI
- Similar sensitivity and specificity as CT for evaluating renal masses
- Clear cell RCC is typically iso or hypointense on precontrast T1 weighted imaging and heterogeneously hyperintense on T2 weighted imaging
- Clear cell RCC is hypervascular and shows prominent enhancement
Radiology images
Prognostic factors
- Grading
- WHO / ISUP grading system is currently validated only for clear cell and papillary RCC
- Grade 1: nucleoli inconspicuous or absent at 400x (objective magnification 40x)
- Grade 2: nucleoli prominent at 400x
- Grade 3: nucleoli prominent at 100x magnification
- Grade 4: extreme nuclear pleomorphism, multinucleated giant cells, sarcomatoid or rhabdoid change
- Sarcomatoid transformation: poor prognostic indicator in other RCC subtypes as well (e.g., chromophobe RCC, SDH deficient RCC); presentation with advanced stage disease and metastases in 45 - 77% of cases (Am J Surg Pathol 2024;48:e65)
- WHO / ISUP grading system is currently validated only for clear cell and papillary RCC
- Staging
- Histologic subtype: see Kidney tumor
- Necrosis: tumor type necrosis (also known as granular necrosis) has been shown to be an independently significant predictor of a less favorable outcome in clear cell RCC (Pathology 2020;52:507, Histopathology 2019;74:284)
- Microscopic coagulative tumor necrosis is also associated with worse outcomes in clear cell and chromophobe RCC (Eur Urol 2021;79:225, Am J Surg Pathol 2013;37:1490)
- Lymphovascular invasion (LVI)
- Conflicting data; according to ISUP 2013 consensus, there is still insufficient evidence to support prognostic significance (Am J Surg Pathol 2013;37:1490)
- Newer studies suggest the detrimental impact of LVI on overall survival in patients with surgically treated renal cell carcinoma (Urol Oncol 2023;41:435.e1)
- 5 year survival: 70% (all histologic types and stages), varies from 94% for stage I versus ~80% for stage II versus ~60% for stage III versus ~5 - 10% for stage IV
Treatment
- Resection (partial or radical nephrectomy) for localized disease
- Active surveillance may be an option for comorbid patients with low stage, histologically confirmed low risk tumors
- Generally limited use for chemotherapy in RCC management but may be offered to patients with collecting duct or SMARCB1 deficient RCC (formerly medullary carcinoma) (Uroweb: Renal Cell Carcinoma [Accessed 8 November 2024])
- Especially in the metastatic setting, it is important to differentiate between clear cell RCC and non-clear cell RCC subtypes, as several HIF1α pathway targeted therapies (e.g., tyrosine kinase inhibitors, anti-VEGF agents) and immune checkpoint inhibitors have been approved for clear cell RCC management in both the U.S. and Europe
- HLRCC associated renal tumors frequently require aggressive treatment with specific chemotherapeutic considerations and follow up regimens even if small and solitary (J Natl Compr Canc Netw 2019;17:1278)
- Dual immune checkpoint inhibitors combinations (anti-CTLA4 and anti-PDL1) are used in the U.S. as first line treatment for patients with intermediate or poor risk metastatic clear cell RCC (JAMA 2024;332:1001)
Handling and staging guide
- Staging according to UICC / AJCC 8th edition
- Risk of downstaging in RCC is greater than in other cancers
- Refer to Grossing topic for sections to take and grossing tips
- Greatest dimension of tumor should be measured after serial sectioning, including tumor extending into extracapsular soft tissue
- Do not include tumor extending into renal vein / inferior vena cava in the measurement
- Measurement of predominantly cystic lesions with focal solid tumor nodules is controversial; some survey data suggests most urological pathologists would measure the entire cyst if cyst lining and solid nodule are composed of similar cells (Am J Surg Pathol 2018;42:1253)
- Significantly greater likelihood for tumors > 7 cm to show invasion into sinus or perirenal fat
- Renal sinus invasion (pT3a)
- If tumor is in direct contact with the sinus fat or the loose connective tissue of the sinus, clearly beyond the renal parenchyma
- If tumor involves any endothelium lined spaces within the renal sinus
- It is recommended to extensively sample tumor sinus interface (> 3 - 5 blocks) if tumor size > 5 cm
- Perinephric fat invasion (pT3a)
- Tumor directly touching fat
- Protruding nodules or irregular tongues / bands of tumor cells into perinephric soft tissue, with or without desmoplasia
- Beware of tangential sectioning of tumor capsule or invasion into but not beyond the capsule (can obtain deeper levels or additional sections)
- Renal vein / segmental branches invasion (pT3a)
- Requirement of gross identification of vein invasion was removed (Amin: AJCC Cancer Staging Manual, 8th Edition, 2016)
- Requirement that venous segmental branches have muscular walls were removed (Amin: AJCC Cancer Staging Manual, 8th Edition, 2016)
- Presence of tumor multinodularity within renal sinus should always raise suspicion for venous invasion, even if nodules are well circumscribed with apparent pseudocapsule
- Invasion of pelvicalyceal system mucosa recently introduced into pT classification (pT3a) (Amin: AJCC Cancer Staging Manual, 8th Edition, 2016)
- Renal vein margin: margin is positive only when tumor is grossly adherent to vessel wall and is confirmed microscopically at the actual margin; if submitted separately as caval thrombus, take 2 or more sections to look for tumor adherent to caval wall
- Microscopic infiltration of vessel wall at renal vein margin is associated with a worse prognosis
- Uninvolved renal parenchyma: should be sampled, distant from tumor for underlying renal disease
- Hilar lymph nodes: should be sought but are found in < 10% of radical nephrectomy specimens; N1 disease is grossly visible in 80% of cases
- Reporting of multifocal tumors
- Provide size range from largest to smallest
- If same histologic subtype, classify as pT(m)
- If different histologic subtypes, stage each tumor separately
- References: Pathology 2021;53:120, Histopathology 2019;74:18
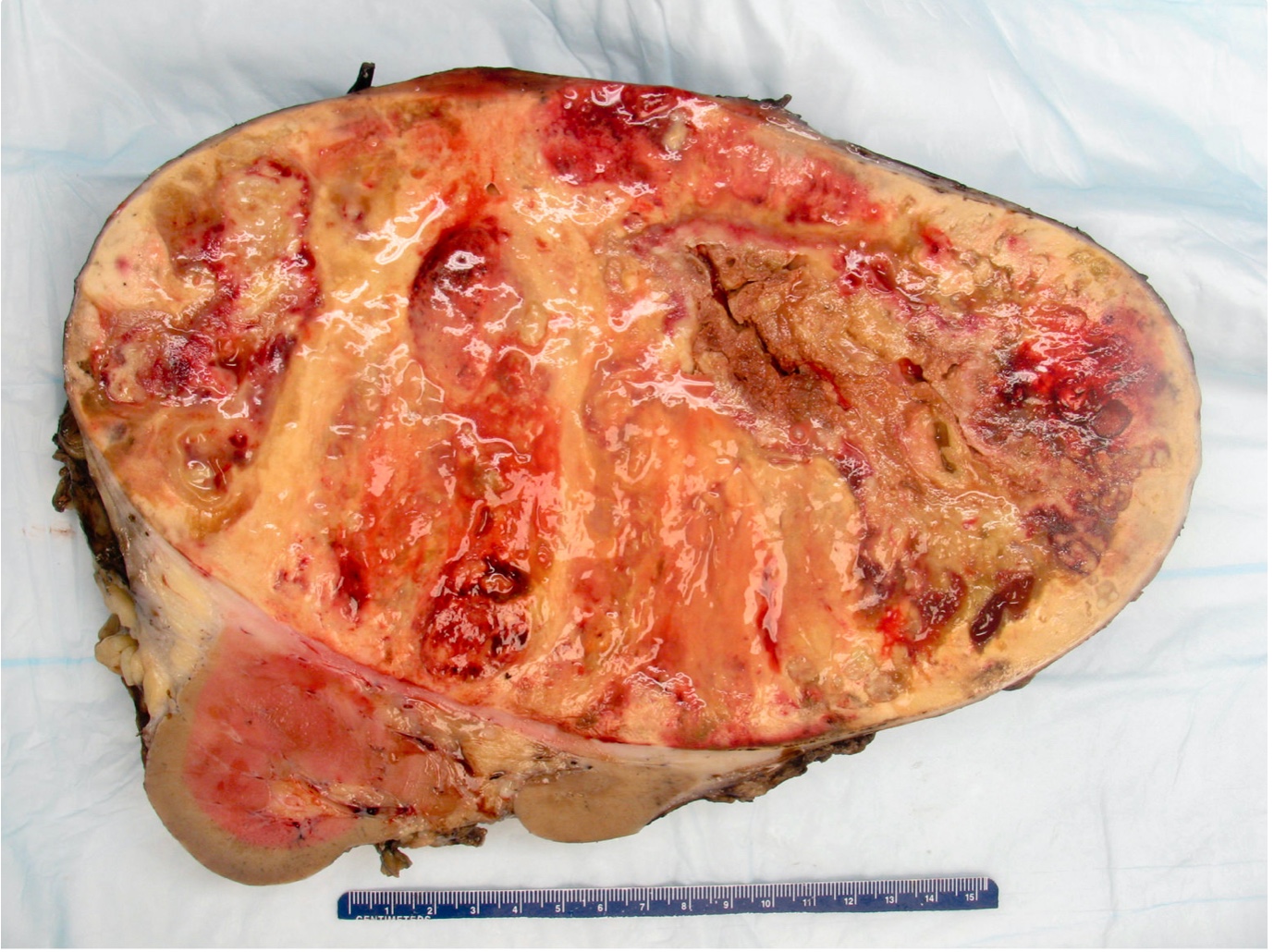
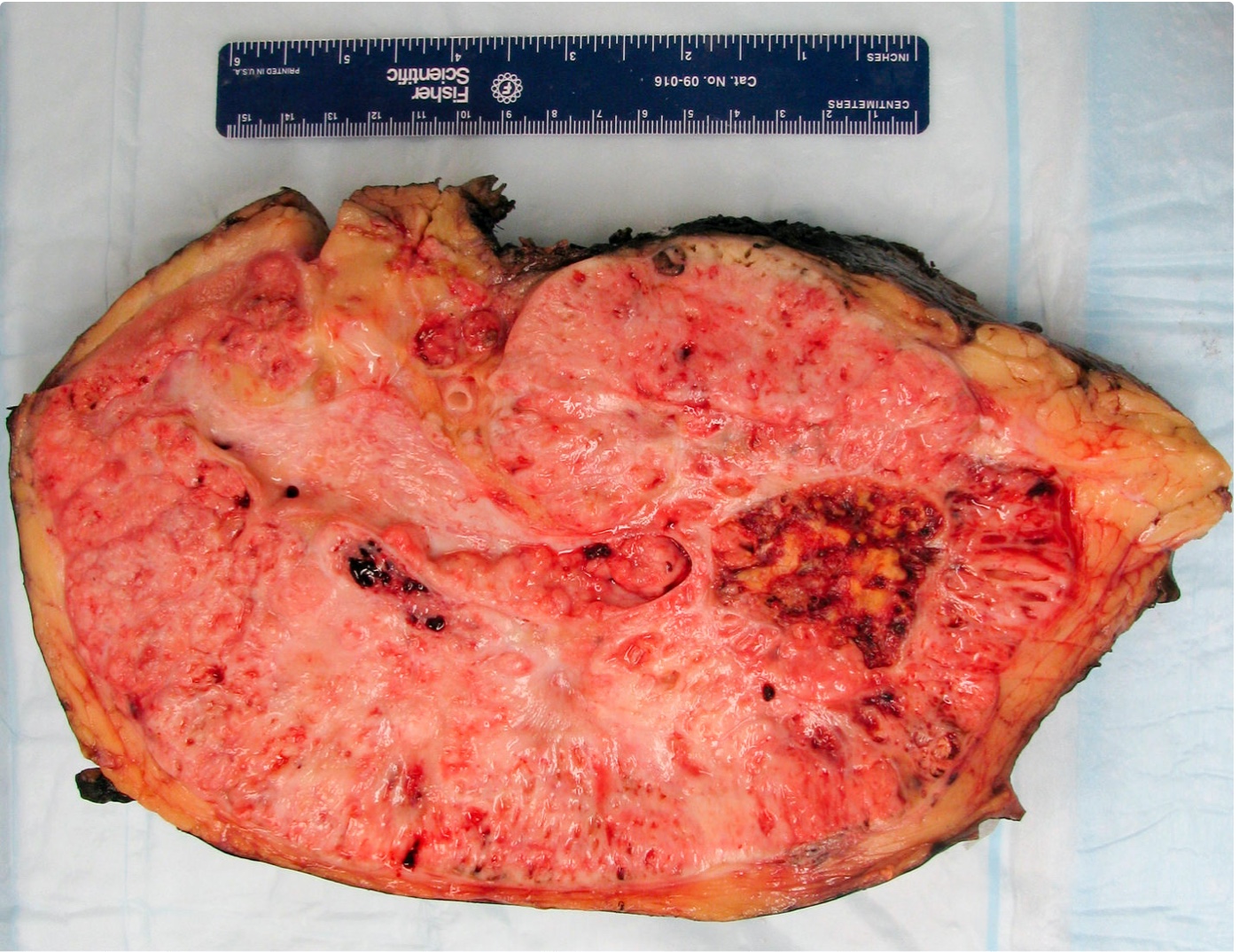
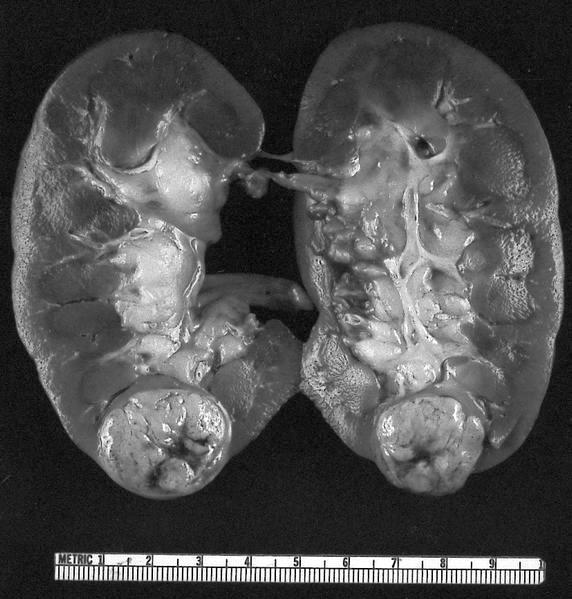
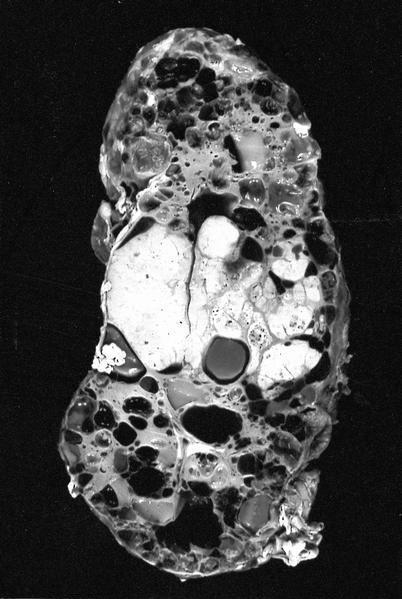
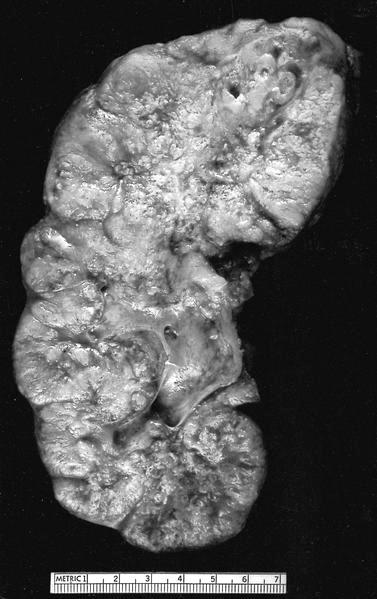
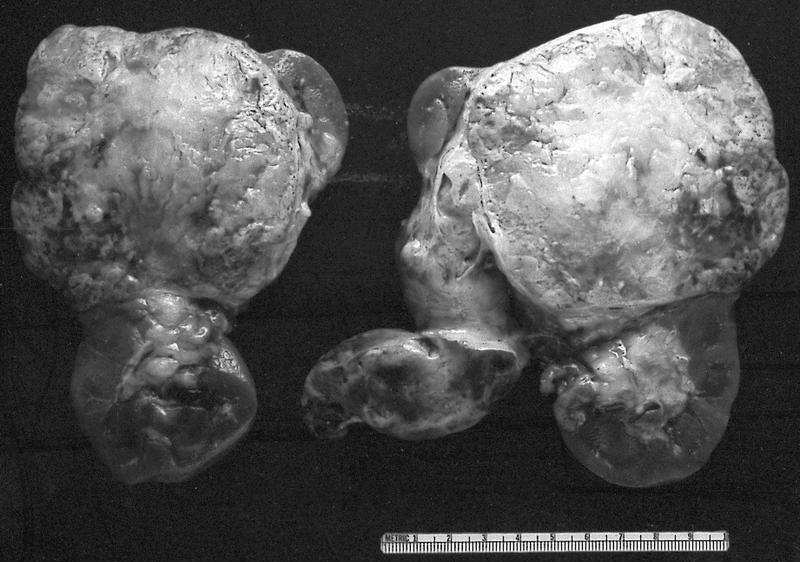
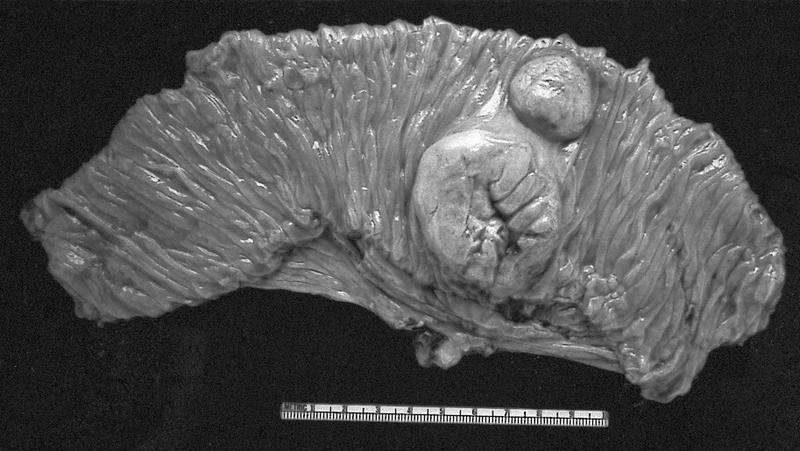
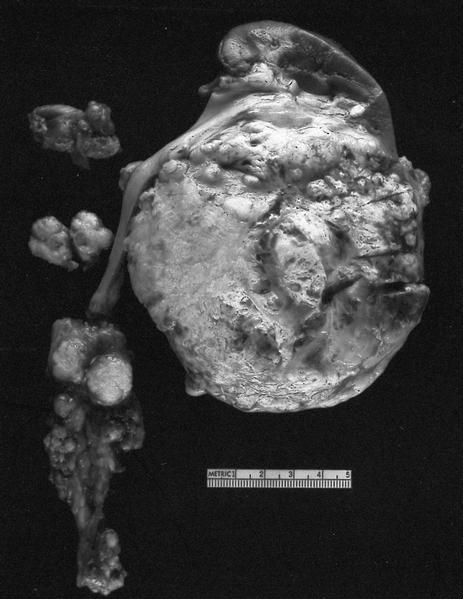
Gross description
- Depends on subtype, with classical golden yellow appearance in clear cell RCC, mahogany brown appearance with central scar in oncocytoma and variants of chromophobe RCC or brown hemorrhagic and granular appearance in papillary RCC
- Usually well circumscribed, centered in cortex
- May bulge / distort kidney contour without invading perirenal fat
- Cut surface often shows hemorrhage, necrosis, calcification, cystic change
- Often extends into the renal vein or vena cava
- Renal sinus invasion is common in large tumors
- May have satellite nodules
- Reference: Cureus 2022;14:e32338
Gross images
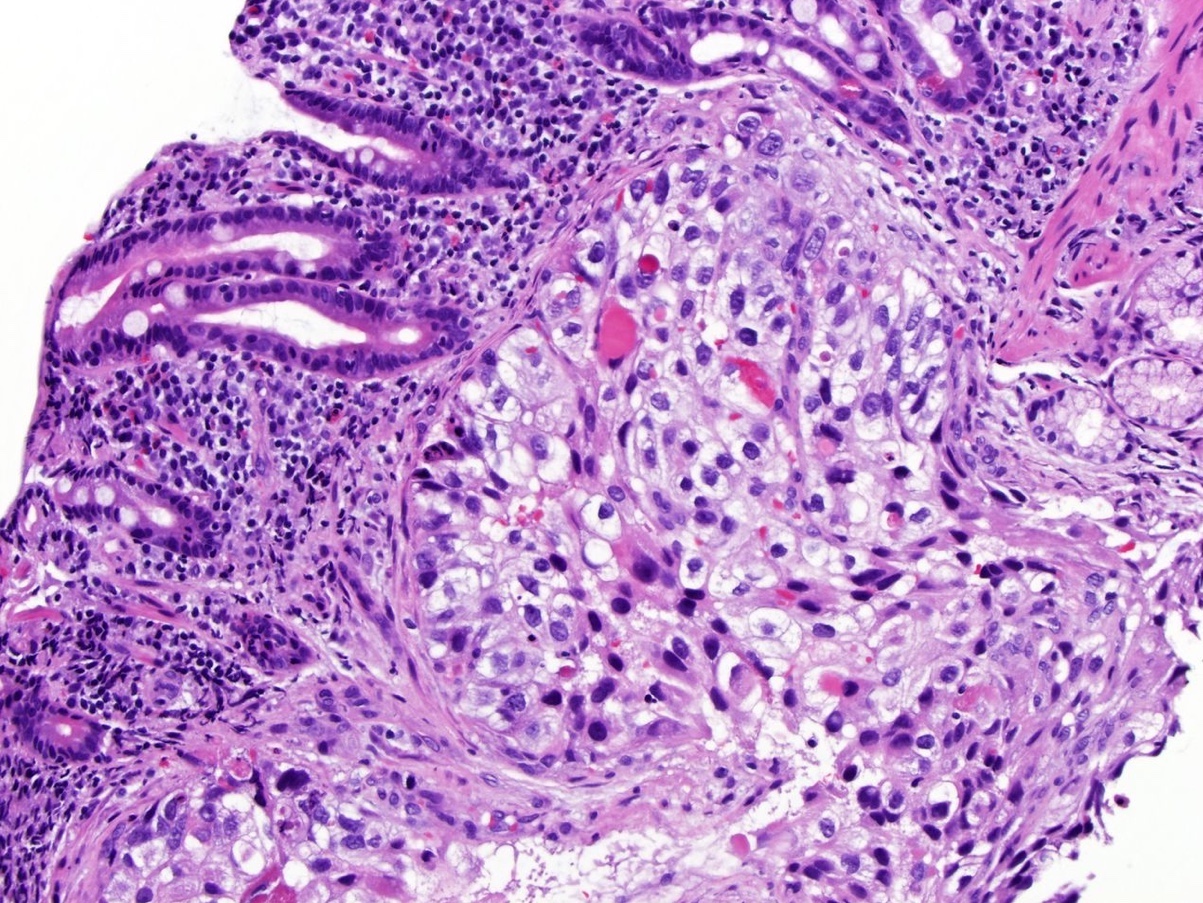
Microscopic (histologic) description
- Depends on Kidney tumor subtype
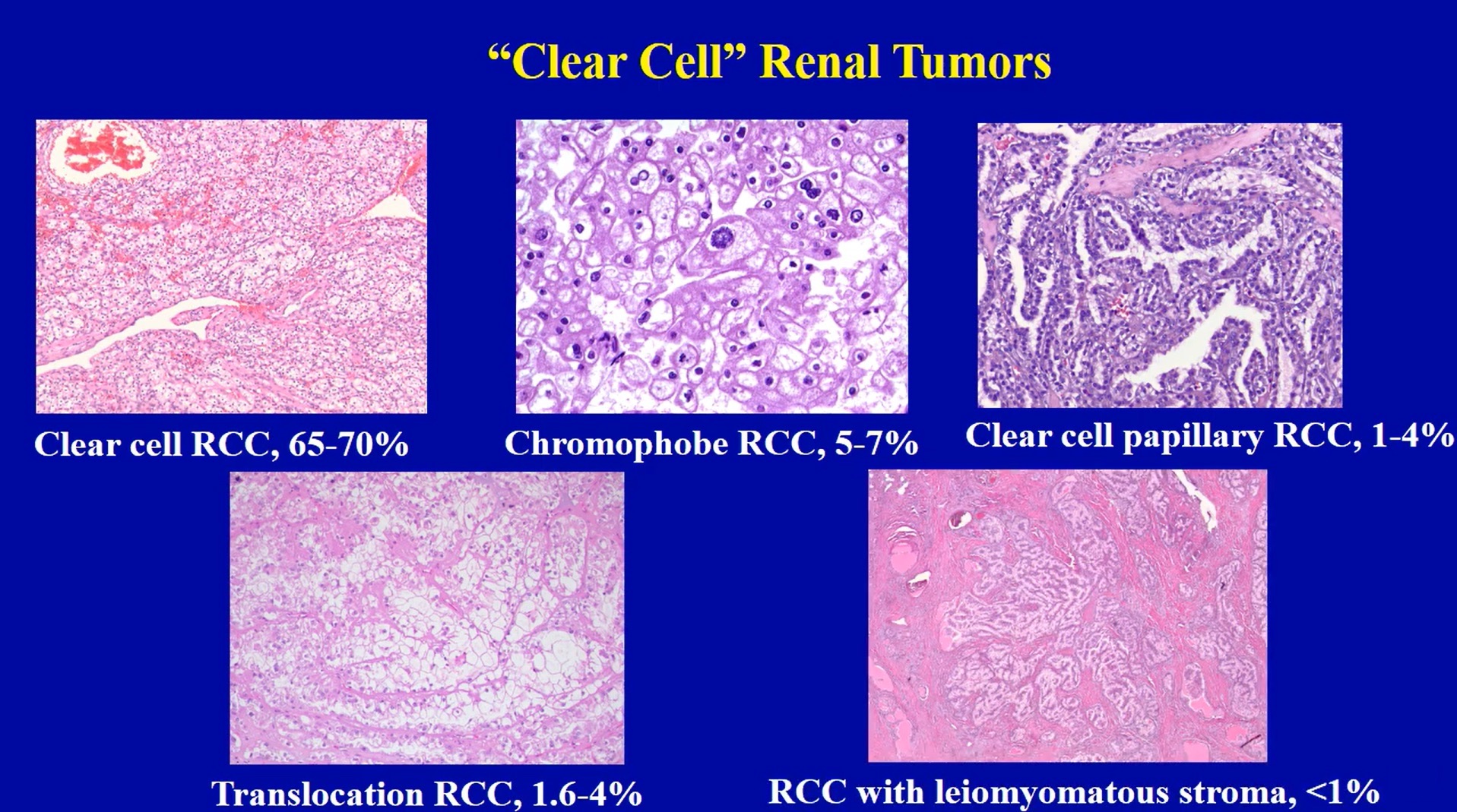
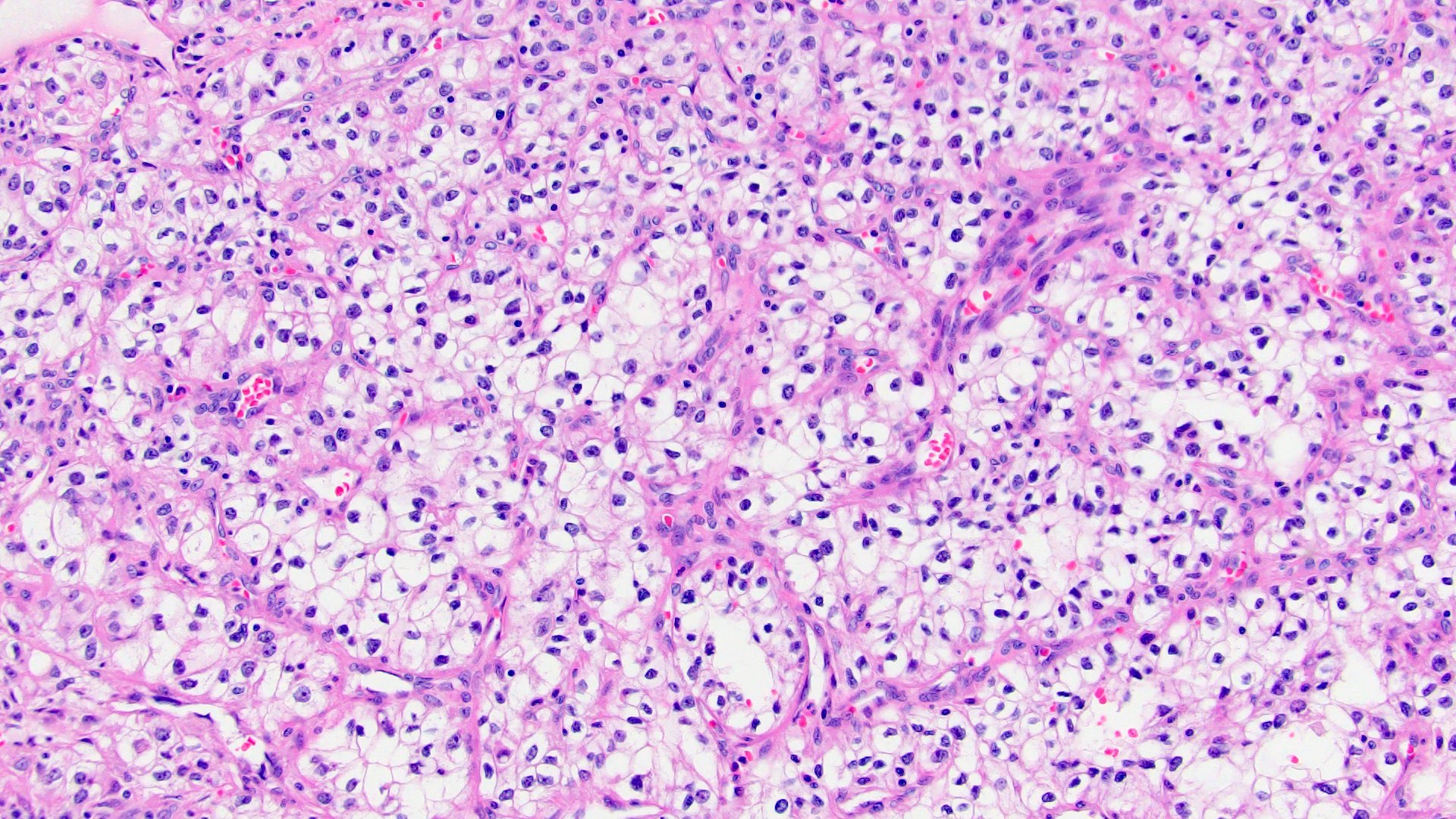
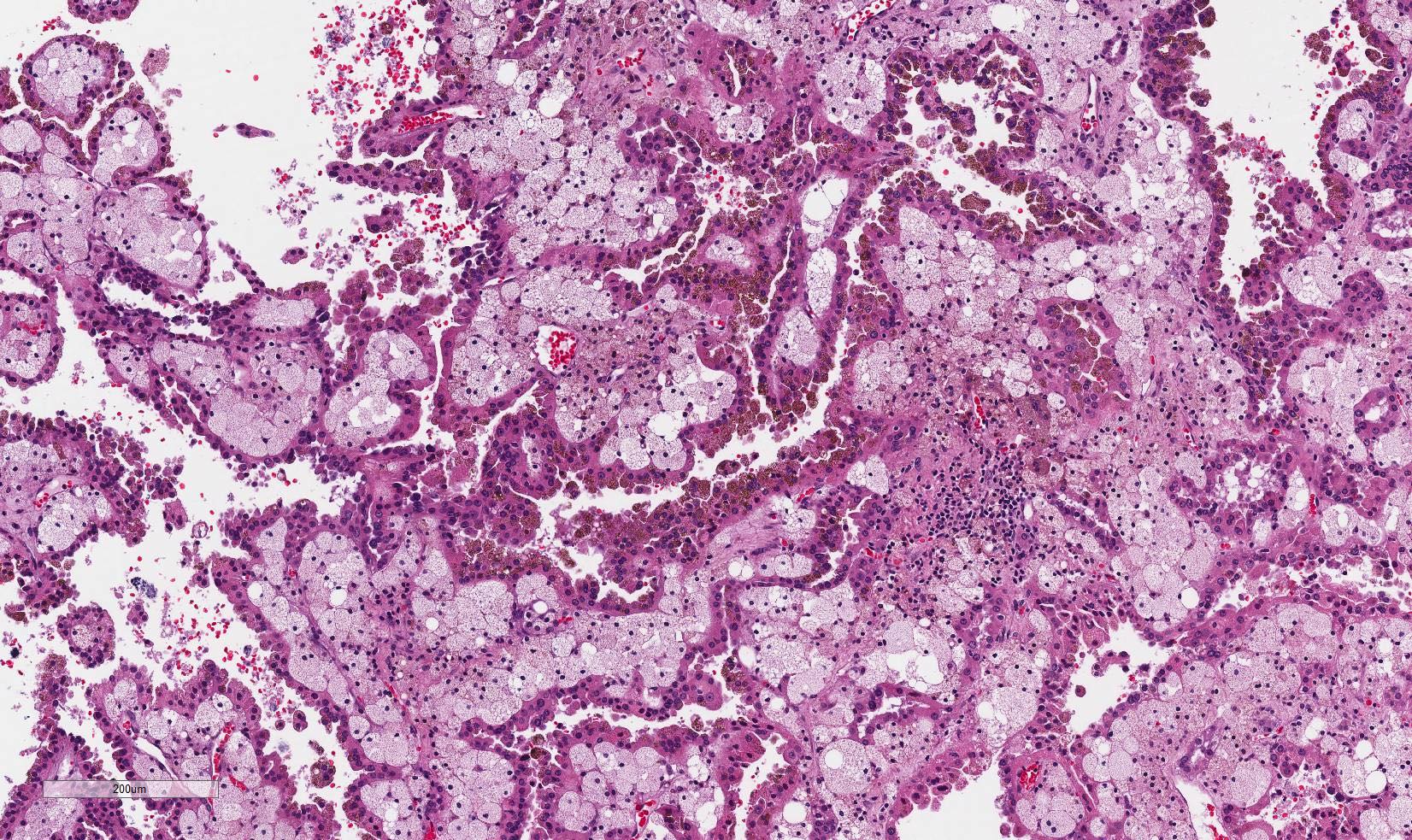
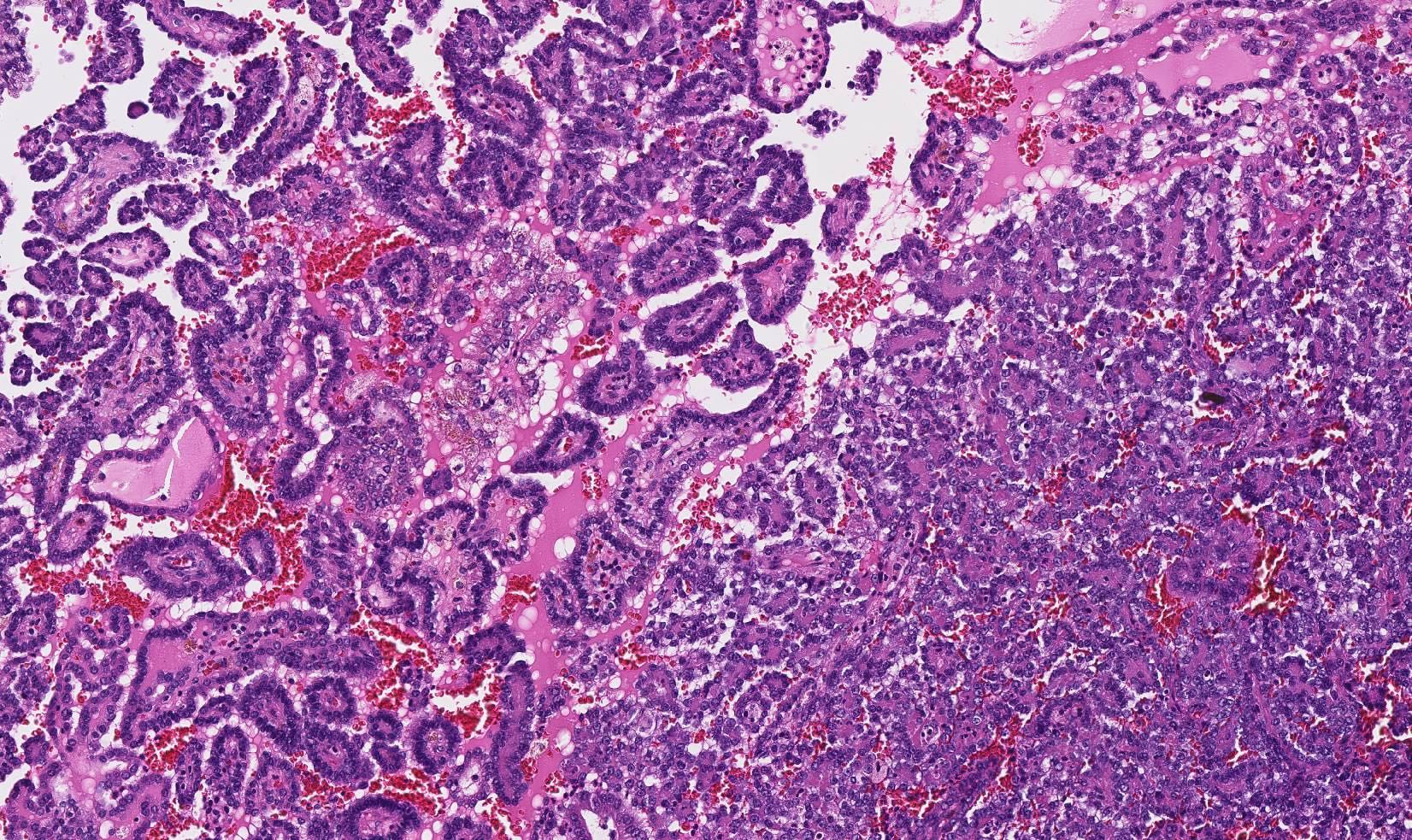
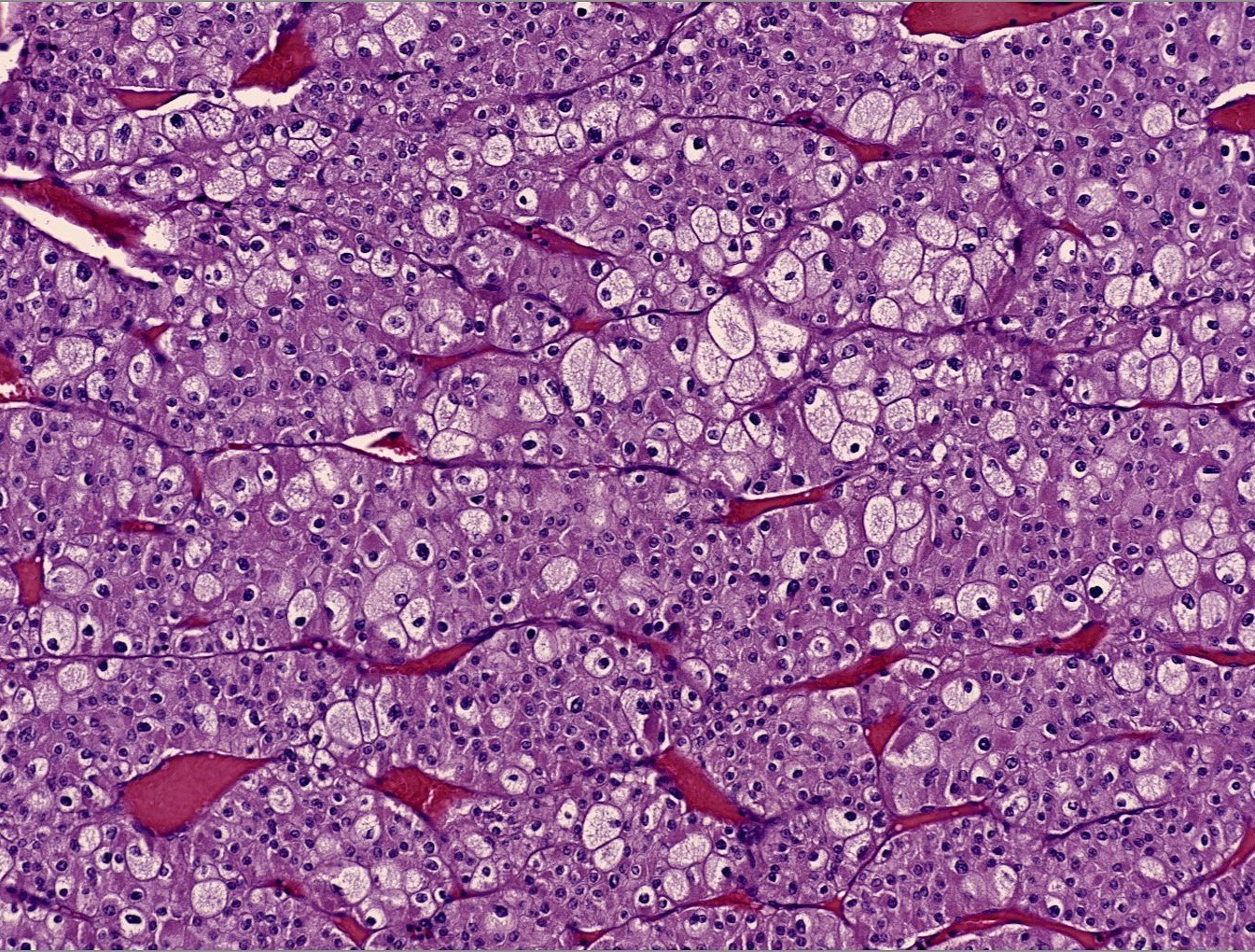
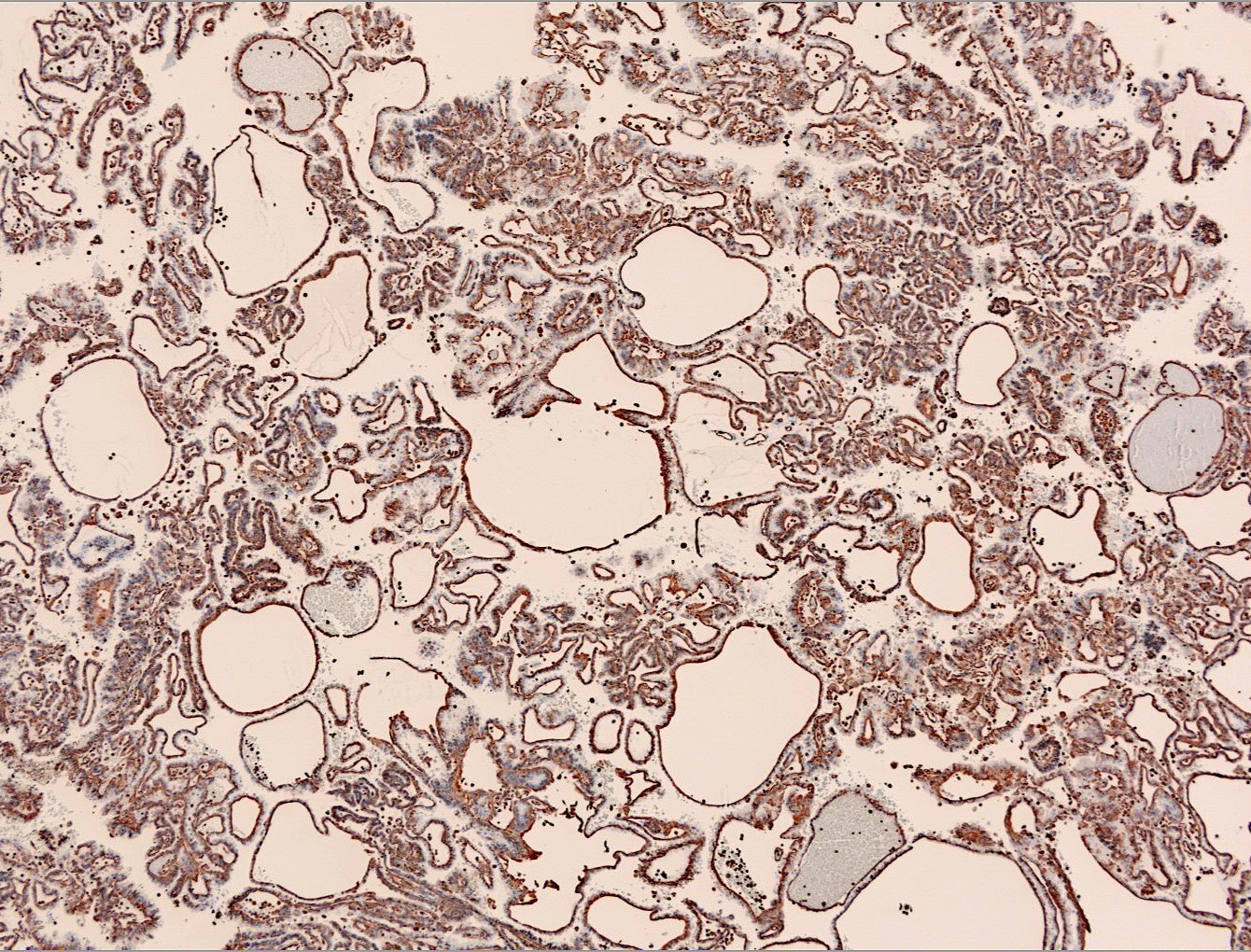
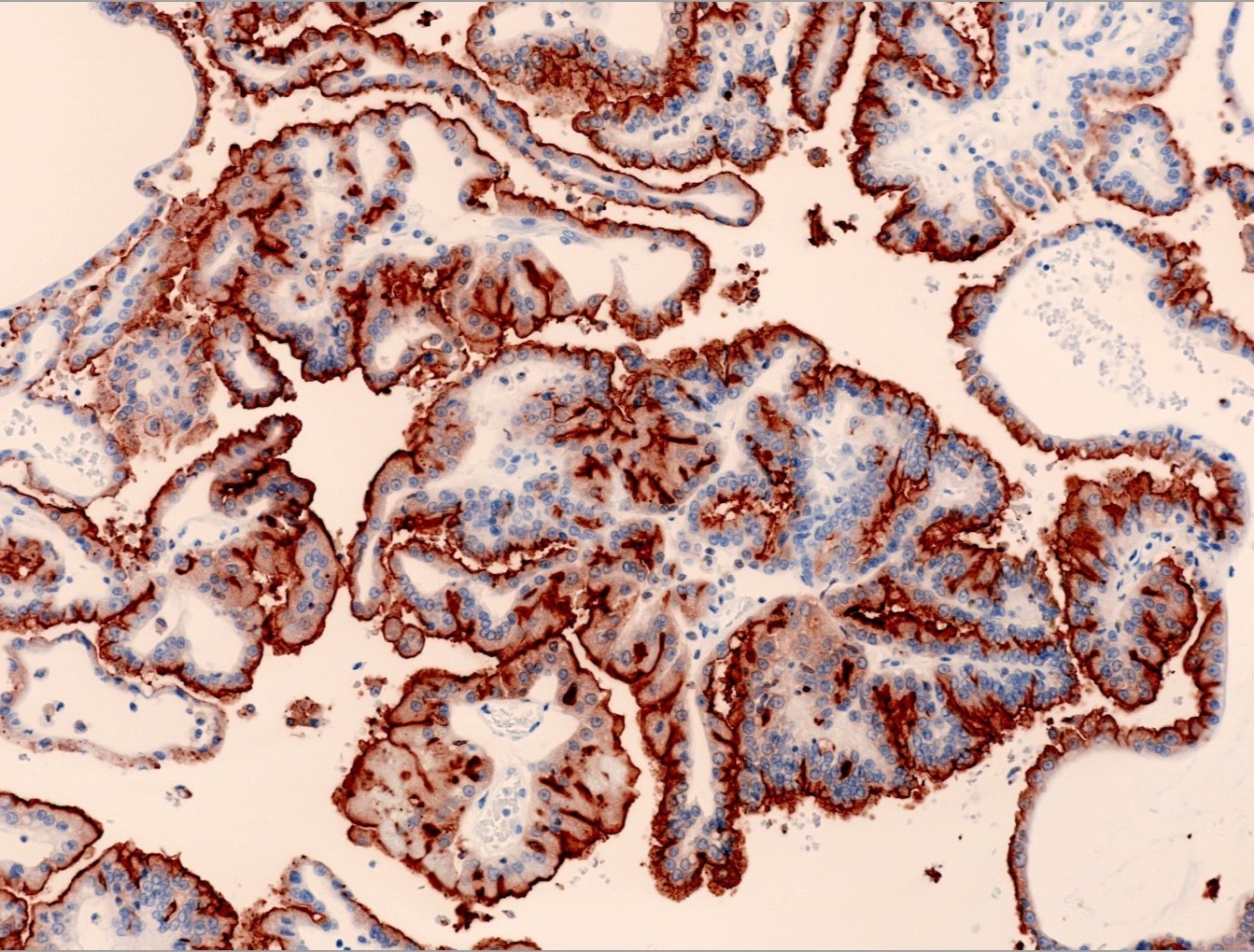
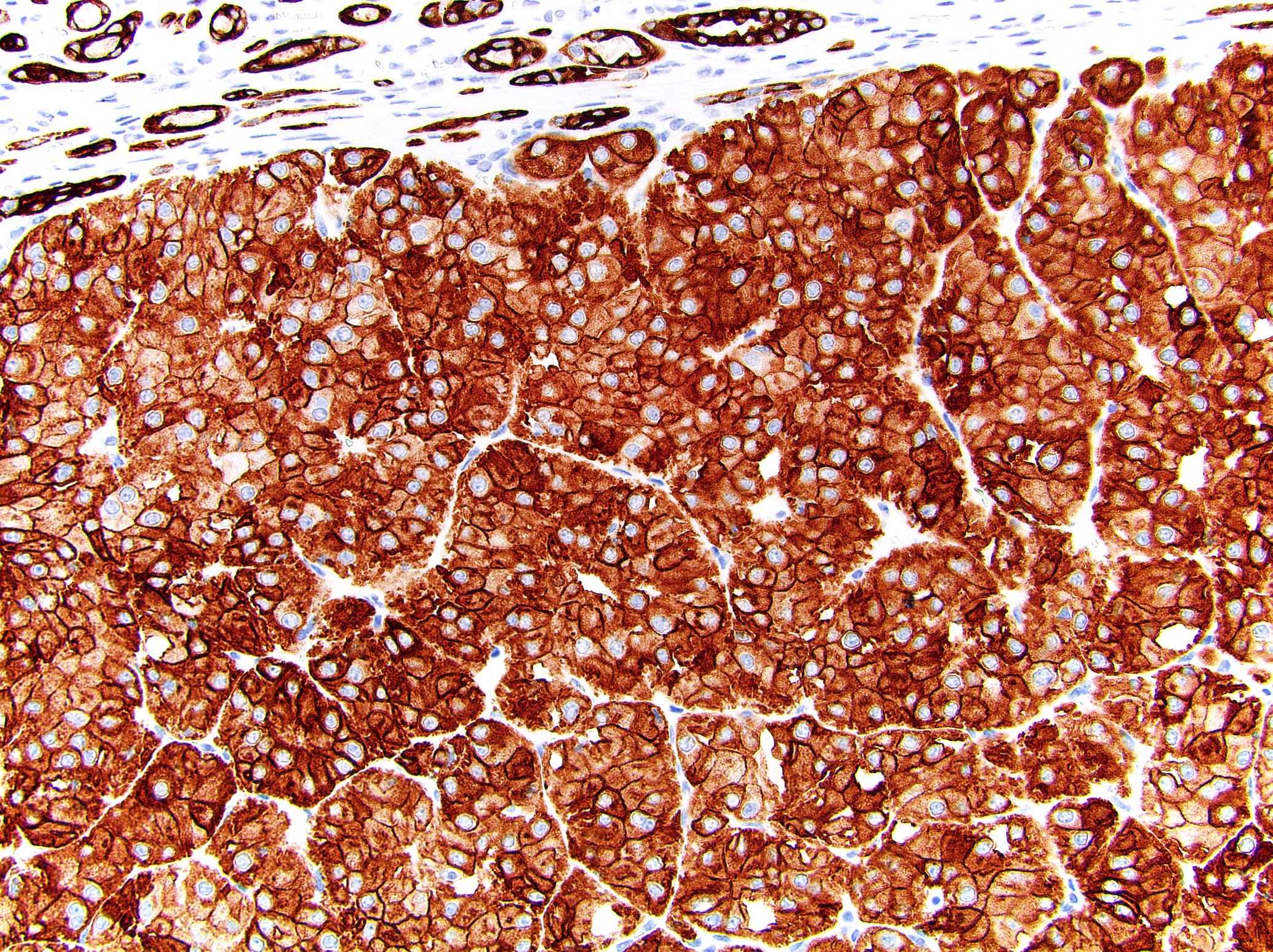
Microscopic (histologic) images
Contributed by Maria Tretiakova, M.D., Ph.D., Miruna Claudia Popescu, M.D. and Katrina Collins, M.D.
Cytology description
- Tumor cells have abundant cytoplasm that is vacuolated, fluffy or granular, usually with indistinct cell borders (chromophobe RCC has distinct borders)
- Tumor nuclei have variable atypia, irregular contours, haphazard orientation with abnormal chromatin, variably prominent nucleoli
- Renal tubular cells have well defined cell borders, homogeneous cytoplasm, round, regular and orderly nuclei
- Important features to distinguish from other neoplasms include heterogeneous cell population, small cytoplasmic vacuoles and hemosiderin deposits
- References: Cancer Cytopathol 2024;132:186, J Am Soc Cytopathol 2023;12:S18
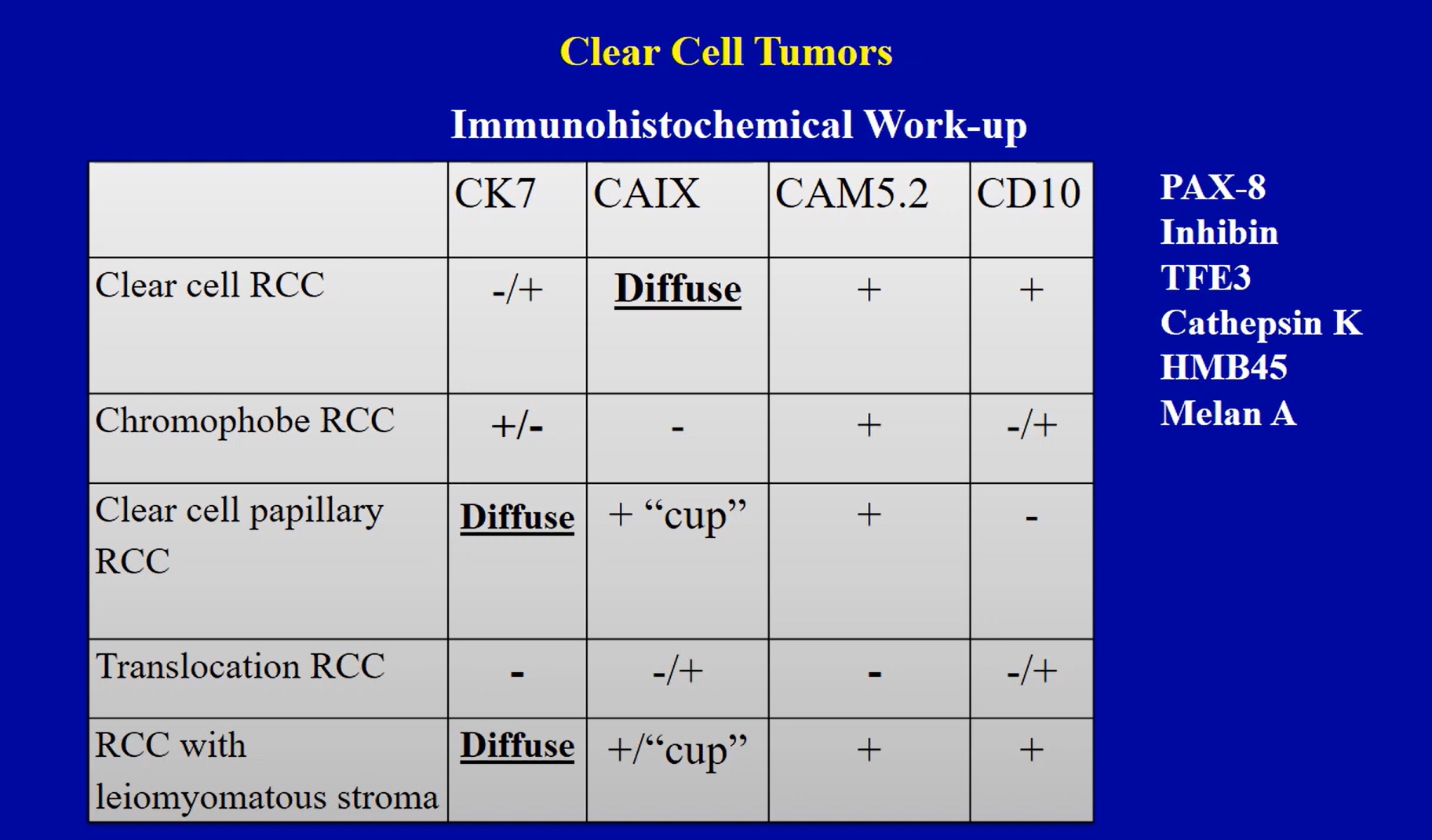
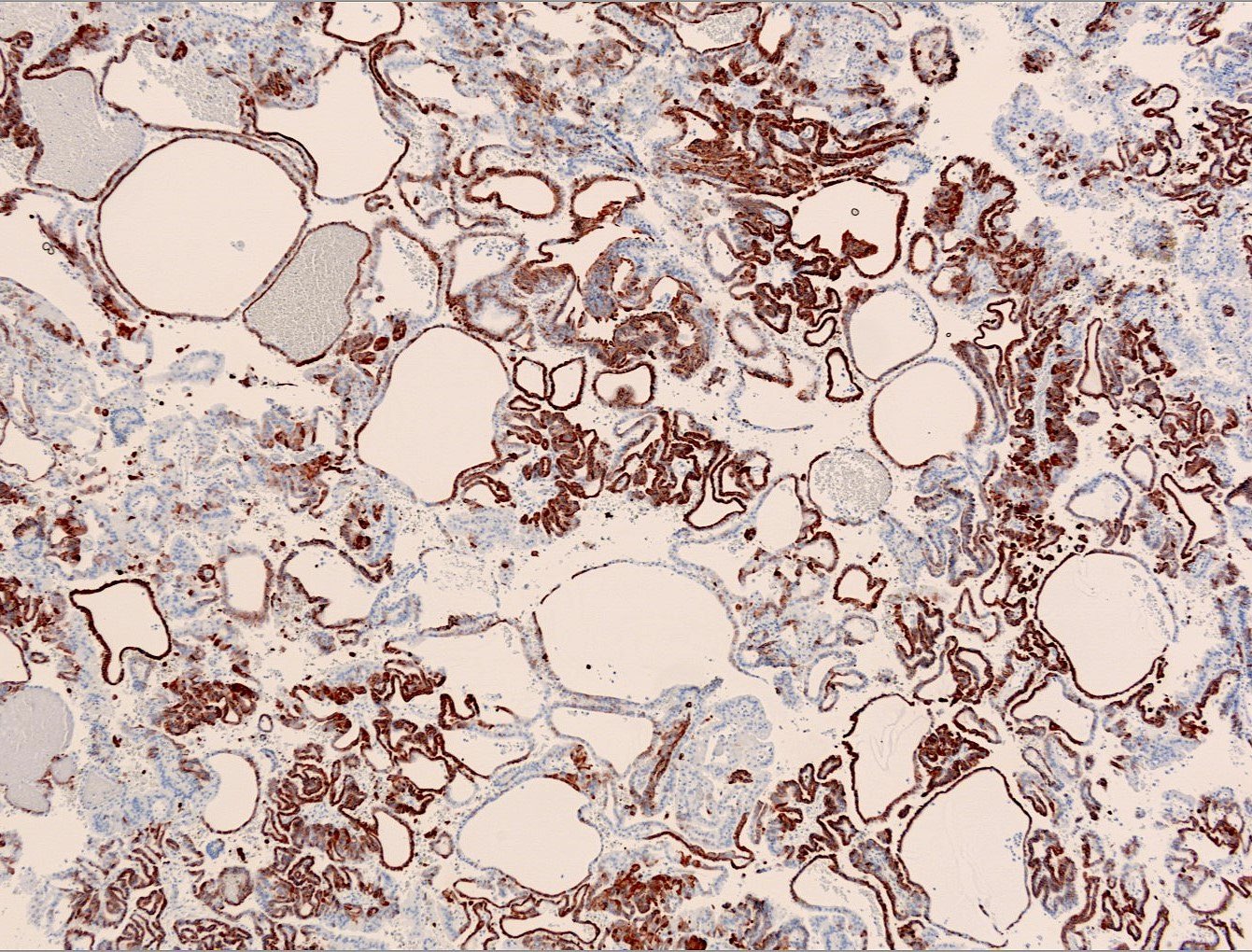
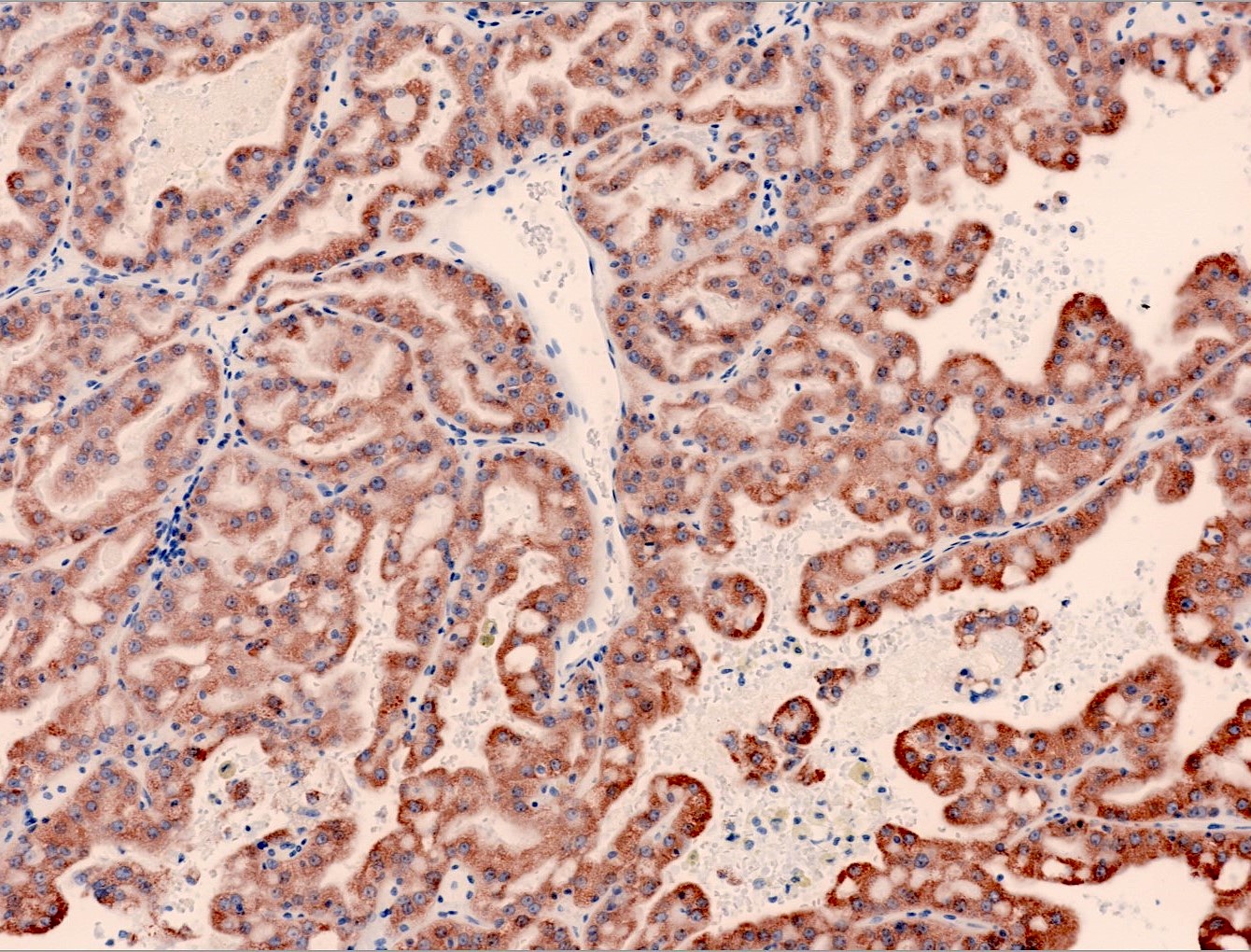
Positive stains
- PAX8 and PAX2: useful to prove renal cell origin
- CAIX
- Circumferential membranous (box shaped) in 75 - 100% of clear cell RCC; useful to support the diagnosis of clear cell RCC
- Also expressed in clear cell papillary renal cell tumor (basolateral, cup shaped) and RCC with fibromyomatous stroma (box or cup shaped)
- Focally expressed in areas of ischemia in non-clear cell RCC (potential pitfall)
- Generally, CD10 (proximal tubular marker), RCC, vimentin and epithelial markers including AE1 / AE3, CAM 5.2, EMA
- Cathepsin K (60% of TFE3 rearranged RCC)
- MelanA and HMB45 (50% of TFEB rearranged RCC)
- KIT (marker of intercalated cells), positive in chromophobe RCC and oncocytoma
- Reference: Semin Diagn Pathol 2022;39:1
Negative stains
- Generally, CK20 (except ESC RCC)
- Inhibin (except rare cases of hemangioblastoma-like clear cell RCC)
- Calretinin, SF1, TTF1, CEA, p63
- Vimentin is consistently negative in almost all oncocytic tumors (pitfall: focal expression in scar areas)
- Reference: Cancers (Basel) 2020;12:602
IHC panels
| CAIX | KIT | CK7 | AMACR | Vimentin | CD10 | HMWCK / GATA3 | FH | SDHB | Other | |
| CCRCC | + (D) (box) | - | - (↑ in cystic areas) | -/+ (P) | + | + | - | + | + | |
| CCPRCT | + (D) (cup) | - | + (D) | - | - | - | + | + | + | |
| RCC FMS | + (D) (box / cup) | - | + (P - D) | -/+ (F) | + | + | - | + | + | MelanA-, HMB45-, cathepsin K- |
| PRCC | - | - | + (D), ↓ when eosinophilic | + (D) | + (D) | + | -, GATA3+ in PRNRP | + | + | |
| MiTF family tRCC | - | - | -, ↓ CK, EMA expression than other RCC types (30 - 50% +) | +/- | -/+ | + | - | + | + | TFE3+ (nuclear), TFEB+ (nuclear and cytoplasmic), cathepsin K+ (60%), MelanA +/-, HMB45 +/- (50% in TFEB), GPNMB+ |
| ChRCC | - | + (D) | + (↓ in eosinophilic variant) | - | - (except scar and sarcomatoid areas) | - | - | + | + | Hale+ |
| Oncocytoma | - | + (D) | - (except rare cells / central scar) | - | - | - | - | + | + | Hale- (or apical bar) |
| LOT | - | - | + (D) | - | - | -/+ (F) | GATA3+ (D) | + | + | Hale- (or apical bar) |
| EVT | - | + (P - D) | -/+ (F) | - | - | + | - | + | + | Cathepsin K +, MelanA-, HMB45- |
| ESC RCC | - | - | -/+ (F) | + | + | -/+ | - | + | + | CK20+ (F - D), CK20 > CK7, cathepsin K+, MelanA variable |
| FH deficient RCC | - | - | - | - | + | - | + | Loss | + | 2SC+ (D) |
| SDH deficient RCC | - | - | - | - | - | - | - | + | Loss | Hale- |
| ALK rearranged RCC | - | - | +/- | +/- | + | -/+ | - | + | + | ALK+, INI1 retained, MelanA-, HMB45-, TFE3+ (10%) |
| SMARCB1 deficient RCC | - | - | + | N/A | N/A | - | + | + | + | INI1 loss, OCT3/4 + (50%), variable p53 |
- F = focal (≤ 25%); P = patchy (25 - 75%); D = diffuse (≥ 75%); +/- = usually positive but can also be negative; -/+ = usually negative but can also be positive
- CCRCC = clear cell renal cell carcinoma; CCPRCT = clear cell papillary renal cell tumor; RCC FMS = renal cell carcinoma with fibromyomatous stroma (to include ELOC mutated RCC); PRCC = papillary renal cell carcinoma; PRNRP = papillary renal neoplasm with reverse polarity; MiTF tRCC = microphthalmia associated transcription factors family translocation associated renal cell carcinoma; ChRCC = chromophobe renal cell carcinoma; LOT = low grade oncocytic tumor; EVT = eosinophilic vacuolated tumor; FH = fumarate hydratase; SDH = succinate dehydrogenase
- References: J Pathol 2022;257:158, Int J Surg Pathol 2023;31:509, Ann Diagn Pathol 2020;44:151448, Arch Pathol Lab Med 2019;143:1455, Transl Androl Urol 2019;8:S123, Hum Pathol 2020;104:18
Electron microscopy description
- Depends on Kidney tumor subtype
Molecular / cytogenetics description
- Various molecular findings depending on Kidney tumor subtype
- Diagnosis of some RCC subtypes requires molecular confirmation
- Current WHO 2022 has introduced a designated chapter on molecularly defined RCC (see What's New in Kidney Tumor Pathology)
Videos
Pathology resident led live unknown slide session: pink renal tumors (part 1)
A dummies guide to diagnosis of renal tumors using pattern based approach by Dr. Rajal B. Shah
A dummies guide to diagnosis of clear renal tumors by Dr. Rajal B. Shah
Differential diagnosis
- Differential diagnosis with primary renal tumors of nonrenal cell origin
- Epithelioid angiomyolipoma (EAML) / epithelioid PEComa of the kidney:
- May resemble high grade clear cell RCC or other eosinophilic renal tumors
- Frequently discohesive or sheet-like growth, lacking characteristic nested growth pattern and chicken wire capillary network of clear cell RCC
- May contain areas of classical AML with smooth muscle, fat and dysmorphic blood vessels
- Negative for cytokeratins, EMA, PAX8 and CAIX
- Positive for MelanA, HMB45, cathepsin K, SMA
- Subset has TFE3 rearrangements
- Upper urinary tract urothelial carcinoma:
- Hemangioblastoma:
- Closely resembles clear cell RCC
- More common in the brain, where it must be differentiated from metastatic clear cell RCC
- As a renal primary, it is overwhelmingly rarer than clear cell RCC but may pose significant diagnostic difficulty in this location because of overlapping features (Biomedicines 2023;11:1467)
- Usually solid, diffuse growth, less well formed epithelial formations
- Flocculent or vacuolated cell cytoplasm
- Focal to negative staining for cytokeratins and EMA
- Positive for inhibin A, S100, NSE
- In the kidney, PAX8 alone should not be used to differentiate hemangioblastoma from clear cell RCC, as PAX8 expression has been reported in the former (Int J Clin Exp Pathol 2015;8:2131, Am J Surg Pathol 2014;38:119, Clin Case Rep 2019;7:2321)
- Rare clear cell RCCs have hemangioblastoma-like morphology with inhibin expression (Asian J Surg 2024;47:1859, Int J Surg Pathol 2019;27:631)
- Renal anastomosing hemangioma:
- May mimic RCC with regressive changes where there is tumor cell dropout with retained prominent vascularity
- Anastomosing vascular spaces with fibrin thrombi
- Usually contains extramedullary hematopoiesis or hyaline globules
- Negative for cytokeratins, EMA, PAX8, CAIX, CD10
- Metanephric adenoma:
- Epithelioid angiomyolipoma (EAML) / epithelioid PEComa of the kidney:
- Differential diagnosis with renal nonneoplastic lesions
- Malakoplakia:
- Solid sheets of eosinophilic histiocytes
- Rare spindle cell morphology may mimic sarcomatoid RCC
- Von Kossa, Prussian blue, PAS stain highlights characteristic Michaelis-Gutmann bodies
- Negative for cytokeratins, EMA and PAX8
- Positive for histiocytic markers CD68, CD163
- Xanthogranulomatous pyelonephritis:
- Histiocytic granulomatous inflammation with admixed lymphocytes, plasma cells and neutrophils in a diffuse distribution
- May mimic clear cell RCC especially if foamy histiocytes predominate
- Spindle cell morphology may mimic sarcomatoid RCC
- Cholesterol clefts and histiocytic giant cells may be seen
- Negative for cytokeratins, EMA and PAX8
- Positive for histiocytic markers CD68, CD163
- Malakoplakia:
- Differential diagnosis with renal metastatic tumors
- Metastatic tumors to the kidney (< 1%) are mostly carcinomas (> 80%), lung being the most common
- 10% of renal masses included in one study were of metastatic origin (Diagn Cytopathol 1999;21:35)
- In > 90% of cases the primary tumor is already known and rarely diagnosed by evaluating the renal mass
- Adrenal cortical carcinoma:
- Foamy cell cytoplasm
- Negative for EMA, CEA, PAX8, CAIX, cathepsin K, mostly negative AE1 / AE3
- Positive for SF1, calretinin, inhibin A, MelanA, neurofilament, synaptophysin, vimentin
- Metastatic tumors to the kidney (< 1%) are mostly carcinomas (> 80%), lung being the most common
Additional references
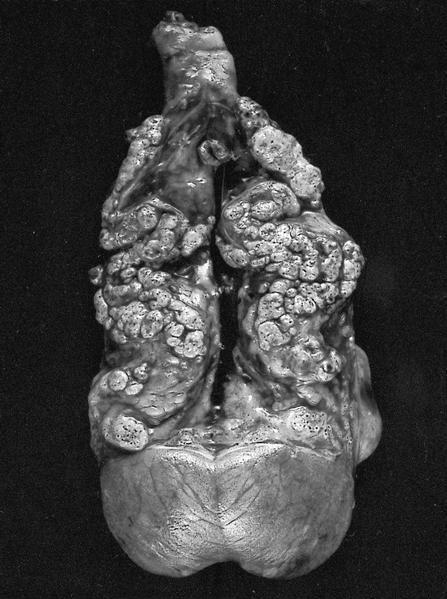
Board review style question #1
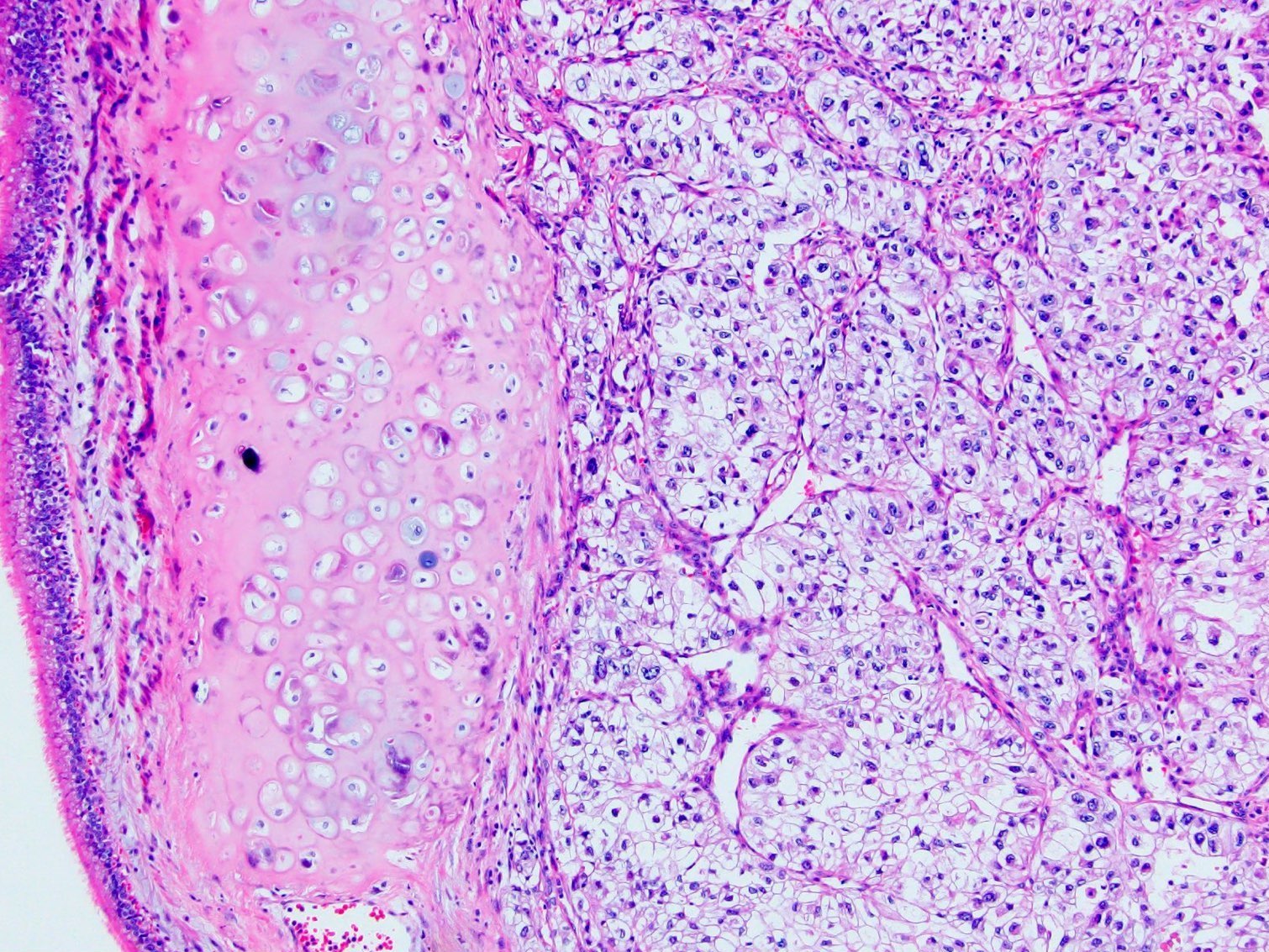
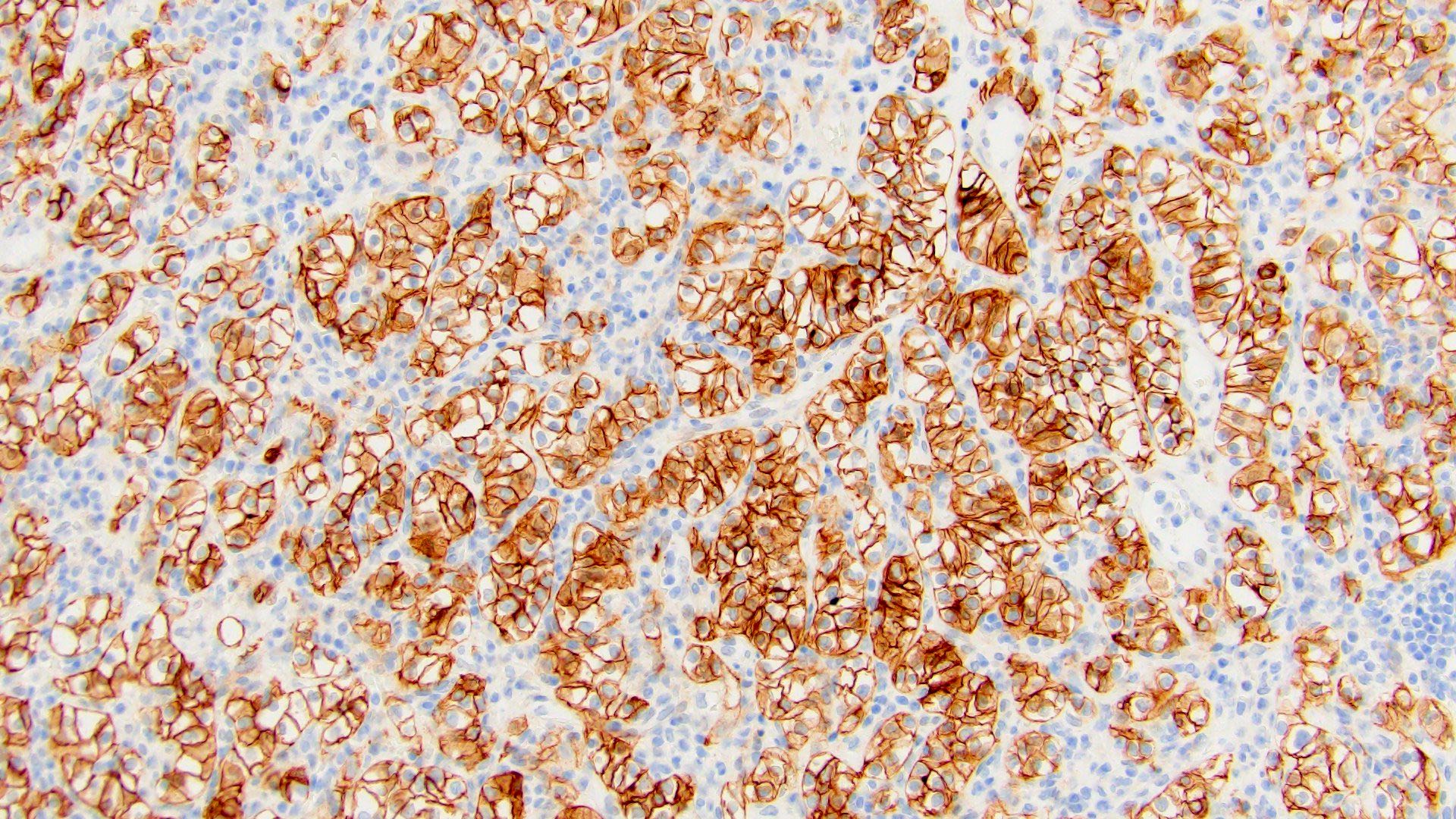
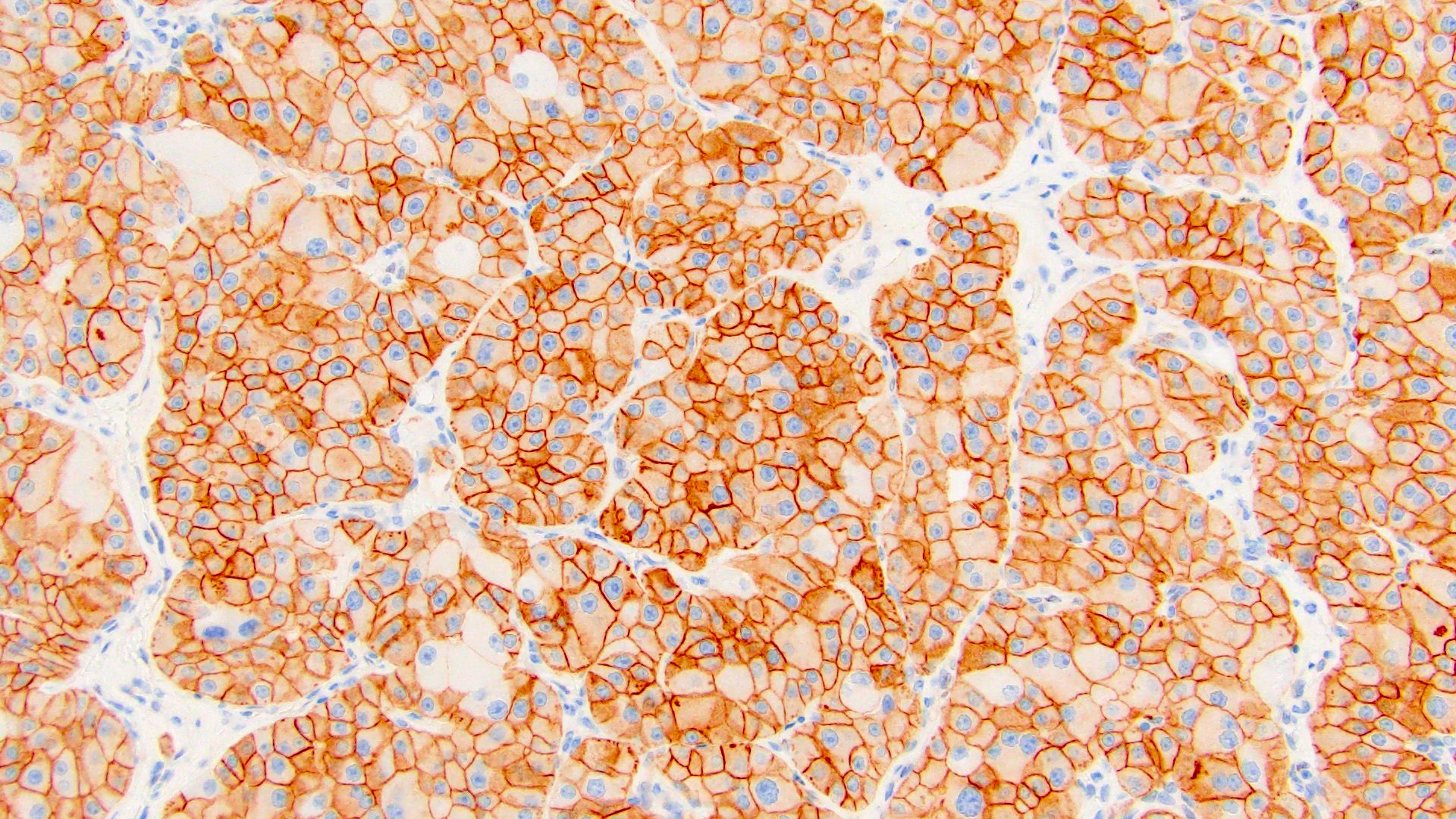
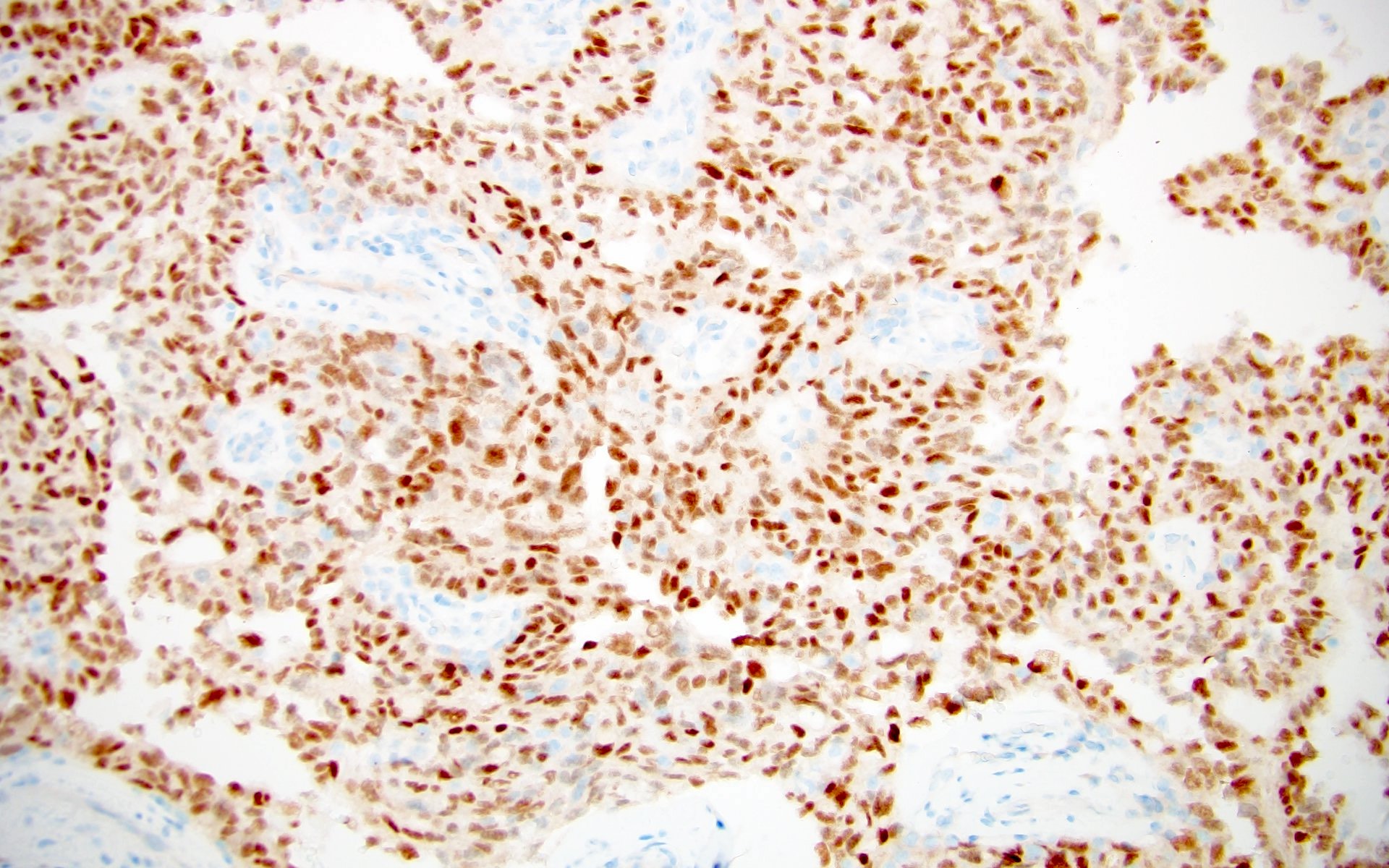
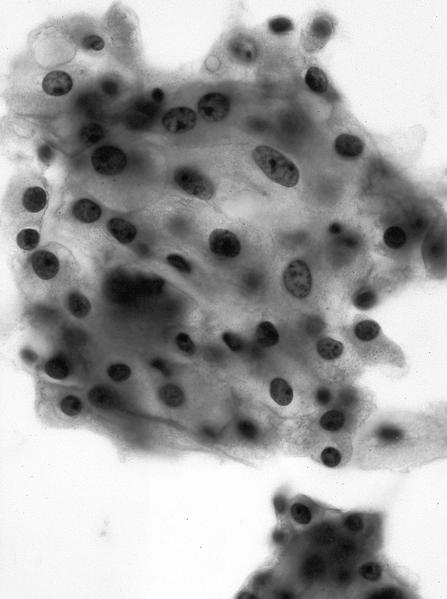
The above image is from a renal tumor. Which of the following statements is true about the immunohistochemical profile of the tumor type shown?
- Consistently negative for CD117
- Diffusely positive for CD117 and CK7 and consistently negative for vimentin
- Diffusely positive for GATA3
- Positive for CAIX, vimentin and CD10
- Positive for CK20 more than CK7
Board review style answer #1
B. Diffusely positive for CD117 and CK7 and consistently negative for vimentin. The image above depicts a classic chromophobe renal cell carcinoma. Chromophobe renal cell carcinomas are oncocytic renal neoplasms that typically show diffuse positivity for CD117 and CK7. Answer A is incorrect because CD117 in this case is diffusely positive not consistently negative. Answer E is incorrect because the pattern of CK20 > CK7 expression refers to eosinophilic solid and cystic renal cell carcinoma. The eosinophilic variant of chromophobe renal cell carcinoma may show reduced expression of CK7. Answer D is incorrect because almost all oncocytic renal neoplasms are negative for vimentin, although a known pitfall is increased vimentin expression in the central scar area of oncocytoma and chromophobe renal cell carcinoma. Answer C is incorrect because GATA3 is consistently positive in low grade oncocytic tumors whereas chromophobe RCC is GATA3 negative (Int J Surg Pathol 2024;32:83, Pathol Oncol Res 2023;29:1610852, Asian J Urol 2022;9:1).
Comment Here
Reference: Renal cell carcinoma overview
Comment Here
Reference: Renal cell carcinoma overview
Board review style question #2
Which of the following statements is true about von Hippel-Lindau syndrome?
- Autosomal dominant syndrome, due to germline mutation of VHL tumor suppressor gene on chromosome 3p25
- Autosomal dominant syndrome, due to heterozygous germline mutations in BAP1 tumor suppressor gene encoding BRCA associated protein 1
- Autosomal dominant syndrome, due to pathogenic germline mutations of FH gene on chromosome 1q42 encoding fumarate hydratase (enzyme of Krebs cycle)
- Autosomal dominant syndrome with incomplete penetrance, due to germline mutations in FLCN (BHD) gene on chromosome 17p12, which codes for folliculin protein
- Autosomal dominant syndrome with variable penetrance, due to mutations in TSC1 on chromosome 9q or TSC2 on chromosome 16p
Board review style answer #2
A. Autosomal dominant syndrome, due to germline mutation of VHL tumor suppressor gene on chromosome 3p25. Associated renal lesions in VHL syndrome are clear cell renal cell carcinoma, benign or atypical renal cysts and numerous microscopic nodules of clear cells. RCC in VHL syndrome can be multifocal or bilateral in 50% of cases and may show branching tubulopapillary growth and apical polarization of nuclei resembling clear cell papillary renal cell tumor but shows characteristic molecular and immunohistochemical profile of clear cell RCC. Other associated lesions are hemangioblastomas of cerebellum and retina, pancreatic or liver cysts, clear cell tumors of other sites, papillary cystadenoma of epididymis, pheochromocytoma. Answer C is incorrect because it refers to hereditary leiomyomatosis and renal cell carcinoma syndrome (HLRCC). Answer D is incorrect because it refers to Birt-Hogg-Dubé syndrome. Answer E is incorrect because it refers to tuberous sclerosis complex. Answer B is incorrect because it refers to BAP1 tumor predisposition syndrome (eMedicine: Von Hippel-Lindau Syndrome Imaging [Accessed 15 November 2024], Semin Diagn Pathol 2024;41:20).
Comment Here
Reference: Renal cell carcinoma overview
Comment Here
Reference: Renal cell carcinoma overview